Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insight into aligned nanofibers improving fuel cell performances: strategies, rationalities, and opportunities
Muhammad
Yusro
 *ab and
Viktor
Hacker
*ab and
Viktor
Hacker
 a
a
aInstitute of Chemical Engineering and Environmental Technology, Graz University of Technology, Inffeldgasse 25/C, 8010 Graz, Austria. E-mail: yusro@student.tugraz.at
bInstitut Teknologi Telkom Purwokerto, Jalan D.I. Panjaitan 128, Purwokerto, 53147, Indonesia
First published on 30th May 2024
Abstract
Nanofibers are advanced materials widely used in fuel cell applications owing to their superior characteristics of large surface area and porosity. Aligned nanofibers, a next-level development in nanofibers, is a promising approach for implementation in fuel cell applications, considering that they enhance specific properties compared to randomly orientated structures. This review presents the current strategies for fabricating aligned nanofibers and explores various methods to obtain targeted assemblies. These methods include increasing the speed of the rotating collector, applying multiple electric fields, and using engineering-defined collectors, for instance, wiring drums, patterned strips, frame shapes, rotating discs, rotating jets, and guide column arrays. Moreover, the rationality behind why this structure can improve fuel cell performance is elaborated, which includes enhanced conductivity, improved mass transport, structural durability, and reduced water flooding. The prospects and challenges of implementing aligned nanofibers in fuel cells are also included.
1. Introduction
Human civilization has always been dependent on energy. Before the industrial revolution, people used wood as an energy source to satisfy their basic needs. Initiated by James Watt with his steam engine in 1769, most energy services in recent centuries have been provided by fossil fuels.1 However, the use of fossil fuels is not sustainable, given that it results in an increase in the carbon dioxide content in the atmosphere, which has already significantly impacted the climate. This condition is a pressing issue that requires urgent attention. Therefore, new approaches for environmentally friendly energy production are urgently needed as a policy to decarbonize energy systems and consequently strengthen regional energy independence through the use of renewable energy sources.2Hydrogen has the advantage of availability compared to other renewable resources. Mainly, natural resources cannot be controlled by humans; often, the sun does not shine, or the wind does not blow during specific periods. Alternatively, hydrogen can be a stable resource because it does not directly depend on natural phenomena, providing continuous energy storage to supply power.
One of the devices that employs hydrogen as an energy source is the fuel cell. This technology applies reverse electrochemical principles to generate electrical current. The inlet used in a fuel cell depends on the type of fuel cell. Generally, the different types of fuel cells have hydrogen on the anode side and oxygen on the cathode side. Compared to other electrochemical technology devices, for instance, batteries, where electricity is produced from internal energy, fuel cell technology has substantial benefits, especially the reactants can be continuously charged.3 Moreover, the electrodes in fuel cells are catalytic and relatively stable. Additionally, their operation can be sustained if the flow is continuously maintained. From an ecological perspective, fuel cells produce water and steam as their product, making these devices a promising approach from an environmental perspective compared to fossil fuel, which produces carbon excess.
It has been reported that the use of nanofibers is a promising approach to enhance the performance of fuel cells. Nanofibers are nanomaterials that have the shape of fibres defined by dimensions in the range of 50–500 nm.4 Owing to these properties, nanofibers can act as membranes with large porosity and surface area. In fuel cell applications, this advanced material successfully upgrades the quality of the product compared to conventional methods such as solution casting.5
Nanofibers can act as a membrane providing proton or anion transfer to the other electrode, enhancing this feature. The prime characteristics of nanofibers applied in fuel cells are providing high surface area and porosity and promoting the establishment of interconnected networks.4 This structure has been reported to have an impact on the performance of fuel cells in terms of preventing their electrochemical degradation4 and facilitating better catalytic performances,6 high stability under repeated cycling,7 and high efficiency in the hydrogen evolution reaction,4 acting as highly efficient and stable bifunctional electrocatalyst for water splitting,4 and enhancing hydroxide conduction.4
The first article related to aligned nanofibers was published in 1993, when Doshi and Reneker reported that an increase in the electric potential can make fibres more oriented in poly(ethylene oxide).8 Since them, strategies to align nanofibers have been continuously developed, with several new approaches reported to date. For instance, rotating disc method,9 use of multiple electric fields,10 tuning the shape of the frame,9 using patterned electrodes,11 rotating jets,12 and guiding column arrays,13 and increasing the speed of the drum collector14 have been reported for the successful fabrication of oriented nanofibers.
Aligned nanofibers have superior characteristics compared to random structures. Consequently, the advantages of these structures facilitate enhanced mass transport,15 electrochemical activity,16 mechanical integrity (durability),17 and electrical conductivity.18 The experimental results provided by characterization support the use of aligned nanofibers.
However, although the nanofiber approach has promising prospects in enhancing the performance of fuel cells, it still needs to be fully exploited to implement oriented nanofibers in fuel cell applications. The research and development of aligned nanofibers for fuel cell applications are still in their infancy. Nonetheless, this strategy can potentially be employed to develop membrane electrode assemblies (MEA) for fuel cell applications.
This review aims to present insight into the use of aligned nanofibers as one prospective strategy for the performance of improving fuel cells. The outlook of this review includes the strategies to fabricate aligned nanofibers, the rationality of the performance of fuel cells and their characterization, and finally the opportunities regarding future research and challenges in fuel cells.
2. Fuel cells
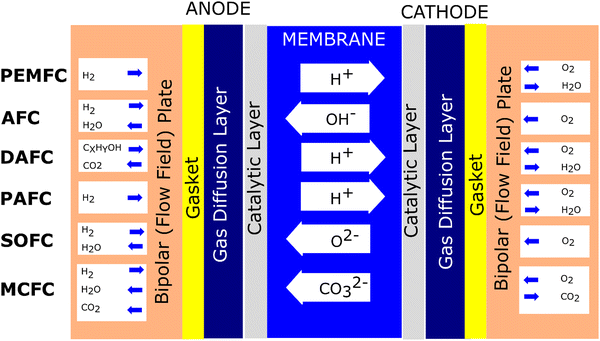
Fuel cells are devices based on the electrochemical principle to convert chemical energy to electrical energy. This process is continuous if fuel and an oxidant are supplied. For instance, in the proton exchange membrane fuel cell (PEMFC), hydrogen (H2) gas is supplied to the anode (−). Meanwhile, oxygen is supplied to the cathode (+). At the anode, the H2 molecule splits into two protons and two electrons by the catalytic process. The protons infiltrate the membrane, and the electron flows through the wire, producing an electrical current. Furthermore, proton particles linked with electrons and oxygen form water at the cathode.Regarding fuel cell development, the type of fuel cell can be classified according to the applied electrolyte.19 To date, at least six types of fuel cells have been established, which are polymer electrolyte fuel cell (PEFC), alkaline fuel cell (AFC), direct alcohol fuel cell (DAFC), phosphoric acid fuel cell (PAFC), molten carbonate fuel cell (MCFC), and solid oxide fuel cell (SOFC).20–22Fig. 1 illustrates the mechanism of the various types of fuel cells.
It has been recognized that PEMFC/PEFC is the leading fuel cell technology due to its characteristics such as high-power density, low weight, and ability to operate at low temperatures (typically at around 80 °C).23 Nonetheless, this type of fuel cell has significant challenges, mainly it employs an expensive catalyst (platinum).24 Thus, to address these limitations, an anion exchange membrane (AEM) can be employed, considering its advantage that it avoids the use of expensive metal catalysts. Moreover, this approach reduces the severe corrosion under alkaline conditions due to the counter-direction between the fuel and OH ions. However, although AEM shows significant promise to overcome the issues associated with PEM, this type of fuel cell must be managed regarding its excessive water absorption, poor mechanical properties, membrane swelling, poor ion conductivity, and poor membrane stability.25 Another promising candidate is the direct alcohol fuel cell (DAFC). This structure is similar to PEMFC. When methanol is used in the fuel in a DAFC uses, it is called a direct methanol fuel cell (DMFC). This is because this fuel can be directly used as the fuel source without a reforming process, and this type of fuel cell has a more compact system regarding its structure and provides a higher energy density.19 PAFC utilizes liquid phosphoric acid and ceramic electrolytes such as silicon carbide or glass mat as membranes.26 This type of fuel cell also employes platinum as a catalyst. Regarding efficiency, the efficiency of PAFC is comparable to that of PEMFC. Nonetheless, this type of fuel cell works at higher temperatures to manage the fuel cell impurities. PAFC is usually applied in stationary applications, satisfying a high energy demand. SOFC employs a ceramic material as the electrolyte membrane. This type of cell operates at 1800 degrees Fahrenheit, making it the highest-temperature fuel cell.27 Compared to the above-mentioned fuel cells, this type of fuel cell does not use platinum-based materials as a catalyst. SOFC is considered to use internal reformation and commonly used natural gas as fuel. Another type of fuel cell that operates at high temperatures and uses non-platinum catalysts through internal reforming is MCFC. This type of fuel cell utilizes molten carbonate salt as a membrane electrolyte,28 reducing the cost. Also, its high working temperature can reduce the requirement of a costly metal catalyst, reducing the cost compared to the fuel cells that use precious materials.
Regarding the structure of fuel cells, they are constructed using several components with distinct materials. From outside to inside, these components include bipolar plates, gaskets, gas diffusion layers (GDL), catalyst layers (CL), and membranes. However, all these components or layers can be degraded, resulting in a decline in the function and performance of fuel cells.29
Table 1 presents a summary of the risk factors and causes for the decline in the performance of fuel cells.
| Components of fuel cells | Risk factors | Causes |
|---|---|---|
| Membrane | Mechanical degradation | Mechanical stress caused by non-uniform pressure |
| Thermal degradation | Thermal stress caused by non-uniform temperature | |
| Chemical electrochemical degradation | Contamination, radical exposure | |
| Membrane thinning | Chemical degradation, high temperature | |
| Pinhole formation | Mechanical tension, accelerated by low and alternating humidification and high temperatures | |
| Catalyst layer | Decrease in mass transport rate of the reactant | Mechanical stress |
| Loss of conductivity | Corrosion | |
| Loss of activation | Sintering or dealloying | |
| Loss of tolerance | Contamination | |
| Decrease in water management | Hydrophobicity change | |
| Carbon corrosion | Fuel starvation, high humidity, quick high load charges | |
| Gas diffusion layer | Decreased ability in water management | Mechanical stress and change in material hydrophobicity |
| Decrease in mass transport | Degradation of backing material | |
| Conductivity loss | Corrosion | |
| Gasket | Mechanical failure | Mechanical stress |
| Corrosion | ||
| Bipolar plate | Fracture/deformation | Mechanical stress |
| Loss of conductivity | Corrosion and oxidation |
The first component, the membrane, is in the central part of the fuel cell structure. The role of the membrane is to ensure that only the required ions pass between the anode and cathode. A membrane is a particularly important material that manages to transfer only positively charged ions from the fuel and prevents the movement of electrons through the membrane structure. An excellent membrane in fuel cells should exhibit durability, chemical stability (against reactive radicals), high thermal stability, and mechanical stability (combat gas crossover).29 In PEM fuel cells, it has been reported that mechanical degradation of the membrane structure is the main factor for their early-stage failure.31
The catalyst layer is a component placed on both sides of the membrane. One side functions as the anode, and the other side acts as the cathode. On the anode side, hydrogen molecules are split into protons and electrons. On the cathode side, water is produced by the reaction between oxygen and the protons generated by the anode. Several factors can damage the catalytic layer in fuel cells, for instance, higher voltages, humidity, and load cycling. Furthermore, in PEM, these factors lead to structural damage and accelerate corrosion.32
GDLs are structures located on the outside of the catalyst layers, which function to transport the reactants to the catalyst layer. This component also plays a role in removing water. In fuel cells, gases diffuse in the pores of the GDL, which is composed of a hydrophobic material to keep the pores open. It has the function of preventing the accumulation of excessive water. The GDL consists of two layers, a microporous layer and a microporous layer. The macroporous layer facilitates an electrical and thermal pathway to the flow-field plates. Moreover, this structure enables the permeation of particles through its macroscopic layer. The microscopic layer manages water, reducing the thermal and electrical contact near the catalyst layer. It has been mentioned that a significant issue in this structure is the hydrophobicity changes on its surface and porous layer caused by degradation.33
The main function of the gasket is to distribute continuous pressure and reduce contact with the components inside the fuel cell. It also secures the gas in a seal-tight condition. This component plays a significant role in the durability of the fuel cell. Thus, the gasket is usually built using a rubbery polymer.34
Bipolar plates have the function of providing electrical conduction between cells. They also provide physical strength to the stack. Bipolar plates have a surface constructed by channels, which allow gases to enter the structures. It has been reported that metallic bipolar plates are recommended due to their small dimensions and weight. Bipolar plates are required to protect against corrosion and enable long-term operation.29Table 1 presents the risk factors and their causes in the components of fuel cells, which refers to.30
3. Electrospinning
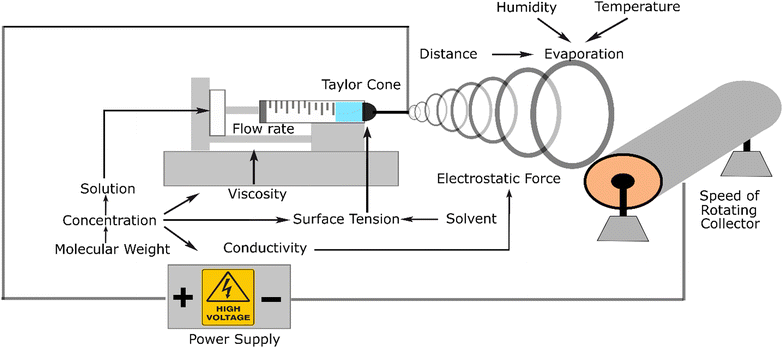
Electrospinning is the most recommended method for the fabrication of nanofibers because of its versatility. This apparatus can be used on the lab and industrial scales and can mix various materials (composites and additives) to achieve the required targeted properties. Electrospinning systems consist of a pump, syringe, polymer solution, needle as a tip, high voltage, and collector. This system applies electrostatic force to stretch and elongate the fibre. This electrostatic force should be sufficient to form a Taylor cone at the tip of the needle due to its positive charge and strong enough to pull out the solution and move it to the collector. During this movement, the solute evaporates, and the fibre gets thinner and thinner until it reaches nanometre dimensions. The source of the electrostatic force comes from a high-voltage power supply.The mechanism of this instrument is that positive charge is converged in a formed Taylor cone, acting as an anode that supplies a high voltage power and gets repelled to the collector, which acts as the cathode. This system could produce three structures, namely, nanofibers, nanobeads, and droplets.
These three varieties originate from the interaction among the parameters influencing the process. At a certain distance, this phenomenon causes the polymer jet to the grounded collector. The jet is a straight line in a short distance because of the jet effect. However, in the next event, the fibre becomes thinner and smoother due to the evaporation of the solute polymer. In this line, the interaction between the fibre, which is charged, and the air occurs, creating a non-woven polymer fibre.
Fig. 2 illustrates the related parameters in the electrospinning process, which depend on or influence each other. From an experimental viewpoint, the events in electrospinning have been examined and influenced by the parameters affected by the electrostatic forces, which lead to successful or failed products. The parameters can be classified into three categories, including fluid parameters, set-up parameters, and ambient parameters. Among these three parameters, the fluid factor consists of viscosity, conductivity, and surface tension, which is the most influential parameter, and thus must be appropriately considered.35
Firstly, viscosity is the parameter that can affect the size and shape of the fibre product. This parameter is an important factor in the fabrication process, which can lead to the presence of solution in the fibre. If the viscosity is not high enough, the stretching and elongating process tends to form beads or a spray. Viscosity is related to the concentration of the material solution. Thus, the solvent can also lead to different viscosities.
The second parameter, conductivity, is related to the positive charge that appears in the solution. The higher the conductivity, the stronger the force in the system. For instance, chitosan exhibits high conductivity with an increase in its concentration. Accordingly, an additional material is required to counter this high conductivity.
The last surface tension parameter affects the Taylor cone, which initiates the process of elongating and stretching the nanofiber. The surface tension is affected by the solvent; it has been reported that a low surface tension increases the failure of nanofiber fabrication. It has been mentioned that in the process of electrospinning, an electrostatic force is applied. The positive charge that converges in the tip forms a Taylor cone, acting as the anode that comes from a high-voltage power supply and gets repelled by the collector, which acts as the cathode. The result of this system can be three structures, namely nanofibers, nanobeads, and droplets. These three varieties originate from the interaction between the influencing parameters.
The devised parameters in the system interact with each other, determining whether a nanofiber is created or not. Based on their source, the parameters can be classified into three main categories, which are fluid parameters, set-up parameters, and environment parameters. Each category has sub-categories that affect the fabrication process.
Table 2 presents the effect of the electrospinning parameters on the size of the fibres. The diameter of the nanofiber reportedly can affect the performance of fuel cells.36,37 It is also interesting to explore the relationship between the geometrical aspect and the fuel cell performance by modulating the influencing parameters.
| Parameters | Effect | Ref. | ||
|---|---|---|---|---|
| Increase | Decrease | |||
| Set up | Flow rate | Decreasing the fibre size. | Increasing the fibre size. | 38–42 |
| Too high flow rate causes the Taylor cone to swell (droplet). | Too low flow rate causes the solution to retreat into the nozzle (unformed Taylor cone). | |||
| Voltage | Increasing the conic angle and decreasing the fibre size result in a smaller Taylor cone, smaller pores, multiple jets, and formation of beads (very high voltage). | Enormous Taylor cone, increase in fibre size, resulting in more prominent pores. Too low applied voltage prevents jet formation. | 38,40,42–44 | |
| Distance tip to the collector | Decreasing the fibre size due to evaporation. | Decrease in distance causes an increase in electrical force (a minimum distance is required). | 40,44–46 | |
| Rotating collector speed | Aligned nanofiber formation. | Isotropic nanofiber formation. | 17,47,48 | |
| Fluid | Viscosity (corresponds to concentration and MW) | Increasing fibre dimensions smoothens the fibre (less beads); a highly viscous solution cannot be injected from the nozzle. | Decreasing fibre size, smaller pores and shallow value generate beads. | 38,41,46,49–51 |
| Conductivity | Higher conductivity increases the limitation of fabrication. | Minimum conductivity is necessary for the electrospinning process. | 35,44 | |
| Surface tension | Higher surface tension results in droplets. | Very low surface tension results in the formation of droplets. | 44,52 | |
| Ambient | Temperature | Higher temperature decreases the nanofiber size. | Higher temperature increases the nanofiber size. | 53–56 |
| Humidity | Higher humidity increases the nanofiber size and the instability of the fiber. | Lower humidity permits faster solvent evaporation. | 54–56 | |
4. Strategies for the fabrication of aligned nanofibers
The fabrication of aligned nanofibers requires a strategy that typically adds special geometrical apparatus to the collector. This approach enables the nanofiber to sit more oriented as soon as it falls on the collector. Interestingly, not only one directional aligned material can be fabricated with electrospinning, but it has been established that by designing a specific patterned strip that has conductive properties,57 two strip directions with two and three axial nanofiber structures can be formed using this electrospinning method. Fig. 3 illustrates the current strategies employed to fabricate aligned nanofibers.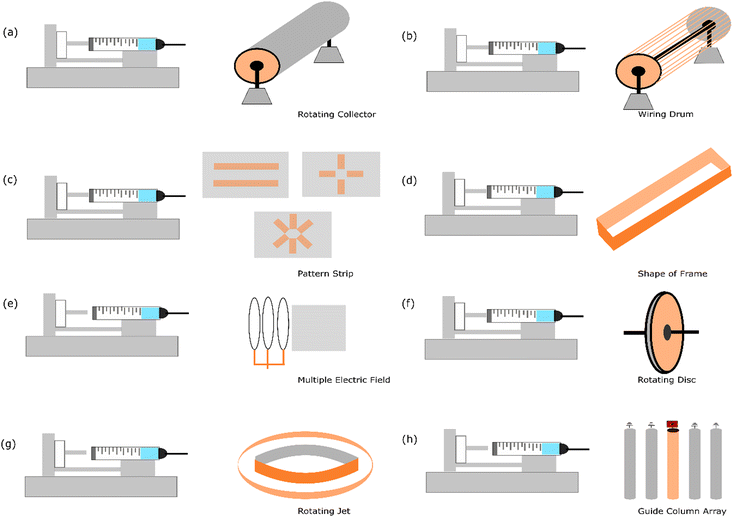 | ||
| Fig. 3 Illustration of the current methods for aligning nanofibers using electrospinning: (a) increasing the speed of the rotating collector,14 (b) wiring drum,58 (c) patterned strip,57 (d) shape of frame,59 (e) multiple electric fields,10 (f) rotating disc collector,9 (g) rotating jet,60 and (h) guide column array.13 | ||
4.1. Increasing the speed of rotating collector
The simplest way to fabricate aligned nanofibers is by increasing the speed of the rotary drum collector. Numerous reports show that this strategy can be employed to successfully fabricate uni-axial nanofibers. It has been reported that a speed in the range of 800–5000 rpm is applied.18,61 The various materials that have been successfully transformed into aligned nanofibers include poly(vinyl alcohol) (PVA),14 metal–organic frameworks (MOFs),18 poly(ε-caprolactone) (PCL),62,63 sulfonated poly(phthalazone ether sulfone ketone) (SPPESK),17,18 chitosan (Cs),61 poly(ethylene oxide) (PEO),61 and polyacrylonitrile (PAN).64It has been noted that increasing the speed of the rotating collector reduces the size of the nanofiber. This phenomenon is attributed to the fact that increasing the speed of the collector will enhance the applied stretching force in the nanofiber induced by the high-speed rotating drum.14 An interesting result has also been published that by increasing the drum speed (800 to 2500 rpm), the modulus also increases (3.83 ± 0.70 to 41.32 ± 1.64) in the parallel nanofiber.61 Regarding the orientation, it has been found that by increasing the speed of the collector gradually, notably 1.000 rpm, 1.500 rpm, 2.000 rpm, and 2.500 rpm, the geometrical angle in the range of 10–0° changes be about 35%, 52%, 57%, and 73%, respectively. This result indicates that the alignment of the nanofibers improves considerably with an increase in the rotary speed.65
4.2. Wiring drum
A wiring drum is a modified rotating collector with parts like a wire similar to a yarn spinner. Theoretically, with these customized structures, the fibre that falls onto the rotating drum can be easily placed constantly, avoiding a random orientation. It has been reported that Nylon-6 was successfully fabricated into an aligned nanofiber using this method.58It has been reported that this technique is robust and can be easily collected without breaking the oriented structure. This experiment used copper wires spaced uniformly in a circular drum formation, which functioned as a collector. This experiment also noted that a proper alignment is achieved in around 15 min of electrospinning, and after that, the fibres begin to condense and entangle. Nonetheless, the process of electrospinning for up to 40 min still produced an aligned structure.
4.3. Patterned strip
In this strategy, two electrodes that can manage the fibre orientation are placed in the plane collector. Metal plates such as aluminum,66 gold,57 and copper67 and conductive silicon68 can be used to build this upgraded collector. It has been reported that this strategy is implemented in proton exchange membranes using sulfonated polyimide material.69 The result of this approach is a one-strip nanofiber alignment.An excellent feature of this strategy is that this plate can be customized given that the electrode is a patterned formation. By making various patterns, the geometry of the nanofiber can be engineered according to the desired goal. It has been reported that poly(vinylpyrrolidone) could be fabricated to become more than uni-axial nanofiber structures using this strategy. This approach is very promising for exploring more comprehensive variables regarding the effect of nanofiber geometry.57
4.4. Shape of frame
The idea behind making frames with a specific shape is to control the deposition process when a fibre is deposited on the collector. When the fibre is deposited at a specific side of the frame, the continued fibre formation must be deposited at the other side. This process can make fibre aligned and uni-axially oriented. The different designs of the frame can based on different geometrical shapes, such as elliptical frame59 and using two strips with an inclined gap.70Due to the presence of two strips or sides configured to create a gap between the deposited side of the nanofiber, the nanofiber product is sequentially deposited across the edges of these two sides in an oriented formation. The materials used and successfully reported using this strategy are polycaprolactone,70 polyamide-6,59 and polylactide.59
4.5. Multiple electric fields
The electric field is one of the parameters in the electrospinning process. It causes the elongated fibre to stretch from the tip to the collector. Normally, this process is formed by a random nanofiber formation. Alternatively, multiple electric fields need to be managed to transform the geometrical shape into a more oriented nanofiber. Simulation has reported that the electrospinning jet spins in the area between the tip and collector.71 The modelling of the electrospinning process is necessary to solve the challenges in nanofiber production, particularly the whipping instability. This obstacle occurs in the chaotic oscillation of polymer jets, producing a random fibre formation.72 In this case, an additional instrument needs to be placed at the appropriate distance.Multiple electric fields using poly(ethylene oxide) material have been used in an experiment. The result showed that an aligned nanofiber was successfully fabricated. This experiment employed a secondary external field with the same polarity to manage the fibre formation. The controlled deposition of sub-micron polymer fibres (<300 nm in diameter) was enabled using an electrostatic lens element customised apparatus.10
4.6. Rotating disc
It has been mentioned in the previous strategy that changing a flat collector to a rotating collector is one of the strategies to fabricate aligned nanofibers. However, the rotating drum is quite large regarding the area of deposition. In this case, the spun fibre affected by the electric field is not sufficiently elongated, and thus this strategy causes a slight angle in fibre formation. Accordingly, to solve this problem, an upgraded geometry needs to be developed with the idea of reducing the area of nanofiber placement in the rotating collector.The rotating disc is a strategy that has a similar concept to the rotating collector but has a narrower area for nanofiber deposition. Hypothetically, when the fibre is placed in a small area (resembling the side of the disc), it can be directly rolled, creating a more oriented form. It has been reported that this strategy was successfully employed to form aligned nanofibers with a disc composed of aluminium. The geometrical design was 200 mm in diameter, and the thickness was only 5 mm. The material that was tested was polyethylene oxide.9
4.7. Rotating jet
Another approach of modifying the collector in electrospinning is the method called rotating jet or centrifugal electrospinning, which is a technique inspired by the cotton candy production concept. This procedure implements a hollow metallic cylinder as a collector with a needle placed in the centre of the cylinder. In this setup, parallel electrodes are inserted with a circular collector around the rotating spinneret, the needle tip acts as the positive electrode, and the collector acts as the negative electrode.73 Due to the opposite charge between the collector and needle, repulsion is caused by the Coulomb force as the jet comes out of the rotating needle, and a nanofiber is forms in the inner surface of the cylinder. It has been reported that this method has advantages regarding high orientation over large collector areas.12It has been reported that three parameters, including the solution properties,74 operating parameters,75 and mechanical objects of the device,76,77 affect the morphology of the nanofibers fabricated by centrifugal spinning. Moreover, based on further study, the effects of surface tension, viscosity, speed of the spinneret, distance to the collector, evaporation rate, and diameter of the orifice on the morphology of the nanofibers are considered the most influencing parameters.75
4.8. Guide column array
The guide column array was reported as a successful method to fabricate ceramic-based aligned nanofiber materials. The guide array was designed using copper tubing columns with a spacing of five 20 cm length, 0.5 cm diameter, and 2 cm apart.13 A high-voltage power supply was applied to the centre column to generate electrostatic forces, while the remaining columns were grounded.This method managed the electrospinning jet via electrostatic interactions between the electrified jet and the electrodes. By implementing an array of five conductive columns separated by an air gap, the electrified jet moves towards the guide column, and the repulsive coulomb forces deflect the fibre to the nearest grounded column.13 In this event, the approaching fibre can disintegrate the charge. Subsequently, the dropped fibre is attracted to the central guide column via the same electrostatic forces that initially repelled it. The fibres could maintain a degree of polarization utilized in repelling due to the constant electric field between columns. It has also been found that the ceramic precursor solution conductivity is also correlated with the optimal operating voltage for the realization of aligned ceramic nanofibers.13
5. Rationality: effect of aligned nanofibers
Engineering nanofibers to be more aligned affects their properties related to fuel cell performances. The theoretical and experimental approaches for explaining why this profile enhances the characteristics of fuel cells will be elaborated in this part including enhanced conductivity, improved mass transport, structural stability, and reduced water flooding.5.1. Enhanced ion conductivity
Aligned nanofibers are promising to enhance the efficiency of fuel cells by improving the electrical conductivity and decreasing the electrical resistance due to the reduced tortuosity. It has been reported that sulfonated block copolymers, which have hydrophobic and hydrophilic parts, could be separated to the outside surface and the inside when fabricated as nanofibers via electrospinning.69,78 This network provided a proton channel structure, which enhanced by the rapid transport of protons. Consequently, an excellent through-plane proton transport performance was observed in the fuel cell operation. Fig. 4 shows a comparison between the conductivity of aligned nanofibers and non-aligned nanofibers. Non-aligned nanofibers can obtained by the solution casting method, which can be random nanofibers or without a nanofiber structure. This method has been successfully modelled using resistors, analogous to the study of fibre conductivity properties. The aligned nanofiber, which has an oriented structure, has superior conductivity, considering that its configuration is less of an obstacle to carrier transport to the other side. The prediction of the conductivity of nanofiber has also been modelled. This approach can approximate the fibre layering and swelling. In this model, the fibre network is considered a resistor and has been confirmed in proton and anion exchange membrane nanofiber-based fuel cell application.79 The effect of aligned nanofibers in fuel cell applications has yet to be highly observed. Considering that this approach can theoretically and hypothetically increase the performance of fuel cells, this point of view is interesting to explore.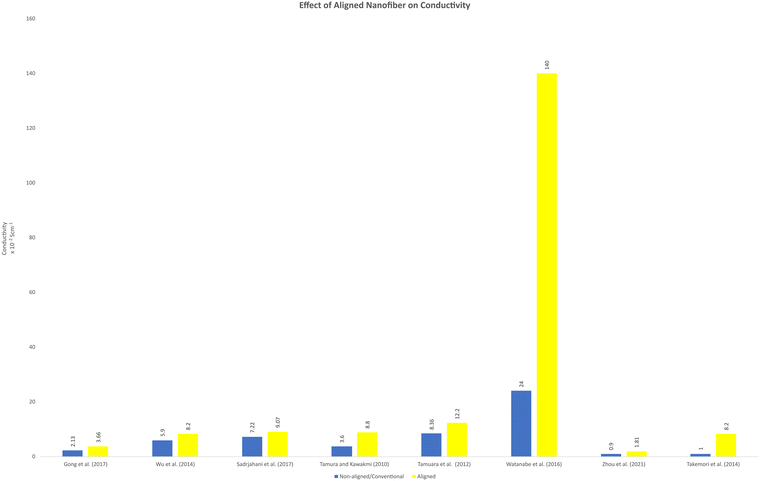 | ||
| Fig. 4 Comparison of the conductivity between aligned and non-aligned nanofiber or other conventional structures reported in the literature. | ||
It has been reported that the ion conductivity increases when aligned nanofibers are implemented. An aligned nanofiber membrane composed of MOFs and SPPESK showed better proton conductivity than the disordered membrane.18 This report also mentioned that this type of nanofiber membrane has a higher conductivity value compared to the conventional method (solvent-casting). The proton conductivity reached (8.2 ± 0.16) × 10−2 S cm−1 at 160 °C temperature operation under anhydrous condition. Moreover, in this study, the methanol permeability also reached up to 0.707 × 10−7 cm2 s−1, which is about 6% lower than that of Nafion-115.18 Another study also reported that an aligned nanofiber could increase the conductivity. The proton conductivity showed a significantly higher value in the parallel direction.
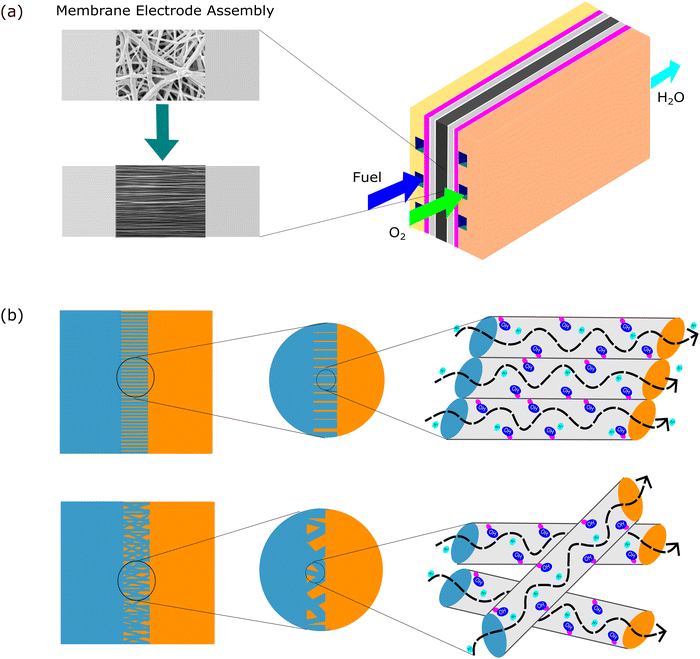
Aligned nanofibers also increased the ion conductivity in an anion exchange membrane. The quaternized-poly(arylene ether sulfone) (Q-PAES) nanofibers fabricated using two strip electrodes were characterized to evaluate their anion conductivity. It was reported that regarding its conductivity, the oriented nanofiber showed a 10–15 times higher value. Based on the experimental data, the anion conductivity significantly increased from 24 mS cm−1 to 140 mS cm−1 (maximum) at an operating temperature of 90 °C for OH− ion species.80 Another study that used aligned nanofibers in anion membrane fuel cells also confirmed that the anion conductivity was successfully enhanced from 0.9 × 10−2 (80 °C) to 1.81 × 10−2 (80 °C).81 The material used in this study was quaternized functional polyketone-based polyelectrolyte (QAFPK). Fig. 5 illustrates the idea of transforming a random nanofiber into an aligned nanofiber. Fig. 5(a) shows the implementation of aligned nanofibers in fuel cells as a membrane. Fig. 5(b) illustrates a comparison of structure-oriented and isotropic membranes. It has been presented that the aligned nanofiber has a straightforward transport path, leading to higher conductivity.
5.2. Improved mass transport
Internal mass transfer limitations can be reduced by maximizing the porosity and lowering the tortuosity of the fibers.16 In this case, an aligned structure leads to faster and more direct diffusion of species (improved mass transport kinetics). The aligned structure of nanofibers provides a better morphology and porosity-correlated membrane function, especially in terms of proton or ion transfer. Moreover, regarding polarity, a chaotic polarity distribution results in disordered mass transport; meanwhile, an oriented structure results in better transfer such as polar area for water transference and non-polar area for gas transport.15Fig. 6 shows an illustration of the comparison between disordered and oriented structures. Regarding electrodes, the nanofiber structure also provides clear pathways for the transport of the reactants and products within the fuel cell electrode. Furthermore, the surface area should be significant to host sufficient active sites per unit of volume.16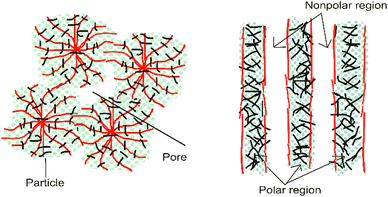 | ||
| Fig. 6 Illustration of the polarity distribution between disordered (left) and more oriented nanofibers (right).15 | ||
Regarding characteristics, ion exchange capacity is one of the properties of fuel cells that is correlated with mass transport, specifically it is correlated with a specific number of ions available in the ion exchange process.82 This characteristic represents the capacity of an insoluble functional group in the membrane to facilitate ion movement.83 The method employed to determine the IEC is back titration.
It has been mentioned that ion exchange is correlated with the membrane properties, including the electrical resistance of the electrolyte, density of fixed charges in the membrane matrix and their distribution, permeability and selectivity of different ions in different membranes, the transport rate of water as a neutral component, stability regarding mechanical and chemical properties and the swelling in various electrolyte solutions.84
The ion exchange capacity is related to the anion conductive characteristic of nanofibers.85 It has been reported that in a quaternized-poly(arylene ether sulfone) (Q-PAES) nanofiber, if the IEC value decreases, the anion conductivity also decreases. It has been tested that in the Q-PAES membrane, when IEC is reduced from 1.72 meq g−1 to 1.58 meq g−1 (almost 20%), the anion conductivity declined by 47%.86
It should be noted that the ion transport characteristic is also affected by chemical structures. This factor is correlated with the flexibility of polymer chains, which is also related to the types of ion exchange groups. The ion species can also contribute to the various ion sizes (radius), electronegativity, and hydration forces. The internal structure of membranes also influences their phase-separation and ion conductive channel formation.86
5.3. Providing structural durability
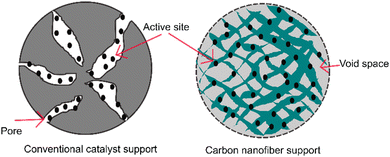
The structure of aligned nanofibers is promising to improve the mechanical integrity and structural stability of fuel cells. This structure supports the endurance to deformation and maintenance of structural integrity during fuel cell operation. An aligned nanofiber assembly is superior to a randomized one, considering that this structure maximizes the exposure of the catalyst surface area to the reactants. This structure facilitates a higher fraction of catalyst, enhancing the catalytic activity, leading to more efficient electrochemical reactions, and enhancing the fuel cell performance. A comparison between a conventional porous support and carbon nanofiber support is shown in Fig. 7. The aligned structure facilitates better adhesion and anchoring of the catalyst material, reducing its detachment or degradation during fuel cell operation, and the improved durability contributes to a longer catalyst duration. Characteristics including mechanical properties and thermal stability can represent the structural durability of fuel cells.Mechanical properties are crucial characteristics concerning the operational condition of fuel cells. The fuel cell membrane must endure the stress and withstand the mechanical degradation generated by physical and chemical stresses.87 Mechanical properties are defined as the properties of materials in response to an applied load.20 It has been reported that the essential mechanical properties to assess fuel cells include the modulus, tensile strength, and elongation at break. The modulus, also known as the elastic modulus, is the elasticity of a material.
Theoretically, by transforming isotropic nanofibers to more oriented nanofibers, for instance, in one axial direction, the mechanical properties of the nanofiber can be enhanced. It has been reported that the geometrical aspects can support the performance of fuel cells. In fuel cell application, this occurrence is also shown in proton exchange membrane application.18,63 It has also been noted that with this approach, the modulus could increase up to 600% (from 1 MPa to > 7 Mpa).62 This observation was found in poly(ε-caprolactone) (PCL), which can be applied as a scaffold in tissue engineering. This experiment strengthened the evidence that changing the geometry of nanofibers to align can increase their mechanical properties. This observation opens a new opportunity to explore the effect of geometry on mechanical properties and other related functional fuel cell characteristics.
Thermal stability is also an important aspect of fuel cells because these devices operate within a certain temperature range, which refers to the stability of the sample when heat is applied. Thermographic analysis (TGA) is a method that can be used to determine this characteristic. This method measures the weight change when a sample is heated.88 It has been mentioned that the combination of TGA and FTIR is reliable for analyzing specific properties.89 TGA is employed to investigate the changes in mass as a function of temperature and time. This test gives data regarding the analysis of thermal decomposition. Nonetheless, this method cannot identify the material during the heating experiment, and thus by combining it with FTIR, which provides the characteristic spectrum of the material, this analytical problem could be addressed.
It has been reported that uniaxial aligned nanofibers containing SPPESK–ZCCH exhibited good thermal stability.18 The result showed that with aligned nanofibers, the stability was higher compared to the starting decomposition temperature of pure ZCCH. This happened because the frameworks of ZCCH are protected by SPPESK wrapped on its surface. It was also mentioned that aligned nanofibers could give a different result regarding thermal stability in proton exchange membrane and anion exchange membrane application. In a proton exchange membrane (sulfonated copolyimide nanofiber), according to the reported results, at 200 °C, the thermal stability was 90% compared to the ion exchange membrane (QAFPK-1-6-E nanofiber), which showed a value of 93%. However, when the temperature increased to 400 °C,90 the proton exchange membrane showed a value of 75%. Meanwhile, the anion exchange membrane presented a value of 70%.81 In high-temperature proton exchange membrane fuel cells (HT-PEMFC), their operating temperatures were reported to be in the range of 120 °C and 200 °C.22 In anion exchange membrane fuel cells (AEMFC), their operating temperature is usually in the range of 50 °C and 80 °C to avoid the degradation of the polymer.91
5.4. Reduced water flooding
Aligned nanofibers show potential for competent water management, preventing water flooding during the fuel cell operation. When excessive liquid water accumulates in the electrode pores, water flooding occurs in fuel cells. This event blocks the reactant gases from accessing the catalyst sites. Aligned nanofibers are promising because their structure can act as capillary channels. Theoretically, aligned nanofibers have better characteristics because of their controlled orientation. This structure allows consistent and reproducible measurements during swelling tests, given that the dimensional changes can be precisely monitored along the aligned direction. Aligned nanofibers also promise to facilitate anisotropic swelling analysis. Fig. 8 illustrates the mechanism of nanofiber suppressing the swelling of the matrix.92,93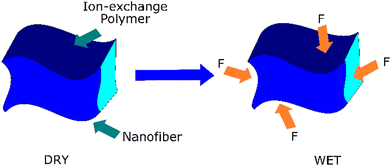 | ||
| Fig. 8 Mechanism of the nanofiber suppressing the swelling of the matrix.92,93 | ||
It has been mentioned that the swelling ratio and water uptake could decrease by using aligned nanofibers. The swelling test could be performed via two approaches, i.e., swelling degree (SD) and water uptake (WU), which was carried out to determine the ratio of fibre expansion. The swelling degree and water uptake were investigated by soaking the samples in water. The swelling degree assesses the changes in sample volume caused by water absorption. The Flory Huggins equation is applied to measure the swelling degree and water uptake.94
In the proton exchange membrane application, the swelling of the aligned nanofibers based on SPESSK material decreased compared to the solution-casted membrane from 21% to 18%. In this experiment, the water uptake decreased from 36% to 34%.17 In alkaline fuel cells, the result also showed a similar outcome. The quaternized-poly(arylene ether sulfone) (Q-PAES) aligned nanofiber exhibited decreased swelling properties. The water uptake was reduced from 7.2% ± 0.4% to 2.8% ± 0.9% according to the chloride anion (Cl−) measurement.80
6. Opportunity: prospects and challenges
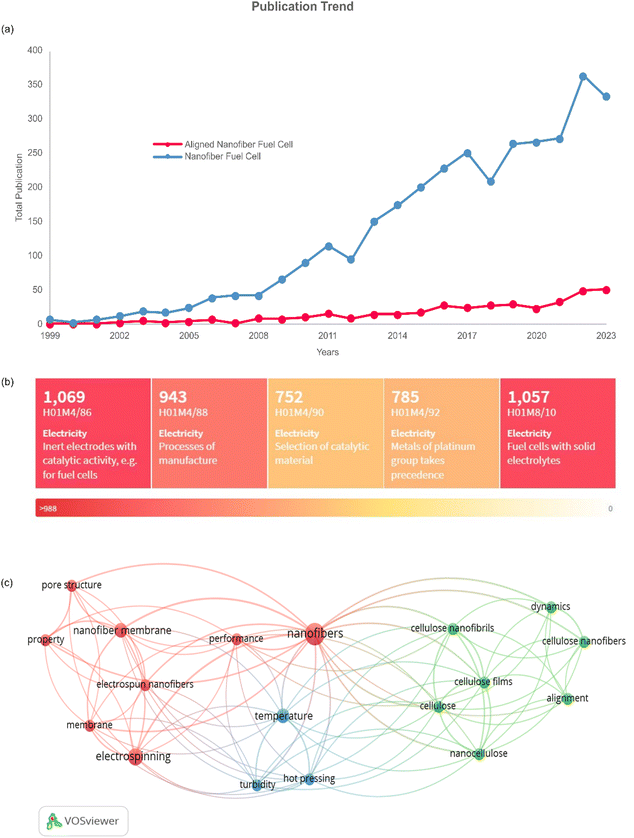
The prospects of aligned nanofiber can be seen in Fig. 9. It can be seen in Fig. 9(a), the journal article publications have a favourable profile in nanofiber application and aligned nanofiber structures. The gap in the number of publications indicates that there is significant opportunity to develop aligned nanofibers, and filling the gap can be one of the approaches to improve the previous research. Based on a heat map of the IPCR classification code, as presented in Fig. 9(b). The main invention of nanofibers in fuel cells is correlated with electrodes with catalytic activity, solid electrolytes, process of manufacture, platinum, and selection of catalytic material. The bibliometric study of this approach is shown in Fig. 9(c), indicating that there are three clusters of nanofiber and alignment. The vital keywords related to aligned nanofibers have co-occurrence, including nanofiber, alignment, performance, membrane, temperature, pore structure, and property.Regarding fuel cell application, the aligned nanofiber enhances the PEMFC performance and can be further exploited in HT-PEMFC applications. In PEMFC, this structure is reported to lead to better proton conductivity, gas permeability, and stability considering chemical, thermal, and mechanical aspects.95Table 3 presents a summary of the current applications of aligned nanofibers, which are dominated by PEMFC. In the case of HT-PMFC, aligned nanofibers are strongly recommended due to their sustained lifespan and prevention of rapid degradation. Furthermore, implementing an aligned nanofiber structure can address the challenges of this type of fuel cell, such as heat management, heat resistance, and providing effective catalysts at high temperatures.
| Method/strategy | Material(s) | Result | App. | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology (nm) | Mechanical strength (MPa) | Conductivity (S cm−1) | IEC (mmol g−1/meq g−1) | Swelling test (%) | Thermal stability (weight loss) | |||||||||
| Non-aligned | Aligned | Non-aligned | Aligned | Non-aligned | Aligned | Non-aligned | Aligned | Non-aligned | Aligned | |||||
| Rotation speed (5000 rpm) | MOFs and SPPESK | 200 | σ = 17,5 | σ = 27 | 5.9 × 10−2 (160 °C) | 8.2 × 10−2 (160 °C) | N/A | N/A | N/A | N/A | ±3% (160) | ±11% (300) | PEMFC | 18 |
| ±4% (300) | ±16% (400) | |||||||||||||
| ±23%(400) | ±2% (160) | |||||||||||||
| *Pure ZCCH | ||||||||||||||
| Rotation speed (>1000 rpm) | SPPESK | 157 ± 52 | E = 15.8 | E = 19.3 | 2.13 × 10−2 | 3.66 × 10−2 (50 °C) | 1.82 | 1.82 | SR = ±21 | SR = ± 18 | N/A | N/A | PEMFC | 17 |
| WU = ± 36 | WU = ± 34 | |||||||||||||
| *Casting | ||||||||||||||
| Two conductive strips | Sulfonated polyimide | 199 ± 37 | N/A | N/A | 3.6 × 10−2 (80 °C) | 8.8 × 10−2 (80 °C) | N/A | N/A | Water uptake | Water uptake | N/A | N/A | PEMFC | 69 |
| *Without nanofiber | 22* | 29 | ||||||||||||
| *Without nanofiber | ||||||||||||||
| Rotation speed (305 m min−1) | SPEEK | 112–131 | N/A | N/A | 7.22 × 10−2 | 9.07 × 10−2 | N/A | N/A | N/A | N/A | N/A | N/A | PEMFC | 66 |
| Two conductive strips | Sulfonated copolyimide | 80–160 | N/A | N/A | 8.36 × 10−2 (90 °C) | 12.2 × 10−2 (90 °C) | 1.65 | 1.65 | Water uptake | Water uptake | ±10% (200) | ±10% (200) | PEMFC | 90 |
| *Without nanofiber | 32% | 42% | ±25% (400) | ±25% (400) | ||||||||||
| *Without nanofiber | ±40%(700) | ±55%(700) | ||||||||||||
| Two conductive strips | Sulfonated polyimide | 108 ± 22 | N/A | N/A | 1 × 10−4 (90 °C) | 8.2 × 10−2 (90 °C) | N/A | 1.65 | N/A | N/A | N/A | N/A | PEMFC | 96 |
| Two conductive strips | Q-PAES | 137 ± 23 | N/A | N/A | 24× 10−2 (90 °C) | 140 × 10−2 (90 °C) | N/A | 1.72 | 7.2 ± 0.4 | 2.8 ± 0.9 Cl_ | N/A | N/A | AFC, air batteries | 80 |
| OH− | OH− | Cl− | ||||||||||||
| Rotation speed (2800 rpm) | QAFPK-1-6-E | 590± 180 | N/A | σ = 54 | 0.9 × 10−2 (80 °C) | 1.81 × 10−2 (80 °C) | 2.14 ± 0.24 (80 °C) | 2.30 ± 0.18 (80 °C) | N/A | ±22% (80 °C) | N/A | ±7% (200) | AEMFC | 81 |
| OH− | OH− | ±11% (300) | ||||||||||||
| ±30% (400) | ||||||||||||||
The challenge of developing aligned nanofibers despite their superior characteristics can be seen from industrialization and scientific engineering aspects. Regarding industrialization, the application of aligned nanofibers sometimes face the cost of manufacturing, considering that in some strategies, additional parts are necessary to fabricate aligned nanofibers, increasing the total cost of manufacturing. In the case of the second point of view, the type of material itself should also be mentioned because some materials act and have to be treated differently considering their unique characteristics; for instance, in a guide column array, a calcination process is conducted to produce aligned nanofibers with ceramic materials.13 However, the uniqueness of materials also has advantages to mix their superior properties. The composition of nanofibers also affects the performance of fuel cells. Composite materials have the advantage of mixing the advantageous properties of each component material. For instance, in the case of a material that needs to be improved, for instance withstanding a higher mechanical load, a material that has good mechanical strength can be added to the composition. The preparation and characterization of polymer-based composite membranes for anion exchange membrane fuel cells using composite PVA-based materials have been reported. This polymer is promising to improve the performances of fuel cells due to the presence of reactive functional groups, which are valuable for improving the properties of the membrane by chemical crosslinking or other chemical modification.87,97 A study aimed to achieve good OH-conductivity, high mechanical properties, and excellent chemical stability. The results showed that the composite could offer new prospects for alkaline polymer electrolyte fuel cells.
Finding the optimum value in some cases also must be considered because bigger is not always better correlated with the characteristic fuel cell itself. For instance, it has been mentioned that the content of electrolyte in the active layer should be at an optimum value. When the substance content is too low, the problem is that not all the catalyst particles are connected to the electrolyte. When the substance content is too high, the gas diffusion is hindered, making the support material electrically isolated.98 Optimization is necessary to find the optimum value for improving the fuel cell performance.
Nanofibers are well known for their significant applications in a wide range of electrochemical devices. It has been reported that this technology is implemented in batteries, sensors, supercapacitors, photovoltaic cells, electrolysis, and fuel cells. Nanofibers are used as electrolytes, cathode materials, anode materials, and separators in batteries.
Electrospinning results in a high surface-to-volume ratio. This characteristic can be used in several electrolytic cell purposes, for instance, dye-degradation applications,99 water dissociation or splitting,100 and disinfection of water (from urea, for example).101 In sensor application, the fibre structure and orientation were reported to lead to high sensitivity.102 In electrochemical solar cell application, dye-sensitized solar cells (DSSCs) or perovskite solar cells can be explored using this approach.103 The application of nanofibers in batteries can improve their cyclic stability104 and enhance their capacity.105 The highly porous structure has the advantage of reducing the degradation rate during charging or discharging.105 Coating methods can also improve their structure, which leads to faster diffusion.103 According to the operating mechanism of the supercapacitor, its performance is affected by surface area and morphology. Numerous studies have illustrated that an optimized pore size and surface area can improve the performance of electrodes. It has been reported that carbon-based nanofibrous materials can improve the ion migration to the active surfaces, leading to enhanced interfacial charge transportation.103,106
Nanofibers have been widely used in fuel cell research due to their advantages, resulting in promising characteristics. In fuel cell application, nanofibers can be used as mats that could be implemented as a membrane, cathode material or anode material. The advantage of the electrospinning manufacturing process is its capability to tailor the morphology, leading to enhanced physical and chemical material properties.
Nanofibers are implemented to improve the cathode function and are successfully constructed in fuel cell applications. Regarding ion transfer properties, the results showed that the advantages of adding these structures are improving the electrical conductivity107 and enhancing the catalytic activity toward oxygen reduction.108–110 Concerning mechanical properties, these structures successfully increased the mechanical strength111 and lowered carbon corrosion.112 Another interesting observation was that by adding nanofibers or transforming structures into nanofibers, the temperature operation could be lower108,113 and the water flooding also decreased.114 The popular materials for building cathodes are composite-based materials using PAN,107,114,115 PVDF,107,112 and PAA.116,117
The anode can also be improved by adding nanofibers. Benefits such as improving activity for oxidation,118,119 showing decent thermal and redox cycling stability,120 reducing the barriers for gas diffusion,121 and enhancing the electrochemical performance at a concurrently lowered platinum122 loading are noted due to the application of nanofiber assemblies. The structured materials utilized to fabricate nanofiber structures for the anode in fuel cells include carbon nanotubes (CNT),120 carbon nanofibers (CNF),123–126 nickel/cadmium,121,127 PVA,118,127 and TiO2.128,129
Regarding the membrane, its main characteristics are also enhanced by adding nanofiber structures. Specific properties such as conductivity, mechanical integrity, and chemical stability also result in better performances. It has been reported that materials such as SPPESK17,18,130,131 and PVDF132–136 can be highly engineered to create a better membrane. Interestingly, the anion exchange membrane has not been highly explored to date compared to the proton exchange membrane. This finding opens the opportunity to seek aligned nanofibers to improve the performance in this type of fuel cell, considering that this type of fuel cell has advantages, especially in terms of reduced cost and high efficiency. Table 4 shows the reports on nanofiber application in fuel cells in detail, and their results are highlighted.
| Application | Material | Type | Result/effect | Ref. |
|---|---|---|---|---|
| Cathode | Hydrophobic graphitized carbon, PAN | PEFC | Decreasing water flooding | 114 |
| Cathode | Nafion/PVDF | PEFC | Lowering carbon corrosion enhanced stress | 112 |
| Cathode | Nafion/PAA, PtCo/C and Pt/C catalyst powders | PEFC | Showing a better initial functioning as well as a superior long-term strength for the electrospun cathodes | 137 |
| Cathode | PAA | PEFC | Decreasing agglomerations of platinum on carbon catalyst elements in catalyst inks | 116 |
| Cathode | PAA/Nafion | PEFC | Performing under low and high feed gas humidification | 117 |
| Cathode | Graphene fixed PAN/PVDF (GPP) | PEFC | Improving electrical conductivity and high porosity. Improving the triple reaction boundary. Stimulate gas and water transport throughout the porous electrode | 107 |
| Cathode | SPEEK fixed with SCNFs | DEFC | Increasing mechanical strength, proton conductivity, and reduced methanol permeability | 111 |
| Cathode | CNx sheet on PAN obliged by the Nafion distribution | DEFC | Performing a power density resembling gold or platinum catalysts. | 138 |
| Cathode | Carbon nitride/polyacrylonitrile nanofibers | DEFC | Improving in oxygen reduction reaction activity | 110 |
| Cathode | PAN, Fe–N/C | DEFC | Assisting active sites, assisted oxygen supply to the active surfaces | 115 |
| Cathode | Lanthanum strontium cobalt ferrite (LSCF) | MCFC | Reducing operation temperature to (750 °C) | 139 |
| Cathode | Yttria-stabilized zirconia with the penetrated LSM | MCFC | Enhancing catalytic activity toward oxygen reduction | 108 |
| Cathode | LSCF | MCFC | Having low operation reduction at (750 °C) | 113 |
| Cathode | (LSCF) tubes/(GDC) nanoparticles | MCFC | Having a reduce operational temperature (650 °C) | 140 |
| Cathode | Sm0.5Sr0.5CoO3−δ and Gd0.2Ce0.8O1.9 | MCFC | Performing major improve of the electrode working | 141 |
| Cathode | Polyacrylonitrile pyro polymer | PAFC | Improving polarization and improved catalytic activity | 109 |
| Cathode | Polyacrylonitrile | PAFC | Being used as the gas diffusion electrodes in high temperature hydrogen | 142 |
| Cathode | FeCo-CNF | AFC | Possessing the equivalent electrocatalytic activity. Higher tolerance to cross overed ethanol compared with Pt/C in the ORR | 143 |
| Anode | TiO2–C/C | Microbial Fuel Cell (MFC) | Having good electrical performance | 128 |
| Anode | CNTs/CNF | MFC | Exhibiting a better conductivity, biocompatibility, hydrophilicity and electrocatalytic activity | 123 |
| Anode | ACNF, with and without CNTs | MFC | Performing the high electrode conductivity, stability, and biocompatibility | 124 |
| Anode | N–CNFs | MFC | Decreasing the process costs simultaneously while retaining excellent properties | 125 |
| Anode | ACNF | MFC | Reporting ACNF exhibited better performance than carbon anodes granular activated carbon, carbon cloth | 126 |
| Anode | NiSn alloy nanoparticle, nickel acetate, tin chloride and PVA | Direct Urea Fuel Cell (DUFC) | Improving activity for oxidation, a high current density for urea oxidation | 118 |
| Anode | PVA, Ni/Pd–C | DUFC | Reporting urea concentration and polarized potential on the impedance behavior | 127 |
| Anode | Ni/Cd-decorated electrospun carbon nanofibers | DUFC | Reporting the low catalytic activity of the anode, extensively increased electrocatalytic activity for urea oxidation, | 119 |
| Anode | LaxSr1−xTiO3–GdyCe1−yO2−δ | SOFC | improve the electrochemical performance | 144 |
| Anode | La0.4Sr0.6TiO3 (LST), YSZ, Gd0.2Ce0.8O1.9, Ni | SOFC | Showing Decent thermal and redox cycling solidity | 120 |
| Anode | Ni-coated yttria-stabilized zirconia | SOFC | Enhancing the electrochemical reaction sites and also to reduce the difficulties for gas diffusion. | 121 |
| Anode | SrCe0.8Y0.2O3−δ–Ni | SOFC | Showing enhancement mechanism in calcined particle properties and proton hopping distance. | 145 |
| Anode | Sr2FeTiO6−δ | SOFC | Indicating that SFT is an actual promising electrode candidate (IT-SOFCs with SDC electrolyte) | 146 |
| Anode | Carbon–CeO2 | DMFC | Enhancing the electrochemical performance at concurrently lowered platinum loading | 122 |
| Anode | Carbon–CeO2, nickel acetate tetrahydrate, (PVA) and urea | DMFC | Proposing approach developed carbon nanofibers containing nickel nanoparticles and fixed by nitrogen. | 147 |
| Anode | PVDF/Pt–Pd/RGO–CeO2 | DMFC | PVDF–Pt–Pd/RGO–CeO2 as the new catalyst material for DMFC. | 148 |
| Anode | TiO2 carbon | DMFC | Ensuring that the best catalytic material focuses on the fabrication of a new composite TiO2 carbon nanofiber anodic catalyst support | 129 |
| Anode | Polyacrylonitrile (PAN), A TiO2-embedded carbon nanofiber (TECNF) | DMFC | TECNF is a promising support of the PtRu nanocatalyst for the methanol oxidation reaction | 149 |
| Anode | Polypyrrole nanowire networks (PPNNs) | DMFC | Showing significantly improves catalyst utilization and mass transfer of methanol on the anode. | 150 |
| Anode | CeO2–C with Pt–Co nanoparticles | DMFC | Proposing the combination of two effective systems, i.e. CeO2–C and Pt–Co | 151 |
| Membrane | Nafion perfluorosulfonic acid/PVDF | SOFC | Providing a fabrication strategy for high-performance electrodes | 132 |
| Membrane | Nafion® PFSA and PVDF | H2Br2 fuel cells | Increasing PVDF content declines in proton conductivity, water/electrolyte swelling and permeability | 152 |
| Membrane | Nafion/polyphenylsulfone | PEFC | Showing excellent water swelling and mechanical properties as well as proton conductivity | 153 |
| Membrane | Nafion® perfluorosulfonic acid (PFSA) ionomer for proton transport and polyvinylidene fluoride (PVDF) for mechanical reinforcement. | Hydrogen/bromine fuel cell | Fabricating and characterizing nanofiber composite for regenerative hydrogen/bromine fuel cell | 154 |
| Membrane | Nafion® PFSA, polyphenylsulfone (PPSU) | H2/Br2-HBr fuel cell | Reporting nanofiber composite membranes can overcome the high cost of PFSA | 155 |
| Membrane | Polyvinylidene fluoride (PVDF)/Nafion | MFC | Producing electricity from a single culture MFC | 135 |
| Membrane | SPPESK | PEFC | Enhancements on open circuit voltage and power density. | 130 |
| Fiberization increases proton conductivity, swelling resistance, and mechanical and thermal stabilities. | ||||
| Membrane | PVDF/Nafion | PEFC | Obtaining via electrospinning experiencing a “reciprocal templating” experience that performs electrical performance | 156 |
| Membrane | SPPESK and poly(phenylene oxide) | Bipolar membrane (BPM) | Performing electrodialysis, hydrogen production, and self-humidifying fuel cells | 131 |
| Membrane | MOFs and SPPESK | PEFC | Increase proton conductivity (aligned nanofiber) | 18 |
| DMFC | ||||
| Membrane | SPPESK | PEFC | Increasing conductivity in the thickness aligned. Enhancing single cell power density and tensile strength | 17 |
| Membrane | Sulfonated polyimide | PEFC | Performing the membrane stability, Decreasing oxygen permeability | 157 |
7. Conclusion
Herein, the use of aligned nanofiber structures was elaborated as a promising strategy to improve the performance of fuel cells. This type of structure can be fabricated in a versatile way using the electrospinning method. To date, although various fabrication techniques have been developed utilizing this apparatus, the simplest way to achieve an aligned structure is by increasing the speed of the rotating drum collector (800–5000 rpm). An exciting approach is modifying the patterned strip (two, four, or six strips) on the collector as an electrode. The results showed that this technique produced one, two, and three axial lines, respectively.Concerning why aligned nanofiber can improve the fuel cell performance, the effect of aligned nanofibers on conductivity was explained. This structure network provides a proton channel structure, enhancing the rapid transport of protons. As a result, an excellent through-plane proton transport performance was shown in the fuel cell operation. It was also reported that the anionic conductivity could be increased by 10–15 times. Regarding mass transport, it should be noted that the ion transport characteristic is also affected by chemical structures. This factor is correlated with the flexibility of the polymer chains and related to the types of ion exchange groups. The most remarkable result is that oriented nanofibers provided structural durability. The mechanical strength was reported to be enhanced by up to 600%, which is an essential property in fuel cells under highly dynamic operating conditions. Aligned nanofibers also promise to reduce the weight loss due to the fuel cell operation. It has been mentioned that the frameworks of certain materials can be protected by nanofibers wrapped on their surface. To reduce water flooding, it has been mentioned that the swelling ratio and water uptake can decrease by using aligned nanofibers. This result is promising for a practical fuel cell operation, considering that an ideal component, such as a membrane, should have highly depressed water absorption.
All the above-mentioned results open new opportunities for research into this promising approach, which improves the performance of fuel cells through the geometry and alignment of nanofibers. The advantage of the electrospinning manufacturing process is that the capability to tailor the morphology leads to enhanced physical and chemical material properties. The complexity and additional manufacturing should also be considered and the characteristics of the material and optimum condition need to be determined as challenges in this approach. Nevertheless, the potential of using aligned nanofiber structures and the key influencing parameters for the development of fuel cells still need to be extensively investigated. Research into future applications of this technology is still in its infancy, but in fuel cells alone, it can be used as the membrane, anode, and cathode materials.
Author contributions
Conceptualization, M. Y. and V. H.; writing – original draft, M. Y.; writing – review and editing, M. Y. and V. H.; visualization, M. Y.; supervision, V. H.; funding acquisition, V. H. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Open Access Funding by TU Graz Open Access Publishing Fund. Additionally, the authors acknowledge the IEA Research Co-operation on behalf of the Austrian Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology, and OeAD (Austria) for the Ernst Mach Grant ASEA-UNINET scholarship.Notes and references
- C. Zou, Q. Zhao, G. Zhang and B. Xiong, Energy revolution: from a fossil energy era to a new energy era, Nat. Gas Ind. B, 2016, 3, 1–11 CrossRef.
- C. Schelly, D. Bessette, K. Brosemer, V. Gagnon, K. L. Arola, A. Fiss, J. M. Pearce and K. E. Halvorsen, Energy policy for energy sovereignty: can policy tools enhance energy sovereignty?, Sol. Energy, 2020, 205, 109–112 CrossRef.
- H. Chen, T. N. Cong, W. Yang, C. Tan, Y. Li and Y. Ding, Progress in electrical energy storage system: a critical review, Prog. Nat. Sci., 2009, 19, 291–312 CrossRef CAS.
- I. S. Chronakis, Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process – A review, J. Mater. Process. Technol., 2005, 167, 283–293 CrossRef CAS.
- X. Gong, G. He, X. Yan, Y. Wu, W. Chen and X. Wu, Electrospun nanofiber enhanced imidazolium-functionalized polysulfone composite anion exchange membranes, RSC Adv., 2015, 5, 95118–95125 RSC.
- S. G. Peera, R. Koutavarapu, S. Akula, A. Asokan, P. Moni, M. Selvaraj, J. Balamurugan, S. O. Kim, C. Liu and A. K. Sahu, Carbon Nanofibers as Potential Catalyst Support for Fuel Cell Cathodes: A Review, Energy Fuels, 2021, 35, 11761–11799 CrossRef CAS.
- C. Klose, M. Breitwieser, S. Vierrath, M. Klingele, H. Cho, A. Büchler, J. Kerres and S. Thiele, Electrospun sulfonated poly(ether ketone) nanofibers as proton conductive reinforcement for durable Nafion composite membranes, J. Power Sources, 2017, 361, 237–242 CrossRef CAS.
- J. Doshi and D. H. Reneker, Electrospinning process and applications of electrospun fibers, Conf. Rec. - IAS Annu. Meet. (IEEE Ind. Appl. Soc.), 1993, 3, 1698–1703 Search PubMed.
- A. Theron, E. Zussman and A. Yarin, Electrostatic field-assisted alignment of electrospun nanofibre, Nanotechnology, 2001, 12, 384–390 CrossRef.
- J. M. Deitzel, J. D. Kleinmeyer, J. K. Hirvonen and N. C. Beck Tan, Controlled deposition of electrospun poly(ethylene oxide) fibers, Polymer, 2001, 42, 8163–8170 CrossRef CAS.
- D. Li, Y. Ru, Z. Chen, C. Dong, Y. Dong and J. Liu, Accelerating the design and development of polymeric materials via deep learning: current status and future challenges, APL Mach. Learn., 2023, 1, 021501 CrossRef.
- F. Dabirian, S. Sarkeshik and A. Kianiha, Production of Uniaxially Aligned Nanofibers Using a Modified Electrospinning Method: Rotating Jet, Curr. Nanosci., 2009, 5, 318–323 CrossRef CAS.
- M. A. K. Budi, A. Kubart and J. S. Andrew, Guide column array: a versatile approach to aligning and patterning ceramic nanofibers, Nanoscale, 2018, 10, 20681–20688 RSC.
- J. Lee and Y. Deng, Increased mechanical properties of aligned and isotropic electrospun PVA nanofiber webs by cellulose nanowhisker reinforcement, Macromol. Res., 2012, 20, 76–83 CrossRef CAS.
- G. Liu, F. Ye, L. Xiong, J. Lee, L. Wang, X. Li, J. Li, J. K. Lee and W. Yang, Cathode catalyst layer with nanofiber microstructure for direct methanol fuel cells, Energy Convers. Manage., 2020, 218, 113013 CrossRef CAS.
- J. K. Chinthaginjala, K. Seshan and L. Lefferts, Preparation and application of carbon-nanofiber based microstructured materials as catalyst supports, Ind. Eng. Chem. Res., 2007, 46, 3968–3978 CrossRef CAS.
- X. Gong, G. He, Y. Wu, S. Zhang, B. Chen, Y. Dai and X. Wu, Aligned electrospun nanofibers as proton conductive channels through thickness of sulfonated poly(phthalazinone ether sulfone ketone) proton exchange membranes, J. Power Sources, 2017, 358, 134–141 CrossRef CAS.
- B. Wu, J. Pan, L. Ge, L. Wu, H. Wang and T. Xu, Oriented MOF-polymer composite nanofiber membranes for high proton conductivity at high temperature and anhydrous condition, Sci. Rep., 2014, 4, 1–7 Search PubMed.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- A. M. Samsudin, M. Bodner and V. Hacker, A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells, Polymers, 2022, 14, 1–26 Search PubMed.
- EG&G Technical Services, Inc., Fuel Cell Handbook, U.S. Department of Energy Office of Fossil Energy National Energy Technology Laboratory, Morgantown, West Virginia, 7th edn, 2004.
- M. Cassir, D. Jones, A. Ringuedé and V. Lair, Electrochemical devices for energy: Fuel cells and electrolytic cells, 2013, 2 Search PubMed.
- R. E. Rosli, A. B. Sulong, W. R. W. Daud, M. A. Zulkifley, T. Husaini, M. I. Rosli, E. H. Majlan and M. A. Haque, A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system, Int. J. Hydrogen Energy, 2017, 42, 9293–9314 CrossRef CAS.
- R. G. Bodkhe, R. L. Shrivastava, V. K. Soni and R. B. Chadge, A review of renewable hydrogen generation and proton exchange membrane fuel cell technology for sustainable energy development, Int. J. Electrochem. Sci., 2023, 18, 100108 CrossRef CAS.
- M. Iravaninia and S. Rowshanzamir, Polysulfone-based Anion Exchange Membranes for Potential Application in Solid Alkaline Fuel Cells, J. Renewable Energy Environ., 2015, 2, 59–65 Search PubMed.
- K. Kargupta, S. Saha, D. Banerjee, M. Seal and S. Ganguly, Performance enhancement of phosphoric acid fuel cell using phosphosilicate gel based electrolyte, Ranliao Huaxue Xuebao, 2012, 40, 707–713 CAS.
- Fuel Cell & Hydrogen Energy Association, Phosporic Acid Fuel Cell, https://www.fchea.org/fc-types/9l1r9u6rfbm891j33hxjiod7el0028.
- E. Antolini, The stability of molten carbonate fuel cell electrodes: A review of recent improvements, Appl. Energy, 2011, 88, 4274–4293 CrossRef CAS.
- M. A. R. S. Al-Baghdadi, Sch. Community Encycl., 2022 Search PubMed.
- M. A. R. S. Al-Baghdadi, Computational Modeling Aspects of Polymer Electrolyte Fuel Cell Durability, Computational Fluid Dynamics: Theory, Analysis and Applications, Nova Science Publishers, Inc, New York USA, 2011, pp. 1–40 Search PubMed.
- M. A. R. S. Al-Baghdadi and H. A. K. S. Al-Janabi, Influence of the design parameters in a proton exchange membrane (PEM) fuel cell on the mechanical behavior of the polymer membrane, Energy Fuels, 2007, 21, 2258–2267 CrossRef CAS.
- M. A. R. S. Al-Baghdadi, Proton exchange membrane fuel cells modeling: a review of the last ten years results of the Fuel Cell Research Center-IEEF, Int. J. Energy Environ., 2017, 8, 1–28 CrossRef.
- M. A. R. S. Al-Baghdadi and H. A. K. S. Al-Janabi, Effect of PEM fuel cell operation on gas diffusion layers and membrane stresses, Int. J. Fluid Mech. Res., 2008, 35, 219–234 CrossRef CAS.
- M. A. R. S. Al-Baghdadi and H. A. K. S. Al-Janabi, Prediction of hygro-thermal stress distribution in proton exchange membranes using a three-dimensional multi-phase computational fluid dynamics model, Proc. Inst. Mech. Eng., Part A, 2007, 221, 941–953 CrossRef CAS.
- M. Yusro and R. Martien, Investigating Fluid Parameters in Nanofiber Biomaterial Fabrication using Electrospinning, J. Energy, Mech. Mater. Manuf. Eng., 2020, 5, 11–24 Search PubMed.
- B. Swanckaert, J. Geltmeyer, K. Rabaey, K. De Buysser, L. Bonin and K. De Clerck, A review on ion-exchange nanofiber membranes: properties, structure and application in electrochemical (waste)water treatment, Sep. Purif. Technol., 2022, 287, 1–45 CrossRef.
- S. Imaizumi, H. Matsumoto, M. Ashizawa, M. Minagawa and A. Tanioka, Nanosize effects of sulfonated carbon nanofiber fabrics for high capacity ion-exchanger, RSC Adv., 2012, 2, 3109–3114 RSC.
- H. V. Mhetre, K. Y. Krishnarao and N. Naik, Optimization of electrospinning process parameters to develop the smallest ZnO + PVP nanofibres using Taguchi experimental design and ANOVA, J. Mater. Sci.: Mater. Electron., 2023, 34, 1–15 CrossRef.
- N. Amini, M. Kalaee, S. Mazinani, S. Pilevar and S. O. Ranaei-Siadat, Morphological optimization of electrospun polyacrylamide/MWCNTs nanocomposite nanofibers using Taguchi's experimental design, Int. J. Adv. Manuf. Technol., 2013, 69, 139–146 CrossRef.
- F. S. Alfares, E. Guler, H. Alenezi, M. E. Cam and M. Edirisinghe, Optimization of Process-Control Parameters for the Diameter of Electrospun Hydrophilic Polymeric Composite Nanofibers, Macromol. Mater. Eng., 2021, 306(12), 2100471 CrossRef CAS.
- M. Elkasaby, H. A. Hegab, A. Mohany and G. M. Rizvi, Modeling and optimization of electrospinning of polyvinyl alcohol (PVA), Adv. Polym. Technol., 2018, 37, 2114–2122 CrossRef CAS.
- H. Albetran, Y. Dong and I. M. Low, Characterization and optimization of electrospun TiO2/PVP nanofibers using Taguchi design of experiment method, J. Asian Ceram. Soc., 2015, 3, 292–300 CrossRef.
- Z. Zhou, X. F. Wu, X. Gao, L. Jiang, Y. Zhao and H. Fong, Parameter dependence of conic angle of nanofibres during electrospinning, J. Phys. D: Appl. Phys., 2011, 44(43), 435401 CrossRef.
- S. Suresh, A. Becker and B. Glasmacher, Impact of apparatus orientation and gravity in electrospinning—a review of empirical evidence, Polymers, 2020, 12, 1–15 CrossRef PubMed.
- C. Akduman and E. P. A. Kumbasar, Electrospun Polyurethane Nanofibers, Aspects Polyurethanes, 2017,(September), 17–52, DOI:10.5772/intechopen.69937.
- J. A. Abbas, I. A. Said, M. A. Mohamed, S. A. Yasin, Z. A. Ali and I. H. Ahmed, Electrospinning of polyethylene terephthalate (PET) nanofibers: optimization study using taguchi design of experiment, IOP Conf. Ser. Mater. Sci. Eng., 2018, 454, 012130 CrossRef.
- J. I. Kim, T. I. Hwang, L. E. Aguilar, C. H. Park and C. S. Kim, A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up, Sci. Rep., 2016, 6, 1–12 CrossRef PubMed.
- C. Ayres, G. L. Bowlin, S. C. Henderson, L. Taylor, J. Shultz, J. Alexander, T. A. Telemeco and D. G. Simpson, Modulation of anisotropy in electrospun tissue-engineering scaffolds: analysis of fiber alignment by the fast Fourier transform, Biomaterials, 2006, 27, 5524–5534 CrossRef CAS PubMed.
- N. E. Zander, Hierarchically structured electrospun fibers, Polymers, 2013, 5, 19–44 CrossRef.
- H. Mohammad Khanlou, B. Chin Ang, S. Talebian, A. Muhammad Afifi and A. Andriyana, Electrospinning of polymethyl methacrylate nanofibers: optimization of processing parameters using the Taguchi design of experiments, Text. Res. J., 2015, 85, 356–368 CrossRef.
- A. Nazir, N. Khenoussi, L. Schacher, T. Hussain, D. Adolphe and A. H. Hekmati, Using the Taguchi method to investigate the effect of different parameters on mean diameter and variation in PA-6 nanofibres produced by needleless electrospinning, RSC Adv., 2015, 5, 76892–76897 RSC.
- M. Yusro, Assessing Beads Generation in Fabricating Nanofiber Bioactive Material-Based Associated with Its Fluid Factors, Lect. Notes Electron. Eng., 2021, 746 LNEE, 173–182 CrossRef PubMed.
- O. Hardick, B. Stevens and D. G. Bracewell, Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency, J. Mater. Sci., 2011, 46, 3890–3898 CrossRef CAS.
- N. Bhardwaj and S. C. Kundu, Electrospinning: a fascinating fiber fabrication technique, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS PubMed.
- A. Haider, S. Haider and I.-K. Kang, A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology, Arab. J. Chem., 2018, 11, 1165–1188 CrossRef CAS.
- S. De Vrieze, T. Van Camp, A. Nelvig, B. Hagström, P. Westbroek and K. De Clerck, The effect of temperature and humidity on electrospinning, J. Mater. Sci., 2009, 44, 1357–1362 CrossRef CAS.
- D. Li, Y. Wang and Y. Xia, Electrospinning Nanofibers as Uniaxially Aligned Arrays and Layer-by-Layer Stacked Films, Adv. Mater., 2004, 16, 361–366 CrossRef CAS.
- P. Katta, M. Alessandro, R. D. Ramsier and G. G. Chase, Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector, Nano Lett., 2004, 4, 2215–2218 CrossRef CAS.
- R. Dersch, T. Liu, A. K. Schaper, A. Greiner and J. H. Wendorff, Electrospun nanofibers: internal structure and intrinsic orientation, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 545–553 CrossRef CAS.
- M. Khamforoush and M. Mahjob, Modification of the rotating jet method to generate highly aligned electrospun nanofibers, Mater. Lett., 2011, 65, 453–455 CrossRef CAS.
- P. Nitti, N. Gallo, L. Natta, F. Scalera, B. Palazzo, A. Sannino and F. Gervaso, Influence of nanofiber orientation on morphological and mechanical properties of electrospun chitosan mats, J. Healthcare Eng., 2018, 2018, 3651480 Search PubMed.
- B. M. Baker and R. L. Mauck, The Effect of Nanofiber Alignment on the Maturation of Engineered Meniscus Constructs, Biomaterials, 2007, 28, 1967–1977 CrossRef CAS PubMed.
- H. M. Paulya, D. J. Kelly, K. C. Popata, N. A. Trujillof, N. J. Dunneg, H. O. McCarthyi and T. L. H. Donahuea, Mechanical Properties and Cellular Response of Novel Electrospun Nanofibers for Ligament Tissue Engineering: Effects of Orientation and Geometry, J. Mech. Behav. Biomed. Mater., 2016, 61, 1–40 CrossRef PubMed.
- L. Li, Y. Liu and Y. F. Li, Electrochemical degradation of methylene blue aqueous solution on electrospinning nanofibers (ESF)electrodes, Adv. Mater. Res., 2013, 807–809, 1362–1367 Search PubMed.
- S. D. Liu, D. Sen Li, Y. Yang and L. Jiang, Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters, Nanotechnol. Rev., 2019, 8, 218–226 CAS.
- M. J. Mehdi Sadrjahani, A. Akbar Gharehaghaji and M. Javanbakht, Aligned Electrospun Sulfonated Poly(ether ether ketone) Nanofiber-Based Proton Exchange Membranes for Fuel Cell Applications, Polym. Eng. Sci., 2017, 789–796 CrossRef.
- C. Y. Huang, K. H. Hu and Z. H. Wei, Comparison of cell behavior on pva/pva-gelatin electrospun nanofibers with random and aligned configuration, Sci. Rep., 2016, 6, 1–8 CrossRef PubMed.
- D. Li, Y. Wang and Y. Xia, Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays, Nano Lett., 2003, 3, 1167–1171 CrossRef CAS.
- T. Tamura and H. Kawakami, Aligned electrospun nanofiber composite membranes for fuel cell electrolytes, Nano Lett., 2010, 10, 1324–1328 CrossRef CAS PubMed.
- S. H. Park and D.-Y. Yang, Fabrication of Aligned Electrospun Nanofibers by Inclined Gap Method, J. Appl. Polym. Sci., 2011, 120, 1800–1807 CrossRef CAS.
- T. A. Kowalewski and S. Barral, Modelling electrospinning of nanofibres, PAMM, 2009, 9, 463–464 CrossRef.
- O. Karatay and M. Dogan, Modelling of electrospinning process at various electric fields, Micro Nano Lett., 2011, 6, 858–862 CrossRef CAS.
- N. A. Norzain and W. C. Lin, Orientated and diameter-controlled fibrous scaffolds fabricated using the centrifugal electrospinning technique for stimulating the behaviours of fibroblast cells, J. Ind. Text., 2022, 51, 6728S–6752S CrossRef CAS.
- Z. Zhang and J. Sun, Research on the development of the centrifugal spinning, MATEC Web Conf., 2017, 95, 07003 CrossRef.
- G. Anusiya and R. Jaiganesh, A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers, Carbohydr. Polym. Technol. Appl., 2022, 4, 41–55 Search PubMed.
- S. M. Taghavi and R. G. Larson, Regularized thin-fiber model for nanofiber formation by centrifugal spinning, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89, 1–9 Search PubMed.
- S. Padron, A. Fuentes, D. Caruntu and K. Lozano, Experimental study of nanofiber production through forcespinning, J. Appl. Phys., 2013, 113(2), 024318 CrossRef.
- L. Wu, Z. Zhang, J. Ran, D. Zhou, C. Li and T. Xu, Advances in proton-exchange membranes for fuel cells: an overview on proton conductive channels (PCCs), Phys. Chem. Chem. Phys., 2013, 15, 4870–4887 RSC.
- M. B. DeGostin, A. A. Peracchio, T. D. Myles, B. N. Cassenti and W. K. S. Chiu, Charge transport in the electrospun nanofiber composite membrane's three-dimensional fibrous structure, J. Power Sources, 2016, 307, 538–551 CrossRef CAS.
- T. Watanabe, M. Tanaka and H. Kawakami, Fabrication and electrolyte characterization of uniaxially-aligned anion conductive polymer nanofibers, Nanoscale, 2016, 8, 19614–19619 RSC.
- Y. C. Zhou, R. Y. Bao, Z. Liu, M. B. Yang and W. Yang, Electrospun Modified Polyketone-Based Anion Exchange Membranes with High Ionic Conductivity and Robust Mechanical Properties, ACS Appl. Energy Mater., 2021, 4, 5187–5200 CrossRef CAS.
- K. Bunzl and B. Sansoni, Determination of the ion exchange capacity of solid ion exchangers by difference weighting, Anal. Chem., 1976, 48, 2279–2280 CrossRef CAS.
- P. Kumar, R. P. Bharti, V. Kumar and P. P. Kundu, in Progress and Recent Trends in Microbial Fuel Cells, ed. P. P. Kundu and K. Dutta, Elsevier, 2018, pp. 47–72 Search PubMed.
- K. S. Spiegler, Transport processes in ionic membranes, Trans. Faraday Soc., 1958, 54, 1408–1428 RSC.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner, P. A. Kohl, A. R. Kucernak, W. E. Mustain, K. Nijmeijer, K. Scott, T. Xu and L. Zhuang, Anion-exchange membranes in electrochemical energy systems, Energy Environ. Sci., 2014, 7, 3135–3191 RSC.
- T. Watanabe, M. Tanaka and H. Kawakami, Fabrication and electrolyte characterization of uniaxially-aligned anion conductive polymer nanofibers, Nanoscale, 2016, 8, 19614–19619 RSC.
- A. M. Samsudin, M. Roschger, S. Wolf and V. Hacker, Preparation and Characterization of QPVA/PDDA Electrospun Nanofiber Anion Exchange Membranes for Alkaline Fuel Cells, Nanomaterials, 2022, 12(22), 3965 CrossRef CAS PubMed.
- S. Ebnesajjad, in Handbook of Adhesives and Surface Preparation Technology, Applications and Manufacturing, ed. S. Ebnesajjad, William Andrew Publishing, Norwich, NY, 2011, pp. 31–48 Search PubMed.
- J. Abraham, A. P. Mohammed, M. P. Ajith Kumar, S. C. George and S. Thomas, in Micro and Nano Technologies, ed. S. Mohan Bhagyaraj, O. S. Oluwafemi, N. Kalarikkal and S. B. T.-C. of N. Thomas, Woodhead Publishing, 2018, pp. 213–236 Search PubMed.
- T. Tamura, R. Takemori and H. Kawakami, Proton conductive properties of composite membranes containing uniaxially aligned ultrafine electrospun polyimide nanofiber, J. Power Sources, 2012, 217, 135–141 CrossRef CAS.
- K. Yassin, I. G. Rasin, S. Willdorf-Cohen, C. E. Diesendruck, S. Brandon and D. R. Dekel, A surprising relation between operating temperature and stability of anion exchange membrane fuel cells, J. Power Sources Adv., 2021, 11, 100066 CrossRef CAS.
- D. M. Yu, S. Yoon, T.-H. Kim, J. Y. Lee, J. Lee and Y. T. Hong, Properties of sulfonated poly(arylene ether sulfone)/electrospun nonwoven polyacrylonitrile composite membrane for proton exchange membrane fuel cells, J. Membr. Sci., 2013, 446, 212–219 CrossRef CAS.
- B. Swanckaert, J. Geltmeyer, K. Rabaey, K. De Buysser, L. Bonin and K. De Clerck, A review on ion-exchange nanofiber membranes: properties, structure and application in electrochemical (waste)water treatment, Sep. Purif. Technol., 2022, 287, 120529 CrossRef CAS.
- D. Archana, J. Dutta and P. K. Dutta, Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies, Int. J. Biol. Macromol., 2013, 57, 193–203 CrossRef CAS PubMed.
- P. Kallem, N. Yanar and H. Choi, Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications, ACS Sustainable Chem. Eng., 2019, 7, 1808–1825 CrossRef CAS.
- R. Takemori, G. Ito, M. Tanaka and H. Kawakami, Ultra-high proton conduction in electrospun sulfonated polyimide nanofibers, RSC Adv., 2014, 4, 20005–20009 RSC.
- A. M. Samsudin and V. Hacker, PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells, Polymers, 2019, 11, 1399 CrossRef CAS PubMed.
- E. Passalacqua, F. Lufrano, G. Squadrito, A. Patti and L. Giorgi, Nafion content in the catalyst layer of polymer electrolyte fuel cells: effects on structure and performance, Electrochim. Acta, 2001, 46, 799–805 CrossRef CAS.
- Y. Sun, Q. Dong, B. Qian, Y. Meng and J. Qiu, Electrolysis removal of methyl orange dye from water by electrospun activated carbon fibers modified with carbon nanotubes, Chem. Eng. J., 2014, 253, 73–77 CrossRef CAS.
- Y. Chen, J. A. Wrubel, W. E. Klein, S. Kabir, W. A. Smith, K. C. Neyerlin and T. G. Deutsch, High-Performance Bipolar Membrane Development for Improved Water Dissociation, ACS Appl. Polym. Mater., 2020, 2, 4559–4569 CrossRef CAS PubMed.
- A. Zaher, W. El Rouby and N. Barakat, Influences of tungsten incorporation, morphology and calcination temperature on the electrocatalytic activity of Ni/C nanostructures toward urea elimination from wastewaters, Int. J. Hydrogen Energy, 2020, 45, 8082–8093 CrossRef CAS.
- S. N. Banitaba, D. Semnani, E. Heydari-Soureshjani, B. Rezaei and A. A. Ensafi, The effect of concentration and ratio of ethylene carbonate and propylene carbonate plasticizers on characteristics of the electrospun PEO-based electrolytes applicable in lithium-ion batteries, Solid State Ionics, 2020, 347, 115252 CrossRef CAS.
- S. N. Banitaba and A. Ehrmann, Application of electrospun nanofibers for fabrication of versatile and highly efficient electrochemical devices: a review, Polymers, 2021, 13(11), 1741 CrossRef CAS PubMed.
- Y. Ou, J. Wen, H. Xu, S. Xie and J. Li, Ultrafine LiCoO2 powders derived from electrospun nanofibers for Li-ion batteries, J. Phys. Chem. Solids, 2013, 74, 322–327 CrossRef CAS.
- F.-D. Yu, L.-F. Que, C.-Y. Xu, M.-J. Wang, G. Sun, J.-G. Duh and Z.-B. Wang, Dual conductive surface engineering of Li-Rich oxides cathode for superior high-energy-density Li-Ion batteries, Nano Energy, 2019, 59, 527–536 CrossRef CAS.
- X. Mao, T. Hatton and G. Rutledge, A Review of Electrospun Carbon Fibers as Electrode Materials for Energy Storage, Curr. Org. Chem., 2013, 17, 1390–1401 CrossRef CAS.
- M. Wei, M. Jiang, L. Xiaobo, M. Wang and S. Mu, Graphene-doped electrospun nanofiber membrane electrodes and proton exchange membrane fuel cell performance, J. Power Sources, 2016, 327, 384–393 CrossRef CAS.
- M. Zhi, N. Mariani, R. Gemmen, K. Gerdes and N. Wu, Nanofiber scaffold for cathode of solid oxide fuel cell, Energy Environ. Sci., 2011, 4, 417–420 RSC.
- K. M. Skupov, I. I. Ponomarev, D. Y. Razorenov, V. G. Zhigalina, O. M. Zhigalina, I. I. Ponomarev, Y. A. Volkova, Y. M. Volfkovich and V. E. Sosenkin, Carbon nanofiber paper cathode modification for higher performance of phosphoric acid fuel cells on polybenzimidazole membrane, Russ. J. Electrochem., 2017, 53, 728–733 CrossRef CAS.
- A. Jindal and S. Basu, Improvement in electrocatalytic activity of oxygen reduction reaction of electrospun carbon nitride/polyacrylonitrile nanofibers by addition of carbon black and Nafion® fillers, Int. J. Hydrogen Energy, 2016, 41, 11624–11633 CrossRef CAS.
- X. Liu, Z. Yang, Y. Zhang, C. Li, J. Dong, Y. Liu and H. Cheng, Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application, Int. J. Hydrogen Energy, 2017, 42(15), 10275–10284 CrossRef CAS.
- J. J. Slack, M. Brodt, D. A. Cullen, K. S. Reeves, K. L. More and P. N. Pintauro, Impact of Polyvinylidene Fluoride on Nanofiber Cathode Structure and Durability in Proton Exchange Membrane Fuel Cells, J. Electrochem. Soc., 2020, 167, 054517 CrossRef.
- A. Enrico, W. Zhang, M. Lund Traulsen, E. M. Sala, P. Costamagna and P. Holtappels, La0.6Sr0.4Co0.2Fe0.8O3−δ nanofiber cathode for intermediate-temperature solid oxide fuel cells by water-based sol–gel electrospinning: synthesis and electrochemical behaviour, J. Eur. Ceram. Soc., 2018, 38, 2677–2686 CrossRef CAS.
- S. Chung, D. Shin, M. Choun, J. Kim, S. Yang, M. Choi, J. W. Kim and J. Lee, Improved water management of Pt/C cathode modified by graphitized carbon nanofiber in proton exchange membrane fuel cell, J. Power Sources, 2018, 399, 350–356 CrossRef CAS.
- R. Mei, J. Xi, L. Ma, L. An, F. Wang, H. Sun, Z. Luo and Q. Wu, Multi-Scaled Porous Fe–N/C Nanofibrous Catalysts for the Cathode Electrodes of Direct Methanol Fuel Cells, J. Electrochem. Soc., 2017, 164, F1556 CrossRef CAS.
- S. Kabir, T. Van Cleve, S. Khandavalli, S. Medina, S. Pylypenko, S. Mauger, M. Ulsh and K. C. Neyerlin, Toward Optimizing Electrospun Nanofiber Fuel Cell Catalyst Layers: Microstructure and Pt Accessibility, ACS Appl. Energy Mater., 2021, 4, 3341–3351 CrossRef CAS.
- W. Zhang, M. W. Brodt and P. N. Pintauro, Nanofiber Cathodes for Low and High Humidity Hydrogen Fuel Cell Operation, ECS Trans., 2011, 41, 891 CrossRef CAS.
- N. A. M. Barakat, M. T. Amen, F. S. Al-Mubaddel, M. R. Karim and M. Alrashed, NiSn nanoparticle-incorporated carbon nanofibers as efficient electrocatalysts for urea oxidation and working anodes in direct urea fuel cells, J. Adv. Res., 2019, 16, 43–53 CrossRef CAS PubMed.
- M. Abdelkareem, Y. Al Haj, M. Al Ajami, H. Alawadhi and N. Barakat, Ni-Cd Carbon Nanofibers as an Effective Catalyst for Urea Fuel Cell, J. Environ. Chem. Eng., 2017, 6(1), 332–337 CrossRef.
- Q. Hu, C. Liu, L. Fan, Y. Wang and Y. Xiong, Nanofiber-based La0.4Sr0.6TiO3-Gd0.2Ce0.8O1.9-Ni composite anode for solid oxide fuel cells, Electrochim. Acta, 2018, 265, 1–9 CrossRef.
- G. Yu, T. S. Li, M. Xu, M. Andersson, B. Li, H. Tang, J. Parbey and J. Shao, Fabrication of nickel-YSZ cermet nanofibers via electrospinning, J. Alloys Compd., 2017, 693, 1214–1219 CrossRef CAS.
- C. Feng, T. Takeuchi, M. A. Abdelkareem, T. Tsujiguchi and N. Nakagawa, Carbon–CeO2 composite nanofibers as a promising support for a PtRu anode catalyst in a direct methanol fuel cell, J. Power Sources, 2013, 242, 57–64 CrossRef CAS.
- T. Cai, M. Huang, Y. Huang and W. Zheng, Enhanced performance of microbial fuel cells by electrospinning carbon nanofibers hybrid carbon nanotubes composite anode, Int. J. Hydrogen Energy, 2019, 44(5), 3088–3098 CrossRef CAS.
- H.-Y. Jung and S.-H. Roh, Carbon Nanofiber/Polypyrrole Nanocomposite as Anode Material in Microbial Fuel Cells, J. Nanosci. Nanotechnol., 2017, 17, 5830–5833 CrossRef CAS.
- G. Massaglia, V. Margaria, M. Re Fiorentin, K. Pasha, A. Sacco, M. Castellino, A. Chiodoni, S. Bianco, F. Pirri and M. Quaglio, Nonwoven mats of N-doped carbon nanofibers as high-performing anodes in microbial fuel cells, Mater. Today Energy, 2020, 16, 100385 CrossRef.
- U. Karra, S. S. Manickam, J. R. McCutcheon, N. Patel and B. Li, Power generation and organics removal from wastewater using activated carbon nanofiber (ACNF) microbial fuel cells (MFCs), Int. J. Hydrogen Energy, 2013, 38, 1588–1597 CrossRef CAS.
- I. Mohamed, K. Palsamy, A. S. Yasin, W. Iqbal and C. Liu, Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells, J. Alloys Compd., 2019, 816, 152513 CrossRef.
- N. A. Garcia-Gomez, I. Balderas-Renteria, D. I. Garcia-Gutierrez, H. A. Mosqueda and E. M. Sánchez, Development of mats composed by TiO2 and carbon dual electrospun nanofibers: a possible anode material in microbial fuel cells, Mater. Sci. Eng., B, 2015, 193, 130–136 CrossRef CAS.
- N. Abdullah, S. K. Kamarudin, L. K. Shyuan and N. A. Karim, Fabrication and Characterization of New Composite TiO2 Carbon Nanofiber Anodic Catalyst Support for Direct Methanol Fuel Cell via Electrospinning Method, Nanoscale Res. Lett., 2017, 12, 613 CrossRef CAS PubMed.
- S. Zhang, G. He, X. Gong, X. Zhu, X. Wu, S. Xinye, X. Zhao and H. Li, Electrospun nanofiber enhanced sulfonated poly (phthalazinone ether sulfone ketone) composite proton exchange membranes, J. Membr. Sci., 2015, 493, 58–65 CrossRef CAS.
- C. Shen, R. Wycisk and P. N. Pintauro, High performance electrospun bipolar membrane with a 3D junction, Energy Environ. Sci., 2017, 10, 1435–1442 RSC.
- M. Ahn, S. Han, J. Lee and W. Lee, Electrospun composite nanofibers for intermediate-temperature solid oxide fuel cell electrodes, Ceram. Int., 2020, 46, 6006–6011 CrossRef CAS.
- J. Woo Park, R. Wycisk, G. Lin, P. Ying Chong, D. Powers, T. Van Nguyen, R. P. Dowd and P. N. Pintauro, Electrospun Nafion/PVDF single-fiber blended membranes for regenerative H2/Br2 fuel cells, J. Membr. Sci., 2017, 541, 85–92 CrossRef.
- J. W. Park, R. Wycisk and P. N. Pintauro, Nafion/PVDF nanofiber composite membranes for regenerative hydrogen/bromine fuel cells, J. Membr. Sci., 2015, 490, 103–112 CrossRef CAS.
- S. Shahgaldi, M. Ghasemi, W. R. Wan Daud, Z. Yaakob, M. Sedighi, J. Alam and A. F. Ismail, Performance enhancement of microbial fuel cell by PVDF/Nafion nanofibre composite proton exchange membrane, Fuel Process. Technol., 2014, 124, 290–295 CrossRef CAS.
- K. Vezzù, G. Nawn, E. Negro, G. Crivellaro, J. W. Park, R. Wycisk, P. N. Pintauro and V. Di Noto, Electric Response and Conductivity Mechanism of Blended Polyvinylidene Fluoride/Nafion Electrospun Nanofibers, J. Am. Chem. Soc., 2020, 142, 801–814 CrossRef.
- J. J. Slack, C. Gumeci, N. Dale, J. Parrondo, N. Macauley, R. Mukundan, D. Cullen, B. Sneed, K. More and P. N. Pintauro, Nanofiber Fuel Cell MEAs with a PtCo/C Cathode, J. Electrochem. Soc., 2019, 166, F3202–F3209 CrossRef CAS.
- A. Jindal, S. Basu, N. Chauhan, T. Ukai, D. S. Kumar and K. T. Samudhyatha, Application of electrospun CNx nanofibers as cathode in microfluidic fuel cell, J. Power Sources, 2017, 342, 165–174 CrossRef CAS.
- M. Zhi, S. Lee, N. Miller, N. H. Menzler and N. Wu, An intermediate-temperature solid oxide fuel cell with electrospun nanofiber cathode, Energy Environ. Sci., 2012, 5, 7066–7071 RSC.
- E. Zhao, C. Ma, W. Yang, Y. Xiong, J. Li and C. Sun, Electrospinning La0.8Sr0.2Co0.2Fe0.8O3−δ tubes impregnated with Ce0.8Gd0.2O1.9 nanoparticles for an intermediate temperature solid oxide fuel cell cathode, Int. J. Hydrogen Energy, 2013, 38, 6821–6829 CrossRef CAS.
- M. Ahn, J. Cho and W. Lee, One-step fabrication of composite nanofibers for solid oxide fuel cell electrodes, J. Power Sources, 2019, 434, 226749 CrossRef CAS.
- I. I. Ponomarev, K. M. Skupov, D. Y. Razorenov, V. G. Zhigalina, O. M. Zhigalina, I. I. Ponomarev, Y. A. Volkova, M. S. Kondratenko, S. S. Bukalov and E. S. Davydova, Electrospun nanofiber pyropolymer electrodes for fuel cells on polybenzimidazole membranes, Russ. J. Electrochem., 2016, 52, 735–739 CrossRef CAS.
- S. Uhm, B. Jeong and J. Lee, A facile route for preparation of non-noble CNF cathode catalysts in alkaline ethanol fuel cells, Electrochim. Acta, 2011, 56, 9186–9190 CrossRef CAS.
- Q. Hu, L. Fan, Y. Wang, Z. Wang and Y. Xiong, Nanofiber-based LaxSr1−xTiO3–GdyCe1−yO2−δ Composite Anode for Solid Oxide Fuel Cells, Ceram. Int., 2017, 43(15), 12145–12153 CrossRef CAS.
- K.-R. Lee, C.-J. Tseng, J.-K. Chang, K.-W. Wang, Y.-S. Huang, T.-C. Chou, K.-C. Chiu, L. D. Tsai and S.-W. Lee, Ba1−xSrxCe0.8−yZryY0.2O3−δ protonic electrolytes synthesized by hetero-composition-exchange method for solid oxide fuel cells, Int. J. Hydrogen Energy, 2017, 42(34), 22222–22227 CrossRef CAS.
- W. Li, Y. Cheng, Q. Zhou, T. Wei, Z. Li, H. Yan, Z. Wang and X. Han, Evaluation of double perovskite Sr2FeTiO6−δ as potential cathode or anode materials for intermediate-temperature solid oxide fuel cells, Ceram. Int., 2015, 41(9), 12393–12400 CrossRef CAS.
- B. M. Thamer, M. H. El-Newehy, N. A. M. Barakat, M. A. Abdelkareem, S. S. Al-Deyab and H. Y. Kim, Influence of Nitrogen doping on the Catalytic Activity of Ni-incorporated Carbon Nanofibers for Alkaline Direct Methanol Fuel Cells, Electrochim. Acta, 2014, 142, 228–239 CrossRef CAS.
- M. F. R. Hanifah, J. Jaafar, M. H. Othman, A. Ismail, M. Rahman, N. Yusof and F. Aziz, Electro-spun of novel PVDF-Pt-Pd/RGO-CeO2 composite nanofibers as the high potential of robust anode catalyst in direct methanol fuel cell: fabrication and characterization, Inorg. Chem. Commun., 2019, 107, 107487 CrossRef CAS.
- Y. Ito, T. Takeuchi, T. Tsujiguchi, M. A. Abdelkareem and N. Nakagawa, Ultrahigh methanol electro-oxidation activity of PtRu nanoparticles prepared on TiO2-embedded carbon nanofiber support, J. Power Sources, 2013, 242, 280–288 CrossRef CAS.
- H. Wu, T. Yuan, Q. Huang, H. Zhang, Z. Zou, J. Zheng and H. Yang, Polypyrrole nanowire networks as anodic micro-porous layer for passive direct methanol fuel cells, Electrochim. Acta, 2014, 141, 1–5 CrossRef CAS.
- Y. Zheng, Z. Zhang, X. Zhang, H. Ni, Y. Sun, Y. Lou, X. Li and Y. Lu, Application of Pt–Co nanoparticles supported on CeO2-C as electrocatalyst for direct methanol fuel cell, Mater. Lett., 2018, 221, 301–304 CrossRef CAS.
- J. Park, R. Wycisk, G. Lin, P. Chong, D. Powers, T. Nguyen, R. Dowd and P. Pintauro, Electrospun Nafion/PVDF Single-fiber Blended Membranes for Regenerative H2/Br2 Fuel Cells, J. Membr. Sci., 2017, 541, 85–92 CrossRef CAS.
- J. Ballengee and P. Pintauro, Preparation of nanofiber composite proton-exchange membranes from dual fiber electrospun mats, J. Membr. Sci., 2013, 442, 187–195 CrossRef CAS.
- J. Park, R. Wycisk and P. Pintauro, Nafion/PVDF Nanofiber Composite Membranes for Regenerative Hydrogen/Bromine Fuel Cells, J. Membr. Sci., 2015, 490, 103–112 CrossRef CAS.
- J. W. Park, R. Wycisk, P. N. Pintauro, V. Yarlagadda and T. Van Nguyen, Electrospun Nafion®/polyphenylsulfone composite membranes for regenerative hydrogen bromine fuel cells, Materials, 2016, 9(3), 143 CrossRef PubMed.
- K. Vezzù, G. Nawn, E. Negro, G. Crivellaro, J. W. Park, R. Wycisk, P. N. Pintauro and V. Di Noto, Electric Response and Conductivity Mechanism of Blended Polyvinylidene Fluoride/Nafion Electrospun Nanofibers, J. Am. Chem. Soc., 2020, 142, 801–814 CrossRef.
- T. Tamura and H. Kawakami, Aligned electrospun nanofiber composite membranes for fuel cell electrolytes, Nano Lett., 2010, 10, 1324–1328 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |