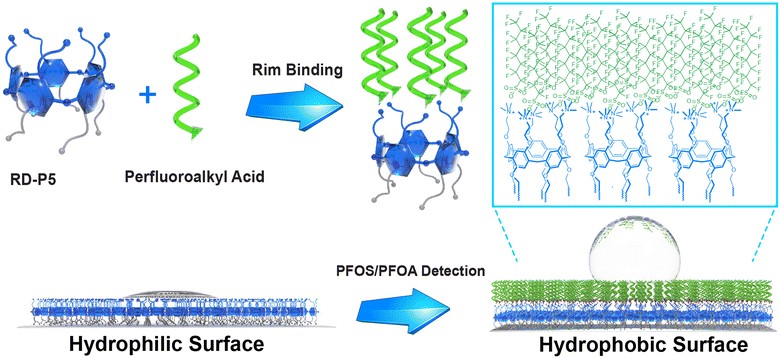
Open Access Article
Open Access ArticleRim-differentiated pillar[5]arene-modified surfaces for rapid PFOA/PFOS detection†
Tu-Nan
Gao
 a,
Zhen
Yang
a,
Zhen
Yang
 ab,
Jesse M. S.
Goed
ab,
Jesse M. S.
Goed
 ac,
Han
Zuilhof
ac,
Han
Zuilhof
 *ad and
Fedor M.
Miloserdov
*ad and
Fedor M.
Miloserdov
 *a
*a
aLaboratory of Organic Chemistry, Wageningen University, Stippeneng 4, 6708WE Wageningen, The Netherlands. E-mail: fedor.miloserdov@wur.nl; han.zuilhof@wur.nl
bImec within OnePlanet Research Center, Bronland 10, 6708 WH Wageningen, The Netherlands
cWetsus, Oostergoweg 4, 8911 MA Leeuwarden, The Netherlands
dSchool of Pharmaceutical Science and Technology, Tianjin University, Weijin Road 92, 300072 Tianjin, China
First published on 26th July 2024
Abstract
A new rim-differentiated pillar[5]arene (RD-P5) has been synthesized and immobilized onto an Al2O3 surface for the rapid detection of perfluoroalkyl acids. This P5-Al2O3 surface provides a novel approach for measuring perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) using contact angle measurements, with limits of detection down to 10 ng L−1.
Per- and polyfluoroalkyl substances (PFAS), known as “forever chemicals”, have emerged as a significant environmental concern worldwide due to their presence in air, water, and soil.1,2 Toxicity studies have demonstrated that the accumulation of PFAS can lead to a variety of health issues.3–5 The US Environmental Protection Agency (EPA) initially set the advisory safe limit for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) at 70 ng L−1 in 2016, and in 2023, lowered this to 4 ng L−1 for each of these chemicals. The updated safe limit (4 ng L−1) presents a great challenge for PFAS detection. Due to the poor molar optical absorption coefficient of PFOA and PFOS, the gold standard detection method is liquid chromatography–mass spectrometry (LC-MS). Although LC-MS is sensitive and accurate, it has drawbacks such as lengthy measurements, requiring expensive equipment, and the need for well-trained analytical chemists for operation. Efforts have been made to develop a more facile and sensitive PFAS detection workflow.6–8 Electrochemical techniques utilizing electrodes modified with molecularly imprinted polymers (MIP) or metal–organic frameworks (MOF) have shown promising results, achieving a limit of detection (LOD) as low as 1.7 ng L−1 for MIP-modified electrodes9 and 1 ng L−1 for MOF-modified electrodes.10 Fluorescence-based techniques can also reach LOD of 80 ng L−1 for PFOA and 350 ng L−1 for PFOS by using amplifying fluorescent polymers (AFP) as sensing element.11 However, while being highly sensitive, both electrochemical and fluorescent techniques again require specific lab-based equipment for their implementation, such as an impedance analyzer or fluorescence spectrometer. This limitation restricts their applicability for on-site PFAS detection. On the other hand, colorimetry, which is convenient to use for on-site applications because of its integration with common smartphones, often exhibits a significantly lower sensitivity,12 with typical LODs from 10 μg L−1 to 1 mg L−1.13–17
While much research is focused on detecting PFAS through changes in either optical or electrochemical properties of probe materials, to the best of our knowledge no investigations have been conducted to detect PFAS by a change of surface contact angle. Contact angle changes can be easily measured on-site using smartphones, and such technology is already used in the detection of ctDNA or metal ions.18,19 In a previous study, we developed a deca-ammonium-functionalized pillar[5]arenes (DAF-P5s) capable of binding to polyfluoroalkyl acids at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 host–guest ratio, and exhibiting high binding constants in aqueous environments of up to 106 M−1.20 Similarly, perfluorinated diacids (HOOC–(CF2)nCOOH) form pillar-like supramolecular structures that can reach macroscopic sizes, again displaying strong and unique interactions with PFAS.21 This high affinity arises from the unique structure of DAF-P5s, which feature five tightly packed amine moieties capable of attaching five PFAS molecules per rim. This attachment leads to the formation of a stable local fluorous phase, resulting in the observed high binding constant. Herein, we report the synthesis of a dual-functionalized rim-differentiated pillar[5]arene RD-P5 and its immobilization on a surface for on-site detection of PFOA and PFOS at ng L−1 levels through a simple, near-instantaneous water contact measurement (Scheme 1).
10 host–guest ratio, and exhibiting high binding constants in aqueous environments of up to 106 M−1.20 Similarly, perfluorinated diacids (HOOC–(CF2)nCOOH) form pillar-like supramolecular structures that can reach macroscopic sizes, again displaying strong and unique interactions with PFAS.21 This high affinity arises from the unique structure of DAF-P5s, which feature five tightly packed amine moieties capable of attaching five PFAS molecules per rim. This attachment leads to the formation of a stable local fluorous phase, resulting in the observed high binding constant. Herein, we report the synthesis of a dual-functionalized rim-differentiated pillar[5]arene RD-P5 and its immobilization on a surface for on-site detection of PFOA and PFOS at ng L−1 levels through a simple, near-instantaneous water contact measurement (Scheme 1).
The DAF-P5s that we previously successfully employed for PFAS binding are not suitable for direct immobilization onto a surface.20 To introduce the unique property of DAF-P5s on a surface, we designed rim-differentiated pillar[5]arene (RD-P5) 2, bearing 5 alkyne moieties for surface immobilization on one rim and 5 positively charged trimethyl ammonium groups for PFAS binding on the other. Since statistical synthesis of RD-P5 is only very low yielding (typically <5%),22 our group previously developed the “pre-orientation” strategy,23,24 that leads to >50% RD-P5 formation and concomitantly decent isolated yields. With this significant improvement, the synthesis of RD-P5s is no longer the bottleneck for their application. However, the number of papers employing RD-P5,25–32 and particularly of RD-P5s with dual-functionalized rims, remains limited.33–36
We synthesized RD-P5 1 following the “pre-oriented” strategy.23 From the 1H NMR spectra of crude product (Fig. S1, ESI†), the presence of P5 constitutional isomers can be observed from the splitting of peaks in the aromatic region. After recrystallization from MeOH/EtOAc, 1 was isolated as a white powder (13% yield). The presence of clean and sharp peaks in the 1H NMR spectrum of 1 (Fig. 1b) indicate its C5-symmetric structure. High-resolution mass spectrometry (HRMS) confirmed the formation of a pentameric product (Fig. 1c). RD-P5 1 was further converted to RD-P5 2via a reaction with trimethylamine (Fig. 1a), maintaining its C5-symmetric structure as confirmed by 1H NMR (Fig. 1b), HRMS (Fig. 1d), and X-ray crystallography (Fig. 1e). The crystal structure of 2 showed both the expected pillar-like shape and the rim-differentiation of appended substituents. The cavity of the pillararene is occupied by disordered solvent molecules (ethanol) and one of the alkyne substituents of the rim.
The host–guest interactions between 2 and PFOS or PFOA were investigated using isothermal titration calorimetry (ITC). The binding constant K of 2 and PFOS was determined to be 2.6 × 106 M−1 with a P5/PFOS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6, analogous in strength and stoichiometry to the interactions of symmetric DAF-P5s in our previous study,20 indicating the same binding mode. RD-P5 2 and PFOA display K = 5.2 × 104 M−1, with a P5/PFOA ratio of 1
5.6, analogous in strength and stoichiometry to the interactions of symmetric DAF-P5s in our previous study,20 indicating the same binding mode. RD-P5 2 and PFOA display K = 5.2 × 104 M−1, with a P5/PFOA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9. This lower K-value is attributed to the better solubility of PFOA in water. For comparison, we also tested the binding constants between 2 and octanoic acid (OA) and octanesulfonic acid (OSA), as non-fluorinated counterparts of PFOA and PFOS; no binding to either could be detected by ITC, confirming the unique involvement of the fluorous phase.21
5.9. This lower K-value is attributed to the better solubility of PFOA in water. For comparison, we also tested the binding constants between 2 and octanoic acid (OA) and octanesulfonic acid (OSA), as non-fluorinated counterparts of PFOA and PFOS; no binding to either could be detected by ITC, confirming the unique involvement of the fluorous phase.21
In order to get a stable surface for PFAS detection, we prepared a self-assembled monolayer of 12-azidododecylphosphonic acid on an Al2O3 surface.37 This was characterized using X-ray photoelectron spectroscopy (XPS), revealing two azide peaks in the N 1s narrow spectrum with the expected 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 404.0 and 400.2 eV, respectively (Fig. 2a). RD-P5 2 was then immobilized onto this Al2O3-N3 surface using a copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. After the CuAAC reaction, the azide N 1s peak at 404.0 eV nearly completely disappeared, and it was replaced by features assigned to the triazole ring at 399.8, 400.7, 401.9 eV and a trimethylammonium group at 403.1 eV (Fig. 2b), in accordance with previous literature, indicating the successful immobilization of 2.37–39 Also the infrared reflection absorption spectroscopy (IRRAS) show strong decrease in the intensity of the azide peak, confirming the successful immobilization of RD-P5.
1 ratio at 404.0 and 400.2 eV, respectively (Fig. 2a). RD-P5 2 was then immobilized onto this Al2O3-N3 surface using a copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. After the CuAAC reaction, the azide N 1s peak at 404.0 eV nearly completely disappeared, and it was replaced by features assigned to the triazole ring at 399.8, 400.7, 401.9 eV and a trimethylammonium group at 403.1 eV (Fig. 2b), in accordance with previous literature, indicating the successful immobilization of 2.37–39 Also the infrared reflection absorption spectroscopy (IRRAS) show strong decrease in the intensity of the azide peak, confirming the successful immobilization of RD-P5.
 | ||
| Fig. 2 XPS spectra of (a) N 1s narrow scan of Al2O3-N3, (b) N 1s narrow scan of Al2O3-2, (c) survey scan of Al2O3-2, and (d) survey scan of Al2O3-2/PFOS. | ||
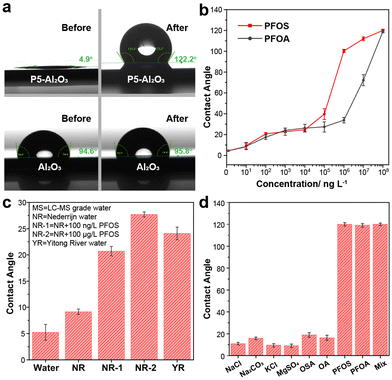
Due to the hydrophilic nature of ammonium groups, the modified Al2O3 surface exhibits superhydrophilicity, with a static water contact angle (CA) <5° (Fig. 3a). After submerging in 100 mg L−1 PFOS aqueous solution, the CA of the 2-modified Al2O3 surface increased from 5° to 120°, indicating a transition from superhydrophilic to hydrophobic behavior. In contrast, the contact angle of a blank Al2O3 surface did not exhibit any significant changes upon exposure to this PFOS solution (Fig. 3a). The XPS spectrum after immersion of Al2O3-2 in a 100 mg L−1 PFOS aqueous solution shows that the F 1s peak corresponding to PFOS has significantly increased in intensity (Fig. 3c and d), leading to a F/N ratio of 15/4. Taking into account that N 1s narrow scan shows trimethylammonium and triazole groups to be in approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 3b and Fig. S11, ESI†), and that PFOS contains 17 F atoms, the 15/4 F/N ratio means that trimethylammonium groups and PFOS are approximately in 1
1 ratio (Fig. 3b and Fig. S11, ESI†), and that PFOS contains 17 F atoms, the 15/4 F/N ratio means that trimethylammonium groups and PFOS are approximately in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, or RD-P5 and PFOS ratio is around 1
1 ratio, or RD-P5 and PFOS ratio is around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, suggesting a (near)-complete coverage of 2-modified surface with PFOS. To investigate the relationship between CA and the concentration of PFOA/PFOS, a series of experiments were conducted using various concentrations of PFOA/PFOS ranging from 0 ng L−1 to 100 mg L−1, leading to easily observable changes in CA (Fig. 3b). For PFOS, the contact angle changed from 5° ± 2° to 10° ± 1° after immersing into a 10 ng L−1 PFOS solution, showing the sensitivity to even trace amounts of PFOS. Further increases in concentration yielded a gradual increase in CA. Once the PFOS concentration exceeded 100 μg L−1, the surface transitioned from hydrophilic to hydrophobic, indicating that the surface was now fully covered with a layer of PFOS. Similar trends were observed for PFOA, for which the surface became hydrophobic when the concentration of PFOA reached 10 mg L−1. The higher sensitivity of 2-modified surfaces towards PFOS compared to PFOA is fully in line with the respective binding constants measured by ITC.
5, suggesting a (near)-complete coverage of 2-modified surface with PFOS. To investigate the relationship between CA and the concentration of PFOA/PFOS, a series of experiments were conducted using various concentrations of PFOA/PFOS ranging from 0 ng L−1 to 100 mg L−1, leading to easily observable changes in CA (Fig. 3b). For PFOS, the contact angle changed from 5° ± 2° to 10° ± 1° after immersing into a 10 ng L−1 PFOS solution, showing the sensitivity to even trace amounts of PFOS. Further increases in concentration yielded a gradual increase in CA. Once the PFOS concentration exceeded 100 μg L−1, the surface transitioned from hydrophilic to hydrophobic, indicating that the surface was now fully covered with a layer of PFOS. Similar trends were observed for PFOA, for which the surface became hydrophobic when the concentration of PFOA reached 10 mg L−1. The higher sensitivity of 2-modified surfaces towards PFOS compared to PFOA is fully in line with the respective binding constants measured by ITC.
One of the biggest challenges for PFOA/PFOS detection methods is their susceptibility to interference from other contaminants commonly found in nature. Factors such as pH, salt concentration, and the presence of other organic compounds can easily influence the results. To therefore further test the applicability of this method (Fig. 3c), an environmental sample was taken from the Dutch river Nederrijn (NR), which was independently confirmed to have [PFOS] < 3 ng L−1.40 To this sample PFOS was added to reach concentrations of 100 ng L−1 (sample NR-1) and 100 μg L−1 (sample NR-2). The CA for NR-1 differed significantly from the original sample, showing that we can easily detect PFOS at 100 ng L−1 in environmental samples. Similarly, samples from the Chinese Yitong River, with a total PFOA + PFOS concentration of 95 ng L−1, showed a significantly higher CA (24° ± 1°) than pure water samples (5° ± 2°). In order to further demonstrate the selectivity of the Al2O3-2 surface, we conducted tests with several salts commonly present in water at 100 mg L−1 concentration. Additionally, we tested octane sulfonic acid (OS), and octanoic acid (OA) which are non-fluorinated counterparts of PFOS and PFOA, respectively (Fig. 2d). The results clearly demonstrate the specificity towards PFOS and PFOA, as no significant increase in CA is observed in the presence of other compounds. We also prepared a “mix” solution containing 100 mg L−1 PFOS, 1 g L−1 NaCl, 1 g L−1 OA and 1 g L−1 OSA in one sample and showed that the CA response by PFOS is not influenced by the presence of such a mixture. In addition, simple sonication in acetone removed most of PFOS from Al2O3-2 surface, allowing PFOS detection being repeated for 10 cycles (10 mg L−1, see ESI† for details).
In conclusion, we have synthesized a novel rim-differentiated pillar-[5]arene (RD-P5) with dual functionality. This RD-P5 features alkyne groups on one rim for easy surface immobilization via various chemistries, while ammonium groups enable host–guest interactions for capturing contaminants such as PFOA and PFOS. By immobilizing RD-P5 on an aluminum oxide surface, we have demonstrated its potential application in the detection of PFOA/PFOS through contact angle measurements requiring only a modified surface and a routine smartphone. These preliminary experiments already show a robust LOD of 100 ng L−1, down to 10 ng L−1 in some cases, thus showing significant promise for detecting PFAS-pollution in water without the need of any laboratory-based equipment. We expect such ‘bringing the lab to the sample’-approaches to make important contributions to environmental monitoring efforts.
Financial support from graduate school VLAG of Wageningen University (graduate fellowship of T.-N. G.), the National Natural Science Foundation of China (grant 22011530163, to H. Z.) and Wetsus (European Centre of Excellence for Sustainable Water Technology, support to J. M. S. G.) is appreciated.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Loos, G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S. Weiss, L. Blaha, M. Bolchi and B. M. Gawlik, Water Res., 2010, 44, 4115–4126 CrossRef CAS.
- M. Sun, E. Arevalo, M. Strynar, A. Lindstrom, M. Richardson, B. Kearns, A. Pickett, C. Smith and D. R. U. Knappe, Environ. Sci. Technol. Lett., 2016, 3, 415–419 CrossRef CAS.
- C. Lau, J. L. Butenhoff and J. M. Rogers, Toxicol. Appl. Pharmacol., 2004, 198, 231–241 CrossRef CAS PubMed.
- I. T. Cousins, R. Vestergren, Z. Wang, M. Scheringer and M. S. McLachlan, Environ. Int., 2016, 94, 331–340 CrossRef CAS.
- V. Barry, A. Winquist and K. Steenland, Environ. Health Perspect., 2013, 121, 1313–1318 CrossRef PubMed.
- Y. Wang, S. B. Darling and J. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 60789–60814 CrossRef CAS.
- S. P. Sahu, S. Kole, C. G. Arges and M. R. Gartia, ACS Omega, 2022, 7, 5001–5007 CrossRef CAS PubMed.
- S. Park, C. T. Gordon and T. M. Swager, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2317300121 CrossRef.
- R. B. Clark, D. C. Wagner, D. T. Holden, J. J. P. Roberts, E. Zumbro, L. Goodnight, K. T. Huynh, R. B. Green, J. A. Grove and J. E. Dick, Environ. Sci. Technol., 2023, 57, 21815–21822 CrossRef.
- Y. H. Cheng, D. Barpaga, J. A. Soltis, V. Shutthanandan, R. Kargupta, K. S. Han, B. P. McGrail, R. K. Motkuri, S. Basuray and S. Chatterjee, ACS Appl. Mater. Interfaces, 2020, 12, 10503–10514 CrossRef PubMed.
- A. Concellón, J. Castro-Esteban and T. M. Swager, J. Am. Chem. Soc., 2023, 145, 11420–11430 CrossRef PubMed.
- M. Zhang, Y. Zhao, B. Bui, L. Tang, J. Xue, M. Chen and W. Chen, Crit. Rev. Anal. Chem., 2023, 2299233 Search PubMed.
- X. Chen, S. Hussain, Y. Tang, X. Chen, S. Zhang, Y. Wang, P. Zhang, R. Gao, S. Wang and Y. Hao, Sci. Total Environ., 2023, 860, 160467 CrossRef CAS PubMed.
- E. E. Harrison and M. L. Waters, Chem. Sci., 2023, 14, 928–936 RSC.
- Q. Chen, P. Zhu, J. Xiong, L. Gao and K. Tan, Spectrochim. Acta, Part A, 2020, 224, 117362 CrossRef CAS.
- A. U. Rehman, M. Crimi and S. Andreescu, Trends Environ. Anal. Chem., 2023, 37, e00198 CrossRef CAS.
- Z. Zheng, H. Yu, W. C. Geng, X. Y. Hu, Y. Y. Wang, Z. Li, Y. Wang and D. S. Guo, Nat. Commun., 2019, 10, 5762 CrossRef CAS.
- J. Zhang, Z. Wang, S. Lv, X. Zeng, Y. Sun, H. Li and R. Zhang, Chem. Commun., 2019, 55, 778–781 RSC.
- C. Song, Z. Zhao, Y. Lin, Y. Zhao, X.-Y. Liu, C. Lin and C. Wu, J. Colloid Interface Sci., 2019, 547, 330–338 CrossRef CAS.
- T.-N. Gao, S. Huang, R. Nooijen, Y. Zhu, G. Kociok-Köhn, T. Stuerzer, G. Li, H. Bitter, G. Salentijn, B. Chen, F. M. Miloserdov and H. Zuilhof, Angew. Chem., Int. Ed., 2024, e202403474 CAS.
- L. W. Honaker, T.-N. Gao, K. R. de Graaf, T. V. M. Bogaardt, P. Vink, T. Stürzer, G. Kociok-Köhn, H. Zuilhof, F. M. Miloserdov and S. Deshpande, Adv. Sci., 2024, 2401807 CrossRef CAS.
- Y. Kou, H. Tao, D. Cao, Z. Fu, D. Schollmeyer and H. Meier, Eur. J. Org. Chem., 2010, 6464–6470 CrossRef CAS.
- M. Guo, X. Wang, C. Zhan, P. Demay-Drouhard, W. Li, K. Du, M. A. Olson, H. Zuilhof and A. C. H. Sue, J. Am. Chem. Soc., 2018, 140, 74–77 CrossRef CAS PubMed.
- P. Demay-Drouhard, K. Du, K. Samanta, X. Wan, W. Yang, R. Srinivasan, A. C. H. Sue and H. Zuilhof, Org. Lett., 2019, 21, 3976–3980 CrossRef CAS PubMed.
- G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS.
- Y. Yao, M. Xue, Z. Zhang, M. Zhang, Y. Wang and F. Huang, Chem. Sci., 2013, 4, 3667–3672 RSC.
- G. Yu, Z. Zhang, C. Han, M. Xue, Q. Zhou and F. Huang, Chem. Commun., 2012, 48, 2958–2960 RSC.
- H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853–7863 CrossRef CAS.
- P. Liu, Y. Deng, J. Lu, X. Gou, Q. Han, Y.-R. Pei and L. Y. Jin, Polym. Int., 2023, 73, 359–367 CrossRef.
- R. Chang, C.-Y. Chen, L. Gao, Y. Li, Z.-H. Lee, H. Zhao, A. C. H. Sue and K.-C. Chang, Org. Biomol. Chem., 2024, 22, 745–752 RSC.
- W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994–3999 CrossRef CAS PubMed.
- L. Luo, G. Nie, D. Tian, H. Deng, L. Jiang and H. Li, Angew. Chem., Int. Ed., 2016, 55, 12713–12716 CrossRef CAS PubMed.
- J. Lu, P. Liu, Y. Deng, N. Zhu and L. Y. Jin, J. Mol. Struct., 2023, 1291, 136054 CrossRef CAS.
- B. Lu, X. Yan, J. Wang, D. Jing, J. Bei, Y. Cai and Y. Yao, Chem. Commun., 2022, 58, 2480–2483 RSC.
- Z. Liu, F. Demontrond, A. Imberty, A. C. H. Sue, S. Vidal and H. Zhao, Chin. Chem. Lett., 2023, 34, 107872 CrossRef.
- J. Zhao, W. Yang, C. Liang, L. Gao, J. Xu, A. C. H. Sue and H. Zhao, Chem. Commun., 2021, 57, 11193–11196 RSC.
- A. Debrassi, E. Roeven, S. Thijssen, L. Scheres, W. M. de Vos, T. Wennekes and H. Zuilhof, Langmuir, 2015, 31, 5633–5644 CrossRef.
- A. R. Kuzmyn, L. W. Teunissen, M. V. Kroese, J. Kant, S. Venema and H. Zuilhof, ACS Omega, 2022, 7, 38371–38379 CrossRef.
- J. Zhao, F. Gao, S. P. Pujari, H. Zuilhof and A. V. Teplyakov, Langmuir, 2017, 33, 10792–10799 CrossRef.
- W. A. Gebbink, L. van Asseldonk and S. P. J. van Leeuwen, Environ. Sci. Technol., 2017, 51, 11057–11065 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2359089. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc02676d |
| This journal is © The Royal Society of Chemistry 2024 |