Microneedle-based transdermal detection and sensing devices
Junxia
Wang
 a,
Ziyi
Lu
a,
Ziyi
Lu
 ab,
Ruisi
Cai
a,
Hanqi
Zheng
a,
Jicheng
Yu
abcd,
Yuqi
Zhang
*ae and
Zhen
Gu
ab,
Ruisi
Cai
a,
Hanqi
Zheng
a,
Jicheng
Yu
abcd,
Yuqi
Zhang
*ae and
Zhen
Gu
 *abcdf
*abcdf
aZhejiang Provincial Key Laboratory for Advanced Drug Delivery Systems, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058, China. E-mail: yqzhang21@zju.edu.cn; guzhen@zju.edu.cn
bDepartment of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, 310016, China
cJinhua Institute of Zhejiang University, Jinhua, 321299, China
dLiangzhu Laboratory, Zhejiang University Medical Center, Hangzhou, 311121, China
eDepartment of Burns and Wound Center, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China
fMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, China
First published on 11th January 2023
Abstract
Microneedles have been expected for the construction of next-generation biosensors towards personalization, digitization, and intellectualization due to their metrics of minimal invasiveness, high integration, and favorable biocompatibility. Herein, an overview of state-of-the-art microneedle-based detection and sensing systems is presented. First, the designs of microneedle devices based on extraction mechanisms are concluded, corresponding to different geometries and materials of microneedles. Second, the targets of equipment-assisted microneedle detections are summarized, as well as the objective significance, revealing the current performance and potential scenarios of these microneedles. Third, the trend towards highly integrated sensors is elaborated by emphasizing the sensing principles (colorimetric, fluorometric and electronic manner). Finally, the key challenges to be tackled and the perspectives on future development are discussed.
1. Introduction
Usage of mobile devices such as sports watches and smart bracelets for monitoring vital signs has exponentially increased, becoming routine for tracing daily exercise as well as heart rate and sleep quality.1 In addition to vital signs, a wearable device that could further indicate and monitor the physiological conditions inside the body is urgently demanded, such as blood glucose and lactate signals. However, many of these are usually associated with blood tests. Statistically, 20–50% of children, and 20–30% of adults, suffer from needle phobia, especially those patients who require frequent blood testing.2 The desire for minimally invasive biosensing devices has increased, posing challenges for the biosafety, patient compliance and multi-functionality of wearable devices.Microneedles, a type of needle mostly shorter than 2 mm, have become an attractive minimally invasive transdermal approach. The original concept of microneedles was described in the 1970s. Along with the development of etching techniques, the first microneedle-structured array was reported by Hashmi et al. in 1995,3 and subsequently, Henry et al. firstly applied them in the transdermal delivery of calcein AM.4 The first microneedle-based sensing method, employing silicon microneedles in combination with a microcuvette for glucose level determination in blood, emerged in 2000.5 In the 2010s, Gu and coworkers integrated a glucose-sensitive component and a drug release component into a microneedle array patch for closed-loop insulin delivery.6,7 Since then, applications of microneedles have been investigated for more than two decades, involving biofluid sampling, instrument-coupled detecting, integrated sensing, and drug delivery.
As an attractive platform, microneedles offer the following advantages in sampling, detection, and sensing areas. Fine designed shapes of microneedles enable minimalized invasive insertion with high patient compliance. Diverse choices of materials and easy manufacturing allow high integration and flexible modification, providing opening scopes for biological sensing. The fabrication of microneedles could be cost-effective and mass-productive, offering vast possibilities for productization, marketization, and terminalization. To date, microneedle-based assays not only facilitate fitness adjustments, daily health management, and disease progression tracking, but also provide new insights into agricultural virus prevention, drug abuse control and food safety inspection.8–12
The majority of detection or sensing-aimed microneedles are still in the clinical trial stage. Only the TAP device,13–15 a microneedle-based minimal blood collection device (∼100 μL), has been approved by the FDA for use in hospitals, clinics, pharmacies, laboratories, and other settings. Taking diabetes monitoring as an example, TAP could be used to perform glycated hemoglobin testing, which is an indicator typically associated with blood glucose levels in diabetic people. In fact, microneedles are currently mostly commercialized in the field of cosmetics. Moreover, there are also several microneedles approved for transdermal injection and delivery of vaccines, insulin, or anesthetics, including Soluvia® (Becton-Dickinson Technologies),16 Intanza® (Sanofi Pasteur),17 MicronJet® (NanoPass Technologies),18etc.
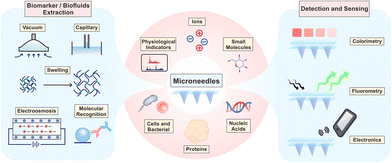
In this review, we focus on state-of-the-art microneedle-based sampling, detection and sensing devices. First, the microanatomy of the skin and biofluids is introduced, and extraction mechanisms are concluded to guide the shape design and material optimization of microneedles. Second, the targets and significances of equipment-assisted microneedle detections are surveyed to reveal the current performance and potential scenarios of these microneedles, including physiological indicators, ions, small molecules, nucleic acids, proteins, cells and bacteria, as well as multiplex assays. Third, the trend towards highly integrated sensors is elaborated by emphasizing the inside sensing principles (colorimetric, fluorometric and electronic manner). Finally, the current challenges to be tackled and the perspectives on future development are also discussed to fulfil the great potential of this technology.
2. Design and fabrication of microneedle devices
Understanding the microanatomy of the skin is important for the design and fabrication of microneedle sensing devices. An ideal device must be capable of successfully penetrating the skin, reaching the targeted biofluid, and accomplishing sensitive detection. The fabrication methods and compositions of microneedles for blood sampling have been elaborately surveyed by Xue et al.19 Thereby, in this section, we mainly focus on the design of microneedle devices in terms of the morphology and extraction mechanisms. The structural basis of skin along with the distributions and components of biofluids (e.g., blood, ISF) will also be discussed.2.1 Skin and biofluids
Human skin consists of two layers, namely the epidermis (50–150 μm thick) and dermis (400–2400 μm thick). The outermost layer of the epidermis is the stratum corneum (10–20 μm thick), which consists of 15–20 layers of horny cells (fully keratinized keratinocytes). The horny cells are filled with keratin and surrounded by lipid-based intercellular matrix, forming a “brick-and-mortar” structure. The stratum corneum is the stiffest part of the skin with an elastic modulus of 1–1000 MPa, depending on the skin source and hydration degree.20–22 Underneath the stratum corneum is the variable epidermis, which is also an avascular region. The variable epidermis is less stiff compared to the stratum corneum, with an elastic modulus of around 10 MPa.22 The innermost layer of the variable epidermis is the stratum basale. It consists of a single layer of keratinocytes, which continuously divide, keratinize, and move outward to form the other layers (stratum spinosum, stratum granulosum, stratum lucidum, and finally stratum corneum). The stratum basale is attached to a basement membrane, which separates the epidermis from the dermis. The dermis consists of a thin superficial papillary layer and a thick deep reticular layer, both being rich in capillaries, lymphatic vessels, free nerve endings and fibers. Unlike that of the epidermis, the elastic modulus of the dermis is only several tens of kPa.23Blood tests have long been the gold standard for clinical diagnosis. The blood in the skin is mainly delivered by the capillaries in the dermis, which consists of two intercommunicating plexuses: the superficial plexus (300–600 μm depth) located at the junction of the papillary layer and reticular layer, and the deeper plexus (1300–1500 μm depth) situated at the dermal–subcutaneous junction.24 Generated by blood transcapillary filtration, ISF is contained in all layers of the skin underneath the stratum corneum and accounts for 45% of the skin volume (blood only 5%).25 There are three dominant pathways for the analyte exchange of ISF: transcellular for analytes around a few hundred Daltons or less and uncharged analytes like glucose; paracellular and transcytosis for larger and charged analytes like proteins.26 Therefore, the ISF concentrations of electrolyte ions (e.g., Na+, K+), small molecules (e.g., glucose), and some diets (e.g., caffeine, alcohol) are comparable to the plasma concentrations.27–31 However, some hormones like cortisol or antibiotics like penicillin and vancomycin are protein bound. Their concentrations in ISF are only equal to the free state concentrations in plasma, excluding the protein-bound ones.32–34 Reported concentrations of common biological analytes in blood and ISF have been surveyed and are presented in Table 1. To make full contact with the capillary plexus, microneedles for blood extraction are generally longer than 1500 μm.19,35,36 However, microneedles for ISF extraction could be as short as 100 μm,37 and the needle length is usually less than 1500 μm to avoid blood drawing.
| Analytes | Molecular weight (Da) | Blood/plasma | ISF | Ref. |
|---|---|---|---|---|
| Na+ | 23.0 | ∼136 mM | Similar | 27 |
| K+ | 39.1 | ∼3.97 mM | Similar | 27 |
| Glucose | 180.2 | 1.6–28.4 mM | Similar | 28 |
| Lactate | 90.1 | 6.9–24.9 mg dL−1 | 20.6–56.6 mg dL−1 | 29 |
| Caffeine | 194.2 | Can reach several tens of μM | Similar | 31 |
| Alcohol | 46.1 | Can reach several tens of mM | Similar to a breathalyzer outcome (a direct estimation of blood alcohol content) | 30 |
| Cortisol | 362.5 | Hundreds of nanomolars (∼90% protein bound) | Similar to unbound in plasma | 32 |
| Penicillin G | 334.4 | ∼60% protein bound | Similar to unbound in plasma | 33 |
| Vancomycin | 1449.3 | ∼55% protein bound | Similar to unbound in plasma | 34 |
2.2 Microneedles for skin penetration
To penetrate the stratum corneum, the microneedle must have a sharp tip and a robust body to avoid bending or breaking. The morphology of a microneedle in a transdermal detection system can be classified as hollow, porous or solid. Generally, hollow microneedles are relatively brittle, and are composed of materials with high mechanical strength. Therefore, long hollow microneedles (>1500 μm long) are usually a single stainless-steel needle,35,36 but short hollow microneedles can be made of various materials (titanium,38 Si,39 glass,40 polymers41) and can be arranged into an array. Clogging is another major concern during biofluid extraction, especially for microneedles with straight sidewalls. Snake-fang-like hollow microneedles can mitigate cell-cutting and avoid clogging issues during insertion, due to the tapered sidewalls and the pore on the edges.39,42 Different from the single-channel hollow microneedles, porous microneedles are featured with internally connected capillary channels. Limited by the stringent conditions in ceramic or metal manufacturing (e.g., processing temperature >1000 °C),43 porous microneedles are usually made of polymers. However, polymer porous microneedles are also physically brittle. One attempt to increase mechanical strength is combining porous structures with solid microneedle substrates. Puttaswamy et al. coated porous PLGA on the surface of malt sugar microneedles.44 Core–shell microneedles could sustain ten times the force required to penetrate the skin.45 Similarly, Samant et al. fixed a porous filter paper between two flat stainless-steel microneedles to ensure efficient penetration.46 Hydrogels, rigid in the dry state, and swellable into porous structures after water absorption, enable both effective skin penetration and biofluid extraction for microneedles. He et al. constructed a microneedle patch made of a polyvinyl alcohol (PVA) hydrogel with 20% chitosan (CS), achieving a 75% porosity with an average diameter of around 20 μm.47 After insertion to rabbit skin, no needle tip was broken although some were slightly blunted. Moreover, hydrogels can be coated around solid microneedles to obtain better mechanical strength.48,49 Solid microneedles are most likely to penetrate the skin for long-term monitoring.30,50 Zheng et al. designed field effect transistor electrode-combined solid polystyrene microneedles.50 This sodium biosensor platform was tested on healthy individuals and achieved excellent on-body monitoring for several hours.2.3 Microneedles for biomarker extraction
For biofluid extraction, mainly five kinds of mechanisms are involved (Fig. 1). First, a vacuum drives the biofluids to flow through pressure differences between the skin and the microneedles. The vacuum-based extraction could be initiated with a vacuum actuator, which is pre-stored35 or press-generated,36 and is usually combined with a long stainless-steel hollow microneedle. Besides direct microneedle extraction, a vacuum could also propel the aspiration of blood or ISF through micropores punched by a solid microneedle array.15,31 To reduce pain and increase penetration efficiency, these microneedles are usually 2D-structured and made of stainless-steel. Interestingly, Miller et al. described a vacuum-free hollow microneedle with a cylinder concentric substrate for pressure-driven ISF extraction. The unique opening design could generate a local pressure at tissues surrounding the insertion point. Consequently, about 1.5 μL of ISF was transported into a single microneedle without the need of additional vacuum.40 Second, capillary force could push the blood or ISF flow along lyophilic solid surfaces (e.g., in the narrow channel in hollow microneedles) based on the biased solid–liquid interfacial interaction. However, the inner diameter of stainless-steel hollow microneedles is often too large (>60 μm) to provide sufficient capillary force. Therefore, Si is mainly used to prepare ultra-fine hollow microneedles.37,42 Strambini et al. fabricated a silicon-based microneedle patch via an etching–oxidation process, achieving an inner diameter of 4 μm and a needle density of 1 × 106 needles per cm2.37 The high-density microchannels with a comparable diameter to porous microneedles provided enough capillary force and reached an ISF extraction rate of 1 μL s−1. Moreover, capillaries in a porous structure (typically made of PLGA with a porogen) can automatically absorb liquids via capillary force. As blood passes through the porous structure, blood cells are trapped, leaving only the plasma.44 Third, swellable hydrogels can extract ISF without additional forces. Once contact is made with ISF, the hydrogel surface will be surrounded by solvent molecules and start expanding, allowing for further molecule transportation into the hydrogel network. Among them, cross-linked hydrogels made of methacrylated hyaluronic acid or gelatin showed the most rapid absorption and the largest swelling ratio, making them ideal for biofluid extraction (Fig. 2A).34,51 The extraction ability is highly dependent on the concentration of monomers and the polymerization time.34 Fourth, electroosmosis on microneedles has been used for ISF extraction.52,53 Electroosmosis refers to the convective solvent flow when an electric field is applied across a solution near a charged surface. The electroosmosis flow direction and rate could be controlled by modifying different charged groups on the porous surface (e.g., sulfonic groups, quaternary amine groups).52 Fifth, specific interaction by antibody or aptamer recognition has also been applied in blood or ISF extraction using microneedles (Fig. 2B). Generally, solid or core–shell microneedles are coated with a high density of recognition molecules, and a porous surface can further increase the contact area.54,55 Wang et al. introduced antibody-coated microneedles to capture desired antigens to realize an ultrasensitive assay.56 A similar strategy was applied for cell capture. Mandal et al. applied adjuvants and specific antigens to attract the surrounding immune cells into microneedles.48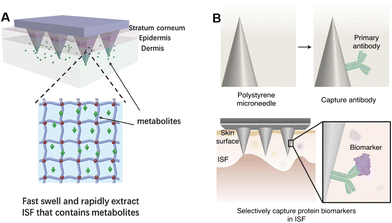 | ||
| Fig. 2 Schematic illustrations of different extraction mechanisms of (A) swelling-based extraction. Reproduced with permission.51 Copyright 2017, Wiley-VCH Verlag. (B) Antibody-based extraction. Reproduced with permission.56 Copyright 2021, Springer Nature Publishing. | ||
3. Equipment-assisted microneedle sensors
With the increased demand for point-of-care testing, microneedles with high patient compliance have become an ideal option for the construction of wearable sensors. Microneedle-assisted detection or sensing systems are featured with rapid responses, wide working ranges, and low detection limits. In some recent cases, microneedles enabled continuous monitoring, multiplex analysis, and even intelligent medication.57 So far, targets of equipment-assisted microneedles have covered physiological indicators, ions, small molecules (glucose, lactate, drugs, and others), nucleic acids, proteins, cells and bacteria, in a single or multiplex analysis manner (Fig. 1). Some recent representative studies in this section are summarized in Table 2, indicating the targets, compositions, working ranges, time consumptions, and the assistant equipment. In this section, we would emphasize the significance and state-of-the-art microneedle assays according to the classification of targets. The validation models (in vitro, ex vivo, in vivo, and human volunteers) and clinical trials are also included.| Targets | Microneedle compositions | Detection ranges | Time consumptions/continuous sensing | Related equipment | Ref. |
|---|---|---|---|---|---|
| Subcellular mechanical force | PDMS-MNs coated with fibronectin or collagen IV | >12 nN | — | Confocal microscopy | 58 |
| Two-degrees of freedom (DOF) force | Stainless steel-MNs with silica epoxy resin optical fibers, and a nitinol tube | 0.25–15 mN | — | Fiber Bragg grating (FBG) interrogator | 59 |
| Three-degrees of freedom (DOF) force | Stainless steel-MNs with silica epoxy resin optical fibers, and a nitinol tube | >0.124 mN for the X/Y direction, >0.74 mN for the Z direction | — | Fiber Bragg grating (FBG) interrogator | 60 |
| K+ | Eshell 300-MNs with PMMA microfluidic channels | 10−5–10−2 M | 20 s for stabilization | Potentiostat | 67 |
| K+, Na+ | A 26-gauge hypodermic needle assembled with a PDMS substrate, and electrodes | 5–200 mM for NaCl, and 1–15 mM for KCl | <100 s | Potentiostat | 69 |
| pH | Epoxy siloxane-MNs with a PDMS substrate | pH = 3–7 | Continuously sensing for 5 h | Electrochemical analyzer | 71 |
| pH | Stainless-steel acupuncture needles | pH = 4–8 | 5 min for a single response, continuously sensing for >40 min | Micro Raman spectrometer | 74 |
| pH | (NOA) 65-MNs | pH = 5–9 | — | Microscopy with extra-long working distance (ELWD) objectives | 73 |
| Lactate | PC–MNs with Au electrodes and nanocarbons | 10–200 μM | — | Potentiostat | 77 |
| Lactate | Pt–MNs with functional electrodes | 0.375–12 mM | — | Potentiostat | 78 |
| β-Hydroxybutyrate, glucose | Carbon paste-filled hollow MNs with electrodes | 0.05–12 mM for β-hydroxybutyrate, 1–10 mM for glucose | Continuously sensing for 4 h | Potentiostat | 99 |
| Alcohol | Stainless steel-MNs with a Pt wire multilayer transducer | 5–80 mM | Continuously sensing for 100 min with 10 min intervals | Potentiostat | 93 |
| H2O2 | Steel-MNs coated with GO/H2PtCl6 | 0.4–20 mM | 5 min | Electrochemical workstation | 95 |
| Urea | c-GelMA-MNs | — | — | Commercial urea kit | 97 |
| Vancomycin | Au-coated Ni–MNs with optofluidics | 0.1–72.6 μM | 10 min | — | 87 |
| Morphine and fentanyl | Hollow MNs with working electrodes and carbon paste transducers | 20–140 μM for morphine, 10–200 μM for fentanyl | 1 min for a single time of response, continuously sensing for 3 h | Potentiostat | 11 |
| Fentanyl | Pt–MNs with electrodes modified by graphene ink | 27.8–160 μM | — | Potentiostat | 89 |
| Apomorphine | Resin-MNs with electrodes | 20–100 μM | Continuous sensing for 2 h with 10-min intervals | Potentiostat | 90 |
| Levodopa | Resin-MNs with electrodes | 5–300 μM | Continuous sensing for 2 h with 10 min intervals | Potentiostat | 91 |
| DNA of Phytophthora infestans | PVA-MNs | 1.2 pg μL−1 to 12 ng μL−1 | 1 h | Thermal cycler | 10 |
| DNA of Epstein–Barr virus | PMVE/MA-MNs | 1.5 × 104–5.0 × 106 copies per μL | 15 min | Microfluidic biosensors with a potentiostat | 102 |
| DNA of Epstein–Barr virus | PMVE/MA-MNs with printed nanotubes | 5.0–1.0 × 104 copies per μL | 10 min | Potentiostat and electrochemical microfluidic biosensor | 103 |
| DNA of Epstein–Barr virus, sepsis-associated cfDNA, and kidney transplantation-associated cfDNA | PMVE/MA-MNs with printed nanotubes | 3 × 10−14–3 × 10−11 M | Continuous sensing for 8 days | Electrochemical workstation | 104 |
| DNA of the allergized domain in milk and shrimps | PVA-MNs | 0.00003 ppm for milk DNA, and 0.00005 ppm for shrimp DNA | <1 h | Thermal cycler | 105 |
| miRNA | PLLA-MNs coated with alginate and peptide nucleic acid | 6–500 nM | <0.5 h | Fluorescence scanner | 49 |
| miRNA | GO-GelMA-MNs | 500 pM–500 nM | 1 h | Confocal microscope | 116 |
| Myoglobin and troponin | Au–MNs with electrodes | 100–1000 ppb | — | Potentiostat | 108 |
| Epidermal growth factor receptor 2 | Si–MNs with Au electrodes | 50–250 ng mL−1 | <1 h | Potentiostat | 9 |
| IL-8 | Au–MNs embedded with microwells | 62 pg mL−1 to 538.6 ng mL−1 | <20 min | Potentiostat | 112 |
| Cocaine antibody, interleukin 6, and periostin | PS-MNs with Fe3O4 nanoparticles in the substrate | 0.33 pg mL−1 −2.5 ng mL−1 for cocaine antibody, n.s. for interleukin 6 and periostin | 5 h | Fluorescence imager | 56 |
| E. coli | TMA/CAA/HEMA-MNs with Au-nanopopcorns | 1.43 × 102 −2.4 × 106 cfu g−f | <10 min | Raman spectrometer | 113 |
| Tissue-resident memory T cells | PLLA-MNs coated with alginate and sucrose | — | 24 h | Flow cytometry | 114 |
| TNF-α, IL-1β, and IL-6 | PEGDA/PEG/ HMPP-MNs coated with photonic crystals | 10–500 ng mL−1 for TNF-α, IL-1β, and IL-6 | <3 h | Spectrometer and luminoscope | 115 |
3.1 Physiological indicators
Physiological indicators mainly include mechanical forces, electromyograms (EMGs), electrocardiograms (ECGs), electroencephalograms (EEGs), etc.Mechanical forces are essential in the interaction of cells with their surrounding extracellular matrix (ECM). To evaluate interactions inside cells, Tan et al. proposed a PDMS-based elastomeric microneedle array. Cells could attach to multiple posts, resulting in deflection in needles, and the degree of deflection observed by confocal microscopy could directly indicate the traction of the subcellular force distributions (Fig. 3A).58 Besides, miniaturized force sensors could offer the surgeon imperceptible force information, improving the accuracy and safety of retinal microsurgery. To assist retinal vein cannulation practice, Gonenc et al. attached two fiber Bragg grating (FBG) sensors on one hollow stainless-steel microneedle. Tests on fertilized eggs showed a 100% success in cannulation, demonstrating the feedback sensing of the microneedle sensors.59 Similarly, while previous studies focused on 2D mechanical monitoring, Zhang et al. took it further into 3D force sensing, by increasing the number of optical fibers into three. Benefiting from this novel FBG configuration, the detection sensitivity was also improved.60
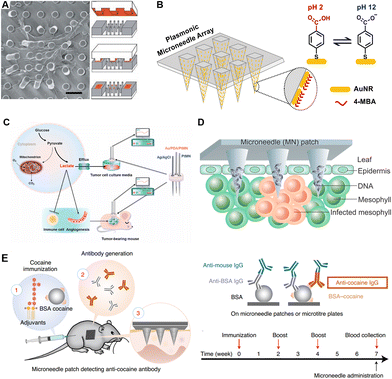 | ||
| Fig. 3 Schematic illustrations of microneedles for detection of different targets. (A) Representative SEM images of cells attached to a microneedle array for single-cell force sensing. Reproduced with permission.58 Copyright 2003, National Academy of Sciences. (B) Plasmonic microneedles coated with gold nanorods functionalized with pH-sensing molecules. Reproduced with permission.73 Copyright 2019, American Chemical Society. (C) Electrochemical sensing platform for quantitative detection of lactate which was correlated with tumor progression. Reproduced with permission.78 Copyright 2021, Elsevier. (D) Rapid extraction of plant DNA using swelling microneedles. Reproduced with permission.10 Copyright 2019, American Chemical Society. (E) Minimally invasive detection of cocaine-specific antibodies in an immunized mouse model after microneedle extraction. Reproduced with permission.56 Copyright 2021, Springer Nature Publishing. Copyright 2020, American Chemical Society. | ||
Biosignals like EMGs, ECGs, and EEGs are all tightly related to physiological status. Particularly, at-home surveillance devices to measure these biosignals are expected to be wearable, miniaturized, highly biocompatible, and stable to meet the needs of long duration monitoring. For the sake of improving biocompatibility, the main body of these MNs is commonly made of titanium, polyimide, and silicon.61
EMGs provide the opportunity to evaluate and record the electrical activity generated by skeletal muscles. Kim et al. proposed a curved microneedle array integrated with a band. The EMG signals before and after 20 min of stair climbing exercise were recorded and compared with those of commercial electrodes, with the microneedles exhibiting improved sweating-tolerance.62 Guvanasen et al. designed stretchable PDMS microneedles to cover and monitor the lateral gastrocnemius muscle in felines. The microneedles could bear repeated stretching of up to 40% tensile strain.63
ECGs can be sensed with microneedle electrodes by measuring minor electrical changes in the skin, which come from the depolarization and repolarization of the heart muscle during heartbeats. Wang et al. fabricated a PDMS microneedle array and coated it with a layer of Ti and a layer of Au, achieving a smaller impedance and higher signal quality during ECG monitoring.64 Long-term monitoring as long as 48 h has been achieved, as well as human volunteer testings.65
EEGs reflect voltage fluctuations caused by ionic currents in neurons, and are commonly used to diagnose epilepsy, sleep disorders, coma, encephalopathy, and even brain death. Microneedles enable the direct collection of an EEG, without the need of hair cutting or gel smearing, providing a user-friendly approach for patients needing long-term monitoring. Wang et al. fabricated a MEMS-based pyramid microneedle array for long-time EEG measurement. The impedance of the microneedle electrode was found to be much more stable than that of a standard electrode within a 5 h monitoring.66
3.2 Ions
Electrolyte imbalances (hyperkalemia, hypernatremia, hypokalemia, and hyponatremia) lead to certain physical disorders, which in turn bring a range of clinical symptoms such as cardiac arrhythmias, muscle spasms, and even death. To determine the concentrations of physiological K+ in the presence of interfering ions, Miller et al. reported a hollow microneedle characterized by ion selective electrodes (ISEs).67 Through ISEs, the dominant ionic activity is selectively converted into potential differences, the value of which is related to the logarithm of the ionic activity on the principle of the Ernst equation.68 Moreover, Li et al. realized the continuous sensing of K+ and Na+ in a chicken skin model. The improved sensor could continuously monitor for over 30 days in an artificial ISF with high accuracy.69Acid/base disorders are closely associated with renal failure, ischemia, multiple sclerosis and psychiatric disorders. Therefore, monitoring the dynamics of pH in the body is also of great significance for health monitoring and disease progression indication.70 To realize pH sensing, Lee et al. fabricated a conformable microneedle array with epoxy siloxane and polydimethylsiloxane. Polyaniline, whose conductivity is strongly influenced by pH, was coated on microneedles, realizing sensitive and persistent skin pH monitoring in a peripheral artery disease (PAD) mouse model.71 Mani et al. proposed an electrode-based microneedle to achieve label-free and real-time pH sensing. The in vivo performance of this microneedle was demonstrated in mouse cerebrospinal fluid (CSF) and bladders, indicating a wide range of potential clinical applications.72 Moreover, specific molecules binding with H+ or responding to various pH levels would improve the specificity of microneedle analysis. Park et al. reported an in situ sensing plasmonic microneedle array that could work in the human skin, functionalized with pH-responsive 4-mercaptobenzoic acid-coated nanorods (Fig. 3B).73 Besides conventional structures, acupuncture-derived microneedles have also been applied in pH sensing. Pan et al. etched two groves on one acupuncture needle, loaded with redox-responsive and pH-responsive SERS probes, respectively. In muscles, the redox and pH could be detected by this acupuncture-derived microneedle within 5 min.74 Besides healthcare, applications of microneedles have been extended to food safety control. Li et al. utilized the above acupuncture-derived microneedle to detect the redox and acid states of peels, bananas, and strawberries, to avoid food exposed to the air.75
3.3 Small molecules
In addition to ions, small molecules (such as lactate, glucose, and drugs) are also highly mobile between the ISF and blood. Many analytes share the same concentration in ISF and blood, with a few exceptions (e.g., lactate). In situ monitoring of small molecules has always been a mutual interest in the healthcare field. The physiological significance differs from one small molecule to another. In this section, we present the significance of detections of these small molecules, classified as lactate, glucose, drugs and others, while the corresponding sensing principles and validation models are also covered.Besides sports medicine, lactate is also a tumor biomarker. Cancer cells increase glucose consumption through glycolysis, while continuously pumping out lactate. This process promotes angiogenesis and immune escape. Thereby, the level of lactate is also an indicator of tumor stage, metastasis, and survival. Li et al. produced a lactate-sensing microneedle array and successfully recorded the lactate levels in tumor-bearing mice. The signal intensity was found to be associated with tumor burden and progression (Fig. 3C).78
According to the International Diabetes Federation, about 537 million adults and 1.2 million children and adolescents suffer from diabetes.82 Hypoglycemia could lead to convulsions or coma, while chronic hyperglycemia causes eye and kidney injury. Long term monitoring of blood glucose levels is of substantial significance for diabetes management. For instance, for each additional day of sensor application per week, the glycated hemoglobin levels could be cut by an average of 0.15%.83 However, the invasive and painful application of these devices limits patient compliance. More recently, glucose sensing devices are heading towards integration, digitalization, and wearability. Tehhrani et al. proposed a fully-integrated wearable microneedle sensor with a wireless transportation function for dual biomarker detection (lactate and glucose, or alcohol and glucose). This wearable device has been verified in volunteers performing common daily activities with robust analytical performances.84 Moreover, Li et al. developed a wearable system to realize closed-loop treatment through sequential glucose sensing, signal transportation, and intelligent drug release. With incorporation with reversible iontophoresis (RI) microneedles and a flexible printed circuit board (PCB) controller, this device exhibited accurate tracking of glucose fluctuations and responsive release of enclosed insulin, successfully regulating the blood glucose levels in a diabetic rat model.53 Besides, to overcome the low-throughput limitation of RI-based sensors, Cheng et al. constructed a fully-integrated touch-actuated glucose microneedle sensor, achieving a 160% enhancement in extraction throughputs.85 In addition, the combination of microneedles and stretchable soft electronic sensors could further improve a diabetic patient's compliance. Lee et al. applied a graphene–Au hybrid sensor with improved electrochemical activities in the construction of a wearable microneedle patch, which could monitor glucose levels in ISF and offer feedback therapeutics accordingly. The sweat-based sensing of glucose and pH was demonstrated in diabetic mouse models and two human volunteers.86
Vancomycin (VAN), a specific antibiotic against Gram-positive bacteria, is the last choice for the treatment of methicillin-resistant Staphylococcus aureus infection.87 However, excess VAN in circulating biofluids would lead to nephrotoxicity (renal failure) and ototoxicity (irreversible deafness). To monitor the VAN levels, Ranamukhaarachchi et al. developed an optofluidics-integrated hollow microneedle and analysed VAN levels through a competitive binding assay. Only sub-nanolitre volumes (∼1 nL) of ISF were required to reach a wide working range.87 Furthermore, Rawson et al. have reported the first in-human study of real-time electric monitoring of phenoxymethylpenicillin using microneedles. This evaluation has shown that the pharmacokinetics obtained by microneedle analysis and microdialysis were highly similar, and the area under the concentration (AUC)–time curve of the drug in ISF is similar to that in serum.88
For abuse management of addictive drugs such as opioids (OPs), a cost-effective and rapidly responsive sensing platform could be availed for drug control. Mishra et al. provided a wearable electronic microneedle device for the simultaneous and continuous monitoring of fentanyl and OP nerve agents. In a skin-mimicking gel model, screening for morphine and fentanyl has been demonstrated.11 Moreover, Joshi et al. proposed an interference-free fentanyl detecting assay using microneedles, the sensing performance of which was verified in diluted serum.89 Similarly, in situ monitoring of apomorphine90 and levodopa91 has also been validated in microneedles, providing attractive tools for drug abuse control.
Hydrogen peroxide (H2O2), a by-product of reactive oxygen metabolism and a hub for the interconversion of reactive oxygen species, is the most common reactive oxygen molecule in living organisms. H2O2 is closely related to apoptosis and cell proliferation, and the related signalling pathways are associated with asthma, inflammatory arthritis, atherosclerosis, neurodegenerative diseases, etc.94 Jin et al. proposed a microneedle-based electrochemical biosensor, and achieved in situ and real-time sensing on pig skin and living mice. This work demonstrated a promising platform for highly sensitive H2O2 sensing, in a minimally invasive manner.95
Urea and uric acid are metabolites of proteins, and are excreted almost exclusively by the kidneys. The urea or uric acid level is clinically significant in assessing kidney function and monitoring kidney diseases.96 To determine urea and indicate renal complaints, Fonseca et al. described a swelling microneedle platform for ex vivo extraction of urea. The subsequent urea detection was conducted with a commercial kit.97 Moreover, He et al. reported a microneedle-based colorimetric tattoo sensor, whose colors changed with pH, glucose, uric acid, and temperature levels in four separated detection areas, realizing the simultaneous detection of the four targets ex vivo.98
Similarly, ketone body monitoring was also realized using microneedles. Since ketone body accumulation leads to hyperglycaemia and metabolic acidosis, complications of diabetic ketoacidosis often happen to diabetic patients, with potentially fatal consequences. Therefore, monitoring both blood glucose and ketone bodies is greatly needed for diabetic patients. Teymourian et al. proposed a real-time monitoring microneedle patch for the continuous detection of ketone bodies and glucose, by measuring β-hydroxybutyrate (a common ketoacidosis biomarker) through β-hydroxybutyrate dehydrogenase.99
3.4 Nucleic acids
Nucleic acids, including DNA, mRNA, and miRNA, play vital roles in genetic information storage and transition, and epigenetic regulation. As biomarkers, nucleic acid analysis has been widely used in the fields of viral or bacterial detection, individualized treatment customization, and social screening for epidemic and infectious diseases.Plant diseases could bring a severe impact on agricultural productivity, while pathogen-induced poor harvest is a widespread issue. In terms of the in-field diagnosis of plant diseases, Paul et al. fabricated a swelling microneedle patch that could extract pathogen DNA (Phytophthora infestans) from field-infected tomato leaves. After extraction, conventional PCR was carried out to quantify the specific DNA fragment (Fig. 3D).10 In order to expand application scenarios, they have also improved this device with an isothermal nucleic acid amplification approach and integrated it with a cellphone-based fluorescence analyzer.100
The Epstein–Barr virus (EBV), a DNA virus of the herpesvirus family, is the pathogen of infectious mononucleosis. EBV is closely associated with the development of nasopharyngeal carcinoma and childhood lymphoma, and has been classified as one of the potentially cancer-causing viruses.101 Rapid sensing of EBV could facilitate the management of individual antiviral therapy. Yang et al. designed microneedles coated with specific oligonucleotides that could respond to EBV genome fragments, which were then sensed with an electrochemical microfluidic platform.102 Furthermore, they combined this device with a reverse iontophoresis approach to realize a wearable EBV-sensing microneedle patch with improved DNA capture. This device could detect EBV DNA from tumor-bearing immunodeficient mice, and the limit of detection was lowered into several copies per μL in vitro, showing great progress for a wearable electronic healthcare device.103 Recently, clustered regularly interspaced short palindromic repeats (CRISPR) technology has also been integrated into microneedle devices for analysis of nucleic acids. Yang et al. demonstrated a CRISPR-activated wearable system consisting of a flexible PDMS substrate, a pair of printed carbon nanotubes, and a three-electrode microneedle prototype functionalized with Cas9/sgRNA.104 The wearable system achieved real-time monitoring of viruses, sepsis, and cell-free DNA with 60% fetal bovine serum interference. The in vivo studies proved its satisfying stability of over 10 days.104
On account of a large number of allergy sufferers, a rapid screening platform for allergens is indispensable for individualized daily healthcare. Li et al. reported a swelling microneedle array to screen the DNA of specific allergy sources (milk, shrimp) in foods, providing a promising daily health control candidate for allergic people.105
MicroRNA, a class of short noncoding RNAs (commonly 19–25 nucleotides), also holds wide potential to become an individual biomarker or in combinations. Sulaiman et al. proposed a hydrogel microneedle patch functionalized with peptide nucleic acid (PNA). The specific miRNA could bind with the conjugated PNA through base pairing, and was subsequently stained by DNA dye, enabling in vivo fluorescence detection of specific miRNAs in a psoriatic mouse model.49
3.5 Proteins
Proteins are the major conductor of life activities. The conformation, modification, and quantity of proteins could be affected by diseases, responses, and body recovery. As one of the broadest spectra of biomarkers, proteins have been the preferred target for terminal diagnostic testing.106 Sensing of protein biomarkers, cytokines, and antibodies has been demonstrated on microneedles.Myoglobin and troponin are the mostly used biomarkers of myocardial damage, which fall off from myocardial fibers during cell injury and then enter the biofluid circulation.107 Miller et al. described a hollow microneedle array containing electrodes for myoglobin and troponin detection. Although not fully integrated, this manuscript offered a proof-of-concept demonstration of on-needle detection of protein biomarkers.108 Cystatin C, a protein marker reflecting the glomerular filtration rate, is an important indicator for the diagnosis of chronic kidney disease, and the prognosis of cardiovascular diseases.109 Puttaswamy et al. provided a lab-on-a-microneedle device for localized surface plasmon resonance (LSPR) of cystatin C in buffer and blood samples.44 Similarly, Dervisevic et al. proposed an electrochemistry microneedle sensor, enabling the quantitation of a breast cancer biomarker (epidermal growth factor receptor 2) in skin mimicking gels.9 Chen et al. proposed a semi-localization microneedle system for detection of the carcinoma embryonic antigen in mice models and human volunteers bearing breast tumor.110
Cytokines, mostly secreted by immune cells, are a type of special small proteins with humoral regulating activities. Along with the expanded clinical immunotherapy, a cytokine-monitoring device could bring insight into subtle changes in the immune system, therefore guiding adaptive clinical treatments.111 Song et al. proposed a microneedle and microwell-based impedance sensor against human interleukin 8 (IL-8). This microneedle in real-time monitored the IL-8 levels in a mouse model that endogenously expressed IL-8, and the results were comparable to those measured by the ELISA gold standard approach.112
Endogenous antibody detection is of particular interest for indication of vaccination efficacies and disease prognosis, and, in some cases, the epidemiological investigation of infectious diseases. Wang et al. proposed an immune sandwich-based microneedle array for ultrasensitive in situ detection of antibodies and cytokines. For the proof-of-concept demonstration, IL-6, periostin and cocaine antibody detection was realized in mice models with high biocompatibility, low detection limitation, and good reproducibility (Fig. 3E).56
3.6 Cells and bacteria
Microbial contamination is a severe global issue in public health. A non-invasive, rapid and accessible bacteria screening device would greatly aid in food safety control. Wang et al. described a zwitterionic microneedle array that could extract E. coli from mutton in 3 min, detect E. coli through SERS nanoparticles, and finally inactivate the bacteria through photothermal conversion.113 Moreover, Kim et al. designed a bacteria-sensing microneedle that could punch commercial food packages, enabling downstream adaption at home. This microneedle could harvest fluids from inside the food through a capillary, and present them on two colorimetric bio-ink patterns, thus indicating contamination and spoilage through detection of E. coli and pH, respectively.12Unlike those of bacteria, extraction and detection of cells using microneedles face practical issues. Most of cells that could be reached by microneedles are keratinocytes, whereas the cells involved in conventional blood tests cannot cross the capillary clusters, and therefore are not available for microneedles. However, there are certain immune cells that prefer to remain in peripheral tissues like skin, rather than circulate in the bloodstream, such as tissue-resident macrophages, natural killer (NK) cells, NK T cells, B cells, and memory T cells. Mandal et al. presented a strategy to monitor the tissue-resident memory T cells (TRM cells) in skin using microneedles. The TRM cells were traced for months in the inoculated mouse, and the sampling from human skin explants was also demonstrated.114
3.7 Multiplex detections
Due to the easy integration virtue of microneedles, multiplex detections have always been demonstrated and innovated. Based on the decoding principle of multiple signals, these can be divided into color- and space-differentiated multiplex detections. Color-based multi-signal differentiation is a user-friendly option, but introduction of colorimetric agents of different colors could be technical. Thereby, different principles for introducing multiple colors are described below. Inspired by the wide color coverage of photonic crystals (PhCs), Zhang et al. loaded PhC barcodes into the tip of microneedles to encode and detect three cytokines (TNF-α, IL-1β, and IL-6) in a sepsis mouse model, which could be read and decoded with a spectrometer and a luminoscope (Fig. 4A).115 Spatial isolation is also an ideal method to distinguish different signal sources. He et al. fabricated four spatially-isolated patterns on one microneedle array, enabling concurrent sensing of pH, glucose, uric acid, and temperature (Fig. 4B).98 Meanwhile, due to the independent electrode fabrication, spatial separation was easier to achieve in the electrode-functionalized microneedles. For example, Tehrani et al. integrated three sensors in one microneedle device to realize simultaneous real-time sensing of pH, glucose and lactate.84 Similarly, synchronous detection of ketone bodies, glucose, and lactate is realized within the same strategy.99 Mishra et al. detected fentanyl and organophosphate nerve agents on a single microneedle patch using two different electrode-containing hollow needles.11 Moreover, the combination of both color- and spatial-differentiation further contributes to a higher throughput. Qiao et al. designed different amplification primers to distinguish 11 kinds of psoriasis-related miRNAs. With labelling of cy3, cy5, and FAM, up to three kinds of miRNAs could be detected simultaneously at the same tip, and the throughput was further enlarged after the separation of the microneedle bodies.116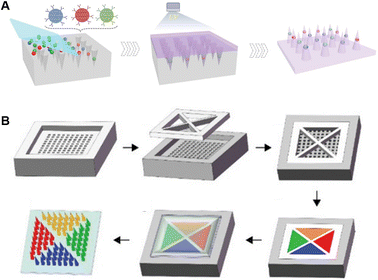 | ||
| Fig. 4 Schematic illustrations of microneedles for multiplex analysis based on different decoding principles. (A) Color-encoded microneedles loaded with photonic crystals. Reproduced with permission.115 Copyright 2019, Wiley-VCH Verlag. (B) Tattoo-patterned microneedles for dermal biosensor delivery. Reproduced with permission.98 Copyright 2021, Wiley-VCH Verlag. | ||
Comprehensive omics information could also be obtained with the aid of microneedles. Tran et al. developed a microneedle array to extract ISF for liquid chromatography mass spectrometry analysis in proteomics. They found that the protein species in ISF were highly similar to those in plasma and serum of the same donors, further supporting the development of ISF biomarkers in the field of in vitro diagnosis.117
4. Integrated microneedle sensing systems
To meet the demands of point-of-care testing, microneedles have been integrated with other devices to allow direct extraction of biomarkers, rapid detection, and on-site readout. So far, targets of integrated microneedle-integrated detection systems have covered ions,50,118 small molecules (glucose,119–122,124 lactate,30,123 cholesterol125 and ROS126,127), and nucleic acids.49,100,116,128,129 In this section, we summarize recently reported integrated microneedle sensing devices, highlighting distinct sensing principles including colorimetric, fluorometric and electronic systems (Fig. 1). The modes of data acquisition, recording, and transmission, and the validated models were also included. The representative highly integrated studies mentioned in this section are summarized in Table 3, indicating the targets, detection principles, assay durations, and working ranges.| Principles | Readouts | Targets | Sensing | Microneedle compositions | Detection ranges | Time consumptions/continuous sensing | Ref. |
|---|---|---|---|---|---|---|---|
| Colorimetric manner | Naked-eye visible | Glucose | GOx-based TMB-HRP colorimetric systems | PVA-MNs with a PVA colorimetric layer | 50–400 mg dL−1 | ∼10 min | 120 |
| Naked-eye visible | Glucose, cholesterol | Oxidase-based MADB/TOPS colorimetric systems | Stainless steel-MNs with a PDMS layer, and two NC membranes | 0–270 mg dL−1 for glucose, 0–320 mg dL−1 for cholesterol | ∼3 min | 125 | |
| Naked-eye visible | E. coli, pH | Polydiacetylene liposomal bio-ink binding with antibodies | Silk-MNs | — | <4 h | 12 | |
| Naked-eye visible | pH, glucose, uric acid, and temperature | pH indicators, GOx/uricase-based TMB-HRP colorimetric systems, and temperature responsive solutions | HA-MNs | 6.0–8.0 for pH; 1–10 mM for glucose, 0.2–1.6 mM for uric acid, 36–39 °C for temperature | 10 min | 98 | |
| Naked-eye visible | Glucose, lactate, cholesterol, and pH | Oxidases/HRP/three chromogenic dye systems, and the pH indicator bromocresol green | HA-MeHA-MNs with a NC membrane | 0–16 mM for glucose, 0–3.2 mM for lactate, 0–12 mM for cholesterol, 5–8 for pH | 10 min | 135 | |
| Cellphone-assisted | Glucose | Glucose-responsive colloidal crystal colorimetric systems | Resin-MNs with colloidal crystals | 100–400 mg/dL | ∼5 min | 121 | |
| Cellphone-assisted | Uric acid | Uricase/polypyrrole nanoparticles/TMB colorimetric systems | PVA-MNs with a PVA colorimetric layer | 200–1000 μM | ∼40 min | 131 | |
| Fluorometric manner | Cellphone-assisted with a handheld LED light | DNA | LAMP amplification with nucleic acid dye | PVA-MNs | 10–100 ng | 30 min | 100 |
| Portable fluorescence reader-assisted | ROS | Redox reaction of PEGylated Cy5 | HA-MNs | 50–250 μM | 20 min | 126 | |
| Electronic manner | Cellphone-assisted | Glucose | Amperometry | Stainless steel-MNs with PCB electrodes | 3–13 mM | Continuous sensing for 21 h | 119 |
| Cellphone- assisted | Glucose, lactate, and alcohol | Chronoamperometry | PMMA-MNs with microelectrodes, an electronic control system, and a Bluetooth package | 0.32–40 mM for glucose, 0.15–28 mM for lactate, and 0.50–100 mM for alcohol | <5 min for a single response, and continuous sensing for 12 h | 84 | |
| Cellphone- assisted | Tyrosinase | Amperometry, CV | Stainless steel-MNs with a bandage sensor (printing stress-enduring inks) | 0.1–0.5 mg mL−1 | <2 min | 145 | |
| Cellphone- assisted | Glucose | CV | Cyclic olefin copolymer-MNs | 0.05–20 mM | Continuous sensing for 72 h | 122 |
4.1 Colorimetric systems
Based on the merits of naked eye visibility, easy fabrication and cost effectiveness, colorimetric assays enable qualitative and quantitative analysis, serving a wide range of point-of-care testing (POCT) devices. Relevant data can be directly distinguished with the naked eye, or captured with cellphones, scanners or cameras, and finally analysed through RGB value processing software. Colorimetric systems have been implemented in detections of small molecules (glucose120,121,130 and uric acid131), proteins,44 and bacteria.113,132 In general, colorimetric signals are induced by changes in chemical structures, densities, orientations, morphologies, or sizes of chromogenic agents upon interaction with the target substance.133 Based on different principles of color development, the chromogenic agents could be classified as redox-responsive agents,120,125,130,134 and nanostructures.121,135In terms of redox-responsive agents, the analytes of interest could be firstly oxidized with a specific oxidase to generate H2O2, which could subsequently react with a H2O2-responsive chromogenic agent. Paper is a major constituent of these colorimetric assays, owing to its merits of hydrophilicity, portability, and cost effectiveness.136 For example, Li et al.125 designed a paper-mediated colorimetric microneedle system to realize dual identification of glucose and cholesterol levels in rabbit models. Glucose and cholesterol were collected via a single microneedle, oxidized via glucose oxidase (GOx) and cholesterol oxidase, and finally reacted with N-ethyl-N-sulfopropyl-m-toluidine (TOPS) and N,N-bis(4-sulfobutyl)-3,5-dimethylaniline disodium salt (MADB) to form purple and green colors, respectively.125 Similarly, Zeng et al. designed paper sensor-integrated microneedles for multi-metabolite analysis in ISF.134 Moreover, direct addition of chromogenic agents into microneedles enables fully integrated sensor fabrication.120,121,131,132 Wang et al. applied GOx, horseradish peroxidase (HRP), and 3,3′,5,5′-tetramethylbenzidine (TMB) into PVA layers to realize the in situ colorimetric detection of glucose in a type 1 diabetic mouse model. The patch could turn blue on the back side once exposed to a high level of glucose. The color intensity of microneedles could be obtained via a miniaturized scanner.120 Moreover, cellphones have been integrated with colorimetry-based microneedle devices for higher accuracy and sensitivity. Zhang et al. described an in situ smartphone-assisted microneedle detection of uric acid. Polypyrrole nanoparticles with HRP-like nanoenzyme activities, urease, and TMB were introduced into microneedles, thus producing a blue color in the absence of uric acid. The color intensity was recorded with a cellphone camera, and finally analysed with the MATLAB software.131
Introduction of colorimetric nanoparticles is an alternative approach for construction of colorimetric microneedles, such as the widely-used Au nanoparticles (AuNPs). The aggregation of AuNPs could induce color changes from red to blue under the principles of local surface plasmon resonance (LSPR). When the distance between nanoparticles was narrowed, one particle could resonate with neighbours to form new vibrational modes, leading to changes of the absorption spectrum.135 For example, Puttaswamy et al. proposed a combined method of extracting ISF using microneedles, and monitoring cysteine C (Cys C) by a lateral flow immunochromatography assay (LFIA). The sample flow in LFIA was driven by capillary force, and the flowing Cys C and antibody-coated AuNPs were immobilized at a specific position. The Cys C levels were quantified under an immune-sandwich principle, when the AuNPs were aggregated into a naked-eye visible band.44 Colloidal crystals, a 2D or 3D oriented arrangement of monodisperse particles (e.g., SiO2 NPs), are featured with photonic band gaps that shift along the array arrangement, thus resulting in macroscopic color changes. Zeng et al. modified SiO2 NP colloidal crystals with glucose-binding fluorophenylboronic acid (FPBA), which was subsequently coated on shells of microneedles. Once in contact, glucose could enter and alter the alignment of FPBA-SiO2 NPs, resulting in a redshift of the microneedle colors from blue to green (Fig. 5A).121
 | ||
| Fig. 5 Schematic diagrams of microneedle-integrated sensing platforms. (A) Colloidal crystal-loaded microneedles for colorimetric detection of glucose. Reproduced with permission.121 Copyright 2020, Elsevier. (B) Multiplexed microneedle sensor with systematic electronic integration. Reproduced with permission.84 Copyright 2022, Springer Nature Publishing. (C) Aptamer-based microneedles for electrochemical signal detection. Reproduced with permission.146 Copyright 2022, American Chemical Society. | ||
4.2 Fluorometric systems
Fluorescence detection has also been demonstrated to have great potential for sensitive analysis, particularly for proteins55,115,137 and nucleic acids.49,100,116,128 Molecules that selectively recognize or interact with targets can be attached to fluorescent labels for an immune sandwich assay, including capturing or detecting antibodies,115 protein ligands,138 DNA intercalators,49 and nucleic acid reporters.116 For example, Wang et al. coated capture antibodies on microneedles for an on-needle fluorophore-linked immunosorbent assay (FLISA). Target proteins were captured by antibodies on the surface of needles, then ex vivo treated with secondary antibody-functionalized fluorescent Au nanorods, and finally imaged via a fluorescence imager.137Besides direct target recognition, trace analytes like nucleic acids often require signal amplification, such as catalytic hairpin assembly (CHA),116,128 post-collection polymerase chain reaction (PCR),8 and loop-mediated isothermal amplification (LAMP).100 Paul et al. designed a microneedle device integrated with a smartphone-based florescence reader and a handheld excited LED light, for detection of pathogen DNA. The microneedles firstly extracted DNA samples by swelling upon insertion into skin, which were then transferred to a reaction chamber to be amplified by LAMP.100
Fluorescent tattooing is an emerging sensing technique with higher integration. Microneedles could serve as a medium to deliver the tattoo sensor to the dermis, enabling the construction of a painless in situ tattoo sensor. Babity et al. loaded hydrocyanines into a dissolvable microneedle array to form a transient ratiometric tattoo, which was non-fluorescent at reduced states, but highly fluorescent in oxidized forms, thus enabling responsive sensing of reactive oxygen species (ROS).126
4.3 Electronic systems
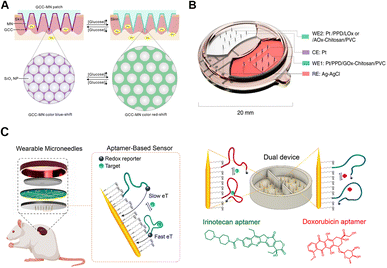
Among all the POCT devices, electrochemical systems offer great prospects for commercialization due to the highly integrated and user-friendly virtues for individuals.139 Meanwhile, the capacities for rapid response, long endurance, high sensitivity and good linearity enable accurate monitoring of trace analytes.140 Typically, electrochemical systems on microneedles consist of sensors (functionalized for specific target recognition), electronics (for controlling and powering), and wired or wireless data transmitters (Fig. 5B).84 Electronic analytical techniques are usually flexibly applied to microneedles, such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), square wave voltammetry (SWV), chronoamperometry, and amperometry.141,142 So far, microneedle-based electronic platforms are mostly applied to detections of ions (pH values,143 Na+,50 and K+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118), small molecules (glucose,30,122,124,144 lactate,123 and ascorbic acid139), nucleic acids,129 and proteins (cytokines112,139 and enzymes145). For example, tyrosinase (TYR) is a polyphenol oxidase involved in melanin production, whose accumulation leads to the biogenesis of melanoma. Ciui et al. designed microneedles for melanoma screening against TYR, functionalized with catechol-coated bandage-shaped electrodes. TYR could oxidize the coated catechol into benzoquinone (BQ), which could be amperometrically quantified at the printed electrodes.145 To expand the real-time monitoring applications, aptamers were combined with microneedles for specific detection of redox inactive molecules like endotoxins, cocaine, and drugs by reversible affinity interactions.146,147 Conformationally reversible aptamers were conjugated with redox reporters on microneedles, so that once the aptamers bound with specific targets, the reporters on microneedles alternately moved. Dynamic electron transmission signals can be obtained through distance changes between redox reporters and golden needles (Fig. 5C).146
118), small molecules (glucose,30,122,124,144 lactate,123 and ascorbic acid139), nucleic acids,129 and proteins (cytokines112,139 and enzymes145). For example, tyrosinase (TYR) is a polyphenol oxidase involved in melanin production, whose accumulation leads to the biogenesis of melanoma. Ciui et al. designed microneedles for melanoma screening against TYR, functionalized with catechol-coated bandage-shaped electrodes. TYR could oxidize the coated catechol into benzoquinone (BQ), which could be amperometrically quantified at the printed electrodes.145 To expand the real-time monitoring applications, aptamers were combined with microneedles for specific detection of redox inactive molecules like endotoxins, cocaine, and drugs by reversible affinity interactions.146,147 Conformationally reversible aptamers were conjugated with redox reporters on microneedles, so that once the aptamers bound with specific targets, the reporters on microneedles alternately moved. Dynamic electron transmission signals can be obtained through distance changes between redox reporters and golden needles (Fig. 5C).146
Besides detection sensitivity, selectivity is essential for electrochemical monitoring devices, especially for blood or ISF samples with complex molecular compositions. To address the issues of endogenous interference, which is caused by direct oxidation on electrodes, the microneedles are generally coated or functionalized with enzymes,148 nano-enzymes,123 antibodies,9 polymer layers,127,129 or permselective membranes.50 For example, Li et al. modified polydopamine nanospheres (PDNS) with AuNPs on Pt microneedles for voltammetric signal detection of lactate. The PDNS served as intermediates between AuNPs and microneedles, enhancing the density of AuNP decoration, and improving the stability of the device.123 Zheng et al. coated a sodium-selective membrane on microneedles for real-time selective monitoring of Na+. The membrane-functionalized microneedles not only penetrated the skin for sampling, but also accommodated selective electrodes and extended gates, greatly facilitating the construction and integration of a flexible electronic biosensor.50
4.4 Device assembly and integration
To meet the growing needs towards high integration and multiple functions, microneedles were assembled with sensing, controlling, and powering components to offer the possibilities of signal conduction, fluidic control, and self-powering. The principles and processes of device integration, especially the electronic components, are discussed below according to different functions.Integration of a sensing component, which can convert extracted biomarkers into transmitted signals, is of vital significance for expansion of terminal applications. Typically, the sensing component is usually attached to the back of the microneedle array.53,125,149,150 Once inserted, the extracted biofluid firstly flows out of the back and subsequently enters the detection or sensing zone, and finally, the corresponding outputs are transmitted to mobile phones via a wireless transmitter.50,53 For example, Cheng et al. developed a microneedle-based wearable system for long-term glucose monitoring. The sensing component was an electrode coated with GOx hydrogels, which could produce H2O2 and induce electron transfer for detection. By combining a touch activator, a wireless electronic detector and a user-friendly app, a miniaturized and user-friendly electrochemical detection platform was constructed (Fig. 6A).85 Meanwhile, microbial contamination during long-term wearing poses biosafety challenges for practical applications of the wearable devices. Cost-effective polymeric microneedles provide a disposable choice to balance the biosafety and cost challenges of electronic devices. For example, Lee et al. designed a microneedle-based strip-type disposable system to realize glucose monitoring and multistage drug delivery. The polymeric microneedles were assembled with a thermal actuator to allow periodic replacement.151 Similarly, Tehrani et al. developed a highly-integrated wearable microneedle sensor for multiple biomarker monitoring, including glucose, lactate, and alcohol.30 Nine distinct subcomponents, including a discardable microneedle array, and reusable electronics, were assembled, enabling convenient replacement of the sampling component.
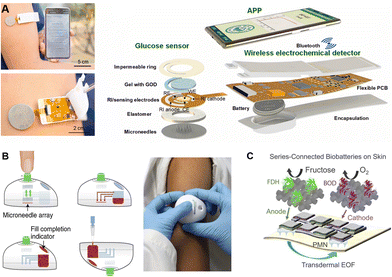 | ||
| Fig. 6 Schematic illustration of different functional components assembled on microneedles. (A) The assembly of a wireless-transited pattern-integrated glucose sensor. Reproduced with permission.85 Copyright 2022, Elsevier. (B) Microneedle-based TAP device with inside microfluidic channels for flow control. Reproduced with permission.15 Copyright 2018, Springer Nature Publishing. (C) A self-powering electronic microneedle array with an enzyme battery. Reproduced with permission.52 Copyright 2021, Springer Nature Publishing. | ||
With the addition of a control component, microneedles are endowed with multiple functions, such as sample handling, and fluidic manipulations. PDMS is widely used to manufacture control components for microneedles, due to the benefits of easy fabrication and flexibility. For example, Li et al. constructed a blood-extraction system consisting of a PDMS-fabricated self-recovery actuator and a long hollow microneedle. By integrating an elastic chamber with several valves, the device achieved blood extraction activated by finger pressing.36 Moreover, the control component could be fabricated by injection-moulding of plastics or rubbers such as acrylonitrile butadiene styrene and thermoplastic elastomers. Blicharz et al. assembled a microneedle-based tap-actuated device (named TAP) for painless collection of blood. A solid microneedle array was integrated into the device to withdraw 100 μL blood by a vacuum after skin puncture. The inner microfluidic channel could generate thorough mixing of blood and lithium heparin anticoagulant to achieve blood preservation (Fig. 6B).15
Power supply determines the level of integration of certain microneedle systems, especially those combined with electro-osmosis extracting components or electronic sensors. Generally, power is supplied with lithium-ion polymer or coin cell batteries.30,53 For example, Cheng et al. connected a 3.7 V rechargeable lithium coin cell battery to the back of a PCB for power supply.85 Besides, enzymatic biobatteries can be an alternative for integrating a fully organic disposable transdermal patch. For example, Kusama et al. integrated series-connected enzymatic biobatteries (fructose/O2 battery) for both ISF extraction and drug delivery.52 Cotton cloth was used as both the reservoir of fructose and the ion conductor in the circuit to drive the transdermal EOF, which demonstrated the feasibility of an entirely organically composited microneedle patch (Fig. 6C).
5. Challenges and perspectives
In this review, we discussed the advances in microneedle-based detection/sensing devices, and the trend towards full integration, digitalization, and intelligent management. Compared to traditional laboratory testing, microneedles offer the merits of minimal invasiveness, rapid readout, patient compliance, miniaturization and high integration, enabling working scopes in community clinics, at-home healthcare, and on-field scenarios. Yet, there is still plenty of room to improve microneedle-based detection/sensing devices to fulfil their great potential in wider applications.Passive and active extraction of biofluids using microneedles has been established, and the surge in microneedle technology has advanced the field of biofluid sampling. However, the latency for sub-millilitre ISF draining calls for minutes, whereas a routine phlebotomy could obtain dozens of millilitres. The sampling using microneedle devices needs to be accelerated. Moreover, small analytes in ISF have been partially investigated (Table 1), while the detailed relationship between ISF and blood in abnormal physiological states (in illness or under treatment) still needs to be systematically explored with extensive clinical validations.
Colorimetric, fluorometric, and electronic signal sensing has been integrated to facilitate the construction of microneedle terminal sensors, especially wearable electronic sensors. Recently, several groups have reported the fabrication of electro-microneedles that could work in vivo for long periods without interference.30,53,85 However, the long-term in vivo implementation is still an issue in the field concerning calibration and biocompability.142 Pipelines including biocompatible testings,152 samplings,153 and threshold settings154 still require careful explorations.
The rising theranostic microneedles, combined with both monitoring and treatment functions, initiated an alternative direction for individual healthcare management.155–157 Yu et al. reported a glucose-sensing insulin patch loaded with nanoscale hypoxia-responsive vesicles (Fig. 7A). The encapsulated GOx could sense the glucose levels, rapidly consume the oxygen under hyperglycemia conditions, and generate a hypoxic environment through enzymatic oxidations, leading to the breaking of vesicles and the release of inside insulin.6 Moreover, the sensing of glucose by phenylboronic acid and the subsequent responsive release of insulin have also been demonstrated on microneedles.7 In addition to microneedles cascaded with sensing and releasing functions, theranostic microneedles capable of outputting signals have also been proposed. Shan et al. constructed a wound microenvironment-responsive colorimetric microneedle array for indication of wound conditions as well as the required treatment. The color would change at bacteria-infected sites, while the inside nanozymes could generate ˙OH for bacteria killing (Fig. 7B).158 Moreover, a closed-loop wearable microneedle array has been proposed to sense glucose levels and accordingly treat diabetic mice with insulin (Fig. 7C). The glucose levels could be determined, and wirelessly transited to cellphones. The pre-loaded insulin could be released, triggered by an integrated flexible PCB, revealing impressive capabilities of a smart carry-on personal health care terminal.53
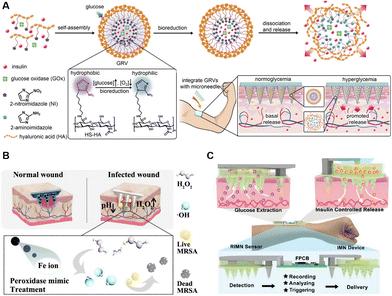 | ||
| Fig. 7 Schematic illustration of intelligent microneedles integrated with diagnosis and treatment functions. (A) A smart glucose-sensing microneedle patch with hypoxia-responsive vesicles for diabetes treatment. Reproduced with permission.6 Copyright 2015, National Academy of Sciences. (B) Wound-healing intelligent colorimetric-sensing microneedles. Reproduced with permission.158 Copyright 2022, Elsevier. (C) Diabetes-treatment electronic-sensing microneedles. Reproduced with permission.53 Copyright 2021, Wiley-VCH Verlag. | ||
In short, as a promising platform, microneedles have been integrated with various detection techniques to realize sampling, sensing, and monitoring capacities. More and more technique crossovers are emerging in conjunction with microneedles to facilitate precise and personalized daily health profiles, such as wireless transitions,50,84,85,122,145 and digital recording.65,159 Further advances in microneedle-based devices could be expected with novel biomarker discovery in ISF, in vivo biocompatibility and stability improvements, and theranostic health management.
Author contributions
Supervision: Z. G. and Y. Z. Writing – original draft: J. W., Z. L., R. C., and H. Z. Writing – review & editing: Z. G., Y. Z., and J. Y.Conflicts of interest
Z. G. is the co-founder of Zenomics Inc., ZCapsule Inc., and μZen Pharm Co., Ltd., and the other authors declare no conflict of interest.Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2021YFA0909900) and the Zhejiang Province “Kunpeng Action” Plan to Z. G., the Startup Packages of Zhejiang University to Z. G. and Y. Z., and the National Natural Science Foundation of China (32101064), the Foundation of National Facility for Translational Medicine (Shanghai) (TMSK-2021-410), and the Fundamental Research Funds for the Central Universities (2021FZZX001-47) to Y. Z.Notes and references
- M. Janssen, J. Scheerder, E. Thibaut, A. Brombacher and S. Vos, PLoS One, 2017, 12, e0181167 CrossRef PubMed.
- J. McLenon and M. A. M. Rogers, J. Adv. Nurs., 2019, 75, 30–42 CrossRef PubMed.
- S. Hashmi, P. Ling, G. Hashmi, M. Reed, R. Gaugler and W. Trimmer, BioTechniques, 1995, 19, 766–770 CAS.
- S. Henry, D. V. McAllister, M. G. Allen and M. R. Prausnitz, J. Pharm. Sci., 1998, 87, 922–925 CrossRef CAS PubMed.
- W. H. Smart and K. Subramanian, Diabetes Technol. Ther., 2000, 2, 549–559 CrossRef CAS PubMed.
- J. Yu, Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse and Z. Gu, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8260–8265 CrossRef CAS PubMed.
- J. Yu, J. Wang, Y. Zhang, G. Chen, W. Mao, Y. Ye, A. R. Kahkoska, J. B. Buse, R. Langer and Z. Gu, Nat. Biomed. Eng., 2020, 4, 499–506 CrossRef CAS PubMed.
- H. Becker, B. L. Gray, T. Minogue, J. Duy, S. Collins and R. Smith, presented in part at the Microfluidics, BioMEMS, and Medical Microsystems XVI, 2018 Search PubMed.
- M. Dervisevic, M. Alba, T. E. Adams, B. Prieto-Simon and N. H. Voelcker, Biosens. Bioelectron., 2021, 192, 113496 CrossRef CAS PubMed.
- R. Paul, A. C. Saville, J. C. Hansel, Y. Ye, C. Ball, A. Williams, X. Chang, G. Chen, Z. Gu, J. B. Ristaino and Q. Wei, ACS Nano, 2019, 13, 6540–6549 CrossRef CAS PubMed.
- R. K. Mishra, K. Y. Goud, Z. Li, C. Moonla, M. A. Mohamed, F. Tehrani, H. Teymourian and J. Wang, J. Am. Chem. Soc., 2020, 142, 5991–5995 CrossRef CAS PubMed.
- D. Kim, Y. Cao, D. Mariappan, M. S. Bono Jr, A. J. Hart and B. Marelli, Adv. Funct. Mater., 2021, 31, 2005370 CrossRef CAS.
- A. Catala, R. Culp-Hill, T. Nemkov and A. D'Alessandro, Metabolomics, 2018, 14, 100 CrossRef PubMed.
- J. Xing, J. Loureiro, M. T. Patel, D. Mikhailov and A. I. Gusev, Bioanalysis, 2020, 12, 919–935 CrossRef CAS PubMed.
- T. M. Blicharz, P. Gong, B. M. Bunner, L. L. Chu, K. M. Leonard, J. A. Wakefield, R. E. Williams, M. Dadgar, C. A. Tagliabue, R. El Khaja, S. L. Marlin, R. Haghgooie, S. P. Davis, D. E. Chickering and H. Bernstein, Nat. Biomed. Eng., 2018, 2, 151–157 CrossRef CAS PubMed.
- P. E. Laurent, S. Bonnet, P. Alchas, P. Regolini, J. A. Mikszta, R. Pettis and N. G. Harvey, Vaccine, 2007, 25, 8833–8842 CrossRef CAS PubMed.
- R. L. Atmar, S. M. Patel and W. A. Keitel, Expert Rev. Vaccines, 2010, 9, 1399–1409 CrossRef CAS PubMed.
- P. Van Damme, F. Oosterhuis-Kafeja, M. Van der Wielen, Y. Almagor, O. Sharon and Y. Levin, Vaccine, 2009, 27, 454–459 CrossRef PubMed.
- P. Xue, L. Zhang, Z. Xu, J. Yan, Z. Gu and Y. Kang, Appl. Mater. Today, 2018, 13, 144–157 CrossRef.
- X. Liu, J. Cleary and G. K. German, Acta Biomater., 2016, 43, 78–87 CrossRef CAS PubMed.
- Y. Yuan and R. Verma, Colloids Surf., B, 2006, 48, 6–12 CrossRef CAS PubMed.
- M. A. Kendall, Y. F. Chong and A. Cock, Biomaterials, 2007, 28, 4968–4977 CrossRef CAS PubMed.
- X. Feng, G.-Y. Li, A. Ramier, A. M. Eltony and S.-H. Yun, Acta Biomater., 2022, 146, 295–305 CrossRef CAS PubMed.
- G. Cevc and U. Vierl, J. Controlled Release, 2007, 118, 18–26 CrossRef CAS PubMed.
- J. N. Roe and B. R. Smoller, Crit. Rev. Ther. Drug Carrier Syst., 1998, 15, 199–241 CAS.
- J. Heikenfeld, A. Jajack, B. Feldman, S. W. Granger, S. Gaitonde, G. Begtrup and B. A. Katchman, Nat. Biotechnol., 2019, 37, 407–419 CrossRef CAS PubMed.
- N. Fogh-Andersen, B. M. Altura, B. T. Altura and O. Siggaard-Andersen, Clin. Chem., 1995, 41, 1522–1525 CrossRef CAS.
- F. J. Service, P. C. O'Brien, S. D. Wise, S. Ness and S. M. LeBlanc, Diabetes Care, 1997, 20, 1426–1429 CrossRef CAS PubMed.
- S. Mitragotri, M. Coleman, J. Kost and R. Langer, Pharm. Res., 2000, 17, 466–470 CrossRef CAS PubMed.
- F. Tehrani, H. Teymourian, B. Wuerstle, J. Kavner, R. Patel, A. Furmidge, R. Aghavali, H. Hosseini-Toudeshki, C. Brown, F. Zhang, K. Mahato, Z. Li, A. Barfidokht, L. Yin, P. Warren, N. Huang, Z. Patel, P. P. Mercier and J. Wang, Nat. Biomed. Eng., 2022, 6(11), 1214–1224 CrossRef CAS PubMed.
- P. P. Samant, M. M. Niedzwiecki, N. Raviele, V. Tran, J. Mena-Lapaix, D. I. Walker, E. I. Felner, D. P. Jones, G. W. Miller and M. R. Prausnitz, Sci. Transl. Med., 2020, 12(571), eaaw0285 CrossRef CAS PubMed.
- J. Cohen, R. Deans, A. Dalley, J. Lipman, M. S. Roberts and B. Venkatesh, J. Crit. Care, 2009, 13, R189 CrossRef PubMed.
- I. M. Cooke, R. P. Bevill, D. R. Nelson and G. D. Koritz, Vet. Res., 1996, 27, 147–159 CAS.
- J. Zhu, X. Zhou, H. J. Kim, M. Qu, X. Jiang, K. Lee, L. Ren, Q. Wu, C. Wang, X. Zhu, P. Tebon, S. Zhang, J. Lee, N. Ashammakhi, S. Ahadian, M. R. Dokmeci, Z. Gu, W. Sun and A. Khademhosseini, Small, 2020, 16, e1905910 CrossRef PubMed.
- C. G. Li, M. Dangol, C. Y. Lee, M. Jang and H. Jung, Lab Chip, 2015, 15, 382–390 RSC.
- C. G. Li, K. Lee, C. Y. Lee, M. Dangol and H. Jung, Adv. Mater., 2012, 24, 4583–4586 CrossRef CAS PubMed.
- L. M. Strambini, A. Longo, A. Diligenti and G. Barillaro, Lab Chip, 2012, 12, 3370–3379 RSC.
- K. Tsuchiya, N. Nakanishi, Y. Uetsuji and E. Nakamachi, Biomed. Microdevices, 2005, 7, 347–353 CrossRef PubMed.
- Y. Li, H. Zhang, R. Yang, Y. Laffitte, U. Schmill, W. Hu, M. Kaddoura, E. J. M. Blondeel and B. Cui, Microsyst. Nanoeng., 2019, 5, 41 CrossRef PubMed.
- P. R. Miller, R. M. Taylor, B. Q. Tran, G. Boyd, T. Glaros, V. H. Chavez, R. Krishnakumar, A. Sinha, K. Poorey, K. P. Williams, S. S. Branda, J. T. Baca and R. Polsky, Commun. Biol., 2018, 1, 173 CrossRef PubMed.
- J. R. Windmiller, N. Zhou, M. C. Chuang, G. Valdés-Ramírez, P. Santhosh, P. R. Miller, R. Narayan and J. Wang, Analyst, 2011, 136, 1846–1851 RSC.
- E. V. Mukerjee, S. D. Collins, R. R. Isseroff and R. L. Smith, Sens. Actuators, A, 2004, 114, 267–275 CrossRef CAS.
- E. M. Cahill, S. Keaveney, V. Stuettgen, P. Eberts, P. Ramos-Luna, N. Zhang, M. Dangol and E. D. O'Cearbhaill, Acta Biomater., 2018, 80, 401–411 CrossRef CAS PubMed.
- S. V. Puttaswamy, G. V. Lubarsky, C. Kelsey, X. Zhang, D. Finlay, J. A. McLaughlin and N. Bhalla, ACS Nano, 2020, 14, 11939–11949 CrossRef CAS PubMed.
- S. Jiang, P. Li, Y. Yu, J. Liu and Z. Yang, J. Biomech., 2014, 47, 3344–3353 CrossRef PubMed.
- P. P. Samant and M. R. Prausnitz, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 4583–4588 CrossRef PubMed.
- R. He, Y. Niu, Z. Li, A. Li, H. Yang, F. Xu and F. Li, Adv. Healthcare Mater., 2020, 9, e1901201 CrossRef PubMed.
- A. Mandal, A. V. Boopathy, L. K. W. Lam, K. D. Moynihan, M. E. Welch, N. R. Bennett, M. E. Turvey, N. Thai, J. H. Van, J. C. Love, P. T. Hammond and D. J. Irvine, Sci. Transl. Med., 2018, 10(467), eaar2227 CrossRef PubMed.
- D. Al Sulaiman, J. Y. H. Chang, N. R. Bennett, H. Topouzi, C. A. Higgins, D. J. Irvine and S. Ladame, ACS Nano, 2019, 13, 9620–9628 CrossRef CAS PubMed.
- Y. Zheng, R. Omar, R. Zhang, N. Tang, M. Khatib, Q. Xu, Y. Milyutin, W. Saliba, Y. Y. Broza, W. Wu, M. Yuan and H. Haick, Adv. Mater., 2022, 34, e2108607 CrossRef PubMed.
- H. Chang, M. Zheng, X. Yu, A. Than, R. Z. Seeni, R. Kang, J. Tian, D. P. Khanh, L. Liu, P. Chen and C. Xu, Adv. Mater., 2017, 29, 1702243 CrossRef PubMed.
- S. Kusama, K. Sato, Y. Matsui, N. Kimura, H. Abe, S. Yoshida and M. Nishizawa, Nat. Commun., 2021, 12, 658 CrossRef CAS PubMed.
- X. Li, X. Huang, J. Mo, H. Wang, Q. Huang, C. Yang, T. Zhang, H. J. Chen, T. Hang, F. Liu, L. Jiang, Q. Wu, H. Li, N. Hu and X. Xie, Adv. Sci., 2021, 8, e2100827 CrossRef PubMed.
- Y. E. Kang, K. Y. Seong, S. G. Yim, Y. Lee, S. M. An, S. C. Kim, K. Kim, B. S. An, K. S. Lee and S. Y. Yang, Biosens. Bioelectron., 2020, 163, 112281 CrossRef CAS PubMed.
- K. Yi, Y. Wang, K. Shi, J. Chi, J. Lyu and Y. Zhao, Biosens. Bioelectron., 2021, 190, 113404 CrossRef CAS PubMed.
- Z. Wang, J. Luan, A. Seth, L. Liu, M. You, P. Gupta, P. Rathi, Y. Wang, S. Cao, Q. Jiang, X. Zhang, R. Gupta, Q. Zhou, J. J. Morrissey, E. L. Scheller, J. S. Rudra and S. Singamaneni, Nat. Biomed. Eng., 2021, 5, 64–76 CrossRef CAS PubMed.
- W. Chen, Z. Wang, L. Wang and X. Chen, Adv. Mater., 2022, 34, e2106701 CrossRef PubMed.
- J. L. Tan, J. Tien, D. M. Pirone, D. S. Gray, K. Bhadriraju and C. S. Chen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1484–1489 CrossRef CAS PubMed.
- B. Gonenc, R. H. Taylor, I. Iordachita, P. Gehlbach and J. Handa, Proc. IEEE Sens., 2014, 2014, 698–701 Search PubMed.
- T. Zhang, B. Chen and S. Zuo, IEEE Trans. Ind. Electron., 2022, 69, 940–949 Search PubMed.
- B. L. Zhang, X. P. Zhang, B. Z. Chen, W. M. Fei, Y. Cui and X. D. Guo, Microchem. J., 2021, 162, 105830 CrossRef CAS.
- M. Kim, T. Kim, D. S. Kim and W. K. Chung, Sensors, 2015, 15, 16265–16280 CrossRef PubMed.
- G. S. Guvanasen, L. Guo, R. J. Aguilar, A. L. Cheek, C. S. Shafor, S. Rajaraman, T. R. Nichols and S. P. DeWeerth, IEEE Trans. Neural Syst. Rehabilitation Eng., 2017, 25, 1440–1452 Search PubMed.
- R. Wang, J. Bai, X. Zhu, Z. Li, L. Cheng, G. Zhang and W. Zhang, Biomed. Microdevices, 2022, 24, 27 CrossRef CAS PubMed.
- A. K. Srivastava, B. Bhartia, K. Mukhopadhyay and A. Sharma, Sens. Actuators, A, 2015, 236, 164–172 CrossRef CAS.
- L.-F. Wang, J.-Q. Liu, X.-X. Yan, B. Yang and C.-S. Yang, Microsyst. Technol., 2013, 19, 269–276 CrossRef.
- P. R. Miller, X. Xiao, I. Brener, D. B. Burckel, R. Narayan and R. Polsky, Adv. Healthcare Mater., 2014, 3, 876–881 CrossRef CAS PubMed.
- H. Teymourian, F. Tehrani, K. Mahato and J. Wang, Adv. Healthcare Mater., 2021, 10, 2002255 CrossRef CAS PubMed.
- H. Li, G. Wu, Z. Weng, H. Sun, R. Nistala and Y. Zhang, ACS Sens., 2021, 6, 2181–2190 CrossRef CAS PubMed.
- P. R. Miller, S. A. Skoog, T. L. Edwards, D. M. Lopez, D. R. Wheeler, D. C. Arango, X. Xiao, S. M. Brozik, J. Wang, R. Polsky and R. J. Narayan, Talanta, 2012, 88, 739–742 CrossRef CAS PubMed.
- W. Lee, S.-h. Jeong, Y.-W. Lim, H. Lee, J. Kang, H. Lee, I. Lee, H.-S. Han, S. Kobayashi, M. Tanaka and B.-S. Bae, Sci. Adv., 2021, 7(48), eabi6290 CrossRef CAS PubMed.
- G. K. Mani, K. Miyakoda, A. Saito, Y. Yasoda, K. Kajiwara, M. Kimura and K. Tsuchiya, ACS Appl. Mater. Interfaces, 2017, 9, 21651–21659 CrossRef CAS PubMed.
- J. E. Park, N. Yonet-Tanyeri, E. Vander Ende, A.-I. Henry, B. E. Perez White, M. Mrksich and R. P. Van Duyne, Nano Lett., 2019, 19, 6862–6868 CrossRef CAS PubMed.
- C. Pan, X. Li, J. Sun, Z. Li, L. Zhang, W. Qian, P. Wang and J. Dong, ACS Appl. Bio Mater., 2019, 2, 2102–2108 CrossRef CAS PubMed.
- Z. Li, C. Pan, J. Sun, W. Qian and J. Dong, ACS Food Sci. Technol., 2021, 1, 1787–1791 CrossRef CAS.
- D. K. Ming, S. Jangam, S. A. N. Gowers, R. Wilson, D. M. E. Freeman, M. G. Boutelle, A. E. G. Cass, D. O'Hare and A. H. Holmes, BMJ Innov., 2022, 8, 87 CrossRef.
- P. Bollella, S. Sharma, A. E. G. Cass and R. Antiochia, Biosens. Bioelectron., 2019, 123, 152–159 CrossRef CAS PubMed.
- Q. Li, Y. Zhang, H. Fan, Y. Gong, Y. Xu, Q. Lv, Y. Xu, F. Xiao, S. Wang, Z. Wang and L. Wang, Biosens. Bioelectron., 2021, 191, 113474 CrossRef CAS PubMed.
- A. El-Laboudi, N. S. Oliver, A. Cass and D. Johnston, Diabetes Technol. Ther., 2012, 15, 101–115 CrossRef PubMed.
- D. Bruen, C. Delaney, L. Florea and D. Diamond, Sensors, 2017, 17(8), 1866 CrossRef PubMed.
- J. Ju, L. Li, S. Regmi, X. Zhang and S. Tang, Biosensors, 2022, 12(8), 606 CrossRef CAS PubMed.
- I. D. Federation, IDF Diabetes Atlas, 10th edn, 2021, https://www.idf.org/ Search PubMed.
- T. Battelino, M. Phillip, N. Bratina, R. Nimri, P. Oskarsson and J. Bolinder, Diabetes Care, 2011, 34, 795–800 CrossRef PubMed.
- F. Tehrani, H. Teymourian, B. Wuerstle, J. Kavner, R. Patel, A. Furmidge, R. Aghavali, H. Hosseini-Toudeshki, C. Brown, F. Zhang, K. Mahato, Z. Li, A. Barfidokht, L. Yin, P. Warren, N. Huang, Z. Patel, P. P. Mercier and J. Wang, Nat. Biomed. Eng., 2022, 6(11), 1214–1224 CrossRef CAS PubMed.
- Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, M. Chen, C. Yi and L. Jiang, Biosens. Bioelectron., 2022, 203, 114026 CrossRef CAS PubMed.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi and D.-H. Kim, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef CAS PubMed.
- S. A. Ranamukhaarachchi, C. Padeste, M. Dübner, U. O. Häfeli, B. Stoeber and V. J. Cadarso, Sci. Rep., 2016, 6, 29075 CrossRef CAS PubMed.
- T. M. Rawson, S. A. N. Gowers, D. M. E. Freeman, R. C. Wilson, S. Sharma, M. Gilchrist, A. MacGowan, A. Lovering, M. Bayliss, M. Kyriakides, P. Georgiou, A. E. G. Cass, D. O'Hare and A. H. Holmes, Lancet Digital Health, 2019, 1, e335–e343 CrossRef PubMed.
- P. Joshi, P. R. Riley, R. Mishra, S. Azizi Machekposhti and R. Narayan, Biosensors, 2022, 12(4), 198 CrossRef CAS PubMed.
- K. Y. Goud, K. Mahato, H. Teymourian, K. Longardner, I. Litvan and J. Wang, Sens. Actuators, B, 2022, 354, 131234 CrossRef CAS.
- K. Y. Goud, C. Moonla, R. K. Mishra, C. Yu, R. Narayan, I. Litvan and J. Wang, ACS Sens., 2019, 4, 2196–2204 CrossRef CAS PubMed.
- D. Nutt, A. Hayes, L. Fonville, R. Zafar, E. O. C. Palmer, L. Paterson and A. Lingford-Hughes, Nutrients, 2021, 13(11), 3938 CrossRef CAS PubMed.
- A. M. V. Mohan, J. R. Windmiller, R. K. Mishra and J. Wang, Biosens. Bioelectron., 2017, 91, 574–579 CrossRef CAS PubMed.
- T. Wang, H. Zhu, J. Zhuo, Z. Zhu, P. Papakonstantinou, G. Lubarsky, J. Lin and M. Li, Anal. Chem., 2013, 85, 10289–10295 CrossRef CAS PubMed.
- Q. Jin, H.-J. Chen, X. Li, X. Huang, Q. Wu, G. He, T. Hang, C. Yang, Z. Jiang, E. Li, A. Zhang, Z. Lin, F. Liu and X. Xie, Small, 2019, 15, 1804298 CrossRef PubMed.
- C. Giordano, O. Karasik, K. King-Morris and A. Asmar, Dis. Markers, 2015, 2015, 382918 Search PubMed.
- D. F. S. Fonseca, P. C. Costa, I. F. Almeida, P. Dias-Pereira, I. Correia-Sá, V. Bastos, H. Oliveira, C. Vilela, A. J. D. Silvestre and C. S. R. Freire, Macromol. Biosci., 2020, 20, 2000195 CrossRef CAS PubMed.
- R. He, H. Liu, T. Fang, Y. Niu, H. Zhang, F. Han, B. Gao, F. Li and F. Xu, Adv. Sci., 2021, 8, e2103030 CrossRef PubMed.
- H. Teymourian, C. Moonla, F. Tehrani, E. Vargas, R. Aghavali, A. Barfidokht, T. Tangkuaram, P. P. Mercier, E. Dassau and J. Wang, Anal. Chem., 2020, 92, 2291–2300 CrossRef CAS PubMed.
- R. Paul, E. Ostermann, Y. Chen, A. C. Saville, Y. Yang, Z. Gu, A. E. Whitfield, J. B. Ristaino and Q. Wei, Biosens. Bioelectron., 2021, 187, 113312 CrossRef CAS PubMed.
- L. S. Young and A. B. Rickinson, Nat. Rev. Cancer, 2004, 4, 757–768 CrossRef CAS PubMed.
- B. Yang, X. Fang and J. Kong, ACS Appl. Mater. Interfaces, 2019, 11, 38448–38458 CrossRef CAS PubMed.
- B. Yang, X. Fang and J. Kong, Adv. Funct. Mater., 2020, 30, 2000591 CrossRef CAS.
- B. Yang, J. Kong and X. Fang, Nat. Commun., 2022, 13, 3999 CrossRef CAS PubMed.
- H. Li, J. Feng, Y. Wang, G. Liu, X. Chen and L. Fu, J. Agric. Food Chem., 2021, 69, 6879–6887 CrossRef CAS PubMed.
- N. Rifai, M. A. Gillette and S. A. Carr, Nat. Biotechnol., 2006, 24, 971–983 CrossRef CAS PubMed.
- S. Aydin, K. Ugur, S. Aydin, İ. Sahin and M. Yardim, Vasc. Health Risk Manage., 2019, 15, 1–10 CrossRef CAS PubMed.
- P. Miller, M. Moorman, R. Manginell, C. Ashlee, I. Brener, D. Wheeler, R. Narayan and R. Polsky, Electroanalysis, 2016, 28, 1305–1310 CrossRef CAS.
- M. G. Shlipak, M. D. Mattes and C. A. Peralta, Am. J. Kidney Dis., 2013, 62, 595–603 CrossRef CAS PubMed.
- L. Chen, C. Zhang, J. Xiao, J. You, W. Zhang, Y. Liu, L. Xu, A. Liu, H. Xin and X. Wang, Mater. Sci. Eng., C, 2020, 109, 110402 CrossRef CAS PubMed.
- M. Wang, X. Zhai, J. Li, J. Guan, S. Xu, Y. Li and H. Zhu, Front. Immunol., 2021, 12, 670391 CrossRef CAS PubMed.
- N. Song, P. Xie, W. Shen, H. Oh, Y. Zhang, F. Vitale, M. Javanmard and M. G. Allen, Microsyst. Nanoeng., 2021, 7, 96 CrossRef CAS PubMed.
- Y. Wang, H. Ni, H. Li, J. Chen, D. Zhang and L. Fu, Chem. Eng. J., 2022, 442, 136140 CrossRef CAS.
- A. Mandal, A. V. Boopathy, L. K. W. Lam, K. D. Moynihan, M. E. Welch, N. R. Bennett, M. E. Turvey, N. Thai, J. H. Van, J. C. Love, P. T. Hammond and D. J. Irvine, Sci. Transl. Med., 2018, 10, eaar2227 CrossRef PubMed.
- X. Zhang, G. Chen, F. Bian, L. Cai and Y. Zhao, Adv. Mater., 2019, 31, e1902825 CrossRef PubMed.
- Y. Qiao, J. Du, R. Ge, H. Lu, C. Wu, J. Li, S. Yang, S. Zada, H. Dong and X. Zhang, Anal. Chem., 2022, 94, 5538–5545 CrossRef CAS PubMed.
- B. Q. Tran, P. R. Miller, R. M. Taylor, G. Boyd, P. M. Mach, C. N. Rosenzweig, J. T. Baca, R. Polsky and T. Glaros, J. Proteome Res., 2018, 17, 479–485 CrossRef CAS PubMed.
- M. Parrilla, M. Cuartero, S. Padrell Sanchez, M. Rajabi, N. Roxhed, F. Niklaus and G. A. Crespo, Anal. Chem., 2019, 91, 1578–1586 CrossRef CAS PubMed.
- Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, M. Chen, C. Yi and L. Jiang, Biosens. Bioelectron., 2022, 203, 114026 CrossRef CAS PubMed.
- Z. Wang, H. Li, J. Wang, Z. Chen, G. Chen, D. Wen, A. Chan and Z. Gu, Biomaterials, 2020, 237, 119782 CrossRef CAS PubMed.
- Y. Zeng, J. Wang, Z. Wang, G. Chen, J. Yu, S. Li, Q. Li, H. Li, D. Wen, Z. Gu and Z. Gu, Nano Today, 2020, 35, 100984 CrossRef CAS.
- K. B. Kim, W.-C. Lee, C.-H. Cho, D.-S. Park, S. J. Cho and Y.-B. Shim, Sens. Actuators, B, 2019, 281, 14–21 CrossRef CAS.
- Q. Li, Y. Zhang, H. Fan, Y. Gong, Y. Xu, Q. Lv, Y. Xu, F. Xiao, S. Wang, Z. Wang and L. Wang, Biosens. Bioelectron., 2021, 191, 113474 CrossRef CAS PubMed.
- O. Heifler, E. Borberg, N. Harpak, M. Zverzhinetsky, V. Krivitsky, I. Gabriel, V. Fourman, D. Sherman and F. Patolsky, ACS Nano, 2021, 15, 12019–12033 CrossRef CAS PubMed.
- C. G. Li, H. A. Joung, H. Noh, M. B. Song, M. G. Kim and H. Jung, Lab Chip, 2015, 15, 3286–3292 RSC.
- S. Babity, F. Couture, E. V. R. Campos, S. Hedtrich, R. Hagen, D. Fehr, M. Bonmarin and D. Brambilla, Adv. Healthcare Mater., 2022, 11, e2102070 CrossRef PubMed.
- Q. Jin, H. J. Chen, X. Li, X. Huang, Q. Wu, G. He, T. Hang, C. Yang, Z. Jiang, E. Li, A. Zhang, Z. Lin, F. Liu and X. Xie, Small, 2019, 15, e1804298 CrossRef PubMed.
- J. Li, H. Lu, Y. Wang, S. Yang, Y. Zhang, W. Wei, Y. Qiao, W. Dai, R. Ge and H. Dong, Anal. Chem., 2022, 94, 968–974 CrossRef CAS PubMed.
- B. Yang, X. Fang and J. Kong, Adv. Funct. Mater., 2020, 30, 2000591 CrossRef CAS.
- D. Nicholas, K. A. Logan, Y. Sheng, J. Gao, S. Farrell, D. Dixon, B. Callan, A. P. McHale and J. F. Callan, Int. J. Pharm., 2018, 547, 244–249 CrossRef CAS PubMed.
- P. Zhang, X. Wu, H. Xue, Y. Wang, X. Luo and L. Wang, Anal. Chim. Acta, 2022, 1212, 339911 CrossRef CAS PubMed.
- D. Kim, Y. Cao, D. Mariappan, M. S. Bono, A. J. Hart and B. Marelli, Adv. Funct. Mater., 2020, 31, 2005370 CrossRef.
- E. Mauriz, Sensors, 2020, 20, 6214 CrossRef CAS PubMed.
- D. D. Zhu, L. W. Zheng, P. K. Duong, R. H. Cheah, X. Y. Liu, J. R. Wong, W. J. Wang, S. T. Tien Guan, X. T. Zheng and P. Chen, Biosens. Bioelectron., 2022, 212, 114412 CrossRef CAS PubMed.
- J. Zeng, Y. Zhang, T. Zeng, R. Aleisa, Z. Qiu, Y. Chen, J. Huang, D. Wang, Z. Yan and Y. Yin, Nano Today, 2020, 32, 100855 CrossRef CAS.
- Y.-Q. Li and L. Feng, Chin. J. Anal. Chem., 2020, 48, 1448–1457 CAS.
- Z. Wang, J. Luan, A. Seth, L. Liu, M. You, P. Gupta, P. Rathi, Y. Wang, S. Cao, Q. Jiang, X. Zhang, R. Gupta, Q. Zhou, J. J. Morrissey, E. L. Scheller, J. S. Rudra and S. Singamaneni, Nat. Biomed. Eng., 2021, 5, 64–76 CrossRef CAS PubMed.
- X. Dong, B. Ma, L. Lei, Y. Chen, C. Xu, C. Zhao and H. Liu, Chem. Eng. J., 2022, 432, 134234 CrossRef CAS.
- E. Skaria, B. A. Patel, M. S. Flint and K. W. Ng, Anal. Chem., 2019, 91, 4436–4443 CrossRef CAS PubMed.
- L. Fang, H. Ren, X. Mao, S. Zhang, Y. Cai, S. Xu, Y. Zhang, L. Li, X. Ye and B. Liang, Biosensors, 2022, 12, 102 CrossRef CAS PubMed.
- Q. Wang, X. Wen and J. Kong, Crit. Rev. Anal. Chem., 2020, 50, 359–375 CrossRef CAS PubMed.
- J. J. García-Guzmán, C. Pérez-Ràfols, M. Cuartero and G. A. Crespo, TrAC, Trends Anal. Chem., 2021, 135, 116148 CrossRef.
- S. R. Chinnadayyala, I. Park and S. Cho, Microchim. Acta, 2018, 185, 250 CrossRef PubMed.
- Y. Liu, Q. Yu, X. Luo, L. Yang and Y. Cui, Microsyst. Nanoeng., 2021, 7, 75 CrossRef PubMed.
- B. Ciui, A. Martin, R. K. Mishra, B. Brunetti, T. Nakagawa, T. J. Dawkins, M. Lyu, C. Cristea, R. Sandulescu and J. Wang, Adv. Healthcare Mater., 2018, 7, e1701264 CrossRef PubMed.
- Y. Wu, F. Tehrani, H. Teymourian, J. Mack, A. Shaver, M. Reynoso, J. Kavner, N. Huang, A. Furmidge, A. Duvvuri, Y. Nie, L. M. Laffel, F. J. Doyle, 3rd, M. E. Patti, E. Dassau, J. Wang and N. Arroyo-Curras, Anal. Chem., 2022, 94, 8335–8345 CrossRef CAS PubMed.
- Y. Wu, I. Belmonte, K. S. Sykes, Y. Xiao and R. J. White, Anal. Chem., 2019, 91, 15335–15344 CrossRef CAS PubMed.
- R. K. Mishra, A. M. Vinu Mohan, F. Soto, R. Chrostowski and J. Wang, Analyst, 2017, 142, 918–924 RSC.
- L. M. Strambini, A. Longo, S. Scarano, T. Prescimone, I. Palchetti, M. Minunni, D. Giannessi and G. Barillaro, Biosens. Bioelectron., 2015, 66, 162–168 CrossRef CAS PubMed.
- P. R. Miller, X. Xiao, I. Brener, D. B. Burckel, R. Narayan and R. Polsky, Adv. Healthcare Mater., 2014, 3, 876–881 CrossRef CAS PubMed.
- H. Lee, C. Song, Y. S. Hong, M. Kim, H. R. Cho, T. Kang, K. Shin, S. H. Choi, T. Hyeon and D.-H. Kim, Sci. Adv., 2017, 3, e1601314 CrossRef PubMed.
- D. Chen, C. Wang, W. Chen, Y. Chen and J. X. Zhang, Biosens. Bioelectron., 2015, 74, 1047–1052 CrossRef CAS PubMed.
- S. Sharma, A. El-Laboudi, M. Reddy, N. Jugnee, S. Sivasubramaniyam, M. El Sharkawy, P. Georgiou, D. Johnston, N. Oliver and A. E. G. Cass, Anal. Methods, 2018, 10, 2088–2095 RSC.
- N. Vasylieva, S. Marinesco, D. Barbier and A. Sabac, Biosens. Bioelectron., 2015, 72, 148–155 CrossRef CAS PubMed.
- J. Yu, Y. Zhang, J. Yan, A. R. Kahkoska and Z. Gu, Int. J. Pharm., 2018, 544, 350–357 CrossRef CAS PubMed.
- P. Makvandi, R. Jamaledin, G. Chen, Z. Baghbantaraghdari, E. N. Zare, C. Di Natale, V. Onesto, R. Vecchione, J. Lee, F. R. Tay, P. Netti, V. Mattoli, A. Jaklenec, Z. Gu and R. Langer, Mater. Today, 2021, 47, 206–222 CrossRef CAS PubMed.
- Y. Lu, A. A. Aimetti, R. Langer and Z. Gu, Nat. Rev. Mater., 2016, 2, 16075 CrossRef.
- J. Shan, X. Zhang, B. Kong, Y. Zhu, Z. Gu, L. Ren and Y. Zhao, Chem. Eng. J., 2022, 444, 136640 CrossRef CAS.
- L. Ren, Z. Chen, H. Wang, Z. Dou, B. Liu and L. Jiang, IEEE Trans. Instrum. Meas., 2020, 69, 8328–8334 CAS.
| This journal is © The Royal Society of Chemistry 2023 |