A water-soluble cationic [2]biphenyl-extended pillar[6]arene: synthesis, host–guest interaction with hemin and application in chemodynamic/photodynamic cancer therapy†
Yan
Cai‡
,
Yue
Zhang‡
,
Xufeng
Liang
,
Chunlin
Deng
,
Jianxia
Zhang
,
Haotian
Wang
,
Hui
Duan
and
Yong
Yao
 *
*
School of Chemistry and Chemical Engineering, Nantong University, Nantong, Jiangsu 226019, P. R. China. E-mail: yaoyong1986@ntu.edu.cn
First published on 10th March 2025
Abstract
A water-soluble cationic [2]biphenyl-extended pillar[6]arene (CBpExP6) was designed and synthesized successfully. It could form a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with hemin, thereby enhancing the stability of hemin in water, and can be further applied in cancer CDT and PDT.
1 complex with hemin, thereby enhancing the stability of hemin in water, and can be further applied in cancer CDT and PDT.
Supramolecular macrocycles possess the unique ability to selectively bind guest molecules through non-covalent interactions (including hydrogen bonding, π–π stacking, electrostatic interactions, van der Waals forces, etc.).1–4 This highly specific selective recognition mechanism is just like a precisely matched key (i.e., the guest molecule) opening the corresponding lock (that is, the macrocyclic host compound), thus laying a solid foundation for the construction of intricate and complex supramolecular systems.5 Taking advantage of molecular recognition ability, many important applications such as the precise separation and sensitive detection of specific molecules can be achieved.6 In the context of biological systems, the recognition process of enzymes for substrates is also essentially a form of molecular recognition. Therefore, the molecular recognition characteristics exhibited by host macrocycles help us to gain a more in-depth and thorough understanding of the intricate interaction principles among biological molecules.7–12
Traditionally macrocycles, such as crown ethers,13 cyclodextrins,14,15 calixarenes,16,17 cucurbiturils,18,19 and pillar[n]arenes,20–24 although they possess molecular recognition abilities to a certain extent, have relatively obvious limitations in terms of selectivity and recognition range.25 With the rapid development and continuous deepening of supramolecular chemistry in cutting-edge fields such as materials science and nanotechnology, there is an urgent need to construct more intricate and diverse supramolecular structures.26–28 Currently, the structures formed by the self-assembly of existing macrocycles can hardly fully meet the requirements under specific situations and application needs.29,30
Therefore, it is particularly crucial to design and synthesize new macrocycles. By skilfully introducing new recognition sites and various functional groups into the macrocycles, the breadth and depth of their molecular recognition can be effectively expanded.31–34 By precisely introducing nitrogen-containing heterocycles with specific coordination abilities onto the macrocyclic host compounds, the recognition efficiency for transition metal ions can be significantly enhanced.35 For example, Prof. Yang designed and prepared an [2]biphenyl-extended pillar[6]arene with larger cavity, which can associate with more guest molecules.36–40 Meanwhile, new macrocycles can also achieve the precise recognition of a wider range of guest molecules (including various organic molecules, biological molecules, etc.) by flexibly adjusting and optimizing key factors such as the size, shape, and flexibility of the ring, which undoubtedly has irreplaceable critical significance and far-reaching value for carrying out efficient molecular recognition and separation work in complex systems and will open up new paths and broad prospects for the further expansion and innovative application of supramolecular chemistry.41–45
Hemin is a kind of porphyrin derivative containing two carboxyl groups. It can be directly absorbed by the human body and is the biological iron with the highest absorption rate currently known. Studies have found that hemin decomposes under light irradiation. Herein, we have designed and synthesized a cationic [2]biphenyl-extended pillar[6]arene (CBpExP6), which has a larger cavity than that of pillar[6]arene and contains eight water-soluble quaternary ammonium cations. This enables it to form a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with hemin in water, thereby enhancing the stability of hemin. Further in vitro experiments have demonstrated that after the formation of CBpExP6 ⊃ hemin, both its ability to generate reactive oxygen species (ROS) and kill tumor cells have been significantly improved.
1 complex with hemin in water, thereby enhancing the stability of hemin. Further in vitro experiments have demonstrated that after the formation of CBpExP6 ⊃ hemin, both its ability to generate reactive oxygen species (ROS) and kill tumor cells have been significantly improved.
As described in Scheme 1, CBpExP6 was synthesized from 4,4′-bis(chloromethyl)-1,1′-biphenyl through three steps with a total yield about 70%. As expected, CBpExP6 exhibited excellent water solubility due to it contains 8 quaternary ammonium cations. The structure of CBpExP6 was confirmed through 1H, 13C NMR, and electrospray ionisation mass spectrometry (ESI-MS) characterisations (Fig. S1–S5, ESI†). As CBpExP6 presents ammonium cations on its macrocyclic framework, it can form a complex with hemin which contains anionic group efficiently. Firstly, the host–guest properties of CBpExP6 with model guest (GM: n-octanoic acid) and hemin were investigated in detail by 1H NMR. All protons on GM and hemin shifted upfield after complexation (Fig. S6 and S15, ESI†), suggested that linear guest GM and hemin were threaded through the cavity of CBpExP6 to form a [2]pseudorotaxane.46 The formation of the complex might be mainly driven by hydrophobic and electrostatic interactions, because the hydrophobic cavity of CBpExP6 could hold the hydrophobic alkyl chain of GM and the cationic trimethylammonium groups of CBpExP6 could bind the anionic carboxylate group of GMvia electrostatic interaction. Then, according to the isothermal titration calorimetry (ITC) results, CBpExP6 and hemin form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, and the association constant (Ka) between CBpExP6 and hemin was calculated to be (2.46 ± 0.18) × 104 M−1, indicted CBpExP6 ⊃ hemin was stable in water (Fig. 1). Further evidence for the formation of the desired 1
1 complex, and the association constant (Ka) between CBpExP6 and hemin was calculated to be (2.46 ± 0.18) × 104 M−1, indicted CBpExP6 ⊃ hemin was stable in water (Fig. 1). Further evidence for the formation of the desired 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was obtained by LRESIMS, revealing peaks at m/z 1239.87 and 799.95, corresponding to [CBpExP6 ⊃ hemin − 2H+ − 4Br−]2+ and [CBpExP6 ⊃ hemin − 2H+ − 5Br−]3+, respectively (Fig. S8, ESI†).
1 complex was obtained by LRESIMS, revealing peaks at m/z 1239.87 and 799.95, corresponding to [CBpExP6 ⊃ hemin − 2H+ − 4Br−]2+ and [CBpExP6 ⊃ hemin − 2H+ − 5Br−]3+, respectively (Fig. S8, ESI†).
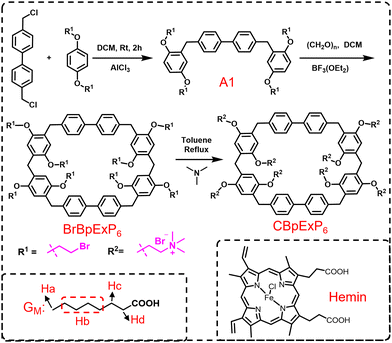 | ||
| Scheme 1 The synthetic route of cationic [2]biphenyl-extended pillar[6]arene (CBpExP6) and chemical structures of model guest and hemin. | ||
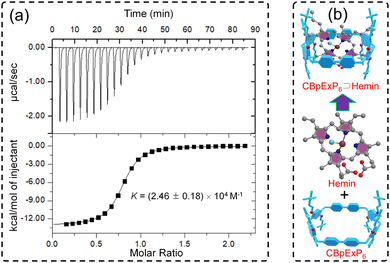 | ||
| Fig. 1 (a) Isothermal titration calorimetry (ITC) studies between CBpExP6 and hemin. (b) Schematic illustration of the formation of CBpExP6 ⊃ hemin. | ||
After confirming the host–guest interaction between CBpExP6 and hemin, we probed the capacity of CBpExP6 to improve the stability of hemin in water under irradiation. From the UV-vis spectrum, we found that under non-illumination conditions, the maximum absorption peak of the aqueous solution of hemin decreased from 2.3 to 1.6 within 60 minutes (Fig. S9, black line, ESI†). However, in the case of CBpExP6 ⊃ hemin under the same conditions, only a slight decrease occurred in the maximum absorption peak (Fig. S9, blue line, ESI†). Even under the irradiation of a 660-nm laser, CBpExP6 ⊃ hemin remained basically unchanged (Fig. S9, red line, ESI†), which demonstrates that CBpExP6 can significantly enhance the stability of hemin in water.
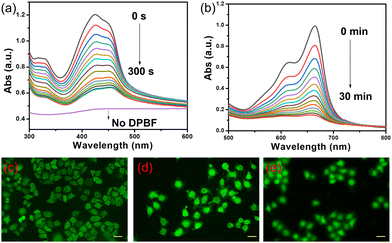
Hemin could generate ROS under 660 nm laser irradiation as it contained the porphyrin core. Firstly, 1,3-diphenyl isobenzofuran (DPBF) was used as a ROS indicator to investigate the ROS generation ability of hemin. Upon irradiated with 660 nm laser, the UV absorbance decreased tremendously within 360s in the presence of CBpExP6 (Fig. 2a), and the degree of decrease is much higher than that in the absence of CBpExP6 (Fig. S14, ESI†), indicating the high efficiency of ROS generation of CBpExP6 ⊃ hemin.47 On the other hand, the structural feature of hemin contains ferrocene units, which can catalyze H2O2 to generate ˙OH via a Fenton-like reaction.48 As shown in Fig. 2b, time-dependent UV-visible spectra for the solution containing CBpExP6 ⊃ hemin, H2O2, and methylene blue (MB) further confirmed the increase in ˙OH generation and hence oxidation of MB. Afterward, the intracellular ROSs generation of CBpExP6 ⊃ hemin in living cells under illumination with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as the probe was investigated by confocal laser scanning microscopy (CLSM). As shown in Fig. 2c, green fluorescence was observed when human cervical carcinoma (HeLa) cells were treated with both CBpExP6 ⊃ hemin and 660 nm laser irradiation, this is because the nonfluorescent DCFH-DA was oxidized into green fluorescence 2′,7′-dichloroflorescein (DCF) by the generated ROSs. Furthermore, the generation of ˙OH, and 1O2 by CBpExP6 ⊃ hemin was confirmed by aminophenyl fluorescein assay (APF), and singlet oxygen sensor green assay (SOSG), respectively (Fig. 2d and e). Electron paramagnetic resonance (EPR) spectra also confirmed the above results (Fig. S12, ESI†).
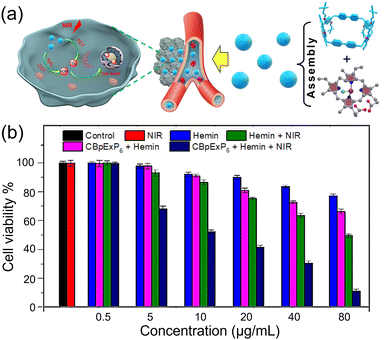
Since CBpExP6 ⊃ hemin can generate ROSs under 660 nm-light irradiation and catalyze overexpressed H2O2 to produce ˙OH in tumor tissue, we then investigated its ability to kill cancer cells upon illumination. Through dynamic light scattering (DLS) and scanning electron microscopy (SEM) studies (Fig. S10, ESI†), it was found that CBpExP6 ⊃ hemin can self-assemble in water to form nanoparticles with diameters around 200 nm. And the zeta potential of CBpExP6 ⊃ hemin was 12.6 ± 0.5 mV (Fig. S13, ESI†). These enabled CBpExP6 ⊃ hemin NPs to be effectively phagocytosed by cancer cells, achieving rapid enrichment of CBpExP6 ⊃ hemin at the tumor site.
Then HeLa cells were also selected to investigate the phototherapy effect of CBpExP6 ⊃ hemin in vitro, after incubating with different groups, its viability was investigated via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. HeLa cells were cultivated with different concentrations (0–80 μg mL−1) of hemin, CBpExP6 ⊃ hemin, then irradiated with 10 min of 660 nm light (1 W cm−2). Fig. 3b showed that the viability of cells in control and near-infrared-radiation (NIR) groups all above 97%, indicating that there was no obvious cytotoxicity of these groups. Hemin exhibits relatively low toxicity in tumor cells. This is because although Fe(II) can catalyze the production of ˙OH from the overexpressed H2O2 in tumor cells, hemin is highly unstable and will quickly become ineffective.
In the hemin + NIR group, the cytotoxicity increases due to 1O2 is generated upon near-infrared light irradiation, which also could kill tumor cells. On the other hand, in the CBpExP6 ⊃ hemin group, the cytotoxicity is also greater than that in the hemin group. This is because CBpExP6 enhances the stability of hemin, enabling it to continuously catalyze the overexpressed H2O2 to produce ˙OH to kill tumor cells. Under the same conditions, the toxicity of hemin and CBpExP6 ⊃ hemin to the normal cells HEK293 is significantly lower than that to HeLa cells (Fig. S11, ESI†). This is caused by the fact that the content of H2O2 in normal cells is lower than that in HeLa cells. Nevertheless, the CBpExP6 ⊃ hemin + NIR group shows the greatest cytotoxicity. When the concentration is 80 μg mL−1, the cell viability is only 8%. This is because it can not only continuously generate ˙OH but also produce 1O2 under NIR. The synergistic effect of the two significantly improves the ability to kill tumor cells.
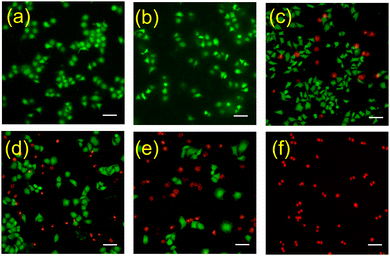
At last, to check the phototherapy effect of CBpExP6 ⊃ hemin more intuitively effect, live (green) and dead (red) cells was differentiated by calceinacetoxymethyl (calcein-AM) and propidium iodide (PI) staining. In control (Fig. 4a) and NIR (Fig. 4b) groups, the cells exhibited green fluorescence, indicating they are living well. On the other hand, when incubated with hemin, CBpExP6 ⊃ hemin, or hemin + NIR, both red and green fluorescence were observed, indicating that partial cells were dead. However, when treated with CBpExP6 ⊃ hemin then irradiated with NIR, almost the cells were dead and showed red fluorescence. These results clearly confirmed the satisfied therapeutic effect of CBpExP6 ⊃ hemin upon irradiation.
In conclusion, we designed and synthesized a cationic [2]biphenyl-extended pillar[6]arene with eight quaternary ammonium salts (CBpExP6). Isothermal titration calorimetry (ITC) studies have shown that CBpExP6 can form a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with hemin in water, thus enhancing the stability of hemin in water. Moreover, CBpExP6 ⊃ hemin can further self-assemble into nanoparticles with a diameter around 200 nm, enabling it to effectively be phagocytosed by tumor cells. Since the Fe(II) in CBpExP6 ⊃ hemin can catalyze the H2O2, which is overexpressed in tumor cells, to generate ˙OH, and on the other hand, under light irradiation, the porphyrin core in CBpExP6 ⊃ hemin can generate 1O2. The synergistic effect of these two processes can effectively kill tumor cells. This work provides a potential supramolecular strategy for enhancing the stability of photosensitizers and thus improving their anti-tumor therapeutic efficacy.
1 complex with hemin in water, thus enhancing the stability of hemin in water. Moreover, CBpExP6 ⊃ hemin can further self-assemble into nanoparticles with a diameter around 200 nm, enabling it to effectively be phagocytosed by tumor cells. Since the Fe(II) in CBpExP6 ⊃ hemin can catalyze the H2O2, which is overexpressed in tumor cells, to generate ˙OH, and on the other hand, under light irradiation, the porphyrin core in CBpExP6 ⊃ hemin can generate 1O2. The synergistic effect of these two processes can effectively kill tumor cells. This work provides a potential supramolecular strategy for enhancing the stability of photosensitizers and thus improving their anti-tumor therapeutic efficacy.
This work was supported by the National Natural Science Foundation of China (22007052), and College Students' Innovation and Entrepreneurship Project (202410304102Y). We also thank Nantong University Analysis & Testing Center for characterization.
Data availability
Data for this article are available at main article of this paper.Conflicts of interest
There are no conflicts to declare.Notes and references
- D. J. Lundberg, C. M. Brown, E. O. Bobylev, N. J. Oldenhuis, Y. S. Alfaraj, J. Zhao, I. Kevlishvili, H. J. Kulik and J. A. Johnson, Nat. Commun., 2024, 15, 3951 CrossRef CAS PubMed.
- D. Núñez-Villanueva, M. A. Jinks, J. G. Magentia and C. A. Hunter, Chem. Commun., 2018, 54, 10874–10877 RSC.
- X. Wu, P. Wang, P. Turner, W. Lewis, O. Catal, D. S. Thomas and P. A. Gale, Chem, 2019, 5, 1210–1222 CAS.
- Q. Wang, Y. Zhong, D. P. Miller, X. Lu, Q. Tang, Z.-L. Lu, E. Zurek, R. Liu and B. Gong, J. Am. Chem. Soc., 2020, 142, 2915–2924 CrossRef CAS PubMed.
- M.-H. Ding, J. Liao, L.-L. Tang, G.-C. Ou and F. Zeng, Chin. Chem. Lett., 2021, 32, 1665–1668 CrossRef CAS.
- C. Rando, J. Vázquez, J. Sokolov, Z. Kokan, M. Nečas and V. Šindelář, Angew. Chem., Int. Ed., 2022, 61, e202210184 CrossRef CAS PubMed.
- T. Chen, J. Wang, R. Tang, Y. Huang, Q. Zhao and Y. Yao, Chin. Chem. Lett., 2023, 34, 108088 Search PubMed.
- N. Song, X.-Y. Lou, L. Ma, H. Gao and Y.-W. Yang, Theranostics, 2019, 9, 3075–3093 CrossRef CAS PubMed.
- R. R. Kashapov, Y. S. Razuvayeva, A. Y. Ziganshina, R. K. Mukhitova, A. S. Sapunova, A. D. Voloshina, V. V. Syakaev, S. K. Latypov, I. R. Nizameev, M. K. Kadirov and L. Y. Zakharova, Molecules, 2019, 24, 1939 Search PubMed.
- H. Zhu, Q. Li, L. E. Khalil-Cruz, N. M. Khashab, G. Yu and F. Huang, Sci. China:Chem., 2021, 64, 688–700 CrossRef CAS.
- Z. Liu, W. Lin and Y. Liu, Acc. Chem. Res., 2022, 55, 3417–3429 Search PubMed.
- J. Li, J. Sun, X. Zhang, R. Zhang, Q. Wang, L. Wang, L. Zhang, X. Xie, C. Li, Y. Zhou, J. Wang, G. Xiao, F. Bai and H. Liu, Chem. Commun., 2023, 59, 868–871 RSC.
- T. Yokoyama and M. Mizuguchi, J. Med. Chem., 2019, 62, 2076–2082 CrossRef CAS PubMed.
- H. Jin, L. Yang, M. J. R. Ahonen and M. H. Schoenfisch, J. Am. Chem. Soc., 2018, 140, 14178–14184 CrossRef CAS PubMed.
- Y. Sugita, D. Aoki, M. Tokita and H. Otsuka, Chem. Commun., 2022, 58, 3067–3070 Search PubMed.
- V. Guérineau, M. Rollet, S. Viel, B. Lepoittevin, L. Costa, P. Saint-Aguet, R. Laurent, P. Roger, D. Gigmes, C. Martini and V. Huc, Nat. Commun., 2019, 10, 113 CrossRef PubMed.
- R. Nag and C. P. Rao, J. Chem. Sci., 2021, 133, 92 CrossRef CAS.
- Y.-H. Liu, Y.-M. Zhang, H.-J. Yu and Y. Liu, Angew. Chem., Int. Ed., 2021, 60, 3870–3880 CrossRef CAS PubMed.
- N. A. Thompson, H. Barbero and E. Masson, Chem. Commun., 2019, 55, 12160–12163 RSC.
- R. Tang, Y. Ye, S. Zhu, Y. Wang, B. Lu and Y. Yao, Chin. Chem. Lett., 2023, 34, 107734 Search PubMed.
- Y. Luo, Y. Yang, Y. Wang, Z. Wu, T. P. Russell and S. Shi, Angew. Chem., Int. Ed., 2022, 61, e202207199 Search PubMed.
- K. Kato, T. Kaneda, S. Ohtani and T. Ogoshi, J. Am. Chem. Soc., 2023, 145, 6905–6913 CrossRef CAS PubMed.
- H. Liang, Y. Yang, L. Shao, W. Zhu, X. Liu, B. Hua and F. Huang, J. Am. Chem. Soc., 2023, 145, 2870–2876 Search PubMed.
- L. Ling, Z. Zhao, L. Mao, S. Wang and D. Ma, Chem. Commun., 2023, 59, 14161–14164 RSC.
- J.-R. Wu and Y.-W. Yang, Chem. Commun., 2019, 55, 1533–1543 RSC.
- D. He, H. Ji, T. Liu, M. Yang, R. Clowes, M. A. Little, M. Liu and A. I. Cooper, J. Am. Chem. Soc., 2024, 146, 17438–17445 Search PubMed.
- P. Karmakar, T. J. Finnegan, D. C. Rostam, S. Taneja, S. Uçar, A. L. Hansen, C. E. Moore, C. M. Hadad, K. Pratumyot, J. R. Parquettea and J. D. Badjić, Chem. Sci., 2024, 15, 10155–10163 Search PubMed.
- R. Fu, Q.-Y. Zhao, H. Han, W.-L. Li, F.-Y. Chen, C. Tang, W. Zhang, S.-D. Guo, D.-Y. Li, W.-C. Geng, D.-S. Guo and K. Cai, Angew. Chem., Int. Ed., 2023, 62, e202315990 CrossRef CAS PubMed.
- S. Ohtani, S. Akine, K. Kato, S. Fa, T. Shi and T. Ogoshi, J. Am. Chem. Soc., 2024, 146, 4695–4703 CrossRef CAS PubMed.
- B. Shi, J. Jiang, H. An, L. Qi, T.-B. Wei, W.-J. Qu and Q. Lin, J. Am. Chem. Soc., 2024, 146, 2901–2906 Search PubMed.
- L. Zhang, Y. Xu and W. Wei, Chem. Commun., 2023, 59, 13562–13570 RSC.
- M.-J. Gu, X.-N. Han, W.-C. Guo, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2023, 62, e202305214 CrossRef CAS PubMed.
- J. Wang, M. Cen, J. Wang, D. Wang, Y. Ding, G. Zhu, B. Lu, X. Yuan, Y. Wang and Y. Yao, Chin. Chem. Lett., 2022, 33, 1475–1478 Search PubMed.
- X. Zheng, S.-N. Lei, Z. Gao, X. Dong, H. Xiao, W. Liu, C.-H. Tung, L.-Z. Wu, P. Wang and H. Cong, Chem. Sci., 2023, 14, 3523–3530 RSC.
- W.-J. Xie, J.-M. Chen, Z.-W. Yang and L.-N. He, Green Chem., 2023, 25, 10366–10371 RSC.
- B. Gao, L.-L. Tan, N. Song, K. Li and Y.-W. Yang, Chem. Commun., 2016, 52, 5804–5807 RSC.
- J.-R. Wu, C.-Y. Wang, Y.-C. Tao, Y. Wang, C. Li and Y.-W. Yang, Eur. J. Org. Chem., 2018, 1321–1325 Search PubMed.
- D. Dai, Z. Li, J. Yang, C. Wang, J.-R. Wu, Y. Wang, D. Zhang and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 4756–4763 Search PubMed.
- D. Dai, J. Yang, Y.-C. Zou, J.-R. Wu, L.-L. Tan, Y. Wang, B. Li, T. Lu, B. Wang and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 8967–8975 CrossRef CAS PubMed.
- D. Li, G. Wu, X. Wang, J.-R. Wu and Y.-W. Yang, Smart Mol., 2023, 1, e20230016 CrossRef.
- G. Wu, J.-R. Wu, Y. Wang and Y.-W. Yang, Chem, 2023, 9, 2918–2930 Search PubMed.
- J.-R. Wu, D. Li, G. Wu, M.-H. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2022, 61, e202210579 CrossRef CAS PubMed.
- J.-R. Wu and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 12280–12287 Search PubMed.
- Y. Hokimoto and T. Nakamura, Chem. Commun., 2024, 60, 1281–1284 RSC.
- F. Balduzzi, P. Stewart, S. K. Samanta, T. J. Mooibroek, T. Hoeg-Jensen, K. Shi, B. D. Smith and A. P. Davis, Angew. Chem., Int. Ed., 2023, 62, e202314373 CrossRef CAS PubMed.
- S. Sun, D. Lu, Q. Huang, Q. Liu, Y. Yao and Y. Shi, J. Colloid Interface Sci., 2019, 533, 42–46 CrossRef CAS PubMed.
- Y. Zhang, X. Yan, L. Shi, M. Cen, J. Wang, Y. Ding and Y. Yao, Inorg. Chem., 2021, 60, 7627–7631 CrossRef CAS PubMed.
- R. Zhang, X. Yan, H. Guo, L. Hu, C. Yan, Y. Wang and Y. Yao, Chem. Commun., 2020, 56, 948–951 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, additional 1H NMR spectra and determination of the association constants. See DOI: https://doi.org/10.1039/d5cc00627a |
| ‡ Y. Cai and Y. Zhang contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2025 |