Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Utilizing cellulose-based conducting hydrogels in iontronics
Kudzanai
Nyamayaro
 ab,
Savvas G.
Hatzikiriakos
b and
Parisa
Mehrkhodavandi
ab,
Savvas G.
Hatzikiriakos
b and
Parisa
Mehrkhodavandi
 *a
*a
aDept. of Chemistry, University of British Columbia, Vancouver, BC, Canada. E-mail: mehr@chem.ubc.ca
bDept. of Chemical and Biological Engineering, University of British Columbia, Vancouver, BC, Canada
First published on 21st July 2023
Abstract
The rising interest in wearable devices has galvanized research into the development of flexible electronics. Flexible iontronics, devices that employ ions as charge carriers, have been accessed by employing ionic conductive hydrogels. While petroleum-based polymers have dominated this field, recently there is a drive towards renewable alternatives to develop more sustainable alternatives. This review focuses on the use of cellulose in ionic conductive hydrogels (IHCs) and their application in fabricating iontronics. The general structure and properties of cellulose and its derivatives are described, highlighting the inherent properties of the material that can be utilized to enhance the performance of IHCs. Subsequently, the basic theory behind ionic conduction is discussed, providing to the reader a brief overview of the origin, process and factors that influence ionic conduction. Thereafter, a discussion is presented on the major factors that influence the performance of ICHs and how the inherent properties of cellulose have been employed to improve the properties of these material. Finally, we discuss examples of devices in which the properties of the ionic conductor are enhanced by the presence of cellulose.
Sustainability spotlightCurrently, over 50 million metric tons of electronic waste is generated worldwide, and a less than 20% of this waste is recycled. As society advances towards more sustainable practices, material selection is critical in the development of new technology such as flexible and stretchable electronics. Material choice is important in the development of soft robotics, artificial electronic skin, medical-healthcare systems and human–machine interface units. Most of the work done in this field has made use of industrial, petrochemically-derived polymers, leading to growing concerns about the accumulation of plastic waste and environmental harm. Biopolymers such as cellulose are alternatives in a variety of applications that require the use of ionic conducting hydrogels. |
1. Introduction: biobased polymers in iontronics
Advancement in technology has sparked interest in wearable devices; as a result, there has been increased research and development of flexible and stretchable electronics. The benefits of wearable electronics include access to devices that are potentially cheaper, more portable, bio-integrateble, shape adaptive, and recyclable.1 These attractive properties have seen flexible and stretchable electronics being used in the development of soft robotics, artificial electronic skin,2 medical-healthcare systems and human–machine interface units.A major branch of flexible devices is ionic conductive bioelectronics that are at the interface between biological systems and electronics. Biological systems use ions to conduct signals, while machines use electrons. The use of ionic conduction in bioelectronic devices has led to the development of iontronics: electronics with hybrid circuits that employ both ionic and electronic conduction.3 Iontronics transmit signals at the interface between an ionic conductor and an electric conductor, differentiating them from a purely electric or ionic conductor. Hybrid circuits have been employed in the development of devices such as diodes,4 capacitors,5 batteries,6 super capacitors,7 and transistors.8
Flexible iontronics can be manufactured by employing ionic conductive hydrogels (ICHs). Hydrogels are three dimensional polymeric networks capable of absorbing large amounts of water into their matrix.9 ICHs are made by incorporating salts or by using charged functional groups in the polymer chain, endowing the hydrogel with ionic conductivity. The mechanical properties of hydrogels can be tuned to obtain Young's moduli in the range between 1 Pa to 0.1 GPa. As such, gels can be used to design electronics with matching properties to biological tissues (Young's moduli between 0.5–500 kPa), which would reduce scarring.10 In addition, the high water content enables enhanced ionic conductivity necessary for good performance of flexible iontronics. Moreover, low toxicity, transparency, elasticity, corrosion resistance and durability of hydrogels make them ideal materials for the development of flexible electronics.
Most of the work done in the field of iontronics has made use of industrial, petrochemically-derived polymers. Some examples of polymers used to make hydrogels for electronics include polyvinyl alcohol (PVA), polyethylene glycol (PEG), poly(acrylic acid) (PAA), poly(methacrylic acid) (PMA), poly(acrylamide) (PAAm), poly(diallyldimethylammonium chloride) (pDADMAC), a polyanion, and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (pAMPSA), a polycation.11 Several excellent reviews have been published on using these polymers in bioelectronics.12 Despite having advantages in the design of flexible electronics, there are growing concerns about the use of non-renewable petrochemical-based materials that would result in accumulation of plastic waste and environmental harm.13
Currently, over 50 million metric tons of electronic waste is generated worldwide annually; less than 20% of this waste is recycled.14 As society advances towards more sustainable practices, material selection is critical in the development of new technology. The new generation of materials should ideally dissolve, breakdown into smaller fragments or decompose into environmentally benign products under physiological conditions.15 Consequently, biodegradable and bio-sourced polymers have become increasingly popular as possible replacements for traditional petrochemical-based materials.16
Biopolymers are attractive for applications in electronics due to their biocompatibility, biodegradability, natural abundance, mechanical flexibility, and lightweight nature.17 The properties possessed by biopolymers are ideal for development of flexible, wearable, implantable electronics. As a result, biopolymers have been used in a variety of applications in ionic conducting electronics that include, flexible sensors and portable energy storage systems, actuators, nanofluidic, flexible nanogenerators.18 The polymers that have been explored for these applications include cellulose, chitosan, lignin, proteins and starch.19
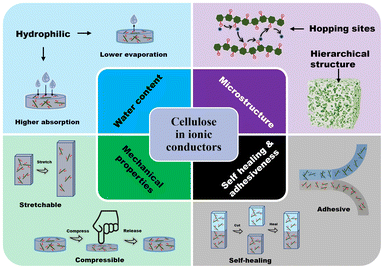
This review aims to serve as an introductory tutorial to the field of cellulose-based iontronics (Fig. 1). For a detailed, thorough review on applications of biopolymer ionic conductive hydrogels in flexible wearable sensors and energy devices, see a recent review by Cui and coworkers.19b For a comprehensive overview on biopolymer-based hydrogel electrolytes for flexible energy storage and conversion devices, see the work of Xu and coworkers.20 For a more specific review on cellulose, Zhao and coworkers describe the use of various nanostructures and forms of cellulose as flexible, functional materials in electronics.21
 | ||
| Fig. 1 Factors influencing the performance of ionic conductive hydrogels and the benefits of incorporating cellulose. | ||
In our contribution, we will focus on the role of cellulose in ionic conductive hydrogels (ICHs) and on the application of ICHs in iontronics. In Section 2, the general structure and properties of cellulose and its derivatives are described, highlighting the inherent properties of the material that can be utilized to enhance the performance of ICHs. In Section 3, the basic theory behind ionic conduction is described, giving the reader a brief overview of its origin, function and factors that influence ionic conduction. In Section 4, a discussion of the major factors that influence the performance of ICHs, and how the inherent properties of cellulose have been employed to improve properties of ICHs, is presented. Finally in Section 5, we present examples of devices in which the properties of the ionic conductor are enhanced by the presence of cellulose.
2. Cellulose: structure and properties
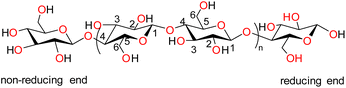
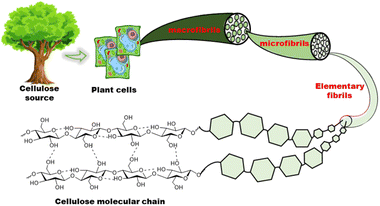
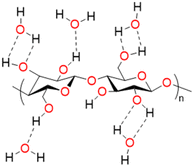
Biopolymers are derived from living organisms such as animals, plants, marine, and microbial sources. Biopolymers can be classified into natural, originating from living organisms, or synthetic biopolymers, manufactured chemically from bio-sourced monomers.22 Synthetic biopolymers can be biodegradable e.g. poly(ε-caprolactone) (PCL) and poly(lactic acid) (PLA) or they can be non-biodegradable e.g. poly(ethylene terephthalate) (PET).23 Natural biopolymers are biodegradable and can be mineralized into carbon dioxide and water by microorganisms under physiological conditions.24 Due to the advancement of extraction methods, natural polysaccharides can be produced at low cost and on a large scale.25Cellulose, the main structural component of plant cell walls, is the most abundant renewable biopolymer with an annual production in the range of giga tons.26 It is a linear homopolysaccharide composed of β-D-glucopyranose repeat units that are connected through glycosidic bonds (Fig. 2).27 The polymer chain end of cellulose is an anomeric carbon bonding via a glycosidic bond and is the nonreducing end. The opposite end of the polymer has a D-glucopyranose unit with a hemiacetal and is the reducing end.28 Due to the presence of three hydroxyl groups on each of the monomers, the chains can associate through hydrogen bonding. The extensive hydrogen bonding and attractive van der Waals forces promote parallel stacking of the cellulose chains. This stacking results in formation of fibrillar and semicrystalline packing in the hierarchical structure.
