Open Access Article
Open Access ArticleSynthesis of mesoporous lanthanum hydroxide with enhanced adsorption performance for phosphate removal†
Kyungmin Kim‡
,
Dujin Kim‡,
Taeyeon Kim ,
Bong-Geun Kim,
Donghyun Ko,
Junsoo Lee,
Yujin Han,
Ji Chul Jung and
Hyon Bin Na
,
Bong-Geun Kim,
Donghyun Ko,
Junsoo Lee,
Yujin Han,
Ji Chul Jung and
Hyon Bin Na *
*
Department of Chemical Engineering, Myongji University, Yongin, Gyeonggi-do 17058, Republic of Korea. E-mail: hyonbin@mju.ac.kr
First published on 16th May 2019
Abstract
Phosphate is a ubiquitous pollutant in aquatic systems, and increasingly stringent post-treatment phosphate effluent standards necessitate increasingly efficient removal techniques. In this study, mesoporous lanthanum hydroxide (MLHO) was synthesized by a hard-template method using ordered mesoporous silica, and its potential as an adsorbent for high-efficiency phosphate removal in aqueous solutions was tested. The porosity characteristics of MLHOs were controlled by adjusting the template structure and synthesis conditions. MLHO adsorbents showed great potential for phosphate removal from solutions containing both high and low initial phosphate concentrations. The phosphate adsorption capacity of MLHO strongly depended on its surface area as this process was governed by monolayer adsorption. Moreover, the phosphate removal performance of MLHO was affected by its structural properties. MLHO showed a high adsorption capacity of 109.41 mg P g−1 at 28 °C (qm by the Langmuir isotherm model). Further, it showed ultrafast adsorption in a solution with low initial concentration of 2 mg P/L; within the first 10 min, 99.8% of phosphate was removed, and the phosphorus concentration remaining in the solution dramatically reduced to 4 μg P/L. These findings suggest that MLHO adsorbent is a good candidate for rapid and efficient low-concentration phosphate removal to meet the increasingly stringent discharge standards for wastewater treatment plants.
Introduction
Phosphorus (P) is an essential element for all life forms, especially plants. The use of P fertilizers has greatly increased agricultural outputs and therefore helped reduce food shortages over the last century. However, overuse of P fertilizers has resulted in shortages of P reserves while also polluting surface water and, in turn, causing eutrophication.1 Therefore, proper management of phosphate, the main P species found in fresh and salt water, is both an environmental and a social issue. The main parameters for assessing the risk of eutrophication of aquatic systems (e.g., lakes; rivers; and transitional, coastal, and marine waters) include total P concentration, chlorophyll a, and Secchi depth. Countries in the European Union have established particularly strict criteria for total P concentrations to prevent eutrophication (lakes: <0.01 mg L−1; rivers: <0.01–0.07 mg L−1).2Thus, wastewater management is crucial before discharging into the environment. Phosphate removal processes are classified into chemical precipitation, biological treatment, ion exchange, and adsorption.3 Although biological processes are widely used in wastewater treatment plants (WWTPs), they are recommended for treating wastewater with only moderate P concentrations of 0.3–2 mg L−1.4 Their efficacy for treating effluents containing low phosphate concentrations remains debatable.5,6 Adsorption is a simple and economical process for high-efficiency phosphate removal even at low P concentrations, and it is considered eco-friendly given the relatively low production of sludge.7,8
Among metal-based adsorbents, lanthanum (La)-based materials are promising for phosphate removal because of their chemical stability and strong affinity for phosphate.9 La-doped or -modified adsorbents show advantageous features including high adsorption capacity and wide operating pH range compared to both host materials and La alone. In particular, recent advances in materials synthesis have enabled the engineering of their properties and functionality via the formation of nanostructures.10 These adsorbents have attracted research attention because of their large surface-area-to-volume ratio and related high reactivity and capacity. Mesoporous silicas, including MCM-41, MCM-48, and SBA-15, have been widely reported as host materials for phosphate adsorption. Although mesoporous silicas themselves remove minimal phosphate from water, they show good phosphate adsorption ability when La is incorporated into them.11 For instance, Huang et al. reported La-doped mesoporous silica spheres that showed high adsorption capacity (42.76 mg P g−1) owing to efficient phosphate transport originating from their small overall size of 300 nm.12 Various other functional materials, including zeolites,13 cellulose fibers,14 polymers,15 hydrogels,16 clays,17 and carbons,18 can also serve as host materials for loading La-based adsorbents.
Owing to rising concerns over eutrophication, many countries are expected to set increasingly stringent P discharge standards for WWTPs of 0.1 mg L−1, or even less.19 Therefore, it is essential to achieve satisfactory P removal to meet the strict standards for secondary effluents from WWTPs. Accordingly, several recent studies have focused on phosphate removal at low P concentrations of <10 mg P/L, which is in the range of P concentrations in WWTPs treating domestic wastewater.11,15,20,21 They suggested that the pore structure of host materials and La dispersity on them determine efficient contact and reaction with phosphate, in turn influencing fast and complete removal of phosphate at low P concentrations. Thus, the new adsorbent should be developed considering both chemical and morphological design for phosphate removal in practice.
Recently, lanthanum oxide (La2O3) and lanthanum hydroxide (La(OH)3) in micro- and nanostructures without any host material were synthesized and applied for phosphate removal.20,22 Compared to La-doped or -incorporated materials, they were expected to have higher adsorption capacities owing to their high La content and rigid structure. Variously shaped La2O3 microparticles were reported to show shape-dependent phosphate removal efficiency.22 Hierarchical La2O3 particles with larger surface areas showed better phosphate removal ability than other microparticles. However, there are obvious structural limits to controlling the pore structure and increasing the surface area of La2O3 particles. Mesoporous materials have much larger surface area than nanoparticles, and the functionalities originating from their unique structures have been applied in various fields including environmental, energy, and biomedical fields.23–25 In particular, mesoporous transition metal oxides with controlled morphologies have attracted much research attention owing to their large surface area and surface-originating activity as catalysts and adsorbents.26,27 Mesoporous materials comprising La2O3 or La(OH)3 are expected to have high adsorption capacities owing to their large surface area and the designed structure of nanopores and channels. However, to the best of our knowledge, mesoporous materials of La2O3/La(OH)3 have never been reported as phosphate adsorbents unlike other metal oxides.
This paper reports the synthesis of mesoporous lanthanum hydroxides (MLHOs) by a hard-template method using ordered mesoporous silica. The pore structure of the resulting La(OH)3 was controlled based on the template and reaction conditions used for synthesis. The resulting material was investigated as a phosphate adsorbent with a large surface area and well-organized pores. Its phosphate adsorption capacity was analysed, and its phosphate removal efficiency under low-phosphate concentrations was further evaluated to assess the potential in practical applications.
Experimental
Synthesis of ordered mesoporous silica
Pluronic P123 (MW: 5800, EO20PO70EO20), tetraethoxysilane (TEOS), lanthanum nitrate hexahydrate (La(NO3)3·6H2O), and citric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid, sodium hydroxide, and ethanol were obtained from Samchun Chemicals (Seoul, South Korea). 1-Butanol was purchased from Junsei Chemicals (Tokyo, Japan).Ordered mesoporous silica (KIT-6) was used as a template to prepare MLHO. Two sets of KIT-6 were prepared at two different hydrothermal temperature via a slight modification of a reported method.28 Briefly, 14.12 g of Pluronic P123 and 22.6 g of concentrated HCl were dissolved in 498 mL of deionized water in a polypropylene bottle, and 14.12 g of 1-butanol was added to the solution with vigorous stirring at 35 °C. After 1 h, 30.36 g of TEOS was added to the solution, and then, the solution was stirred at this temperature for 24 h. The capped bottle was stored at the designated temperature of 35 °C (KIT-6-35C) or 100 °C (KIT-6-100C) for another 24 h. The solid was filtered and dried at 100 °C overnight, and then, the surfactant was extracted from the solid in an ethanol solution containing HCl (5 vol%). KIT-6 powder was finally obtained after calcination at 550 °C for 6 h.
Synthesis of mesoporous lanthanum hydroxide
MLHO was prepared by the hard-template approach using KIT-6, as described originally by Taguchi and Schüth.29 In a typical synthesis, 2 mmol of La(NO3)3·6H2O and an equimolar amount of citric acid were dissolved in 2 mL of anhydrous ethanol. The solution was added dropwise to 1 g of KIT-6, and the slurry was mixed thoroughly until ethanol was evaporated. The mixed powder was dried at 80 °C for 6 h, and then, calcination was performed at 500 °C for 4 h. Additional ethanol solution containing La(NO3)3·6H2O and an equimolar amount of citric acid was infiltrated into the prefilled powder, and then, calcination was performed at 750 °C for 6 h. The silica template was removed with 3 M aqueous NaOH solution at 80 °C for 24 h. The sample was collected after several washing steps with water and ethanol and then dried at 80 °C. We prepared a series of samples using two KIT-6 templates and different amounts of La precursor. They were named as MLHO-X-Y, where X represents the template synthesized at the designated hydrothermal temperature (KIT-6-X) and Y, the amount of reacted La precursor for 1 g of template. For instance, MLHO-35C-3 and MLHO-35C-4 were synthesized from 3 mmol and 4 mmol of La(NO3)3·6H2O, respectively, using the KIT-6-35C template. MLHO-35C-2 and MLHO-100C-2 were synthesized with only a single calcination step at 750 °C for 6 h.Characterization
The morphology of the materials was characterized by transmission electron microscopy (TEM; JEM-2011, JEOL) with an Oxford X-MaxN-80T detector for energy-dispersive X-ray spectroscopy analysis. The phase and structural parameters were characterized by X-ray diffraction (XRD) patterns using a PANalytical X'pert-Pro (Cu Kα radiation, λ = 0.15406 nm). X-ray photoelectron spectroscopy (XPS) was performed using Thermo Scientific K-Alpha with Al Kα radiation as the X-ray source. The Brunauer–Emmett–Teller (BET) surface area and pore size distribution were determined using a Tristar 2 (Micromeritics Inc.) through nitrogen adsorption/desorption experiments.Phosphate adsorption experiments
Batch adsorption and continuous fixed-bed column experiments were conducted to investigate the phosphate adsorption properties of the prepared samples, as outlined below.
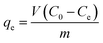 | (1) |
The isotherm data were fitted to the Langmuir and Freundlich isotherm models given by eqn (2) and (3), respectively:
Langmuir model:
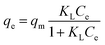 | (2) |
Freundlich model:
| qe = KFCe1/n | (3) |
Pseudo-first order model:
| qt = qe(1 – e−k1t) | (4) |
Pseudo-second order model:
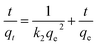 | (5) |
Phosphate removal experiments with low initial P concentration were conducted using a 2 mg P/L solution; the experimental method was the same as described above.
Results and discussion
Characterization of materials
MLHO samples were synthesized by the hard-template method using KIT-6 as the template to prepare an ordered structure with nanometer-sized pores. Two sets of KIT-6 were prepared at two temperatures, 35 °C (KIT-6-35C) and 100 °C (KIT-6-100C), to alter the pore structure. Table S1† lists the structural characteristics of the KIT-6 templates (e.g., BET specific surface area (SBET), pore diameter (dpore), and total pore volume (Vt)); further, Fig. S1† shows TEM images and N2 sorption isotherms. A previous study31 showed that a lower aging temperature of KIT-6 resulted in a smaller pore volume and pore size with poor interconnectivity among channel systems; similarly, our study showed that KIT-6-100C had larger surface area, pore size, and total pore volume than KIT-6-35C.Incipient wetness impregnation of the La precursor inside the pores of each template was performed, following which the citrate sol–gel process was performed.32 After calcination, the silica template was removed via etching with NaOH. By varying the amount of loaded precursor, a series of MLHOs were synthesized. Fig. 1 shows XRD patterns of synthesized MLHOs. Most MLHOs had a mixture phase of amorphous oxide and crystalline La(OH)3 with well-resolved peaks. La2O3 is hygroscopic in nature and quickly converts into La(OH)3 in a moist atmosphere. Before the etching process, XRD analysis was used to confirm that the composites contained the La2O3 phase (Fig. S2†). Prolonged reaction (24 h) with aqueous NaOH solution in the etching process resulted in transformation into La(OH)3. Further, the XRD patterns of MLHO samples prepared using a small amount of La precursor (2 mmol g−1) revealed a largely amorphous structure without distinct crystalline peaks.
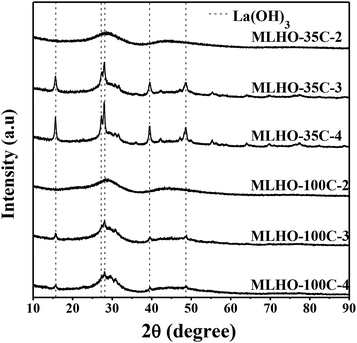 | ||
| Fig. 1 X-ray diffraction (XRD) patterns of the synthesized mesoporous lanthanum hydroxides (MLHOs). Dashed lines represent the reference XRD pattern of La(OH)3 (JCPDS#31-1481). | ||
The morphologies of the synthesized MLHOs were characterized by TEM. The MLHO samples had well-defined pore structures within the nanometer size range (Fig. 2 and S3†). In particular, MLHOs prepared with high loading of La precursors (3 or 4 mmol g−1) had organized pore and wall structures comprising arrays of nanoparticles. This ordered mesopore structure was expected given the use of KIT-6 as the mold. As expected from the XRD results, MLHO-35C-2 and MLHO-100C-2 showed relatively imperfect development of ordered structures. Further, STEM images with elemental mapping showed the presence of silicon within the wall even after long-term etching (72 h) using 3 M NaOH (Fig. S3d†). The calcination of La precursor with silica at high temperatures (>850 °C) has been reported to yield a lanthanum silicate phase.33,34 In this study, we calcinated samples at a lower temperature (750 °C) to avoid the formation of a lanthanum silicate phase. However, some lanthanum silicate phase was evidently formed, and it resisted etching. This lanthanum silicate became amorphous under relatively low-temperature and short-duration calcination, resulting in less-distinguishable XRD patterns.
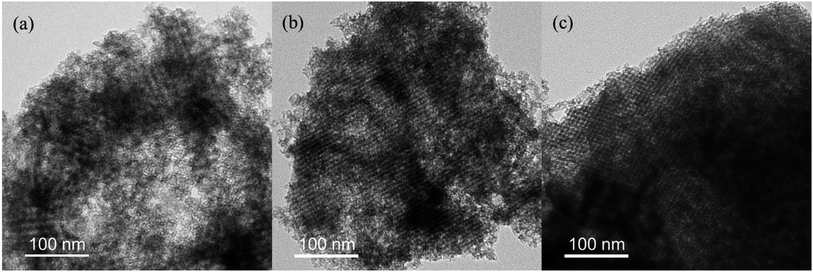 | ||
| Fig. 2 Transmission electron microscopy images of MLHO-100Cs: (a) MLHO-100C-2, (b) MLHO-100C-3, and (c) MLHO-100C-4. | ||
XPS analysis was conducted to evaluate the surface composition and valence state of materials (Fig. 3 and S4†). High-resolution spectra around the binding energy of the La 4d region (Fig. 3a and S4a†) revealed the existence of La(III) with binding energies at 102.4 eV (4d5/2) and 105.6 eV (4d3/2). The profiles in the La 3d region showed split peaks of La 3d3/2 and La 3d5/2 as multiplet splitting, representing the state of La(III) (Fig. 3b and S4b†).35 In particular, the splitting in La 3d5/2 was found to be DE = ∼3.7 eV; this is characteristic of La(OH)3.36 In addition to the XPS profiles in the La 4d and La 3d regions, peaks in the La 4p region were examined to determine the state of La (Fig. 3c and S4c†). The main peak of 4p3/2 was at 196.1 eV. The position of the satellite peak and its intensity can be used to distinguish La2O3 and La(OH)3. The satellite peak represented La(OH)3 because its relative position to the main peak was ∼3.6 eV with low intensity.35,36 Overall, XPS analysis confirmed that the surface of MLHOs did not contain La2O3; instead, they contained La(OH)3. From the XRD and TEM results, we concluded that MLHOs had a stable surface of crystalline La(OH)3 on a less crystalline backbone of La2O3/lanthanum silicate after aqueous-phase etching.
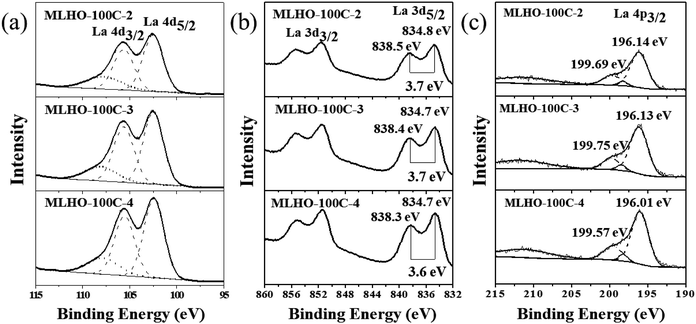 | ||
| Fig. 3 X-ray photoelectron spectroscopy spectra of MLHO-100Cs: (a) La 4d region, (b) La 3d region, and (c) La 4p region. | ||
The pore structure was characterized by N2 adsorption/desorption isotherms. The surface area of MLHOs showed higher dependency based on the amount of La precursor used (Table 1). Smaller amounts of precursor resulted in larger BET surface area (SBET) of MLHOs from both KIT-6-35C and KIT-6-100C. In particular, MLHO-35C-2 and MLHO-100C-2 had large surface area of 429.8 and 348.3 m2 g−1, respectively; these areas were much larger than those of the bulk counterpart,9,37 nanosized particles,20 and hierarchical microparticles.22 Further, the pore structure was affected by both the template and the amount of precursor used. Fig. 4 and S5† show the N2 adsorption/desorption isotherms and calculated pore distributions of the MLHOs. Most MLHOs exhibited a bimodal distribution as a characteristic structure of replicated materials from KIT-6 aged at low temperatures. The first peak was at 4–5 nm, in agreement with the wall thickness of KIT-6 (KIT-6-35C: 4.4 nm; KIT-6-100C: 4.7 nm) as characterized by TEM (Fig. S1†). As mentioned previously, KIT-6 has two sets of mesopore channels, and the size and interconnectivity among channels depend on the hydrothermal treatment temperature. KIT-6 treated hydrothermally at low temperature (<100 °C) showed less development of micropores among mesopore channels, resulting in poor infiltration of the La precursor. This explained why MLHOs had pores of 8–10 nm size; this is equivalent to the dimension of two walls and a pore of KIT-6.38,39 Thus, the MLHOs from KIT-6-35C had a more distinct bimodal pore distribution relative to those from KIT-6-100C owing to less filling of La precursor into the mesopores in the KIT-6-35C matrix. Interestingly, the isotherms of MLHO-100Cs showed a hysteresis loop at a high relative pressure (P/P0 > 0.9), suggesting the presence of macroporosity. Pore size distribution analysis (Fig. 4d) also confirmed that MLHO-100Cs had irregular pores of 15–50 nm size. The presence of large pores accounts for the large pore volume of MLHO-100Cs as well as their relatively smaller surface area compared to those of MLHO-35Cs. Overall, both the structure characteristics of templates and the amount of La precursor induced the variation of growth of La(OH)3 in mesopore channels of templates, and this affected the pore structure of the resulting MLHOs.
| Sample | SBET (m2 g−1) | da (nm) | Vt (cm3 g−1) | qm (mg g−1) |
|---|---|---|---|---|
| a Pore diameter, d, was calculated from the N2 desorption isotherm by the Barrett–Joyner–Halenda (BJH) method. SBET, Vt, and qm are the BET surface area, total pore volume, and maximum adsorption capacity by Langmuir model, respectively. | ||||
| MLHO-100C-2 | 348.3 | 5.6/9.9 | 1.05 | 109.41 |
| MLHO-100C-3 | 241.7 | 5.2/9.5 | 0.74 | 93.90 |
| MLHO-100C-4 | 221.3 | 4.2/8.9 | 0.62 | 90.91 |
| MLHO-35C-2 | 429.8 | 5.0/8.2 | 0.76 | 98.62 |
| MLHO-35C-3 | 263.1 | 5.3/9.1 | 0.57 | 80.32 |
| MLHO-35C-4 | 150.4 | 3.9/7.6 | 0.44 | 76.10 |
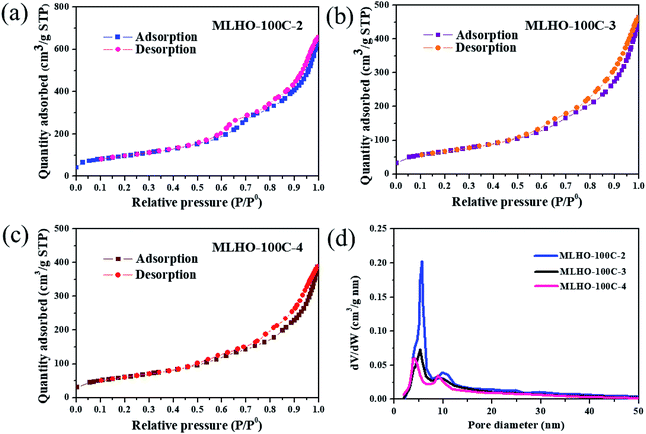 | ||
| Fig. 4 N2 adsorption/desorption isotherms of (a) MLHO-100C-2, (b) MLHO-100C-3, and (c) MLHO-100C-4. (d) Pore size distributions of MLHO-100Cs as calculated by the Barrett–Joyner–Halenda (BJH) method. | ||
Adsorption of phosphate by MLHOs
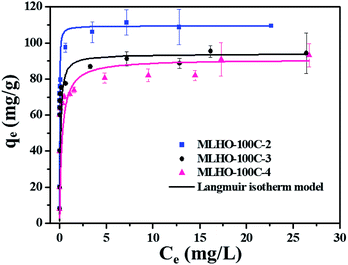 | ||
| Fig. 5 Adsorption isotherms of phosphate on MLHO-100Cs. Solid lines represent plots fitted to Langmuir isotherm model. | ||
Within the same KIT-6 set, MLHOs with large surface areas had high adsorption capacities. Further, MLHO-100Cs from KIT-6-100C had larger adsorption capacity but smaller surface area than MLHO-35Cs from KIT-6-35C. These results indicate that, in addition to surface area, the pore structure is also an important parameter of adsorbents. As shown in Fig. 4d, MLHO-100Cs had large pores of 15–50 nm size along with large pore volume; these could result in efficient contact with phosphate during the adsorption process and promote the transformation into lanthanum phosphate. This result agrees with previous studies on La-doped adsorbents indicating that support materials with macropores had high adsorption capacity owing to the enhanced accessibility of phosphate to La.40,41 Our results indicate that when designing phosphate adsorbents, both the surface area and the pore structure should be considered in terms of the balance between mesopores and macropores.
Based on these findings, we selected MLHO-100C-2, with the highest adsorption capacity, for further investigating the phosphate adsorption performance.
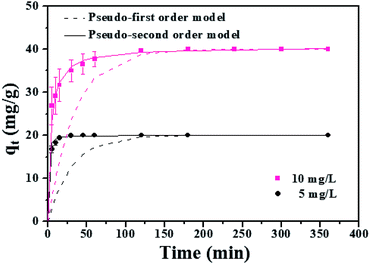 | ||
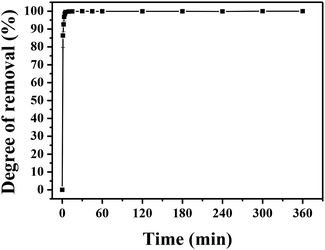
| Fig. 6 Adsorption kinetics of MLHO-100C-2. Initial phosphate concentrations were 5 and 10 mg P/L and dosage of MLHO-100C-2 was 0.25 g L−1. | ||
The kinetic study revealed that the MLHO adsorbent achieved very fast phosphate removal. Phosphate adsorption by MLHO adsorbent reached equilibrium within 30 min and 2 h at initial concentrations of 5 and 10 mg P/L, respectively. Accordingly, the adsorption rate constant, k2, in solutions of 5 and 10 mg P/L was calculated to be 0.106 and 0.006 g mg−1 min−1, respectively; these values were higher than those of previously reported La-based nanostructures.41 The calculated k2 for the solution with initial concentration of 5 mg P/L was approximately two orders of magnitude greater than that for the solution with initial concentration of 10 mg P/L. This result shows that phosphate removal occurs more rapidly at lower concentrations.
The MLHO adsorbent exhibited very fast adsorption, with 86.3% and 99.5% of phosphate in the initial solution removed within just 1 and 5 min, respectively (Fig. 7). After 10 min, 99.8% of phosphate was removed and the P concentration of the solution dramatically decreased to 4 μg P/L. After adsorption, the residual P concentration of the solution was much smaller than the maximum allowable concentration for urban WWTPs in countries such as the United Kingdom (1 mg P/L),42 Korea (0.2 mg P/L),43 and South America (0.02 mg P/L).44 These results indicate that MLHO is an excellent candidate for phosphate removal via high-speed adsorption to achieve levels low enough to prevent eutrophication.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 kg wastewater per kg MLHO. MLHOs showed superior performance comparing to La-doped adsorbents.5,16,45 This indicates the great potential of MLHOs for long-term treatment of wastewater containing low phosphate concentrations.
700 kg wastewater per kg MLHO. MLHOs showed superior performance comparing to La-doped adsorbents.5,16,45 This indicates the great potential of MLHOs for long-term treatment of wastewater containing low phosphate concentrations.MLHOs showed more effective phosphate removal in both batch and continuous fixed-bed column experiments than previously reported La-doped adsorbents. Further, they showed rapid and complete removal of low phosphate concentrations as well as promising long-term treatment ability. This outstanding performance of MLHOs for phosphate adsorption resulted from the chemically controlled and rigid surface of La(OH)3 as well as the optimized porosity in the nanometer regime for enabling enhanced accessibility to phosphate. This result implies that mesoporous adsorbents comprising active materials could show better performance than doped or host/guest platforms for the removal of other adsorbates.
Conclusions
MLHOs were fabricated by the hard-template method. This enabled the control of their microstructures by adjusting reaction conditions including the template and amount of La precursor. The synthesized MLHO adsorbent showed great potential for phosphate removal from solutions containing various P concentrations owing to their nanosized pores and La(OH)3 composition. The surface area of the MLHOs was closely related to their phosphate removal capacity as this process was governed by monolayer adsorption of phosphate on the MLHO surface. Further, it was important for the adsorbent to have a proper pore structure. Accordingly, MLHO-100C-2 synthesized using KIT-6 aged at 100 °C with 2 mmol g−1 of La precursor exhibited the highest adsorption capacity. It also showed rapid adsorption of phosphate in a 2 mg P/L solution, which is a common standard for WWTP effluents. Most phosphate was removed within 10 min, after which the P concentration in the solution dramatically decreased to 4 μg P/L. In conclusion, MLHO is an excellent adsorbent for phosphate removal from solutions containing both high and low P concentrations, and it can be used in WWTPs to meet increasingly stringent discharge standards.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by C1 Gas Refinery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015M3D3A1A01064908) and also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2018R1A2B6001415).Notes and references
- K. G. Sellner, G. J. Doucette and G. J. Kirkpatrick, J. Ind. Microbiol. Biotechnol., 2003, 30, 383–406 CrossRef CAS PubMed.
- European Commission, Guidance document on eutrophication assessment in the context of European water policies, Office for Official Publications of the European Communities, Luxembourg, 2009 Search PubMed.
- G. K. Morse, S. W. Brett, J. A. Guy and J. N. Lester, Sci. Total Environ., 1998, 212, 69–81 CrossRef CAS.
- Commission Implementing Decision (EU) 2018/1147 of 10 August 2018 establishing best available techniques (BAT) conclusions for waste treatment, under Directive 2010/75/EU of the European Parliament and of the Council, Official Journal of the European Union, 2018, vol. L208, pp. 38–90 Search PubMed.
- Y. Wu, X. Li, Q. Yang, D. Wang, Q. Xu, F. Yao, F. Chen, Z. Tao and X. Huang, J. Environ. Manage., 2019, 231, 370–379 CrossRef CAS PubMed.
- E. Rott, H. Steinmetz and J. W. Metzger, Sci. Total Environ., 2018, 615, 1176–1191 CrossRef CAS PubMed.
- X. Luo, X. Wang, S. Bao, X. Liu, W. Zhang and T. Fang, Sci. Rep., 2016, 6, 39108 CrossRef CAS PubMed.
- H.-S. Yoon, K. W. Chung, C.-J. Kim, J.-H. Kim, H.-S. Lee, S.-J. Kim, S.-I. Lee, S.-J. Yoo and B.-C. Lim, Korean J. Chem. Eng., 2018, 35, 470–478 CrossRef CAS.
- J. Xie, Z. Wang, S. Lu, D. Wu, Z. Zhang and H. Kong, Chem. Eng. J., 2014, 254, 163–170 CrossRef CAS.
- P. Huang, Y. Zhao, J. Zhang, Y. Zhu and Y. Sun, Nanoscale, 2013, 5, 10844–10848 RSC.
- W. Huang, Y. Zhang and D. Li, J. Environ. Manage., 2017, 193, 470–482 CrossRef CAS PubMed.
- W. Huang, X. Yu, J. Tang, Y. Zhu, Y. Zhang and D. Li, Microporous Mesoporous Mater., 2015, 217, 225–232 CrossRef CAS.
- J. Xie, Z. Wang, D. Fang, C. Li and D. Wu, J. Colloid Interface Sci., 2014, 423, 13–19 CrossRef CAS PubMed.
- E. W. Shin, K. G. Karthikeyan and M. A. Tshabalala, Environ. Sci. Technol., 2005, 39, 6273–6279 CrossRef CAS PubMed.
- J. He, W. Wang, F. Sun, W. Shi, D. Qi, K. Wang, R. Shi, F. Cui, C. Wang and X. Chen, ACS Nano, 2015, 9, 9292–9302 CrossRef CAS PubMed.
- S. Dong, Y. Wang, Y. Zhao, X. Zhou and H. Zheng, Water Res., 2017, 126, 433–441 CrossRef CAS PubMed.
- W.-Y. Huang, D. Li, Z.-Q. Liu, Q. Tao, Y. Zhu, J. Yang and Y.-M. Zhang, Chem. Eng. J., 2014, 236, 191–201 CrossRef CAS.
- L. Zhang, Q. Zhou, J. Liu, N. Chang, L. Wan and J. Chen, Chem. Eng. J., 2012, 185–186, 160–167 CrossRef CAS.
- S. K. Zheng, J. J. Chen, X. M. Jiang and X. F. Li, Chem. Eng. J., 2011, 169, 194–199 CrossRef CAS.
- L. Fang, B. Wu, J. K. M. Chan and I. M. C. Lo, Chemosphere, 2018, 192, 209–216 CrossRef CAS PubMed.
- T. Liu, X. Chen, X. Wang, S. Zheng and L. Yang, Chem. Eng. J., 2018, 335, 443–449 CrossRef CAS.
- J. Liu, G. Wang, L. Lu, Y. Guo and L. Yang, RSC Adv., 2017, 7, 40965–40972 RSC.
- E. Lim, C. Jo and J. Lee, Nanoscale, 2016, 8, 7827–7833 RSC.
- P. Bhanja and A. Bhaumik, Chem. Rec., 2019, 19, 333–346 CrossRef CAS PubMed.
- B. G. Cha and J. Kim, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1515 Search PubMed.
- Y. Ren, Z. Ma and P. G. Bruce, Chem. Soc. Rev., 2012, 41, 4909–4927 RSC.
- K. An, S. Alayoglu, N. Musselwhite, S. Plamthottam, G. Melaet, A. E. Lindeman and G. A. Somorjai, J. Am. Chem. Soc., 2013, 135, 16689–16696 CrossRef CAS PubMed.
- F. Kleitz, S. H. Choi and R. Ryoo, Chem. Commun., 2003, 2136–2137, 10.1039/B306504A.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1–45 CrossRef CAS.
- Standard Methods for the Examination of Water and Wastewater, ed. E. W. Rice and L. Bridgewater, American Public Health Association, Washington, D.C., 22nd edn, 2012 Search PubMed.
- H. Tüysüz, C. W. Lehmann, H. Bongard, B. Tesche, R. Schmidt and F. Schüth, J. Am. Chem. Soc., 2008, 130, 11510–11517 CrossRef PubMed.
- A. E. Danks, S. R. Hall and Z. Schnepp, Mater. Horiz., 2016, 3, 91–112 RSC.
- R. Zhang, P. Li, N. Liu, W. Yue and B. Chen, J. Mater. Chem. A, 2014, 2, 17329–17340 RSC.
- B. Ballinger, J. Motuzas, C. R. Miller, S. Smart and J. C. Diniz da Costa, Sci. Rep., 2015, 5, 8210 CrossRef CAS PubMed.
- D. F. Mullica, H. O. Perkins, C. K. C. Lok and V. Young, J. Electron Spectrosc. Relat. Phenom., 1993, 61, 337–355 CrossRef CAS.
- M. F. Sunding, K. Hadidi, S. Diplas, O. M. Løvvik, T. E. Norby and A. E. Gunnæs, J. Electron Spectrosc. Relat. Phenom., 2011, 184, 399–409 CrossRef CAS.
- J. Xie, Y. Lin, C. Li, D. Wu and H. Kong, Powder Technol., 2015, 269, 351–357 CrossRef CAS.
- F. Jiao, A. H. Hill, A. Harrison, A. Berko, A. V. Chadwick and P. G. Bruce, J. Am. Chem. Soc., 2008, 130, 5262–5266 CrossRef CAS PubMed.
- D. Klaus, S. Amrehn, M. Tiemann and T. Wagner, Microporous Mesoporous Mater., 2014, 188, 133–139 CrossRef CAS.
- W. Huang, Y. Zhu, J. Tang, X. Yu, X. Wang, D. Li and Y. Zhang, J. Mater. Chem. A, 2014, 2, 8839–8848 RSC.
- J. Yang, P. Yuan, H.-Y. Chen, J. Zou, Z. Yuan and C. Yu, J. Mater. Chem., 2012, 22, 9983–9990 RSC.
- UK legislation, The Urban Waste Water Treatment (England and Wales) Regulations 1994, UK Legislation, 1994, http://www.legislation.gov.uk/uksi/1994/2841/contents/made Search PubMed.
- Ministry of Government Legislation in Korea, Enforcement Decree of Sewerage Act, Ministry of Government Legislation, 2018, http://www.law.go.kr Search PubMed.
- A. F. D. Sousa, T. P. Braga, E. C. C. Gomes, A. Valentini and E. Longhinotti, Chem. Eng. J., 2012, 210, 143–149 CrossRef.
- Q. Zhang, J. Teng, G. Zou, Q. Peng, Q. Du, T. Jiao and J. Xiang, Nanoscale, 2016, 8, 7085–7093 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00895k |
| ‡ These authors (K. Kim, D. Kim) have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |