Unprecedented catalyst-free lignin dearomatization with hydrogen peroxide and dimethyl carbonate
Lotte Wiermans,
Hannah Schumacher,
Christian-Marvin Klaaßen and
Pablo Domínguez de María†
*
Institut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany. E-mail: dominguez@itmc.rwth-aachen.de; dominguez@sustainable-momentum.net
First published on 4th December 2014
Abstract
Lignin can be efficiently dearomatized and oxidized under catalyst-free conditions in dimethyl carbonate as solvent and hydrogen peroxide as oxidant at 20–80 °C. Based on H2O2 loadings, modified fibres with different aromatic content and sizes can be achieved (from 100% to 0% aromatics). At loadings > 0.2 mol H2O2 g lignin−1 almost complete dearomatized lignin can be achieved, resulting in a pale oily gel. Analyses of the derivatives show increase in carbonylic bonds (FTIR) and oxygen (elemental analysis) and smaller size fibres (ESI-MS). Changes were also in-depth monitored by 2D 1H-13C HSQC NMR. Depending on a desired application (e.g. resins, additives, coatings, etc.), tailored dearomatized lignin fibres can be straightforwardly produced. Being a novel reaction with relatively high peroxide concentrations, experiments must be conducted under strong safety conditions to avoid possible explosions and other risks associated to peroxides and/or in situ formed derivatives.
Introduction
Upon selective fractionation and separation in its main components (e.g. xylose, cellulosic pulp and lignin), lignocellulose may deliver a broad array of fuels and platform chemicals.1 Lignin valorization is an increasingly important area, as lignin may account for up to 40 wt% of lignocellulose, and is typically discarded or merely incinerated as a source of energy.1 Thus, the development of (bio)catalytic approaches for lignin cracking and valorization is gaining momentum.2One option for lignin processing is its oxidation to afford a number of different platform chemicals.2,3 Herein, the use of peracids for the oxidative delignification of wood has been studied, especially for pulp and paper industries.4 Peracids attack lignin via oxidation of hydroxyl groups in side chains – to form carbonyl groups – cleavage of β-aryl bonds and reducing the size of lignin fibers, as well as by modifications in the aromatic rings.5 Overall, a selective delignification of lignocelluloses is achieved, remaining intact the polysaccharide fractions (hemicellulose and cellulose). However, the storage, transport and handling of peracids may be hazardous, and thus peracids must be in situ formed (e.g. MILOX process, using aqueous formic acid and hydrogen peroxide), typically using mineral acids (e.g. sulphuric acid) as catalysts.4,5 Also hydrolases have been assessed as biocatalysts to in situ produce peroxycarboxylic acids using diluted hydrogen peroxide and carboxylic acids, what subsequently leads to the oxidative delignification of different biomasses such as aspen or beech wood.5,6
Recently6a we reported on the peracid-assisted formation of a fully dearomatized oxidized lignin oil by using beech wood as raw material and lipases suspended in dimethyl carbonate (DMC) as non-aqueous solvent. Lipases catalyzed the peracid formation under mild conditions, by adding diluted hydrogen peroxide, and lignin was depolymerized and dearomatized through oxidation. Performing the reaction in non-aqueous conditions led to the isolation of an unexpected yellowish lignin–oil with significantly low molecular weight and complete absence of aromatics.6a The dearomatization of lignin may be produced by peracids through different oxidative pathways, leading to more flexible fibers by opening the aromatic rings (Scheme 1).5
 | ||
| Scheme 1 Postulated oxidative and dearomatization pathways in lignin mediated by peracids.5 | ||
The development of processes that may deliver more or less dearomatized lignins might open new bulk applications, since fibers could become more or less flexible and tailored depending on dearomatization level (Scheme 1). Thus, that fibers might in principle be envisaged for different composites, paintings, coatings, resins, to cite some potential uses. However, to our knowledge the application of peracids to add value to lignin has not been fully explored yet, presumably because in this area peracids have been typically used in aqueous solutions for pulp and paper processing. Herein we report on the unexpected catalyst-free lignin dearomatization in DMC and H2O2 under mild conditions (80 °C, ambient pressure), leading to the “on-demand” production of lignin fibers with different dearomatization degrees.
Results & discussion
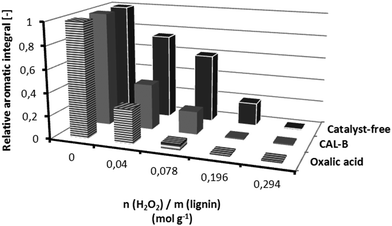
Lignin was obtained from beech wood through the OrganoCat pretreatment recently developed by us.7 Having observed that the OrganoCat lignin could be dissolved in DMC (up to 10 g L−1), its dearomatization by addition of diluted H2O2 (35% v/v) was assessed using lipase B from Candida antarctica (CAL-B)6a or oxalic acid as catalysts, as well as under catalyst-free conditions (Fig. 1).In agreement with literature for beech wood,5,6a both oxalic acid and CAL-B led to analogous results for the lignin dearomatization, and fully dearomatized fibers were obtained after several additions of H2O2. Surprisingly, and contrary to observations with suspended beech wood,8 under catalyst-free conditions a significant lignin dearomatization also occurred, leading to full dearomatization at higher amounts of H2O2. The yields in lignin fibers, together with the dearomatization degree were assessed as a function of the actual H2O2 concentration in the reactive mixture, working under catalyst-free conditions (Fig. 2).
Consistent with literature,5 peracids produce some volatile by-products from lignin (e.g. methanol and CO2) – thus leading to less yields – while dearomatization is proceeding simultaneously. Under catalyst-free conditions (adding 1.5 M H2O2) a solid pale-orange fully dearomatized lignin fibre could be achieved with yields around 40–50 wt% at 80 °C. After storage at room temperature for some hours, the solid turned into more gelatinous oil. At higher H2O2 proportions (>1.5 M) fully dearomatized lignin oils were directly achieved (Fig. 2). Conversely, with less addition of oxidant, solid lignin fibers keeping 40–70% of the initial aromatics could be afforded in higher yields (Fig. 2). With regard to the reaction temperature, the catalyst-free dearomatization proceeded at room temperature as well, albeit at lower rates than at 80 °C (data not shown). It must be noted that working with peroxides and peracids must be always be handled with extreme caution, especially under novel conditions whereby the actual reactive specie(s) must be still characterized.
Moreover, different solvents were assessed (ethanol, n-propanol, ethyl acetate, dioxane and DMC). The presence of DMC resulted crucial for the catalyst-free dearomatization, and only by using other carbonyl-containing solvents like ethyl acetate some slightly dearomatized samples were observed. Given that in DMC a dissolution limit of OrganoCat lignin of 10 g lignin L−1 was observed, mixtures of ethanol and DMC were applied, as beech wood OrganoCat lignin resulted largely soluble in ethanol. Under these ethanol–DMC mixtures (e.g. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), up to 40 g L−1 of lignin were fully dearomatized in 1 h upon addition of 0.2 mol H2O2 g lignin−1. To our knowledge, these results represent the first catalyst-free oxidation of lignin under such mild reaction conditions.
1 v/v), up to 40 g L−1 of lignin were fully dearomatized in 1 h upon addition of 0.2 mol H2O2 g lignin−1. To our knowledge, these results represent the first catalyst-free oxidation of lignin under such mild reaction conditions.
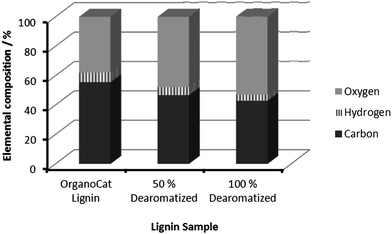
To further assess the changes in the obtained derivatives, analytic studies were subsequently conducted. In Fig. 3 FTIR analysis of OrganoCat beech wood lignin and samples with 50% and 100% dearomatization degrees are shown.
Consistent with the literature (Scheme 1),5 the treatment of lignin with peracids led to a significant increase in the proportion of carbonylic bonds (esters, acids, aldehydes, etc.), as well as the decrease of the aromatic stretching bands in the region of 1400–1600 cm−1. The oxidation profile of the peracid-based treatment became further evident when the elementary analysis of the lignin derivatives was conducted, observing a decrease of the C and H content in the fibres along the oxidation pattern (Fig. 4).
To study the actual fractionation pattern that peracids could be creating along the lignin fibres, ESI-MS studies of different samples (pure lignin, 50% and 0% aromatics) were performed (Fig. 5). OrganoCat lignin showed a rather narrow distribution, with two main sizes of fibres of 1017 and 1142 g mol−1. As expected, the peracid treatment led to a decrease in the molecular weight size of the fibres, in agreement with previous literature as well.6a
 | ||
| Fig. 5 ESI-MS studies of OrganoCat lignin from beech wood, and samples of 50 and 100% dearomatized fibres. | ||
Moreover 2D 1H-13C HSQC (Heteronuclear Single Quantum Coherence) NMRs were recorded on all three lignins. To assign (part of) the cross-peaks, the aromatic (6–8 1H-NMR) and oxygenated-aliphatic (3–6 1H-NMR) regions were analyzed in detail. The main linkages that could be assigned from the cross-peaks in the aromatic and in the oxygenated-aliphatic regions are depicted in Fig. 6.
To perform the assessment, three samples, the pure OrganoCat lignin (100% aromatics), a sample with 50% dearomatization and another one with 0% dearomatization were used. The 2D 1H-13C HSQC NMRs of them were recorded and divided into three main sections, namely the aliphatic, oxygenated-aliphatic and aromatic regions (Fig. 7).
As it can be observed, the cross-peaks in the aromatic section were disappearing along the oxidative processing, fully confirming the previous analytic data of the dearomatization. Taking into account the previous cross-peaks assignation (Fig. 6), further characterizations of the detailed sections were conducted with pure OrganoCat lignin (100% aromatics), and thus compared with the other two samples.
Starting with the oxygenated-aliphatic region, the assessment of that part for the pure OrganoCat lignin is depicted in Fig. 8.
 | ||
| Fig. 8 2D 1H-13C HSQC NMR spectrum from OrganoCat lignin, focused on the aliphatic oxygenated region (3–6 ppm in 1H-NMR). | ||
The β-O-4′ linkage (A) can be identified with its Cα–Hα correlation at δC/δH 72.1/5.02 ppm. The Cβ–Hβ correlation for the syringyl unit (S) at δC/δH 86.1/4.26 ppm and the Cγ–Hγ correlation at δC/δH 60.1/3.76. Also clearly assignable can be the phenyl-coumaran (β-5′) unit (B) with its Cα–Hα, Cβ–Hβ and Cγ–Hγ correlations at δC/δH 87.4/5.55 ppm, 54.5/3.54 ppm and 63.3/3.87 ppm respectively. Furthermore, the resinol (β–β′) substructure (C) may be visible with its Cα–Hα, Cβ–Hβ and the double Cγ–Hγ correlations at δC/δH 85.4/4.75 ppm, 54.1/3.11 ppm; and 71.4/3.87 & 4.22. Moreover, a very broad signal for –OMe groups can be expected at δC/δH 56.1/3.76 (Fig. 8).
A subsequent comparison with the oxygenated-aliphatic regions of fibers with 50% and 0% aromatics followed (Fig. 9). Surprisingly, even in the samples where 50% of aromatics are left, none of the cross-peaks could be assigned to the ones found in the OrganoCat lignin, except the broad signal for –OMe groups (yet narrower than in the spectrum for OrganoCat lignin). The broad cross-peak at δC/δH 51.6/3.70 ppm and the region between δC/δH 64.3/4.13 to δC/δH 66.1/4.21 (shown in circles in the figure), most probably arise from aliphatic acids, alcohols or esters. In these samples also in the aliphatic region, more peaks occur. Interestingly, two peaks at δC/δH 41.6/3.43 ppm, δC/δH 60.5/3.43 ppm were found, typically assigned to the C–H correlation between the two carbons before an alcohol group or the two carbons on both sides of an ester group.
 | ||
| Fig. 9 2D 1H-13C HSQC NMR spectrum from lignins with 50% and 0% aromatics, focused on the aliphatic oxygenated region (3–6 ppm in 1H-NMR). | ||
Likewise, the aromatic region of the pure OrganoCat lignin was studied, and the guaiacyl unit (G) was identified with its C2–H2 linkage at δC/δH 111.1/6.96, its C5–H5 linkage at δC/δH 115.2/6.73 and its C6–H6 linkage at δC/δH 119.1/6.79. Besides, also the C2,6–H2,6 linkages in both the etherified syringyl units (S) and the double C2,6–H2,6 correlations of oxidized syringyl units (S′) can be found, at δC/δH 103.8/6.69 (S) and δC/δH 106.2/7.08 & 7.25 (Fig. 10).
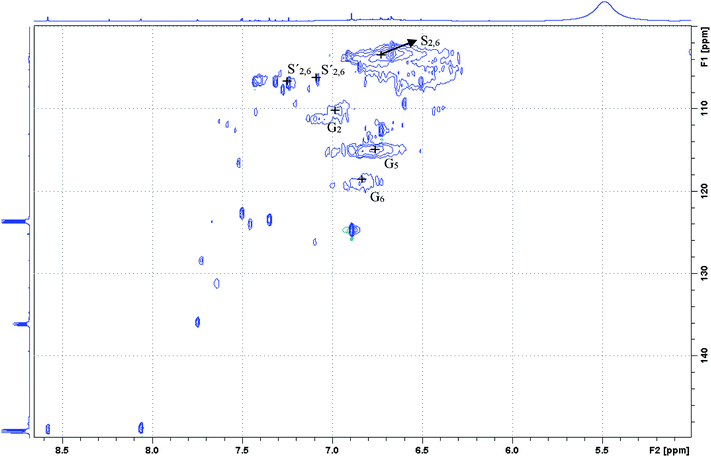 | ||
| Fig. 10 2D 1H-13C HSQC NMR spectrum from pure OrganoCat lignin, focused on the aromatic region (6–8 ppm in 1H-NMR). | ||
When compared with the region of samples with 50% and 0% aromatics, it can be observed (Fig. 11) that many cross-peaks disappeared and none of the cross-peaks could be assigned as in the OrganoCat lignin NMR. For the 50% aromatics sample, the largest cross-peaks – like the ones at δC/δH 106.9/7.30 ppm, δC/δH 115.1/6.90 ppm, δC/δH 124.8/6.90 ppm, δC/δH 126.8/6.70 ppm – can all come from oxygenated aromatic compounds. For the 0% aromatics sample, almost no peaks occur. The largest peaks that are left can be seen at δC/δH 123.7/7.37 ppm, δC/δH 133.9/6.73 ppm, δC/δH 134.4/6.21 ppm and δC/δH 136.1/7.77 and could come from oxygenated aromatic compounds as well. No couplings between the peaks occur, which probably means that they originate from individual (fragmented) compounds.
 | ||
| Fig. 11 2D 1H-13C HSQC NMR spectrum from OrganoCat lignin with 50% and 0% aromatics, focused on the aromatic region (6–8 ppm in 1H-NMR). | ||
Conclusions
An unprecedented catalyst-free efficient dearomatization of lignin in mixtures of dimethyl carbonate and hydrogen peroxide under rather mild conditions has been reported. Depending on the amount of oxidant added, lignin fibres with controlled aromatic content may be produced – in decreasing yields – what may open novel bulk uses for lignin. If the oxidant amount was higher, fully dearomatized fibres of lignin could be delivered, which turned into oil if even stronger conditions were set. Being a novel process dealing with relatively high peroxide concentrations in which the actual reactive species are not known yet, safety precautions must be always kept.Experimental section
Chemicals
DMC, H2O2 (35% v/v), oxalic acid, styrene, cyclohexene and NaN3 were purchased from Sigma-Aldrich and used without further modification. All solvents were purchased from commercial suppliers and used without further purification. CAL-B was kindly donated by c-LEcta GmbH.Standard catalyst-free dearomatization of lignin
Lignin obtained from the OrganoCat process7 was dissolved in DMC (10 g lignin L−1) and the reaction was heated up to 80 °C and stirred. Diluted hydrogen peroxide was added in variable amounts to start the reaction. Samples (typically 100 μL) were taken and dried at 100–110 °C before 1H-NMR analysis on a Bruker DPX -300, using DMSO as a solvent.Acknowledgements
This work was performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded by the Excellence Initiative of the German Research Foundation to promote science and research at German universities. Furthermore, financial support from DFG training group 1166 “BioNoCo” (“Biocatalysis in Non-conventional Media”) is gratefully acknowledged as well. We thank Dr Philipp Grande for his valuable assistance in lignin analysis.References
- Biorefineries – Industrial processes and products. Status quo and future directions, ed. B. Kamm, P. R. Gruber, and M. Kamm, Wiley-VCH, Weinheim, 2010 Search PubMed.
- (a) P. Picart, C. R. Müller, J. Mottweiler, L. Wiermans, C. Bolm, P. Domínguez de María and A. Schallmey, ChemSusChem, 2014, 11, 3164–3171 CrossRef PubMed; (b) S. Sun, R. Bai and Y. Gu, Chem.–Eur. J., 2014, 20, 549–558 CrossRef CAS PubMed; (c) C. Xu, R. A. Arancon, J. Labidi and R. Luque, Chem. Soc. Rev., 2014, 43, 7485–7500 RSC; (d) V. Martínez, M. Mitjans and M. P. Vinardel, Curr. Org. Chem., 2012, 16, 1863–1870 CrossRef; (e) G. Yang, M. Sarwar and Y. Ni, Curr. Org. Chem., 2013, 17, 1589–1595 CrossRef CAS; (f) J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed; (g) H. Lange, S. Decina and C. Crestini, Eur. Polym. J., 2013, 49, 1151–1173 CrossRef CAS.
- A. Rahimi, A. Azapira, H. Kim, J. Ralph and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 6415–6418 CrossRef CAS PubMed.
- See, for instance: (a) P. Ligero, A. Vega and J. J. Villaverde, Bioresour. Technol., 2010, 101, 3188–3193 CrossRef CAS PubMed; (b) D. da Silva Perez, M. G. H. Terrones, S. Grelier, A. Nourmamode, A. Castellan, R. Ruggiero and A. E. H. Machado, J. Wood Chem. Technol., 1998, 18, 333–365 CrossRef CAS; (c) A. Seisto and K. Poppius-Levlin, Tappi J., 1997, 80, 215–221 CAS; (d) B. Hortling, K. Poppius and J. Sundquist, Holzforschung, 1991, 45, 109–120 CrossRef CAS.
- Pathways for peracid-assisted lignin dearomatization: (a) L. B. Brasileiro, J. L. Colodette and D. Pilo-Veloso, Quim. Nova, 2001, 24, 819–829 CrossRef; (b) J. Gierer, Holzforschung, 1982, 36, 55–64 CrossRef CAS.
- (a) L. Wiermans, M. Pérez-Sánchez and P. Domínguez de María, ChemSusChem, 2013, 6, 251–255 CrossRef CAS PubMed; (b) D. T. Yin, Q. Jing, W. W. AlDajani, S. Duncan, U. Tschirner, J. Schilling and R. J. Kazlauskas, Bioresour. Technol., 2011, 102, 5183–5192 CrossRef CAS PubMed; (c) S. Duncan, Q. Jing, A. Katona, R. J. Kazlauskas, J. Schilling, U. Tschirner and W. W. AlDajani, Appl. Biochem. Biotechnol., 2010, 160, 1637–1652 CrossRef CAS PubMed.
- T. vom Stein, P. M. Grande, H. Kayser, F. Sibilla, W. Leitner and P. Domínguez de María, Green Chem., 2011, 13, 1772–1777 RSC.
- No significant catalyst-free dearomatization was observed when suspended beech wood was treated, instead of fully dissolved lignin.
Footnote |
| † Present address: Sustainable Momentum, SL Ap. Correos 3517. 35004, Las Palmas de Gran Canaria, Canary Islands, Spain, Tel: +34 609565237. |
| This journal is © The Royal Society of Chemistry 2015 |