Individual cellulose chains associate in an ordered parallel layered fashion to form elementary fibrils. Each of these fibrils is made of alternating ordered crystalline regions and disordered amorphous regions. These further interact to form microfibrils that are 5–50 nm in diameter and up to several microns in length which, in turn, assemble into cellulose fibers (Fig. 3). The hydroxyl groups on the surface of cellulose nanomaterials impart chemical reactivity, enabling moderately easy surface modification. Some of the functional groups grafted on cellulose include sulfate, carboxyl, aldehyde, phosphate, amino, and urethane groups.29 Since modification can target surface hydroxyl groups, the desired functionality can be achieved without adversely affecting the intrinsic chemical and physical properties of the material. This ability to retain its properties while being easily modified, is crucial in expanding the possible functionality of cellulose, enabling access to properties that are beneficial for ICH.
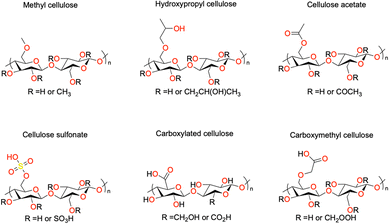
The chemical derivatization of cellulose to obtain specialized cellulose polymers is often achieved using etherification and esterification. The derivatives vary in terms of surface chemical structure, moisture sorption, water interaction, surface activity, and solubility.30 The structure of some common or commercially available derivatives are shown in Fig. 4. Cellulose ethers are a class of industrially relevant materials and are usually water soluble.31 For example, methyl cellulose (MC) is used as a thickener in the food industry, as an admixture for concrete in construction, and in controlled drug delivery applications in the pharmaceutical industry.32 Hydroxyethyl cellulose (HEC) is used in a wide range of industries including food, oil recovery, cosmetics, pharmaceuticals, adhesives, printing, textile, construction, paper, and agriculture.33 Carboxymethyl cellulose (CMC) is a water-soluble ether derivative in which some of the hydroxyl groups have been replaced with carboxymethyl groups and has been used as a detergent and food additive.34 In addition, it has been used as a supporting material in electronics such battery electrodes.35
2.1. Properties of cellulose
Nanocelluloses such as cellulose nanofibrils (CNF), also referred to as microfibrillated cellulose, and cellulose nanocrystals (CNCs) prepared from wood and other plant celluloses, have emerged as important cellulose derivatives with a wide range of applications in composites, packaging, coatings, biomedical, construction, and electronics to name a few.26,36 Due to the versatile properties that can be accessed through modification, cellulose has potential use in ionic conductive hydrogels for flexible electronics. The properties of cellulosic material can differ depending on the treatment of the fibers during the extraction process (Fig. 5). Mechanical shearing results in a fibrous material referred to as cellulose nanofibrils (CNF), whereas chemical treatment with acid will result in highly crystalline rod like material called cellulose nanocrystals (CNC).37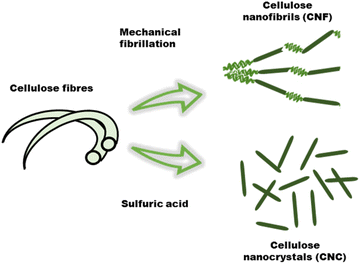 | ||
| Fig. 5 Isolation of cellulose nanocrystals and cellulose nanofibrils via of sulfuric hydrolysis and mechanical fibrillation of cellulose fibers, respectively. | ||
In contrast, sulfuric acid mediated selective hydrolysis of the amorphous regions of the cellulose fibers results in rod like particles called cellulose nanocrystals (CNC). These have aspect ratios ranging from 5–20 (3–5 nm wide, 50–500 nm in length) and are highly crystalline (54–88%).28,37a,40 The high strength and dimensional anisotropy of CNC's has made them excellent candidates for exploration as reinforcing agent in hydrogels.41
Despite its hydrophilicity, cellulose is insoluble in water and other common solvents due to the strong intra- and intermolecular hydrogen bonds, as well as hydrophobic interactions between the cellulose chains.42 Despite this, different cellulose forms can be dispersed in solutions, enabling the material to transfer desired properties to the hydrogel. However, this dispersion poses a challenge to fabrication of bioelectronics that require the material to be transparent. Therefore, there has been development of solvents that are capable of dissolving cellulose under mild conditions. Ionic liquids such as 1-butyl-3-methylimidazolium formate (BMIMFmO), 1-butyl-3-methylimidazolium chloride (BmimCl), N,N-dimethylacetamide/lithium chloride (DMAc/LiCl), NaOH/thiourea, LiOH/urea, and NaOH/urea have been employed to dissolve cellulose.43 Dissolution of cellulose enables the expansion of potential benefits in fabricating ionic conducting hydrogels for bioelectronics.
The number of surface accessible hydroxyl groups might be dependent on the degree of crystallinity of the cellulose,46 or by the size of cellulose crystallites.47 Water molecules can penetrate the structure of cellulose in the disordered region more readily compared to the crystalline regions,46 suggesting that the more amorphous the cellulose the higher the water absorption will be. Another factor that influences water uptake is the presence of charged group on the surface of functionalized cellulose. These functional groups contribute to the osmotic pressure within the hydrogel. The higher the charge density on the surface of the material, the higher the osmotic pressure which in turn increases water absorption.48
3. Theory of ionic conductivity
As highlighted above, contrary to electronic conductors, ionic conductors employ positive and negative ions as charge carriers. This mode of conduction is similar to that of living organisms and designing efficient iontronics opens up avenues for bio-interfaced devices. To modulate the performance of ionic conductors, a brief background of ionic conduction is necessary.Ionic conduction is a result of ionic drift under an applied electric field. In biological systems, ionic transport is generated by ion pumps or ion channels, while in synthetic devices it is generated by electric fields.49 The general set up of a circuit containing an ionic conductor is two electronic conducting electrodes sandwiching the ionic conductor. If the voltage applied across the electrode-ionic conductor interphase is small, electrons and ions do not cross the interface and no electrochemical reaction occurs. Instead, there is formation of an electrical double layer and so the interface between the electrode and the ionic conductor behaves as a capacitor.50 As a result, impedance spectroscopy is commonly used to determine the mobility and charge density in ionic conductors and can be used to calculate conductivity.51 The magnitude of ionic conductivity is dependent on the sum of charge carriers according to eqn (1) (ref. 52)
| σ = ΣiZieniui | (1) |
The mobility (μ) of different ions is dependent on the temperature of the system (T), the diffusion coefficient (D), charge of the ion (q), and the Boltzmann constant (KB) as shown in eqn (2).
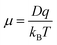 | (2) |
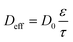 | (3) |
In ionic conduction, cations and anions correspond to holes and electrons in electronic conduction. Ionic conductors can be categorized as nonrestrictive and restrictive ionic conductors. In nonrestrictive ionic conduction both cations and anions can migrate under an applied electric field, while in restrictive ionic conduction only one type of ion migrates.49
4. Factors influencing ionic conductivity in hydrogels
Ionic conducting hydrogels are composed of a polymer network and a liquid phase.54 The charged ions can be introduced into the matrix by employing charged monomers in the polymer network or by dissolving ionic salts into the hydrogel solutions.12a The performance and application of these hydrogels is dependent on several factors. Here, we highlight the factors that influence ionic conductivity and discuss how cellulose materials have been used to tune these factors to improve the performance.4.1. Water content
Stretchable ionic conductors are constructed from hydrogels which are composed mainly of water. The absorption and evaporation of water from the hydrogels would significantly influence performance and the long-term practical application of these ionic conductors in devices. Therefore, it becomes important to modulate the water absorption and evaporation from ionic conductive hydrogels. The ability of the hydrogel to absorb water is defined as water uptake or swelling ratio and plays an important role in affecting ionic conductivity in hydrogels.55 Higher water content increases the ionic conductivity of polyelectrolytes since the increased number of water molecules not involved in hydration shells enhances the μ and Deff leading to higher conductivity.56 With an increase in water content, the activation barrier for the ion transport is lowered and ionic dynamics accelerate.57 Due to the presence of hydroxyl groups on cellulose, this material can form hydrogen bonds with water and in the process influence the water content of the hydrogel greatly (Fig. 6). The amount of water a hydrogel holds is dependent on its ability to absorb water into the polymer matrix and how well the polymer matrix can interact with water molecules to prevent evaporation. Thus, Sections 4.1.1 and 4.1.2 highlight the role of cellulose on water absorption and water retention, respectively.Due to the presence of hydroxyl groups on cellulose, it can form hydrogen bonds with water, in the process enabling the water absorption properties of the hydrogel to be improved. Li and coworkers made a poly(acrylic acid) (PAA) ionic conductive hydrogel reinforced by cellulose nanofibrils (CNF) (Fig. 7a).58 They investigated the alkaline absorption property of the hydrogel with varying CNF content. The final equilibrium-swelling ratio increased with increasing CNF content; the final swelling ratio of the PAA hydrogel with 3 wt% CNF was ∼2100%, which was higher compared to the PAA hydrogel without CNF which had a swelling ratio of 1600%. The rate of water absorption was also enhanced by the presence of CNF. It was postulated that the hydroxyl groups of CNF resulted in more pathways for ions and water to move and thus increased the number of sites for ion incorporation, which resulted in higher solution content and faster absorption. The increase in water content increased the ionic conductivity as well, increasing from ∼0.07 S cm−1 at a swelling ratio of 50% to ∼0.3 S cm−1 at a swelling ratio of 300%.
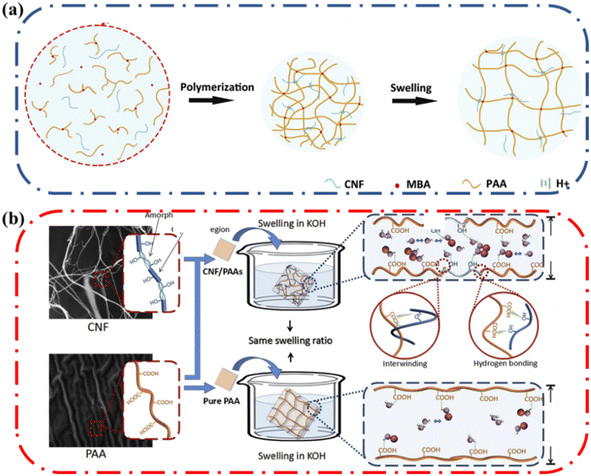 | ||
| Fig. 7 (a) Schematic of polymerization and swelling of the cellulose nanofibrils (CNF)/poly(acrylic acid) (PAA) IC hydrogel (b) schematic picture of the mechanism of CNF/PAAs gel electrolyte and PAA electrolyte swelling in KOH in same swelling ratio. Reprinted with permission from Li et al.58 Copyright 2018 Elsevier. | ||
Dimensional change of ionic conducting hydrogels due to water absorption poses a challenge as excessive change in dimensions results in lowered mechanical properties. Li and coworkers noted that CNF could aid in controlling the extent of dimensional change and thus maintain the attractive properties of the composite ionic conductor.58 As the content of CNF increased, the changes in length and width decreased but the changes in height increased. PAA is a flexible polymer that can swell as the polymer chains straighten, whereas CNF is a rigid polymer that cannot. In the composite ionic conductor hydrogel, the CNF chains are intertwined with PAA chains through hydrogen bonding and physical entanglement in the polymer system. Consequently, the over swelling was suppressed by the rigid CNF (Fig. 7b).
The advantage of adding cellulose to a hydrogel system was clearly demonstrated by Yao and coworkers.59 They designed an ionic conductive hydrogel from the polymerization of acrylamide and phenylboronic acid-ionic liquid (PBA-IL) in the presence of TEMPO-oxidized CNFs (Fig. 8a). The resulting polymer network was polyacrylamide (PAM)/poly(phenylboronic acid-ionic liquid) (PBA-IL)/cellulose nanofibrils (CNF) i.e., PAM/PBA-IL/CNF. They observed that the pure PAM hydrogel loses water at a fast rate, losing ∼70% original weight after 14 days at 25 °C and 40% relative humidity. In contrast, the PAM/PBA-IL/CNF hydrogel maintained ∼85% of its initial weight under the same conditions. Low-field nuclear magnetic resonance (LF-NMR) was used to show that the amount of bound water was higher, and the mobility of water molecules was reduced in PAM/PBA-IL/CNF hydrogel. To support this observation, the authors employed density functional theory (DFT) to quantify the interaction energy between the different components of the hydrogel individually (Fig. 8b). They determined that water interacts more favorably with CNF compared to the other components. This study clearly indicates that the surface properties of cellulose are beneficial in reducing water loss in ionic conductive hydrogels.
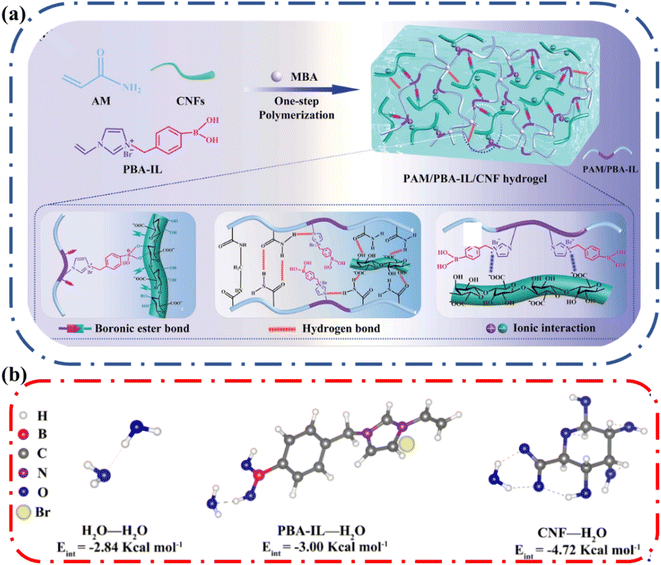 | ||
| Fig. 8 (a) Fabrication mechanism and hierarchical dynamic networks in the construction of the polyacrylamide (PAM)/phenylboronic acid-ionic liquid (PBA-IL)/cellulose nanofibril (CNF) hydrogel. (b) DFT analysis of the interaction energy between PBA-IL, CNF, and water. Reprinted with permission from Yao et al.59 Copyright 2022 Wiley. | ||
4.2. Microstructure
The microstructure of the hydrogel has been shown to influence the ionic conductivity.60 The easier it is for the ions to migrate in the hydrogel, the higher the conductivity. Cellulose can enhance movement of ions through two mechanisms. Hydroxyl groups and charged groups such as the carboxyl and sulfonate groups on the surface of cellulose provide coordination and hopping sites for ions (Fig. 9a).61 Additionally, the hierarchical nature of cellulose materials enables formation of a porous network (Fig. 9b). This porous structure has a larger surface area and provides more room for ions to migrate.61b,c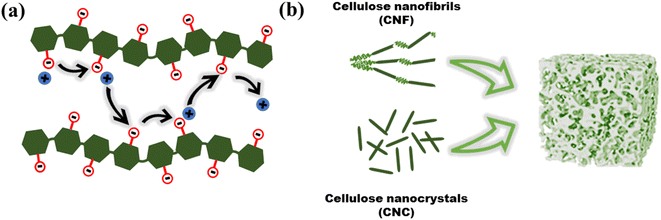 | ||
| Fig. 9 Influence of cellulose on hydrogel microstructure that affects ionic conductivity. (a) Introduces ion hopping sites and (b) enables hierarchical structure that increases porosity. | ||
In general, ionic conductivity increases in the presence of cellulose. Mittal and coworkers designed a sodium ion battery that employed a mixture of cellulose nanocrystals and cellulose nanofibers (Fig. 10a) to prepare a gel polymer electrolyte that offers a high liquid electrolyte uptake of 2985% and an ionic conductivity of 2.32 mS cm−1.62 The role of nanocellulose in enhancing ionic conductivity in the gel electrolyte (ionic conductive hydrogel) was discussed. All the cellulose containing gels had good ionic conductivity which was attributed to the presence of hydroxyl groups on the surface of CNF and CNC that provided coordination sites for the mobile Na+ ions (Fig. 10b). In addition, they observed the ionic conductivity increases from 1.467 to 2.323 mS cm−1 by increasing CNF fraction from 20 to 50 weight%. This increase in conductivity suggested that the presence of sodium carboxylate (–COONa) groups on the CNFs enhanced the counterion mobility. They concluded that the presence of cellulose enhanced ionic conductivity. The same justification has been provided by several authors63 and highlights the importance of incorporating cellulose in ionic conductive hydrogels.
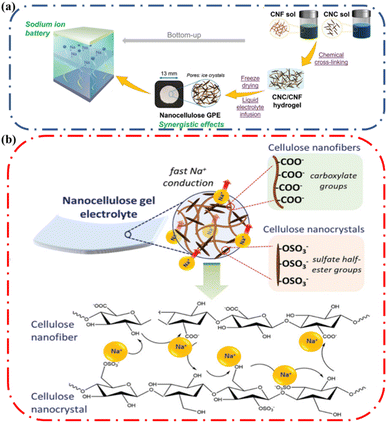 | ||
| Fig. 10 (a) Schematic representation of the nanocellulose gel electrolyte fabrication. (b) Schematic illustration of the proposed Na+ conduction mechanism across the nanocellulose Gel Polymer Electrolytes (GPE). Reprinted with permission from Mittal et al.62 Copyright 2022 Wiley. | ||
Soluble cellulose derivatives can also achieve enhanced ionic conductivity. Gan and coworkers describe a hydroxyl propyl cellulose which enhances ionic conductivity in a polyvinyl alcohol-hydroxyl propyl cellulose–sodium alginate–calcium (PVA-HPCT/SA-Ca) ionic conductive double network hydrogel.64 As the HPC content of hydrogel increased from 0 to 6.67 wt%, the ionic conductivity increased from 2.17 S m−1 to 3.39 S m−1. In this case, the authors suggested that the increase in conductivity was due to an increase in porosity as the HPC content increased. More pores would result in higher absorption of the conductive ions and reduce the resistance to ion migration by providing ion coordination sites. As such, by taking advantage of the hierarchical structure of cellulosic material, the porosity of ionic conductors can be fine-tuned to enhance ionic conductivity of ICHs.
4.3. Mechanical properties
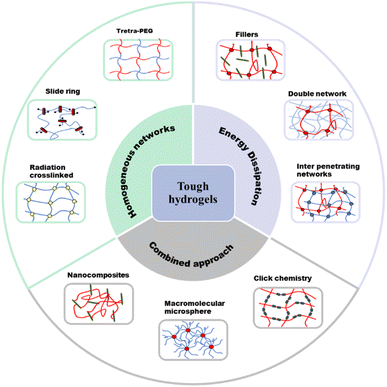
Conventional hydrogels have attractive characteristics such as being flexible and transparent.65 However, they are usually very brittle, making them difficult to apply in functional and durable devices.66 Improving the mechanical properties (e.g., toughness, stretchability, adhesiveness, and self-healing properties) of hydrogels has enabled the application of hydrogels in ionic devices.The strength of hydrogels can be impacted by crack formation and the resulting propagation.67 The occurrence of fracture in hydrogel systems is due to the inhomogeneous nature of the polymer network. This non uniform arrangement results in stress being concentrated in certain parts of the network, and once a fracture appears, it can propagate to the rest of the network.68 To suppress the appearance and propagation of cracks, it is necessary to introduce energy dissipation mechanisms into the hydrogel to reduce stress concentration. There are several mechanisms that have been reported for improving the mechanical properties of hydrogels (Fig. 11).67 The mechanical properties of these hydrogels are evaluated in terms of tensile strength and modulus, compression strength and modulus, fracture stress and strain, fracture energy, as well as storage and loss moduli.
Cellulose-only hydrogels usually display less desirable mechanical properties, which impact their use in bioelectronics negatively. In some cases, chemical crosslinking strategies have been employed in cellulose-only hydrogels to obtain good performance in ionic conductors. For instance, Tong and coworkers reported a cellulose ionic conductive hydrogel made by dissolving allyl cellulose in a NaOH/urea aqueous solution then chemically cross-linking through free radical polymerization (Fig. 12a).69 The mechanical properties of the hydrogel were strongly influenced by the extent of crosslinking. The resulting tensile, compression, and conductivity properties were satisfactory for a self-supporting cellulose only hydrogel (Fig. 12b–d). After optimization, the resulting hydrogels exhibited good stretchability (tensile strain ∼ 126%) and compressibility (compression strain ∼ 80%) properties and maintained good ionic conductivity of ∼0.16 mS cm−1.
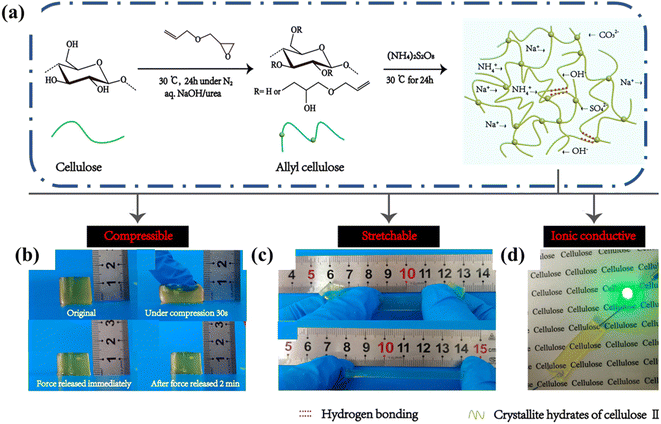 | ||
| Fig. 12 (a) Schematic illustration of synthesis of cellulose hydrogels. Desirable properties include (b) compressibility, (c) stretchability, (d) ionic conductivity and transparency. Reprinted with permission from Tong et al.69 Copyright 2019 American Chemical Society. | ||
Despite the relatively good mechanical properties achieved by Tong and coworkers, the mechanical properties of hydrogels should be significantly enhanced for utility in functional devices. The various strategies to do this include formation of double network (DN) gels, topological gels, and nanocomposite gels. DN hydrogels have been effectively formed using cellulose as one of the components. A DN hydrogel consists of at least two components that are intertwined and crosslinked. This results in hydrogels with better mechanical performance. The strength and toughness can be tuned by manipulating the inter/intramolecular interactions and the structures of the components of the hydrogel. Thus, DN hydrogels enable the development of extremely tough and yet well-hydrated materials.70 This strategy has been employed to make cellulose containing ionic conductive hydrogels with very good mechanical properties.
Ma and coworkers reported an alkaline-tolerant dual-network hydrogel electrolyte-based sodium polyacrylate (PANa) and cellulose which had an optimal conductivity of 0.28 S cm−1.71 The resulting PANa-cellulose dual-network ionic conductive hydrogel was physically and chemically cross-linked (Fig. 13a). The hydrogel matrix had cross-linking between PANa and MBAA and physical cross-linking due to hydrogen bonding and chain entanglements between PANa and cellulose chains. The elongation at break and tensile strength were enhanced by the introduction of cellulose. The PANa–cellulose hydrogel stretched to over 1000% strain, which was a significant improvement compared to the pure PANa (300% maximum strain). In addition, the hydrogel exhibited good flexibility; it could be rolled, folded, twisted, and crumpled without any mechanical failure or visible cracks. The superior stretchability and softness were attributed to the celluloses and MBAA-assisted toughening and hydrogen bond cross-linking mechanism (Fig. 13b). The semi rigid cellulose chains were uniformly dispersed in the intrinsically flexible PANa polymer networks. The intertwining of the cellulose chains with the PANa chains endowed extraordinary flexibility. Also, hydrogen bonding could behave as dynamic crosslinks that could break and re-form to dissipate mechanical energy during strain. These combined factors resulted in enhancement of mechanical properties, highlighting the benefits of having cellulose as the additional component in double network ionic conductive hydrogels.
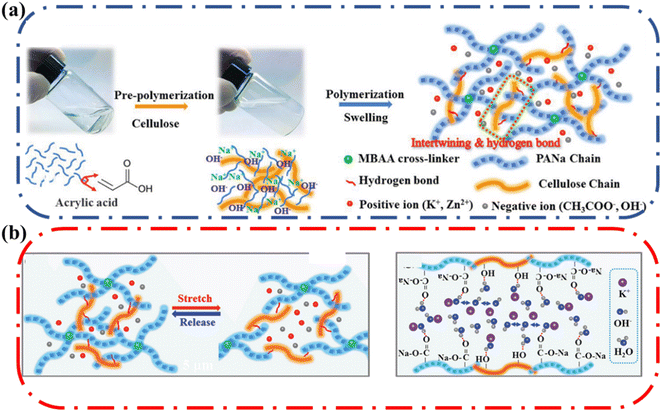 | ||
| Fig. 13 (a) Synthetic procedure of the PANa–cellulose hydrogel electrolyte using MBAA (cross-linker), acrylate (AA, main monomer) and cellulose (enhancer). (b) Schematic illustration for origin of ultra-stretchability and structure of PANa-cellulose hydrogel showing entrapped KOH and water via hydrogen bond interactions. Reprinted with permission from Ma et al.71 Copyright 2019 Wiley. | ||
4.4. Self-healing and adhesiveness
To expand the applicability of ionic conductive hydrogels, the mechanical properties can be enhanced even further to include properties such as self-healing and adhesiveness. However, to obtain these properties, the mechanical properties usually suffer. To obtain self-healing and adhesiveness, dynamic crosslinks must be used which inherently compromises the mechanical strength.Self-healing in hydrogels can be achieved by employing covalent or noncovalent crosslinking (Fig. 14). Covalent self-healing hydrogels employ dynamic covalent bonds such as disulfide bonds, borate ester bonds, imine bonds (Schiff base), Diels–Alder reactions, hydrazone bonds and reversible free radical reaction.72 In contrast, noncovalent self-healing hydrogels are accessed by employing intermolecular interactions that include hydrogen bonds, host–guest interactions, metal coordination, π–π stack, and hydrophobic associations.72 Given that cellulose has a large number of hydroxyl groups on the surface, these can directly enhance the hydrogen bonding to access self-healing properties. In addition, these hydroxyl groups can be modified to attach different functional groups that can enable both covalent and non-covalently crosslinked hydrogels.72a,73
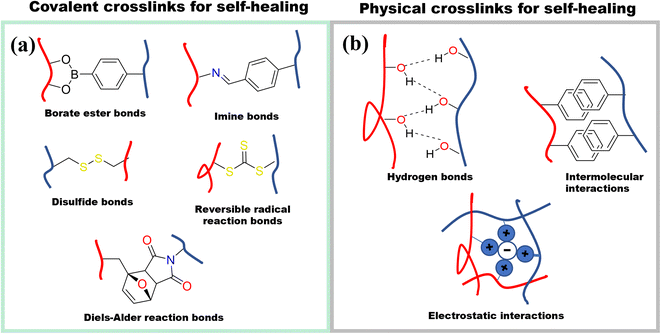 | ||
| Fig. 14 Different strategies for designing self-healing hydrogels. (a) Chemical crosslinking self-healing mechanisms (b) physically crosslinking self-healing mechanisms. | ||
Shao and coworkers designed an ionic conductive hydrogel that retained good mechanical strength along with good self-healing, and self-adhesive properties. The designed gel employed multiple coordination bonds between tannic acid-coated cellulose nanocrystals (TA@CNCs), poly(acrylic acid) chains, and metal ions in a covalent polymer network (Fig. 15a).74 The researchers took advantage of the facile modification of cellulose to attach tannic acid onto the surface. The resulting ionic conductive hydrogels had good mechanical properties, and these could be tailored by controlling the TA@CNC content. Notably, the hydrogel exhibited elasticity and had an elongation at break of 2900% without fracture.
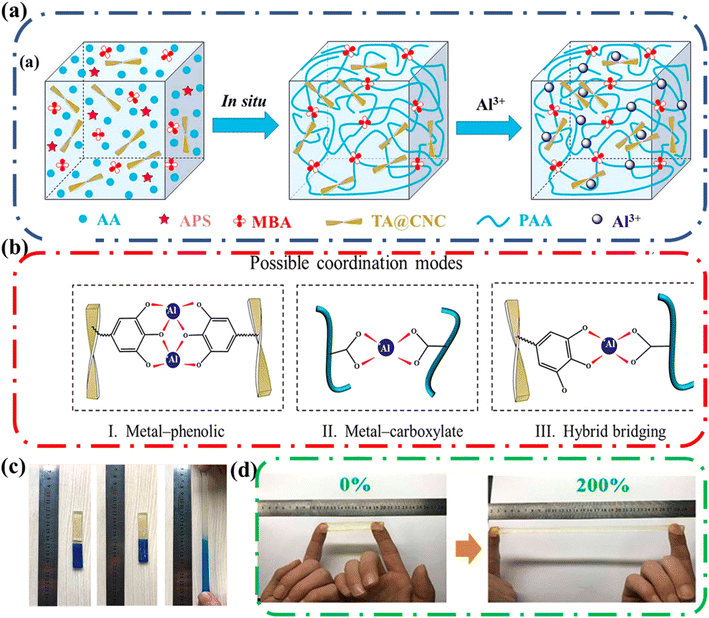 | ||
| Fig. 15 (a) Schematic illustration of ionic gel synthetic process that includes the in situ polymerization to form nanocomposite hydrogels and then immersion in Al3+ solution to produce ionic coordination. (b) Possible coordination modes among TA@CNC, PAA, and Al3+: (I) metal-phenolic coordination between TA@CNCs; (II) metal-carboxylate coordination between PAA chains; (III) hybrid bridging between TA@CNCs and PAA chains. Possible coordination modes. (c) Self-healing properties of the TA@CNC ionic gels at 25 °C. Ionic gel is cut into two pieces and recombined. Then the fractured gel can self-healed automatically into a complete gel and sustain stretching without failure after 30 min healing. (d) Adhesive properties of the TA@CNC ionic gels. Ionic gel can adhere to fingers without the assistance of additional adhesive tapes and withstand the recoverable stretch of 200%. Reprinted with permission from Shao et al.74 Copyright 2018 American Chemical Society. | ||
The hydrogel also demonstrated self-healing behavior; fresh cut pieces of the hydrogel could recombine without applied stress (Fig. 15c). The hydrogel regained its tensile properties with increased healing time. Additionally, the efficiency of the healing increased as the content of TA@CNC increased, highlighting the importance of the TA@CNC to self-healing process. The TA@CNC motifs associated by coordination bonds (Fig. 15b) which prevented crack propagation, enhancing the stability of the network and allowing for greater mechanical strength and energy dissipation during deformation (imparting good recovery). Finally, this gel exhibited good self-adhesiveness to different surfaces including polytetrafluoroethylene, glass slides, rubbers, wood, and carnelian. Remarkably, the hydrogel was adhesive enough to adhere to fingers and withstand the recoverable stretch of 200% (Fig. 15d).
5. Applications of ionic conductive hydrogels containing cellulose
As described above, cellulose offers unique features of biodegradability, biocompatibility, low production cost, abundance, nontoxicity, and excellent mechanical properties. We have also described how these properties can be utilized to enhance the performance of ionic conductive hydrogels. We now describe a few examples in which ionic conductive hydrogels have been employed to fabricate functional electronics.5.1. Strain sensor
Strain sensors transduce external mechanical stimuli into electrical signals. They are particularly interesting due to their potential applications in personalized health-monitoring, human motion detection, human–machine interfaces, and soft robotics.76 Wang and coworkers reported a polyacrylamide (PAAm), cellulose nanocrystal (CNC) and phytic acid (PA) biocompatible hydrogel that had satisfactory mechanical properties and ionic conductivity well below zero degrees Celsius (Fig. 16a).75 The inclusion of cellulose nanocrystals into the hydrogel aided energy dissipation, increasing the strength of the hydrogel. Also, the CNC and PA enabled good retention of water due to increased hydrogen bonding in the system. Consequently, since the ionic conducting hydrogel could retain all these attractive properties under harsh conditions, it enabled the hydrogel to be used to fabricate a strain sensor. The gauge factor (GF) is used as an index to judge the strain sensitivity of hydrogels, the higher the GF the greater the resistance to change when a strain is applied. The GF can be determined by the change in relative resistance (ΔR/R0) in linear strain. The GF of the PAAm–PA–CNC hydrogel increased as the strain increased and reached a maximum GF of 4.1. As such, the hydrogel shows high sensitivity under both small and large deformations. In addition, the response was frequency dependent. These properties indicate that the hydrogel is a good candidate for strain sensing applications.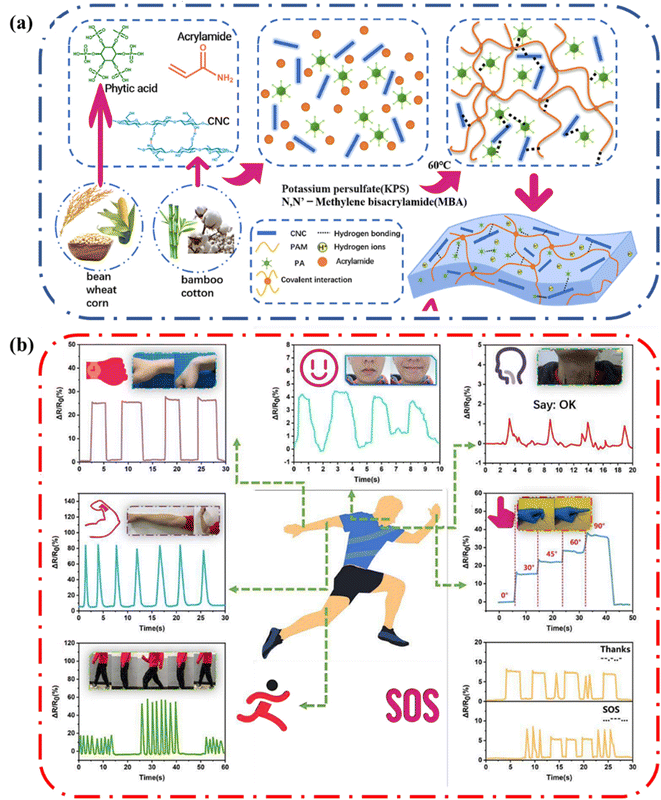 | ||
| Fig. 16 (a) Illustration showing the formation of the biomass-reinforced hydrogels (b) variation of ΔR/R0 of the hydrogel at room temperature during palm movement, smiling and talking, arm movement, finger bending, walking, and running. Information delivery using the hydrogel as a strain sensor via Morse codes. Reprinted with permission from Wang et al.75 Copyright 2022 Elsevier. | ||
As a proof-of-concept demonstration, Wang and coworkers applied the hydrogel as a wearable strain sensor to detect human body movement (Fig. 16b). The sensor could detect large strains such as straightening and bending of the wrist, walking, and running. In addition, it could detect small strains from as smiling, chewing, talking. These results show that the ionic conductive hydrogels containing cellulose can be applied to fabricate sensing and communication devices.
5.2. Humidity sensor
A humidity sensor is an electronic device capable of responding to the presence of moisture and converting the response into an electrical signal. Yu and coworkers fabricated a CNF-reinforced and ionic conductive organogel with enhanced, mechanical, anti-freezing and anti-dehydration properties and utilized it to make a humidity sensor.77 The gel was synthesized in a two-step process as illustrated in Fig. 17a. The inclusion of a rigid CNF as a dynamic hydrogen bonding component bestowed the organogels with a hierarchical honeycomb-like cellular structure, leading to enhanced mechanical properties. The one-pot solvent displacement method was utilized to introduce sorbitol and CaCl2 into the hydrogel, leading to anti-freezing tolerance, anti-dehydration ability, and ionic conductivity.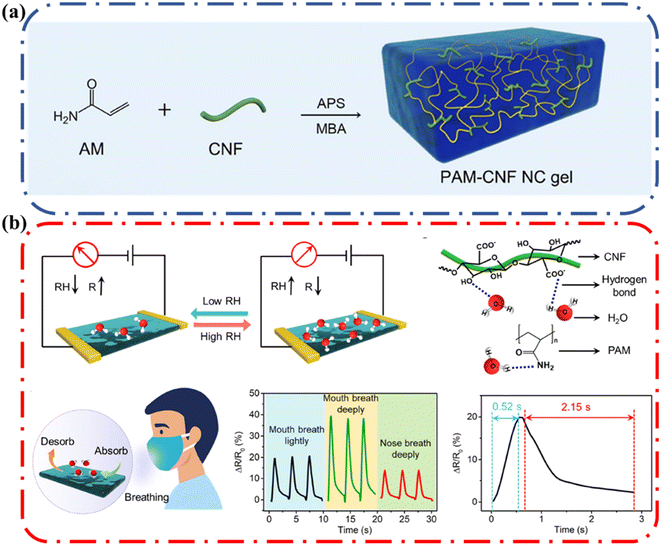 | ||
| Fig. 17 (a) Schematic illustration of the fabrication of the PAM-CNF NC gel via in situ radical polymerization (b) schematic illustrating the humidity sensing mechanism of the CS–NC gel, the formation of hydrogen bonds between water and CNF, as well as water and PAM, schematic diagram of breath monitoring and resulting relative resistance changes of CS–NC sensors under different breath conditions. Reprinted with permission from Yu et al.77 Copyright 2022 American Chemical Society. | ||
The CaCl2/sorbitol (CS)–CNF nanocomposite (NC) gel was assembled into a humidity sensor to detect the relative humidity (RH) of the environment. The sensing performances of the CS–NC humidity sensors were evaluated by monitoring their resistance change under varying RH (Fig. 17b). The humidity response is defined as relative resistance change (ΔR/R0), where ΔR is the resistance change with respect to its initial resistance under dry air. When the sensors absorbed moisture from the surrounding environment it led to an increase in resistance change. Once the sample was dried, the resistance decreased, indicating that the changed resistance in humid conditions was due to the dynamic absorption of water molecules. The high sensitivity of the CS–NC humidity sensor was attributed to the generated hydrogen bonds between the water molecules and a preponderance of hydrophilic groups in the organohydrogels. This demonstrates how one can take advantage of the surface functionality of cellulose to obtain a functional device where the formation of hydrogen bonds promotes the adsorption and condensation of water molecules.
5.3. Ionic diode
Single ion conductors are another class of ionic conductors that can be used to make functional devices. In these polyelectrolyte hydrogels, a polymer with a fixed positive charge with negative counterions that can migrate is referred to as an n-type ionic conductor. Conversely, if the hydrogel has a fixed negative charge and free positive counterions that are free to migrate it is referred to as a p-type ionic conductor. These ionic conductors have been used in complex devices such as ionic diodes. In an ionic diode, the device permits unidirectional flow of current i.e., rectification. Rectification is achieved when there is an anisotropic distribution of counterions at the interface between the two gels having a p-type ionic conductor on one side and a n-type ionic conductor on the other.4a Polymers with high fixed charge densities, such as cationic poly(diallyldimethylammonium chloride) (PDAC) and anionic poly(styrene sulfonate) (PSS), have been used in conjunction with hydrogels to construct ionic diodes.An alternative approach was previously employed to fabricate an ionic diode paper made from two oppositely charged microfibrillated cellulose (MFC) layers. The MFC diode used up to 50% cellulose and achieved a rectification ratio of ∼15. To improve on these results, Nyamayaro and coworkers employed cellulose nanocrystals (CNCs), a highly crystalline form of cellulose with nanoscale dimensions, exceptional mechanical properties, large surface areas, and tremendous rigidity. Due to the higher surface area, they achieved a rectification ratio of ∼70 (Fig. 18).4b,78 These examples demonstrate the potential of cellulose as the active component of complex biodegradable ionic devices.
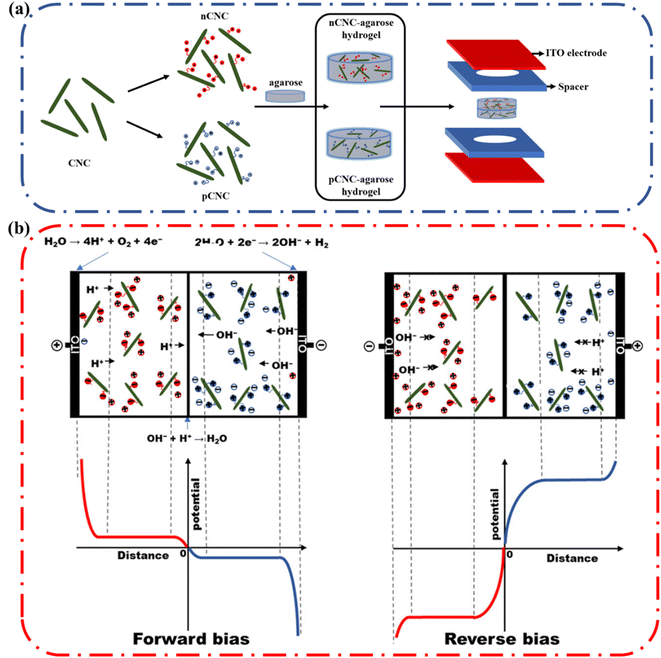 | ||
| Fig. 18 (a) Schematic representation of the ionic diode fabrication. Cationic CNC (nCNC) and anionic CNC (pCNC) were incorporated into two separate slabs of agarose. The agarose gels with oppositely charged CNCs were placed into spacers and sandwiched between ITO electrodes. (d) Illustration of the rectification mechanism of the nCNC/pCNC–agarose ionic diode under forward bias and negative bias. Reprinted with permission from Nyamayaro et al.75 Copyright 2018 American Chemical Society. | ||
6. Conclusions
The rising interest in wearable electronic devices has led to increased research into the development of flexible electronics. As such, there is need for renewable and environmentally friendly alternatives to move towards more sustainable electronics. Cellulose and its composites have been shown to degrade under environmental, composting, and marine conditions, opening a pathway to biodegradable electronics.79 This review introduces cellulose as a good alternative material with attractive properties for inclusion in iontronics. From the general principles of ionic conductivity, we summarized how water microstructure, absorption and retention properties of a hydrogel influence ionic conductivity. In addition, it was seen that for long term performance of iontronics, the mechanical properties of the hydrogel are important.The ability of cellulose to tailor the water retention and absorption properties was discussed. It has been seen that due to the hydroxyl group present on the surface of cellulose, the hydrogen bonding capability of the material endows hydrogels with good water absorption and retention of water due to increased interaction. Cellulose has also been employed to increase ionic conductivity by influencing the microstructure of the hydrogel. Firstly, by influencing assembly of polymer chains, cellulose increases the porosity of a hydrogel, enabling easier movement of ions. Also, due to the different possible functionality on cellulose, the material can provide coordination site that can enhance ionic conductivity through ion hoping. In addition, the presence of cellulose in composite ICHs enables the enhancement of mechanical properties such as stretchability by inhibiting crack propagation. Lastly, by manipulating the surface chemistry of cellulose incorporated into ICHs, the hydrogels could be imparted with adhesive and self-healing properties.
Despite the above-mentioned advantages of employing cellulose materials in ICHs, there are still some challenges that must be overcome to increase the applicability of cellulose in iontronics. Although cellulosic material can be regarded as non-toxic, biocompatible, and biodegradable, these materials are usually used for ICHs in conjunction with other components that are non-degradable and toxic. As such, it is important to develop further strategies to make mechanically robust and highly conductive cellulose only hydrogels. In addition, the processes occurring at the interface between the hydrogel and electrode have to be studied in order to better understand the efficiency of signal transmission between the electrical conductor into the ionic conductor. Despite having some drawbacks, cellulose has proven to be an exciting prospect for the design of high functioning, sustainable and biodegradable ICHs for iontronics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
KN thanks the UBC Department of Chemistry for the Award for Graduate Research Excellence. This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada.Notes and references
- Z. Wang, Z. Ma, J. Sun, Y. Yan, M. Bu, Y. Huo, Y.-F. Li and N. Hu, Polymers, 2021, 13, 813 CrossRef CAS PubMed.
- J. C. Yang, J. Mun, S. Y. Kwon, S. Park, Z. Bao and S. Park, Adv. Mater., 2019, 31, 1904765 CrossRef CAS PubMed.
- (a) H. Chun and T. D. Chung, Annu. Rev. Anal. Chem., 2015, 8, 441–462 CrossRef CAS; (b) S. Z. Bisri, S. Shimizu, M. Nakano and Y. Iwasa, Adv. Mater., 2017, 29, 1607054 CrossRef PubMed; (c) P. Zhang, W. Guo, Z. H. Guo, Y. Ma, L. Gao, Z. Cong, X. J. Zhao, L. Qiao, X. Pu and Z. L. Wang, Adv. Mater., 2021, 33, 2101396 CrossRef CAS; (d) S. H. Han, S. I. Kim, H.-R. Lee, S.-M. Lim, S. Y. Yeon, M.-A. Oh, S. Lee, J.-Y. Sun, Y.-C. Joo and T. D. Chung, ACS Appl. Mater. Interfaces, 2021, 13, 6606–6614 CrossRef CAS PubMed.
- (a) O. J. Cayre, S. T. Chang and O. D. Velev, J. Am. Chem. Soc., 2007, 129, 10801–10806 CrossRef CAS PubMed; (b) K. Nyamayaro, V. Triandafilidi, P. Keyvani, J. Rottler, P. Mehrkhodavandi and S. G. Hatzikiriakos, Phys. Fluids, 2021, 33, 032010 CrossRef CAS.
- P. Janson, E. O. Gabrielsson, K. J. Lee, M. Berggren and D. T. Simon, Adv. Mater. Technol., 2019, 4, 1800494 CrossRef.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- M. F. El-Kady, Y. Shao and R. B. Kaner, Nat. Rev. Mater., 2016, 1, 16033 CrossRef CAS.
- (a) H.-J. Koo, S. T. Chang and O. D. Velev, Small, 2010, 6, 1393–1397 CrossRef CAS PubMed; (b) U.-J. Kim, S. Kuga, M. Wada, T. Okano and T. Kondo, Biomacromolecules, 2000, 1, 488–492 CrossRef CAS PubMed; (c) K. Tybrandt, E. O. Gabrielsson and M. Berggren, J. Am. Chem. Soc., 2011, 133, 10141–10145 CrossRef CAS; (d) K. Tybrandt, K. C. Larsson, A. Richter-Dahlfors and M. Berggren, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9929–9932 CrossRef CAS.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS.
- O. Wichterle and D. LÍM, Nature, 1960, 185, 117–118 CrossRef.
- (a) M. Jia and M. Rolandi, Adv. Healthcare Mater., 2020, 9, 1901372 CrossRef CAS PubMed; (b) T. Arbring Sjöström, M. Berggren, E. O. Gabrielsson, P. Janson, D. J. Poxson, M. Seitanidou and D. T. Simon, Adv. Mater. Technol., 2018, 3, 1700360 CrossRef.
- (a) C. Yang and Z. Suo, Nat. Rev. Mater., 2018, 3, 125–142 CrossRef CAS; (b) T. Someya, Z. Bao and G. G. Malliaras, Nature, 2016, 540, 379–385 CrossRef CAS PubMed; (c) S. Inal, J. Rivnay, A.-O. Suiu, G. G. Malliaras and I. McCulloch, Acc. Chem. Res., 2018, 51, 1368–1376 CrossRef CAS PubMed; (d) Z. Deng, R. Yu and B. Guo, Mater. Chem. Front., 2021, 5, 2092–2123 RSC.
- (a) C. M. Rochman, M. A. Browne, B. S. Halpern, B. T. Hentschel, E. Hoh, H. K. Karapanagioti, L. M. Rios-Mendoza, H. Takada, S. Teh and R. C. Thompson, Nature, 2013, 494, 169–171 CrossRef CAS PubMed; (b) R. C. Thompson, C. J. Moore, F. S. v. Saal and S. H. Swan, Philos. Trans. R. Soc., B, 2009, 364, 2153–2166 CrossRef CAS PubMed.
- Y. Cao and K. E. Uhrich, J. Bioact. Compat. Polym., 2019, 34, 3–15 CrossRef CAS.
- V. R. Feig, H. Tran and Z. Bao, ACS Cent. Sci., 2018, 4, 337–348 CrossRef CAS PubMed.
- (a) M. A. Hillmyer and W. B. Tolman, Acc. Chem. Res., 2014, 47, 2390–2396 CrossRef CAS PubMed; (b) T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Angew. Chem., Int. Ed., 2019, 58, 50–62 CrossRef CAS PubMed; (c) Y. Zhong, P. Godwin, Y. Jin and H. Xiao, Adv. Ind. Eng. Polym. Res., 2020, 3, 27–35 Search PubMed.
- H. Xiang, Z. Li, H. Liu, T. Chen, H. Zhou and W. Huang, npj Flexible Electron., 2022, 6, 15 CrossRef.
- S. Ling, W. Chen, Y. Fan, K. Zheng, K. Jin, H. Yu, M. J. Buehler and D. L. Kaplan, Prog. Polym. Sci., 2018, 85, 1–56 CrossRef CAS PubMed.
- (a) M. Jia, J. Kim, T. Nguyen, T. Duong and M. Rolandi, Biopolymers, 2021, 112, e23433 CrossRef CAS PubMed; (b) C. Cui, Q. Fu, L. Meng, S. Hao, R. Dai and J. Yang, ACS Appl. Bio Mater., 2021, 4, 85–121 CrossRef CAS PubMed.
- T. Xu, K. Liu, N. Sheng, M. Zhang, W. Liu, H. Liu, L. Dai, X. Zhang, C. Si, H. Du and K. Zhang, Energy Storage Mater., 2022, 48, 244–262 CrossRef.
- D. Zhao, Y. Zhu, W. Cheng, W. Chen, Y. Wu and H. Yu, Adv. Mater., 2021, 33, 2000619 CrossRef CAS PubMed.
- (a) G. Babak and A. Hadi, in Biodegradation, ed. C. Rolando and R. Francisca, IntechOpen, Rijeka, 2013, ch. 6 Search PubMed; (b) A. Das, T. Ringu, S. Ghosh and N. Pramanik, Polym. Bull., 2023, 80, 7247–7312 CrossRef CAS PubMed.
- A. Mtibe, M. P. Motloung, J. Bandyopadhyay and S. S. Ray, Macromol. Rapid Commun., 2021, 42, 2100130 CrossRef CAS PubMed.
- S. J. Christian, in Nonconventional and Vernacular Construction Materials, ed. K. A. Harries and B. Sharma, Woodhead Publishing, 2016, pp. 111–126 Search PubMed.
- M. Okada, Prog. Polym. Sci., 2002, 27, 87–133 CrossRef CAS.
- Y. Habibi, Chem. Soc. Rev., 2014, 43, 1519–1542 RSC.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- M. Tavakolian, S. M. Jafari and T. G. M. van de Ven, Nano Micro Lett., 2020, 12, 73 CrossRef CAS.
- H. Seddiqi, E. Oliaei, H. Honarkar, J. Jin, L. C. Geonzon, R. G. Bacabac and J. Klein-Nulend, Cellulose, 2021, 28, 1893–1931 CrossRef CAS.
- D. N. S. Hon, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan and P. Veyssière, Elsevier, Oxford, 2001, pp. 1039–1045 Search PubMed.
- (a) R. G. P. Viera, G. R. Filho, R. M. N. de Assunção, C. d. S. Meireles, J. G. Vieira and G. S. de Oliveira, Carbohydr. Polym., 2007, 67, 182–189 CrossRef CAS; (b) P. L. Nasatto, F. Pignon, J. L. M. Silveira, M. E. R. Duarte, M. D. Noseda and M. Rinaudo, Polymers, 2015, 7, 777–803 CrossRef CAS.
- B. Orhan, C. A. Ziba, M. H. Morcali and M. Dolaz, Sustainable Environ. Res., 2018, 28, 403–411 CrossRef CAS.
- A. Aravamudhan, D. M. Ramos, A. A. Nada and S. G. Kumbar, in Natural and Synthetic Biomedical Polymers, ed. S. G. Kumbar, C. T. Laurencin and M. Deng, Elsevier, Oxford, 2014, pp. 67–89 Search PubMed.
- J. Profili, S. Rousselot, E. Tomassi, E. Briqueleur, D. Aymé-Perrot, L. Stafford and M. Dollé, ACS Sustain. Chem. Eng., 2020, 8, 4728–4733 CrossRef CAS.
- V. Thakur, A. Guleria, S. Kumar, S. Sharma and K. Singh, Mater. Adv., 2021, 2, 1872–1895 RSC.
- (a) R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC; (b) E. Niinivaara and E. D. Cranston, Carbohydr. Polym., 2020, 247, 116664 CrossRef CAS PubMed.
- (a) A. Isogai and L. Bergström, Curr. Opin. Green Sustainable Chem., 2018, 12, 15–21 CrossRef; (b) O. Nechyporchuk, M. N. Belgacem and J. Bras, Ind. Crops Prod., 2016, 93, 2–25 CrossRef CAS.
- (a) S. Iwamoto, W. Kai, A. Isogai and T. Iwata, Biomacromolecules, 2009, 10, 2571–2576 CrossRef CAS; (b) P. Tingaut, T. Zimmermann and G. Sèbe, J. Mater. Chem., 2012, 22, 20105–20111 RSC.
- H.-M. Ng, L. T. Sin, T.-T. Tee, S.-T. Bee, D. Hui, C.-Y. Low and A. R. Rahmat, Composites, Part B, 2015, 75, 176–200 CrossRef CAS.
- K. J. De France, T. Hoare and E. D. Cranston, Chem. Mater., 2017, 29, 4609–4631 CrossRef CAS.
- (a) M. Bergenstråhle, J. Wohlert, M. E. Himmel and J. W. Brady, Carbohydr. Polym., 2010, 345, 2060–2066 CrossRef; (b) B. Lindman, B. Medronho, L. Alves, C. Costa, H. Edlund and M. Norgren, Phys. Chem. Chem. Phys., 2017, 19, 23704–23718 RSC.
- (a) S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC; (b) A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed; (c) J. Zhang, J. Wu, J. Yu, X. Zhang, J. He and J. Zhang, Mater. Chem. Front., 2017, 1, 1273–1290 RSC.
- P. Bettotti and M. Scarpa, Adv. Mater. Interfaces, 2022, 9, 2101593 CrossRef CAS.
- L. Solhi, V. Guccini, K. Heise, I. Solala, E. Niinivaara, W. Xu, K. Mihhels, M. Kröger, Z. Meng, J. Wohlert, H. Tao, E. D. Cranston and E. Kontturi, Chem. Rev., 2023, 123, 1925–2015 CrossRef CAS.
- S. Väisänen, R. Pönni, A. Hämäläinen and T. Vuorinen, Cellulose, 2018, 25, 6923–6934 CrossRef.
- C. Driemeier and J. Bragatto, J. Phys. Chem. B, 2013, 117, 415–421 CrossRef CAS PubMed.
- J. Grignon and A. M. Scallan, J. Appl. Polym. Sci., 1980, 25, 2829–2843 CrossRef CAS.
- H.-R. Lee, C.-C. Kim and J.-Y. Sun, Adv. Mater., 2018, 30, 1704403 CrossRef PubMed.
- C. Keplinger, J.-Y. Sun, C. C. Foo, P. Rothemund, G. M. Whitesides and Z. Suo, Science, 2013, 341, 984–987 CrossRef CAS PubMed.
- F. H. Muhammad, R. H. Y. Subban and T. Winie, Mater. Today: Proc., 2017, 4, 5130–5137 CrossRef.
- B. D. Paulsen, K. Tybrandt, E. Stavrinidou and J. Rivnay, Nat. Mater., 2020, 19, 13–26 CrossRef CAS PubMed.
- H. Dechiraju, M. Jia, L. Luo and M. Rolandi, Adv. Sustainable Syst., 2022, 6, 2100173 CrossRef CAS.
- S. K. De, N. R. Aluru, B. Johnson, W. C. Crone, D. J. Beebe and J. Moore, J. Microelectromech. Syst., 2002, 11, 544–555 CrossRef CAS.
- C.-J. Lee, H. Wu, Y. Hu, M. Young, H. Wang, D. Lynch, F. Xu, H. Cong and G. Cheng, ACS Appl. Mater. Interfaces, 2018, 10, 5845–5852 CrossRef CAS PubMed.
- (a) S. De, C. Cramer and M. Schönhoff, Macromolecules, 2011, 44, 8936–8943 CrossRef CAS; (b) P. Pissis and A. Kyritsis, Solid State Ionics, 1997, 97, 105–113 CrossRef CAS.
- Y. Guo, X. Zhou, Q. Tang, H. Bao, G. Wang and P. Saha, J. Mater. Chem. A, 2016, 4, 8769–8776 RSC.
- L. Li, L. Liu, Y. Qing, Z. Zhang, N. Yan, Y. Wu and C. Tian, Electrochim. Acta, 2018, 270, 302–309 CrossRef CAS.
- X. Yao, S. Zhang, L. Qian, N. Wei, V. Nica, S. Coseri and F. Han, Adv. Funct. Mater., 2022, 32, 2204565 CrossRef CAS.
- M. Jia, L. Luo and M. Rolandi, Macromol. Rapid Commun., 2022, 43, 2100687 CrossRef CAS PubMed.
- (a) H. Qin, K. Fu, Y. Zhang, Y. Ye, M. Song, Y. Kuang, S.-H. Jang, F. Jiang and L. Cui, Energy Storage Mater., 2020, 28, 293–299 CrossRef; (b) J. Hu, Y. Wu, Q. Yang, Q. Zhou, L. Hui, Z. Liu, F. Xu and D. Ding, Carbohydr. Polym., 2022, 275, 118697 CrossRef CAS PubMed; (c) H. Wang, Z. Li, M. Zuo, X. Zeng, X. Tang, Y. Sun and L. Lin, Carbohydr. Polym., 2022, 280, 119018 CrossRef CAS PubMed.
- N. Mittal, S. Tien, E. Lizundia and M. Niederberger, Small, 2022, 18, 2107183 CrossRef CAS.
- X. Wu, W. Pi, X. Hu, X. He, Y. Zhu, J. Wang and S. Yang, J. Colloid Interface Sci., 2022, 608, 2158–2168 CrossRef CAS PubMed.
- S. Gan, S. Bai, C. Chen, Y. Zou, Y. Sun, J. Zhao and J. Rong, Int. J. Biol. Macromol., 2021, 181, 418–425 CrossRef CAS PubMed.
- H. Yuk, B. Lu and X. Zhao, Chem. Soc. Rev., 2019, 48, 1642–1667 RSC.
- W. Wang, R. Narain and H. Zeng, Front. Chem., 2018, 6, 497 CrossRef CAS PubMed.
- X. Zhang, J. Xiang, Y. Hong and L. Shen, Macromol. Rapid Commun., 2022, 43, 2200075 CrossRef CAS.
- (a) C. Yang, T. Yin and Z. Suo, J. Mech. Phys. Solids, 2019, 131, 43–55 CrossRef CAS; (b) H. Furukawa, K. Horie, R. Nozaki and M. Okada, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 031406 CrossRef; (c) A. M. S. Costa and J. F. Mano, Eur. Polym. J., 2015, 72, 344–364 CrossRef CAS; (d) J. Guo, M. Liu, A. T. Zehnder, J. Zhao, T. Narita, C. Creton and C.-Y. Hui, J. Mech. Phys. Solids, 2018, 120, 79–95 CrossRef CAS; (e) A. Lucantonio, G. Noselli, X. Trepat, A. DeSimone and M. Arroyo, Phys. Rev. Lett., 2015, 115, 188105 CrossRef PubMed.
- R. Tong, G. Chen, D. Pan, H. Qi, R. a. Li, J. Tian, F. Lu and M. He, Biomacromolecules, 2019, 20, 2096–2104 CrossRef CAS.
- (a) X. Xu, V. V. Jerca and R. Hoogenboom, Mater. Horiz., 2021, 8, 1173–1188 RSC; (b) T. Zhu, Y. Ni, G. M. Biesold, Y. Cheng, M. Ge, H. Li, J. Huang, Z. Lin and Y. Lai, Chem. Soc. Rev., 2023, 52, 473–509 RSC.
- L. Ma, S. Chen, D. Wang, Q. Yang, F. Mo, G. Liang, N. Li, H. Zhang, J. A. Zapien and C. Zhi, Adv. Energy Mater., 2019, 9, 1803046 CrossRef.
- (a) Y. Yang, L. Xu, J. Wang, Q. Meng, S. Zhong, Y. Gao and X. Cui, Carbohydr. Polym., 2022, 283, 119161 CrossRef CAS PubMed; (b) W. Wang, R. Narain and H. Zeng, Front. Chem., 2018, 6, 497 CrossRef CAS PubMed.
- H. An, Y. Bo, D. Chen, Y. Wang, H. Wang, Y. He and J. Qin, RSC Adv., 2020, 10, 11300–11310 RSC.
- C. Shao, M. Wang, L. Meng, H. Chang, B. Wang, F. Xu, J. Yang and P. Wan, Chem. Mater., 2018, 30, 3110–3121 CrossRef CAS.
- Z. Wang, Z. Ma, S. Wang, M. Pi, X. Wang, M. Li, H. Lu, W. Cui and R. Ran, Carbohydr. Polym., 2022, 298, 120128 CrossRef CAS.
- (a) M. Amjadi, K.-U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS; (b) H. Souri, H. Banerjee, A. Jusufi, N. Radacsi, A. A. Stokes, I. Park, M. Sitti and M. Amjadi, Adv. Intell. Syst., 2020, 2, 2000039 CrossRef.
- J. Yu, Y. Feng, D. Sun, W. Ren, C. Shao and R. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 10886–10897 CrossRef CAS.
- K. Nyamayaro, P. Keyvani, F. D'Acierno, J. Poisson, Z. M. Hudson, C. A. Michal, J. D. W. Madden, S. G. Hatzikiriakos and P. Mehrkhodavandi, ACS Appl. Mater. Interfaces, 2020, 12, 52182–52191 CrossRef CAS.
- (a) A. K. Jaiswal, V. Kumar, E. Jansson, O.-H. Huttunen, A. Yamamoto, M. Vikman, A. Khakalo, J. Hiltunen and M. H. Behfar, Adv. Electron. Mater., 2023, 9, 2201094 CrossRef CAS; (b) B. P. Frank, C. Smith, E. R. Caudill, R. S. Lankone, K. Carlin, S. Benware, J. A. Pedersen and D. H. Fairbrother, Environ. Sci. Technol., 2021, 55, 10744–10757 CrossRef CAS PubMed; (c) A. Dey, P. Jali, A. K. Behera, A. B. Das and C. Pradhan, Polym. Compos., 2020, 41, 1428–1434 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |