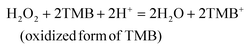Open Access Article
Open Access ArticleRecent progress of noble metal-based nanozymes: structural engineering and biomedical applications
Xiao
Wang†
,
Chenhao
Shu†
,
Gang
Wang
,
Peng
Han
,
Long
Zheng
,
Lei
Xu
and
Ye
Chen
 *
*
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China. E-mail: yechen@cuhk.edu.hk
First published on 19th March 2025
Abstract
Due to their tunable catalytic activity, high chemical stability, and favorable electronic structure, noble metal-based nanozymes that can mimic important biocatalytic processes have attracted great attention. Rational structural design of noble metal-based nanozymes can endow them with excellent enzyme-like activities, enhanced sensitivity and stability, as well as unique physicochemical functionalities towards various biomedical applications such as sensing, diagnostics, and disease treatment. This review summarizes the recent progress in structural engineering of noble metal-based nanozymes and emphasizes the relationship between key structural factors of nanozymes and their enzyme-like properties in various enzyme-mimicking reactions. The diverse applications of noble metal-based nanozymes in biosensors, antibiosis, and disease treatment are further introduced. Finally, current challenges and future research directions in noble metal-based nanozymes are discussed. This review could offer scientific guidance to design and fabricate advanced nanozymes with enhanced functionality and performance towards clinical, environmental and biomedical applications.
1. Introduction
Natural enzymes are efficient catalysts for organisms and play key roles in various metabolisms, but their shortcomings such as susceptibility, complex preparation, and high price limit their practical applications in industrial, medical, and biological fields.1 To overcome these disadvantages, researchers have devoted themselves to the exploration of artificial enzyme mimics.2 Traditionally, various proteins and organic molecules have been explored as stable and low-cost alternatives to natural enzymes, but they still suffer from unsatisfactory performances compared with natural enzymes.3 In recent years, nanozymes made of nanomaterials have received great interest owing to their tunable catalytic activities in mimicking the enzyme-like substrate-to-product conversion.4 Notably, nanozymes can integrate their excellent catalytic activities with other unique physicochemical properties (e.g., magnetic, optical, electrical properties) of nanomaterials, which often endows them with multiple functionalities.5 Among the various kinds of nanozymes developed to date, noble metals such as gold (Au),6–8 silver (Ag),9,10 platinum (Pt),11–13 palladium (Pd),14–16 iridium (Ir),17,18 ruthenium (Ru),19,20 and rhodium (Rh)12,21 are recognized as promising candidates due to their unique features such as excellent intrinsic catalytic activities,22 high chemical stability, and outstanding optical and photothermal conversion properties, as well as remarkable local surface plasmon resonance (LSPR) effect. To date, noble metal based-nanozymes have been reported effective in mimicking a variety of enzyme-like processes, like those of peroxidase (POD),23,24 catalase (CAT),25–27 oxidase (OxD),28,29 and superoxide dismutase (SOD).30,31 More importantly, noble metal-based nanomaterials often possess unique plasmonic,32,33 electrical,18 magnetic,34 and thermal35 properties that can be smartly integrated into multifunctional nanozymes by structural engineering. For example, plasmonic noble metal nanomaterials (Au, Ag, and Pd), whose optical spectra closely rely on their size, shape, and compositions, are widely used in photothermal therapy (PTT) and photodynamic therapy (PDT).35–38 Meanwhile, owing to the X-ray attenuation capability of noble metals (Au, Pt, Ir, etc.), noble metals are also ideal imaging contrast agents for X-ray computed tomography (CT) and photoacoustic imaging (PAI).39–41 Therefore, noble metal-based nanozymes have great potential to integrate the enzyme-like function with imaging, biosensing, and treatment via leveraging the synergistic effects of their unique optical and catalytic properties. As a result, noble metal-based nanozymes have proved very promising in a variety of biomedical applications such as biosensors,42,43 antioxidation,44,45 cancer therapy,46,47 immunoassays,28etc.The integrated enzyme-like behaviors of noble metal-based nanozymes were found to be closely related to their size,48 surface structure (e.g., facet, phase, and defect),49,50 ligand,51 and composition.52,53 For instance, reducing the size of nanozymes is an efficient strategy to optimize the catalytic efficiency with an enlarged specific surface area15,48 and optimized biocompatibility.54 Surface engineering is another important aspect to functionalize nanozymes by modulating their exposed facets,49 crystal phase,23,55 type of ligand,51,56 and defects,35,57 which would distinctly affect the interaction between the nanozyme surface and catalytic intermediates. With rational design and precise synthesis, the atom utilization efficiency, selectivity, activity, and multi-functionality of surface-engineered noble metal nanozymes could be greatly optimized.34,52,53 Moreover, constructing noble metal-based nanoheterostructures can further regulate the enzymatic activity and expand their potential bioapplications. By introducing a second metal or nonmetal components, nanoheterostructures can achieve various effects such as theragnostics, targeted therapy, and cascade biocatalysis.
Altogether, structural engineering is crucial for developing promising noble metal-based nanozymes with tailorable catalytic performances and multi-functionalities as well as expanded potential applications. In this review, we present an overview of the latest progress in the structural design, enzymatic behaviors, and biomedical applications of noble metal-based nanozymes (Fig. 1). First, the structural factors of noble metal nanozymes that affect enzymatic activity, selectivity, and stability are introduced. The additional functions brought by these structural factors are also discussed. Representative strategies to construct various noble metal-based heterostructures, including single-atom nanozymes (SAzymes), towards further enhanced enzyme-like properties and multiple functionalities are presented. Subsequently, the enzyme-like catalytic behaviors of noble metal-based nanozymes are summarized. Then, the applications of noble metal-based nanozymes in biosensors, antibiosis, and disease treatment are demonstrated. Finally, some major challenges and prospective opportunities in this research field are discussed.
 | ||
| Fig. 1 Schematic overview of the structural engineering, enzyme-like activities, and biomedical applications of noble metal-based nanozymes. | ||
2. Structural engineering for noble metal-based nanozymes
Understanding the roles played by structural factors of noble metal-based nanomaterials plays a fundamental role in revealing their structure–property–performance relationship. Great advances have been achieved in the past few years in understanding the effects of several structural factors, including size, facet, strain, crystallinity, alloying, and ligands, of noble metal nanozymes on enhancing their activity, selectivity, and multi-functionalities. In addition, heterostructures synthesized by incorporating a second component into noble metals have also emerged as promising nanozymes. Noble metal-based nanozyme heterostructures can induce geometric, compositional, and electronic effects, thereby optimizing the activity and bringing more intriguing possibilities. In section 2, we systematically summarize these strategies to realize structural engineering of noble metal-based nanozymes.2.1 Structural factors in noble metal nanozymes
 | ||
| Fig. 2 Transmission electron microscopy (TEM) (scale bar: 50 nm) and high-resolution TEM (HRTEM) images (scale bar in insets: 5 nm) of (a) 2 nm Ru NPs (sRuNP), (b) 3.9 nm (mRuNP), and (c) 5.9 nm Ru NPs (lRuNP). (d) The correlation fitting curve between the ratio of Ru4+ (oxidized Ru) and the surface to volume ratio of different Ru NPs. Catalase-like (CAT-like) catalytic behaviors of different-sized Ru NPs in terms of (e) degree of intracellular oxidative stress of liver cells (L02) after being treated with H2O2 (200 μm) for 4 h, and (f) flow cytometry analysis of reactive oxygen species (ROS) in the cells. (g) The percentage of Treg cells recorded in liver tissues of mice with acetaminophen-induced liver injury after different treatments, showing advantageous internalization of sRuNP by Treg cells in vivo therapy (control: without any treatment. PBS: treated with phosphate buffered saline solution. NAC: treated with N-acetylcysteine solution. sRuNP: treated with sRuNP). Reproduced with permission from ref. 48. Copyright 2022, Wiley-VCH. | ||
Meanwhile, researchers found that the poorly defined shapes of nanomaterials make it difficult to establish a clear structure–property–performance relationship. Thanks to continuous research efforts, colloidal synthesis has been successful in producing noble metal nanocrystals bearing well-defined shapes, leading to the identification of specific facets. In the past decade, both experimental and computational studies have been established to prove that enzyme-like performances are highly sensitive to the exposed facets. For instance, concave Au/Cu nanorods (NRs) with high-index (200) facets were synthesized via etching Au NRs with Cu2+ ions.71 The catalytic activity of Au/Cu NRs was confirmed by the oxidation of colorless 3,3′,5,5′-tetramethylbenzidine (TMB) to blue oxidized TMB by H2O2. With the presence of more tips and high-index facets, the Kcat/Km (Kcat, catalytic constant; Km, Michaelis constant) value of Au/Cu NRs was 781.3 μM−1 min−1, which is 4.5 times higher than that of Au NRs (172.8 μM−1 min−1). Furthermore, the facet-dependent OxD-like property of Pd nanocrystals was studied by Zhou's group.49,72 The (100)-faceted Pd cubes nanozymes exhibited higher activities for H2O2 generation than (111)-faceted Pd octahedra (Fig. 3a–g),72 while the Pd concave nanocubes with high-index (730) facets were reported to have better OxD-like activity than (111) facets due to the undulating surface composed of highly unsaturated Pd atoms (Fig. 3h–k).49 Recently, holey Pd nanosheets with highly active Pd(100) facets were prepared by anisotropic oxidative etching, which showed enhanced CAT-like activity compared with the intact Pd nanosheets only exposing (111) facets. The high efficiency for the decomposition of H2O2 to O2 and the ability to generate singlet oxygen (1O2) from O2 make holey Pd nanosheets great nanozyme candidates against hypoxic tumors.73 In addition to these experimental efforts, theoretical calculations also provide evidence for facet-dependent activities of noble metal nanozymes. The facet-dependent OxD-like properties of Au, Ag, Pd and Pt were predicted via the density functional theory (DFT) method,74 in which the dissociation of O2 on various metal surfaces was evaluated to predict their OxD-like activities. Ag(111) and Au(111) were found to possess high activation and reaction energies, therefore were both thermodynamically and kinetically unfavored for dissociative adsorption of O2. Thus, the predicted sequence of OxD activities of the (111) facet is Pd > Pt ≫ Au and Ag. Facets with higher energies like (211) of Au were also studied. Compared with the Au(111) facet, the high-index (211) facet had a better performance due to the strong dissociative adsorption of O2.
 | ||
| Fig. 3 (a) TEM image (scale bar: 20 nm), (b and c) HRTEM images (scale bar: 2 nm), and corresponding fast Fourier transformation (FFT) pattern (inset) of (100)-faceted Pd cubes. (d) TEM image (scale bar: 20 nm), (e and f) HRTEM images (scale bar: 2 nm), and corresponding FFT pattern (inset) of (111)-faceted Pd octahedra. (g) Comparison of concentrations of H2O2 generated using different Pd nanozymes, showing a facet-dependent oxidase-like (OxD-like) activity. AA (ascorbic acid), as a physiologically relevant antioxidant, was used as substrate to assess the OxD-like activities of Pd nanocrystals. Reproduced with permission from ref. 72. Copyright 2018, Springer Nature Limited. (h) TEM image of (730)-faceted Pd concave nanocubes (Pd CNCs) (scale bar: 50 nm), (i) HRTEM image of Pd CNCs (scale bar: 5 nm), and (j) model of the atomic arrangement on the (730) surface of Pd CNCs. (k) Comparison of absorbance changes of substrate using Pd nanocubes (NCs) or Pd CNCs, showing a facet-dependent OxD-like activity. Reproduced with permission from ref. 49. Copyright 2018, Springer Nature Limited. | ||
 | ||
| Fig. 4 TEM images of (a) Pd octahedra and (b) Pd icosahedra nanozymes. (c) A histogram comparing the peroxidase-like (POD-like) efficiency and surface strain mappings (inset) of Pd octahedron and icosahedron. (d) X-ray diffraction (XRD) patterns of Pd octahedra and icosahedra. The dashed line compares the peak shift of (111) between the Pd icosahedron and octahedron, indicating a possible tensile stress in the icosahedron. The small hump indicated by the black arrow was believed to reflect the presence of compressive stress in the icosahedron. Reproduced with permission from ref. 82. Copyright 2019, American Chemical Society. HRTEM images of (e) Pd nanosheets (Pd NSs) and (f) strained Pd NSs (SPd NSs), as well as (g) their lattice spacing measurements. (h) Comparison of CAT-like catalytic behaviors of Pd NSs and SPd NSs. The O2 generation upon the addition of 10 mM H2O2 and 5 μg mL−1 nanozymes in a neutral buffer shows a clear difference. Reproduced with permission from ref. 57. Copyright 2022, Wiley-VCH. | ||
While the crystalline phase is mostly obtained in noble metal nanomaterials, the amorphous phase with a highly disordered atom arrangement, which is thermodynamically unfavorable, has also been obtained. Crystallinity control has been realized by annealing,87,88 tuning reaction temperature,89 choosing appropriate reducing agents,90 and doping.91–94 For example, ultrathin Ru nanosheets with different crystallinity were synthesized by using different potassium salts during annealing treatment, resulting in amorphous (Fig. 5a–c) and crystalline (Fig. 5e–g) Ru nanosheets of similar thickness.88 The randomly distributed atoms with blurred diffraction ring verified the successful synthesis of amorphous Ru nanosheets (Fig. 5b), while crystalline Ru nanosheets showed a clear lattice with multiple diffraction rings (Fig. 5f). The degree of amorphousness of Ru nanosheets exhibited a positive relationship with the POD-like activity (Fig. 5d). The author explained that the enhanced POD-like activity and selectivity of amorphous Ru nanosheets were related to the increased H2O2 affinity caused by the amorphous surface. Additionally, amorphous noble metal compounds including IrTe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 and RuTe2
55 and RuTe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 also have been reported to exhibit enhanced enzymatic performance compared with their crystalline counterparts. Briefly, the amorphous RuTe2 NRs POD-like nanozymes showed a 3.77-fold higher Kcat and better substrate affinity than their crystalline structure counterparts.95 Defect-rich glassy IrTe2 NRs with weak Ir–Te bond strength exhibited excellent photothermal conversion efficiency and enzymatic performance. These properties could significantly contribute to synergistic oncotherapy, effectively achieving outstanding antitumor efficiency.55
95 also have been reported to exhibit enhanced enzymatic performance compared with their crystalline counterparts. Briefly, the amorphous RuTe2 NRs POD-like nanozymes showed a 3.77-fold higher Kcat and better substrate affinity than their crystalline structure counterparts.95 Defect-rich glassy IrTe2 NRs with weak Ir–Te bond strength exhibited excellent photothermal conversion efficiency and enzymatic performance. These properties could significantly contribute to synergistic oncotherapy, effectively achieving outstanding antitumor efficiency.55
 | ||
| Fig. 5 TEM images of (a) amorphous Ru (A-Ru) and (e) crystalline Ru (C-Ru) nanosheets (NSs). Insets: digital photos of the corresponding aqueous solutions. HRTEM images of (b) A-Ru and (f) C-Ru NSs. Insets: the corresponding selected area electron diffraction (SAED) patterns. Atomic force microscope images of (c) A-Ru and (g) C-Ru NSs. (d) Comparisons of POD-like and OxD-like activity of Ru NSs with different degrees of crystallinity. Inset: schematic illustration of the POD-like activity of Ru NSs. Reproduced with permission from ref. 88. Copyright 2021, American Chemical Society (h) TEM image and (i) spherical aberration corrected (CS-corrected) high-angle annular dark-field scanning TEM (HAADF-STEM) image of amorphous-crystalline heterophase RhRu NSs, with corresponding (j1 and k1) FFT patterns and (j2 and k2) HR-STEM images taken from the red and cyan squares in (i). (l) Comparisons of CAT-, OxD-, and POD-like activities of crystalline RhRu (c-RhRu) and amorphous-crystalline heterophase RhRu (denoted as la-RhRu) nanozymes, showing strain-enhanced effect and photoenhanced effect on enzymatic performances. NIR: near-infrared. Reproduced with permission from ref. 35. Copyright 2024, Wiley-VCH. | ||
Besides the fully amorphous phase, an amorphous–crystalline heterophase can also modify the surface properties and catalytic efficiency of noble metal-based nanomaterials. For instance, RuCu nanosheets composed of crystalline Ru and amorphous Cu exhibited a wider pH range for the oxidation of POD substrate H2O2 and demonstrated POD-like activity with high catalytic efficiency (Kcat/Km = 177.2 m−1 s−1).19 Additionally, Guo et al. synthesized amorphous–crystalline heterophase PtRuTe nanomaterials with dominant amorphous phase, which consisted of a Pt/Te-enriched core and a Ru-enriched shell.23 Compared with its less amorphous PtRuTe and fully crystalline PtRuTe counterparts, the highly amorphous PtRuTe could efficiently accelerate the oxidation of TMB by H2O2, indicating excellent POD-like activity. The authors supposed that enriched catalytically active sites as well as the electron redistribution induced by amorphous structure and multiple contents promoted the excellent POD-like property of the highly amorphous PtRuTe. Moreover, Wu et al. synthesized a heterophase RhRu bimetallene nanozyme (Fig. 5h–k).35 Compared with crystalline RhRu, the amorphous–crystalline heterophase RhRu bimetallene possessed strong ultraviolet-visible (UV)-NIR absorption in a broad spectral range, leading to an exceptional intrinsic photothermal effect and photoenhanced CAT-, OxD-, and POD-like activities (Fig. 5(l)).35
2.1.5.1 Random alloy. Random alloys, also known as solid-solution alloys, feature a random distribution of atoms throughout the lattice.102 Compared with monometallic nanozymes, multi-metallic alloy nanozymes can achieve improved chemical stability and enzymatic activity. For example, Meng et al. recently synthesized a AuPd alloy nanozyme with enhanced SOD- and myeloperoxidase-like activities compared with its pure Au and Pd counterparts. Furthermore, the enzymatic activities of the AuPd alloy nanozymes remained stable over a wide temperature range from 25 to 80 °C, indicating their high stability.46 Additionally, several studies have confirmed that the enhancement of enzyme-like activities results from the electronic state modification of the active metal through alloying.103–106 For example, Yan et al. doped a p-block metal Sn into Pd nanocrystals and investigated the enzymatic behaviors of PdSn nanozymes.105 The energy dispersive spectroscopy (EDS) mappings demonstrated a homogeneous distribution of Sn and Pd (Fig. 6a). Projected density of states (PDOS) analysis indicated strong p–d orbital hybridization with the peaks of Pd 4d orbitals matching well with Sn 5p orbitals (Fig. 6b). The authors explained that the orbital hybridization could raise the d-band energy level, reducing antibonding electron filling and enhancing substrate adsorption. Consequently, PdSn nanozymes exhibited excellent POD-like activity with high Vmax values for both H2O2 and TMB compared with pure Pd nanozymes. Furthermore, the PdSn nanozymes exhibited outstanding catalytic specificity (Fig. 6c). The activation energy calculated from the Arrhenius equation was used to demonstrate the enzyme-like performance. The PdSn nanozymes showed an activation energy value of 1.03 kJ mol−1 for POD-like activity and −4.64 kJ mol−1 for OxD-like activity, which illustrated the excellent specificity. Additionally, introducing functional compositions into noble metals can lead to the formation of a multifunctional nanoalloys with intriguing properties.107 For example, oxophilic metals, such as Bi, Sn, and Ni, are widely incorporated into noble metals to enhance enzymatic activities by forming oxygen-containing species on the catalyst surface.108,109 Xia et al. reported that the POD-activity of bimetallic PdBi alloy was closely correlated with Bi content on the surface, which was beneficial for strong adsorption of H2O2 on Bi and had a low potential barrier for further decomposition.108 Similarly, the oxophilic metal Sn has been studied in a bimetallic PtSn nanozyme, where oxygen vacancy sites provided by SnO2−x enhanced H2O2 adsorption, further boosting enzymatic activities to catalyze H2O2 into O2 and hydroxyl radical (˙OH).109
 | ||
| Fig. 6 (a) TEM image and energy dispersive spectroscopy (EDS) mapping of PdSn nanozymes. (b) Calculated projected density of state (PDOS) of PdSn nanozymes. Inset: calculated density of states of Pd 4d and Sn 5p orbitals. (c) Comparison of POD-like and OxD-like activities of Pd and PdSn nanozymes. Reproduced with permission from ref. 105. Copyright 2024, American Chemical Society. (d) HRTEM image of high-indexed Pt3Sn (H-Pt3Sn) intermetallic nanozymes. (e) The derived structure models and corresponding 2D atomic models of H-Pt3Sn intermetallic nanozymes. (Pt: blue balls; Sn: gray balls). (f) Comparison of absorbance spectra (indicating the POD-like activities) of H-Pt3Sn, Pt3Sn, and Pt nanozymes, in which 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate to catalyze H2O2. Reproduced with permission from ref. 13. Copyright 2022, American Chemical Society. (g) STEM images of the ultra-small PtPdRuRhIr high-entropy alloy nanoparticles (US-HEANPs) (inset: size distribution of US-HEANPs). Steady-state catalytic kinetics of POD-like activities of high-entropy alloy nanozymes with different concentrations of (h) H2O2 and (i) TMB, revealing the fast POD-like reaction rate and strong affinity between nanozymes and substrate. (j) EDS mapping of US-HEANPs. Reproduced with permission from ref. 121. Copyright 2023, Wiley-VCH. | ||
2.1.5.2 Intermetallic alloy. Intermetallic alloys refer to solid-state metallic alloys with an ordered atomic arrangement, which can lead to significant changes in their catalytic performance.110–114 Recently, Zhu et al. reported a high-indexed intermetallic Pt3Sn nanozyme with high activity and specificity, synthesized by a one-step solvothermal approach.13 The lattice spacing of 0.283 and 0.179 nm could be assigned to (110) and (210) crystal planes of Pt3Sn, respectively, confirming the formation of a high-indexed Pt3Sn intermetallic structure (Fig. 6d and e). Consequently, the high-indexed Pt3Sn (H-Pt3Sn) exhibited the highest absorbance of ox-TMB at 371 and 652 nm compared with Pt3Sn cubic and Pt nanozymes, indicating its superior POD-like activity (Fig. 6f). As expected, the H-Pt3Sn demonstrated approximately twice the specific activity value (345.32 U mg−1) compared with that of Pt nanozymes (190.12 U mg−1). In another work, Pd2Sn intermetallic NRs were identified as multifunctional nanozymes for anti-tumor immunity.115 In addition to enhancing catalytic activity, the Pd2Sn intermetallics were further functionalized with soybean phospholipid and glucose oxidase (GOx), demonstrating a uniform hydrated particle size over 7 days in phosphate buffer, which indicated excellent stability. Notably, Pd2Sn intermetallic NRs, featuring a plasmonic Pd component, exhibited high light absorption and photothermal conversion efficiency, making them suitable contrast agents for PAI due to the LSPR effect.
2.1.5.3 High-entropy alloy. To date, HEAs have demonstrated tremendous potential in energy, engineering, catalysts fields.116–118 Compared with traditional alloys, HEAs consist of five or more principal elements in nearly equal percentages, typically ranging from 5 to 35 at%.118,119 The variable element compositions of HEAs offer greater flexibility in adjusting the coordination environment, thereby optimizing reaction activities. On the other hand, the high mixing entropy of HEAs contributes to the stability of the material system.120 Recently, HEAs have also been applied in the field of nanozymes.121–123 Ai et al. prepared ultra-small PtPdRuRhIr high-entropy alloy NPs by a general metal–ligand cross-linking strategy.121 These HEANPs have an average size of approximately 1.5 nm and consist of Pt, Pd, Ru, Rh, and Ir (Fig. 6g and j). The POD-like activities of high-entropy alloy nanozymes (HEAzymes) with H2O2 and TMB were observed with a small Km value and high Vmax, indicating their exceptional POD-like activity (Fig. 6h and i). The authors claimed that the presence of random mismatches and occupancies of five different atoms in the alloys could accelerate the transfer of electrons by HEAzymes, which may contribute to their high enzymatic performance. Additionally, the slow diffusion effect of HEAs and the increased mixed conformational entropy contributed to the excellent stability of PtPdRuRhIr HEANPs. Beyond noble metal-dominant HEAs, FeCuAgCeGd HEAzymes122 and MnFeCoNiCu HEAzymes123 have also been reported recently.
 | ||
| Fig. 7 (a) TEM images of Ru NPs modified with different ligands, including polystyrene sulfonate (PSS) (a1), poly(vinyl pyrrolidone) (PVP) (a2), poly(vinyl alcohol) (PVA) (a3), and poly(acrylic acid) (PAA) (a4). Insets: the corresponding SAED patterns of Ru NPs with PSS ligands (Ru@PSS), Ru NPs with PVA ligand (Ru@PVA), Ru NPs with PVP ligand (Ru@PVP), and Ru NPs with PAA ligand (Ru@PAA). (b) High-resolution X-ray photoelectron spectroscopy spectra of Ru 3p for Ru@PSS, Ru@PVA, Ru@PVP, and Ru@PAA nanozymes, respectively. (c) The specific activities (U mg−1) of horseradish peroxidase (HRP) and various Ru nanozymes, among which Ru@PSS exhibits the highest slope (POD-like activity) in the linear fitting curves. Reproduced with permission from ref. 51. Copyright 2023, Wiley-VCH. | ||
2.2 Structural engineering of noble metal-based nanozyme heterostructures
Construction of heterostructures based on noble metal nanozymes can effectively enhance their dispersity and stability during storage and catalysis, while the presence of various heterostructure interfaces may promote electron transfer and change atomic coordination environments, thereby further regulating the enzyme-like behaviors.6,131,132 Meanwhile, the incorporation of multiple components in the nanozymes endow them with more possible functions for integrated applications such as synergistic therapy,133–135 theragnostic agents for both diagnosis and therapy,45 as well as multiple imaging modalities.21 Herein, we discuss the construction of various heterostructures based on noble metal nanozymes with the addition of different second components, including metals, metal compounds, carbon materials, metal–organic frameworks (MOFs), covalent–organic frameworks (COFs), etc. | ||
Fig. 8 (a) TEM image of Ni–Pt NPs and photograph (inset) of the aqueous suspension. (b) HAADF-STEM image of a typical Ni–Pt heterostructure. Red circles highlight regions containing Ni atoms. (c) EDS mapping of Ni–Pt NPs showing Ni-rich core and Pt-rich shell. (d–f) POD-like catalytic efficiencies for Ni–Pt NPs with different Ni/Pt ratios. (d) Plots of initial reaction velocity against TMB concentration, (e) Kcat (catalytic constant) values, and (f) Kcat-specific (Kcat normalized to the surface area) values of different Ni–Pt NPs. The NPs with Ni/Pt ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the highest catalytic activity. Reproduced with permission from ref. 52. Copyright 2021, American Chemical Society. (g) HRTEM images and (h) EDS mapping of Pd@Pt–Ru nanozyme. (i) Scheme of electron migration in Pd@Pt–Au and Pd@Pt–Ru NPs. The Ru deposition on PdPt core induces electron transfer from Pd/Pt to Ru, whereas the Au deposition induces electron transfer toward Pd and Pt. Reproduced with permission from ref. 20. Copyright 2024, American Chemical Society. 1 exhibited the highest catalytic activity. Reproduced with permission from ref. 52. Copyright 2021, American Chemical Society. (g) HRTEM images and (h) EDS mapping of Pd@Pt–Ru nanozyme. (i) Scheme of electron migration in Pd@Pt–Au and Pd@Pt–Ru NPs. The Ru deposition on PdPt core induces electron transfer from Pd/Pt to Ru, whereas the Au deposition induces electron transfer toward Pd and Pt. Reproduced with permission from ref. 20. Copyright 2024, American Chemical Society. | ||
Plasmonic metal-based heterostructure nanozymes are another representative group of nanozyme heterostructures that have attracted significant attention due to their unique photoresponsive properties.32 The decay of LSPR from a plasmonic metal can induce the near-field enhancement and generate hot carriers (hot electrons and hot holes) and local heating (photothermal effect), which could be utilized by adjacent components to extend their free path and enhance their lifetime and efficiency.135 For example, Sang et al. designed a Pd–Au dimer with a clear boundary and independently exposed Pd and Au surfaces.136 Under light irradiation, the TMB oxidation capability of the Pd–Au dimer increased by 2.3 times compared with dark conditions. After excluding the photothermal effects, the authors attributed the catalytic activity enhancement to the LSPR effect of Au. Specifically, LSPR-induced electron–hole pairs in Au were transferred to Pd, facilitating the decomposition of H2O2 on the Pd surface into reactive oxygen species (ROS) including ˙OH, ˙O2− and 1O2. A different work studied the local photothermal effect of heterostructures induced by LSPR excitation, which could also regulate the enzyme-like performance.132 Fan et al. synthesized plasmon-enhanced OxD-like rod-shaped nanozymes with Pd coating and Au core (Au@PdNRs).132 Under 808 nm irradiation (close to the LSPR absorption wavelength of Au NRs), the catalytic performance of Au@PdNRs was significantly enhanced. Based on the slight change in the activation energy of Au@PdNRs before and after irradiation, the authors suggested that the contribution of hot electrons to the activity enhancement was minimal and the primary contribution came from the local photothermal effect induced by LSPR. The advantages of plasmonic metal-based heterostructure nanozymes have also been demonstrated in other enzyme-mimicking reactions. For example, Pt-tipped Au NRs exhibited excellent CAT-like performance under NIR light irradiation,133 Au@Pt0.46Ag0.54 nanozymes displayed enhanced CAT-, OxD-, and POD-like activities alongside efficient photothermal conversion under an 808 nm laser,131 while 2D Pd@Au core–shell nanosheets demonstrated robust CAT-like activity by stably producing O2 under NIR-II laser excitation.41 Due to their outstanding catalytic and optical properties, plasmonic nanozymes have found extensive applications in advanced synergistic therapeutic methods, such as photodynamic and photothermal therapy.12,40,134
In addition, metal compounds with unique physicochemical properties bring new functions to the noble metal nanozymes. For example, Fe3O4 is a typical magnetic and recyclable material that can be used in magnetic resonance imaging and magnetic thermal therapy. He et al. synthesized monodisperse Fe3O4-Au@mesoporous SiO2 microspheres with cascade catalytic ability.143 The superparamagnetic Fe3O4 cores not only benefitted from POD-like activity but also enabled a recyclable enzymatic system. Combined with the GOx-like activity of Au, the catalyst achieved cascade catalytic activity from glucose oxidation to TMB oxidation. Different from Fe3O4, Ti3C2 nanosheets were found to possess high absorption in the NIR-II range and are thus excellent materials for PTT. A Ti3C2Tx-Pt-PEG heterostructure nanozyme was synthesized by anchoring Pt NPs with POD-like activity onto Ti3C2,141 which achieved synergistic cancer cell killing under low-energy density NIR-II laser irradiation through both photothermal effects and enzyme-like catalysis. Additionally, due to the excellent optical absorption of Ti3C2, this integrated nanozyme could also serve as PAI agent to accurately display the treatment site, facilitating imaging-guided synergistic cancer therapy. Similarly, a PtFe@Fe3O4 heterostructure nanozyme constructed by Li et al. also exhibited the ability to kill tumor cells through the synergistic effect of photothermal and enzymatic therapy.101 The authors believed that redistribution of electrons in each component enhanced the POD-like activity of Pt and the CAT-like activity of Fe3+, while the photothermal effect shared by both Pt and Fe3O4 enabled the PTT and PAI functionalities. In another work, a heterostructure nanozyme assembled from zinc phthalocyanine, Pt, and the semiconductor TiO2 also demonstrated synergistic activities in enzyme catalysis, PDT, and PTT.144
COFs, known for their consistent porosity and substantial carbon and nitrogen backbone, have also been effectively paired with noble metals to stabilize nanozymes.157,158 For example, Liu et al. synthesized COFs with a hierarchical flower-like hollow structure and subsequently produced COF-Ag nanozymes through in situ reduction of Ag NPs on the COF.159 They found that COFs, as carriers, could effectively regulate the dispersion and size of Ag NPs, thereby enhancing the catalytic stability of COF-Ag nanozymes. The COF-Ag nanozymes showed high sensitivity and selectivity in catalysis-based colorimetric detection of Hg2+ ions in blood due to the formation of the Ag–Hg metallic bond and the enhanced OxD-like activity of COF-Ag nanozymes in the presence of Hg2+ ions. Moreover, a Pt–Pd alloy/COFs nanozyme was obtained through a simple aldimine condensation and in situ reduction method.160 This heterostructure provided abundant anchor sites for Pt–Pd bimetallic NPs, preventing the aggregation of Pt–Pd NPs, and enhancing both stability and activity in POD-like and OxD-like catalysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Au1.0Pd2.0/MoS2), demonstrated synergistically enhanced POD-like activity due to strong metal–metal and metal–support interactions. In another example, by immobilizing Au NPs on hollow silica microspheres (HSM), the obtained HSM-AuNPs possessed dual enzymatic (GOx and POD-like activities) activity to promote the cascade reactions.162 The HSMs not only enabled Au to be uniformly anchored, exposing more active sites, but also assisted in the diffusion of reaction substrates and products, thereby enhancing the stability of the reaction environment.
2 (Au1.0Pd2.0/MoS2), demonstrated synergistically enhanced POD-like activity due to strong metal–metal and metal–support interactions. In another example, by immobilizing Au NPs on hollow silica microspheres (HSM), the obtained HSM-AuNPs possessed dual enzymatic (GOx and POD-like activities) activity to promote the cascade reactions.162 The HSMs not only enabled Au to be uniformly anchored, exposing more active sites, but also assisted in the diffusion of reaction substrates and products, thereby enhancing the stability of the reaction environment.
Different from the above examples, carbon nitride (C3N4) can not only improve the dispersity of nanozymes, but also act as POD mimics by themselves.163 Ding et al. prepared highly dispersed ultrasmall Ru NPs anchored onto C3N4 nanosheets for colorimetric detection. With excellent carrier capability, p-conjugated framework and the abundant pyridinic nitrogen, the C3N4 support provided uniform binding sites for Ru NPs, thereby promoting their POD-like catalytic performance.164 Au NPs grown on the surface of C3N4 have also been reported to show an excellent improvement in POD-like activity.165 Benefiting from the synergistic effect of Au NPs and g-C3N4 nanosheets, the catalytic activity of the AuNPs@g-C3N4 nanosheets was 6 times that of the physical mixture of naked AuNPs and g-C3N4 nanosheets.165 In addition, other noble metals such as Pt166 and Ag167 have also been used to construct heterostructures with C3N4 nanosheets for nanozyme applications.
Single-atom based nanoheterostructures have emerged as another promising nanozyme candidate.168–171 With optimized atomic utilization efficiency and strong metal–support interactions, the enzyme-like activity of active sites in SAzymes could be precisely regulated in terms of the coordination environment and charge transfer.168,169 Chang et al. developed a nitrogen-coordinated carbon-supported Pd SAzyme by pyrolyzing Pd NPs supported on MOFs.172 The developed SAzyme exhibited excellent POD-like activity as well as a glutathione oxidase-like activity, which reduced overproduced intracellular glutathione in cancer cells. By limiting glutathione, the SAzyme effectively prevented the depletion of ˙OH, a key product of the POD-like reaction. Combined with the photothermal effect, the Pd SAzyme demonstrated effective PTT with mild treatment conditions. SAzymes have also shown promise in PDT. For example, Wang et al. partially replaced Co in Mn3 [Co(CN)6]2 MOFs with Ru to synthesize an SAzyme containing Ru single-atom sites.173 The authors proposed that the six unsaturated Ru–C6 coordination sites in SAzymes enhanced enzyme-like activity, enabling the rapid decomposition of H2O2. Furthermore, due to coordination and other noncovalent interactions, this SAzyme could self-assemble with the chlorin e6 photosensitizer, releasing highly cytotoxic 1O2 species to enable PDT. Additionally, Yan et al. reported a Pt SAzyme-based bandage for brain trauma.174 By reducing Pt onto CeO2 clusters, they doped single-atom Pt in CeO2 with a 2% lattice expansion of CeO2. The Pt SAzyme exhibited glutathione peroxidase-like activity along with 10 times higher POD-like activity, 10 times higher CAT-like activity, and 4 times higher SOD-like activity compared with CeO2 clusters. The Pt SAzyme was then incorporated into a bandage for noninvasive brain trauma treatment, which could prevent neuronal damage by effectively reducing reactive oxygen and nitrogen species.
3. Enzyme-like catalytic behaviors of noble metal-based nanozymes
In this section, we will introduce representative enzyme-like processes in which noble metal-based nanozymes have been applied and studied. Their enzyme-like catalytic behaviors including substrate adsorption, electron transfer, and product release in specific catalytic processes will be discussed.3.1 POD
The POD family is the most common type of enzyme present in virtually all living species, and includes peroxidase, glutathione peroxidase, and haloperoxidase.4 A typical reaction catalyzed by POD mimics, using TMB as substrate for the oxidation of H2O2, can be written as follows:175In 2010, cysteamine-encapsulated Au NPs were unexpectedly discovered as mimics of POD for the oxidation of H2O2. Since then, the POD-like behaviors of noble metal-based nanozymes have been further explored.24 He et al. reported that the absorbance peak of Au NPs in UV-vis spectra remained unchanged during the POD-like reaction, indicating that Au NPs acted as the catalyst rather than the reactant.176 Furthermore, several studies demonstrated that the intermediate ˙OH was observed during the catalytic reaction when noble metal-based nanomaterials were used as POD mimics.72,177 Mechanism studies showed that the adsorption of H2O2 on Au, Ag, Pd, and Pt is the first step to initiate the reaction. The predicted pathway is that H2O2 first breaks the O–O to form two OH* (‘*’ is utilized to indicate adsorbed species on the metal surface), followed by the formation of H2O* and O*, or alternatively the formation of H*, O* and OH*.178 Recently, Shen et al. calculated the adsorption energy of OH* via DFT, which showed that the hydroxyl adsorption energies of Au, Ag, Pt, Pt, Ir, Rh, and Ru were located within the POD-like activity window, predicting their good POD-like activity.179 This theoretical prediction matched well with the currently experimental confirmation of POD activities of noble metals.
3.2 OxD
OxD-like nanozymes exhibit a chemical catalytic function similar to oxidases, promoting the oxidation process of organic substrates such as TMB, glucose, glutathione, ascorbic acid, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).180 Taking TMB as an example, two possible enzyme-driven oxidation reactions of TMB are written as follows:175| O2 + 2TMB + 2H+ = H2O2 + 2TMB+ |
| O2 + 4TMB + 4H+ = 2H2O + 4TMB+ |
Unlike POD-like nanozymes, OxD-like nanozymes produce H2O2 with O2 by acting as the hydrogen acceptor for substrate oxidation.68,172,181–184 Long et al. demonstrated the oxidation reactions of TMB with Pd NPs as the OxD mimics, finding that O2 could be adsorbed on the surface of Pd NPs and subsequently activated into O21 through electron transfer from metal atoms to O2.183 Subsequent reports have identified several ROS in OxD-like processes, including ˙OH,391O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 and O2˙−,183 which promote the oxidation of various substrates. Furthermore, Shen et al. investigated the catalytic mechanism of noble metal OxD-like nanozymes through a combination of DFT calculations and experimental investigations.74 They proposed that noble metal nanomaterials facilitated the dissociation of O2, producing O adatoms that subsequently could oxidize substrates (such as TMB and ascorbic acid) by extracting hydrogen from substrates.74 Additionally, it is worth noting that noble metal-based nanozymes have also been utilized in other reactions in the OxD family. For instance, Chen et al. studied the catalytic mechanism of noble metal nanozymes as glucose oxidase, showing that Au NPs can catalyze glucose oxidation by reducing glucose to H2O2, while Pt, Pd, Ru, Rh, and Ir can preferentially reduce O2 to H2O.186 Moreover, reports showed that PdPtCu nanocomposite, Pt NPs, and Pt/CeO2 nanozyme can act as glutathione oxidase,11 ascorbate oxidase,187 and uricase mimics,188 respectively. These findings broaden the types of OxD mimics achievable by the noble metal-based nanozyme family.
185 and O2˙−,183 which promote the oxidation of various substrates. Furthermore, Shen et al. investigated the catalytic mechanism of noble metal OxD-like nanozymes through a combination of DFT calculations and experimental investigations.74 They proposed that noble metal nanomaterials facilitated the dissociation of O2, producing O adatoms that subsequently could oxidize substrates (such as TMB and ascorbic acid) by extracting hydrogen from substrates.74 Additionally, it is worth noting that noble metal-based nanozymes have also been utilized in other reactions in the OxD family. For instance, Chen et al. studied the catalytic mechanism of noble metal nanozymes as glucose oxidase, showing that Au NPs can catalyze glucose oxidation by reducing glucose to H2O2, while Pt, Pd, Ru, Rh, and Ir can preferentially reduce O2 to H2O.186 Moreover, reports showed that PdPtCu nanocomposite, Pt NPs, and Pt/CeO2 nanozyme can act as glutathione oxidase,11 ascorbate oxidase,187 and uricase mimics,188 respectively. These findings broaden the types of OxD mimics achievable by the noble metal-based nanozyme family.
3.3 CAT
CAT-like nanozymes catalyze the decomposition of H2O2 according to the following reaction:25| 2H2O2 = 2H2O + O2 |
O2 can be effectively produced with CAT-like nanozymes, which are widely applied in overcoming tumor hypoxia.25,27,189 In 2009, AuPt NPs stabilized with pectin were reported to exhibit CAT-like properties, which can decompose H2O2 to produce O2.190 Furthermore, Li et al. investigated the enzymatic performance mechanisms of Ag, Au, Pd, and Pt NPs.178 In a basic environment, the CAT-like activity of noble metals involves H2O2 adsorbing onto the surfaces with pre-adsorbed OH groups, which then gives rise to H2O* and HO2*. Subsequently, HO2* reacts with another H2O2 to yield O2*, OH*, and H2O*. Interestingly, Ag, Au, Pd, and Pt NPs exhibit pH-switchable POD- and CAT-like activities. The authors claimed that pH value affects the reaction energy barriers and adsorption energies of noble metal NPs, resulting in POD-like activity at low pH and CAT-like activity at high pH, respectively. Indeed, the typical reaction condition of noble metal-based CAT-like mimics is a neutral or slightly alkaline environment, with commonly used buffers including phosphate buffer solution and sodium carbonate/sodium bicarbonate buffer solution.191
3.4 SOD
The SOD-like reaction for catalyzing O2˙− can be written as follows:31| 2O2˙− + 2H+ = H2O2 + O2 |
By clearing O2˙− and generating O2, SOD-like nanozymes effectively reduce oxidative stress in cells while mitigating apoptosis and inflammation.31 Shen et al. utilized DFT calculations to predict the SOD-like activities of noble metals.74 By analyzing the reaction pathway and energy changes on different noble metal surfaces, they proposed that O2˙− can easily capture protons from water, forming HO2˙ and OH−. The adsorption of HO2˙ on the surfaces of Au, Ag, Pd, and Pt is a strongly exothermic process that occurs easily. Once HO2˙ is adsorbed on the noble metal surface, it readily converts to O2* and H2O2. Subsequently, O2 and H2O2* are transformed into O2 and H2O2. As a result, noble metals have great potential as SOD-like nanozymes.74
3.5 Multiple and specific enzyme mimics
Due to the high activity of noble metal nanomaterials in multiple enzyme-like reactions, most noble metal-based nanozymes exhibit two or more types of enzyme-like activities, enabling self-cascade synergistic catalysis.192,193 For example, Li et al. constructed a PtFe@Fe3O4 nanozyme with dual POD–CAT enzyme-like activities, which could catalyze endogenous H2O2 to generate highly toxic ˙OH and supply O2, effectively killing tumor cells and overcoming the tumor hypoxia.101 In another work, by combining OxD-like activity to reduce O2 to H2O2 with POD-like ability to consume H2O2 and produce ˙OH, Pd nanozymes could form a cascade catalytic system that produced abundant ROS.72 It has been reported that the Pt@CNDs nanozymes with dual SOD–CAT activities could suppress oxidative damage by utilizing their SOD-like activity to clear O2˙− and produce H2O2, as well as their CAT-like activity to decompose H2O2 into O2.149 Moreover, noble metal-based nanozymes with more than two enzyme-like activities have also been reported. For instance, a AuPt3Cu trimetallic nanozyme with Au-rich cores and Pt/Cu outer shells possessed OxD-, POD-, and CAT-like activities, displaying an excellent chemodynamic therapy performance towards cancerous MCF-7 cells without damage to normal cells.6 Yang et al. synthesized ultrathin RuCu nanosheets that possessed POD-like activities, SOD-like activities and glutathione POD-like activities, demonstrating high catalytic efficiency in oxidizing H2O2 at a wide pH range and therefore their great potential as a chemodynamic therapy reagent.19While high catalytic activity is desired in nanozyme applications, specificity is another important factor to be considered, which is essential for achieving selective catalysis, accurate detection, and efficient disease treatment.192 In recent years, significant efforts have been made to enhance the specificity of nanozymes.14,105 To optimize the specificity of noble metal-based nanozymes, the enzymatic pathway can be adjusted through substrate binding, substrate conversion, and product release.192 Recently, Pd@PtBi2 nanospheres were synthesized and exhibited selectively enhanced POD-like activity, enabling the establishment of colorimetric biosensors with high reliability and sensitivity.14 Additionally, PdSn nanozymes were prepared and showed specific POD-like activity, which could improve the sensitivity and accuracy of the colorimetric immunoassays compared with pure Pd nanozymes.105 Moreover, Liu et al. reported that amine-terminated poly(amidoamine) dendrimer-encapsulated gold nanoclusters could generate O2 through their intrinsic and specific CAT-like activity, thereby enhancing the efficiency for cancer treatment.36 It remains highly crucial to further explore more specific nanozymes to meet various application requirements.
4. Biomedical applications of noble metal-based nanozymes
With controllable structure, high activity, and excellent stability, noble metal-based nanozymes have great potential to replace natural enzymes in biomedical applications in the field of biosensing,26,31 diagnosis,54 therapeutics,28,121 and antibacterials.72,194 In this section, we classified and summarized noble metal-based nanozymes according to their types of applications.4.1 Sensor
Noble metal-based nanozyme sensors are widely employed for detecting drugs, food processing, and biomolecules because of their high sensitivity, stability, and enzyme-mimicking activity.195–197 ELISA is a widely used technology for medical diagnostics, in which an enzyme catalyzes a colorimetric reaction, producing a measurable color change (e.g., diamine converted from oxTMB has a characteristic λmax = 450 nm) that reflects the concentration of the target molecule in the sample. Recently, several nanozymes with enhanced POD-like activities have been reported for detecting biomolecules.18,83,96 For example, Jv et al. used Au NPs as POD mimics for colorimetric detection of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and Wu et al. used them for glucose detection.165 In another example, Xi et al. compared the LOD of Pd icosahedra and octahedra to illustrate the strain effect on POD-like activities. From the calibration curves of ELISAs to detect carcinoembryonic antigen (CEA, a typical cancer biomarker), the LOD of Pd icosahedra and octahedra were calculated to be 34 pg mL−1 and 98 pg mL−1, respectively (Fig. 9a).82 The LOD of Ir/WO2.72 heterostructure for ELISA was 2.2 pg mL−1 to CEA, which is 174-fold lower than that of the conventional horseradish peroxidase-based ELISA.17 In another work, amorphous RuTe2 NRs were synthesized for detecting prostate-specific antigen. A LOD of 32.6 pg ml−1 with linear range from 50 pg mL−1 to 5 ng mL−1 was achieved due to the negligible OxD-like activity, which eliminates the interference of O2 for colorimetric assays.95
24 and Wu et al. used them for glucose detection.165 In another example, Xi et al. compared the LOD of Pd icosahedra and octahedra to illustrate the strain effect on POD-like activities. From the calibration curves of ELISAs to detect carcinoembryonic antigen (CEA, a typical cancer biomarker), the LOD of Pd icosahedra and octahedra were calculated to be 34 pg mL−1 and 98 pg mL−1, respectively (Fig. 9a).82 The LOD of Ir/WO2.72 heterostructure for ELISA was 2.2 pg mL−1 to CEA, which is 174-fold lower than that of the conventional horseradish peroxidase-based ELISA.17 In another work, amorphous RuTe2 NRs were synthesized for detecting prostate-specific antigen. A LOD of 32.6 pg ml−1 with linear range from 50 pg mL−1 to 5 ng mL−1 was achieved due to the negligible OxD-like activity, which eliminates the interference of O2 for colorimetric assays.95
 | ||
| Fig. 9 (a) Scheme of the colorimetric enzyme-linked immunosorbent assay (ELISA) of carcinoembryonic antigen (CEA) and photographs showing the different detection sensitivity Pd icosahedra-ELISA (top), Pd octahedra-ELISA (middle), and HRP-ELISA (bottom) using standard CEA concentrations. The yellow-colored product comes from the oxidized TMB. Reproduced with permission from ref. 80. Copyright 2019, American Chemical Society. (b) Schematic diagram of lateral flow immunoassays (LFIA) application of Pd@Pt–Ru NPs. Antibody and Pd@Pt–Ru NPs were conjugated and assembled into a LFIA for human chorionic gonadotropin detection. Reproduced with permission from ref. 20. Copyright 2024, American Chemical Society (c) Comparison of OxD-like activities with and without the addition Pd@Ir nanostructures and humic acid (HA). A significant increase in the H2O2 concentration was observed with Pd@Ir nanostructures and HA, indicating HA could enhance the OxD-like activity of Pd@Ir nanostructures. (d) Typical SEM images of bacterial cells after different treatments, showing more severe damage to the bacterial membrane in the presence of HA. (e) Possible mechanisms for the potentiated antibacterial activity of Pd@Ir nanostructures in the presence of HA. Reproduced with permission from ref. 194. Copyright 2019, American Chemical Society. | ||
Lateral flow immunoassays (LFIAs) have attracted considerable interest in point-of-care testing.20 Recently, Pd@Pt–Ru-LFIA were fabricated to detect human chorionic gonadotropin.20 Thanks to the enhanced POD-like activity, with the presence of TMB and H2O2, the Pd@Pt–Ru-LFIA exhibited a LOD of 0.1 IU L−1 with amplified color responses (Fig. 9b).20 In addition to hormone detection, Pd@Ir nanocubes were reported as effective nanozymes to detect the gastric cancer biomarkers, pepsinogen I and pepsinogen II.53 By catalyzing chromogenic substrate deposition, the Pd@Ir NPs were used as a label to produce stronger signals and achieved high sensitivity with a cutoff value as low as 10 pg ml−1.
OxD-like nanozymes can also be used in colorimetric detection, which efficiently transform colorless substrates (e.g., TMB) into colorful products (e.g., TMB+). For example, Pt nanozymes were used to detect isoniazid because the unique pyridine ring of isoniazid inhibited the high OxD-like activity of Pt nanozymes and showed a colorimetric response.181 Other biosensors based on OxD-like reactions have also been explored for detecting glucose,186 acetaminophen,187etc. Additionally, the enzymatic activity can be regulated by surrounding biological substances such as nucleic acid and protein, thus noble metal-based OxD-like nanozymes have also shown potential in detecting a wider library of substances like cytokine interleukin 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and DNA hybridization.198
29 and DNA hybridization.198
4.2 Antibiosis
Pathogenic infections caused by bacterial, viral, and fungal pathogens pose major global health challenges, further aggravated by the rise of antimicrobial resistance and the growing scarcity of effective antibiotics.199 Recently, noble metal-based nanozymes have emerged as promising alternatives for combating bacterial infections due to their ability to mimic OxD and POD activities for ROS generation or oxidative modulation.199 For instance, the antibacterial ability of OxD-mimic Pd@Ir octahedra against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Salmonella enteritidis has been verified.194 The bactericidal activity of Pd@Ir octahedra was significantly enhanced in the presence of humic acid, leading to elevated levels of ROS. The ROS induced oxidative damage to essential biomacromolecules, including DNA, proteins, and lipids, effectively contributing to bacterial eradication (Fig. 9c–e).194 Similarly, by generating high-level ROS, mesoporous silica-supported AuNPs possessing dual enzyme activities (POD-like and OxD-like activities) were prepared to kill the pathogenic bacteria.200 Additionally, an antibacterial agent was developed via doping Ag into oxygen-vacancy-enriched glucose-modified MoOx.185 The resultant nanozyme heterostructure exhibited NIR-enhanced OxD-like activity, which helped to generate ROS and greatly enhanced antimicrobial activities against multidrug-resistant bacteria.1854.3 Disease treatment
With their intrinsic antioxidant or pro-oxidant activity, noble metal-based nanozymes are also widely used in disease treatment. Nanozymes with antioxidant activities, like CAT and SOD mimics, regulate ROS to treat diseases related to oxidative stress, while pro-oxidant nanozymes, such as OxD and POD mimics, generate ROS in vivo, enabling applications in anticancer, anti-inflammatory, etc.60 For example, PtMo-Au nanozyme with CAT-like activity was developed for tumor therapy by continuously catalyzing the decomposition of H2O2 to O2.8 Apart from tumor therapy, anti-oxidant activities are also used to protect cells and tissues by decreasing ROS and relieving oxidative stress. For instance, by scavenging UV-induced cellular ROS, surface-functionalized Au–Pt NCs nanozymes protected cells in vivo from the UV-induced oxidative stress damage.201 Ming et al. designed and fabricated a Pd–Ru/uricase@red blood cell nanoreactor that could efficiently degrade H2O2 through its CAT-like activity,189 facilitating uricase oxidation and enabling the safe and effective treatment of hyperuricemia with minimal side effects. In a study model of lipopolysaccharide-induced brain injury, the SOD-like activity of PdPtMo nanozymes helped to treat the lipid peroxidation, with increased survival rate of injured mice. With their ability in regulating ROS, nanozymes with antioxidant activities are also applied in H2O2-associated inflammation.202 Chen et al. synthesized a CeO2/Pt nanozyme in cross-linked zwitterionic vesicles, which could resist protein adsorption efficiently and hold a good therapeutic effect in ROS-induced ear inflammation.142 In addition to therapeutic function, Pt-based nanozyme heterostructures, like the Pt@PCN222-Mn with SOD- and CAT-like activities, were also used for anti-inflammatory therapy, which could effectively relieve two forms of inflammatory bowel disease, ulcerative colitis and Crohn's disease, by catalyzing the anti-ROS cascade reaction in vivo.155 In another example, bovine serum albumin-coated RuO2 NPs were reported as SOD- and CAT-like nanozymes to transform O2˙− into H2O2 and subsequently decompose H2O2 into oxygen, which remarkably reduced the infarcted area and restored cardiac function in ischemic myocardium.203Different from antioxidant nanozymes, pro-oxidant nanozymes play a role by generating a large amount of ROS for disease treatment. For example, biocompatible Zr-based MOFs with an Ir-porphyrin linker exhibited remarkable stability, strong H2O2 adsorption energy, and high POD-like catalytic activity, inducing abundant ROS for an effective antitumor effect.204 In another work, Jia et al. reported an AgPd bimetallic nanozyme with significantly enhanced POD-like activity which efficiently decomposed H2O2 to generate a large number of ˙OH in an acidic environment for ROS-killing-tumor therapy.67
Apart from the excellent enzyme-like activities of noble metal-based nanozymes, their unique physicochemical properties can also be integrated for multifunctional disease treatment. For instance, noble metal-based nanozymes can convert captured light energy into thermal energy, thereby increasing temperature and acting as PTT agents for synergistic treatment.205 Zhu et al. synthesized Ti3C2Tx-Pt-PEG nanocomposites, which exhibited a significant temperature increase to 48.1 °C within 5 minutes under irradiation with NIR-II light at 1064 nm. The high photothermal conversion efficiency (η = 31.78%) and stability of Ti3C2Tx-Pt-PEG nanocomposites ensure their potential in killing cancer cells.141 As expected, the POD-like activities were enhanced with temperature, generating more highly toxic ˙OH to kill breast cancer cells (Fig. 10a). In vitro experiments confirmed their efficacy in phototheragnostics, demonstrating synergistic ablation of cancer cells (Fig. 10b and c). In another similar work, OxD-like activities of RhRu nanozymes were enhanced upon exposure to a 1064 nm laser that resulted in a temperature increase. The OxD-like activities were found to be 1.33-fold higher than those of counterparts without irradiation. Additionally, noble metal-based nanozymes have been reported as photosensitizers for PDT. During the PDT process, non-toxic photosensitizers are activated by irradiation to generate ROS, such as ˙OH (type I PDT against hypoxia) and 1O2 (type II PDT against hypoxia). For instance, a Ru–Te nanorod-based enzymatic platform showed a synergistic effect of PDT and enzymatic treatment, showing high efficiency in hypoxic pancreatic cancer phototherapy both in vitro and in vivo. The O2 produced by the CAT-like Ru–Te NRs could promote the generation of 1O2via the type II photodynamic reaction, while the POD- and SOD-like reactions of Ru–Te NRs helped overcome the rapid quenching of ˙OH and H2O2 species generated from the type I photodynamic process under hypoxic conditions (Fig. 10d).206 Similar to PDT, sonodynamic therapy (SDT) is also a promising non-invasive therapeutic modality, based on sonosensitizers activated by low-intensity ultrasound to produce ROS for cancer therapy. Recently, PtCu3 nanocages were synthesized and utilized as sonosensitizers to generate ROS, including ˙OH and 1O2.47In vitro and in vivo studies of PtCu3 nanocages confirmed their ability to generate ROS under ultrasound irradiation, which effectively delayed the tumor growth.47 In another work, multifunctional nanozymes composed of Au NPs, carbon dots, and TiO2 hollow nanospheres showed multiple enzyme-like activities and ultrasonic response, which could form sufficient oxygen to alleviate the hypoxia condition in the tumor microenvironment and generate abundant ROS.207
 | ||
| Fig. 10 (a) Schematic illustration of photothermal-enhanced enzymatic catalytic activities for cancer treatment using Ti3C2Tx-Pt-PEG nanocomposites, where the temperature increase significantly enhances the POD-like activity. (b) The calcein acetoxymethyl ester (green) and propidium iodide (red) double-staining assay and (c) flow cytometry analysis of 4T1 cells incubated with different treatments (T1: control group without any treatment; T2: laser only; T3: Ti3C2Tx-PEG nanocomposites; T4: Ti3C2Tx-PEG nanocomposites + laser; T5: Ti3C2Tx-Pt-PEG nanocomposites; T6: Ti3C2Tx-Pt-PEG nanocomposites + laser). Reproduced with permission from ref. 141, Copyright 2022, American Chemical Society. (d) Schematic illustration showing the synergistic SOD-, CAT-, and POD-like enzymatic cycle (indicated by straight arrows) and the photocatalytic cycle (indicated by curved arrows) for cancer treatment. (e) Schematic illustration showing how the simultaneous SOD-, CAT-, and POD-like enzymatic activities of Ru–Te nanorods generate O2 and ROS to overcome hypoxia in photodynamic therapy. The table records the positive and negative feedback of O2 or ROS in different enzymatic and photodynamic processes. Reproduced with permission from ref. 206, Copyright 2020, American Chemical Society. | ||
Immunotherapy is a unique treatment methodology that fights disease by activating or suppressing the patient's immune system. For example, cGAS-STING, short for cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS)-stimulator of interferon genes, has been identified as an innate immune signaling pathway to induce interferon production and therapeutic effects.18 Li et al. found that the Mn2+ in immune-responsive PtMnIr nanozymes could activate the cGAS-STING pathway with increased expression of interferons, which helped to prevent tumor metastasis.18 In addition, AuPtAg-GOx nanozymes were developed for synergistic tumor immunotherapy, demonstrating their potential in cancer treatment.208 Furthermore, PdPtCu trimetallic nanozymes were reported to trigger immunogenic cell death and activate the antitumor immune response, highlighting the role they can play in advancing immunotherapeutic strategies.11
5. Conclusion and perspective
In this review, we systematically summarize recent progress in the field of noble metal-based nanozymes, focusing on their structural regulations and biomedical applications. As structural factors are closely correlated with the exposed surface atoms and active sites, a broad spectrum of examples are used to illustrate that the enzyme-like activities of noble metal-based nanozymes can be tuned by size, morphologies, strain, crystallinity, alloying, ligand, and chemical compositions. Furthermore, the roles of noble metal-based nanozymes as catalysts are discussed for enzyme-like reactions including POD, CAT, OxD, SOD, and so on. Synergistic catalysis involving multi-enzyme mimics as well as highly specific selective catalysis are also critical for enzyme mimics in advanced biomedical applications. Consequently, noble metal-based nanozymes have shown great potential to replace natural enzymes in sensors, antibiosis, and disease treatment. Several multimodal treatments utilizing unique physicochemical properties of noble metal-based nanozymes, including PTT, PDT, and SDT, are also discussed. Despite remarkable progress in this area, many challenges and opportunities are present in the further development of noble metal-based nanozymes.5.1 Fine structural design of noble metal-based nanozymes
While significant advancements in structural control of noble metal nanomaterials have been made over the past decade, precisely controlled nanostructures with specific facets, crystallinity, and components remain limited in the field of nanozymes. For example, noble metal-based nanomaterials with tailorable atomic arrangements, including intermetallic compounds and unconventional-phase metals, have rarely been applied as nanozymes. Currently, there is insufficient fundamental research focusing on the design and synthesis of fine noble metal-based nanocrystals towards enhanced enzymatic activity, stability, and/or dispersity. More efforts are desired to develop novel noble metal-based nanozymes and to gain deeper insights into their rational structural design towards specific nanozyme applications. Thanks to the development of computational technology, artificial intelligence (AI) may further assist in novel nanozyme prediction, design and performance optimization. Through AI-driven design and experimental validation, we can further accelerate the creation and optimization of nanozyme structures to greatly widen the nanozyme library.5.2 Structure–“enzyme-like property”–performance relationships
Although some investigations have been made into the structural effects on enzymatic performances of noble metal-based nanozymes, the interpretation of the structure–performance relationship is generally vague, and sometimes even contradictory. For example, alloying Au into Ag(111) facet has been reported to enhance OxD-like activities, whereas the opposite effect occurs when alloying Au into Pd(111) facets.74 The enzyme-like properties, such as binding strength of substrates, electron transfer, and product release, lack specific investigation. Therefore, experimental designs to systematically investigate the structure–“enzyme-like property”–performance relationships are crucial for the development of noble metal-based nanozymes. Besides, the exploration of enzymatic performance primarily focuses on POD- and OxD-like activities, while research on the other types of enzyme activity is relatively limited.Meanwhile, the commonly existing multiple enzyme-like activities and specificity in nanozymes have become key issues in disease diagnosis and treatment. To take full advantage of the self-cascade synergistic catalysis, the synergy between different enzymatic activities must be sorted out and correlated to specific structural factors of a nanozyme. Additionally, research on specificity of noble metal-based nanozymes is still in the early stage and needs more in-depth research focus.
5.3 In-depth catalytic mechanisms study
Despite considerable efforts, studies on the working mechanisms of noble metal-based nanozymes are still limited to specific microenvironments and idealized conditions, making theoretical results deviate from experimental ones. Additionally, limitations in characterization techniques make it challenging to obtain direct experimental evidence to verify the key catalytic sites of nanozymes, the dynamic changes during the catalytic process, or the critical intermediates. An integration of high-throughput experiments, theoretical calculations, and advanced characterizations (e.g., in situ characterizations) will provide deeper mechanistic insights in various biochemical reactions.5.4 Exploration of multifunctional nanozymes
Noble metal-based nanozymes integrate their unique physicochemical properties with enzyme-like activity, endowing them with multiple functionalities. Further expanding the functionalities of noble metal-based nanozymes by introducing more functional components could be a promising research direction. For example, noble metal nanozymes can be easily magnetically recycled by introducing magnetic components, or can act as SDT agents when combined with sonosensitizers. Future exploration of noble metal-based nanozymes with expanded functionalities could enable innovative applications in more practical scenarios.5.5 Biosafety
The biosafety of nanozymes has been a critical issue in biomedical applications. Although noble metal-based nanozymes have shown great potential in antioxidation, antibiosis, disease treatment, biosensing, and bioimaging, their biosafety, which may be affected by various factors such as concentration, temperature, and pH value, remains a significant concern. While some structural regulation strategies (e.g., reducing size and surface modification) have been developed to mitigate the toxicity of noble metal based-nanozymes, their biosafety in practical applications has not been comprehensively assessed.5.6 Towards practical applications
Although various noble metal-based nanozymes have been reported with significant potential in biomedical applications, further breakthroughs are essential towards their practical applications. Firstly, large-scale, commercial synthesis of these nanozymes has not yet been achieved, and their structural control is often sensitive to reaction conditions, limiting the conversion of products from laboratory to industry. Secondly, while a diverse range of noble metal-based nanozymes have been reported, there is a lack of a comprehensive database covering their key information, such as cost, synthetic methods, and functionality. AI-based machine learning holds great potential for information integration in this field. By using AI-assisted technology, we can establish a database that includes the classification, fabrication, and performance evaluation of nanozymes, including noble metals and others. The database would be beneficial for academic research and large-scale production of nanozymes. Finally, to facilitate the widespread application of noble metal-based nanozymes, it is crucial to develop a standardized evaluation system that quantitatively assesses key parameters of nanozymes such as morphology, dispersibility, stability, enzyme-like activity, and biosafety.In summary, we believe that noble metal-based nanozymes possess great potential for biomedical applications, relying not only on their inherently catalytic activity but also on their unique physicochemical properties. Structural regulation is an effective method for modulating enzyme-like activity, specificity, and multifunctionality in noble metal-based nanozymes. Despite the challenges and obstacles, future development of noble metal-based nanozymes is expected to address these issues and unlock new possibilities in biomedical applications and beyond.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. C. is grateful for the funding support from the NSFC Young Scientists Fund (project no. 22305203), the support from the Start-up Fund (project no. 4930977) from The Chinese University of Hong Kong, and the support from the Hong Kong Branch of the National Precious Metals Material Engineering Research Center (NPMM) at City University of Hong Kong.References
- X. T. Ding, Z. Zhao, Y. F. Zhang, M. L. Duan, C. Z. Liu and Y. H. Xu, Small, 2023, 19, 2207142 CrossRef CAS PubMed.
- M. Wittwer, U. Markel, J. Schiffels, J. Okuda, D. F. Sauer and U. Schwaneberg, Nat. Catal., 2021, 4, 814–827 Search PubMed.
- R. F. Zhang, X. Y. Yan and K. L. Fan, Acc. Mater. Res., 2021, 2, 534–547 CrossRef CAS.
- Y. Y. Huang, J. S. Ren and X. G. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- J. J. X. Wu, S. R. Li and H. Wei, Nanoscale Horiz., 2018, 3, 367–382 RSC.
- L. Li, Y. Hu, Y. Shi, Y. Liu, T. Liu, H. Zhou, W. Niu, L. Zhang, J. Zhang and G. Xu, Chem. Eng. J., 2023, 463, 142494 CrossRef CAS.
- M. W. Ji, M. Xu, W. Zhang, Z. Z. Yang, L. Huang, J. J. Liu, Y. Zhang, L. Gu, Y. X. Yu, W. C. Hao, P. F. An, L. R. Zheng, H. S. Zhu and J. T. Zhang, Adv. Mater., 2016, 28, 3094–3101 CrossRef CAS PubMed.
- M. J. Chao, S. M. Tai, M. X. Mao, W. B. Cao, C. F. Peng, W. Ma, Y. W. Feng and Z. P. Wang, Aggregate, 2025, 6, e644 CrossRef CAS.
- F. Wei, X. Y. Cui, Z. Wang, C. C. Dong, J. D. Li and X. J. Han, Chem. Eng. J., 2021, 408, 127240 CrossRef CAS PubMed.
- Y. Y. Zhao, J. Yang, G. Y. Shan, Z. Y. Liu, A. Cui, A. L. Wang, Y. W. Chen and Y. C. Liu, Sens. Actuators, B, 2020, 305, 127420 CrossRef CAS.
- Y. L. Xie, M. Wang, Y. R. Qian, L. Li, Q. Q. Sun, M. H. Gao and C. X. Li, Small, 2023, 19, 2303596 CrossRef CAS PubMed.
- G. Q. Song, X. Y. Shao, C. Qu, D. H. Shi, R. Jia, Y. F. Chen, J. P. Wang and H. L. An, Chem. Eng. J., 2023, 477, 147161 CrossRef CAS.
- Y. J. Tang, Y. J. Chen, Y. Wu, W. Q. Xu, Z. Luo, H. R. Ye, W. L. Gu, W. Y. Song, C. Z. Zhu and S. J. Guo, Nano Lett., 2023, 23, 267–275 CrossRef CAS PubMed.
- M. D. Lei, X. L. Ding, J. Liu, Y. J. Tang, H. X. Chen, Y. Zhou, C. Z. Zhu and H. Y. Yan, Anal. Chem., 2024, 96, 6072–6078 CrossRef CAS PubMed.
- Z. Xi, W. W. Gao and X. H. Xia, ChemBioChem, 2020, 21, 2440–2444 CrossRef CAS PubMed.
- S. S. Li, B. L. Xu, H. K. Yang, C. Zhang, J. L. Chen, S. Liu, Z. J. Huang and H. Y. Liu, Small, 2024, 20, 2309704 CrossRef CAS PubMed.
- Z. Xi, W. W. Gao, A. Biby, A. Floyd and X. H. Xia, ACS Appl. Nano Mater., 2022, 5, 6089–6093 CrossRef CAS.
- D. Y. Li, E. Ha, Z. L. Zhou, J. G. Zhang, Y. Y. Zhu, F. J. Ai, L. Yan, S. Q. He, L. Li and J. Q. Hu, Adv. Mater., 2024, 36, 2308747 Search PubMed.
- J. Yang, L. Fang, R. B. Jiang, L. B. Qi, Y. T. Xiao, W. X. Wang, I. Ismail and X. H. Fang, Adv. Healthcare Mater., 2023, 12, 2300490 CrossRef CAS PubMed.
- W. G. Wang, Q. Q. Cao, J. He, Y. F. Xie, Y. Zhang, L. Yang, M. H. Duan, J. K. Wang and W. Li, Nano Lett., 2024, 24, 8311–8319 CrossRef CAS PubMed.
- J. P. Wang, J. Y. Sun, W. Hu, Y. H. Wang, T. M. Chou, B. L. Zhang, Q. Zhang, L. Ren and H. J. Wang, Adv. Mater., 2020, 32, 2001862 CrossRef CAS PubMed.
- C. Yang and L. F. Cui, Green Energy Environ., 2023, 8, 1–3 Search PubMed.
- C. S. Shang, Q. Q. Wang, H. Tan, S. Y. Lu, S. G. Wang, Q. H. Zhang, L. Gu, J. Li, E. R. Wang and S. J. Guo, JACS Au, 2022, 2, 2453–2459 CrossRef CAS PubMed.
- Y. Jv, B. X. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- D. T. Xu, L. Y. Wu, H. D. Yao and L. N. Zhao, Small, 2022, 18, 2203400 CrossRef CAS PubMed.
- Z. F. Wang, Y. Yan, C. Li, Y. Yu, S. Cheng, S. Chen, X. J. Zhu, L. P. Sun, W. Tao, J. W. Liu and F. Wang, ACS Nano, 2022, 16, 9019–9030 CrossRef CAS PubMed.
- Y. M. Sun, S. D. Mu, Z. Y. Xing, J. S. Guo, Z. H. Wu, F. Y. Yu, M. R. Bai, X. L. Han, C. Cheng and L. Ye, Adv. Mater., 2022, 34, 2206208 CrossRef CAS PubMed.
- Y. Wu, W. Q. Xu, L. Jiao, Y. J. Tang, Y. F. Chen, W. L. Gu and C. Z. Zhu, Mater. Today, 2022, 52, 327–347 CrossRef CAS.
- W. W. He, Y. Liu, J. S. Yuan, J. J. Yin, X. C. Wu, X. N. Hu, K. Zhang, J. B. Liu, C. Y. Chen, Y. L. Ji and Y. T. Guo, Biomaterials, 2011, 32, 1139–1147 CrossRef CAS PubMed.
- J. Lee, M. H. Kim, H. Lee, J. Kim, J. Seo, H. W. Lee, C. Hwang and H. K. Song, Angew. Chem., Int. Ed., 2023, 62, e202312928 CrossRef CAS PubMed.
- H. Q. Zhao, R. F. Zhang, X. Y. Yan and K. L. Fan, J. Mater. Chem. B, 2021, 9, 6939–6957 RSC.
- G. P. Xu, X. C. Du, W. J. Wang, Y. Y. Qu, X. D. Liu, M. W. Zhao, W. F. Li and Y. Q. Li, Small, 2022, 18, 2204131 CrossRef CAS PubMed.
- N. A. Bakar, N. N. Yusoff, F. S. N. Azmi and J. G. Shapter, Aggregate, 2023, 4, e339 CrossRef.
- S. Fiorito, N. Soni, N. Silvestri, R. Brescia, H. GavilOn, J. S. Conteh, B. T. Mai and T. Pellegrino, Small, 2022, 18, 2200174 CrossRef CAS PubMed.
- J. D. Wu, Q. H. Liu, D. X. Jiao, B. Tian, Q. Wu, X. Chang, H. Y. Chu, S. Jiang, Q. Yang, T. Liu, Y. Zhang, W. Zhang, J. C. Fan, X. Q. Cui and F. F. Chen, Angew. Chem., Int. Ed., 2024, 63, e202403203 CrossRef CAS PubMed.
- C. P. Liu, T. H. Wu, C. Y. Liu, K. C. Chen, Y. X. Chen, G. S. Chen and S. Y. Lin, Small, 2017, 13, 1700278 CrossRef PubMed.
- C. Y. Cao, H. Zou, N. Yang, H. Li, Y. Cai, X. J. Song, J. J. Shao, P. Chen, X. Z. Mou, W. J. Wang and X. C. Dong, Adv. Mater., 2021, 33, 2106996 CrossRef CAS PubMed.
- Z. Q. Lv, S. J. He, Y. F. Wang and X. Y. Zhu, Adv. Healthcare Mater., 2021, 10, 2001806 CrossRef CAS PubMed.
- X. Y. Cui, M. H. Li, L. Tong, M. J. Li, X. F. Tang and X. J. Han, Colloids Surf., B, 2023, 223, 113168 CrossRef CAS PubMed.
- Y. M. Ju, H. L. Zhang, J. Yu, S. Y. Tong, N. Tian, Z. Y. Wang, X. B. Wang, X. T. Su, X. Chu, J. Lin, Y. Ding, G. J. Li, F. G. Sheng and Y. L. Hou, ACS Nano, 2017, 11, 9239–9248 CrossRef CAS PubMed.
- Y. Yang, M. Chen, B. Z. Wang, P. Wang, Y. C. Liu, Y. Zhao, K. Li, G. S. Song, X. B. Zhang and W. H. Tan, Angew. Chem., Int. Ed., 2019, 58, 15069–15075 CrossRef CAS PubMed.
- W. Duan, J. L. Wang, X. M. Peng, S. F. Cao, J. J. Shang, Z. W. Qiu, X. Q. Lu and J. B. Zeng, Biosens. Bioelectron., 2023, 223, 115022 CrossRef CAS PubMed.
- Y. Huang, Y. Q. Gu, X. Y. Liu, T. T. Deng, S. Dai, J. F. Qu, G. H. Yang and L. L. Qu, Biosens. Bioelectron., 2022, 209, 114253 CrossRef CAS PubMed.
- M. Xu, X. Zhang, B. W. Dong, W. J. Wang and Z. H. Zhao, Part. Part. Syst. Charact., 2023, 41, 2300135 Search PubMed.
- L. Ding, Y. Chen, B. Zhang, R. Z. Hu, C. Dai, C. H. Dong and H. Huang, ACS Nano, 2022, 16, 15959–15976 CrossRef PubMed.
- X. Meng, H. Fan, L. Chen, J. He, C. Hong, J. Xie, Y. Hou, K. Wang, X. Gao, L. Gao, X. Yan and K. Fan, Nat. Commun., 2024, 15, 1626 CrossRef CAS PubMed.
- X. Y. Zhong, X. W. Wang, L. Cheng, Y. A. Tang, G. T. Zhan, F. Gong, R. Zhang, J. Hu, Z. Liu and X. L. Yang, Adv. Funct. Mater., 2020, 30, 1907954 CrossRef CAS.
- F. Xia, X. Hu, B. Zhang, X. Wang, Y. A. Guan, P. H. Lin, Z. Y. Ma, J. P. Sheng, D. S. Ling and F. Y. Li, Small, 2022, 18, 2201558 CrossRef CAS PubMed.
- Y. Chong, X. Dai, G. Fang, R. F. Wu, L. Zhao, X. C. Ma, X. Tian, S. Y. Lee, C. Zhang, C. Y. Chen, Z. F. Chai, C. C. Ge and R. H. Zhou, Nat. Commun., 2018, 9, 4861 CrossRef PubMed.
- S. Yougbare, H. L. Chou, C. H. Yang, D. I. Krisnawati, A. Jazidie, M. Nuh and T. R. Kuo, J. Hazard. Mater., 2021, 407, 124617 CrossRef CAS PubMed.
- H. Z. Fan, J. J. Zheng, J. Y. Xie, J. W. Liu, X. F. Gao, X. Y. Yan, K. L. Fan and L. Z. Gao, Adv. Mater., 2023, 36, 2300387 Search PubMed.
- Z. Xi, K. C. Wei, Q. X. Wang, M. J. Kim, S. H. Sun, V. Fung and X. H. Xia, J. Am. Chem. Soc., 2021, 143, 2660–2664 CrossRef CAS PubMed.
- X. M. Meng, W. C. Zuo, P. C. Wu, Y. H. Song, G. J. Yang, S. B. Zhang, J. Yang, X. P. Zou, W. L. Wei, D. H. Zhang, J. J. Dai and Y. M. Ju, Nano Lett., 2023, 24, 51–60 CrossRef PubMed.
- S. H. Tang, M. Chen and N. F. Zheng, Small, 2014, 10, 3139–3144 CrossRef CAS PubMed.
- D. Wen, K. Li, R. P. Deng, J. Feng and H. J. Zhang, J. Am. Chem. Soc., 2023, 145, 3952–3960 CrossRef CAS PubMed.
- Y. Y. Li, Z. H. Zeng, J. J. Tong, T. Yang, G. H. Liu, B. Feng, P. Zhang, X. F. Liu and T. P. Qing, Appl. Surf. Sci., 2024, 655, 159695 CrossRef CAS.
- S. S. Li, B. L. Xu, M. Z. Lu, M. X. Sun, H. K. Yang, S. Liu, Z. J. Huang and H. Y. Liu, Adv. Mater., 2022, 34, 2202609 CrossRef CAS PubMed.
- Y. F. Shi, Z. H. Lyu, M. Zhao, R. H. Chen, Q. N. Nguyen and Y. N. Xia, Chem. Rev., 2021, 121, 649–735 CrossRef CAS PubMed.
- H. L. Liu, F. Nosheen and X. Wang, Chem. Soc. Rev., 2015, 44, 3056–3078 RSC.
- L. Su, S. N. Qin, Z. J. Xie, L. Wang, K. Khan, A. K. Tareen, D. F. Li and H. Zhang, Coord. Chem. Rev., 2022, 473, 214784 CrossRef CAS.
- C. Ma, W. W. Chen, Y. J. Wu, W. B. Wang, L. Xu, C. S. Chen, L. Zheng, G. Wang, P. Han, P. Gu, X. Wang, Y. Zhu, Z. Y. Zeng, H. Y. He, Q. Y. He, Z. H. Ke, D. Su and Y. Chen, Nano Lett., 2025, 25, 3212–3220 CrossRef CAS PubMed.
- R. F. Wu, Y. Chong, G. Fang, X. M. Jiang, Y. Pan, C. Y. Chen, J. J. Yin and C. C. Ge, Adv. Funct. Mater., 2018, 28, 1801484 CrossRef.
- G. T. Yuan, S. T. Zhang, Z. X. Yang, S. J. Wu, H. J. Chen, X. Tian, S. Cheng, Y. Pan and R. H. Zhou, Biomater. Sci., 2022, 10, 7067–7076 RSC.
- Y. N. Liu, D. L. Huo, X. F. Zhu, X. Chen, A. G. Lin, Z. Jia and J. Liu, Nanoscale, 2021, 13, 14900–14914 RSC.
- H. F. Zhang, J. Tan, X. Q. Yang, Y. Y. Ma, H. Y. Zou, Y. Wang, P. Zhang and Y. Q. Zheng, ACS Appl. Nano Mater., 2022, 5, 10818–10828 CrossRef CAS.
- H. H. Ye, J. Mohar, Q. X. Wang, M. Catalano, M. J. Kim and X. H. Xia, Sci. Bull., 2016, 61, 1739–1745 CrossRef CAS.
- T. Jia, D. Li, J. R. Du, X. K. Fang, V. Gerasimov, H. Ågren and G. Y. Chen, J. Nanobiotechnol., 2022, 20, 424 CrossRef CAS PubMed.
- X. Wang, S. J. Chen, X. M. Tang, D. Q. Lin and P. Qiu, RSC Adv., 2019, 9, 36578–36585 RSC.
- R. T. P. da Silva, M. P. D. Rodrigues, G. F. B. Davilla, A. M. R. P. da Silva, A. H. B. Dourado and S. I. C. de Torresi, ACS Appl. Nano Mater., 2021, 4, 12062–12072 CrossRef CAS.
- Z. Xi, J. Xie, J. Hu, Q. C. Wang, Z. Y. Wang, X. Q. Yang, L. Y. Zong, M. Y. Zhang, X. H. Sun, S. H. Sun and J. Han, Nano Lett., 2024, 24, 3432–3440 CrossRef CAS PubMed.
- S. Y. Xu, X. J. Dong, S. Q. Chen, Y. Y. Zhao, G. Y. Shan, Y. C. Sun, Y. W. Chen and Y. C. Liu, Sens. Actuators, B, 2019, 281, 375–382 CrossRef CAS.
- G. Fang, W. F. Li, X. M. Shen, J. M. Perez-Aguilar, Y. Chong, X. F. Gao, Z. F. Chai, C. Y. Chen, C. C. Ge and R. H. Zhou, Nat. Commun., 2018, 9, 129 CrossRef PubMed.
- S. S. Li, K. Gu, H. Wang, B. L. Xu, H. W. Li, X. H. Shi, Z. J. Huang and H. Y. Liu, J. Am. Chem. Soc., 2020, 142, 5649–5656 Search PubMed.
- X. M. Shen, W. Q. Liu, X. J. Gao, Z. H. Lu, X. C. Wu and X. F. Gao, J. Am. Chem. Soc., 2015, 137, 15882–15891 CrossRef CAS PubMed.
- J. S. Zhang, Y. N. Liu, L. S. Cui, S. J. Hao, D. Q. Jiang, K. Y. Yu, S. C. Mao, Y. Ren and H. Yang, Adv. Mater., 2020, 32, 1904387 Search PubMed.
- D. B. Kim, J. Y. Kim, J. Han and Y. S. Cho, Nano Energy, 2024, 125, 109551 Search PubMed.
- X. B. Yang, Y. Y. Wang, X. L. Tong and N. J. Yang, Adv. Energy Mater., 2022, 12, 2102261 CrossRef CAS.
- L. Wang, Z. H. Zeng, W. P. Gao, T. Maxson, D. Raciti, M. Giroux, X. Q. Pan, C. Wang and J. Greeley, Science, 2019, 363, 870–874 Search PubMed.
- H. Chen, B. Zhang, X. Liang and X. X. Zou, Chin. J. Catal., 2022, 43, 611–635 CrossRef CAS.
- G. G. Liu, W. Zhou, Y. R. Ji, B. Chen, G. T. Fu, Q. B. Yun, S. M. Chen, Y. X. Lin, P. F. Yin, X. Y. Cui, J. W. Liu, F. Q. Meng, Q. H. Zhang, L. Song, L. Gu and H. Zhang, J. Am. Chem. Soc., 2021, 143, 11262–11270 CrossRef CAS PubMed.
- C. R. Kao, A. H. Yeh, B. H. Chen, L. M. Lyu, Y. C. Chuang, B. T. Sneed and C. H. Kuo, Chem. Mater., 2022, 34, 2282–2291 Search PubMed.
- Z. Xi, X. Cheng, Z. Q. Gao, M. J. Wang, T. Cai, M. Muzzio, E. Davidson, O. Chen, Y. Jung, S. H. Sun, Y. Xu and X. H. Xia, Nano Lett., 2020, 20, 272–277 CrossRef CAS PubMed.
- X. H. Xia, J. T. Zhang, N. Lu, M. J. Kim, K. Ghale, Y. Xu, E. McKenzie, J. B. Liu and H. H. Yet, ACS Nano, 2015, 9, 9994–10004 Search PubMed.
- Y. Chen, Z. C. Lai, X. Zhang, Z. X. Fan, Q. Y. He, C. L. Tan and H. Zhang, Nat. Rev. Chem., 2020, 4, 243–256 CrossRef CAS PubMed.
- L. Zheng, Y. J. Wu, K. K. Li, G. Wang, C. Ma, C. S. Chen, P. Han, Y. Zhu, Z. H. Ke and Y. Chen, ACS Mater. Lett., 2024, 6, 4149–4157 CrossRef CAS.
- J. J. Ge, P. Q. Yin, Y. Chen, H. F. Cheng, J. W. Liu, B. Chen, C. L. Tan, P. F. Yin, H. X. Zheng, Q. Q. Li, S. M. Chen, W. J. Xu, X. Q. Wang, G. Wu, R. B. Sun, X. H. Shan, X. Hong and H. Zhang, Adv. Mater., 2021, 33, 2006711 CrossRef CAS PubMed.
- G. Wu, X. S. Zheng, P. X. Cui, H. Y. Jiang, X. Q. Wang, Y. T. Qu, W. X. Chen, Y. Lin, H. Li, X. Han, Y. M. Hu, P. G. Liu, Q. H. Zhang, J. J. Ge, Y. C. Yao, R. B. Sun, Y. Wu, L. Gu, X. Hong and Y. D. Li, Nat. Commun., 2019, 10, 4855 CrossRef PubMed.
- Y. J. Tang, Y. Wu, W. Q. Xu, L. Jiao, Y. F. Chen, M. Sha, H. R. Ye, W. L. Gu and C. Z. Zhu, Anal. Chem., 2022, 94, 1022–1028 CrossRef CAS PubMed.
- N. L. Yang, H. F. Cheng, X. Z. Liu, Q. B. Yun, Y. Chen, B. Li, B. Chen, Z. C. Zhang, X. P. Chen, Q. P. Lu, J. T. Huang, Y. Huang, Y. Zong, Y. H. Yang, L. Gu and H. Zhang, Adv. Mater., 2018, 30, 1803234 CrossRef PubMed.
- Y. Y. Ge, J. J. Ge, B. Huang, X. X. Wang, G. G. Liu, X. H. Shan, L. Ma, B. Chen, G. H. Liu, S. M. Du, A. Zhang, H. F. Cheng, Q. Wa, S. Y. Lu, L. J. Li, Q. B. Yun, K. Yuan, Q. X. Luo, Z. J. Xu, Y. H. Du and H. Zhang, Nano Res., 2023, 16, 4650–4655 CrossRef CAS.
- Z. Lyu, J. L. Cai, X. G. Zhang, H. Q. Li, H. P. Huang, S. P. Wang, T. Y. Li, Q. X. Wang, Z. X. Xie and S. F. Xie, Adv. Mater., 2024, 36, 2314252 CrossRef CAS PubMed.
- W. C. Wang, X. T. Shi, T. N. He, Z. R. Zhang, X. L. Yang, Y. J. Guo, B. Chong, W. M. Zhang and M. S. Jin, Nano Lett., 2022, 22, 70287033 Search PubMed.
- P. F. Yin, M. Zhou, J. Z. Chen, C. L. Tan, G. G. Liu, Q. L. Ma, Q. B. Yun, X. Zhang, H. F. Cheng, Q. P. Lu, B. Chen, Y. Chen, Z. C. Zhang, J. T. Huang, D. Y. Hu, J. Wang, Q. Liu, Z. Y. Luo, Z. Q. Liu, Y. Y. Ge, X. J. Wu, X. W. Du and H. Zhang, Adv. Mater., 2020, 32, 2000482 CrossRef CAS PubMed.
- W. C. Wang, T. O. He, X. L. Yang, Y. M. Liu, C. Q. Wang, J. Li, A. D. Xiao, K. Zhang, X. T. Shi and M. S. Jin, Nano Lett., 2021, 21, 3458–3464 CrossRef CAS PubMed.
- H. Y. Yan, Y. F. Chen, L. Jiao, W. L. Gu and C. Z. Zhu, Sens. Actuators, B, 2021, 341, 130007 CrossRef CAS.
- X. F. Huang, G. X. Yang, S. Li, H. J. Wang, Y. H. Cao, F. Peng and H. Yu, J. Energy Chem., 2022, 68, 721–751 Search PubMed.
- X. W. Wei, G. X. Zhu, Y. J. Liu, Y. H. Ni, Y. Song and Z. Xu, Chem. Mater., 2008, 20, 6248–6253 CrossRef CAS.
- A. Amiri, V. Yurkiv, A. H. Phakatkar, T. Shokuhfar and R. Shahbazian-Yassar, Adv. Funct. Mater., 2024, 34, 2304685 CrossRef CAS.
- H. Y. Cheng, C. X. Wang, D. Qin and Y. A. Xia, Acc. Chem. Res., 2023, 56, 900–909 CrossRef CAS PubMed.
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845–910 CrossRef CAS PubMed.
- S. S. Li, L. Shang, B. L. Xu, S. H. Wang, K. Gu, Q. Y. Wu, Y. Sun, Q. H. Zhang, H. L. Yang, F. R. Zhang, L. Gu, T. R. Zhang and H. Y. Liu, Angew. Chem., Int. Ed., 2019, 58, 12624–12631 CrossRef CAS PubMed.
- M. Zhou, C. Li and J. Y. Fang, Chem. Rev., 2021, 121, 736–795 CrossRef CAS PubMed.
- L. Kong, D. Chen, X. Zhang, L. Zhou, Y. Deng and S. Wei, ACS Appl. Nano Mater., 2023, 6, 3618–3626 CrossRef CAS.
- J. Liu, S. M. Dong, S. L. Gai, Y. S. Dong, B. Liu, Z. Y. Zhao, Y. Xie, L. L. Feng, P. P. Yang and J. Lin, ACS Nano, 2023, 17, 20402–20423 CrossRef CAS PubMed.
- D. B. Yan, L. Jiao, C. J. Chen, X. K. Jia, R. M. Li, L. J. Hu, X. T. Li, Y. L. Zhai, P. E. Strizhak, Z. J. Zhu, J. G. Tang and X. Q. Lu, Nano Lett., 2024, 24, 2912–2920 CrossRef CAS PubMed.
- X. Zong, X. R. Xu, D. W. Pang, X. L. Huang and A. A. Liu, Adv. Healthcare Mater., 2024, 2401836 Search PubMed.
- L. Zheng, L. Xu, P. Gu and Y. Chen, Nanoscale, 2024, 16, 8672–8672 RSC.
- S. Y. Xia, F. X. Wu, L. Cheng, H. B. Bao, W. P. Gao, J. Duan, W. X. Niu and G. B. Xu, Small, 2023, 19, 2205997 CrossRef CAS PubMed.
- Y. L. Zhu, R. X. Zhao, L. Feng, C. Wang, S. M. Dong, M. V. Zyuzin, A. Timin, N. S. Hu, B. Liu and P. P. Yang, ACS Nano, 2023, 17, 6833–6848 CrossRef CAS PubMed.
- B. Huang, Y. Y. Ge, A. Zhang, S. Q. Zhu, B. Chen, G. X. Li, Q. B. Yun, Z. Q. Huang, Z. Y. Shi, X. C. Zhou, L. J. Li, X. X. Wang, G. Wang, Z. Q. Guan, L. Zhai, Q. X. Luo, Z. J. Li, S. Y. Lu, Y. Chen, C. S. Lee, Y. Han, M. H. Shao and H. Zhang, Adv. Mater., 2023, 35, 2300490 Search PubMed.
- J. W. Liu, C. R. Lee, Y. Hu, Z. S. Liang, R. Ji, X. Y. D. Soo, Q. Zhu and Q. Y. Yan, SmartMat, 2023, 4, e1210 CrossRef CAS.
- H. S. Chen, T. M. Benedetti, V. R. Goncales, N. M. Bedford, R. W. J. Scott, R. F. Webster, S. Cheong, J. J. Gooding and R. D. Tilley, J. Am. Chem. Soc., 2020, 142, 3231–3239 CrossRef CAS PubMed.
- S. M. Han, C. H. He, Q. B. Yun, M. Y. Li, W. Chen, W. B. Cao and Q. P. Lu, Coord. Chem. Rev., 2021, 445, 214085 CrossRef CAS.
- S. Furukawa and T. Komatsu, ACS Catal., 2017, 7, 735–765 CrossRef CAS.
- Y. L. Zhu, X. X. Wang, L. L. Feng, R. X. Zhao, C. Yu, Y. L. Liu, Y. Xie, B. Liu, Y. Zhou and P. P. Yang, Nat. Commun., 2024, 15, 8696 CrossRef CAS PubMed.
- C. H. Zhan, Y. Xu, L. Z. Bu, H. Z. Zhu, Y. G. Feng, T. Yang, Y. Zhang, Z. Q. Yang, B. L. Huang, Q. Shao and X. Q. Huang, Nat. Commun., 2021, 12, 6261 CrossRef CAS PubMed.
- F. X. Lin, M. G. Li, L. Y. Zeng, M. C. Luo and S. J. Guo, Chem. Rev., 2023, 123, 12507–12593 CrossRef CAS PubMed.
- J. T. Ren, L. Chen, H. Y. Wang and Z. Y. Yuan, Chem. Soc. Rev., 2023, 52, 8319–8373 RSC.
- X. J. Chang, M. Q. Zeng, K. L. Liu and L. Fu, Adv. Mater., 2020, 32, 1907226 CrossRef CAS PubMed.
- Y. Q. Zhang, D. D. Wang and S. Y. Wang, Small, 2022, 18, 2104339 CrossRef CAS PubMed.
- Y. J. Ai, M. Q. He, H. Sun, X. M. Jia, L. Wu, X. Y. Zhang, H. B. Sun and Q. L. Liang, Adv. Mater., 2023, 35, 2302335 CrossRef CAS PubMed.
- R. Sheng, Y. Liu, T. M. Cai, R. Wang, G. Yang, T. Wen, F. J. Ning and H. L. Peng, Chem. Eng. J., 2024, 485, 149913 CrossRef CAS.
- J. X. Feng, X. W. Yang, T. Du, L. Zhang, P. F. Zhang, J. C. Zhuo, L. P. Luo, H. Sun, Y. R. Han, L. Z. Liu, Y. Z. Shen, J. L. Wang and W. T. Zhang, Adv. Sci., 2023, 10, 2303078 CrossRef CAS PubMed.
- M. Sha, L. Rao, W. Q. Xu, Y. Qin, R. A. Su, Y. Wu, Q. Fang, H. J. Wang, X. W. Cui, L. R. Zheng, W. L. Gu and C. Z. Zhu, Nano Lett., 2023, 23, 701–709 CrossRef CAS PubMed.
- Y. Y. Liang, F. Y. Sun, S. H. Qu, X. M. Zhou and L. Shang, Sens. Actuators, B, 2024, 417, 136069 CrossRef CAS.
- Y. Chen, X. R. Yang, K. Li, J. K. Feng, X. Y. Liu, Y. X. Li, K. Y. Yang, J. H. Li and S. H. Ge, ACS Nano, 2024, 18, 7024–7036 CrossRef CAS PubMed.
- K. Y. Wang, Q. Hong, C. X. Zhu, Y. Xu, W. Li, Y. Wang, W. H. Chen, X. Gu, X. H. Chen, Y. F. Fang, Y. F. Shen, S. Q. Liu and Y. J. Zhang, Nat. Commun., 2024, 15, 5705 CrossRef CAS PubMed.
- Y. H. Lin, Z. H. Li, Z. W. Chen, J. S. Ren and X. G. Qu, Biomaterials, 2013, 34, 2600–2610 CrossRef CAS PubMed.
- J. S. Fan, X. Y. Zhang, W. L. Tan, Z. Z. Feng and K. Li, Nano Lett., 2024, 24, 7800–7808 CrossRef CAS PubMed.
- H. T. Shan, J. F. Shi, T. K. Chen, Y. T. Cao, Q. F. Yao, H. An, Z. C. Yang, Z. H. Wu, Z. Y. Jiang and J. P. Xie, ACS Nano, 2023, 17, 23682377 Search PubMed.
- J. Zhou, X. J. Yang, Q. Yu, X. L. Li, H. Y. Chen and J. J. Xu, ACS Appl. Nano Mater., 2022, 5, 7009–7018 CrossRef CAS.
- H. Z. Fan, Y. Y. Li, J. B. Liu, R. Cai, X. S. Gao, H. Zhang, Y. L. Ji, G. J. Nie and X. C. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 45416–45426 CrossRef CAS PubMed.
- X. Liu, Y. L. Wan, T. Jiang, Y. F. Zhang, P. Huang and L. H. Tang, Chem. Commun., 2020, 56, 1784–1787 RSC.
- Y. Kang, C. Li, H. L. Shi, A. Zhang, C. S. Huang, C. H. Zhou and N. Q. Jia, Chin. J. Chem., 2023, 41, 3189–3196 CrossRef CAS.
- C. Wang, Y. Shi, Y. Y. Dan, X. G. Nie, J. Li and X. H. Xia, Chem. – Eur. J., 2017, 23, 6717 CrossRef CAS PubMed.
- X. Q. Sang, S. Y. Xia, L. Cheng, F. X. Wu, Y. Tian, C. X. Guo, G. B. Xu, Y. L. Yuan and W. X. Niu, Small, 2024, 20, 2305369 CrossRef CAS PubMed.
- J. K. Zhang, Y. Yang, F. M. Qin, T. T. Hu, X. S. Zhao, S. C. Zhao, Y. Q. Cao, Z. Gao, Z. Zhou, R. Z. Liang, C. L. Tan and Y. Qin, Adv. Healthcare Mater., 2023, 12, 2302056 CrossRef CAS PubMed.
- L. Zhao, Z. Q. Sun, Y. Wang, J. Huang, H. T. Wang, H. Li, F. Chang and Y. Y. Jiang, Acta Biomater., 2023, 170, 496–506 CrossRef CAS PubMed.
- M. S. Xu, R. X. Zhao, B. Liu, F. Geng, X. D. Wu, F. Zhang, R. F. Shen, H. M. Lin, L. L. Feng and P. P. Yang, Chem. Eng. J., 2024, 491, 151776 CrossRef CAS.
- X. R. Song, J. F. Liu, W. R. Wang, L. Ding, W. Feng, T. T. Zhang, Y. Chen and X. J. Ni, Adv. Funct. Mater., 2024, 34, 2312172 Search PubMed.
- Y. L. Zhu, Z. Wang, R. X. Zhao, Y. H. Zhou, L. L. Feng, S. L. Gai and P. P. Yang, ACS Nano, 2022, 16, 3105–3118 CrossRef CAS PubMed.
- Y. Chen, J. B. Tan, Q. Zhang, T. Xin, Y. L. Yu, Y. Nie and S. Y. Zhang, Nano Lett., 2020, 20, 6548–6555 Search PubMed.
- X. L. He, L. F. Tan, D. Chen, X. L. Wu, X. L. Ren, Y. Q. Zhang, X. W. Meng and F. Q. Tang, Chem. Commun., 2013, 49, 4643–4645 Search PubMed.
- C. C. Wang, Y. Q. Li, W. J. Yang, L. Zhou and S. H. Wei, Adv. Healthcare Mater., 2021, 10, 2100601 CrossRef CAS PubMed.
- S. H. Ku, M. Lee and C. B. Park, Adv. Healthcare Mater., 2013, 2, 244–260 CrossRef CAS PubMed.
- Y. Sun, B. L. Xu, X. T. Pan, H. Y. Wang, Q. Y. Wu, S. S. Li, B. Y. Jiang and H. Y. Liu, Coord. Chem. Rev., 2023, 475, 214896 CrossRef CAS.
- H. Wang, K. W. Wan and X. H. Shi, Adv. Mater., 2019, 31, 1805368 CrossRef CAS PubMed.
- M. J. Liang, Y. B. Wang, K. Ma, S. S. Yu, Y. Y. Chen, Z. Deng, Y. Liu and F. Wang, Small, 2020, 16, 2002348 CrossRef CAS PubMed.
- Y. J. Zhang, W. H. Gao, Y. A. Ma, L. L. Cheng, L. Zhang, Q. G. Liu, J. Y. Chen, Y. R. Zhao, K. S. Tu, M. Z. Zhang and C. Liu, Nano Today, 2023, 49, 101768 CrossRef CAS.
- L. Fan, X. D. Xu, C. H. Zhu, J. Han, L. Z. Gao, J. Q. Xi and R. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 4502–4511 CrossRef CAS PubMed.
- L. Ma, F. B. Jiang, X. Fan, L. Y. Wang, C. He, M. Zhou, S. Li, H. R. Luo, C. Cheng and L. Qiu, Adv. Mater., 2020, 32, 2003065 CrossRef CAS PubMed.
- Y. R. Xu, Y. W. Ren, X. L. Liu, H. P. Li and Z. Y. Lu, Acta Phys.-Chim. Sin., 2024, 40, 2403032 CrossRef.
- G. L. Kuang, Z. C. Wang, M. Bilal, Z. Y. Wang, Y. X. Feng, Y. J. Du and J. D. Cui, Aggregate, 2024, e724 Search PubMed.
- Y. Huang, M. T. Zhao, S. K. Han, Z. C. Lai, J. Yang, C. L. Tan, Q. L. Ma, Q. P. Lu, J. Z. Chen, X. Zhang, Z. C. Zhang, B. Li, B. Chen, Y. Zong and H. Zhang, Adv. Mater., 2017, 29, 1700102 CrossRef PubMed.
- Y. F. Liu, Y. Cheng, H. Zhang, M. Zhou, Y. J. Yu, S. C. Lin, B. Jiang, X. Z. Zhao, L. Y. Miao, C. W. Wei, Q. Y. Liu, Y. W. Lin, Y. Du, C. J. Butch and H. Wei, Sci. Adv., 2020, 6, eabb2695 CrossRef CAS PubMed.
- Y. Liu, L. Y. Zhang, Q. G. Li, H. Dai, T. Xiang, G. Y. Yang and L. Li, Anal. Chim. Acta, 2021, 1146, 24–32 CrossRef CAS PubMed.
- J. He, F. J. Xu, J. Hu, S. L. Wang, X. D. Hou and Z. Long, Microchem. J., 2017, 135, 91–99 CrossRef CAS.
- L. Zhang, S. L. Wang, G. H. Zhang, N. Shen, H. Chen, G. H. Tao, G. H. Tao, F. Yong, J. Fu, Q. H. Zhu and L. He, Cell Rep. Phys. Sci., 2022, 3, 101114 CrossRef CAS.
- Q. Q. Liu, C. C. Xu, S. Chu, S. Li, F. X. Wang, Y. M. Si, G. J. Mao, C. F. Wu and H. Wang, J. Mater. Chem. B, 2022, 10, 10075–10082 CAS.
- Y. Y. Yuan, X. X. Xi, T. Bao, P. G. Bian, F. Pei, X. H. Zhang, S. F. Wang and W. Wen, J. Anal. Test., 2024, 8, 278287 Search PubMed.
- Z. Sun, Q. S. Zhao, G. H. Zhang, Y. Li, G. L. Zhang, F. B. Zhang and X. B. Fan, RSC Adv., 2015, 5, 10352–10357 CAS.
- Y. Q. Xu, J. B. Fei, G. L. Li, T. T. Yuan, X. Xu and J. B. Li, Angew. Chem., Int. Ed., 2019, 58, 5572–5576 CrossRef CAS PubMed.
- T. R. Lin, L. S. Zhong, J. Wang, L. Q. Guo, H. Y. Wu, Q. Q. Guo, F. F. Fu and G. N. Chen, Biosens. Bioelectron., 2014, 59, 89–93 CAS.
- Z. Y. Ding, Z. Li, X. X. Zhao, Y. R. Miao, Z. F. Yuan, Y. Y. Jiang and Y. Z. Lu, J. Colloid Interface Sci., 2023, 631, 86–95 CAS.
- N. Wu, Y. T. Wang, X. Y. Wang, F. N. Guo, H. Wen, T. Yang and J. H. Wang, Anal. Chim. Acta, 2019, 1091, 69–75 CAS.
- G. G. Yang, Y. Chen, R. Shi, R. R. Chen, S. S. Gao, X. Zhang, Y. Rao, Y. Lu, Y. C. Peng, Z. H. Qing and C. X. Song, Molecules, 2023, 28, 3736 Search PubMed.
- S. R. Tang, M. L. Wang, G. W. Li, X. Li, W. Chen and L. Zhang, Microchim. Acta, 2018, 185, 273 Search PubMed.
- C. Peng, R. Y. Pang, J. Li and E. R. Wang, Adv. Mater., 2024, 36, 2211724 CAS.
- L. Jiao, H. Y. Yan, Y. Wu, W. L. Gu, C. Z. Zhu, D. Du and Y. H. Lin, Angew. Chem., Int. Ed., 2020, 59, 2565–2576 CrossRef CAS PubMed.
- L. Huang, J. X. Chen, L. F. Gan, J. Wang and S. J. Dong, Sci. Adv., 2019, 5, eaav5490 CrossRef CAS PubMed.
- S. F. Ji, B. Jiang, H. G. Hao, Y. J. Chen, J. C. Dong, Y. Mao, Z. D. Zhang, R. Gao, W. X. Chen, R. F. Zhang, Q. Liang, H. J. Li, S. H. Liu, Y. Wang, Q. H. Zhang, L. Gu, D. M. Duan, M. M. Liang, D. S. Wang, X. Y. Yan and Y. D. Li, Nat. Catal., 2021, 4, 407–417 CrossRef CAS.
- M. Y. Chang, Z. Y. Hou, M. Wang, C. Z. Yang, R. F. Wang, F. Li, D. L. Liu, T. L. Peng, C. X. Li and J. Lin, Angew. Chem., Int. Ed., 2021, 60, 12971–12979 CrossRef CAS PubMed.
- D. D. Wang, H. H. Wu, S. Z. F. Phua, G. B. Yang, W. Q. Lim, L. Gu, C. Qian, H. B. Wang, Z. Guo, H. Z. Chen and Y. L. Zhao, Nat. Commun., 2020, 11, 357 CrossRef CAS PubMed.
- R. J. Yan, S. Sun, J. Yang, W. Long, J. Y. Wang, X. Y. Mu, Q. F. Li, W. T. Hao, S. F. Zhang, H. L. Liu, Y. L. Gao, L. F. Ouyang, J. C. Chen, S. J. Liu, X. D. Zhang and D. Ming, ACS Nano, 2019, 13, 11552–11560 CrossRef CAS PubMed.
- X. M. Shen, Z. Z. Wang, X. J. J. Gao and X. F. Gao, Adv. Mater., 2024, 36, 2211151 CrossRef CAS PubMed.
- W. W. He, Y. T. Zhou, W. G. Warner, X. N. Hu, X. C. Wu, Z. Zheng, M. D. Boudreau and J. J. Yin, Biomaterials, 2013, 34, 765–773 CrossRef CAS PubMed.
- Y. Cheng, Y. D. Xia, Y. Q. Sun, Y. Wang and X. B. Yin, Adv. Mater., 2024, 36, 2308033 CrossRef CAS PubMed.
- J. N. Li, W. Q. Liu, X. C. Wu and X. F. Gao, Biomaterials, 2015, 48, 37–44 CrossRef CAS PubMed.
- X. M. Shen, Z. Z. Wang, X. F. Gao and Y. L. Zhao, ACS Catal., 2020, 10, 12657–12665 CrossRef CAS.
- Y. Chong, Q. Liu and C. C. Ge, Nano Today, 2021, 37, 101076 CrossRef CAS.
- S. B. He, L. Yang, X. L. Lin, L. M. Chen, H. P. Peng, H. H. Deng, X. H. Xia and W. Chen, Talanta, 2020, 211, 120707 CrossRef CAS PubMed.
- P. Cheng, H. Wang and X. H. Shi, Nanoscale, 2020, 12, 3050–3057 RSC.
- R. Long, K. K. Mao, X. D. Ye, W. S. Yan, Y. B. Huang, J. Y. Wang, Y. Fu, X. S. Wang, X. J. Wu, Y. Xie and Y. J. Xiong, J. Am. Chem. Soc., 2013, 135, 3200–3207 CrossRef CAS PubMed.
- W. Q. Jiang, Y. Y. Feng, C. Jiang, H. Li, Z. Wang, Y. Q. Xiao, W. Lan and Y. N. Liu, Sens. Actuators, B, 2024, 412, 135861 CrossRef CAS.
- M. Zhang, W. H. Yue, W. S. Ma, X. N. Wang, Y. H. Xu and A. H. Li, Adv. Healthcare Mater., 2024, 2401602 Search PubMed.
- J. X. Chen, Q. Ma, M. H. Li, D. Y. Chao, L. Huang, W. W. Wu, Y. X. Fang and S. J. Dong, Nat. Commun., 2021, 12, 3375 CrossRef CAS PubMed.
- S. B. He, L. Yang, M. T. Lin, H. A. A. Noreldeen, R. X. Yu, H. P. Peng, H. H. Deng and W. Chen, Sens. Actuators, B, 2021, 347, 130627 CrossRef CAS.
- A. Q. Lin, Z. Y. Sun, X. Q. Xu, S. Zhao, J. W. Li, H. Sun, Q. Wang, Q. Jiang, H. Wei and D. Q. Shi, Nano Lett., 2022, 22, 508–516 CrossRef CAS PubMed.
- J. Ming, T. B. Zhu, J. C. Li, Z. C. Ye, C. R. Shi, Z. D. Guo, J. J. Wang, X. L. Chen and N. F. Zheng, Small, 2021, 17, 2103645 CrossRef CAS PubMed.
- M. Kajita, K. Hikosaka, M. Iitsuka, A. Kanayama, N. Toshima and Y. Miyamoto, Free Radical Res., 2007, 41, 615–626 CrossRef CAS PubMed.
- C. C. Ge, G. Fang, X. M. Shen, Y. Chong, W. G. Wamer, X. F. Gao, Z. F. Chai, C. Y. Chen and J. J. Yin, ACS Nano, 2016, 10, 10436–10445 CrossRef CAS PubMed.
- H. Z. Fan, R. F. Zhang, K. L. Fan, L. Z. Gao and X. Y. Yan, ACS Nano, 2024, 18, 2533–2540 CrossRef CAS PubMed.
- J. Y. Sheng, Y. H. Wu, H. Ding, K. Z. Feng, Y. Shen, Y. Zhang and N. Gu, Adv. Mater., 2024, 36, 2211210 CrossRef CAS PubMed.
- T. T. Cai, G. Fang, X. Tian, J. J. Yin, C. Y. Chen and C. C. Ge, ACS Nano, 2019, 13, 12694–12702 CrossRef CAS PubMed.
- X. Y. Liu, X. Y. Liang, J. Yu, K. Y. Xu, J. W. Shen, W. Duan and J. B. Zeng, TrAC, Trends Anal. Chem., 2023, 169, 117386 CrossRef CAS.
- F. Arshad, N. F. Mohd-Naim, R. Chandrawati, D. Cozzolino and M. U. n. Ahmed, RSC Adv., 2022, 12, 26160–26175 RSC.
- J. N. Xia, Z. Li, Y. P. Ding, L. A. Shah, H. B. Zhao, D. X. Ye and J. J. Zhang, Anal. Chem., 2024, 96, 8221–8233 CrossRef CAS PubMed.
- X. X. Zheng, Q. Liu, C. Jing, Y. Li, D. Li, W. J. Luo, Y. Q. Wen, Y. He, Q. Huang, Y. T. Long and C. H. Fan, Angew. Chem., Int. Ed., 2011, 50, 11994–11998 CrossRef CAS PubMed.
- C. Y. Zhou, Q. Wang, H. L. Cao, J. Jiang and L. Z. Gao, Adv. Mater., 2024, 36, 2403362 CrossRef CAS PubMed.
- Y. Tao, E. G. Ju, J. S. Ren and X. G. Qu, Adv. Mater., 2015, 27, 1097–1104 CrossRef CAS PubMed.
- B. Xiong, R. L. Xu, R. Zhou, Y. He and E. S. Yeung, Talanta, 2014, 120, 262–267 CrossRef CAS PubMed.
- X. Y. Mu, J. Y. Wang, Y. H. Li, F. J. Xu, W. Long, L. F. Ouyang, H. L. Liu, Y. Q. Jing, J. Y. Wang, H. T. Dai, Q. Liu, Y. M. Sun, C. L. Liu and X. D. Zhang, ACS Nano, 2019, 13, 1870–1884 CAS.
- X. Li, X. Y. Ren, M. D. Xie, M. L. Zhu, Y. B. Zhang, T. Li, M. F. Huo and Q. Li, Adv. NanoBiomed. Res., 2023, 3, 2200144 CrossRef CAS.
- D. J. Wang, J. Wang, X. J. Gao, H. Ding, M. Yang, Z. H. He, J. Y. Xie, Z. X. Zhang, H. B. Huang, G. H. Nie, X. Y. Yan and K. L. Fan, Adv. Mater., 2024, 36, 2310033 CrossRef CAS PubMed.
- C. H. Wang, H. Y. Wang, B. L. Xu and H. Y. Liu, VIEW, 2021, 2, 20200045 CrossRef CAS.
- S. Kang, Y. G. Gil, D. H. Min and H. Jang, ACS Nano, 2020, 14, 4383–4394 CrossRef CAS PubMed.
- N. Tao, H. H. Li, L. Deng, S. F. Zhao, J. Ouyang, M. Wen, W. S. Chen, K. Zeng, C. W. Wei and Y. N. Liu, ACS Nano, 2022, 16, 485–501 CrossRef CAS PubMed.
- M. Wang, M. Y. Chang, P. Zheng, Q. Q. Sun, G. Q. Wang, J. Lin and C. X. Li, Adv. Sci., 2022, 9, 2202332 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this review. |
| This journal is © The Royal Society of Chemistry 2025 |