Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lysosomal tracking with a cationic naphthalimide using multiphoton fluorescence lifetime imaging microscopy†
Meng
Li‡
*ab,
Haobo
Ge‡
*a,
Vincenzo
Mirabello
 a,
Rory L.
Arrowsmith
a,
Gabriele
Kociok-Köhn
a,
Stanley W.
Botchway
c,
Weihong
Zhu
a,
Rory L.
Arrowsmith
a,
Gabriele
Kociok-Köhn
a,
Stanley W.
Botchway
c,
Weihong
Zhu
 *d,
Sofia I.
Pascu
*d,
Sofia I.
Pascu
 *a and
Tony D.
James
*a and
Tony D.
James
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.pascu@bath.ac.uk
bDepartment of Environmental Science and Engineering, North China Electric Power University, 689 Huadian Road, Baoding, 071003, P. R. China. E-mail: mli201509@163.com
cCentral Laser Facility, Rutherford Appleton Laboratory, Research Complex at Harwell, STFC Didcot, OX11 0QX, UK
dShanghai Key Laboratory of Functional Materials Chemistry, Key Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science & Technology, Shanghai 200237, P. R. China. E-mail: whzhu@ecust.edu.cn
First published on 1st September 2017
Abstract
A naphthalimide-based chemosensing motif turns ON the fluorescence emission in solution in the presence of aqueous iron(III) chloride, and maintains this property in living cancer cells. The emission response to Fe(III) ions occurs simultaneously with a change in pH. The protonation of methyl piperazine-conjugated naphthalimide promotes its lysosomal localisation as assessed by co-localisation tests and fluorescence lifetime imaging microscopy (FLIM) in vitro.
The development of highly sensitive and selective probes for the recognition and measurement of transition metal ions in vitro are necessary tools in biological,1,2 chemical3–5 and environmental science.6–9 Iron is one of the essential metal ions in biological systems since it plays a fundamental role in many biochemical processes.7,10–15 Both its deficiency and overload may lead to biological disorders in vivo, such as anaemia, liver and kidney damage, heart failure and diabetes onset.16–18 Due to its biological significance, fluorescence probes for the precise detection of iron(III) are sought after.19–23 Since in aqueous conditions at neutral pH, Fe(III) halides form insoluble Fe(OH)3 and, in the lysosomes (pH 4.5), species such as [Fe(OH)2]+ and [Fe(OH)]2+ with release of protons,24 there are only a few Fe3+ fluorescent sensors that can directly detect this ion, especially with turn-on fluorescence response.25–27 It is generally accepted that simple and effective chemosensors that involve fluorescence turn-on are advantageous.28,29 By using a “turn on” probe, it is possible to measure low-concentrations because measuring a small increase relative to a dark background, reduces the likelihood of false positive signals and increases sensitivity.30,31 Therefore, we aimed to design and deliver to cells a biocompatible fluorescent probe that turns into a highly fluorescent, protonated species, only in the presence of trivalent cations such as Fe(III) in vitro. We also investigated the effect of an excess of aqueous tricationic chlorides resembling the Fe(III) charge and size ratios and the coupled pH variation caused on a simple naphthalimide-based fluorescent probe tagged with a Lewis base methyl piperazine as the receptor of choice. Photo-induced electron transfer (PET) chemosensors with the ‘fluorophore-spacer-receptor’ format, are one of the most important classes for the design of fluorescent sensors.32 Naphthalamide was the fluorophore tag of choice due to its excellent photophysical properties, including high extinction coefficient, photo-stability and relatively long emission wavelength (500–600 nm).33,34 The intermediate TN was first synthesised from 4-bromo-1,8-naphthalic anhydride and 5-methylthiazol-2-amine by refluxing in absolute acetic acid (60% yield, ESI,† Scheme S1). The bromine atom of TN was then substituted by methyl-piperazine in 2-methoxyethanol yielding TNP, which was treated with either an excess of aqueous FeCl3 or HCl to give [TNPH]+ species (Scheme 1). The coordination chemistry of FeCl3 with various modifications of N-aryl or N-alkyl-piperazine ligands has been reported.35,36 When coordinated to the Fe3+ metal centre, the ligand likely adopts a boat configuration acting as a bidentate chelator, however the [(TNP)2FeCl2]+ intermediate proposed in Scheme 1 (on the basis of mass spectrometry) could not be isolated on a laboratory scale from wet solvents used for the sensing and imaging experiments as it rapidly decomposed to form [TNPH]Cl (vide infra). This new compound was fully characterized by 1H and 13C NMR spectroscopies, HRMS, and single crystal X-ray diffraction (Fig. 1 and ESI†). The photo-physical properties of the free TNP were investigated using UV-vis absorption and fluorescence spectroscopies including titration studies with aqueous FeCl3 under an aqueous environment (Fig. S1 and S6, ESI†). In HEPES buffer, free TNP showed one main absorption band centred at 410 nm (ε = 1.3 × 104 M−1 cm−1) assigned as a π–π* transition (10 mM HEPES; ethanol/H2O = 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v; pH 7.4, Fig. S1, ESI†).
20, v/v; pH 7.4, Fig. S1, ESI†).
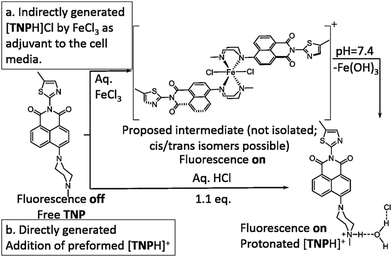 | ||
| Scheme 1 Proposed pathway for the TNP protonation leading to the fluorescent [TNPH]Cl in situ in aqueous FeCl3 media. | ||
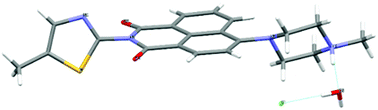 | ||
Fig. 1 Molecular structure of [TNPH]Cl·H2O by single crystal X-ray diffraction. Crystals were grown from a mixture of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TNP and FeCl3 in wet DMSO. Colour code: yellow: sulfur, red: oxygen, grey: carbon, light grey: hydrogen, green: chlorine. Details are given in the ESI† and Fig. S13, S14. 1 TNP and FeCl3 in wet DMSO. Colour code: yellow: sulfur, red: oxygen, grey: carbon, light grey: hydrogen, green: chlorine. Details are given in the ESI† and Fig. S13, S14. | ||
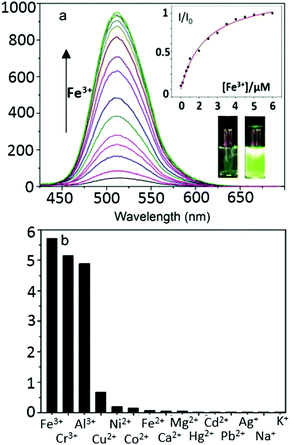
Upon the addition of Fe3+ (0–10 eq. FeCl3), the maximum absorption peak exhibits a distinct shift from 410 to 381 nm. When excited in the proximity of its isosbestic point (403 nm in single-photon, or 810 nm in two-photon mode), TNP shows only weak fluorescence emission due to PET. In the presence of FeCl3, in a variety of (wet) organic solvents, a strong fluorescence emission with a maximum at 515 nm was observed, as shown in Fig. 2. Furthermore, the fluorescence intensities at 515 nm display a nonlinear relationship towards Fe3+ concentrations from 0.2 to 10 μM, and the binding constant is (3.75 ± 0.31) × 105 M−1, indicating that TNP is particularly sensitive to the detection of Fe3+ (ESI,† Fig. S6). An intense peak at m/z 910.1347 in the electron-spray MS ionization (ESI) exhibited the correct isotopic pattern corresponding to the species [TNPFeCl2]+ and also several related fragments indicative of the formation of an [TNP]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [iron] complex with a 2
[iron] complex with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Scheme 1 and ESI,† Fig. S4). The selectivity of TNP toward Lewis acids such as Fe3+, Cr3+, Al3+ was evaluated by adding 10 eq. of various metal ions, including those essential ions of relevance to biological processes in vivo (Cu2+, Co2+, Ni2+, Fe2+, Na+, K+, Ca2+ and Mg2+) and toxic metals such as Hg2+, Cd2+, Pd2+, Ag+. Under the same conditions, no obvious fluorescence changes were observed for the M2+ ions tested (Fig. 2b). A Job's plot analysis indicated that the binding mode of TNP donors to Fe3+ was 2
1 stoichiometry (Scheme 1 and ESI,† Fig. S4). The selectivity of TNP toward Lewis acids such as Fe3+, Cr3+, Al3+ was evaluated by adding 10 eq. of various metal ions, including those essential ions of relevance to biological processes in vivo (Cu2+, Co2+, Ni2+, Fe2+, Na+, K+, Ca2+ and Mg2+) and toxic metals such as Hg2+, Cd2+, Pd2+, Ag+. Under the same conditions, no obvious fluorescence changes were observed for the M2+ ions tested (Fig. 2b). A Job's plot analysis indicated that the binding mode of TNP donors to Fe3+ was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
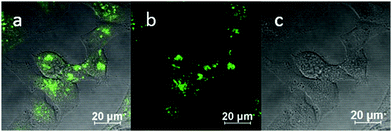
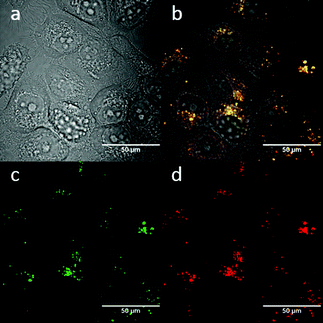
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ESI,† Fig. S3). TNP is therefore very selective and sensitive for aqueous Fe3+ which is essential to life as well as being earth abundant, and we hypothesised that this is due to the initial formation of the corresponding metal complex. In the case of aqueous FeCl3 (Scheme 1) this was followed by its decomposition to [TNPH]Cl in the wet solvents used (ESI†). [TNPH]Cl was also isolated by reacting TNP with 1.1 eq. of concentrated HCl (Scheme 1). The quantum yield of [TNPH]Cl in DMSO was measured to be 0.017, comparable with other naphtyl-based chromophores (ESI†). Taken together, data suggests that the initial capture of Fe3+ by the receptor results in a decrease of the electron-donating ability of the methyl-nitrogen, thus resulting in a reduction of the PET effect, and with time, (in the presence of H2O) in the formation of the protonated species [TNPH]Cl (as well as presumably [Fe(H2O)3(OH)3] and Fe(OH)3 and aqueous HCl). The protonated species could well form under differing condition too: specimens with the same unit cell parameters were isolated and characterised by X-ray diffraction from several different aqueous solvent mixtures (EtOH/H2O, DMSO, wet CHCl3) (Fig. 1 and ESI†). The onset of enhanced fluorescence upon treatment with an excess of aqueous FeCl3 was also observed in experiments carried out using 2-photon excitation in wet DMSO for steady-state emission spectra and excited state lifetime measurements. While the fluorescence emissions were remarkably different (ESI†), lifetime measurement via time correlated single photon counting (TCSPC) indicated that the lifetime point decays recorded in solutions (giving rise to data which was modelled to 1 component multi-exponential fitting) are in the region of ca. 5 ns for both [TNP] and [TNPH]Cl (the latter being generated in situ, from the TNP in the presence of excess FeCl3 in wet DMSO). Specifically, free [TNP] in solution showed a 4.92 ns (χ2 = 1.28) and, in the presence of aqueous FeCl3 (in situ protonation) giving rise to the protonated species [TNPH]+ increased to 5.56 ns (χ2 = 1.19) (ESI,† Fig. S24–S26). These results confirmed the iron-mediated protonation of methyl piperazine unit disrupts the PET turning on the fluorescence of the probe. Studies of the pH-dependent response of TNP were also carried out over a pH range of 3.0–9.0 (ESI,† Fig. S7). We explored the ability of TNP towards in vitro conversion to [TNPH]+ in the acid environment of lysosomes. The cellular environment of the new probe was determined in HeLa and PC-3 cancer cells using confocal fluorescence spectroscopy and fluorescence lifetime imaging microscopy (FLIM) (Fig. S27 and S28, ESI†), which is used as a sensitive analytical tool for the probes' environment with added advantages when using multiphoton imaging techniques, i.e. the use of tissue-friendly low energy near infra-red photon rather than UV excitation for live cells. The pH evaluation showed that between pH 7.4–9.0 (well within the biologically relevant pH range 6.5–8.5) the emission of free TNP is turned off in aqueous solutions. On the contrary, at lower pH, [TNPH]Cl, a highly fluorescent and kinetically stable organic molecule, was formed. This new lipophilic cation may be employed further to measure pH changes. However, such sensing experiments are beyond the scope of this study. Cells were incubated at 37 °C with TNP and visualised using single photon, epi- and confocal-fluorescence microscopies (Fig. 3 and ESI†) and two-photon confocal fluorescence microscopy coupled with fluorescence lifetime imaging microscopy (FLIM Fig. 4 and ESI.† Cellular assays were prepared according to standard methods:9,34,37–41 generally, cells were incubated with TNP in the presence or absence of FeCl3 as well as with [TNPH]Cl solutions for 15 minutes in serum free medium (SFM). After washing with PBS, the cells were imaged using laser scanning confocal fluorescence microscopy (in single- or two-photon excitation modes). Control experiments (Fig. S15 and S16, ESI†) were carried out in cells incubated with serum free medium alone, or with 1 to 2% DMSO in SFM). The confocal imaging results regarding the iron-mediated fluorescence enhancement in cells were most promising using an excitation wavelength of 405 nm (single-photon excitation) or 810 nm (under two-photon excitation). The imaging studies also indicated that TNP alone passes through cell membranes but its fluorescence is extremely weak. Single-photon, as well as two-photon microscopy experiments, were repeated in a PC-3 (a prostate cancer cell line), also incubated at 37 °C (ESI†). In both HeLa and PC-3, in the presence of an excess of aqueous FeCl3 (minimum 1 mM concentration), the characteristic and intense fluorescence emission and the corresponding lifetime of [TNPH]+ can be reliably observed away from that of cellular auto fluorescence. The experiments carried out at 4 °C did not show reliable uptake of TNP, either alone or in the presence of FeCl3. Imaging experiments of TNP alone with incubation times longer than 1 h or at concentrations higher than 100 μM (with 1–2% DMSO) did not show reliable results due to the lack of fluorescence emission and were not pursued. The ability of [TNPH]+ to localise in the acidic intracellular organelles in vitro was investigated in both HeLa and PC-3 cells using 1P- and 2P-confocal fluorescence microscopies and FLIM. Co-localisation studies were carried out using LysoTracker Red CMXRos, and confirm the ability of [TNPH]+ to target the acidic organelles and be co-localised in the lysosomes (Fig. 5 and ESI†). Control experiments were carried out on cells incubated with DMSO and TNP alone (10 μM) at various temperatures and compared with probe-free control experiments in cells (incubated with SFM, or with 1 to 2% DMSO in SFM). In conclusion, imaging experiments in cancer cells reliably showed that a strong fluorescence emission is detectable for TNP only when this is converted to [TNPH]+: we call this acido-modulation of the fluorescence response of a Lewis base in cells. Imaging experiments in cancer cells suggested that a strong fluorescence emission is detectable for the protonated species TNPH+ in the presence of iron(III) at moderate concentrations (50 μM), within a comparable order of magnitude with that of commercial chemosensors based on chelators, and of relevance to the detection of free ion on concentration scales, of ca. 18 μM in plasma ion concentrations.
1 (ESI,† Fig. S3). TNP is therefore very selective and sensitive for aqueous Fe3+ which is essential to life as well as being earth abundant, and we hypothesised that this is due to the initial formation of the corresponding metal complex. In the case of aqueous FeCl3 (Scheme 1) this was followed by its decomposition to [TNPH]Cl in the wet solvents used (ESI†). [TNPH]Cl was also isolated by reacting TNP with 1.1 eq. of concentrated HCl (Scheme 1). The quantum yield of [TNPH]Cl in DMSO was measured to be 0.017, comparable with other naphtyl-based chromophores (ESI†). Taken together, data suggests that the initial capture of Fe3+ by the receptor results in a decrease of the electron-donating ability of the methyl-nitrogen, thus resulting in a reduction of the PET effect, and with time, (in the presence of H2O) in the formation of the protonated species [TNPH]Cl (as well as presumably [Fe(H2O)3(OH)3] and Fe(OH)3 and aqueous HCl). The protonated species could well form under differing condition too: specimens with the same unit cell parameters were isolated and characterised by X-ray diffraction from several different aqueous solvent mixtures (EtOH/H2O, DMSO, wet CHCl3) (Fig. 1 and ESI†). The onset of enhanced fluorescence upon treatment with an excess of aqueous FeCl3 was also observed in experiments carried out using 2-photon excitation in wet DMSO for steady-state emission spectra and excited state lifetime measurements. While the fluorescence emissions were remarkably different (ESI†), lifetime measurement via time correlated single photon counting (TCSPC) indicated that the lifetime point decays recorded in solutions (giving rise to data which was modelled to 1 component multi-exponential fitting) are in the region of ca. 5 ns for both [TNP] and [TNPH]Cl (the latter being generated in situ, from the TNP in the presence of excess FeCl3 in wet DMSO). Specifically, free [TNP] in solution showed a 4.92 ns (χ2 = 1.28) and, in the presence of aqueous FeCl3 (in situ protonation) giving rise to the protonated species [TNPH]+ increased to 5.56 ns (χ2 = 1.19) (ESI,† Fig. S24–S26). These results confirmed the iron-mediated protonation of methyl piperazine unit disrupts the PET turning on the fluorescence of the probe. Studies of the pH-dependent response of TNP were also carried out over a pH range of 3.0–9.0 (ESI,† Fig. S7). We explored the ability of TNP towards in vitro conversion to [TNPH]+ in the acid environment of lysosomes. The cellular environment of the new probe was determined in HeLa and PC-3 cancer cells using confocal fluorescence spectroscopy and fluorescence lifetime imaging microscopy (FLIM) (Fig. S27 and S28, ESI†), which is used as a sensitive analytical tool for the probes' environment with added advantages when using multiphoton imaging techniques, i.e. the use of tissue-friendly low energy near infra-red photon rather than UV excitation for live cells. The pH evaluation showed that between pH 7.4–9.0 (well within the biologically relevant pH range 6.5–8.5) the emission of free TNP is turned off in aqueous solutions. On the contrary, at lower pH, [TNPH]Cl, a highly fluorescent and kinetically stable organic molecule, was formed. This new lipophilic cation may be employed further to measure pH changes. However, such sensing experiments are beyond the scope of this study. Cells were incubated at 37 °C with TNP and visualised using single photon, epi- and confocal-fluorescence microscopies (Fig. 3 and ESI†) and two-photon confocal fluorescence microscopy coupled with fluorescence lifetime imaging microscopy (FLIM Fig. 4 and ESI.† Cellular assays were prepared according to standard methods:9,34,37–41 generally, cells were incubated with TNP in the presence or absence of FeCl3 as well as with [TNPH]Cl solutions for 15 minutes in serum free medium (SFM). After washing with PBS, the cells were imaged using laser scanning confocal fluorescence microscopy (in single- or two-photon excitation modes). Control experiments (Fig. S15 and S16, ESI†) were carried out in cells incubated with serum free medium alone, or with 1 to 2% DMSO in SFM). The confocal imaging results regarding the iron-mediated fluorescence enhancement in cells were most promising using an excitation wavelength of 405 nm (single-photon excitation) or 810 nm (under two-photon excitation). The imaging studies also indicated that TNP alone passes through cell membranes but its fluorescence is extremely weak. Single-photon, as well as two-photon microscopy experiments, were repeated in a PC-3 (a prostate cancer cell line), also incubated at 37 °C (ESI†). In both HeLa and PC-3, in the presence of an excess of aqueous FeCl3 (minimum 1 mM concentration), the characteristic and intense fluorescence emission and the corresponding lifetime of [TNPH]+ can be reliably observed away from that of cellular auto fluorescence. The experiments carried out at 4 °C did not show reliable uptake of TNP, either alone or in the presence of FeCl3. Imaging experiments of TNP alone with incubation times longer than 1 h or at concentrations higher than 100 μM (with 1–2% DMSO) did not show reliable results due to the lack of fluorescence emission and were not pursued. The ability of [TNPH]+ to localise in the acidic intracellular organelles in vitro was investigated in both HeLa and PC-3 cells using 1P- and 2P-confocal fluorescence microscopies and FLIM. Co-localisation studies were carried out using LysoTracker Red CMXRos, and confirm the ability of [TNPH]+ to target the acidic organelles and be co-localised in the lysosomes (Fig. 5 and ESI†). Control experiments were carried out on cells incubated with DMSO and TNP alone (10 μM) at various temperatures and compared with probe-free control experiments in cells (incubated with SFM, or with 1 to 2% DMSO in SFM). In conclusion, imaging experiments in cancer cells reliably showed that a strong fluorescence emission is detectable for TNP only when this is converted to [TNPH]+: we call this acido-modulation of the fluorescence response of a Lewis base in cells. Imaging experiments in cancer cells suggested that a strong fluorescence emission is detectable for the protonated species TNPH+ in the presence of iron(III) at moderate concentrations (50 μM), within a comparable order of magnitude with that of commercial chemosensors based on chelators, and of relevance to the detection of free ion on concentration scales, of ca. 18 μM in plasma ion concentrations.
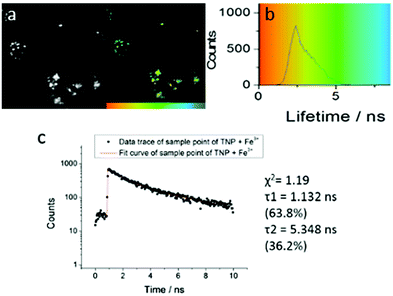 | ||
| Fig. 4 Two-photon FLIM of TNPHCl (showing the same cluster of HeLa cells as above, albeit with slightly shifted field of view due to instrument alignment) formed from TNP in the presence of excess aqueous FeCl3 (37 °C, 50 μM, 1% DMSO, 15 minutes incubation, 1% DMSO of TNP, 5 eq. FeCl3). Images show: (a) 2P fluorescence lifetime FLIM map (λex = 810 nm); (b) corresponding lifetime distribution curve (ns) showing an average lifetime distribution (τm) of 2.40 ± 1.25 ns, (c). A representative point decay trace corresponding to the blue cursor whereby the value of the τ2 component (ca. 5 ns, similar to the lifetime of free TNP in solution) is suggestive of a TNP core present within the lysosomal cells' regions (ESI†). | ||
In conclusion, we showed the dual capability of the naphthalimide-based probe in the sensitive detection Fe(III) in vitro, and its application in the selective visualization of the acidic lysosomes using FLIM. This highlights the potential of TNP as a chemosensor for lysosomal tracking, confirmed by co-localisation tests. Further studies are in progress to identify the likely cytotoxicity: ESI† gives initial MTT tests in a range of cells and conditions. Our ongoing interest is to establish a trend for TNP behaviour in the presence of other trivalent ions e.g. of relevance to molecular imaging in vivo such as 67Ga or 68Ga species for nuclear medicine as diagnostics, as these would likely mimic the behaviour of trivalent iron. The responsiveness of TNP upon protonation within intracellular compartments as well as in tumour microenvironments are our ongoing investigations.
S. I. P. is grateful to the EC for the ERC Consolidator fellowship O2SENSE (617107). T. D. J. and S. I. P. thank the Royal Society for funding. T. D. J. thanks ECUST for a Guest Professorship. T. D. J. and M. L. are grateful for financial support from China Scholarship Council (CSC) and University of Bath Full Fees Scholarship. The Catalysis and Sensing for our Environment (CASE) network is thanked for research exchange opportunities. W. H. Z. is grateful for financial support from the Oriental Scholarship and the Fundamental Research Funds for the Central Universities (WK1013002). M. L. thanks the NSF of China (21607044), NSF of Hebei Province (B2017502069) and Fundamental Research Funds for the Central Universities (2016MS108). SWB acknowledges Laserlab-Europe EU-H2020 654148. We thank Yue Wu (ECUST) for contributions in preliminary stages. We thank the EPSRC Mass spectrometry (Swansea) and the National X-ray Crystallography (Southampton) Services.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Zhou, Z. Zhou, A. Gong, Y. Zhang and Q. Li, Talanta, 2015, 143, 107–113 CrossRef CAS PubMed.
- B. Sui, S. Tang, T. Liu, B. Kim and K. D. Belfield, ACS Appl. Mater. Interfaces, 2014, 6, 18408–18412 CAS.
- B.-Y. Chen, C.-C. Kuo, Y.-S. Huang, S.-T. Lu, F.-C. Liang and D.-H. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 2797–2808 CAS.
- G. Mun, S. H. Jung, A. Ahn, S. S. Lee, M. Y. Choi, D. H. Kim, J.-Y. Kim and J. H. Jung, RSC Adv., 2016, 6, 53912–53918 RSC.
- K. Zheng, K. L. Lou, C. H. Zeng, S. S. Li, Z. W. Nie and S. Zhong, J. Photochem. Photobiol., 2015, 91, 814–818 CrossRef CAS PubMed.
- Q. Zou, X. Li, J. Zhang, J. Zhou, B. Sun and H. Tian, Chem. Commun., 2012, 48, 2095–2097 RSC.
- Z.-Q. Guo, W.-Q. Chen and X.-M. Duan, Org. Lett., 2010, 12, 2202–2205 CrossRef CAS PubMed.
- Y. Ma, W. Luo, P. J. Quinn, Z. Liu and R. C. Hider, J. Med. Chem., 2004, 47, 6349–6362 CrossRef CAS PubMed.
- M. Li, H. Ge, R. L. Arrowsmith, V. Mirabello, S. W. Botchway, W. Zhu, S. I. Pascu and T. D. James, Chem. Commun., 2014, 50, 11806–11809 RSC.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- D. Buccella, J. A. Horowitz and S. J. Lippard, J. Am. Chem. Soc., 2011, 133, 4101–4114 CrossRef CAS PubMed.
- P. A. Gale, S. E. García-Garrido and J. Garric, Chem. Soc. Rev., 2008, 37, 151–190 RSC.
- D. R. Richardson and P. Ponka, Biochim. Biophys. Acta, 1997, 1331, 1–40 CrossRef CAS.
- E. L. Mackenzie, K. Iwasaki and Y. Tsuji, Antioxid. Redox Signaling, 2008, 10, 997–1030 CrossRef CAS PubMed.
- Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC.
- F. Bousejra-ElGarah, C. Bijani, Y. Coppel, P. Faller and C. Hureau, Inorg. Chem., 2011, 50, 9024–9030 CrossRef CAS PubMed.
- J. Morrissey and M. L. Guerinot, Chem. Rev., 2009, 109, 4553–4567 CrossRef CAS PubMed.
- L. A. Ba, M. Doering, T. Burkholz and C. Jacob, Metallomics, 2009, 1, 292–311 RSC.
- J. Huang, Y. Xu and X. Qian, Org. Biomol. Chem., 2009, 7, 1299–1303 CAS.
- R. K. Jackson, Y. Shi, X. Yao and S. C. Burdette, Dalton Trans., 2010, 39, 4155–4161 RSC.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS PubMed.
- J. Cao, C. Zhao, X. Wang, Y. Zhang and W. Zhu, Chem. Commun., 2012, 48, 9897–9899 RSC.
- Y. Wei, Z. Aydin, Y. Zhang, Z. Liu and M. Guo, ChemBioChem, 2012, 13, 1569–1573 CrossRef CAS PubMed.
- D. Ghernaout, A. I. Al-Ghonamy, A. Boucherit, B. Ghernaout, M. W. Naceur, N. A. Messaoudene, M. Aichouni, A. A. Mahjoubi and N. A. Elboughdiri, Am. J. Environ. Prot., 2015, 4, 1–15 CrossRef CAS.
- R. Wang, F. Yu, P. Liu and L. Chen, Chem. Commun., 2012, 48, 5310–5312 RSC.
- B. Wang, J. Hai, Z. Liu, Q. Wang, Z. Yang and S. Sun, Angew. Chem., Int. Ed., 2010, 49, 4576–4579 CrossRef CAS PubMed.
- Z.-Q. Hu, X.-M. Wang, Y.-C. Feng, L. Ding, M. Li and C.-S. Lin, Chem. Commun., 2011, 47, 1622–1624 RSC.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed.
- H. Weizman, O. Ardon, B. Mester, J. Libman, O. Dwir, Y. Hadar, Y. Chen and A. Shanzer, J. Am. Chem. Soc., 1996, 118, 12368–12375 CrossRef CAS.
- K. Rurack, M. Kollmannsberger, U. Resch-Genger and J. Daub, J. Am. Chem. Soc., 2000, 122, 968–969 CrossRef CAS.
- Y. Liu, Y. Liu, Z. Xu, L. Yang, Z. Wang, D. Zhang and Y. Ye, Monatshefte für Chemie-Chemical Monthly, 2016, 147, 311–317 CrossRef CAS.
- Y. Xiao and X. Qian, Tetrahedron Lett., 2003, 44, 2087–2091 CrossRef CAS.
- J. J. P. Desvergne and A. W. Czarnik, Chemosensors of ion and molecule recognition, Springer, 1997 Search PubMed.
- S.-Y. Xu, X. Sun, H. Ge, R. L. Arrowsmith, J. S. Fossey, S. I. Pascu, Y.-B. Jiang and T. D. James, Org. Biomol. Chem., 2015, 13, 4143–4148 CAS.
- M. Ostermeier, C. Limberg and B. Ziemer, Z. Anorg. Allg. Chem., 2006, 632, 1287–1292 CrossRef CAS.
- A. Marzotto, D. A. Clemente and G. Valle, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 43–46 Search PubMed.
- Z. Hu, G. D. Pantoş, N. Kuganathan, R. L. Arrowsmith, R. M. Jacobs, G. Kociok-Köhn, J. O'Byrne, K. Jurkschat, P. Burgos and R. M. Tyrrell, Adv. Funct. Mater., 2012, 22, 503–518 CrossRef CAS.
- S. I. Pascu, P. A. Waghorn, T. D. Conry, B. Lin, H. M. Betts, J. R. Dilworth, R. B. Sim, G. C. Churchill, F. I. Aigbirhio and J. E. Warren, Dalton Trans., 2008, 2107–2110 RSC.
- S. I. Pascu, P. A. Waghorn, B. W. Kennedy, R. L. Arrowsmith, S. R. Bayly, J. R. Dilworth, M. Christlieb, R. M. Tyrrell, J. Zhong and R. M. Kowalczyk, Chem. – Asian J., 2010, 5, 506–519 CrossRef CAS PubMed.
- I. S. Alam, R. L. Arrowsmith, F. Cortezon-Tamarit, F. Twyman, G. Kociok-Köhn, S. W. Botchway, J. R. Dilworth, L. Carroll, E. O. Aboagye and S. I. Pascu, Dalton Trans., 2015, 45, 144–155 RSC.
- P. A. Waghorn, M. W. Jones, M. B. Theobald, R. L. Arrowsmith, S. I. Pascu, S. W. Botchway, S. Faulkner and J. R. Dilworth, Chem. Sci., 2013, 4, 1430–1441 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Syntheses, spectroscopy, ESI-MS, NMR, X-ray crystal data, cell culture, confocal and fluorescence lifetime data. CCDC 1542385. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc05166b |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2017 |