 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in the chemical imaging of human fingermarks (a review)
Qianhui
Wei
a,
Meiqin
Zhang
*a,
Božidar
Ogorevc
b and
Xueji
Zhang
*a
aResearch Center for Bioengineering and Sensing Technology, School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: zhangmeiqin@ustb.edu.cn; zhangxueji@ustb.edu.cn; Tel: (+)86-10-82375840
bDepartment of Analytical Chemistry, National Institute of Chemistry, Hajdrihova 19, 1000 Ljubljana, Slovenia
First published on 12th September 2016
Abstract
This review highlights the considerable advances in the chemical imaging of human fingermarks that provide more chemical information, including numerous endogenous and exogenous constituents. Despite remarkable development in DNA analysis and recognition, human fingermark analysis remains one of the priority approaches available for obtaining reliable forensic evidence. Additional information about the donor can be obtained from the chemical composition of latent fingermarks in addition to the ridge pattern, such as the age, gender, medical history, and possible drug habits. The analytical approaches reviewed here include spectroscopy, mass spectrometry, immuno-labelling and electrochemical methods. Each method has different capabilities with respect to sensitivity, reproducibility, selectivity, reliability and ultimately applicability, either for use in routine forensic practice or in academic research work. The advantages of spectroscopic techniques, including infrared, Raman and micro-X-ray fluorescence spectroscopy, are the capabilities of a rapid and non-destructive imaging of fingermarks by providing spectral information on chemical composition. In addition, mass spectrometry imaging can provide spatially specific information on fingermark chemical composition. Recently, the use of immuno-labelling in latent fingermark detection has attracted significant attention because it can overcome the sensitivity and selectivity problems experienced with other existing methods. The electrochemical method has also been employed to image latent fingermarks by measuring the electric current changes with the spatial chemical composition from the ridges and valleys at high resolution to provide a third level of detail, which is especially useful for multicoloured background surfaces or for surfaces contaminated with blood or other bodily fluids.
Introduction
Human fingermarks have been, and will continue to be, one of the most powerful types of forensic evidence available because they are left at a crime scene once suspects touch a surface. The characteristic patterns on the finger pads are unique to each individual and remain unchanged throughout a person's lifetime. Most fingermarks we encounter in daily life are called latent fingermarks (LFMs), which are not readily visible to the naked eye, so they require some specific treatments to visualize them for further forensic personal identification. Up to now, various chemical, physical and optical methods have been developed to enhance LFMs.1–3 However, most of the existing conventional methods can only enhance the physical ridge patterns of LFMs and sometimes fail to provide molecular information, such as the chemical composition, age and gender of the fingermark donor, personal hygiene, diet, the properties of the substrate and the environmental conditions, etc. Recently, more and more forensic researchers have begun to examine specific chemical information in fingermarks in addition to just the ridge patterns because of the possibility that a fingermark can provide more information about a person than just their identity.In order to improve the current techniques and to develop novel ones for detecting and identifying a LFM, it is highly required to understand that the mechanism of some visualization techniques react with specific chemical components in the natural secretions from the skin as well as with contaminants from the environment. There are three types of secretion glands in a human body, each of them produce a different kind of sweat, i.e. the eccrine, sebaceous and apocrine glands. Eccrine sweat glands are located all over the skin of the body but are also located in particular higher density at the palmar and plantar surfaces.4 The sweat secreted from eccrine glands consists of 98–99% water, various inorganic salts, such as chloride, bromide, iodide and phosphate, and of different organic compounds, such as amino and fatty acids, urea etc.5 Sebaceous glands are also found all over the skin except on the friction ridges of the palmar and plantar surfaces. They excrete sebum fluid, which is mainly composed of saturated fats, waxes and squalene.6 The apocrine glands are always located in the axillae and anogenital areas of the human body and they primarily excrete a viscous milky fluid.6 In fact, the chemical composition of fingermark residues varies qualitatively and quantitatively from one to another individual and depends on several aspects, such as sex, age, diet, physical condition, etc. These are referred to as endogenous composition factors, while numerous contaminants, such as cosmetics, food residues and drugs and their metabolites are considered as exogenous composition factors.
Spectroscopic techniques for the chemical imaging of human fingermarks
Spectroscopic imaging is an emerging technology that combines digital imaging and molecular spectroscopy, where high quality spectral and spatial information are collected over a period of time. It has been proven to have a wide variety of applications in scientific and industrial fields, one of which is forensic science. Of particular interest is the potential of chemical imaging to detect treated or even untreated latent fingermarks.7 Moreover, spectroscopic imaging can effectively serve to identify microscopic particles deposited within LFMs. For example, the application of chemical imaging would aid in identifying a suspect accused of building bombs by finding bomb-making substances in that person's LFMs. In another case where the accused person has used a gun, investigators would be able to prove who was holding the gun when it was fired by finding gunpowder deposits in the fingermarks. This could also help the positive identification of drug dealers and users by finding the illicit drugs in the LFMs of the accused individual. The main limitations of spectroscopic imaging as applied to the enhancement of fingermarks are its long imaging time (in the order of four hours) and the small LFM sample size, so it is not yet suitable to analyze a whole fingermark at present.8Herein, we mainly review the recent advances in the chemical imaging of LFMs by three types of techniques, including infrared absorption (mid-infrared and near-infrared radiation), Raman spectroscopy and micro-X-ray fluorescence (MXRF).
Infrared spectroscopy
Infrared (IR) spectroscopy is based on molecular absorptions that are characteristic of a specific molecular structure due to the chemical composition of the compound being studied. IR is an attractive detection technique in forensic applications for analyzing specific chemical components in fingermark residues because it is non-destructive and very often requires no additional sample treatment after collection.9 It can be used to identify the intrinsic chemical composition of materials from spectra produced by the radiation interaction with the samples.Williams et al. utilized IR spectromicroscopy, which is usually referred to as microscopic IR spectroscopy in the forensic community, for the analysis of latent fingermark deposits.10 In addition, Fourier transform infrared (FTIR) microspectroscopy is a non-invasive technology and is used to characterize the chemical composition in fingermark ridge residues over time, which is significant for investigating the difference between LFMs from children and adults. Grant et al. demonstrated that IR spectromicroscopy could locate and identify microscopic particles from a mixture of common substances in the LFMs by analyzing their unique infrared spectral signatures.11 The researchers believed that IR spectromicroscopy could be a powerful investigative tool not only to identify who was present at a crime scene, but also to relate a specific person to specific acts according to the foreign materials left in their fingermarks.
Among the available spectroscopic techniques, FTIR spectroscopy has been proven to be a powerful tool for forensic applications.12 Typically, FTIR chemical imaging uses a focal plane array detector to obtain images that consist of thousands of pixels, with an infrared spectrum at each pixel. Tahtouh et al. showed that FTIR spectroscopy could image larger fingermark samples up to several centimetres in size by combining it with an expanded field of view optics. They also reported that FTIR chemical imaging was more suitable for collecting the latent fingermark images from various difficult surfaces (including polymer banknotes, many types of paper and masking tapes) than conventional fingermark detection methods. The reason for this is that the contrast in FTIR chemical imaging is based on spectrochemical signal differences rather than the response of the surface to lighting conditions.13 Ricci et al. proved that attenuated total reflection (ATR) FTIR chemical imaging could obtain simultaneously physical visualization and chemical information of the fingermarks and could also be used to monitor the change in fingermark chemistry with temperature and time.14 Crane et al. developed a data-processing method of FTIR imaging for the non-invasive detection of both un-pretreated LFMs and trace evidence on challenging porous and non-porous substrates.15 They preserved the trace evidence contained in the fingermarks, which enabled the following analyst to make a direct connection between the fingermark and the trace evidence without changing the state of the evidence.
At some crime scenes, LFMs on textured, multicoloured or patterned substrates can be very difficult to be developed in situ, so it requires using an adhesive tape to lift them for further examination to be carried out in a lab or in a fingerprint bureau. Ricci et al. first obtained the IR chemical imaging of LFMs lifted with gelatin tapes from different kinds of surfaces (for example, door handles, mug handles, curved glass surfaces and computer screens).16 They minimized the interference from the gel lifters by utilizing ATR-FTIR imaging from different depths of the same sample with a variable angle ATR accessory.
Because conventional globar-sourced IR spectrometers cannot offer adequate sensitivity and spatial resolution required in fingermark analysis, synchrotron radiation-based FTIR (SR-FTIR) micro-imaging has been developed for exploring the molecular chemistry within the microstructures of microscopic particles owing to its high spatial resolution and brightness of the synchrotron source.17,18 Banas et al. demonstrated the potential of SR-FTIR micro-imaging to accurately identify solid microscopic particles with a diameter even down to 4 μm deposited within LFMs on various substrates commonly encountered in everyday life.17 They also imaged contaminated fingermarks left on hard-to-reach places using commercial foil as the lifting medium.
It is well known that the chemical compositions of fingermarks highly depend on age and differ obviously between children and adults. For example, it was found by using gas chromatography-mass spectrometry (GC-MS). That the fingermark compositions from children contained higher concentrations of volatile unesterified free fatty acids while adults had higher concentrations of less volatile fatty acids esterified with long-chain alcohols.19 Such findings that fingermark chemical compositions change with age led scientists to examine whether changes in composition could possibly be a function of age or not. In 2008, Hemmila et al. successfully demonstrated FTIR reflectance spectra in combination with a partial least-square regression and proved a linear relationship between a combination of constituents and age, with a significant shift around puberty.20 This means this technique could be effectively employed to provide the age of a person leaving a LFM at a crime scene even if no other information is known, for example, even if database searches do not show a match. However, this methodology is only suitable for cases where the LFM was first located by a light source without damage. Later, Williams et al. examined children's LFMs as a function of time and temperature by means of FTIR microspectroscopy.21 The results revealed that the salts in their fingermark residues were more stable than the esters due to the thermal stability. Hence, the salts could easily be found and measured over time at crime scenes.
Furthermore, it was reported that by using IR spectroscopic imaging, it was also possible to separate superimposed latent fingermarks and detect inter-ridge trace evidence non-invasively.22Fig. 1 displays two overlaid fingermark images developed by using cyanoacrylate fuming. Due to the difference in chemical composition of the two fingermarks, the spectral variations in the absorbance of the CH stretching and other vibrational modes can be easily observed. In particular, the fingermark on the left-hand side contains an excess of one oily substance (ester), while the fingermark on the right does not demonstrate any unique peaks and shows only a variation in peak intensities. Therefore, this shows that the spectra could be used to distinguish and identify overlaid fingermarks due to the different ratios of their chemical components by setting specific modes.
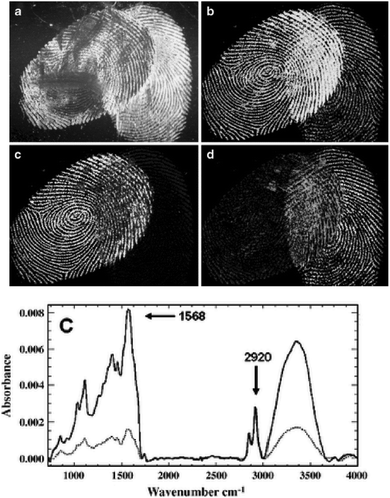 | ||
| Fig. 1 (a) Cyanoacrylate-developed latent fingermarks that are superimposed. (b) Infrared absorbance distribution using the CH stretching mode at nearly 2940 cm−1. (c) The carbonyl band shown at nearly 1730 cm−1. (d) The ratio of the carbonyl band at nearly 1730 cm−1 and the amide band at nearly 1640 cm−1. (e) Examples of spectra using different vibrational modes from the oil-rich region (top, dark line) and flake rich region.22 Reproduced from ref. 22 with permission from Springer. | ||
Raman spectroscopy
Raman spectroscopy is a powerful spectroscopic technique used to observe vibrational, rotational and other low-frequency modes in molecular systems.23 It is non-destructive and requires little or no sample pre-treatment. The narrow Raman bands allow straightforward spectral identification and the subtraction of background bands without loss of analyte information. Day et al. reported the application of Raman spectroscopy for the detection of illicit drugs and other exogenous substances in latent fingermarks.24 Later, they also extended this technique to detect exogenous substances in a fingermark beneath a layer of cyanoacrylate polymer.25 Illicit drugs within latent fingermarks, which have been first developed with powder and then lifted with tape, have been successfully detected by Raman spectroscopy, even inside thin plastic evidence bags.26 These combined techniques, that is Raman spectral mapping, tape-lifting and multivariate analysis, have also been used to detect chemical information and trace amounts of materials secreted from latent fingermarks.27 The challenge in conventional detection is that some lifting media can induce strong spectral interferences that may cover the weak spectral signals of the trace materials contained in the fingermarks. To overcome this shortcoming, the band-target entropy minimization (BTEM) method was proposed. Here, all pure component spectra of both the lifting media and tape-lifted materials can be recovered via BTEM and identified by subsequent comparison with the known spectra from the corresponding libraries. Tripathi et al. demonstrated that an automated background subtraction algorithm could overcome the background interference for the detection of trace amounts of exogenous explosive material in fingermark residues, so it is then possible to detect explosives on most surfaces encountered in forensic applications.28The sensitivity of conventional Raman spectroscopy is still not high enough for its routine application due to the small Raman scattering cross-section. With the objective of improving its performance, the surface-enhanced Raman scattering (SERS) technique was developed for detecting and identifying a wide range of substances and showed an enhancement of over 100-fold over the conventional technique.29 SERS imaging can provide chemical information for clean, aged or fresh, latent fingermarks, especially on difficult surfaces or on LFMs exposed to conditions that would make a latent fingermark undetectable via the traditional visualization techniques.30 The distinction of SERS imaging from FTIR imaging and other conventional methods is that the amino acids in eccrine fingermarks can be used rather than basing the analysis on the response of the oils’ common hydrocarbon signals to provide the image. In 2012, Song et al. demonstrated for the first time that proteins can be readily detected within latent fingermarks by using the SERS imaging.31 An antibody-bound Raman probe modified with silver nanoparticles (ASNRPC) enabled binding to specific proteins present in fingermarks to afford high-definition SERS images of the fingermark pattern. This study proved that SERS imaging was capable of sensitively determining different proteins within a fingermark. Fig. 2 shows SEM images of the fingermark with human IgG incubated by the ASNRPC. The SEM and XPS measurements show that Ag nanoparticles have successfully linked to the fingermark ridges, which is due to there being more human IgG deposited on the ridges of the fingermarks than in the valleys.
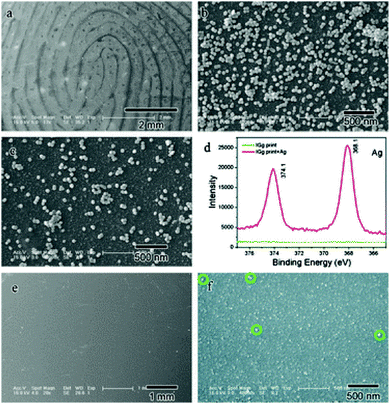 | ||
| Fig. 2 SEM images of a fingermark with human IgG incubated by the ASNRPC: (a) with a lower and (b) higher magnification on the ridges and (c) beside the ridges; (d) XPS spectra of the fingermark with human IgG before and after incubation with the ASNRPC; SEM images of the fingermark with human IgG before incubation with the ASNRPC: (e) with a lower and (f) higher magnification.31 Reproduced from ref. 31 with permission from the Royal Society of Chemistry. | ||
In addition, a compact Raman spectrometer assembled by a green laser pointer, an air cooled intensified charged coupled device and an x–y motorized translation stage was well developed and applied for the detection of liquids and the solid particles of explosives and for the chemical imaging of residues contained in latent fingermarks in a short acquisition time (in a few seconds).32 This system was proven to be sensitive enough to identify masses as low as ∼1 ng, which make it a potential candidate for taking the place of the existing Raman microscopes.
Raman and IR-based spectroscopy can provide information on the functional groups of the molecules analyzed; however, identification of the species may prove difficult without consulting a database or if the compound is not present in the database. Therefore, further testing with more recent innovations in spectral similarity measures is required.
Micro-X-ray fluorescence
The spatial elemental imaging characteristics of micro-X-ray fluorescence (MXRF) have been exploited to enhance the visual contrast between a fingermark and the substrate surface based on the detection of the inorganic elements (potassium, chlorine, etc.) contained in the print residue. A main advantage of MXRF is the capability to image fingermark ridges and examine valuable chemical information simultaneously, which is beneficial for forensic analysis. Some prepubescent children's fingermarks are particularly difficult to be detected as a function of time because they do not contain sebum and consist of relatively volatile free fatty acids.33 In addition, fingermarks left on dark or multicoloured backgrounds can be problematic for their sensitive visualization with conventional development methods, owing to their poor contrast with the surface colour.7,10 Thus, the imaging of fingermarks based on their elemental composition by utilizing MXRF could be valuable for detecting these problematic types of fingermarks, because the surface colour is not an interference in MXRF imaging. Worley et al. reported that MXRF could image the fingermark residues collected by tape lifters at a crime scene without disturbing their subsequent chemical analysis.34 Moreover, some of the organic compounds present in fingermarks can volatilize or bleed into porous surfaces, which make it difficult for the conventional methods to work, especially when the fingermarks have been left for a long time. In contrast, a fingermark 7.5 months old containing lotion could still be analyzed by detecting the chlorine and potassium maps of the friction ridges using MXRF.34Mass spectrometry techniques for chemical imaging of human fingermarks
Mass spectrometry techniques are more spatially specific than the above-mentioned spectroscopy through measuring the molecular weight of the molecules. They can select the species of interest and obtain their structural information for compound identification in both targeted and untargeted approaches. Mass spectrometry imaging (MSI) has been proven to provide complementary information to conventional fingerprint development methods.35 Up to now, a number of analytical approaches have been developed for providing chemical corroborative information of fingermarks by mass spectrometry, including gas chromatography-mass spectrometry (GC-MS), desorption electrospray ionization-mass spectrometry (DESI-MS), surface-assisted laser desorption ionization-mass spectrometry (SALDI-MS), matrix-assisted laser desorption ionisation-mass spectrometry (MALDI-MS), time-of-flight-secondary ion mass spectrometry (ToF-SIMS), direct analysis in real time-mass spectrometry (DART-MS), and capillary electrophoresis-mass spectrometry (CE-MS). MSI can not only show the physical shapes but can also provide information on the endogenous (such as free fatty acids, cholesterol esters and squalene) and exogenous substances of fingermarks, which should be useful for the identification of known or unknown molecules.19 However, MS facilities are expensive and often incompatible with some other fingermark developers at a crime scene.Desorption electrospray ionization-mass spectrometry
Ifa et al. reported the first MS chemical imaging of latent fingermarks providing information on the spatially specific chemical composition on surfaces.36 DESI-MS imaging provided the analysis of contact residues and endogenous components by mapping the distribution of such analytes over the surface of the fingermark.36 In DESI, a solvent is electrosprayed onto the surface. Secondary scattered droplets are generated and evaporated off, and their constituents mass analyzed. A mass spectrum is recorded in turn at each point on the surface by rastering the stream of charged droplets across the surface. Fig. 3 displays the image of a fingermark contaminated with cocaine on a substrate. Fig. 3a shows the distribution of cocaine (monitored as the mass-to-charge ratio, m/z, 304) in a latent fingermark on glass, showing clearly the distinction of the ridges and minutiae. Fig. 3c is an ink fingermark of the same individual pressed on paper. The similar patterns show that the MS analysis allows the physical identification of a subject. Mirabelli et al. reported the application of DESI-MS for the analysis and imaging of latent fingermarks containing condom-derived traces.37 The application of this approach in the analysis of trace compounds associated with condom use in sexual assault cases could provide evidence for a victim's claims that an assault has taken place. | ||
| Fig. 3 (a) DESI-MS image of the distribution of cocaine on an LFM blotted on glass. (b) Computer-generated fingermark from the DESI image. (c) Ink fingermark blotted on paper and optically scanned. (d) Computer-generated fingermark from the optical image. Some of the automatically detected points of interest (minutiae) are represented by dots in (b) and (d).36 Reproduced from ref. 36 with permission from the American Association for the Advancement of Science. | ||
Surface-assisted laser desorption ionization-mass spectrometry
As mentioned before, one of the critical tasks is to locate latent fingermarks at a crime scene. The dusting of surfaces with fingermark powder is the most commonly used methods. Surface-assisted laser desorption ionization (SALDI)-MS was introduced to address these circumstances. Laser desorption ionization (LDI) is an energy-transfer process that can create small inorganic and organic gaseous ions when an analyte is interrogated with a laser. SALDI can absorb the laser energy and transfer this energy to the analyte for desorption. It also shields the analyte from direct laser impact, reducing analyte fragmentation and aiding in the ionization of the analyte, thus enabling detection.38 It was reported that SALDI-time-of-flight-mass spectrometry (ToF-MS) could be employed for detecting latent fingermarks pre-dusted with magnetized carbon black doped silica nanoparticles.39 The excreted drugs, namely nicotine and their metabolites, were able to be detected in the pre-dusted lifted fingermark and the distribution of such contact residues could also be visualized over the surface of the lifted fingermark using SALDI-ToF-MS, although there did not seem to be a simple correlation between the number of cigarettes consumed per day and the nicotine peak intensity.39,40 The nicotine peak at m/z 163 is shown in Fig. 4. This was recorded from a dusted and then lifted fingermark from a smoker donor.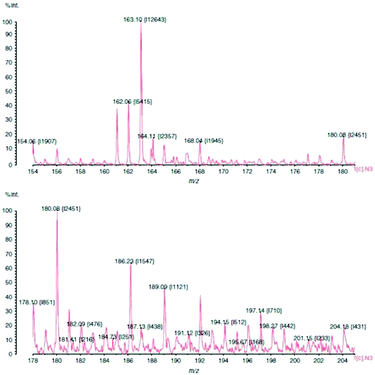 | ||
| Fig. 4 SALDI-MS plot of a smoker's fingermark showing a nicotine peak at m/z 163. The print was dusted using hydrophobic silica powder and lifted with a conductive adhesive tape.40 Reproduced from ref. 40 with permission from Wiley. | ||
Exogenous pharmaceutical drugs, such as terbinafine – an anti-fungal medicine – in latent fingermarks could also be detected and imaged by SALDI-ToF-MS after first being developed using carbon black doped silica particles.41 Medicine signatures in latent fingermarks provide clues to the lifestyle and other useful information of a donor individual. In cases when pre-dusted lifted fingermarks cannot be analyzed for certain drugs via the SALDI-MS method, the re-dusting or spraying of the prints with carbon black doped silica particles can be successfully applied.42 The development of latent fingermarks using cyanoacrylate fuming is a widely used forensic tool when the effects of a thick and rigid layer adhering to the substrate makes it difficult to visualize the developed prints. SALDI-ToF-MS can both enhance these developed latent fingermarks and detect drug residues by means of exposing them to acetone vapour after dusting with a commercial black powder.43 The use of this enhancement agent provides a unique superiority over other techniques, which enables the fingermarks to be located and lifted prior to the analysis.
Matrix-assisted laser desorption ionization-mass spectrometry
Among the different mass spectrometry techniques, MALDI-MS is a particularly suitable technique with the capability of detecting high molecular weight molecules (up to 1 million Da).44 MALDI enlists the use of a matrix to aid in the desorption and ionization process of LDI, which enhances the yield of the analyte ions. With the increased analyte ion yield, tandem MS can be subsequently carried out to provide structural information on the molecule of interest. For the first time in 2009, MALDI-MS imaging was used to detect endogenous lipids (such as cholesterol, palmitoleic acid, etc.) present in fingermarks, allowing images of the ridge patterns to be obtained by mapping the distribution of components in groomed and ungroomed (not sebum-enriched) fingermarks, thus demonstrating the sensitivity required for analyzing latent fingermarks found at real crime scenes.45 The researchers also detected several exogenous substances (dimethyldioactadecylammonium contaminants originating from personal and household products). Francese et al. applied this technique to map the fingermark ridge pattern and detect condom lubricant within the fingermark itself simultaneously for the identification of sexual assault suspects.46 The results showed the powerful ability of MALDI-MS to relate a suspect by identification through imaging the fingermark ridge pattern with the crime by detecting the exogenous substances, including cocaine and condom lubricants, left in the fingermark in one analysis. Further elaboration led to a two-step matrix application process, where α-cyano-4-hydroxycinnamic acid (α-CHCA) acts as a dusting powder in the first step, allowing additional fluorescence images to be taken and fingermarks to be lifted and making the technique amenable to real crime scene investigations.47 Thereafter, this group further extended MALDI-MS imaging to separate overlapping fingermarks using ion signals from endogenous or exogenous species in nature.48Fig. 5 demonstrates that MALDI-MS is able to distinguish between the fingermarks of two different fingermark donors and can even provide information as to which donor's fingermark resulted from fingertip contamination with the condom lubricant. As can be observed, the spectrum of the groomed fingermark (donor 2) does not show the presence of nonoxynol-9, which is a known spermicidal contained in Trojan Enz condoms (Fig. 5c). The complete image of this impression (on the left-hand side in Fig. 5a) was obtained by imaging many other molecular species that were absent in the condom-contaminated fingermark. The mass spectra in Fig. 5b and c were visualized by simply selecting, during the processing stage, a raster point in the overall image acquired for each of the two ridge impressions. This technique was able to distinguish the marks from the two donors and to identify which donor's fingermark resulted from fingertip contamination with the condom lubricant. This intelligence would be valuable in such a criminal investigation. In 2015, Francese et al. investigated a large range of drugs and their metabolites in fingermarks by MALDI-MS detection and by using mapping capabilities.49 Some prior treatment, such as cyanoacrylate fuming or vacuum metal deposition, does not prevent the detection of illicit drugs and their metabolites.49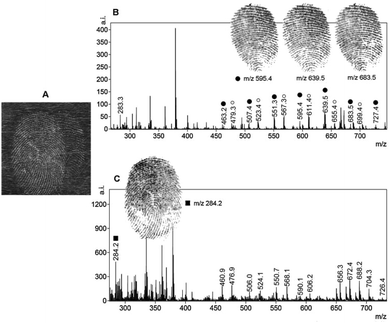 | ||
| Fig. 5 MALDI-MS separation of overlapping fingermarks achieved through the presence of an exogenous contaminant. (a) The optical image of the dusted overlapping fingermarks deposited by two different donors. Mass spectrum image of a “condom-contaminated fingermark” (donor 1) and a groomed fingermark (donor 2). (b) The spermicidal lubricant ion signals at m/z 595.4, 639.5 and 683.5 shown as images (they are distinctive of the fingermark whose donor has contaminated his/her fingertips with the condom). (c) The spectral profile of the groomed (non-contaminated) fingermark is apparently different and the ion signal at m/z 284.2 was selected to achieve separation from the condom-contaminated fingermark.48 Reproduced from ref. 48 with permission from Elsevier. | ||
Time-of-flight secondary ion-mass spectrometry
Recently, ToF-SIMS has gradually become one of the most sensitive analytical techniques in surface science. With this technique, the sample surface is bombarded with a focused high energy primary ion beam (1–40 keV), causing the desorption of secondary ions. A mass spectrometry-based image is then produced by rastering the ion beam across the sample surface. Depending on the ion dose, the technique can be very surface sensitive, i.e. down to 1–2 monolayers, and with a finely focused primary ion beam, can have a high lateral resolution of the order of a few tens of nanometres. It has also been introduced in the forensic field to link ToF-SIMS fingermarks to the evidence found at a crime scene, such as reliable age prediction of a latent fingermark.50 Szynkowska et al. visualized and analyzed fingermarks that were contaminated by different drugs left on four kinds of surfaces.51 The results demonstrated that the ToF-SIMS technique can be a very effective tool in the chemical investigations of fingermarks, and can help ascertain both the identification of a drug and its distribution over the fingermark. It is also suitable for the observation of very small regions of the fingermark examined and for the analysis of micro-objectives within fingermarks. Later, they also applied ToF-SIMS to detect the exogenous contaminants of fingermarks.52Fig. 6 shows the fingermark images obtained with ToF-SIMS, which illustrates its capability of visualizing a general arrangement of dermal ridges, terminals and sweat pores. | ||
| Fig. 6 The 5 × 5 mm SIMS images of fingermark friction ridges showing that the minutiae required for forensic fingermark analysis are observable.52 Reproduced from ref. 52 with permission from Wiley. | ||
ToF-SIMS has also been developed to identify the order of depositions. For example, in the case of a fingermark overlapping with an ink line on the paper, it is possible to determine whether the fingermark had been laid down before or after the ink was deposited.53 The basic principle of this approach is to take line scans of the fragment ions characteristic for the ink molecules (m/z 358.2 and 372.2), where the fingermark and ink overlap, and to calculate the normalized standard deviation of the intensity variations across the line scan. In addition, the mapping of selected endogenous elements and molecular species within fingermarks, such as sodium, potassium and C3H5 positive ions, can reveal the friction ridge patterns in fingermarks.54 Imaging ToF-SIMS could be used as a final step to enhance the visualization of LFMs in cases where samples have not been fully recovered using conventional reagents, such as by cyanoacrylate fuming.55
However, a drawback of ToF-SIMS is the necessity to introduce samples into a vacuum chamber, which is known to cause changes to fingermark chemistry (e.g. reduction of several fatty acids and changes of the concentration of lipids and squalene) and to increase the time (and cost) per analysis.55
Other mass spectrometry approaches
In 2011, a study using laser desorption/ionization time-of-flight-mass spectrometry (LDI/TOF-MS) established a list of triglycerides (TAGs), which are esters derived from glycerol and three fatty acids, found in fingermark residue.56 Gender differences in the TAGs composition were assessed; however, as isomeric triglycerides have the same mass, an exact identification could not be determined based only on the m/z value obtained through LDI/TOF-MS. In addition, illicit drug residues from fingermarks were determined via direct analyte-probed nano-extraction coupled to nanospray ionization-mass spectrometry (DAPNE-NSI-MS) without damaging the ridge details.57 Furthermore, some tandem high-resolution MS approaches can provide useful information in the chemical imaging of latent fingermarks in forensic field. For example, multiplex MS imaging using the hybrid linear iontrap–orbitrap mass spectrometry methodology can detect and image the distribution of both endogenous and exogenous compounds, even when sample specimens are very limited.58,59 Forbes and co-worker utilized desorption electro-flow focusing ionization (DEFFI) mass spectrometry to image the chemical distributions of endogenous (fatty acids) and exogenous compounds (explosives, narcotics and lotions) in deposited and lifted artificial fingermarks.60 As compared to DESI, DEFFI provides a unique combination of low applied pressure (atmospheric conditions) and applied potential operation, electrospray and chemical ionization, and minimal sample preparation. Tang et al. applied laser-activated electron tunnelling (LAET) MS to image latent fingermarks by visualization of the spatial distribution of these molecular ions and structural information-rich fragment ions on a special tight and shining film of semiconductors.61 LAET provides higher spatial resolution than that of regular MALDI by overcoming the interferences of the MALDI matrix materials, which cause ion suppression and limited spatial resolution. The researchers demonstrated the potential capability of the LAET-MS approach, including the identification of physical shapes, detection of endogenous metabolites and deconvolution of overlapped fingermarks as well as investigation of the contacts with drugs or other prohibited substances. Fig. 7 shows that LAET-MS imaging is able to resolve overlapped latent fingermarks based on chemical differences. Fig. 7(B) is the LAET-MS imaging of an ion extracted at m/z 164.0427 of an unknown molecule that has higher abundance in the left person's fingermark. Fig. 7C is the LAET-MS imaging of an oestrogen dienestrol extracted at m/z 265.1240. It is shown that the right person has a much higher level of dienestrol than that of the left person. Moreover, two estrogens, namely dienestrol and diethylstilbestrol, have been identified in female fingermarks with intensities comparatively higher than those in male fingermarks. Although sex determination based on the hormonal level in LFMs is not that reliable because of its variation with physiological status, diet and age, this technique offers the possibility of monitoring prohibited hormone abuses in cosmetic skin care products.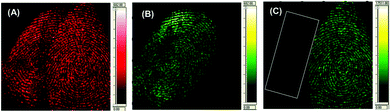 | ||
| Fig. 7 (A) LAET-MS imaging of two overlapped latent fingermarks at m/z 311. (B) Resolved imaging at m/z 164.0427 for an unknown molecule. (C) Resolved imaging at m/z 265.1240 for dienestrol hormone.61 Reproduced from ref. 61 with permission from American Chemical Society Publications. | ||
Recently, Walton et al. utilized soft-landing ion mobility (SLIM) to deposit silver clusters as a MALDI matrix instead of traditional α-CHCA for the detection and imaging of low mass illicit chemical compounds without causing damage to the ridge patterns.62 This novel method improved the signal-to-noise ratio compared with conventional CHCA for melatonin and tryptamine and showed great promise for fingermark-imaging applications. Laser desorption imaging (LDI)-MS is able to detect and image numerous endogenous and exogenous compounds by an approach involving nanometre-sputtered silver on fingermarks on conductive and non-conductive substrates.63 The silver layer renders the analysis surface conductive, which allows the detection of latent fingermarks on non-conductive surfaces such as paper. This approach allows the detection of [M + Ag]+ ionized endogenous and also [M + Na]+ exogenous substances, providing high-resolution imaging MS and a higher sensitivity for several endogenous substances, such as fatty acids. The result shows the possibility to identify pathogens or other biological agents carried by suspects directly from their fingermarks.
Non-imaging mass spectrometry techniques
GC-MS, direct analysis in real time mass spectrometry (DART-MS) and capillary electrophoresis-mass spectrometry (CE-MS) are not capable of providing the imaging detection of the chemical information of a fingermark; however, they play a significant role in providing chemical information in forensic science.GC-MS was one of the most commonly used approaches in most of the early works for studying the endogenous substances and chemical changes within latent fingermark deposits (such as amino and fatty acids) that happen over time.64,65 It was found that the amount of materials deposited in latent fingermarks was significantly different between individuals and also changes with time for the same individual.64 The detection of the relative amounts of intrinsic residue components can benefit the development of a reliable method for predicting the age of fingermarks. For example, squalene and cholesterol compositions were investigated as possible indicators for the age of fingermarks.14,66 The variations of some other components may be used to uniquely identify an individual. Via GC-MS analysis, different ratios of several fatty acid methyl esters were found for individuals from different races and genders.67 Michalski et al. proposed that these data could also be due to the small sample size and that a larger sample set was needed to determine the true legitimacy of the observed trends.67 GC-MS typically requires dissolution of the analyte, which consumes a large portion of the sample and precludes subsequent imaging, microscopic examination and preservation of the structure of the fingermark.68
DART-MS is a powerful tool that can be applied for the identification of non-DNA trace evidence associated with sexual assault since it is extremely fast and samples are processed immediately, without the need for sample preparation or other procedures that might compromise the future analysis. DART has been applied to the analysis of Ranitidine, which is used to treat a sour stomach, in less than 30 s in urine.69 DART is a prominent ambient ionization method that employs a gas-phase ionization mechanism, and by its very nature consumes a minimal amount of sample. In 2012, Musah et al. demonstrated the ability of DART-MS to identify lubricant compounds used in condom manufacturing (such as the spermicide nonoxynol-9) and numerous other trace components associated with sexual assault.70 They also applied this technique to identify fatty acids in latent fingermarks. This methodology offered a comprehensive, rapid and sensitive fingermark analysis for forensic use.
CE-MS, a hyphenated MS system, is also available for detecting chemical compositions in fingermarks.71 Amino acids are suitable for CE separation due to their zwitterionic nature.72 There are many ways of measuring amino acids and other components by CE-MS or CE in various biological matrices, including in tissue and sweat.73,74 Although the amounts of total amino acids within fingermark samples are very low, ranging from about 21 to 345 ng per fingermark, the residues can be successfully analyzed by CE-MS.71
Immuno-labelling methods for the chemical imaging of human fingermarks
Nanoparticles functionalized with antibodies
Antibodies are soluble proteins that belong to the glycoprotein family. An antigen can be any substance where the capacity to bind specifically to constituents of an immune response. Antibodies are highly specific to individual antigens due to the unique three-dimensional structures developed at the antigen binding site.The detection of a drug present in a fingermark may indicate that an individual has come into contact with that drug, but this is not enough to prove the use of that drug because it cannot be excluded that the drug was deposited on the latent fingermark at a certain time after it had been generated. An effective solution to solve this problem was proposed by Russell et al. that involved using gold nanoparticles functionalized with antibodies.75 In this research, it was demonstrated that the gold nanoparticles functionalized with an antibody specific to cotinine, the main metabolite of nicotine, and could be used to detect cotinine present in the fingermarks of smokers and to simultaneously obtain an image of the fingermark. The anti-cotinine antibody was bound to the nanoparticles functionalized with protein A, which was present in the fingermark, and then a secondary antibody fragment, tagged with a fluorescent dye, was used to fluorescently label the fingermark. The high-resolution image was obtained using a fluorescence stereomicroscope after the fingermark was deposited by an individual who smoked cigarettes. A simple preparation of antibody-functionalized gold nanoparticles is illustrated in Fig. 8.75 In 2008, Russell et al. showed that, through the use of the antibody–magnetic–particle conjugates, various drugs and drug metabolites (such as Δ9-tetrahydrocannabinol, methadone and its metabolites) could be readily detected in latent fingermarks imaged by using either brightfield and/or fluorescence microscopy.76 This ability to examine a change in colour of a fingermark by using brightfield microscopy offers an option for the detection of latent fingermarks to use a simple white light source instead of expensive instrumentation, thereby providing a simple, portable method for in situ crime investigations. Later, Russell et al. further reported the use of magnetic particles functionalized with anti-cotinine antibodies to image cotinine present in latent fingermarks deposited by smokers and demonstrated that it was possible to establish the identity of an individual within only 15 minutes.77 Then, they illustrated that metabolites from two different types of drugs, for example, morphine and benzoylecgonine, can be detected simultaneously in a single fingermark using white light and/or a fluorescence light source.78 In a further development, antibody–magnetic particle conjugates for the detection of cotinine were successfully applied for the development of latent fingermarks on a highly reflective white porcelain surface, which is difficult to be enhanced by conventional techniques.79 They also reported that these antibody–particle conjugates could be an excellent technique for developing aged fingermarks as well.80Fig. 9 displays fingermarks aged for up to and including 4 weeks at room temperature in the presence of light after being developed using the anti-cotinine magnetic conjugates. The research carried out by Russell's group showed great promise for the development of latent fingermarks as well as for gaining extra information on drug use. However, the targeting drug metabolites limit the use of this method as a general approach since these compounds are not universal components present in latent fingermark deposits.
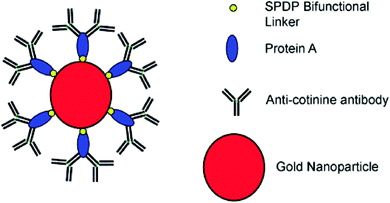 | ||
| Fig. 8 Representation of the antibody–nanoparticle conjugates. The conjugates are formulated via depositing the protein A, which acts as a biological linker to orientate the anti-cotinine antibody on the gold particle surface. SPDP = N-succinimidyl 3-(2-pyridyldithio) propionate is served as a bifunctional linker.75 Reproduced from ref. 75 with permission from Wiley. | ||
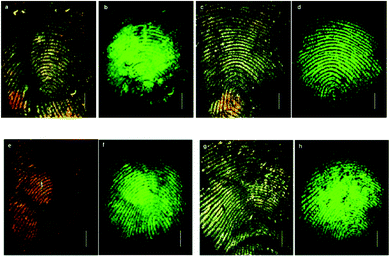 | ||
| Fig. 9 Detection of fingermarks after ageing at room temperature in the presence and absence of light. Brightfield (a) and fluorescence (b) images of fingermarks after ageing for 3 days in the presence of light. Brightfield (c) and fluorescence (d) images after ageing for 4 weeks in the presence of light. Brightfield (e) and fluorescence (f) images of fingermarks after ageing for 3 weeks in the absence of light. Brightfield (g) and fluorescence (h) images after ageing for 4 weeks in the absence of light.80 Reproduced from ref. 80 with permission from the Royal Society of Chemistry. | ||
Some other investigations in this field have greatly improved the immuno-labelling visualization of fingermarks. Spindler et al. targeted free amino acids, such as enantioselective anti-L-amino acid, to detect latent fingermarks on non-porous surfaces.81 In this work, anti-L-amino acid antibodies were conjugated to gold nanoparticles (16 nm in diameter) by either direct electrostatic adsorption or by a covalent amide-bonding to a thioether. This antibody-based system is particularly effective for enhancing aged and degraded fingermarks. van Dam et al. described for the first time that the multiple immune-labelling of antibodies allowed the simultaneous detection of components in a single fingermark.82 They applied the simultaneous multiple immuno-labelling of antibodies such as anti-dermcidin and the antibody of human serum albumin, that can identify specific components in the secretions present in fingermarks on both porous and non-porous substrates. This group was also successful in finding the compatibility of immuno-labelling with two different fingermark visualization techniques: magnetic powdering and ninhydrin staining, which was then investigated with fingermarks deposited on glass and on nitrocellulose membranes.83 They concluded that both the fingermark visualization techniques examined, i.e. magnetic powdering and ninhydrin staining, did not influence the immuno-labelling of fingermarks. Very recently, van Dam et al. reported an investigation on the applicability of immuno-labelling for developing fingermarks left on a variety of substrates, such as non-porous (aluminium foil, stainless steel keys, plastic sheets, different coloured garbage bags, sandwich bags, Ziploc bags), semi-porous (tiles, laminated chipboard) and porous (thermal and copy paper) surfaces.84 Except for the laminated chipboard and copy paper, the immuno-labelling of specific components in fingermarks was performed well on most of the forensic relevant surfaces.
Multi-metal-deposition (MMD), which was introduced to the forensic field by Saunders, is a very sensitive method for visualizing latent fingermarks on both porous and non-porous surfaces.85 Recently, Su et al. reported a novel technique that integrates the advantages of the conventional MMD method with the immunoassay technique (iMMD).86 Compared to the conventional MMD method, iMMD is faster (less than an hour), facile, not expensive to operate and can provide additional chemical information than just identification.
Drapel et al. proposed a method of using an antibody to detect and visualize latent fingermarks by targeting certain skin proteins.87 In order to identify these proteins, three antibodies directed against human proteins were tested using western blots of the fingermark residues: anti-keratin 1 and 10 (K1/10), anti-cathepsin-D (Cat.D) and anti-dermcidin (Derm.). These antigens are three of the most abundant proteins involved in the desquamation process during skin regeneration. As a result, fingermarks were detected on all the substrate types including the three antibodies. It was noted that the antigens from the desquamation process (keratins 1 and 10; cathepsin-D), the polyvinylidene fluoride (PVDF) membranes, as depicted in Fig. 10, and the standard whitened and non-whitened papers all produced prints with very precise ridge edges, while the antigens from sweat (cathepsin-D; dermcidin) produced marks with strong dotted patterns, which could be attributed to the pores of the skin.
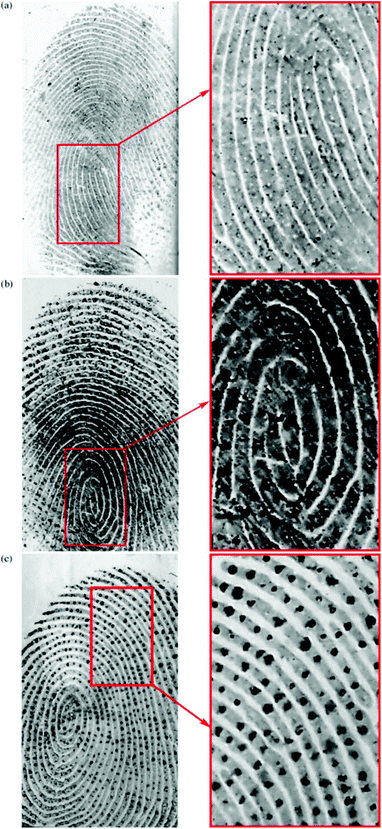 | ||
| Fig. 10 Mixed fingermarks apposed on a PVDF membrane and immuno-detected with (a) anti-keratin 1/10, (b) anti-cathepsin-D and (c) anti-dermcidin.87 Reproduced from ref. 87 with permission from Elsevier. | ||
Aptamer-based methods
Aptamers are oligonucleotide or peptide molecules that bind to a specific target molecule. They were initially developed in 1990 to isolate rare ribonucleic acids from random sequence pools that showed a strong affinity to specifically chosen targets.88 Through the formation of specific and stable three-dimensional structures, aptamers are able to bind a number of targets.89 The affinity and specificity shown by aptamer–target binding complexes have been found to be higher than those of antibody–antigen complexes.90Wood et al. first developed this technique for the detection and visualization of latent fingermarks by using lysozyme-specific aptamer-based reagents on a PVDF membrane.91 This aptamer-based reagent is highly selective and sensitive compared to conventional methods. In the presence of lysozyme, the aptamers fold into their specific three-dimensional structures, thus allowing them to bind to the lysozyme present in the latent fingermarks. Fig. 11 presents the enhancement of a fingermark, which can provide first and second level detail by using an aptamer-based reagent.
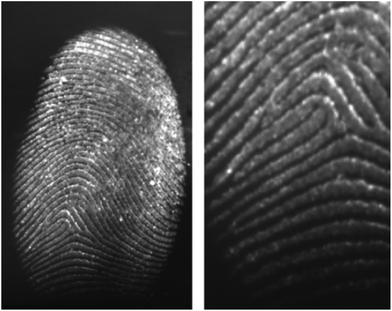 | ||
| Fig. 11 A charged latent fingermark on a PVDF substrate developed by using the aptamer-based reagent. The fingermark was aged for 24 hours before it was developed with the lysozyme aptamer 1 reagent containing CAL Fluor® Orange 560 as the fluorescent tag.91 Reproduced from ref. 91 with permission from Wiley. | ||
Li et al. demonstrated a nanoplasmonic method to visualize latent fingermarks by using the localized surface plasmon resonance (LSPR) property of aptamer-bound gold nanoparticles.92Fig. 12 indicates the detection principle. They found that the presence of cocaine in latent fingermarks could induce the aggregation of gold nanoparticles, which resulted in true colour changes of the scattered light in dark-field microscopy (DFM) images, thus providing colourful images of LFMs. As the LSPR images of gold nanoparticles are much more intense than the fluorescent, luminescent and electrochemiluminescent signals, they enable higher spatial resolution in the imaging of latent fingermarks. The researchers then expanded the idea of nanoplasmonic imaging to identify 1,3,5-trinitro-1,3,5-triazinane (RDX) explosive residues in LFMs by exploring the competitive reduction of Cu2+ and RDX by a reduced form of nicotinamide-adenine dinucleotide (NADH).93
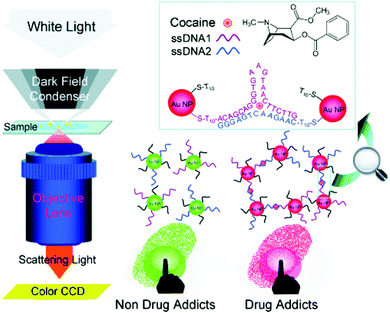 | ||
| Fig. 12 Principle of the nanoplasmonic imaging of latent fingermarks and the identification of cocaine in latent fingermarks by DFM.92 Reproduced from ref. 92 with permission from Wiley. | ||
In addition, some other nanoparticles can also be functionalized with aptamers, such as upconversion nanoparticles (UCNPs). UCNPs are able to convert two or more near-infrared pump photons into a higher energy output photon through sequential electronic excitation and energy-transfer processes. Because the compositions in various substrates cannot be excited by near-infrared light, the imaging of latent fingermarks with UCNPs suffers little from background fluorescence interference, thus offering apparent optical contrast to allow high detection sensitivity. In 2014, Wang et al. combined the advantages of using UCNPs functionalized with an aptamer and near-infrared-light-mediated imaging to detect lysozyme and cocaine present in fingermarks and to develop a general and easy-to-perform strategy for latent fingermark detection based on molecular recognition.94 UCNPs possess the ability of suppressing the background fluorescence and thus making it possible for fingermark imaging to be performed on some problematic surfaces. This design possesses the potential to be used for various kinds of exogenous substance detection by simply changing the type of aptamer.94
Recently, Ran and co-workers reported the use of DNA-modulated silver nanoclusters (AgNCs) combined with molecule-binding aptamers to visualize and detect endogenous and exogenous components on LFMs for the first time.95 The emissions of the AgNCs are regulated by the nearby DNA region, resulting in a multi-colour image of the LFMs that can be visualized under excitation by the naked eye and recorded by fluorescence microscopy. This promising protocol is simple and cost-effective as the AgNCs do not need be further functionalized when the analytes are changed. Zhao et al. developed a sensitive and universal protocol to image various types of fingermarks (including sebaceous, eccrine, fresh LFMs and aged LFMs) on different substrates (such as smooth, scratching, semi-porous and porous surfaces) by combining sandwiched SERS probes with aptamers.96 The structures of the nanoprobes (Au/pNTP/SiO2-lysozyme-binding aptamer) were helpful for avoiding environmental interference of the SERS signal and for improving the efficiency of the fingermark identification.
However, sample treatment with immunogenic and nucleic acid reagents requires careful operation to inhibit the non-specific adsorption, and also the quality of the eventual ridge development is strongly dependent on the substrate surface where the fingermark is deposited. The major disadvantage of the targeted-methodology though is that it can only provide relative single information per analysis that may be not applicable at the crime scene.
Electrochemical detection methods
Scanning electrochemical microscopy (SECM) is a technique that has been designed for observing the local electrochemical behaviours at liquid/solid, liquid/gas and liquid/liquid interfaces.97,98 It is capable of imaging the chemical activities present at a substrate surface to provide chemical information that is complementary to the information obtained from some other microscopic techniques that display mainly the topography of the sample surface, such as scanning electron microscopy (SEM) and atomic force microscopy (AFM). Moreover, the conventional optical methods of visualizing latent fingermarks do not work at their optimum level in some cases; for example, in the case of object surfaces with unique characteristics: surfaces with multicoloured backgrounds, surfaces contaminated with blood or other body fluids and other porous or non-absorbent surfaces.In 2007, Zhang et al. first applied SECM for imaging latent fingermarks based on the detecting of silver stained proteins on the PVDF membrane.99 The operating principle of the SECM imaging of treated latent fingermarks on a PVDF membrane is presented in Fig. 13. In the amperometric feedback mode, the SECM probe signal used for fingermark imaging is the Faradaic current resulting from the oxidation of the mediator IrCl63−, at the tip potential of +0.8 V. When the probe is scanned near the black ridges of the silver stained fingermark, an electrochemical recycling of IrCl63− becomes possible by a heterogeneous bimolecular electron transfer reaction between IrCl62− and the silver particles. This process induces an increase in the probe current signal (the positive feedback effect), while the current response monotonically decreases when the probe approaches to the valley part of the sample because IrCl63− diffusion is then prevented (the negative feedback effect). These changes in the tip current response during lateral scans in SECM imaging reflect the topology of the fingermark on the surface. This high-resolution SECM imaging of latent fingermarks can provide the third level of valuable information for confirming forensic identifications. Moreover, this method is also compatible with the previous detection techniques for bloody and protein-contained LFMs. At present, the main shortcomings of SECM lie in its long imaging time (a few hours) and small LFM sample size and the fact that it only provides relative single information per analysis. The SECM technique is not applicable to imaging fingermarks after treatment with conventional development methods.
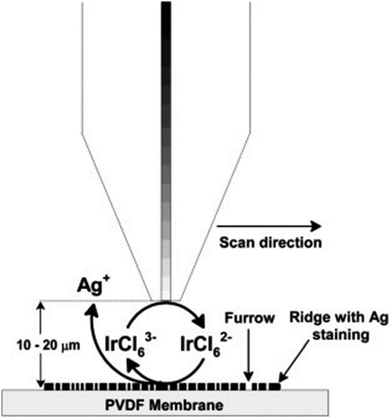 | ||
| Fig. 13 Schematic (not to scale) of the operating principle for SECM imaging of silver stained fingermarks on the PVDF membrane using IrCl63− oxidation (Etip = 0.8 V vs. Ag QRE) at the tip.99 Reproduced from ref. 99 with permission from the Elsevier. | ||
Electrochemiluminescence (ECL) is a controllable form of chemiluminescence where light emission is initiated via an electrochemical reaction, which is a superior way of imaging electrochemical and biosensing events on different surfaces.100 In conjunction with the enzyme-linked immuno-sensing methodologies, ECL can enhance the visualization of latent fingermarks through the recognition of specific proteins/polypeptides, such as epidermal growth factor (EGF), lysozyme and dermcidin.101 Moreover, the approach described in this work could easily be adapted to fabricate portable hand-held devices. The main limitation of ECL is its low image resolution and the fact it can only offer relative single information per analysis compared with MS-based chemical imaging methods. Also, the ECL technique is not applicable to imaging fingermarks after treatment with conventional development methods.
Conclusions
The identification of human fingermarks has long been a vital point of criminal investigation and forensic science. Forensic researchers are continually aiming to develop novel methods that are fast, precise, simple, low-cost, friendly, non-invasive, portable and compatible with conventional fingerprint development for obtaining personal chemical information and identification. Accurate fingermark identification is of great significance in providing further chemical analysis about a fingermark donor's age, race, gender, diet habit, etc. In general, extracting chemical information from a fingermark is far more important than just physically enhancing the fingermark. Herein, we reviewed the recent developments in the chemical imaging of latent fingermarks by using various methods, i.e. spectroscopy, mass spectrometry, immuno-labelling and electrochemistry. Several MS techniques without imaging capabilities mentioned above (e.g. GC-MS, CE-MS, DART-MS) have also been covered in this review as they play a significant role in providing information on the chemical components in fingermarks.As might be expected, all of these techniques have advantages and disadvantages depending on how the analysis needs to be performed and what or how the analytes can be detected. In Table 1, a schematic overview is presented, describing the main techniques, including their advantages and disadvantages. Raman and IR-based methods can provide information on the functional groups of the molecules analyzed, but identification of the species may prove difficult without consulting a library or if the compound is not present in the library. Mass spectrometry is a powerful way to identify compounds with high accuracy compared to other methods, but MS-based methods still suffer from some disadvantages that could restrict their wide use, such as high initial investment costs and lack of portability.108 Even so, MS techniques will continue to play an important role in this field because of their unique characteristic of providing chemical and imaging information simultaneously. The use of immuno-labelling for the detection of latent fingermarks is a relatively new area that has been developed as a promising tool to overcome the sensitivity and selectivity issues currently encountered with the existing methods. However, the tagging technique is complicated due to it requiring different tagged particles to be prepared for each target molecule. Electrochemical methods have shown great potential to provide high resolution and sensitive images of latent fingermarks or of bloody fingermarks on multicoloured surfaces, which are often problematic for optical techniques.
| Advantages | Disadvantages | Spatial resolution | Compounds | ||
|---|---|---|---|---|---|
| Spectroscopy | Infrared spectroscopy | - Can be applied after lifting the fingermarks16 | - Proven to be difficult to do without consulting a library or if the compound is not present in the library102 | 18 μm (ref. 103) | - Endogenous (amino acids), exogenous (drug abuse) compounds and lipids14,104 |
| Raman spectroscopy | - Non-destructive and requires no sample preparation24 | - Intense fluorescence signals exhibited by many substrates, which can cover the desired chemical information106 | 10 μm (ref. 28) | - Endogenous (amino acids and proteins) compounds, explosives, drugs of abuse and polymer lubricants24,28,30,31,104 | |
| - Can be applied after lifting the fingermarks24 | |||||
| - Portable105 | |||||
| Micro-X-ray fluorescence | - Can be applied on aged fingermarks33 | - Relatively slow pixel acquisition rate107 | 10 μm (ref. 105) | - Inorganic elemental constituents, such as Na, Mg, Si, S, Cl, etc.34 | |
| - Complements the current spectroscopy and mass spectrometry techniques used by forensic investigators34 | |||||
| Mass spectrometry | MALDI-MS | - The matrix CHCA can be used as a dusting agent for latent fingerprint recovery during an investigations47 | - Need vacuum conditions55 | 10 μm (ref. 102) | - Endogenous (such as fatty acids, peptides, proteins) and exogenous (drugs, polymer lubricants and beauty product contaminants) compounds45,46,49 |
| - Can be applied on lifted fingermarks40 | - Strong background peaks in the low mass region108 | ||||
| - Uneven distribution of matrix crystals with different sizes and shapes61,102 | |||||
| SALDI-MS | - The agent can be used as a dusting agent for latent fingerprint recovery during an investigation39 | - The surface influences the analysis | Down to 10 μm | - Exogenous (pharmaceutical drugs, such as terbinafine, illicit drugs, such as cocaine and nicotine) species39–41 | |
| - Can be applied on lifted dusting fingermarks39 | |||||
| DESI-MS | - DESI flexibility and high-speed analysis can help to speed-up crime scene forensic investigation37 | - The high pressure gas can result in unwanted redistribution or smearing of analytes on certain surfaces60 | 150 μm (ref. 36) | - Drugs, explosives, inks on documents, polymer lubricants and endogenous compounds (fatty acids)36,37 | |
| - No sample pre-processing prior to analysis37 | |||||
| ToF-SIMS | - Superior spatial resolution and sensitivity52,108 | - High vacuum conditions may result in changes to the fingerprint chemistry, also, an increased time per analysis109 | Down to 1 μm (ref. 53) | - Endogenous (fatty acids and squalene) and exogenous (gunshot residues, drugs, toxic substances, such as As2O3) compounds52,110 | |
| - No sample pre-processing prior to analysis52 | |||||
| LAET-MS | - High spatial resolution and quantitative capability to detect small molecules61 | - Not applicable at crime scenes61 | 5 μm (ref. 61) | - Small molecule endogenous compounds (fatty acids, hormones)61 | |
| DEFFI-MS | - Minimal sample pre-processing in atmospheric conditions60 | - Difficulty in repairing the source if it becomes damaged or clogged111 | - Endogenous (fatty acids) and exogenous (explosives, narcotics and lotions) compounds60 | ||
| SLIM-MALDI-MS | - Improved signal-to-noise ratio compared with conventional CHCA62 | - The technique costs a lot due to coinage metal clusters62 | - Low mass chemical compounds (fatty acids, beauty product contaminants and illicit and pharmaceutical drugs)62 | ||
| - Silver cluster powder can be used as a dusting agent for latent fingerprint recovery during investigations62 | |||||
| Immunoassay method | Immuno-labelling | - Compatible with fingermark techniques76,83 | - Background staining101 | 1 μm (ref. 113) | - Endogenous (amino acids) compounds and exogenous (cotinine, methadone, etc.) compounds75,76,113 |
| - Not applicable at crime scenes112 | |||||
| Antigen | - Has an affinity and specificity to detect components in fingermarks91 | - Surfaces of the detection basements need complex pre- and post-treatments96 | A few hundred nanometres94 | - Endogenous (proteins) and exogenous (explosive residues, illicit drugs) compounds91–93 | |
| Electrochemical method | Scanning electrochemical microscopy | - Sensitive and overcomes the issue of problematic backgrounds114 | - Not applicable at crime scenes | 20 μm (ref. 114) | - Proteins and polypeptides114 |
Although a number of techniques exist that allow for the detection of specific components in fingermarks, there is still a gap between forensic research in the lab and forensic practice at a crime scene. In future, methods that are reliable, portable, fast, low-cost, sensitive, selective and non-destructive are highly needed for visualizing latent fingermarks physically and for imaging them chemically.
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (No. 21127007), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (The 45th) and the Fundamental Research Funds for the Central Universities (No. 06199046). B. Ogorevc also thanks for the support of the Slovenian Research Agency (P1-0034).References
- M. J. Choi, T. Smoother, A. A. Martin, A. M. McDonagh, P. J. Maynard, C. Lennard and C. Roux, Forensic Sci. Int., 2007, 173, 154–160 CrossRef CAS PubMed.
- D. T. Burns, J. K. Brown, A. Dinsmore and K. K. Harvey, Anal. Chim. Acta, 1998, 362, 171–176 CrossRef CAS.
- G. Qin, M. Zhang, Y. Zhang, Y. Zhu, S. Liu, W. Wu and X. Zhang, J. Electroanal. Chem., 2013, 693, 122–126 CrossRef CAS.
- K. Saga, Prog. Histochem. Cytochem., 2002, 37, 323–386 CrossRef CAS PubMed.
- R. Byard and J. Payne-James, Encyclopedia of Forensic and Legal Medicine, Academic Press, 2015 Search PubMed.
- E. B. Beckett, G. H. Bourne and W. Montagna, J. Physiol., 1956, 134, 202–206 CrossRef CAS.
- D. L. Exline, C. Wallace, C. Roux, C. Lennard, M. P. Nelson and P. J. Treado, J. Forensic Sci., 2003, 48, 1047–1053 CAS.
- B. J. Reedy, C. Lennard, C. Roux, J. R. Kalman and M. Tahtouh, J. Forensic Sci., 2005, 50, JFS2004213–JFS2004219 Search PubMed.
- H. U. Gremlich, Infrared and Raman spectroscopy, in Ullmann's encyclopedia of industrial chemistry, 2000 Search PubMed.
- D. K. Williams, R. L. Schwartz and E. G. Bartick, Appl. Spectrosc., 2004, 58, 313–316 CrossRef CAS PubMed.
- A. Grant, T. Wilkinson, D. R. Holman and M. C. Martin, Appl. Spectrosc., 2005, 59, 1182–1187 CrossRef CAS PubMed.
- M. Tahtouh, J. R. Kalman, C. Roux, C. Lennard and B. J. Reedy, J. Forensic Sci., 2005, 50, 64–72 CrossRef CAS PubMed.
- M. Tahtouh, P. Despland, R. Shimmon, J. R. Kalman and B. J. Reedy, J. Forensic Sci., 2007, 52, 1089–1096 CrossRef CAS PubMed.
- C. Ricci, P. Phiriyavityopas, N. Curum, K. Chan, S. Jickells and S. G. Kazarian, Appl. Spectrosc., 2007, 61, 514–522 CrossRef CAS PubMed.
- N. J. Crane, E. G. Bartick, R. S. Perlman and S. Huffman, J. Forensic Sci., 2007, 52, 48–53 CrossRef CAS PubMed.
- C. Ricci, S. Bleay and S. G. Kazarian, Anal. Chem., 2007, 79, 5771–5776 CrossRef CAS PubMed.
- A. Banas, K. Banas, M. B. Breese, J. Loke, B. Heng Teo and S. K. Lim, Analyst, 2012, 137, 3459–3465 RSC.
- P. Fritz, W. van Bronswjik, K. Lepkova, S. W. Lewis, D. E. Martin and L. Puskar, Microchem. J., 2013, 111, 40–46 CrossRef CAS.
- A. Girod, R. Ramotowski and C. Weyermann, Forensic Sci. Int., 2012, 223, 10–24 CrossRef CAS PubMed.
- A. Hemmila, J. McGill and D. Ritter, J. Forensic Sci., 2008, 53, 369–376 CrossRef CAS PubMed.
- D. K. Williams, C. J. Brown and J. Bruker, Forensic Sci. Int., 2011, 206, 161–165 CrossRef CAS PubMed.
- R. Bhargava, R. S. Perlman, D. C. Fernandez, I. W. Levin and E. G. Bartick, Anal. Bioanal. Chem., 2009, 394, 2069–2075 CrossRef CAS PubMed.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957–2976 CrossRef CAS PubMed.
- J. S. Day, H. G. Edwards, S. A. Dobrowski and A. M. Voice, Spectrochim. Acta, Part A, 2004, 60, 563–568 CrossRef.
- J. S. Day, H. G. Edwards, S. A. Dobrowski and A. M. Voice, Spectrochim. Acta, Part A, 2004, 60, 1725–1730 CrossRef PubMed.
- M. J. West and M. J. Went, Spectrochim. Acta, Part A, 2009, 71, 1984–1988 CrossRef PubMed.
- E. Widjaja, Analyst, 2009, 134, 769–775 RSC.
- A. Tripathi, E. D. Emmons, P. G. Wilcox, J. A. Guicheteau, D. K. Emge, S. D. Christesen and A. W. Fountain III, Appl. Spectrosc., 2011, 65, 611–619 CrossRef CAS PubMed.
- D. Cialla, A. März, R. Böhme, F. Theil, K. Weber, M. Schmitt and J. Popp, Anal. Bioanal. Chem., 2012, 403, 27–54 CrossRef CAS PubMed.
- R. M. Connatser, S. M. Prokes, O. J. Glembocki, R. L. Schuler, C. W. Gardner, S. A. Lewis Sr. and L. A. Lewis, J. Forensic Sci., 2010, 55, 1462–1470 CrossRef CAS PubMed.
- W. Song, Z. Mao, X. Liu, Y. Lu, Z. Li, B. Zhao and L. Lu, Nanoscale, 2012, 4, 2333–2338 RSC.
- I. Malka, A. Petrushansky, S. Rosenwaks and I. Bar, Appl. Phys. B: Lasers Opt., 2013, 113, 511–518 CrossRef CAS.
- K. M. Antoine, S. Mortazavi, A. D. Miller and L. M. Miller, J. Forensic Sci., 2010, 55, 513–518 CrossRef CAS PubMed.
- C. G. Worley, S. S. Wiltshire, T. C. Miller, G. J. Havrilla and V. Majidi, Powder Diffr., 2006, 21, 136–139 CrossRef CAS.
- B. Prideaux and M. Stoeckli, J. Proteomics, 2012, 75, 4999–5013 CrossRef CAS PubMed.
- D. R. Ifa, N. E. Manicke, A. L. Dill and R. G. Cooks, Science, 2008, 321, 805–805 CrossRef CAS PubMed.
- M. F. Mirabelli, A. Chramow, E. C. Cabral and D. R. Ifa, J. Mass Spectrom., 2013, 48, 774–778 CrossRef CAS PubMed.
- A. Y. Lim, J. Ma and Y. C. F. Boey, Adv. Mater., 2012, 24, 4211–4216 CrossRef CAS PubMed.
- F. Rowell, K. Hudson and J. Seviour, Analyst, 2009, 134, 701–707 RSC.
- M. Benton, F. Rowell, L. Sundar and M. Jan, Surf. Interface Anal., 2010, 42, 378–385 CrossRef CAS.
- A. Y. Lim and J. Seviour, Anal. Methods, 2012, 4, 1983–1988 RSC.
- A. Y. Lim, F. Rowell, C. G. Elumbaring-Salazar, J. Loke and J. Ma, Anal. Methods, 2013, 5, 4378–4385 RSC.
- L. Sundar and F. Rowell, Analyst, 2014, 139, 633–642 RSC.
- D. C. Schriemer and L. Li, Anal. Chem., 1996, 68, 2721–2725 CrossRef CAS PubMed.
- R. Wolstenholme, R. Bradshaw, M. R. Clench and S. Francese, Rapid Commun. Mass Spectrom., 2009, 23, 3031–3039 CrossRef CAS PubMed.
- R. Bradshaw, R. Wolstenholme, R. D. Blackledge, M. R. Clench, L. S. Ferguson and S. Francese, Rapid Commun. Mass Spectrom., 2011, 25, 415–422 CrossRef CAS PubMed.
- L. Ferguson, R. Bradshaw, R. Wolstenholme, M. Clench and S. Francese, Anal. Chem., 2011, 83, 5585–5591 CrossRef CAS PubMed.
- R. Bradshaw, W. Rao, R. Wolstenholme, M. R. Clench, S. Bleay and S. Francese, Forensic Sci. Int., 2012, 222, 318–326 CrossRef CAS PubMed.
- G. Groeneveld, M. de Puit, S. Bleay, R. Bradshaw and S. Francese, Sci. Rep., 2015, 5, 11716 CrossRef CAS PubMed.
- S. Muramoto and E. Sisco, Anal. Chem., 2015, 87, 8035–8038 CrossRef CAS PubMed.
- M. I. Szynkowska, K. Czerski, J. Rogowski, T. Paryjczak and A. Parczewski, Forensic Sci. Int., 2009, 184, 24–26 CrossRef PubMed.
- M. Szynkowska, K. Czerski, J. Rogowski, T. Paryjczak and A. Parczewski, Surf. Interface Anal., 2010, 42, 393–397 CrossRef CAS.
- N. A. Montalto, J. J. Ojeda and B. J. Jones, Sci. Justice, 2013, 53, 2–7 CrossRef PubMed.
- N. Attard Montalto, J. J. Ojeda and B. J. Jones, Sci. Justice, 2013, 53, 2–7 CrossRef CAS PubMed.
- N. J. Bright, T. R. Willson, D. J. Driscoll, S. M. Reddy, R. P. Webb, S. Bleay, N. I. Ward, K. J. Kirkby and M. J. Bailey, Forensic Sci. Int., 2013, 230, 81–86 CrossRef CAS PubMed.
- B. Emerson, J. Gidden, J. O. Lay and B. Durham, J. Forensic Sci., 2011, 56, 381–389 CrossRef CAS PubMed.
- K. Clemons, R. Wiley, K. Waverka, J. Fox, E. Dziekonski and G. F. Verbeck, J. Forensic Sci., 2013, 58, 875–880 CrossRef CAS PubMed.
- D. C. Perdian and Y. J. Lee, Anal. Chem., 2010, 82, 9393–9400 CrossRef CAS PubMed.
- G. B. Yagnik, A. R. Korte and Y. J. Lee, J. Mass Spectrom., 2013, 48, 100–104 CrossRef CAS PubMed.
- T. P. Forbes and E. Sisco, Analyst, 2014, 139, 2982–2985 RSC.
- X. Tang, L. Huang, W. Zhang and H. Zhong, Anal. Chem., 2015, 87, 2693–2701 CrossRef CAS PubMed.
- B. L. Walton and G. F. Verbeck, Anal. Chem., 2014, 86, 8114–8120 CrossRef CAS PubMed.
- N. Lauzon, M. Dufresne, V. Chauhan and P. Chaurand, J. Am. Soc. Mass Spectrom., 2015, 26, 878–886 CrossRef CAS PubMed.
- N. E. Archer, Y. Charles, J. A. Elliott and S. Jickells, Forensic Sci. Int., 2005, 154, 224–239 CrossRef CAS PubMed.
- R. S. Croxton, M. G. Baron, D. Butler, T. Kent and V. G. Sears, J. Forensic Sci., 2006, 51, 1329–1333 CrossRef CAS PubMed.
- C. Weyermann, C. Roux and C. Champod, J. Forensic Sci., 2011, 56, 102–108 CrossRef CAS PubMed.
- S. Michalski, R. Shaler and F. L. Dorman, J. Forensic Sci., 2013, 58(Suppl 1), S215–S220 CrossRef CAS PubMed.
- H. C. Lee and R. E. Gaensslen, Advances in Fingermark Technology, CRC Press, Boca Raton, 2nd edn, 2001, pp. 63–104 Search PubMed.
- R. B. Cody, J. A. Laramée and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS PubMed.
- R. A. Musah, R. B. Cody, A. J. Dane, A. L. Vuong and J. R. Shepard, Rapid Commun. Mass Spectrom., 2012, 26, 1039–1046 CrossRef CAS PubMed.
- T. Atherton, R. Croxton, M. Baron, J. Gonzalez-Rodriguez, L. Gamiz-Gracia and A. M. Garcia-Campana, J. Sep. Sci., 2012, 35, 2994–2999 CrossRef CAS PubMed.
- V. Poinsot, V. Ong-Meang, P. Gavard and F. Couderc, Electrophoresis, 2014, 35, 50–68 CrossRef CAS PubMed.
- N. Shama, S. W. Bai, B. C. Chung and B. H. Jung, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2008, 865, 18–24 CrossRef CAS PubMed.
- T. Hirokawa, H. Okamoto, Y. Gosyo, T. Tsuda and A. R. Timerbaev, Anal. Chim. Acta, 2007, 581, 83–88 CrossRef CAS PubMed.
- R. Leggett, E. E. Lee-Smith, S. M. Jickells and D. A. Russell, Angew. Chem., Int. Ed., 2007, 119, 4178–4181 CrossRef.
- P. Hazarika, S. M. Jickells, K. Wolff and D. A. Russell, Angew. Chem., Int. Ed., 2008, 47, 10167–10170 CrossRef CAS PubMed.
- P. Hazarika, S. M. Jickells and D. A. Russell, Analyst, 2009, 134, 93–96 RSC.
- P. Hazarika, S. M. Jickells, K. Wolff and D. A. Russell, Anal. Chem., 2010, 82, 9150–9154 CrossRef CAS PubMed.
- A. M. Boddis and D. A. Russell, Anal. Methods, 2011, 3, 519–523 RSC.
- A. M. Boddis and D. A. Russell, Anal. Methods, 2012, 4, 637 RSC.
- X. Spindler, O. Hofstetter, A. M. McDonagh, C. Roux and C. Lennard, Chem. Commun., 2011, 47, 5602–5604 RSC.
- A. van Dam, M. C. Aalders, K. van de Braak, H. J. Hardy, T. G. van Leeuwen and S. A. Lambrechts, Forensic Sci. Int., 2013, 232, 173–179 CrossRef CAS PubMed.
- A. van Dam, M. C. Aalders, T. G. van Leeuwen and S. A. Lambrechts, J. Forensic Sci., 2013, 58, 999–1002 CrossRef PubMed.
- A. van Dam, K. A. van Nes, M. C. G. Aalders, T. G. van Leeuwen and S. A. G. Lambrechts, Anal. Methods, 2014, 6, 1051 RSC.
- B. Schnetz and P. Margot, Forensic Sci. Int., 2001, 118, 21–28 CrossRef CAS PubMed.
- Y. He, L. Xu, Y. Zhu, Q. Wei, M. Zhang and B. Su, Angew. Chem., Int. Ed., 2014, 126, 12817–12820 CrossRef.
- V. Drapel, A. Becue, C. Champod and P. Margot, Forensic Sci. Int., 2009, 184, 47–53 CrossRef CAS PubMed.
- A. D. Ellington and J. W. Szostak, Nature, 1990, 346, 818–822 CrossRef CAS PubMed.
- R. Stoltenburg, C. Reinemann and B. Strehlitz, Biomol. Eng., 2007, 24, 381–403 CrossRef CAS PubMed.
- Y. Yang, D. Yang, H. J. Schluesener and Z. Zhang, Biomol. Eng., 2007, 24, 583–592 CrossRef CAS PubMed.
- M. Wood, P. Maynard, X. Spindler, C. Lennard and C. Roux, Angew. Chem., Int. Ed., 2012, 51, 12272–12274 CrossRef CAS PubMed.
- K. Li, W. Qin, F. Li, X. Zhao, B. Jiang, K. Wang, S. Deng, C. Fan and D. Li, Angew. Chem., Int. Ed., 2013, 52, 11542–11545 CrossRef CAS PubMed.
- T. Peng, W. Qin, K. Wang, J. Shi, C. Fan and D. Li, Anal. Chem., 2015, 87, 9403–9407 CrossRef CAS PubMed.
- J. Wang, T. Wei, X. Li, B. Zhang, J. Wang, C. Huang and Q. Yuan, Angew. Chem., Int. Ed., 2014, 53, 1616–1620 CrossRef CAS PubMed.
- X. Ran, Z. Wang, Z. Zhang, F. Pu, J. Ren and X. Qu, Chem. Commun., 2016, 52, 557–560 RSC.
- J. Zhao, K. Zhang, Y. Li, J. Ji and B. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 14389–14395 CAS.
- A. L. Barker, M. Gonsalves, J. V. Macpherson, C. J. Slevin and P. R. Unwin, Anal. Chim. Acta, 1999, 385, 223–240 CrossRef CAS.
- P. Sun, F. O. Laforge and M. V. Mirkin, Phys. Chem. Chem. Phys., 2007, 9, 802–823 RSC.
- M. Zhang and H. H. Girault, Electrochem. Commun., 2007, 9, 1778–1782 CrossRef CAS.
- A. B. Nepomnyashchii, A. J. Bard, J. K. Leland, J. D. Debad, G. B. Sigal, J. L. Wilbur and J. N. Wohlstadter, in Encyclopedia of Analytical Chemistry, Wiley, 2000 Search PubMed.
- L. Xu, Z. Zhou, C. Zhang, Y. He and B. Su, Chem. Commun., 2014, 50, 9097–9100 RSC.
- S. Francese, R. Bradshaw, L. S. Ferguson, R. Wolstenholme, M. R. Clench and S. Bleay, Analyst, 2013, 138, 4215–4228 RSC.
- R. Bradshaw, R. Wolstenholme, L. S. Ferguson, C. Sammon, K. Mader, E. Claude, R. D. Blackledge, M. R. Clench and S. Francese, Analyst, 2013, 138, 2546–2557 RSC.
- C. Ricci, K. A. Chan and S. G. Kazarian, Appl. Spectrosc., 2006, 60, 1013–1021 CrossRef CAS PubMed.
- E. L. Izake, Forensic Sci. Int., 2010, 202, 1–8 CrossRef CAS PubMed.
- A. Braz, M. López-López and C. García-Ruiz, Forensic Sci. Int., 2013, 232, 206–212 CrossRef CAS PubMed.
- D. L. Howard, M. D. de Jonge, D. Lau, D. Hay, M. Varcoe-Cocks, C. G. Ryan, R. Kirkham, G. Moorhead, D. Paterson and D. Thurrowgood, Anal. Chem., 2012, 84, 3278–3286 CrossRef CAS PubMed.
- X. Dong, J. Cheng, J. Li and Y. Wang, Anal. Chem., 2010, 82, 6208–6214 CrossRef CAS PubMed.
- M. J. Bailey, M. Ismail, S. Croxton, N. Bright, M. L. Elad, Y. Cohen, B. Geller, D. Everson, C. Costa, R. P. Webb, J. F. Watts and M. de Puit, Analyst, 2013, 138, 6246–6250 RSC.
- M. J. Bailey, N. J. Bright, R. S. Croxton, S. Francese, L. S. Ferguson, S. Hinder, S. Jickells, B. J. Jones, B. N. Jones, S. G. Kazarian, J. J. Ojeda, R. P. Webb, R. Wolstenholme and S. Bleay, Anal. Chem., 2012, 84, 8514–8523 CrossRef CAS PubMed.
- E. R. Sisco, Methods and standards for the analysis and imaging of latent fingerprints and trace contraband using ambient ionization mass spectrometry and secondary ion mass spectrometry, Doctoral dissertation, University of Maryland, College Park, 2014 Search PubMed.
- A. van Dam, F. T. van Beek, M. C. Aalders, T. G. van Leeuwen and S. A. Lambrechts, Sci. Justice, 2016, 56, 143–154 CrossRef PubMed.
- P. Hazarika and D. A. Russell, Angew. Chem., Int. Ed., 2012, 51, 3524–3531 CrossRef CAS PubMed.
- M. Zhang and H. H. Girault, Analyst, 2009, 134, 25–30 RSC.
| This journal is © The Royal Society of Chemistry 2016 |




