Use of dicationic ionic liquids as a novel liquid platform for dielectrophoretic cell manipulation
Rajeshwari Taruvai Kalyana Kumara,
Izabelle De Mello Gindrib,
David Kinnamona,
Danieli C. Rodriguesb,
Clarissa P. Frizzoc and
Shalini Prasad*a
aBiomedical Microdevices and Nanotechnology Laboratory, Department of Bioengineering, University of Texas at Dallas, Richardson, Texas 75080, USA. E-mail: shalini.prasad@utdallas.edu
bBiomaterials for Osseointegration and Novel Engineering Laboratory, Department of Bioengineering, University of Texas at Dallas, Richardson, Texas 75080, USA
cNúcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria, UFSM, CEP: 97105-900 Santa Maria, RS, Brazil
First published on 9th February 2016
Abstract
Separation, characterization and analysis of target cells demonstrate critical cues for diagnosis and monitoring of chronic diseases. Electrokinetic cell separation methods have been previously established to have greater efficiency when compared to traditional flow cytometry methods. Ionic liquids show promise in the design of conductive buffers with required electrical properties suitable for electrokinetic manipulation of cells with an enhanced signal to noise ratio (SNR). The goal of this project is to design and test tailored ionic liquid compositions with the hypothesis that dielectrophoretic forces are enhanced on cells while creating an environment for retaining cell integrity. We analysed two uniquely synthesized methylimidazolium based ionic liquids with a low toxicity as conductive suspension buffers for cell separation. These dicationic ionic liquids possess slight electrical and structural differences with high thermal stability. The two ionic liquids were tested for cytotoxicity and their ability to enhance SNR. We validated our hypothesis using osteosarcoma cells Saos-2 and MC3T3-E1 osteoblast cells. The tests were compared against commonly used dielectrophoretic sucrose-isotonic solution. The effects of electrical neutrality, charged particle effects, free charge screening due to ionic liquids from cells were studied using a single-shell model. Effects of ionic liquid and isotonic medium on electrokinetic signal from cells were studied through dielectrophoretic force profiles as a function of non-linear displacement of cells in the two ionic liquids and control media. We observed significant differences in electrokinetic responses between healthy and cancerous cells and steady increase in signal magnitude resulting in enhanced SNR using ILs when compared against sucrose buffer.
1. Introduction
Live cell separation is key for downstream molecular analysis.1 Cell stabilization in their native state is necessary for all applications pertinent to cell manipulation.2 Current state of the art technologies for cell separation can effectively identify and quantify cells of a specific sub-type through fluorophore tagging.3 Post separation, these cells lose viability making the downstream analysis of cell sequencing or genetic analysis ineffective and reducing the efficacy of these techniques for disease diagnosis and detection.4 It has been previously shown that tagging with fluorescent markers or other chemical reagents often result in cell instability and programmed cell death, also known as apoptosis.5Traditionally the choice of separation techniques are made based on properties of cells such as cell size, density and surface charges. Some of the standard cell separation techniques include centrifugation, magnetic bead separation, and dielectric isolation.6 Amongst these standard procedures, electrokinetics for cell studies has shown tremendous promise in the past two decades.7,8 This methodology enables identification and isolation of cells with distinct dielectric properties using externally applied alternating electric fields with specific signal magnitude and frequency.9 Electrokinetic techniques such as dielectrophoresis for cell separation and electrorotation for cell characterization greatly depend on input electrical signal and also the nature of the cell suspension buffer.10 Characterization of cells using electrokinetics require low conductivity buffer solution and free floating ions for polarization of cells under the application of electric fields.11
For achieving maximum cell separation efficiency, several electrokinetic techniques use selective formulations of the suspension medium to either increase the solution isotonicity or decrease the conductivity of the buffer to achieve physiological balance.4 In order to adjust the cell suspension medium, Food and Drug Administration (FDA) approves the addition of standard excipients such as sugars, salts, mannitol, HEPES, amino acids and other non-reactive compounds which are known to preserve cellular function.12 Although these additivities have been shown to improve results in cell separation and characterization, they add complexity to the sample solution and dramatically increase processing time thereby decreasing cell life time.13,14 Identifying biocompatible excipients that satisfy regulatory requirements would enhance existing electrokinetic cell separation methods to enhance cell purity and yield.15
Ionic Liquids (ILs) are salt structures which are characterized by melting temperatures below 100 °C, highly variable conductivity, high thermal stability and negligible volatility making these compounds of great interest in various applications.16 One important property of the ionic liquids are the presence of large cation and anion structures that allow for wide range of liquid viscosities and moderate specific conductivities, usually in the range same as those of aqueous electrolytes.17 Additionally, the cationic and anionic structures of ILs can be strategically modified to obtain ILs with desired features.18 Quinn and Shirota et al., have discussed the possibility of changing IL polarity, hydrophobicity and solvent miscibility to suit specific applications.16,18 The tailoring of ionic conductivity of ionic liquids (ILs), have been explored as an alternative solution for biological, biomimetic, electrolytic and chemical processes.19–21
Although ILs have attracted great interest in the field of biology and biotechnology as a highly reactive buffer with distinct properties, certain ILs can have toxic effects on human cell lines.22–25 It has been reported that ILs such as monocationic ILs upon interaction with cell membrane result in cytotoxic reactions and cell death.26 In order to overcome the deleterious effects, symmetrical dicationic imidazolium-based ILs have recently emerged as an alternative non-toxic candidate material for use as biological buffers. Dicationic ILs have two cationic moieties linked by an alkyl chain. Interestingly, these compounds have higher thermal stability than monocationic ILs. The influence of structural elements like the type of head group and anion, length of side or linkage chain and symmetry on the physicochemical properties such as conductivity, glass transition temperature, melting point have been investigated by many research groups.18 Gindri et al. investigated the difference in toxicity between monocationic and dicationic imidazolium based-ILs on bone osteoblast cells (MC3T3-E1). It was observed that the introduction of a new cationic head in the IL structure (dicationic), as opposed to one cationic head in monocationic ILs, reduced the toxicity of the compound by 20 fold.26 These recent findings motivated the study presented in this article, where dicationic imidazolium ILs were used as cell suspension medium to characterize cells of interest using electrokinetic biophysics.
In this study, our focus was on investigating the dielectrophoretic force profiles as a function of non-linear displacement movement by cells when subjected to alternating electric fields. This phenomenon is known as dielectrophoresis (DEP), in which polarized cells are synchronized with applied electric fields and experience external force on cells thus resulting in lateral displacement either towards or away from strong electric field regions. Phenomenon in which cells move towards strong electric fields is known as positive dielectrophoresis (pDEP) and cells that move away from strong fields are known as negative dielectrophoresis (nDEP).27
In this paper we have integrated the use of dicationic imidazolium based ILs as a suspension buffer for targeting cells without damaging their inherent cellular properties. Traditional dielectrophoretic isotonic buffer with allowed excipients such as sucrose and dextrose28 was used as a control buffer to compare dielectrophoretic responses. We used Saos-2 osteosarcoma cells (cancerous cell line) and MC3T3-E1 pre-osteoblasts cells (non-cancerous cell line) as study model to observe differences in electrokinetic signature using DEP technique and identified amplification in the electrokinetic response with the new IL buffers with adjusted conductivity as opposed to the control isotonic buffer.
2. Experimental and theory
2.1. Dielectrophoresis theory and cell model
Theory for dielectrophoresis has been very well established by many research groups.7,10,29,30 Time averaged dielectrophoretic force acting on a spherical dielectric particle in non-uniform electric field is given by eqn (1)
 | (1) |
 | (2) |
 and ‘ω’ = 2πf is the angular frequency.
and ‘ω’ = 2πf is the angular frequency.
 | (3) |
The distance travelled by cells is directly proportional to the force exerted on cells when subjected to optimal electrical fields.30 Different types of cells experience different magnitude of forces which can be defined by real and imaginary parts of CM factor. The real part provides the magnitude of lateral displacement experienced by cells under optimal non-uniform electric fields, whereas the imaginary part defines the ability of cells to rotate along its central axis when subjected to uniform rotational electric fields.31
In this study we used single-shell model (shown in Fig. 1), in which the dielectric nature of cells are averaged to define the overall dielectric properties of cells. For each cell type with specific suspension medium, there exists unique frequency at which the cells change the direction of displacement (towards or away from strong electric fields). This frequency is known as cross-over frequency (fc). At this frequency, the real part of CM factor equals zero indicating there is not net force acting on the cells. Change in cross-over frequency for different suspension buffer with varying conductivity was integrated and discussed in Section 3.3.
Single-shell model is primarily used to interpret the unique dielectric signature of cells over a spectrum of frequencies. It provides a combination of dielectric properties of cells and relation to determine specific capacitance of cell membrane Cmem per unit area, which is defined by eqn (4),
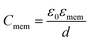 | (4) |
Capacitance per unit area, Cmem, is a fundamental parameter which helps in understanding signal transduction and propagation of charges. During polarization, cells undergo constant charging and discharging to form dipoles thereby creating a moment inside and outside of cell and as a result, experience translational dielectrophoretic forces. Determining membrane capacitance of individual cell type is based on its membrane thickness, dielectric properties and constituents. Hence, Cmem can provide insightful information of how cells behave under applied electric fields. For example, increase in the value of Cmem, correlates to the cell under study with increase in number of protrusions, folds and blebs along the membrane.32–34 This parameter can provide vital information on predicting cell movement and determining their unique dielectric signature as a function of applied electric fields. Cmem for both Saos-2 and MC3T3-E1 cells were calculated and presented in Section 3.3.
2.2. Materials
All chemicals were of analytical grade and used as received without further purification. Silicone elastomer kit containing base and curing agent to prepare polydimethylsiloxane based microfluidic well was purchased from Dow Corning (MI, US). Dulbecco's Modified Eagle Medium (DMEM) and HyClone medium with added nutrients for cell growth was received from Life Technologies (CA, US) and GE Lifesciences (PA, US) respectively. Deionized water and 0.15 M phosphate buffer solution (PBS, pH 7.0) was purchased from Sigma Aldrich (MO, US). 1-Methylimidazole, 1,10-dibromodecane and diethyl ether from Across Organics (NJ, US), 1,8-dibromooctane from Alfa Aesar (MA, US) and acetonitrile from Fisher Scientific (MA, US).2.3. ILs synthesis and characterization
Synthesis and characterization of 1,8-bis(3-methylimidazolium-1-yl)octane dibromide (IL-1) and 1,10-bis(3-methyl-imidazolium-1-yl)decane dibromide (IL-2) were performed according to the methodology described in the literature.35,38,50 50 mM of 1-methylimidazole and 50 ml of acetonitrile was mixed under inert atmosphere (N2) with continuous stirring for 2 minutes. Consecutively 25 mmol of dibromide alkyl were slowly added and reaction was kept at 70 °C during 72 h. Subsequently the product was dried under reduced pressure and washed with diethyl ether. Final products were dried at 70 °C under reduced pressure during 48 h. The chemical structures were confirmed using mass spectrometry and nuclear magnetic resonance (1H and 13C).26,38 Control isotonic medium with sucrose and dextrose cocktail was prepared using protocol established by ref. 28.2.4. Synthetically derived cell lines and culture
Osteosarcoma cell line, Saos-2 and mouse pre-osteoblast cell line, MC3T3-E1 were chosen for this study. These cell lines were specifically selected to provide differential understanding in cell response from a healthy non-cancerous cell line (MC3T3-E1) versus cancerous cell line (Saos-2). The culturing process involved temperature and humidity controlled culture space with the following process. Cells were maintained at 37 °C with 100% humidity and 5% CO2 throughout the process. Saos-2 cells were grown in McCoy's, modified DMEM medium supplemented with 0.1 mM of DMEM non-essential amino acids. Osteoblast cells were cultured with HyClone, MEM Alpha modification medium with 10% FBS solution. The medium was supplemented with Earle's balanced salts. The cells were cultured on a 75 cm2 plate and incubated for 48 hours under the conditions previously mentioned. To perform real-time electrokinetic experiments, cells were trypsinized with trypsin–EDTA solution and freshly suspended in medium to obtain 90% confluence. Prior to experiments, cells were washed with deionized water and suspended in test medium at appropriate conductivities for subsequent tests.2.5. MTT cytotoxicity assay
Cytotoxicity of IL-1 and IL-2 was assessed in vitro using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which is based on the conversion of MTT into purple formazan in the respiratory mitochondrial chain of viable cells. Each cell line (MC3T3-E1 and Saos-2) was seeded at a density of 5000 cells per well in 96-well microtiter plates at 37 °C, 5% CO2, and humidified atmosphere. After 24 hours of incubation the medium was removed and a fresh medium containing ILs at the concentration range of 10−4 mM to 103 mM was added. A control was maintained with only fresh medium. The plate was incubated for 24 hours and after this period 10 μL of MTT was added to each microtiter plate well and cells returned to incubation for 4 hours. Then, 100 μL of detergent solution was added to each well to dissolve the formazan crystals and the plate was incubated overnight. Absorption was measured at 570 nm with a Spectrophotometer (Biotek, Winooski, VT). The percentage cell viability was calculated relative to control wells at each concentration after blank subtraction.2.6. DEP device fabrication and assembly
We used interdigitated microelectrodes (IDE, 250 μm space between digits) to generate non-uniform electric fields for producing dielectrophoretic displacement. IDE with 6 sets of digits were fabricated by metal deposition of 250 nm Au per 50 nm Cr on glass substrates using shadow masks. A thin layer of photosensitive polyimide was spin coated as passivation layer between microelectrode digits using negative mask. A microfluidic channel of 40 μm depth and 100 μm wide using polydimethyl siloxane (PDMS) was fabricated using photolithography. Microfluidic inlets and outlets (Ø = 500 μm) connectors were manually fixed using water resistant silicone sealant. Flow rate of 100 μL was maintained throughout the experiments. Prior to experiments, the assembled device was washed with 2% Bovine Serum Albumin (BSA) solution in DI water to test for leakages. This provided a thin lamination coating to prevent cells from adhering to the sides of the walls. The tail of the interdigitated microelectrodes were attached to electrical connectors using conducting silver epoxy. AC signal of voltage ranging between 5 Vpp and 8 Vpp and frequency from 100 mHz to 100 MHz was supplied by a function generator (Agilent, CA).2.7. Conductivity and limiting molar conductivity measurement
Solution conductivity was measured using Accumet conductivity meter (Cole Parmer, IL). Conductivity measurements provided basis for understanding solvent purity, relative ionic strength, dissolution kinetics and equilibrium for partially soluble salts. Conductivity of solution was compared against cell constant value of 10 and 1000 μS cm−1 for accuracy. Critical micelle concentrations (CMC) of each ILs were previously studied to determine the maximum concentration of ILs that can be used without the formation of aggregates.26,51 However, in order to determine limiting molar conductance (LMC), we measured conductivities of various concentrations of the two dissimilar compounds, which in this were IL solutions and the corresponding diluent media (DI water). This parameter provided the molar conductivity for solute compound (ILs) at infinite dilution.36 For experimental data, series of dilutions of ILs were performed to understand the equivalent molar conductance.2.8. Lateral dielectrophoretic displacement experiments
Saos-2 and MC3T3-E1 cells were centrifuged at room temperature at 3000 rpm for 5 minutes. The pellets were washed with DI water for 3 times and suspended in IL-1 and IL-2 solutions. The medium conductivity of the buffer was adjusted to 0.1 S m−1 using DI water. The concentration of ILs did not exceed the limiting molar conductance when diluted with DI water. The final cell population was counted with a hemocytometer and was found to be approximately 3000 cells per μL ± 20% for both Saos-2 and MC3T3-E1 cells. The DEP system described in Section 2.6 was used to perform experiments with different compositions of ILs and standard DEP medium with known additives such as sucrose and dextrose isotonic solution. The device was placed beneath the objective of an optical microscope for visualization. The sample was allowed to flow through the device via microfluidic tubing (diameter ∼ 0.02 inches) at both inlet and outlet. The biophysical response of cells (i.e., dielectrophoretic force profile) was measured in terms of the maximum distance moved per minute by the cells under applied conditions from the electrode. Using respective IL solution and the control isotonic buffer at their optimal electrical field intensity, the experiment was repeated n = 3 times with different cell samples of the same cell line to decrease the effect of variation in cell density per experiment.3. Results and discussion
3.1. Cytotoxicity studies
Primary reason behind this study is to provide further information about the toxicological effects of ILs on the cell line. It is conventional to define the effects through half-maximal inhibitory concentration (IC50) at which cell growth is inhibited at 50% relative to controlled exposure of ILs.37 The results (n = 3 replicates) for both the Saos-2 cells as well as for MC3T3-E1 cells are shown in Table 1. This response is typically evaluated after 24 hours of exposure to the respective ILs. It was observed that the synthesized ILs triggered a low toxic effect against the investigated cell lines, and thus considerable amounts of these compounds can be used without causing cell damage. The increase in toxicity of IL-2 (Cn = 10) due to lower IC50 values in comparison to IL-1 (Cn = 8) can be attributed to the longer alkyl chain connecting the imidazolium cationic heads, which confers higher hydrophobicity and cell permeability.26,38 Higher the IC50, lower is the toxicity level of particular IL sample resulting in greater tolerance for cells to be suspended in much higher concentrations. As previously observed by Gindri et al. for MC3T3-E1 cells in IL-1 with IC50 of 24.6 ± 3.5 mM as opposed to 12.3 ± 0.1 mM in IL-2.26 In this study, for osteosarcoma cells Saos-2 in IL-1 has IC50 of 12.6 ± 1.0 mM and 18.7 ± 1.3 mM for cells in IL-2. In particular, increasing in side chain length in the methylimidazolium based ILs comes with a higher inhibitory potential (lower IC50s).35 The IC50 values indicate the suitability of the medium for cell viability and sustainability. This is important for performing any kind of electrokinetic or experimental analysis of cell lines.| Ionic liquid | IC50 (mM) | |
|---|---|---|
| Saos-2 | MC3T3-E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
|
| IL-1 | 18.7 ± 1.3 | 24.6 ± 3.5 |
| IL-2 | 12.6 ± 1.0 | 12.3 ± 0.1 |
3.2. Limiting molar conductivity (LMC) studies
Fig. 2 shows the plot for conductivity of IL-1 and IL-2 in water solutions as a function of IL concentration. The plot represents data obtained using varying concentrations of the buffer solution and respective conductivity measurements. Data plotted was obtained from n = 5 measurements (SD shown in error bars) on samples with IL concentrations in the range of 1–250 mM and the values were in accordance with those already reported in literature.51 The inset values represent the slope corresponding the limiting equivalent molar conductance of IL-1 and IL-2 in water. The resultant values of LMC obtained were 11.7 S m−1 mol−1 for IL-1 and 11.9 S m−1 mol−1 for IL-2. The overall conductivity of the ILs from this experiment was observed to be between 0.1 and 1.6 S m−1, which is greater than other buffer medium used for cell separation.39 However, LMC for any electrolyte is obtained by expressing them as the sum of independent contributions from the constituent cations and anions. In this case, conductivity of IL-1 and IL-2 was absorbed to be well below LMC, hence 1 mM concentration of ILs were chosen to perform DEP experiments. However control sugar solution with equimolar concentration of sodium chloride, sucrose and dextrose provided a balanced solution without triggering water efflux in cells. Hence, 8.5% w/v sucrose and 0.3% w/v dextrose combined with NaCl will have no effect on the surrounding cells.3.3. Dielectrophoretic spectrum
A dielectric particle suspended in conductive media under an AC electric field will undergo polarization and experience a net force, Section 2.1. Upon experiencing net force, the cells respond by moving away from strong electric fields. Conductivity is a measure of the ability to conduct an electric field while permittivity is a measure of the ability to transmit an electric field. The Saos-2 cells have cytoplasmic conductivity of 0.52 S m−1, while MC3T3-E1 cells have 0.39 S m−1. Since the cells are suspended in high conductivity physiological medium (∼0.1 S m−1) when compared to control isotonic buffer (∼0.05 S m−1), cells exhibit strong negative DEP forces over a very large range of frequency. By substituting measured cell radius, known permittivity and conductivity of Saos-2/MC3T3-E1 cells and buffer(s), specific membrane capacitance, membrane thickness and applied electric field as mentioned in Table 2 respectively, Clausius–Mossotti (CM) factor for different concentrations of ILs can be predicted as a function of applied signal frequency. The real part of CM factor for IL-1, IL-2 (1 mM each) and isotonic medium for cell lines are shown in Fig. 3.| Parameter | Saos-2 cells | MC3T3-E1 |
|---|---|---|
| a ε0 is the absolute permittivity with a constant value of 8.85 × 10−12 F m−1. | ||
| Cell diameter | 8 μm | 12 μm |
| Cell conductivity (σ) S m−1 | 0.52 | 0.39 |
| Membrane thickness (d) | 9 nm | 6 nm |
| Specific membrane capacitance (mF m−2) | 13.3 | 3.2 |
| Parameter | IL-1 (1 mM) | IL-2 (1 mM) | Isotonic |
|---|---|---|---|
| Medium conductivity (σ) S m−1 | 0.104 | 0.022 | 0.05 |
| Medium permittivity (εm) | 25 × ε0 | 32 × ε0 | 78 × ε0 |
The cross-over frequencies (fc-IL1, fc-IL2, fc-iso) were found to be between 2 MHz and 1.6 MHz for Saos-2 cells in σm = 0.1 S m−1, 0.02 S m−1 and 0.05 S m−1. For MC3T3-E1 cells fc was observed to be between 1.1 MHz and 800 kHz in σm = 0.1 S m−1, 0.02 S m−1 and 0.05 S m−1. For a fixed medium conductivity, cell's response to applied voltage varies from the applied frequency due to different CM factor values. However, with increase in medium conductivity, both cellular response voltage and cross over frequency increases due to differences in polarization within and outside of cells.40 The Fig. 3 shows dielectrophoretic spectrum calculated for both the cell lines. When the frequency is bigger than fc, the cells experience positive DEP. When the frequency is smaller than fc, the cells experience negative DEP force and a very high voltage is required to trap the cells. It could be due to the fact that cells are pushed to the top surface of the electrodes, where the electric field is much weaker than electric fields at the edges of the electrodes. The trend is similar for concentrations well within LMC (1 mM and 10 mM), which alter solution conductivity. These results can be associated with the aggregation behaviour of ILs in aqueous solution. ILs with higher number of carbon atoms have lower critical aggregation concentration (CAC).41 It is also known that the aggregation of IL-2 is more thermodynamically favoured than for IL-1. As a result, physico-chemical properties of the solution is also altered due to the formation of aggregates.51 Our results also demonstrates at very high concentrations, both ILs are more susceptible for forming aggregates thus having similar electrokinetic behaviour. However, at higher concentrations (100 mM), IL-1 and IL-2 performed similar for a broad range of frequencies.
Furthermore, it can be observed that, cells suspended in IL-2 have greater probability to be trapped for separation at optimal voltage than IL-1 at a constant frequency. As a result, 1 mM of IL-1 and IL-2 was used to perform dielectrophoretic studies. Measurements at lower frequencies were not conducted due to electrode polarization and α-dispersion, which is the tangential flow of ions on the cell surface. This indicates, higher voltages are required to manipulate cells. This could also indicate that cells having lower cell membrane thickness resulting in greater permeability for the flow of polarized ions.
3.4. Dielectrophoretic response of cells
Saos-2 and MC3T3-E1 cells in 1 mM of IL-1 and IL-2 were used to perform DEP experiments. Cells in isotonic sucrose medium was used as control studies. Using single-shell model, the effective membrane capacitance and cytoplasmic conductivity of both cells were determined in the previous Section 3.3. A voltage sweep from 10 mV to 10 Vpp was performed to determine optimal voltage. The presence of polyimide passivation layer provides high electrical resistance and proper channel to ground excess charges thus preventing the electrodes from delamination. Cells when suspended in IL-2 required 10 Vpp of voltage to produce comparable electric fields effects over 8 Vpp using IL-1 as suspension buffer.The interaction between the charges within the dipole and the applied non-uniform electric field generates a force. And when this dielectric particle (cells) is suspended in a conductive medium and a spatially uniform electric field is applied, the magnitude of the force exerted on the two poles of the induced dipole is equal and opposite thereby, cancelling the net force acting on the particle. We applied electric fields in a spatially non-uniform manner, resulting in charge imbalance within the dielectric particle (cells). The strength of the electric field on one pole of the dipole is greater than on the other. Therefore, the imbalance of Coulomb forces exerts on the particle in the form of translational force during which the particle or cells will undergo displacement. In our present case, both the cell types were less polarizable than the suspending ionic liquid media thus the cells were repelled from the high electric field strength regions. In order to quantify the dielectrophoretic force for the different types of cells suspended in different medium with variable ionic strength, at each respective voltage, frequency scans ranging from 100 mHz to 10 MHz were applied for 5 minutes. For each frequency decade cells responses were recorded using CCD camera. Five frequency points were identified, which showed maximum response within their narrow range. At low frequencies, DC to 10 kHz, the lateral displacement of cells was negligible due to electrode polarization and tangential dispersion of ions on cell surface thus resulting in bubbling effects. However, at higher frequencies (>100 kHz) the cells displaced rapidly due to cell's electrical permeability and polarization of ions. Cells were pushed from the edges of the electrode towards the center of the microfluidic channel. This indicates that at higher frequencies a strong nDEP force was exerted on cells (applied frequency f < fc). When frequency greater than fc was applied (f > 2 MHz), the cells became more polarizable than the suspending medium and decreased nDEP activity was observed.
With a view to understand the role of ionic liquids as potential candidate for cell suspension buffer, we sought to assess the potential for DEP to be used as a tool in cellular characterization. The effective membrane capacitance Cmem and cytoplasmic conductivity of both cancerous and non-cancerous cells have been evaluated for better understanding the effect of ionic liquids on the cells. In order to obtain quantifiable data lateral displacement of cells was used to obtain dielectrophoretic force profiles of cells. Fig. 4 shows the relative change in force parameter as a function of applied signal frequency. Significant differences between non-cancerous cells MC3T3-E1 and cancerous cells Saos-2 can be observed over the frequency spectrum. Distinguishable trend differences were achieved throughout the applied signal frequency. Although this was only statistically significant for IL-1 medium with cells. But for IL-2 medium, relative low change in DEP force was observed when compared with IL-1 medium. The DEP force spectrum was determined by finding the best-fit combination of values corresponding to membrane and cytoplasmic properties of the cells taking the displacement values into account. Non-linear curve fit model was used to estimate signal saturation and behaviour of Saos-2 and MC3T3-E1 cells as indicated in figure below. Other factors include cell radius, measured medium properties using single-shell dielectric mathematical model. The magnitude of movement and relative change in DEP response experienced by cancerous cells (Saos-2) was greater and antagonistic response than that of healthy cells (MC3T3-E1) over the applied frequency window, as shown in Fig. 4.
The Cmem, function of membrane surface area, permittivity and thickness has been estimated to be 13.3 mF m−2 and 3.2 mF m−2 for Saos-2 and MC3T3-E1 respectively. It has been estimated that a cell with a completely smooth membrane has a Cmem of approximately 0.6 μF cm−2.46 The Cmem determination assumes a sphere with a smooth membrane, therefore an increase in membrane roughness due to folding, blebbing and ruffles would result in an increase in Cmem values. Thus Cmem is a good indicator of change in surface and other morphological changes of cells. High values of Cmem would correlate to higher number of blebs and folds in the cell membrane which is often observed in the cell membrane morphology with disease progression. This is consistent with observations that increased surface roughness is a vital component of the cancer cell phenotype and correlates well with metastatic potential cell type and invasiveness.47–49 The cancer cells have approximately 75.9% higher mean Cmem than normal osteoblast cells. Increase in Cmem of cancer cells due to increased surface protrusions could result in more charges and polarizable ions.
The displacement of cells in microns is tabulated in Table 3. Cells suspended in IL-1 moved farther away from electrodes indicating very strong nDEP over cells in IL-2. Cancer cells (Saos-2) exhibited almost 12 times increase in displacement magnitude versus MC3T3-E1 (as shown in Table 3). Displacement by MC3T3-E1 cells in IL-2 was observed to be opposite in trend with no statistical significance between experimental data. Relatively low change in DEP response from cells were observed over the range of frequency with cells in IL-2. This could also be indicative of increase in polarization of suspension medium due to increase in alkyl side chain length than the cell itself. Increase in side chain length comes with high inhibitory potential resulting in lower IC50 thus providing an environment with charge imbalance and varying tonicity. The overall change in lateral displacement of cells from baseline was minimal for all frequency points, indicating that IL-2 less reactive with cells. This also connects DEP properties of cells to the dielectric nature of the suspension medium. Additionally, cells in IL-2 did not visibly remain stable as cells started to form colonies and experienced strong attractive forces and moved towards electrodes, which could account for the decrease in cross-over frequency. As a control study, similar experiments were performed with sucrose based isotonic solution as suspension medium. The cells exhibited uniform movement but experienced comparatively low magnitude nDEP. This also aids in the initial hypothesis that ionic liquids can provide flexible medium to take advantage of cells in suspension and amplify dielectrophoretic signature of cells. However, changes in cellular metabolism and modifications to inherent cell functions for the observed biophysical responses are subject of further investigation in our laboratory, where future work must be done for validation.
| Applied frequency (kHz) | Lateral displacement (d) observed by cells (in μm) | |||||
|---|---|---|---|---|---|---|
| Isotonic solution | IL-1 (1 mM) | IL-2 (1 mM) | ||||
| MC3T3-E1 | Saos-2 | MC3T3-E1 | Saos-2 | MC3T3-E1 | Saos-2 | |
| a The values represented are absolute maximum of the displacement observed over n = 3 replicates with least-square regression fit carried out for each frequency points. | ||||||
| 10 | 7.9 | 48.1 | 15.1 | 180 | 13.2 | 6.4 |
| 100 | 4.0 | 76.7 | 36.1 | 114.8 | 7.4 | 15.6 |
| 500 | 14.4 | 34.5 | 23.5 | 100.1 | 5.1 | 19.8 |
| 1000 | 20.4 | 48.7 | 74.6 | 90.2 | 4.5 | 25.3 |
3.5. Cell viability tests
Saos-2 and MC3T3-E1 cells were suspended in respective IL solution with concentration of 1 mM, which is much lower than LMC and IC50 value. Cells post dielectrophoretic experiments in ILs were studied under optical microscope. Standard cell viability Trypan blue exclusion assay was performed and compared against the control population, and 90% of cells were estimated as live cell population. Continuous time lapse images were captured using a CCD camera for 60 minutes. Physical characteristics of cells was also observed to confirm cell viability. The cells outer membrane was observed to be round and intact without any shrinkage. Also, cells that were collected from the microfluidic well outlet was cultured under ambient environment as explained in Section 2.4 to study cell proliferation and growth pattern. Post DEP processing Saos-2 and MC3T3-E1 cell culture shows 87% and 83% confluence over 48 h period.4. Conclusion
In this study, two dicationic imidazolium ionic liquids were investigated to understand their role as suspension buffer medium for performing dielectrophoretic studies on cells. DEP spectrum for individual cell type in respective ionic liquid solution were computed using single-shell model. From this spectrum, we found that IL-1 (1,8-bis(3-methylimidazolium-1-yl)octane dibromide) exhibited highest amplitude of dielectrophoretic force response. Whereas IL-2 (1,10-bis(3-methyl-imidazolium-1-yl)decane dibromide) solution for the same cell types under similar conditions, required significantly higher input energy to produce comparable effects on cells. Ionic liquids performed comparable and/or better within frequency range between 100 and 1000 kHz. These differences may be due to the chemical structure of the ILs with varying number of carbon chain. This effectively results in IL-2 forming aggregates at much lower concentration than IL-1 leading to altered physico-chemical properties. Also, specific membrane capacitance was calculated using dielectrophoretic biophysics. These results indicate that DEP using ILs can be used to study cell biophysics and their morphology and function when suspended in ILs solutions that leverages the advantages for cell separation and manipulation. These results provides a better understanding for tracking cells of interest in a complex heterogeneous sample solution. This study also provided us with an insight into understanding cellular differences between membranes of different cell lines and a non-subjective, easy to prepare experimental procedure for complex functioning. This research can further be applied to understand different stages of cell cycle and the effective changes to cell functioning based on their electrical properties, which may lead to a more effective screening mechanism for early detection or selection of cells for disease diagnosis.Acknowledgements
This work is supported by Cecil H. and Ida Green Endowment in Systems Biology Science. Authors would like to acknowledge Duy Huu Bui and Pradyotha Kanchustambham for helping with image analysis. The fellowship from Coordination for the Improvement of Higher Education Personnel (I. M. G.) is also acknowledged.References
- M. J. Tomlinson, et al., Cell separation: terminology and practical considerations, J. Tissue Eng., 2013, 4, 2041731412472690 CrossRef PubMed.
- R. M. Vrikkis, et al., Biocompatible ionic liquids: a new approach for stabilizing proteins in liquid formulation, J. Biomech. Eng., 2009, 131(7), 074514 CrossRef PubMed.
- S. Basu, et al., Purification of specific cell population by fluorescence activated cell sorting (FACS), J. Visualized Exp., 2010(41), e1546 Search PubMed.
- X.-B. Wang, et al., Cell separation by dielectrophoretic field-flow-fractionation, Anal. Chem., 2000, 72(4), 832–839 CrossRef CAS PubMed.
- D. Davies, Cell sorting by flow cytometry, in Flow Cytometry, Springer, 2007, pp. 257–276 Search PubMed.
- P. T. Sharpe, Methods of cell separation, Elsevier, 1988 Search PubMed.
- G. H. Markx, P. A. Dyda and R. Pethig, Dielectrophoretic separation of bacteria using a conductivity gradient, J. Biotechnol., 1996, 51(2), 175–180 CrossRef CAS PubMed.
- M. Radisic, R. K. Iyer and S. K. Murthy, Micro-and nanotechnology in cell separation, Int. J. Nanomed., 2006, 1(1), 3 CrossRef CAS PubMed.
- T. B. Jones, Basic theory of dielectrophoresis and electrorotation, IEEE Engineering in Medicine and Biology Magazine, 2003, 22(6), 33–42 CrossRef PubMed.
- P. R. Gascoyne, et al., Dielectrophoretic separation of cancer cells from blood, IEEE Trans. Ind. Appl., 1997, 33(3), 670–678 CrossRef PubMed.
- S. V. Puttaswamy, et al., Enhanced cell viability and cell adhesion using low conductivity medium for negative dielectrophoretic cell patterning, Biotechnol. J., 2010, 5(10), 1005–1015 CrossRef CAS PubMed.
- H. Tsutsui, et al., Efficient dielectrophoretic patterning of embryonic stem cells in energy landscapes defined by hydrogel geometries, Ann. Biomed. Eng., 2010, 38(12), 3777–3788 CrossRef PubMed.
- A. Nakano and A. Ros, Protein dielectrophoresis: advances, challenges, and applications, Electrophoresis, 2013, 34(7), 1085–1096 CrossRef CAS PubMed.
- M. D. Pysher and M. A. Hayes, Electrophoretic and dielectrophoretic field gradient technique for separating bioparticles, Anal. Chem., 2007, 79(12), 4552–4557 CrossRef CAS PubMed.
- E. D. Pratt, et al., Rare cell capture in microfluidic devices, Chem. Eng. Sci., 2011, 66(7), 1508–1522 CrossRef CAS PubMed.
- B. M. Quinn, et al., Novel electrochemical studies of ionic liquids, Langmuir, 2002, 18(5), 1734–1742 CrossRef CAS.
- K. E. Johnson, What's an ionic liquid?, J. Electrochem. Soc., 2007, 16(1), 38–41 CAS.
- H. Shirota, et al., Comparison between dicationic and monocationic ionic liquids: liquid density, thermal properties, surface tension, and shear viscosity, J. Chem. Eng. Data, 2011, 56(5), 2453–2459 CrossRef CAS.
- M. Armand, et al., Ionic-liquid materials for the electrochemical challenges of the future, Nat. Mater., 2009, 8(8), 621–629 CrossRef CAS PubMed.
- J. K. Shah and E. J. Maginn, Molecular dynamics investigation of biomimetic ionic liquids, Fluid Phase Equilib., 2010, 294(1), 197–205 CrossRef CAS.
- W. R. Pitner, et al., Applications of ionic liquids in electrolyte systems, Handbook of Green Chemistry, 2010 Search PubMed.
- K. M. Docherty and C. F. Kulpa Jr, Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids, Green Chem., 2005, 7(4), 185–189 RSC.
- W. Gouveia, et al., Toxicity of ionic liquids prepared from biomaterials, Chemosphere, 2014, 104, 51–56 CrossRef CAS PubMed.
- M. Cvjetko, et al., Cytotoxic effects of imidazolium ionic liquids on fish and human cell lines, Arch. Ind. Hyg. Toxicol., 2012, 63(1), 15–20 CAS.
- J. Arning and M. Matzke, Toxicity of ionic liquids towards mammalian cell lines, Curr. Org. Chem., 2011, 15(12), 1905–1917 CrossRef CAS.
- I. M. Gindri, et al., Dicationic imidazolium-based ionic liquids: a new strategy for non-toxic and antimicrobial materials, RSC Adv., 2014, 4(107), 62594–62602 RSC.
- R. Pethig, Review article—dielectrophoresis: status of the theory, technology, and applications, Biomicrofluidics, 2010, 4(2), 022811 CrossRef PubMed.
- L. M. Broche, et al., Early detection of oral cancer–Is dielectrophoresis the answer?, Oral Oncol., 2007, 43(2), 199–203 CrossRef CAS PubMed.
- H. Mulhall, et al., Cancer, pre-cancer and normal oral cells distinguished by dielectrophoresis, Anal. Bioanal. Chem., 2011, 401(8), 2455–2463 CrossRef CAS PubMed.
- H. Morgan and N. G. Green, AC electrokinetics: colloids and nanoparticles, Research Studies Press, 2003 Search PubMed.
- R. Pethig, et al., Electrokinetic measurements of membrane capacitance and conductance for pancreatic β-cells, IEE Proc.: Nanobiotechnol., 2005, 189–193 CrossRef CAS PubMed.
- X.-B. Wang, et al., Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation, Biochim. Biophys. Acta, Biomembr., 1994, 1193(2), 330–344 CrossRef CAS.
- K. Ratanachoo, P. R. Gascoyne and M. Ruchirawat, Detection of cellular responses to toxicants by dielectrophoresis, Biochim. Biophys. Acta, Biomembr., 2002, 1564(2), 449–458 CrossRef CAS.
- F. H. Labeed, H. M. Coley and M. P. Hughes, Differences in the biophysical properties of membrane and cytoplasm of apoptotic cells revealed using dielectrophoresis, Biochim. Biophys. Acta, Gen. Subj., 2006, 1760(6), 922–929 CrossRef CAS PubMed.
- S. Steudte, et al., Toxicity and biodegradability of dicationic ionic liquids, RSC Adv., 2014, 4(10), 5198–5205 RSC.
- D. Electrolyte, Conductance Measurements Part 1: Theory, Curr. Sep., 1999, 18(3), 92 Search PubMed.
- E. Liwarska-Bizukojc, Influence of imidazolium ionic liquids on dehydrogenase activity of activated sludge microorganisms, Water, Air, Soil Pollut., 2011, 221(1–4), 327–335 CrossRef CAS PubMed.
- I. M. Gindri, et al., Preparation of TiO2 Nanoparticles Coated with Ionic Liquids: A Supramolecular Approach, ACS Appl. Mater. Interfaces, 2014, 6(14), 11536–11543 CAS.
- M. G. Moisescu, et al., Changes of cell electrical parameters induced by electroporation. A dielectrophoresis study, Biochim. Biophys. Acta, Biomembr., 2013, 1828(2), 365–372 CrossRef CAS PubMed.
- L. Wu, L.-Y. L. Yung and K.-M. Lim, Dielectrophoretic capture voltage spectrum for measurement of dielectric properties and separation of cancer cells, Biomicrofluidics, 2012, 6(1), 014113 CrossRef PubMed.
- J. Wang and H. Wang, Aggregation in Systems of Ionic Liquids, in Structures and Interactions of Ionic Liquids, Springer, 2014. pp. 39–77 Search PubMed.
- C.-T. Huang, C.-H. Weng and C.-P. Jen, Three-dimensional cellular focusing utilizing a combination of insulator-based and metallic dielectrophoresis, Biomicrofluidics, 2011, 5(4), 044101 CrossRef PubMed.
- E. Niebur, Electrical properties of cell membranes, Scholarpedia, 2008, 3(6), 7166 CrossRef.
- A. Salmanzadeh, et al., Investigating dielectric properties of different stages of syngeneic murine ovarian cancer cells, Biomicrofluidics, 2013, 7(1), 011809 CrossRef PubMed.
- J. Chen, et al., Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells, Lab Chip, 2011, 11(18), 3174–3181 RSC.
- R. Pethig and D. B. Kell, The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology, Phys. Med. Biol., 1987, 32(8), 933–970 CrossRef CAS PubMed.
- W. Jiang, Focus on science—membrane ruffling of cancer cells: a parameter of tumour cell motility and invasion, Eur. J. Surg. Oncol., 1995, 21(3), 307–309 CrossRef CAS PubMed.
- N. A. van Larebeke, M. E. Bracke and M. M. Mareel, Invasive epithelial cells show more fast plasma membrane movements than related or parental non-invasive cells, Cytometry, 1992, 13(1), 9–14 CrossRef CAS PubMed.
- A. W. Partin, et al., Early cell motility changes associated with an increase in metastatic ability in rat prostatic cancer cells transfected with the v-Harvey-ras oncogene, Cancer Res., 1988, 48(21), 6050–6053 CAS.
- M. A. Martins, et al., Update 1 of: ionic liquids in heterocyclic synthesis, Chem. Rev., 2014, 114(20), PR1–PR70 CrossRef PubMed.
- C. P. Frizzo, et al., Effect on aggregation behavior of long-chain spacers of dicationic imidazolium-based ionic liquids in aqueous solution, Colloids Surf., A, 2015, 468, 285–294 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |




