Research on controllable degradation of sulfonylurea herbicides†
Xue-Wen Huaa,
Ming-Gui Chena,
Shaa Zhoua,
Dong-Kai Zhanga,
Ming Liua,
Sha Zhoua,
Jing-Bo Liua,
Kang Leia,
Hai-Bin Songa,
Yong-Hong Lia,
Yu-Cheng Gub and
Zheng-Ming Li*a
aState Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Room 518, Bo-Ling Building, Balitai, Nankai District, Tianjin 300071, China. E-mail: nkzml@vip.163.com; Fax: +86-22-23505948; Tel: +86-22-23503732
bJealott's Hill International Research Centre, Syngenta Ltd, Berkshire RG42 6EY PO BOX 163, Bracknell, UK
First published on 23rd February 2016
Abstract
In order to seek ecologically safer and environmentally benign sulfonylurea herbicides (SU), insight into the structure/bioassay/soil degradation tri-factor relationship was first established. With the introduction of various groups (alkyl, nitro, halogen, cyano etc.) at the 5th position of its benzene ring, structural derivatives of chlorsulfuron were designed, synthesized, and evaluated for their herbicidal activity. The structures of the title compounds were confirmed by infrared spectroscopy, ultraviolet spectroscopy, 1H and 13C NMR, mass spectrometry, elemental analysis and X-ray diffraction. Bioassay results confirmed that most derivatives retained their superior herbicidal activities in comparison with chlorsulfuron. After investigating the soil degradation behavior of each molecule under set conditions, it was found that structures with electron-withdrawing substituents at the 5th position of the benzene ring retained their long degradation half-lives, yet the introduction of electron-donating substituents accelerated the degradation rate. These results will provide a valuable clue to further explore the potential controllable degradation of SU and other herbicides, and to discover novel herbicides that are favorable for environmentally and ecologically sustainable development.
Introduction
In the past two decades, an important class of herbicides, sulfonylureas (SU), has been developed. These structures interfere with an unique enzyme existing only in plants, acetolactate synthase (ALS),1,2 which results in blocking of the biosynthesis of three branched-chain amino acids that have outstanding properties such as ultra-low application rate, good selectivity and negligible mammalian toxicity.3,4In recent years, with large scale application of sulfonylurea herbicides, sometimes the residues have showed up certain phytotoxicity in crop rotation which are not conducive to environmental protection and ecologically sustainable development.5,6 Experiments,7 as well as evidence from farmers in China and Australia, have suggested that under certain conditions, a few sulfonylurea herbicides persist long enough to affect the growth of sensitive crops such as grain legumes, field peas and sunflower in the following season.8,9 In 2013, three sulfonylurea herbicides, chlorsulfuron, metsulfuron-methyl, and ethametsulfuron (Fig. 1), have been prohibited in China due to their persistent existence in soil which is harmful to the next rotation crop. Due to the huge population and comparatively rather limited arable land in China, a traditional intensive cultivation practice has been undertaken to rear 2–3 different crops annually on the same piece of land,10 which requires that herbicides used should degrade efficiently in soil during different period of this particular crop rotation system. Sulfonylurea herbicides if degrade too slow than expected, there will pose a phytotoxicity problem to the next crop. In summary, our cultivation system (various crops per land per year) requires a special approach to integrate sulfonylurea herbicides into our plant protection practices. During our innovation program, it is anticipated that “green herbicides” should be highly active as well as to possess a suitable degradation rate in which residues will not persist in soil to hurt the next crop seedlings.
The researches about the relationship of degradation behaviors and structural modification can contribute to development of environment-friendly pesticides which are favorable for ecological protection. Recently, several reports mentioned that by introducing a special moiety onto the 5th position of its classical benzene ring, the revised sulfonylurea structure could exert influence on its degradation rate, i.e. iodosulfuron-methyl-sodium, foramsulfuron, and flupyrsulfuron-methyl-sodium (Fig. 1).10,11 It inspired us to further explore the relationship about degradation behaviors and structural modification of SU molecules. Based on our previous experience, the introduction of a few substituents at 5th position of the benzene ring in sulfonylurea structures was favorable to remain the herbicidal activity, on the other hand, can accelerate their hydrolysis in water with different pH values.10,12 To our knowledge, the current researches about the degradation on sulfonylurea herbicides mostly focused individually on soil degradation, hydrolysis or photodegradation under different conditions,13–16 such as pH values, temperature, light, sterile or non-sterile, and organic amendments. However, there are no reports available about the systemic relationship between the soil degradation and structural modification of sulfonylurea herbicides by introducing different substituents onto the 5th position of their benzene ring. Therefore, this relationship should be studied to guide the search for ecologically safer and environmentally benign sulfonylureas.
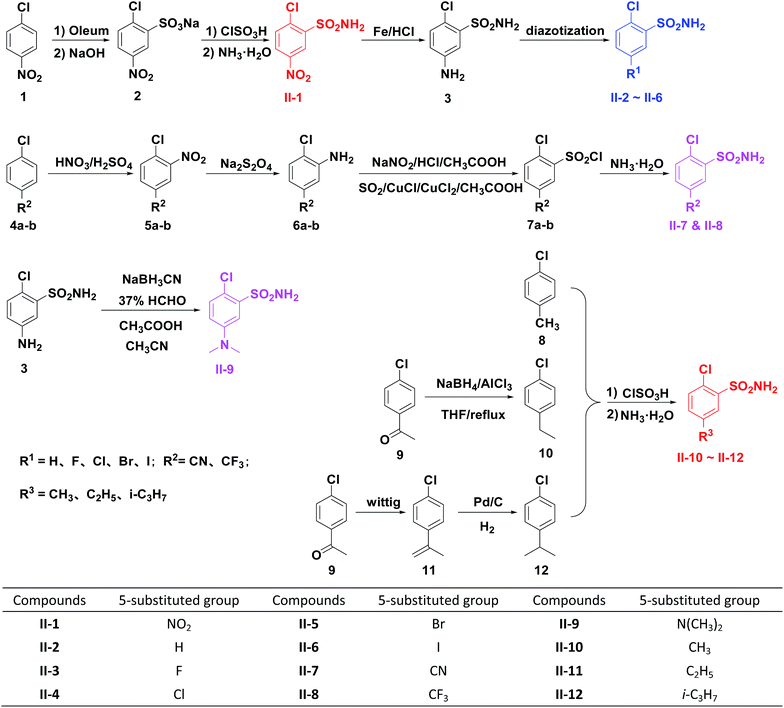
The classical chlorsulfuron had been considered once to be a popular sulfonylurea herbicide widely applied in grain fields in China. In this study, a series of chlorsulfuron derivatives were designed and synthesized with introducing various groups (alkyl, nitro, halogen, cyano etc.) onto the 5th position of its benzene ring (Fig. 2). The corresponding synthetic routes towards intermediates II-1–II-12 and target compounds I-1–I-12 were designed and carried out according to Schemes 1 and 2 respectively. Followed by evaluation of herbicidal activity and investigation of soil degradation under set conditions, an insight of structure/bioassay/soil degradation tri-factor relationship was firstly established, which will provide us important information on environment and ecological impact during future SU global application.
Materials and methods
Soil
A soil from the plain of Ji'an city (Jiangxi Province, China) was sampled from the upper layer (0–25 cm), air-dried in the shade and passed through a 2 mm sieve. It is a red acid soil, with pH 5.41 (soil/water ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), soil organic matter content 6.85 g kg−1 and 25% water-holding capacity (WHC).
5), soil organic matter content 6.85 g kg−1 and 25% water-holding capacity (WHC).
Reagents and instruments
All reaction reagents were analytical grade, while all analytical reagents for high performance liquid chromatograph (HPLC) were HPLC grade, including methanol, acetonitrile etc. Melting points of all compounds were determined on an X-4 binocular microscope (Gongyi Tech. Instrument Co., Henan, China), and the temperatures were uncorrected. 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained on a Bruker AV-400 spectrometer (400 MHz), and chemical-shift values (δ) were reported as parts per million (ppm) with tetramethylsilane as the internal standard. Elemental analyses (EA) were measured on a vario EL CUBE elemental analyzer. Infrared (IR) spectra were recorded on a Bruker Vector 22 Fourier transform infrared (FTIR) spectrometer using KBr pellets. Ultraviolet (UV) spectra were performed on a TU-1810 ultraviolet-visible spectrophotometer. Mass spectra were recorded on a Thermo-Finnigan LCQ-Advantage LC/mass detector instrument. HPLC data were obtained on a SHIMADZU LC-20AT. Column chromatography purification was carried out using silica gel (200–300 mesh).General synthetic procedure for title compounds I-1–I-12
1-(2-Chloro-5-nitrophenylsulfonyl)-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea (I-1) was prepared as follows. To a mixture of intermediate II-1 (1.15 g, 4.8 mmol) and a catalytic amount of triethylenediamine (0.06 g, 0.5 mmol) in anhydrous toluene (50 mL) was added dropwise oxalyl chloride (3.05 g, 24 mmol) at room temperature. The solution was heated to 60 °C and stirred for 8 h, then heated up to 100 °C to continue reacting for another 12 h. The excess oxalyl chloride and a little toluene was distilled to remove, followed by the addition of 4-methoxy-6-methyl-1,3,5-triazin-2-amine. The mixture was stirred for 8 h at 70 °C, and then concentrated to be purified through chromatograph on silica gel using dichloromethane/ethyl acetate (v/v 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give white solid I-1. Compounds I-2–I-12 were synthesized similarly.
1) as eluent to give white solid I-1. Compounds I-2–I-12 were synthesized similarly.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1354 and 1173 (SO2). δH (400 MHz; (CD3)CO) 2.40 (3H, s, CH3), 3.90 (3H, s, OCH3), 7.88 (1H, d, J 8.7, Ph-H), 8.45 (1H, d, J 8.6, Ph-H), 8.83 (1H, s, Ph-H), 9.81 (1H, s, NH), 13.15 (1H, s, NH). δC (101 MHz; (CD3)CO) 25.5, 55.9, 128.4, 130.3, 134.5, 138.6, 139.0, 147.4, 149.1, 165.1, 171.8, 179.8. m/z (ESI) [M–H]− found: 400.9. Calc. for C12H10ClN6O6S−: 401.0.
O), 1354 and 1173 (SO2). δH (400 MHz; (CD3)CO) 2.40 (3H, s, CH3), 3.90 (3H, s, OCH3), 7.88 (1H, d, J 8.7, Ph-H), 8.45 (1H, d, J 8.6, Ph-H), 8.83 (1H, s, Ph-H), 9.81 (1H, s, NH), 13.15 (1H, s, NH). δC (101 MHz; (CD3)CO) 25.5, 55.9, 128.4, 130.3, 134.5, 138.6, 139.0, 147.4, 149.1, 165.1, 171.8, 179.8. m/z (ESI) [M–H]− found: 400.9. Calc. for C12H10ClN6O6S−: 401.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1350 and 1165 (SO2). δH (400 MHz; (CD3)CO) 2.53 (3H, s, CH3), 4.04 (3H, s, OCH3), 7.62–7.69 (2H, m, Ph-H), 7.75 (1H, t, J 7.6, Ph-H), 8.26 (1H, d, J 7.9, Ph-H), 9.77 (1H, s, NH), 12.96 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 127.5, 131.4, 131.8, 132.7, 135.2, 136.5, 148.2, 164.3, 170.8, 178.9. m/z (ESI) [M–H]− found: 355.9. Calc. for C12H11ClN5O4S−: 356.0.
O), 1350 and 1165 (SO2). δH (400 MHz; (CD3)CO) 2.53 (3H, s, CH3), 4.04 (3H, s, OCH3), 7.62–7.69 (2H, m, Ph-H), 7.75 (1H, t, J 7.6, Ph-H), 8.26 (1H, d, J 7.9, Ph-H), 9.77 (1H, s, NH), 12.96 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 127.5, 131.4, 131.8, 132.7, 135.2, 136.5, 148.2, 164.3, 170.8, 178.9. m/z (ESI) [M–H]− found: 355.9. Calc. for C12H11ClN5O4S−: 356.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1362 and 1166 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 4.03 (3H, s, OCH3), 7.44–7.61 (1H, m, Ph-H), 7.67–7.76 (1H, m, Ph-H), 7.81–8.01 (1H, m, Ph-H), 9.81 (1H, s, NH), 13.03 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 119.7 (d, J 26.7), 122.3 (d, J 23.2), 126.8 (d, J 3.6), 133.8 (d, J 7.8), 138.1 (d, J 7.4), 148.2, 160.5 (d, J 249.2), 164.2, 170.8, 178.9. δF (376 MHz, (CD3)CO) −114.1. m/z (ESI) [M–H]− found: 373.9. Calc. for C12H10ClFN5O4S−: 374.0.
O), 1362 and 1166 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 4.03 (3H, s, OCH3), 7.44–7.61 (1H, m, Ph-H), 7.67–7.76 (1H, m, Ph-H), 7.81–8.01 (1H, m, Ph-H), 9.81 (1H, s, NH), 13.03 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 119.7 (d, J 26.7), 122.3 (d, J 23.2), 126.8 (d, J 3.6), 133.8 (d, J 7.8), 138.1 (d, J 7.4), 148.2, 160.5 (d, J 249.2), 164.2, 170.8, 178.9. δF (376 MHz, (CD3)CO) −114.1. m/z (ESI) [M–H]− found: 373.9. Calc. for C12H10ClFN5O4S−: 374.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1368 and 1171 (SO2). δH (400 MHz; (CD3)CO) 2.53 (3H, s, CH3), 4.04 (3H, s, OCH3), 7.73 (1H, d, J 8.6, Ph-H), 7.80 (1H, dd, J 8.6, 2.5, Ph-H), 8.19 (1H, d, J 2.5, Ph-H), 9.88 (1H, s, NH), 13.13 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 130.1, 132.1, 132.7, 133.4, 134.9, 138.0, 148.3, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 389.9. Calc. for C12H10Cl2N5O4S−: 390.0.
O), 1368 and 1171 (SO2). δH (400 MHz; (CD3)CO) 2.53 (3H, s, CH3), 4.04 (3H, s, OCH3), 7.73 (1H, d, J 8.6, Ph-H), 7.80 (1H, dd, J 8.6, 2.5, Ph-H), 8.19 (1H, d, J 2.5, Ph-H), 9.88 (1H, s, NH), 13.13 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 130.1, 132.1, 132.7, 133.4, 134.9, 138.0, 148.3, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 389.9. Calc. for C12H10Cl2N5O4S−: 390.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1369 and 1169 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 4.03 (3H, s, OCH3), 7.64 (1H, d, J 8.5, Ph-H), 7.92 (1H, d, J 8.5, Ph-H), 8.32 (1H, s, Ph-H), 9.86 (1H, s, NH), 13.10 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 120.1, 130.8, 133.6, 134.9, 137.9, 138.1, 148.3, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 433.8. Calc. for C12H10BrClN5O4S−: 433.9.
O), 1369 and 1169 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 4.03 (3H, s, OCH3), 7.64 (1H, d, J 8.5, Ph-H), 7.92 (1H, d, J 8.5, Ph-H), 8.32 (1H, s, Ph-H), 9.86 (1H, s, NH), 13.10 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 120.1, 130.8, 133.6, 134.9, 137.9, 138.1, 148.3, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 433.8. Calc. for C12H10BrClN5O4S−: 433.9.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1358 and 1170 (SO2). δH (400 MHz; (CD3)CO) 2.51 (3H, s, CH3), 4.02 (3H, s, OCH3), 7.47 (1H, d, J 8.3, Ph-H), 8.08 (1H, d, J 8.1, Ph-H), 8.48 (1H, d, J 1.8, Ph-H), 9.67 (1H, s, NH), 13.02 (1H, s, NH). m/z (ESI) [M–H]− found: 481.8. Calc. for C12H10ClIN5O4S−: 481.9.
O), 1358 and 1170 (SO2). δH (400 MHz; (CD3)CO) 2.51 (3H, s, CH3), 4.02 (3H, s, OCH3), 7.47 (1H, d, J 8.3, Ph-H), 8.08 (1H, d, J 8.1, Ph-H), 8.48 (1H, d, J 1.8, Ph-H), 9.67 (1H, s, NH), 13.02 (1H, s, NH). m/z (ESI) [M–H]− found: 481.8. Calc. for C12H10ClIN5O4S−: 481.9.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1356 and 1166 (SO2). δH (400 MHz; (CD3)CO) 2.54 (3H, s, CH3), 4.05 (3H, s, OCH3), 7.95 (1H, d, J 8.3, Ph-H), 8.18 (1H, dd, J 1.9, 8.3, Ph-H), 8.56 (1H, d, J 1.9, Ph-H), 9.86 (1H, s, NH), 13.21 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 111.6, 116.4, 133.2, 136.0, 136.5, 138.0, 148.6, 164.3, 170.8, 178.8. m/z (ESI) [M–H]− found: 380.9. Calcd for C13H10ClN6O4S−: 381.0.
O), 1356 and 1166 (SO2). δH (400 MHz; (CD3)CO) 2.54 (3H, s, CH3), 4.05 (3H, s, OCH3), 7.95 (1H, d, J 8.3, Ph-H), 8.18 (1H, dd, J 1.9, 8.3, Ph-H), 8.56 (1H, d, J 1.9, Ph-H), 9.86 (1H, s, NH), 13.21 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 111.6, 116.4, 133.2, 136.0, 136.5, 138.0, 148.6, 164.3, 170.8, 178.8. m/z (ESI) [M–H]− found: 380.9. Calcd for C13H10ClN6O4S−: 381.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1361 and 1177 (SO2). δH (400 MHz; (CD3)CO) 2.54 (3H, s, CH3), 4.05 (3H, s, OCH3), 7.97 (1H, d, J 8.4, Ph-H), 8.13 (1H, ddd, J 0.5, 2.2, 8.4, Ph-H), 8.49 (1H, d, J 2.1, Ph-H), 9.89 (1H, s, NH), 13.21 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 123.2 (q, J 273.2), 129.0 (q, J 34.1), 129.5 (q, J 3.9), 131.8 (q, J 3.4), 133.3, 135.9, 137.6, 148.3, 164.2, 170.8, 178.9. δF (376 MHz, (CD3)CO) −63.32. m/z (ESI) [M–H]− found: 424.0. Calc. for C13H10ClF3N5O4S−: 424.0.
O), 1361 and 1177 (SO2). δH (400 MHz; (CD3)CO) 2.54 (3H, s, CH3), 4.05 (3H, s, OCH3), 7.97 (1H, d, J 8.4, Ph-H), 8.13 (1H, ddd, J 0.5, 2.2, 8.4, Ph-H), 8.49 (1H, d, J 2.1, Ph-H), 9.89 (1H, s, NH), 13.21 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 55.0, 123.2 (q, J 273.2), 129.0 (q, J 34.1), 129.5 (q, J 3.9), 131.8 (q, J 3.4), 133.3, 135.9, 137.6, 148.3, 164.2, 170.8, 178.9. δF (376 MHz, (CD3)CO) −63.32. m/z (ESI) [M–H]− found: 424.0. Calc. for C13H10ClF3N5O4S−: 424.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1355 and 1164 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 3.05 (6H, s, N(CH3)2), 4.04 (3H, s, OCH3), 6.98 (1H, d, J 8.9, Ph-H), 7.38 (1H, d, J 8.9, Ph-H), 7.49 (1H, s, Ph-H), 9.74 (1H, s, NH), 12.84 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 39.5, 54.9, 115.0, 116.3, 117.4, 131.9, 136.5, 148.1, 149.1, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 399.0. Calc. for C14H16ClN6O4S−: 399.1.
O), 1355 and 1164 (SO2). δH (400 MHz; (CD3)CO) 2.52 (3H, s, CH3), 3.05 (6H, s, N(CH3)2), 4.04 (3H, s, OCH3), 6.98 (1H, d, J 8.9, Ph-H), 7.38 (1H, d, J 8.9, Ph-H), 7.49 (1H, s, Ph-H), 9.74 (1H, s, NH), 12.84 (1H, s, NH). δC (101 MHz; (CD3)CO) 24.6, 39.5, 54.9, 115.0, 116.3, 117.4, 131.9, 136.5, 148.1, 149.1, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 399.0. Calc. for C14H16ClN6O4S−: 399.1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1353 and 1165 (SO2). δH (400 MHz; (CD3)CO) 2.33 (3H, s, Ph-CH3), 2.38 (3H, s, CH3), 3.90 (3H, s, OCH3), 7.40 (2H, s, Ph-H), 7.90 (1H, s, Ph-H), 9.64 (1H, s, NH), 12.80 (1H, s, NH). δC NMR (101 MHz; (CD3)CO) 20.7, 25.5, 55.8, 129.2, 132.4, 133.7, 136.6, 137.0, 138.9, 149.0, 165.2, 171.7, 179.7. m/z (ESI) [M–H]− found: 369.9. Calc. for C13H13ClN5O4S−: 370.0.
O), 1353 and 1165 (SO2). δH (400 MHz; (CD3)CO) 2.33 (3H, s, Ph-CH3), 2.38 (3H, s, CH3), 3.90 (3H, s, OCH3), 7.40 (2H, s, Ph-H), 7.90 (1H, s, Ph-H), 9.64 (1H, s, NH), 12.80 (1H, s, NH). δC NMR (101 MHz; (CD3)CO) 20.7, 25.5, 55.8, 129.2, 132.4, 133.7, 136.6, 137.0, 138.9, 149.0, 165.2, 171.7, 179.7. m/z (ESI) [M–H]− found: 369.9. Calc. for C13H13ClN5O4S−: 370.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1354 and 1168 (SO2). δH (400 MHz; (CD3)CO) 1.27 (3H, t, J 7.6,
O), 1354 and 1168 (SO2). δH (400 MHz; (CD3)CO) 1.27 (3H, t, J 7.6,  ), 2.52 (3H, s, CH3), 2.78 (2H, q, J 7.6,
), 2.52 (3H, s, CH3), 2.78 (2H, q, J 7.6,  ), 4.03 (3H, s, OCH3), 7.56 (1H, d, J 8.2, Ph-H), 7.59 (1H, dd, J 1.9, 8.2, Ph-H), 8.06 (1H, d, J 1.7, Ph-H), 9.76 (1H, s, NH), 12.91 (1H, s, NH). δC (101 MHz; (CD3)CO) 14.7, 24.6, 27.7, 55.0, 128.4, 131.7, 131.8, 134.6, 136.2, 144.1, 148.2, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 384.0. Calc. for C14H15ClN5O4S−: 384.0.
), 4.03 (3H, s, OCH3), 7.56 (1H, d, J 8.2, Ph-H), 7.59 (1H, dd, J 1.9, 8.2, Ph-H), 8.06 (1H, d, J 1.7, Ph-H), 9.76 (1H, s, NH), 12.91 (1H, s, NH). δC (101 MHz; (CD3)CO) 14.7, 24.6, 27.7, 55.0, 128.4, 131.7, 131.8, 134.6, 136.2, 144.1, 148.2, 164.2, 170.8, 178.8. m/z (ESI) [M–H]− found: 384.0. Calc. for C14H15ClN5O4S−: 384.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1357 and 1164 (SO2). δH (400 MHz; (CD3)CO) 1.29 (6H, d, J 6.9, CH(CH3)2), 2.52 (3H, s, CH3), 3.01–3.19 (1H, m, CH), 4.03 (3H, s, OCH3), 7.57 (1H, d, J 8.2, Ph-H), 7.63 (1H, dd, J 2.1, 8.2, Ph-H), 8.09 (1H, d, J 2.1, Ph-H), 9.80 (1H, s, NH), 12.97 (1H, s, NH). δC (101 MHz; (CD3)CO) 23.0, 24.6, 33.4, 55.0, 128.5, 130.6, 131.7, 133.1, 136.2, 148.2, 148.5, 164.3, 170.8, 178.8. m/z (ESI) [M–H]− found: 398.0. Calc. for C15H17ClN5O4S−: 398.1.
O), 1357 and 1164 (SO2). δH (400 MHz; (CD3)CO) 1.29 (6H, d, J 6.9, CH(CH3)2), 2.52 (3H, s, CH3), 3.01–3.19 (1H, m, CH), 4.03 (3H, s, OCH3), 7.57 (1H, d, J 8.2, Ph-H), 7.63 (1H, dd, J 2.1, 8.2, Ph-H), 8.09 (1H, d, J 2.1, Ph-H), 9.80 (1H, s, NH), 12.97 (1H, s, NH). δC (101 MHz; (CD3)CO) 23.0, 24.6, 33.4, 55.0, 128.5, 130.6, 131.7, 133.1, 136.2, 148.2, 148.5, 164.3, 170.8, 178.8. m/z (ESI) [M–H]− found: 398.0. Calc. for C15H17ClN5O4S−: 398.1.X-ray diffraction analysis
The target compound I-12 was recrystallized by slow evaporation from a mixture of ethyl acetate and n-hexane to afford a colourless single crystal with dimensions of 0.20 × 0.18 × 0.12 mm, which was mounted on a glass fiber for X-ray diffraction analysis. The data were collected at 113(2) K on a Rigaku Saturn 724 CCD diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 Å), θmax = 27.91°. The molecular formula is C15H18ClN5O4S and the formula weight is 399.85. The crystal was a monoclinic system, space group P2(1)/n, with unit cell parameters: a = 9.4336 (19) Å; b = 11.524 (2) Å; c = 16.608 (3) Å, V = 1773.1 (6) Å3, Z = 4, density (calculated) = 1.498 g cm−3, and linear absorption coefficient 0.366 mm−1. In total, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 integrated reflections were collected, reducing to a data set of 4240 unique with Rint = 0.0549, and completeness of data (to theta = 27.91°) of 99.7%. Data were collected and processed using CrystalClear (Rigaku). An empirical absorption correction was applied using CrystalClear (Rigaku). The structure was solved by direct methods with the SHELXS-97 program.17 Full-matrix least-squares refinement based on F2 using the weight of 1/[σ2(Fo2) + (0.0567P)2 + 0.3384P] gave final values of R = 0.0439, wR = 0.1090. Hydrogen atoms were observed and refined with a fixed value of their isotropic displacement parameter. The correction for absorption was multi-scan, Tmin = 0.9304, Tmax = 0.9574.
832 integrated reflections were collected, reducing to a data set of 4240 unique with Rint = 0.0549, and completeness of data (to theta = 27.91°) of 99.7%. Data were collected and processed using CrystalClear (Rigaku). An empirical absorption correction was applied using CrystalClear (Rigaku). The structure was solved by direct methods with the SHELXS-97 program.17 Full-matrix least-squares refinement based on F2 using the weight of 1/[σ2(Fo2) + (0.0567P)2 + 0.3384P] gave final values of R = 0.0439, wR = 0.1090. Hydrogen atoms were observed and refined with a fixed value of their isotropic displacement parameter. The correction for absorption was multi-scan, Tmin = 0.9304, Tmax = 0.9574.
Herbicidal activity screening
Herbicidal activities were tested in greenhouse (25 ± 2 °C) according to the following method.11 The emulsions of purified compounds (10.0 mg) were respectively prepared by dissolving them in N,N-dimethylformamide (1 mL) with the addition of a certain amount of Tween 80 (1.0 g) in distilled water (1000 mL). The mixture of the same amount of N,N-dimethylformamide, Tween 80, and distilled water was used as a negative control and chlorsulfuron was employed as a positive control. Each experiment was performed in triplicate. The solutions of tested compounds were sprayed with a laboratory belt sprayer delivering a 750 L ha−1 (1 ha = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2) spray volume.
000 m2) spray volume.
Soil treatment: sandy clay (100.0 g) in a plastic box (11.0 cm × 7.5 cm × 6.0 cm) was wetted with distilled water. Fifteen sprouting seeds of the test weeds were planted in the fine earth (0.6 cm depth) in the glasshouse and sprayed with the test compound solution. After spraying for 28 days, the ground fresh weight was measured and compared to the negative group to calculate the inhibition percent of fresh weight. The test weed involves Brassica campestris, Amaranthus tricolor, Echinochloa crusgalli and Digitaria adscendens.
Foliage spray: seedlings (one leaf and one stem) of the test weeds were sprayed with the test compound solution at the same rate as used for the soil treatment test. The calculated method and test materials were as same as soil treatment.
Soil degradation investigating
The soil degradation of chlorsulfuron derivatives were investigated in acid soil (pH 5.41) with the initial additive concentration of 5 mg a.i. per kg under laboratory conditions at 25 °C and a moisture content corresponding to 70% field capacity.The samples were analysed by a high-performance liquid chromatograph (HPLC) technique. The methods employed a Shimadzu HPLC (series LC-20AT), equipped with a binary pump (Shimadzu, LC-20AT), an UV/VIS detector (Shimadzu, SPD-20A), an auto sampler (Shimadzu, SIL-20A), a Shimadzu shim-pack VP-ODS column (5 μm, 250 mm × 4.6 mm) connected to a Shimadzu shim-pack GVP-ODS (10 mm × 4.6 mm) precolumn, and a computer (model Dell) for carrying out the analysis. The mobile phases consisted of methanol (A) and phosphoric acid solution in double distilled water (B) (pH = 3.00) with a flow rate of 0.8–1.0 mL min−1. The injection volume was set at 10 μL, and the detector wavelength was adjusted at 235 nm according to the UV spectra of the target compounds. The specific HPLC analytical conditions, which could ensure good separation between soil contaminants and standard samples, were listed in Table 1. From the results, the HPLC analytical methods were adjusted according to the different molecular characteristics. Therefore, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for target compounds were calculated with prediction system of log
P values for target compounds were calculated with prediction system of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (CISOC-log
P (CISOC-log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) as previously reported,18 and displayed in Fig. 3. Then the appropriate extraction solvent was selected in accordance with log
P) as previously reported,18 and displayed in Fig. 3. Then the appropriate extraction solvent was selected in accordance with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values by measuring soil recovery rates and coefficients of variation (Table 1). When all the analytical methods were ready, the soil degradation behaviors were investigated under set conditions.
P values by measuring soil recovery rates and coefficients of variation (Table 1). When all the analytical methods were ready, the soil degradation behaviors were investigated under set conditions.
| Compounds | R | HPLC analysis conditions (wavelength, flow rate, mobile phase (v/v)) | Extraction solvents (v/v) | Adding mass fraction (mg kg−1) | Average recovery rate/% | Coefficient of variation/% |
|---|---|---|---|---|---|---|
| a Compound I-2 represent chlorsulfuron, and the determination of soil recovery rates was performed in quintuplet at each adding mass fraction to calculate the average recovery rate and coefficient of variation. | ||||||
| I-1 | NO2 | 235 nm, 1.0 mL min−1, CH3OH/H2O (pH 3.0) = 60/40 | Acetone/DCM/phosphoric acid solution (pH 2.0) = 40/5/5 | 5 | 86.71 | 1.34 |
| 2 | 83.56 | 1.89 | ||||
| 0.5 | 79.43 | 4.14 | ||||
| I-2 | H | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 60/40 | Acetone/THF/DCM = 30/5/5 | 5 | 81.81 | 1.30 |
| 2 | 82.35 | 4.04 | ||||
| 0.5 | 80.23 | 1.55 | ||||
| I-3 | F | 235 nm, 1.0 mL min−1, CH3OH/H2O (pH 3.0) = 60/40 | Acetone/DCM/phosphoric acid solution (pH 2.0) = 40/5/5 | 5 | 86.40 | 1.44 |
| 2 | 82.79 | 1.60 | ||||
| 0.5 | 75.57 | 2.55 | ||||
| I-4 | Cl | 235 nm, 1.0 mL min−1, CH3OH/H2O (pH 3.0) = 65/35 | Acetone/THF/DCM = 30/10/10 | 5 | 84.51 | 2.77 |
| 2 | 81.75 | 1.40 | ||||
| 0.5 | 72.08 | 1.64 | ||||
| I-5 | Br | 235 nm, 1.0 mL min−1, CH3OH/H2O (pH 3.0) = 65/35 | Acetone/THF/DCM = 30/10/10 | 5 | 75.50 | 3.84 |
| 2 | 73.80 | 3.27 | ||||
| 0.5 | 72.26 | 1.84 | ||||
| I-6 | I | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 70/30 | Acetone/THF/DCM = 30/5/5 | 5 | 84.09 | 1.80 |
| 2 | 82.07 | 1.46 | ||||
| 0.5 | 83.58 | 2.47 | ||||
| I-7 | CN | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 60/40 | Acetone/THF/DCM/phosphoric acid solution (pH 2.0) = 30/10/10/10 | 5 | 91.06 | 1.03 |
| 2 | 87.79 | 4.03 | ||||
| 0.5 | 73.44 | 4.22 | ||||
| I-8 | CF3 | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 70/30 | Acetone/THF/DCM = 30/20/10 | 5 | 81.25 | 1.84 |
| 2 | 83.41 | 3.43 | ||||
| 0.5 | 88.05 | 3.41 | ||||
| I-9 | N(CH3)2 | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 65/35 | Acetone/THF/DCM = 30/10/10 | 5 | 74.53 | 1.67 |
| 2 | 72.91 | 1.31 | ||||
| 0.5 | 72.80 | 2.59 | ||||
| I-10 | CH3 | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 65/35 | Acetone/THF/DCM = 30/10/10 | 5 | 83.90 | 4.41 |
| 2 | 84.59 | 1.20 | ||||
| 0.5 | 88.69 | 1.32 | ||||
| I-11 | C2H5 | 235 nm, 1.0 mL min−1, CH3OH/H2O (pH 3.0) = 65/35 | Acetone/THF/DCM = 30/5/5 | 5 | 84.95 | 4.35 |
| 2 | 83.66 | 1.50 | ||||
| 0.5 | 75.10 | 2.35 | ||||
| I-12 | i-C3H7 | 235 nm, 0.8 mL min−1, CH3OH/H2O (pH 3.0) = 70/30 | Acetone/THF/DCM = 30/5/5 | 5 | 93.52 | 1.10 |
| 2 | 91.40 | 1.62 | ||||
| 0.5 | 91.31 | 3.63 | ||||
Testing soil (20.00 g, air-dry weight) passed through 2 mm sieve was weighed into six groups of 150 mL Erlenmeyer flasks, three in each group respectively, and added standard solutions at a concentration of 5 mg a.i. per kg. Followed the solvent volatilizing completely under a fume hood, the soil was thoroughly mixed, and an appropriate volume of distilled water was added to adjust the soil moisture content to approximately 70% field capacity. The bottles were then stoppered with a cotton plug and put into an ecological incubator (temperature 25 ± 1 °C, humidity 80%) to incubate in the dark prior to treatment. In the cultivating process, the moisture content in Erlenmeyer flask was adjusted regularly to maintain the original water-holding state. The samples were taken periodically and added the extraction solvent for extraction. The mixture was shaken for 2 h at 200 rpm with an oscillator, and then centrifuged for 2 min at 6500 rpm with a Thermo Scientific Legend Mach 1.6 R centrifuge to obtain supernatant which was concentrated later in vacuum at room temperature. After the residue was extracted by dichloromethane (30 mL × 2), the combined organic layer was dried by anhydrous sodium sulfate, filtered and concentrated up to dryness. The product was dissolved in acetonitrile (10 mL), and filtered through a filter membrane (0.22 μm) to HPLC analysis. Each sample was taken six times for drawing first-order kinetic curves, and every time was performed in triplet for statistical analysis.
Results and discussion
Synthetic chemistry
Herein, the important intermediates II were designed and synthesized according to the methods in Scheme 1. Firstly, intermediate II-1 was prepared from 1-chloro-4-nitrobenzene 1 through sulfonation and nucleophilic substitution reactions as previously reported,19 and then reduced to provide 2-chloro-4-aminobenzenesulfonamide 3 with Fe/HCl in ethanol which was diazotized to get intermediates II-2–II-6 with diffident diazo reagents as reported literatures.20–24 Compound II-7 (or II-8) was synthesized by a process starting from nitration of material 4a (or 4b) with fuming nitric acid/concentrated sulfuric acid,25,26 followed by reduction of nitro group with Na2S2O4, diazotization of amino group27 and then nucleophilic substitution reaction. Compound 3 was transformed to intermediate II-9 by reaction with NaBH3CN, formalin, CH3COOH in acetronitrile.28 4′-Chloroacetophenone 9 was reduced to produce 1-chloro-4-ethylbenzene 10 with NaBH4/AlCl3 in tetrahydrofuran.29 Synthesis of 1-chloro-4-isopropylbenzene 12 from material 9 usually involves Wittig reaction, reduction of olefin group with Pd/C/H2 in methanol. Intermediate II-10 was synthesized by reaction of 1-chloro-4-methylbenzene 8 with chlorosulfonic acid,30,31 ammonium hydroxide, and compounds II-11, II-12 were prepared as the same manner, which all the structures were also confirmed by 13C-HMBC. The synthetic route towards the target compounds was summarized in Scheme 2. Subsequently, twelve molecules were synthesized from intermediates II. Compounds III were prepared by reaction of materials II with oxalyl chloride and a catalytic amount of triethylenediamine (DABCO), and converted to products I through reaction with 4-methoxy-6-methyl-1,3,5-triazin-2-amine in anhydrous toluene. All target compounds were purified by chromatography on silica gel using dichloromethane/ethyl acetate as eluent. The synthesized compounds were identified and characterized by infrared spectroscopy (IR), ultraviolet (UV) spectra, 1H NMR, 13C NMR, mass spectra and elemental analysis (EA). Several unique structural characteristics were also revealed via the crystal structure of compound I-12 (Fig. 4).Crystal structure analysis
Several molecular characteristics were apparently presented upon crystal structure analysis of compound I-12 (CCDC no. 1426721). From the data, the bond angles of O(1)–S(1)–C(1), O(2)–S(1)–C(1), and N(1)–S(1)–C(1) were 107.86(8)°, 109.27(8)°, and 106.65(8)° respectively, indicating the sp3 hybridization state of the S(1) atom. The sum of O(3)–C(10)–N(1), O(3)–C(10)–N(2), and N(1)–C(10)–N(2) angles was 359.99°, indicating the sp2 hybridization state of the C(10) atom. The torsion angle of O(3)–C(10)–N(2)–H(2) was 0.083(14)°, while 176.539(16)° for O(3)–C(10)–N(1)–H(1). The dihedral angle between two planes containing N(1)–C(10)–O(3)–N(2) and C(11)–N(3)–C(12)–N(4)–C(13)–N(5) was 13.379(70)°, which showed both planes were non-planar. In the meantime, the dihedral angle between the benzene ring and the triazine ring was 66.914(52)°, demonstrating their non-plane.Biological assay
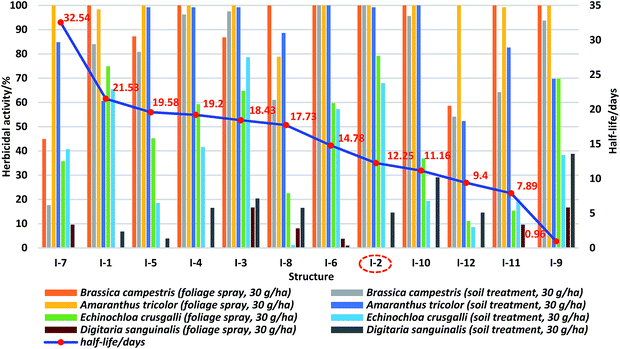
The herbicidal activities of target compounds, with chlorsulfuron as a positive control, against four weeds representing monocotyledonous and dicotyledonous plants at 150 g ha−1 and 30 g ha−1 respectively, were shown in Table 2 and comprehensively evaluated. From the data, in a given category, no matter soil treatment or foliage spray, the herbicidal activities of the title compounds against dicotyledonous plants were higher than those against monocotyledons, including positive control chlorsulfuron (compound I-2). In addition, no compounds displayed good inhibition rate against Digitaria sanguinalis regardless of which spraying methods adopted whereas, on the other hand, almost all of the target compounds exhibited excellent herbicidal activity against Amaranthus tricolor at 30 g ha−1. Interestingly, the structure I-9 containing dimethylamino group at 5th position exhibited superior herbicidal activities under soil treatment at 150 g ha−1 against Brassica campestris, Amaranthus tricolor, Echinochloa crusgalli, and Digitaria sanguinalis with the inhibition percent of 100, 98.5, 92.6, and 91.3% respectively. In the meantime, the introduction of halo, nitro, dimethylamino, and methyl groups at 5th position was favorable to remain or improve the herbicidal activities of the target compounds, while ethyl, isopropyl, cyano, and trifluoromethyl substituents decreased the inhibition rates. For example, the herbicidal activities of compounds I-1 (5-nitro), I-3 (5-fluoro), I-4 (5-chloro), I-5 (5-bromo), I-6 (5-iodo), I-9 (5-dimethylamino), and I-10 (5-methyl) were similar to the positive control chlorsulfuron (I-2), however, the products I-7 (5-cyano), I-8 (5-trifluoromethyl), I-11 (5-ethyl), and I-12 (5-isopropyl) displayed relatively weak herbicidal activities when compared with chlorsulfuron. From the above results, it was concluded that most compounds in general showed good herbicidal activities in comparison with chlorsulfuron except structures I-7 and I-12.| Compounds | R | Concentration (g a.i. per ha) | Herbicidal activity (inhibition percent)/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil treatment | Foliage spray | |||||||||
| Brassica campestris | Amaranthus tricolor | Echinochloa crusgalli | Digitaria sanguinalis | Brassica campestris | Amaranthus tricolor | Echinochloa crusgalli | Digitaria sanguinalis | |||
| a Compound I-2 represent chlorsulfuron. | ||||||||||
| I-1 | NO2 | 30 | 84.0 | 60.6 | 65.4 | 6.8 | 100 | 98.3 | 74.9 | 0 |
| 150 | 98.1 | 98.5 | 86.2 | 12.6 | 100 | 100 | 85.1 | 0 | ||
| I-2 | H | 30 | 100 | 99.2 | 67.9 | 14.6 | 100 | 100 | 79.2 | 0 |
| 150 | 100 | 100 | 88.0 | 69.9 | 100 | 100 | 84.7 | 0 | ||
| I-3 | F | 30 | 97.5 | 99.2 | 78.6 | 20.4 | 86.8 | 100 | 64.8 | 16.7 |
| 150 | 99.7 | 99.8 | 81.1 | 72.8 | 100 | 100 | 89.8 | 29.7 | ||
| I-4 | Cl | 30 | 96.2 | 99.2 | 41.6 | 16.5 | 100 | 100 | 59.3 | 0 |
| 150 | 99.7 | 100 | 62.1 | 59.2 | 100 | 100 | 66.7 | 12.4 | ||
| I-5 | Br | 30 | 80.8 | 99.2 | 18.5 | 3.9 | 87.2 | 100 | 45.2 | 0 |
| 150 | 99.1 | 100 | 49.8 | 33.0 | 100 | 100 | 64.0 | 0 | ||
| I-6 | I | 30 | 100 | 100 | 57.2 | 1.0 | 100 | 100 | 59.7 | 3.8 |
| 150 | 100 | 100 | 72.8 | 8.7 | 100 | 100 | 64.4 | 8.1 | ||
| I-7 | CN | 30 | 17.6 | 84.8 | 40.7 | 0 | 44.9 | 100 | 35.8 | 9.6 |
| 150 | 32.7 | 88.6 | 50.6 | 10.7 | 62.1 | 100 | 47.5 | 13.9 | ||
| I-8 | CF3 | 30 | 61.0 | 88.6 | 1.2 | 16.5 | 100 | 78.8 | 22.5 | 8.1 |
| 150 | 95.6 | 98.5 | 25.1 | 20.4 | 100 | 100 | 38.1 | 27.5 | ||
| I-9 | N(CH3)2 | 30 | 93.7 | 69.7 | 38.3 | 38.8 | 100 | 100 | 69.8 | 16.7 |
| 150 | 100 | 98.5 | 92.6 | 91.3 | 100 | 100 | 88.3 | 38.3 | ||
| I-10 | CH3 | 30 | 95.6 | 100 | 19.3 | 29.1 | 100 | 100 | 36.9 | 0 |
| 150 | 97.5 | 100 | 59.7 | 39.8 | 100 | 100 | 55.7 | 16.7 | ||
| I-11 | C2H5 | 30 | 64.2 | 82.6 | 20.2 | 0 | 100 | 99.2 | 15.4 | 9.6 |
| 150 | 87.7 | 97.0 | 33.3 | 36.9 | 100 | 100 | 33.8 | 39.7 | ||
| I-12 | i-C3H7 | 30 | 54.1 | 52.3 | 8.6 | 14.6 | 58.6 | 100 | 11.1 | 0 |
| 150 | 95.6 | 80.3 | 29.2 | 30.1 | 75.8 | 100 | 18.1 | 16.7 | ||
Soil degradation
The soil degradation for target compounds was investigated under set conditions, and should follow first-order kinetic equation. The appropriate HPLC analytical conditions were confirmed through comparing HPLC analyses of blank soil, test soil and standard samples. In the process of extraction solvent selection, methanol, acetonitrile, acetone, tetrahydrofuran (THF), dichloromethane (DCM) and mixed solutions, like acetone/dichloromethane, acetone/tetrahydrofuran, and acetone/tetrahydrofuran/dichloromethane were chosen to measure the soil recovery rates respectively. Interestingly, a ternary mixed system, acetone/tetrahydrofuran/dichloromethane, was found to be favorable to extract the standard samples. At last, the disappearance of SU was reported by plotting the concentration as a function of the degradation time. Each experiment was performed in triplicate to measure the standard deviation. The corresponding first-order kinetic equations and half-life (t1/2) periods were shown in Table 3 respectively. From the data, it was concluded that the introduction of electron-donating substituents at 5th position of the benzene ring inclined to accelerate the degradation rates, while with the electron-withdrawing groups to prolong the half-life periods of target compounds in comparison with chlorsulfuron (I-2). For example, the half-life data of I-9 (5-dimethylamino), I-11 (5-ethyl), I-12 (5-isopropyl), and I-10 (5-methyl) were 0.96, 7.89, 9.4, and 11.16 days respectively, which were shorter than that of chlorsulfuron (t1/2 = 12.25 days), however, those of compounds I-6 (5-iodo), I-8 (5-trifluoromethyl), I-3 (5-fluoro), I-4 (5-chloro), I-5 (5-bromo), I-1 (5-notro), and I-7 (5-cyano) were 14.78, 17.73, 18.43, 19.2, 19.58, 21.53, and 32.54 days respectively. Surprisingly, the half-life of compound I-9 was less than one day. Furthermore, the degradation rates among the target compounds introduced halo groups at 5th position had no significant difference. For instance, the degradation half-life data of compounds I-6, I-3, I-4, and I-5 were 14.78, 18.43, 19.2, and 19.58 days respectively. From the above experimental results, it was confirmed that the introduction of different substitutes at 5th position do influence the soil degradation rates of target compounds, which could provide valuable information to explore potential controllable degradation of other herbicides.| Compounds | R | First-order kinetic equation | Correlation coefficient/R2 | Half-life t1/2/days |
|---|---|---|---|---|
| a Compound I-2 represent chlorsulfuron. | ||||
| I-7 | CN | Ct = 4.698![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0213t e−0.0213t |
0.9978 | 32.54 |
| I-1 | NO2 | Ct = 4.445![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0322t e−0.0322t |
0.9958 | 21.53 |
| I-5 | Br | Ct = 3.842![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0354t e−0.0354t |
0.9983 | 19.58 |
| I-4 | Cl | Ct = 3.845![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0361t e−0.0361t |
0.9982 | 19.20 |
| I-3 | F | Ct = 4.542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0376t e−0.0376t |
0.9998 | 18.43 |
| I-8 | CF3 | Ct = 3.911![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0391t e−0.0391t |
0.9944 | 17.73 |
| I-6 | I | Ct = 3.824![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0469t e−0.0469t |
0.9989 | 14.78 |
| I-2 | H | Ct = 3.914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0566t e−0.0566t |
0.9979 | 12.25 |
| I-10 | CH3 | Ct = 4.284![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0621t e−0.0621t |
0.9978 | 11.16 |
| I-12 | i-C3H7 | Ct = 4.392![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0737t e−0.0737t |
0.9973 | 9.40 |
| I-11 | C2H5 | Ct = 4.466![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.0878t e−0.0878t |
0.9975 | 7.89 |
| I-9 | N(CH3)2 | Ct = 3.982![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.725t e−0.725t |
0.9945 | 0.96 |
Conclusions
In summary, a structural derivation of chlorsulfuron was designed and synthesized by introducing various groups (alkyl, nitro, halogen, cyano etc.) onto the 5th position of its benzene ring. Identities of the target compounds were confirmed by IR, UV, 1H and 13C NMR, MS, EA and X-ray diffraction. Bioassay results indicated that most of synthesized sulfonylureas showed superior herbicidal activities when compared with chlorsulfuron. After their soil degradation behaviors were investigated, an insight of structure/bioassay/soil degradation tri-factor relationship was firstly established and summarized in Fig. 5. As observed from the figure, the introduction of various substituents at 5th position of the benzene ring can remain or vary the herbicidal activities, such as nitro, halo, methyl, and dimethylamino etc., on the other hand, can influence the soil degradation rates where electron-donating groups are favorable, especially dimethylamino group. These results will provide a valuable clue to further explore the potential controllable degradation of SU and other herbicides to seek ecologically safer and environmentally benign herbicides. It will also provide us a new strategy to decrease the relevant impact on our environment and ecology during future herbicides research program.Acknowledgements
This work was supported by the National National Science Foundation of China (No. 21272129), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), and Syngenta Doctorate Scholarship.Notes and references
- J. G. Wang, P. K. M. Lee, Y. H. Dong, S. S. Pang, R. G. Duggleby, Z. M. Li and L. W. Guddat, FEBS J., 2009, 276, 1282 CrossRef CAS PubMed.
- R. G. Duggleby and S. S. Pang, J. Biochem. Mol. Biol., 2000, 33, 1 CAS.
- Z. M. Li, Y. Ma, L. W. Guddat, P. Q. Cheng, J. G. Wang, S. S. Pang, Y. H. Dong, C. M. Lai and L. X. Wang, et al., Pest Manage. Sci., 2012, 68, 618 CrossRef CAS PubMed.
- E. Vulliet, C. Emmelin, J. M. Chovelon, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2002, 38, 127 CrossRef CAS.
- D. M. Laura and A. Peña, J. Agric. Food Chem., 2007, 55, 6213 CrossRef PubMed.
- P. Lu, L. Jin, B. Liang, J. Zhang, S. P. Li, Z. Z. Feng and X. Huang, Curr. Microbiol., 2011, 62, 1718 CrossRef CAS PubMed.
- P. R. Stork, Aust. J. Agric. Res., 1995, 46, 1445 CrossRef CAS.
- A. K. Sarmah, R. S. Kookana and A. M. Alston, Weed Res., 1999, 39, 83 CrossRef CAS.
- I. D. Black, R. N. Pederson, A. Flynn, M. Moerkerk, C. B. Dyson, R. Kookana and N. Wihelm, Aust. J. Exp. Agric., 1999, 39, 465 CrossRef CAS.
- M. Y. Wang, X. L. Mu, W. C. Guo, Y. H. Li and Z. M. Li, Chem. Res. Chin. Univ., 2007, 23, 674 CrossRef CAS.
- S. R. Teaney, L. Armstrong, K. Bentley, D. Cotterman, D. Leep, P. H. Liang, C. Powley, J. Summers and S. Cranwell, Brighton Crop Prot. Conf.–Weeds, 1995, 1, 49 Search PubMed.
- G. Cao, M. Y. Wang, M. Z. Wang, S. H. Wang, Y. H. Li and Z. M. Li, Chem. Res. Chin. Univ., 2011, 27, 60 CAS.
- J. Kim, K. H. Liu, S. H. Kang, S. J. Koo and J. H. Kim, J. Agric. Food Chem., 2003, 51, 710 CrossRef CAS PubMed.
- D. Vega, J. P. Cambon and J. Bastide, J. Agric. Food Chem., 2000, 48, 3733 CrossRef CAS PubMed.
- S. Saha and G. Kulshrestha, J. Agric. Food Chem., 2002, 50, 4572 CrossRef CAS PubMed.
- I. Braschi, L. Calamai, M. A. Cremonini, P. Fusi, C. Gessa, O. Pantani and A. Pusino, J. Agric. Food Chem., 1997, 45, 4495 CrossRef CAS.
- G. M. Sheldrick, SHELXTL (Version 5.0), University of Göttingen, Göttingen, Germany, 2001 Search PubMed.
- Q. Liao, J. H. Yao and S. G. Yuan, Mol. Diversity, 2006, 10, 301 CrossRef CAS PubMed.
- L. Pan, Y. Jiang, Z. Liu, X. H. Liu, Z. Liu, G. Wang, Z. M. Li and D. Wang, Eur. J. Med. Chem., 2012, 50, 18 CrossRef CAS PubMed.
- C. Görl, N. Beck, K. Kleiber and H. G. Alt, J. Mol. Catal. A: Chem., 2012, 352, 110 CrossRef.
- Z. Q. Yu, Y. W. Lv, C. M. Yu and W. K. Su, Tetrahedron Lett., 2013, 54, 1261 CrossRef CAS.
- P. H. Fries and D. Imbert, J. Chem. Eng. Data, 2010, 55, 2048 CrossRef CAS.
- C. X. Zhao, J. W. Malecha, S. A. Noble, S. G. Duron, A. K. Lindstrom and A. K. Shiau, US Patent, 0234046, 2005.
- L. Shi, L. P. Zhou, G. K. Dai, N. Y. Wang, D. L. An and Q. Y. Cai, Talanta, 2013, 115, 386 CrossRef CAS PubMed.
- J. B. Adams, US Patent, 4452628, 1984.
- T. W. Ly and G. T. Potter, US Patent, 0218207, 2011.
- J. W. Leahy, C. A. Buhr, H. W. Johnson, B. G. Kim, T. G. Baik and J. Cannoy, et al., J. Med. Chem., 2012, 55, 5467 CrossRef CAS PubMed.
- G. M. Dull, J. M. Wagner, W. S. Caldwell, C. H. Miller, J. D. Schmitt, B. S. Bhatti and S. B. Hadimani, US Patent, 6337351, 2002.
- A. Ono, N. Suzuki and J. Kamimura, Synthesis, 1987, 736 CrossRef CAS.
- S. Samanta, K. Srikanth, S. Banerjee, B. Debnath, S. Gayen and T. Jha, Bioorg. Med. Chem., 2014, 12, 1413 CrossRef PubMed.
- G. Bocelli, A. Cantoni, Y. A. Simonov, M. S. Fonari and E. V. Ganin, J. Inclusion Phenom. Mol. Recognit. Chem., 1995, 20, 105 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Containing the crystal data for compound I-12, soil degradation curves and structure spectra of all target compounds, including 1H and 13C NMR, IR, UV and MS. CCDC 1426721. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra25765d |
| This journal is © The Royal Society of Chemistry 2016 |