pH-sensitive ternary nanoparticles for nonviral gene delivery†
Ming-Hua Zhanga,
Zhi-Peng Gub,
Xi Zhang*a and
Min-Min Fan*a
aThe State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, China. E-mail: zhangxi6352@163.com; fanminmin@scu.edu.cn
bDepartment of Neurosurgery, West China Hospital, Sichuan University, Chengdu 610065, China
First published on 11th May 2015
Abstract
PEGylation, which is reversed after the therapeutic agent reaches the target cell, presents attractive features for drug, protein or gene delivery. Herein, a tumor acidity-responsive PEGylated anionic polymer was synthesized for bioreversible surface shielding of DNA complexes. The pH-sensitive and non-pH-sensitive ternary nanoparticles were respectively fabricated by introducing tumor acidity-responsive PEGylated anionic polymer and its corresponding pH stable analog to the surface of positively charged PEI25K/DNA complexes via electrostatic interaction. We show clear evidence that introducing the PEGylated anionic polymer to the surface of a nanoparticle markedly reduces its nonspecific interactions with protein. We further demonstrate that the pH-sensitive ternary nanoparticle versus non-pH-sensitive analog is capable of reversing its surface charge from neutral to positive at the slightly acidic tumor extracellular microenvironment to facilitate the delivery of DNA. Such delivery system with the ability to deshield the PEG layer at the target tissues has remarkable potential in gene delivery.
1. Introduction
Among various synthetic vectors, polyethyleneimines (PEIs) are most widely used for gene delivery due to their high nucleic acid condensing capability, ability for endosomal escape,1–3 and nuclear localization capability.4 Positively charged PEIs can compact negatively charged DNA into condensed nanoparticles by electrostatic interaction, which can protect DNA from digestion in serum and then enter cells.5–9 In addition, PEI can act as a buffer or “proton sponge” to induce osmotic swelling and thus lead to endosomal escape,1–3 which can protect DNA from degradation in the lysosome.10 However, undesirable interactions of the positively charged PEI/DNA complexes with blood components due to the high positive charge limit their in vivo applications.9–12Usually, to achieve high transfection efficiency, excess polycation is usually complexed with nucleic acid resulting in complexes with a net positive charge.13 It is now understood that neutral particles are preferred since body enzymes recognize the charge on foreign particles and eliminate them.14 To inhibit the interaction of serum proteins with DNA-carrier complexes, introduction of hydrophilic polymers like polyethylene glycol (PEG) have been widely studied to reduce positive surface charge and toxicity, to prevent aggregation, protect from uptake by the mononuclear phagocytic system, increase circulation time, and, hence, improve systemic gene delivery.5,15–21 However, at the same time, PEGylation may lower gene transfer efficiency due to reduced cell surface and/or endosomal lipid membrane interactions.22,23 Ideally, these PEGlated particles should circulate until they reach the target cells, either by specific targeting or by passive accumulation, and be triggered to remove PEG and release their therapeutic content.
The extracellular pH of tumor tissue (pH 6.5) is significantly lower than that of normal tissue and blood (pH 7.4) due to the hypoxia-induced production of excess lactate and protons in tumor extracellular microenvironments.24,25 Thus, shielding of the DNA/PEI complex when in the systemic circulation with PEG, which is capable of detaching at lowered pH values exposing cationic DNA complex only at the tumor sites could serve as an attractive solution.13 The use of cleavable pH-sensitive PEG shields has already been demonstrated as an effective strategy for overcoming the PEG-dilemma.26–29 For instance, Sethuraman et al. have demonstrated the enhanced transfection efficiency of a tumor acidity-responsive sulfonamide/poly(ethylenimine)/pDNA delivery in vitro.29
Our current work describes the design of reversibly shielded ternary nanoparticular system for tumor acidity-targeted gene delivery by introducing a tumor acidity-responsive PEGylated anionic polymer (Boc-PEOn/PAGEm-Cys-DMMA, PPC-DA, where the subscript number represents degree of polymerization of each block) layer to the surface of positively charged PEI25K/DNA complexes through electrostatic interaction. It has demonstrated that the amide bond formed between an amino and DMMA is cleavable under slightly acidic conditions of tumors.30,31 PPC-DA/PEI25K/DNA nanoparticles were thus expected to release the PEG from the stabilized nanoparticles, revealing the cationic entities at their surface. This would in turn facilitate nanoparticles internalization in the targeted cells, thus favouring gene delivery and, ultimately, protein expression. Here, we evaluated the pH-sensitive PPC-DA for the delivery of plasmid DNA and characterized it for its various physicochemical properties, cell cytotoxicity, and transfection efficiency in three cell lines. In comparison to analogous stable shielded nanoparticles, pH-triggered PEG deshielding and higher transfection efficiency was demonstrated.
2. Experimental
2.1 Materials
Branch PEI25K (Mw 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), allyl glycidyl ether (AGE), 2,3-dimethylmaleic anhydride (DMMA), 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), agarose and trypsin were purchased from Sigma (St Louis, MO, USA). Gold-view was purchased from Solarbio (Beijing, China). The pORF-lacZ and plasmid DNAs coding green fluorescence protein (pEGFP) were amplified in DH5-αEscherichia coli and purified with Qiagen Giga Endofree plasmid purification kit (Valencia, CA, USA). Cell culture medium 1640 was bought from Gibco (Grand Island, NY, USA). β-Gal assay kit and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen (Carlsbad, CA, USA). Fluorescently labelled FAM-DNA was synthesized by Suzhou Ribo Life Science Co. (Kunshan, China). All the other chemicals and reagents used were of the analytical grade obtained commercially.
000), allyl glycidyl ether (AGE), 2,3-dimethylmaleic anhydride (DMMA), 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), agarose and trypsin were purchased from Sigma (St Louis, MO, USA). Gold-view was purchased from Solarbio (Beijing, China). The pORF-lacZ and plasmid DNAs coding green fluorescence protein (pEGFP) were amplified in DH5-αEscherichia coli and purified with Qiagen Giga Endofree plasmid purification kit (Valencia, CA, USA). Cell culture medium 1640 was bought from Gibco (Grand Island, NY, USA). β-Gal assay kit and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen (Carlsbad, CA, USA). Fluorescently labelled FAM-DNA was synthesized by Suzhou Ribo Life Science Co. (Kunshan, China). All the other chemicals and reagents used were of the analytical grade obtained commercially.
2.2 Instruments
The structure of Boc-aminoethanol, Boc-PEOn/PAGEm, Boc-PEOn/PAGEm-Cys (PPC), Boc-PEOn/PAGEm-Cys-DA (PPC-DA) and Boc-PEOn/PAGEm-Cys-SA (PPC-SA) were characterized by 1H NMR (600 MHz; Bruker Corporation, Germany). Samples were negatively stained with 2% phosphotungstic acid and analyzed under a JEM-100CX transmission electron microscope (TEM) at an accelerating voltage of 80 kV. Both the particle size and zeta potential of PEI25K/DNA, PPC-DA/PEI25K/DNA and PPC-SA/PEI25K/DNA nanoparticles were measured by laser-light scattering (Zetasizer 3000, Malvern Instruments, UK). Molecular weight distribution of PPC was estimated by GPC performed using Shodex KB-803 column (Shoko Co, Tokyo, Japan), Waters 515 pump, and a Waters 2410 Refractive Index Detector. PPC was dissolved in ultrapure water, and PEG was applied as the molecular standard. The cells transfected with PEI25K/EGFP and PPC-DA/PEI/EGFP were monitored by a fluorescence microscope (Axiovert 40 CFL; Carl Zeiss, Germany). Intracellular localization and expression of labelled plasmid DNA were monitored by confocal laser scanning microscope (510 META; Carl Zeiss, Germany).2.3 Cell culture
A549 (human lung carcinoma), HepG2 (hepatocellular carcinoma), and HT1080 (human fibrosarcoma) cell lines were kindly given by Shanghai Cell Institute, China Academy of Sciences. The cells were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere.2.4 Synthesis
Detailed synthesis procedure and analysis data of tumor acidity-responsive PEGylated anionic polymer are shown in ESI.†2.5 DNA retardation assay
To assess the effect of introducing the anionic PPC-DA polymer on the surface of PEI25K/DNA, the PPC-DA/PEI25K/DNA nanoparticles at various mass ratios (0/1/1, 5/1/1, 10/1/1, 15/1/1, and 20/1/1) in a final volume of 10 μL were prepared respectively. Then the above nanoparticles were analyzed on 1% agarose gel containing 0.5 μg mL−1 gold-views. Gel electrophoresis was carried out in TAE running buffer (40 mM Tris–acetate, 1 mM EDTA) at 80 V for 40 min in a Sub-Cell system (Bio-Rad Laboratories, CA). DNA bands were visualized and photographed by a UV transilluminator and BioDoc-It imaging system.2.6 Preparation of the pH-sensitive ternary nanoparticles
According to the literature,32 when the mass ratio of PEI25K/DNA complex is 1/1, DNA can be compacted by PEI25K tightly and protected from DNase degradation. Moreover, the PEI25K/DNA complex is stable in the mass ratio of 1/1, so we choose 1/1 as the mass ratio of PEI25K/DNA. To prepare series of pH-sensitive ternary nanoparticles with various mass ratios (PPC-DA/PEI25K/DNA = X/1/1, X = 0, 5, 10, 15, and 20), the aqueous solutions of plasmid (40 μg mL−1) were mixed with PEI25K (40 μg mL−1) and incubated for 30 min at room temperature, and then different volumes of PPC-DA solution (5 mg mL−1) were mixed with the PEI25K/DNA complexes respectively. These PPC-DA/PEI25K/DNA nanoparticles were further denoted as pH-sensitive ternary nanoparticles. After standing for 15 min at room temperature, the solution was used for further study.2.7 Preparation of the non-pH-sensitive ternary nanoparticles
The non-pH-sensitive ternary nanoparticles (PPC-SA/PEI25K/DNA) were prepared by a similar procedure to the pH-sensitive ternary nanoparticles, by replacing the PPC-DA with PPC-SA.2.8 Characterization of the stability of nanoparticles
The PEI25K/DNA complexes, non-pH-sensitive ternary nanoparticles, or pH-sensitive ternary nanoparticles were gently mixed with BSA (Sigma-Aldrich) solution in phosphate buffered saline (PBS, pH 7.4, 0.01 M). The final concentrations of DNA and BSA were 0.04 and 0.25 mg mL−1, respectively. The mean diameters of nanoparticles after different periods of incubation were measured by laser-light scattering (Zetasizer 3000, Malvern Instruments, UK).2.9 Zeta-potential tests of pH-sensitive and non-pH-sensitive ternary nanoparticles at different pH
The pH-sensitive or non-pH-sensitive ternary nanoparticles were incubated in PBS (0.01 M) at pH 6.8 or 7.4 at 37 °C. The final concentration of DNA was 1.0 μM. At different time intervals, aliquot of the nanoparticle solution was withdrawn and the zeta-potential was measured with a Malvern Zetasizer 3000.2.10 Cellular uptake analyses after treatment with pH-sensitive and non-pH-sensitive ternary nanoparticles at different pH
For flow cytometric analysis, HepG2 cells (1 × 105 cells per well) were seeded on 24 well plate in 0.5 mL complete RPMI 1640 media and cultured at 37 °C in 5% CO2 humidified atmosphere for 24 h. Afterward, the medium was replaced with complete RPMI 1640 media (pH 7.4 or 6.8) containing pH-sensitive or non-pH-sensitive ternary nanoparticles. The final concentration of FAM-DNA in the culture medium was 200 nM in all of the experiments. The cells were incubated at 37 °C for 2 h at either pH 7.4 or 6.8, and then the cells were rinsed twice with cold PBS (pH 7.4). The cells were then trypsinized, washed three times with cold PBS (pH 7.4), and subjected to flow cytometric analysis with FC500 flow cytometer (Beckman Coulter, Brea, CA, USA).For confocal analysis, HepG2 cells (1 × 105 cells per well) were seeded on poly-D-lysine treated glass coverslips in a 24 well plate and incubated for 24 h. The medium was replaced with complete RPMI 1640 media (pH 7.4 or 6.8) containing pH-sensitive or non-pH-sensitive ternary nanoparticles. The final concentration of FAM-DNA in the culture medium was 200 nM in all of the experiments. After incubated for 2 h at 37 °C in complete RPMI 1640 media, the cells were fixed on the coverslips with 4% formaldehyde in PBS for 20 minutes at room temperature. After fixing, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, 100 ng mL−1), rinsed three times with PBS and mounted on the ends of two partial glass slides, and one drop of PBS was placed on top to keep the cells from drying out. Confocal analysis was performed on each sample of cells for no more than 30 minutes.
2.11 Cell viability assay (MTT assay)
MTT assays were performed according to a minor medication on A549, HepG2, and HT1080 cells to evaluate the cytotoxicity of pH-sensitive or non-pH-sensitive ternary nanoparticles.33 The indicated cells (1 × 104 cells per well) were seeded on 96 well plates and incubated for 24 h before the application of serial dilutions of polymer solutions.After 24 h of incubation, filtered MTT reagent (20 μL) at a concentration of 5 mg mL−1 was added to each well and the cells were incubated for 4 h at 37 °C. The unreacted dye was removed by aspiration. The formazan crystals were dissolved in 150 μL of DMSO and measured spectrophotometrically at 570 nm using a microplate reader (Model-550; Biorad Co., USA). Cells treated with ddH2O containing 5% glucose were used as the negative control with 100% cell viability, and PEI25K/DNA was used as the positive control. Experiments were performed in triplicate. The relative cell growth (%) related to control cells cultured in media without polymer was calculated by [A]test/[A]control × 100%.
2.12 In vitro transfection assay
Transfection experiments were performed on A549, HepG2, and HT1080 cells with plasmid pORF-LacZ and pEGFP as the reporter genes. Briefly, cells (1 × 105 cells per well) were seeded on 24 well plates in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. Directly before transfection, the medium was replaced with 0.2 mL of fresh complete RPMI 1640 media, and each formed PEI25K/DNA complexes, non-pH-sensitive ternary nanoparticles, or pH-sensitive ternary nanoparticles containing 2 μg of plasmid was added to the cells. After 4–8 h incubation at 37 °C, medium was again replaced with 1 mL of fresh complete medium, and cells were incubated for another 48 h under the same conditions. For cells transfected with pEGFP, the transfection efficiency was analyzed with the inverted fluorescence microscope by observing the fluorescent density of the cells treated with plasmid DNAs coding EGFP. For cells transfected with pORF-LacZ, the transfection efficiency was assayed by quantifying the LacZ gene expression, in which a β-galactosidase enzyme assay system was used to measure the activity of β-galactosidase and BCA assay was used to measure the total protein content of the lysates. Transfection efficiency was expressed as a ratio of β-galactosidase activity to total cellular protein (mU mg−1 protein). The data represented the mean SD of three wells and was representative of three independent experiments.3. Results and discussion
The design and concept of the pH-sensitive ternary gene delivery system is shown in Fig. 1A. The pH-sensitive ternary nanoparticles for tumor acidity-targeted DNA delivery were prepared by introducing a tumor acidity-responsive PEGylated anionic polymer PPC-DA to the surface of positively charged PEI25K/DNA complexes through electrostatic interaction. The polymeric gene carrier can respond to the tumor extracellular pH gradients through chemically defined mechanisms, which is expected to simultaneously improve targeted accumulation at the tumor site via EPR effect and facilitate the cell internalization, accordingly enhancing the gene delivery efficiency.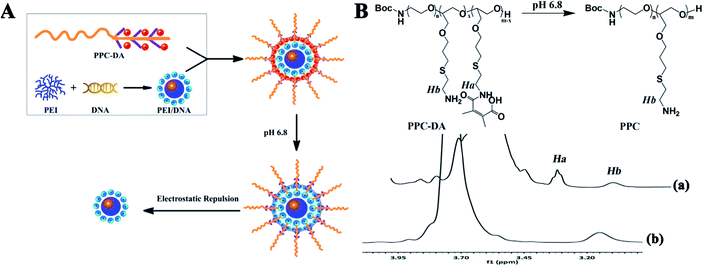 | ||
| Fig. 1 (A) pH-triggered unshielding of PEG ternary nanoparticles. (B) 1H NMR spectra of PPC-DA after incubation at pH 6.8 in D2O/DCl (25 °C) for (a) 0 min and (b) 120 min (ppm). | ||
The PPC-DA was obtained through a multiple synthesis process as shown in Scheme S1, and the details are described in the ESI.† First, the copolymer (PPC) was synthesized by copolymerized the ethylene oxide (EO) and allyl glycidyl ether (AGE) with Boc-aminoethanol as the initiator, then added cysteamine to the double bond side chains of the pendant. The PPC was further reacted with 2,3-dimethylmaleic anhydride (DMMA) to obtained the tumor acidity-responsive PPC-DA. As a control, Boc-PEOn/PAGEm-Cys-SA (PPC-SA), which is stable under the acidity of the tumor, was synthesized by reacting PPC with succinic anhydride (SA). The synthesis of Boc-PEOn/PAGEm, PPC, PPC-DA and PPC-SA were confirmed by 1H NMR spectra, while the results were shown in ESI Fig. S1–S4.† The peaks at 1.250 ppm indicated the Boc group had been conjugated to amine group of β-aminoethanol. The peaks at 3.509–3.991 ppm showed the polymerization of EO, and the polymerization of AGE was confirmed by the peaks at 5.320 and 5.962 ppm belonging to protons of carbon–carbon double bond. The GPC measurement showed that the weight-average molecular weight (Mw) of BOC-PEOn/PAGEm was 4450 Da. With the Mw and 1H NMR spectra of BOC-PEOn/PAGEm, it can be calculated that n was 70 and m was 10, respectively. The complete disappearance of peaks of double bond suggested the addition of cysteamine. Then, the pH-sensitive polymer PPC-DA and non-pH-sensitive polymer PPC-SA were characterized by the typical peak at 3.40 ppm (–SCH2CH2NHC(O)–) (the chemical shift of hydrogen atoms was shown in bold).
According to the literature,30,31 the DMMA-modified amine is extremely acid labile. To verify the acid-responsive cleavage of the amide bond in current study, the 1H NMR spectra of PPC-DA at different time points after incubation at pH 6.8 were recorded (Fig. 1B). Apparently, about 75% of the amino groups were converted to carboxyl groups in PPC-DA according to the integral ratio of Ha to Hb. However, after longer incubation at pH 6.8, the integral ratio of Ha to Hb gradually decreased, finally approaching zero by 2 h, corresponding to complete transformation of the carboxyl groups to amino groups, which proved the rapid acid-responsive cleavage of the amide bonds.
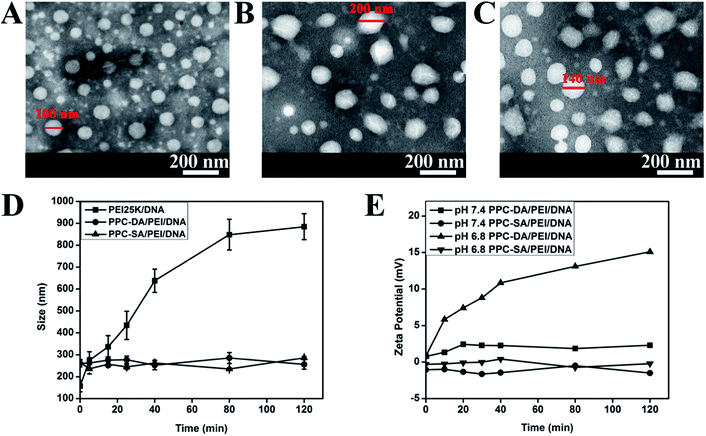
To investigate the effect of PEGylated anionic polymer incorporation on the shielding of PEI25K/DNA complexes, particle size and zeta potential were measured. As shown in Fig. 2, freshly prepared PEI25K/DNA complexes without PPC-DA were positively charged (∼25 mV) and exhibited a small size (∼160 nm), while the PPC-DA/PEI25K/DNA ternary nanoparticles were larger (∼250 nm) and showed neutral surface charge at a mass ratio of 10/1/1. This suggests that the PEGylated anionic polymer PPC-DA in the formulation are suitable for shielding the PEI25K/DNA complex surface at optimal mass ratio (PPC-DA/PEI25K/DNA = 10/1/1).
 | ||
| Fig. 2 The size (A) and zeta potential (B) assay of the pH-sensitive ternary nanoparticles (PPC-DA/PEI25K/DNA). | ||
Since the electrostatic interaction between the negatively charged PEG and PEI might cause the release of DNA, the agarose retardation assay was performed. As shown in Fig. S5,† the introduction of the anionic polymer PPC-DA did not interfere with the formation of pH-sensitive nanoparticle. No significant release of DNA from the resulting ternary nanoparticles was observed with the addition of different amounts of PPC-DA.
The PEI25K/DNA complexes, pH-sensitive and non-pH-sensitive ternary nanoparticles at optimal ratio observed by the transmission electronic microscopic image all showed compact and spherical morphology (Fig. 3A–C). It should be noticed that diameters of the PEI25K/DNA complexes, pH-sensitive and non-pH-sensitive ternary nanoparticles in Fig. 3 were little smaller than from Dh, which may result from the shrinkage of particles during the process of solvent evaporation in the sample preparation. These results further demonstrated that the introduction of anionic polymer PPC-DA or PPC-SA had no effect on the self-assembly of PEI25K and DNA, and PEI25K/DNA complexes could be shielded by the PEGylated anionic polymer.
Since the surface of PEI25K/DNA complex usually has cationic charge, it can strongly interact with blood cells and negatively charged components such as albumin. Introduction of hydrophilic polymers like PEG is suitable to reduce positive surface charge of PEI25K/DNA and diminish those undesirable interactions. In order to demonstrate this, the PEI25K/DNA complexes, pH-sensitive or non-pH-sensitive ternary nanoparticles were incubated in phosphate buffered saline (PBS, pH 7.4, 0.01 M) with bovine serum albumin (BSA, 0.25 mg mL−1), and the particle sizes were recorded at different time intervals. As shown in Fig. 3D, the particle sizes of pH-sensitive or non-pH-sensitive ternary nanoparticles remained constant with increasing incubation time, whereas the size of the PEI25K/DNA complexes exhibited significantly large particles and rapid aggregation, demonstrated improved serum stability of ternary nanoparticles by introducing PEG layer onto PEI25K/DNA complexes.
It has been demonstrated that the resultant amide bond formed between an amino and DMMA is relatively stable at neutral and alkali pH values, but degrades promptly under slightly acidic conditions to expose positively charged amino groups again.31,34 To further demonstrate the fact that pH-sensitive ternary nanoparticles can deshield PEG segments under slightly acidic conditions of tumors, the charge-conversional behaviour of the nanoparticles was monitored on the basis of the change in zeta potential after incubation at pH 7.4 and 6.8. As shown in Fig. 3E, the zeta potential of the pH-sensitive ternary nanoparticles gradually became positive with a prolonged incubation time. In contrast, the zeta potential also increased at pH 7.4, but at a relatively slow rate. Nevertheless, the non-pH-sensitive ternary nanoparticles were not affected by the pH of PBS buffer, exhibiting no visible sign of charge-conversional behaviour. This significant difference confirmed the pH sensitivity of amide bonds of PPC-DA and the formation of pH-sensitive ternary nanoparticles with the charge conversional ability by deshielding the PEG layer at tumor acidity.
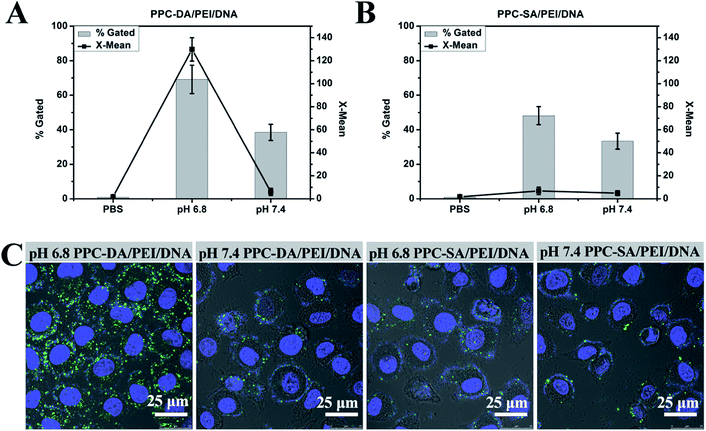
Since PEGylation may lower gene transfer efficiency due to reduced cell surface and/or endosomal lipid membrane interactions.22 The ability of the nanoparticle system to reverse its surface charge from neutral or negative to positive at tumor extracellular pH could thus facilitate cell internalization. To verify this hypothesis, the HepG2 cells were cultured with pH-sensitive or non-pH-sensitive ternary nanoparticles containing FAM-labelled DNA at pH 7.4 or 6.8. As shown in Fig. 4A, there was a much stronger cellular fluorescence when the cells were cultured with pH-sensitive ternary nanoparticles for 2 h at pH 6.8 compared to pH 7.4. In contrast, there was no significant difference in terms of mean fluorescence intensities when the cells were cultured with non-pH-sensitive ternary nanoparticles for 2 h at pH 6.8 and 7.4 (Fig. 4B). For further characterization of pH-sensitive or non-pH-sensitive ternary nanoparticles in their interaction with cells, the cellular uptake was observed by the confocal microscopy. As shown in Fig. 4C, more fluorescent dots appeared inside the cells after incubation of cells with the pH-sensitive ternary nanoparticles for 2 h at pH 6.8 when compared with cells treated at other conditions. These results, together with the zeta potential data, clearly establish that the pH-sensitive ternary nanoparticles becoming positively charged upon hydrolysis at pH 6.8, which strengthens the interaction of the nanoparticles with cells and enhances cellular internalization. In vivo, the PPC-DA is thus expected to shield the PEI/DNA complexes in the systemic circulation and expose the complex only at the tumor sites where the pH is slightly acidic and can facilitate the delivery of DNA to the tumor cells after accumulation at the tumor site.
Cytotoxicity of polymeric gene vector is an important factor that affects the transfection efficiency. Therefore, the cell toxicity of the pH-sensitive or non-pH-sensitive ternary nanoparticles at optimal ratio was measured by MTT assay on A549, HepG2, and HT1080 cells. The relative viabilities of cells treated with PEI25K/DNA, PPC-DA/PEI25K/DNA and PPC-SA/PEI25K/DNA were measured after 24 h by the MTT assay. As shown in Fig. 5, PPC-DA/PEI25K/DNA or PPC-SA/PEI25K/DNA nanoparticles showed no appreciable toxicity at any of the concentrations compared to PEI25K/DNA complexes. Thus the pH-sensitive or non-pH-sensitive ternary nanoparticles were almost nontoxic, which is highly suitable for further transfection experiments.
The influence of PEGylation on transfection efficiency was examined by delivering plasmid pORF-LacZ into A549, HepG2, and HT1080 cells. As shown in Fig. 6A, in case of non-pH-sensitive ternary nanoparticles, transfection efficiency was reduced at a 10/1/1 ratio of PPC-SA/PEI25K/DNA as compared with the pH-sensitive ternary nanoparticles. Such a PEG-triggered reduction in gene transfer has been previously shown for similar stable PEG shielded formulations.14,23,35,36 In contrast, enhanced transfection efficiency was achieved at the optimal PPC-DA/PEI25K/DNA ratio of 10/1/1, demonstrating the advantage of acid-triggered deshielding. Moreover, prolongation of incubation time didn't result in improved transfection efficiency. Therefore following experiments were done according to these optimal transfection conditions unless otherwise specified. It is noteworthy that, since highly positively charged PEI25K/DNA complexes interact with negatively charged serum proteins to trigger the dissociation of the complexes and/or diminished endocytosis resulting in the reduction in transfection efficacy. Thus, the encapsulation of PEI/DNA complexes into pH-sensitive PPC-DA coat increased the stability of DNA in complete medium.
To further investigate the serum compatibility of the pH-sensitive ternary nanoparticles, subsequent experiments were done in the medium with serum concentrations from 10% to 40% (Fig. 6B and C). For unshielded PEI25K/DNA complexes, the transfection efficiency decreased drastically, while in the case of pH-sensitive ternary nanoparticles, reduction was much lower, demonstrated improved transfection efficiency over the unshielded PEI standard. In summary, such delivery system with the ability to deshield the PEG layer at the slightly acidic tumor extracellular microenvironment presents an encouraging step towards improved gene transfer efficiency, at least as compared to the non pH-sensitive analogs.
4. Conclusions
In conclusion, we reported here the synthesis and characterization of an anionic pH-sensitive polymer PPC-DA, which was explored for gene delivery in vitro. Mixing of the PEI/DNA complexes with PPC-DA produced the pH-sensitive ternary nanoparticles with near-neutral surface charge, resistance to nonspecific interactions with protein, and transfection activity unaffected by the presence of serum. In vitro results showed a significant increase in transfection with pH-sensitive ternary nanoparticles versus non-pH-sensitive analogs. Moreover, the pH-sensitive ternary nanoparticles can respond to the extracellular pH of tumor tissue to enhance cellular uptake but not to the pH of normal blood, which has a promise for site-specific transfection of tumor cells in vivo. In addition, work is in progress to validate the use of the pH-sensitive ternary nanoparticles for gene delivery to tumors in vivo.Acknowledgements
This Project was supported by China Postdoctoral Science Foundation (Grant no. 2013M531974).Notes and references
- A. Akinc, M. Thomas, A. M. Klibanov and R. Langer, J. Gene Med., 2005, 7, 657 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J.-P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297 CrossRef CAS.
- N. D. Sonawane, F. C. Szoka and A. Verkman, J. Biol. Chem., 2003, 278, 44826 CrossRef CAS PubMed.
- S. Brunner, E. Fürtbauer, T. Sauer, M. Kursa and E. Wagner, Mol. Ther., 2002, 5, 80 CrossRef CAS PubMed.
- W.-C. Tseng and C.-M. Jong, Biomacromolecules, 2003, 4, 1277 CrossRef CAS PubMed.
- R. Kircheis, L. Wightman and E. Wagner, Adv. Drug Delivery Rev., 2001, 53, 341 CrossRef CAS.
- C. L. Gebhart and A. V. Kabanov, J. Controlled Release, 2001, 73, 401 CrossRef CAS.
- U. Lungwitz, M. Breunig, T. Blunk and A. Göpferich, Eur. J. Pharm. Biopharm., 2005, 60, 247 CrossRef CAS PubMed.
- M. Neu, D. Fischer and T. Kissel, J. Gene Med., 2005, 7, 992 CrossRef CAS PubMed.
- X.-Q. Zhang, J. Intra and A. K. Salem, J. Microencapsulation, 2008, 25, 1 CrossRef CAS PubMed.
- M. Ogris and E. Wagner, Somatic Cell Mol. Genet., 2002, 27, 85 CrossRef CAS.
- G. Lemkine and B. Demeneix, Curr. Opin. Mol. Ther., 2001, 3, 178 CAS.
- R. R. Sawant, S. K. Sriraman, G. Navarro, S. Biswas, R. A. Dalvi and V. P. Torchilin, Biomaterials, 2012, 33, 3942 CrossRef CAS PubMed.
- P. Erbacher, T. Bettinger, P. Belguise-Valladier, S. Zou, J. L. Coll, J. P. Behr and J. S. Remy, J. Gene Med., 1999, 1, 210 CrossRef CAS.
- R. Qi, S. Liu, J. Chen, H. Xiao, L. Yan, Y. Huang and X. Jing, J. Controlled Release, 2012, 159, 251 CrossRef CAS PubMed.
- K. Itaka, K. Yamauchi, A. Harada, K. Nakamura, H. Kawaguchi and K. Kataoka, Biomaterials, 2003, 24, 4495 CrossRef CAS.
- H. Xiao, R. Qi, S. Liu, X. Hu, T. Duan, Y. Zheng, Y. Huang and X. Jing, Biomaterials, 2011, 32, 7732 CrossRef CAS PubMed.
- A. Malek, F. Czubayko and A. Aigner, J. Drug Targeting, 2008, 16, 124 CrossRef CAS PubMed.
- V. Trubetskoy, S. Wong, V. Subbotin, V. Budker, A. Loomis, J. Hagstrom and J. Wolff, Gene Ther., 2003, 10, 261 CrossRef CAS PubMed.
- C.-H. Ahn, S. Y. Chae, Y. H. Bae and S. W. Kim, J. Controlled Release, 2002, 80, 273 CrossRef CAS.
- M. Sakae, T. Ito, C. Yoshihara, N. Iida-Tanaka, H. Yanagie, M. Eriguchi and Y. Koyama, Biomed. Pharmacother., 2008, 62, 448 CrossRef CAS PubMed.
- Y. Nie, M. Günther, Z. Gu and E. Wagner, Biomaterials, 2011, 32, 858 CrossRef CAS PubMed.
- M. Ogris, S. Brunner, S. Schüller, R. Kircheis and E. Wagner, Gene Ther., 1999, 6, 595 CrossRef CAS PubMed.
- G. Helmlinger, A. Sckell, M. Dellian, N. S. Forbes and R. K. Jain, Clin. Cancer Res., 2002, 8, 1284 CAS.
- M. G. Vander Heiden, L. C. Cantley and C. B. Thompson, science, 2009, 324, 1029 CrossRef CAS PubMed.
- M. Oishi, Y. Nagasaki, K. Itaka, N. Nishiyama and K. Kataoka, J. Am. Chem. Soc., 2005, 127, 1624 CrossRef CAS PubMed.
- G. F. Walker, C. Fella, J. Pelisek, J. Fahrmeir, S. Boeckle, M. Ogris and E. Wagner, Mol. Ther., 2005, 11, 418 CrossRef CAS PubMed.
- A. A. Kale and V. P. Torchilin, J. Liposome Res., 2007, 17, 197 CrossRef CAS PubMed.
- V. A. Sethuraman, K. Na and Y. H. Bae, Biomacromolecules, 2006, 7, 64 CrossRef CAS PubMed.
- Z. Zhou, Y. Shen, J. Tang, M. Fan, E. A. Van Kirk, W. J. Murdoch and M. Radosz, Adv. Funct. Mater., 2009, 19, 3580 CrossRef CAS PubMed.
- J. Z. Du, T. M. Sun, W. J. Song, J. Wu and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 3621 CrossRef CAS PubMed.
- D. Zhao, T. Gong, D. Zhu, Z. Zhang and X. Sun, Int. J. Pharm., 2011, 413, 260 CrossRef CAS PubMed.
- B. R. Schroeder, M. I. Ghare, C. Bhattacharya, R. Paul, Z. Yu, P. A. Zaleski, T. C. Bozeman, M. J. Rishel and S. M. Hecht, J. Am. Chem. Soc., 2014, 136, 13641 CrossRef CAS PubMed.
- M. Meyer, A. Philipp, R. Oskuee, C. Schmidt and E. Wagner, J. Am. Chem. Soc., 2008, 130, 3272 CrossRef CAS PubMed.
- D. Oupicky, M. Ogris, K. A. Howard, P. R. Dash, K. Ulbrich and L. W. Seymour, Mol. Ther., 2002, 5, 463 CrossRef CAS PubMed.
- J.-Y. Legendre and F. C. Szoka Jr, Pharmaceut. Res., 1992, 9, 1235 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04745e |
| This journal is © The Royal Society of Chemistry 2015 |