Synthesis, characterization and photocatalytic activity of Ag–TiO2 nanoparticulate film
P. V. R. K. Ramacharyulu*,
J. Praveen kumar,
G. K. Prasad* and
A. R. Srivastava
Defence R & D Establishment, Jhansi Road, Gwalior, India. E-mail: gkprasad2001@gmail.com; ramamsc2007@gmail.com; Tel: +07512390169
First published on 26th November 2014
Abstract
Ag–TiO2 nanoparticulate film was synthesized by dip coating followed by adsorption and photoreduction in UVA light, characterized by transmission electron microscopy, scanning electron microscopy, energy dispersive analysis of X-rays, glancing angle X-ray diffractometry and UV-Vis absorption spectrophotometry techniques. The data indicated the presence of TiO2 particles of anatase phase of size varying from 5–15 nm, Ag nanoparticles of size varying from 10–20 nm, and also indicated the added visible light activity in Ag–TiO2 nanoparticle films. Photocatalytic degradation of methyl parathion (O,O-dimethyl O-(4-nitrophenyl) phosphorothioate), a well known pesticide in aqueous solution was studied using Ag–TiO2 nanoparticulate film and the data was compared with TiO2 nanoparticulate film. Photocatalytic degradation reactions demonstrated pseudo first order behaviour. Methyl parathion was found to be degraded initially to paraoxon which further was degraded to p-nitrophenol, trimethyl ester of phosphoric acid, trimethyl ester of phosphothioic acid, and finally to phosphate ion. Minute amounts of carbon dioxide and acetaldehyde were also detected.
Introduction
Organophosphorous compounds (OPC) have been extensively used as insecticides, pesticides, and acaricides to protect the crops. They inhibit acetylcholinesterase enzyme in mammalians and were found to be highly toxic to human beings.1–3 Methyl parathion (MP) is one such OPC which is being used in many countries worldwide as a fumigant and acaricide for protective treatment of a wide variety of crops. It is classified as an acutely toxic pesticide by the US EPA (2003). It is slowly hydrolyzed to other toxic chemical species in aqueous solution and is quite persistent in environment posing threat to people who get exposed. Therefore, it is important to develop methods to eliminate the OPC like MP from contaminated water streams.4–6Photocatalysis is one of the advanced oxidation processes that have been used to degrade toxic OPCs in contaminated water streams. When a photocatalyst is irradiated with light whose energy is greater than band gap, charge carriers are generated and react with water and surrounding oxygen species to form highly reactive hydroxyl (˙OH) and superoxide anion (O2−˙) radicals.7–9 These species in turn react with pollutant molecules like OPCs and degrade them to innocuous products. Recently, titania materials were used to photocatalytically degrade organophosphorous pesticides such as malathion, diazon, fenitrothion, and parathion.10 Doong and Chang have studied photocatalytic degradation of organophosphorous pesticides and attributed the decomposition of P–S and C–S bonds as a cause for their degradation.11 Zhang has reported the photocatalytic degradation of methamidophos by UV radiation in the presence of nano TiO2.12 More recently, acephate, a well known insecticide was decomposed by photocatalysis treatment assisted by nano TiO2 and UV light.13 Vorontsov has studied the photocatalytic oxidation of dimethyl methyl phosphonate, trimethyl phosphate and diethyl phosphoramidate using suspension of TiO2 particles in aqueous media.14 Subsequently, Moctezuma et al. have studied photocatalytic degradation of MP using aqueous suspension of titania particles. They also have investigated pathways of MP degradation along with its reaction products.15 Evgenidou et al. have studied photocatalytic oxidation of MP over TiO2 and ZnO suspensions.16 Titanium dioxide was found to be more promising photocatalyst since the degradation of the insecticide was observed to proceed at relatively higher reaction rate. Kim et al. have explored degradation mechanism of MP using TiO2 photocatalysis and photolysis.17
Although TiO2 photocatalyst exhibited promising activity towards degradation of above OPCs, removal of the catalyst from solutions after use is expensive and technologically difficult. TiO2 thin films have been used for the purpose of solving these technical problems. Compared to powder TiO2 particles, immobilized TiO2 films are more promising in practical applications, especially in water treatment.18–20 In this connection, Prasad et al. have studied photocatalytic degradation of paraoxon-ethyl using titania nanoparticulate film.21 On the other hand, modifications of TiO2 photocatalyst with noble metal such Au, Ag, and Pt was found to enhance its photocatalytic efficiency towards environmental pollutants. These small metal particles were found to accelerate the scavenging of photoelectrons and assist the separation of charge carrier species, and in turn lead to generation of superoxide anion and hydroxyl radical species.22–26
Although, many researchers have reported the degradation of MP using titania suspensions, there were scanty of literature on the photocatalytic degradation of MP using TiO2 and Ag–TiO2 nanoparticulate films which is technologically important. Hence, we have prepared the TiO2 and Ag–TiO2 films and characterized them by transmission electron microscopy (TEM), glancing angle X-ray diffractometry (GAXRD), scanning electron microscopy coupled with energy dispersive analysis of X-rays (SEM-EDAX), UV-visible spectrophotometry and studied the degradation of MP under photocatalytic conditions.
Experimental
Materials
Silver nitrate, titanium tetraisopropoxide (TTIP), and MP of more than 90% purity, 1.27 g mL−1 density of Aldrich chemicals, USA make were used for photocatalytic degradation experiments. Isopropanol, dichloromethane, acetonitrile, bistrimethyl silyl trifluoroacetamide (BSTFA) were procured from E. Merck India Ltd. Double distilled water having pH 6.0 ± 1.0 was used for making MP solutions.Fabrication of Ag–TiO2 nanoparticulate film
Before the fabrication of Ag–TiO2 nanoparticulate film, TiO2 nanoparticle dispersion was synthesized in our laboratory by sol–gel method followed by hydrothermal treatment at 80 °C by a reported method.21 5 mL of titania nanoparticle dispersion (1 mg mL−1) was diluted to 149 mL by double distilled water, and 1 mL of concentrated hydrochloric acid was added to it. Resulting dispersion was sonicated for 60 min before the preparation of the films. Films were prepared by dip coating technique. Quartz and glass substrates with 50 mm length, 1.3 mm thickness, and 13 mm width were used for fabricating TiO2 and Ag–TiO2 nanoparticulate films. Substrate was immersed in dispersion for 5 min, and then lifted slowly from the solution with a withdrawal speed around 10 cm min−1. Subsequently, the coated films were dried in an oven at 100 °C for 2 h and this immersion, withdrawal and drying cycle was repeated for 5 times to obtain films. Subsequently, the film was immersed in 50 mL of aqueous solution of silver nitrate (1 mM) for 30 min and then it was dried at 100 °C for 2 h. Followed by this, it was photoreduced under UV irradiation of 0.3 mW cm−2 for 30 min to obtain Ag–TiO2 nanoparticulate film.27Characterization
TEM measurements were done on Tecnai transmission electron microscope of FEI, The Netherlands make. Ag–TiO2 film was peeled off and the sample was suspended in 30 mL acetone, and the suspension was sonicated for 30 min. After that, suspension was placed on carbon coated copper grids of 3 mm dia and dried at room temperature prior to the analysis. Prepared films were characterized by X pert pro diffractometer (Panalytical, Netherlands) using glancing angle X-ray diffraction (GAXRD, angle of incidence 1°, penetration depth <300 nm). Measurements on scanning electron microscopy coupled with energy dispersive analysis of X-rays (SEM-EDAX) were done on FEI Quanta 400 model equipment of FEI, The Netherlands make at 20 kV operating voltage. Lambda 35 ultraviolet and visible spectrophotometer of Perkin Elmer, USA was used for recording the absorbance of MP at different intervals of time. MP showed the absorption maxima at 274 nm.The amount of MP remaining in solution at different intervals of time was determined following calibration of the absorption maxima at 274 nm according to Beer–Lambert law. UV-vis spectrometry was also used for recording the absorbance of Ag–TiO2, TiO2 nanoparticles immobilized on quartz substrates prepared by dip coating for 5 cycles. The pH of the solution was measured by using cyber Scan pH 1500 Eutech instrument of Thermo Scientific Company, Italy. Reaction products were characterized by gas chromatography-mass spectrometry (GC-MS) system after concentration followed by silylation with BSTFA. GC (model no. 6890N), MS (5975B) of Agilent technologies, USA was used for this purpose. Temperature program from 50 to 250 °C, at a rate of 15 °C min−1 was used for the analysis. DB 5 MS capillary column of 0.25 mm i.d, 0.25 μm film thickness and 30 meters length was used for the purpose of analysis of reaction products.
Photocatalyitc degradation of MP
Stock solution was prepared by adding ∼19 μL of MP in 1000 mL of double distilled water. 50 mL of aqueous solution of MP was taken in quartz round bottom (QRB) flask. Subsequently, Ag–TiO2 or TiO2 nanoparticulate film was immersed in QRB flask containing MP solution, and was kept in front of 175 Watt xenon illuminator of Luzchem, Canada having a range of wavelength of 280–800 nm. Intensity of irradiance was adjusted by the regulator provided with xenon illuminator. Intensity for the position of QRB flask was measured by digital light meter (SLM 110 model) of A.W. Sperry Instruments, USA with help of adopters provided. Intensity of UV light was found to be 0.3 W cm−2. The solution was magnetically stirred to facilitate proper mixing of reactant species. Calibrated equipment based on infrared absorption and electrochemical principle manufactured by Drager, Germany was used to sniff the released CO2 and acetaldehyde from headspace. GC-MS analysis of reaction products extracted from the reaction mixture after silylation with BSTFA, semi-micro qualitative analysis of concentrated water samples and sniffing the headspace gases by Drager chemical sensor were used to ascertain the reaction pathways of photocatalytic degradation of MP.Results and discussion
Characterization of films
Titania nanoparticles of 5–15 nm size range were used for making TiO2 and Ag–TiO2 nanoparticulate films. Simple dip coating method was used for making films on glass as well as quartz substrates. SEM image of TiO2 nanoparticulate film illustrate uniformly deposited titania nanoparticles on the substrate.TEM image of Ag–TiO2 nanoparticles peeled off from Ag–TiO2 nanoparticulate film and SEM image of Ag–TiO2 film made by dip coating of titania nanoparticles followed by adsorption and photo reduction (UV) of silver ions are shown in Fig. 1. The SEM and TEM images of TiO2 and Ag–TiO2 nanoparticulate films showed similar morphologies except that in the case of Ag–TiO2 nanoparticulate film, slightly bigger aggregates of silver nanoparticles were found in addition to the TiO2 nanoparticles. Relatively brighter particles of nanodimensions shown in SEM image of Ag–TiO2 film were actually found to be the aggregates of silver nanoparticles. These observations are consistent with TEM data of Ag–TiO2 film which indicated TiO2 nanoparticles of size at around 5–15 nm and silver nanoparticles of size at around 10–20 nm. The aggregation of these particles can be ascribed to van der Waals forces. The EDAX result confirmed the presence of silver on Ag–TiO2 film. Due to limited resolution power of SEM, it could not give exact indication of the size of particles however illustrated the titania and silver particles of nanodimensions.
 | ||
| Fig. 1 TEM image of Ag–TiO2 nanoparticles (a) peeled off from the film and (b) SEM image of Ag–TiO2 nanoparticulate film. | ||
GAXRD data
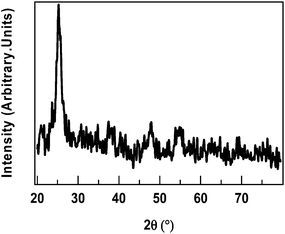
GAXRD data of Ag–TiO2 film depicted the presence of titania nanoparticles on the substrate as shown in Fig. 2.GAXRD pattern showed peaks at 2θ = 25.2° (101), 2θ = 37.8° (004), 2θ = 48.0 (200), 2θ = 53.8 (106), 2θ = 62.7 (215) and illustrated the presence of titania nanoparticles of anatase phase. Silver nanoparticles did not give any indication of their presence. This can be attributed to the amorphous nature of film. The Scherer formula was used to calculate crystallite size of titania particles present on the substrate. The average crystallite size of titania particles deposited on the substrate was found to be 11 nm. XRD pattern of TiO2 film was already reported.21
TiO2 nanoparticulate film showed absorption maximum at around 240 nm indicating the presence of TiO2 nanoparticles. Ag–TiO2 nanoparticulate film showed two absorption maxima at around 240 nm and 420 nm. Maxima at around 240 nm can be attributed to TiO2 nanoparticles and the maxima at around 420 nm can be ascribed to the presence of silver nanoparticles (Fig. 3). Apparently, Ag–TiO2 film was found to possess enhanced visible light absorbance when compared to TiO2 nanoparticulate film due to the presence of silver nanoparticles.
Photocatalytic degradation of MP using TiO2 and Ag–TiO2 nanoparticulate films
Photocatalytic activity of TiO2 and Ag–TiO2 nanoparticulate films were evaluated against MP and the data was compared to that obtained under photolytic degradation conditions. MP was observed to decompose slowly by photolysis under sole treatment of light radiation ranging from 280–800 nm. Only 81% of MP was found to be degraded in 420 min due to photolysis. Whereas, 90% of MP was found to be degraded in the presence of TiO2 nanoparticulate film due to photocatalysis and 100% of MP was found to be degraded in the presence of Ag–TiO2 nanoparticulate film in 420 min. Only 0.7% of methyl parathion was observed to be degraded in dark reaction in the absence of light when only TiO2 or Ag–TiO2 nanoparticulate film was used (Fig. 4). This can be ascribed to negligible adsorption capacity of the films.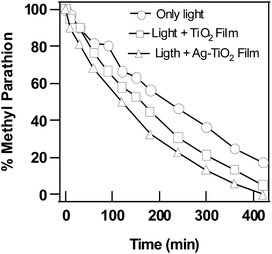 | ||
| Fig. 4 Degradation of MP in the presence of only light, in the presence of light along with TiO2 and Ag–TiO2 nanoparticulate films. | ||
Concentration of MP was found to be decreasing exponentially and logarithmic plots were observed to be linear when plotted versus time depicting pseudo first order kinetics. Kinetic analysis of above data indicated the value of rate constant to be 2.3 × 10−3 min−1 for photolytic degradation of MP in aqueous solution. The rate of degradation reaction was found to be enhanced by the presence of TiO2 nanoparticulate film and the value of rate constant was calculated to be 4.6 × 10−3 min−1. The increase of value of rate constant from 2.3 × 10−3 to 4.6 × 10−3 can be attributed to the additive effect of presence of TiO2 nanoparticulate film which facilitated the photocatalytic degradation of MP (Fig. 5). It further increased to 6.9 × 10−3 min−1 when Ag–TiO2 film was used for photocatalysis. Blank experiments conducted by using TiO2 or Ag–TiO2 nanoparticulate film in absence of light indicated negligible degradation of MP. Data showed that there is no change of MP concentration in the absence of light. When TiO2 and Ag–TiO2 films were compared, the increase of the value of rate constant from 4.6 × 10−3 to 6.9 × 10−3 min−1 can be attributed to the effective separation of photo-generated charge carriers which was found to be promoted by silver nanoparticles present on Ag–TiO2 film. In addition to this, addition of silver nanoparticles to TiO2 film extended its absorbance to visible light region which in turn extended the photoactivity of Ag–TiO2 film into visible light region, where TiO2 absorb only in UV region. This also could be a reason for the slightly larger activity of Ag–TiO2 film when compared to TiO2 film.
 | ||
| Fig. 5 Kinetics of degradation of MP in the presence of only light, TiO2 and Ag–TiO2 nanoparticulate films along with R2 values in brackets. | ||
In order to examine the stability and regenerability of Ag–TiO2 nanoparticulate film, four successive experiments were conducted under the same experimental conditions using the same film. Film was rinsed with water thoroughly after every cycle of photocatalysis experiment. It was observed that, 100% of methyl parathion (Fig. 6) was found to be degraded in each reaction cycle in 420 min of reaction time and in each reaction cycle value of rate constant was found to be same, i.e., 6.9 × 10−3 min−1.
 | ||
| Fig. 6 Regenerability of Ag–TiO2 nanoparticulate film used for photocatalytic degradation of methyl parathion. | ||
This observation clearly demonstrated the stability and regenerability of titania nanoparticulate film. Reaction products formed on surface of the film were found to be removed instantaneously by water molecules which collide the surface, and this way the surface poisoning seemed to be avoided. Perhaps the negligible chemical adsorption of reaction products formed on the surface seemed to have influenced the availability of active sites and regeneration of surface. In addition to this, to understand whether any loss of titania nanoparticles was there due to peeling off the film, absorbance of the film was recorded which was re-used for 4 cycles. Interestingly there is no significant change in absorbance at 240 nm which further confirmed the stability of titania nanoparticulate film. However, the absorbance at 420 nm decreased after each cycle due to oxidation of silver nanoparticles to silver ions. However, after washing thoroughly and subsequently exposing it to UV irradiance for 30 min regained the absorbance value indicating that although silver nanoparticles were oxidized to silver ions after each cycle of the experiment and they were not peeled off from the Ag–TiO2 film surface.
Short discussion on photocatalytic degradation mechanism
Table 1 shows the main products detected by GC-MS after silylation during photocatalytic oxidation of MP on TiO2 and Ag–TiO2 nanoparticulate film. Carbon dioxide and acetaldehyde were detected by sniffing the head space gas by using a Drager chemical sensor. GC-MS data indicated the presence of paraoxon-methyl, silylated product of p-nitrophenol, and trimethyl silyl phosphate ester in the reaction mixture. In addition to these, trimethyl ester of phosphoric acid, and phosphorothioic acid O,O,S-trimethyl ester were also observed in the reaction mixture.| Compounds | Retention time | Characteristic ions (m/z) |
|---|---|---|
| Phosphoric acid, trimethyl ester | 5.04 | 140, 110, 95, 79, 65, 47, 29, 15 |
| Phosphorothioic acid O,O,S-trimethyl ester | 7.03 | 156, 141, 126, 110, 109, 95, 79 |
| Trimethyl silyl phosphate | 8.85 | 314, 299, 283, 211, 133, 73 |
| Trimethyl silyl(4-nitrophenoxy) | 10.87 | 213, 196, 150, 73, 45 |
| Methyl paraxon | 13.29 | 247, 230, 170, 152, 109, 93, 79, 63 |
Phosphoric acid dimethyl ester further underwent reaction with hydroxyl radical and formed phosphoric acid monomethyl ester, phosphoric acid and then lead to the formation of phosphate ion in final stage. As mentioned above, formation of phosphate ion was confirmed by the presence of trimethyl silyl phosphate in the reaction mixture. It was understood that, photocatalytic degradation of MP started with cleavage of P–O–C bond. Subsequently, P–O bond, C–C bond etc., and P and C atoms were found to be oxidized gradually and finally lead to the formation of final degradation products such as carbon dioxide and phosphate ion.28
Based on the above results, reaction mechanism of photocatalytic degradation of MP is presented in Fig. 7. In aqueous solution of MP, holes seemed to have reacted with water molecules and hydroxide ions and produced hydroxyl radicals. Electrons observed to have reacted with dissolved oxygen molecules and generated super oxide anion radicals. They further reacted with water molecules and facilitated the formation of hydroxyl radicals. These strongly electrophilic ˙OH radicals found to have contributed to degradation of MP in aqueous solutions. Formation of phosphate ion was also confirmed by the semi micro qualitative analysis of concentrated water. Water was acidified with nitric acid and then treated with ammonium heptamolybdate solution.
The solution had turned to yellow precipitate due to the formation of ammonium molybdophosphoric acid thus confirming the presence of phosphate ions by semi micro qualitative analysis. This observation is consistent with reported results by Vorontsov et al.29 Subsequently, oxidation of P–O–C and C–H bonds by ˙OH radicals observed to have assisted the formation of acetaldehyde, and then finally carbon dioxide.
Silver nanoparticles seemed to have contributed to enhanced photocatalysis by trapping the charge carriers. Visible light activity of Ag–TiO2 film aroused due to the presence of silver nanoparticle also seemed to have contributed to enhanced photocatalytic degradation of MP. Collectively, studies on photocatalytic degradation of MP, a toxic pesticide using Ag–TiO2 nanoparticulate film provides a promising technology for purification of water contaminated with toxic OPCs avoiding several logistic problems such as removal of catalysts after treatment when used for water purification.
Conclusions
Studies have revealed that Ag–TiO2 film has demonstrated enhanced rate of photocatalytic degradation of MP when compared to TiO2 nanoparticulate film. Silver nanoparticles were found to have contributed to photocatalysis by trapping the charge carriers. Oxidation or cleavage of P–O–C was found to be predominant relative to oxidation of C–C or C–H bonds. Some amount of MP was completely mineralized to phosphate ion and the same was confirmed by GC-MS data.Notes and references
- J. Fournier, Chemie des Pesticides, Editions Cultures et Techniques, Nantes, 1988 Search PubMed.
- J. R. Chambers and H. W. Chambers, in Pesticide transformation products, ed. L. Somasundaraman and J. R. Coats, ACS Symp. Ser, 1991 Search PubMed.
- K. Harada, T. Hisanaga and K. Tanaka, Water Res., 1990, 24, 1415 CrossRef CAS.
- H. D. Burrows, M. L. Canle, J. A. Santaballa and S. Steenken, J. Photochem. Photobiol., B, 2002, 67, 71 CrossRef CAS.
- H. Roques, Chemical Treatment: Principles and Practice, VCH, New York, 1996 Search PubMed.
- M. Eto, Organophosphorous pesticides: organic and biological chemistry, CRC Press, Cleveland, 1974 Search PubMed.
- A. Topalov, D. Molnar-Gabor, B. Abramovic, S. Korom and D. Pericin, J. Photochem. Photobiol., A, 2003, 160, 195 CrossRef CAS.
- A. Fujishima, D. A. Tryk and T. N. Rao, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., Part 1, 2005, 44, 8269 CrossRef CAS.
- F. Denny, E. Permana, J. Scott, J. Wang, D. Y. H. Pui and R. Amal, Environ. Sci. Technol., 2010, 44, 5558 CrossRef CAS PubMed.
- R.-a. Doong and W.-h. Chang, J. Photochem. Photobiol., A, 1997, 107, 239 CrossRef CAS.
- L. Zhang, F. Yan, Y. Wang, X. Guo and P. Zhang, Inorg. Mater., 2006, 42, 1379 CrossRef CAS.
- S. Han, J. Li, H. Xi, D. Xu, Y. Zuo and J. Zhang, J. Hazard. Mater., 2009, 163, 1165 CrossRef CAS PubMed.
- E. A. Kozlova, P. G. Smirniotis and A. V. Vorontsov, J. Photochem. Photobiol., A, 2004, 162, 503 CrossRef CAS.
- E. Moctezuma, E. Leyva, G. Palestino and H. D. Lasa, J. Photochem. Photobiol., A, 2007, 186, 71 CrossRef CAS PubMed.
- E. Evgenidou, I. Konstantinou, K. Fytianos, I. Poulios and T. Albanis, Catal. Today, 2007, 124, 156 CrossRef CAS PubMed.
- T.-S. Kim, J.-K. Kim, K. Choi, M. K. Stenstrom and K.-D. Zoh, Chemosphere, 2006, 62, 926 CrossRef CAS PubMed.
- Y. Lei, L. D. Zhang and J. C. Fan, Chem. Phys. Lett., 2001, 338, 231 CrossRef CAS.
- K. Esquivel, L. G. Arriaga, F. J. Rodriguez, L. Martinez and L. A. Godinez, Water Res., 2009, 43, 3593 CrossRef CAS PubMed.
- H. Choi, S. R. Al-Abed and D. D. Dionysio, in Nanotechnology applications for clean water, ed. M. Diallo, J. Duncan, N. Savage, A. Street and R. Sustich, William Andrew Inc., Norwich, NY, 2009, p. 39 Search PubMed.
- G. K. Prasad, P. V. R. K. Ramacharyulu, J. PraveenKumar, A. R. Srivastava and B. Singh, Thin Solid Films, 2012, 520, 5597 CrossRef CAS PubMed.
- C. Yogi, K. Kojima, T. Takai and N. Wada, J. Mater. Sci., 2009, 44, 821 CrossRef CAS.
- Y. Wu, H. Liu, J. Zhang and F. Chen, J. Phys. Chem. C, 2009, 113, 14689 CAS.
- L. Sun, J. Li, C. Wang, S. Li, Y. Lai, H. Chen and C. Lin, J. Hazard. Mater., 2009, 171, 1045 CrossRef CAS PubMed.
- H.-Y. Chuang and D.-H. Chen, Nanotechnology, 2009, 20, 105704 CrossRef PubMed.
- M. Qamar, Desalination, 2010, 254, 108 CrossRef CAS PubMed.
- L. C. Courrol, F. R. de Oliveira Silva and L. Gomes, Colloids Surf., A, 2007, 305(1–3), 54 CrossRef CAS PubMed.
- B. Sun, A. V. Vorontsov and P. G. Smirniotis, J. Hazard. Mater., 2011, 186, 1147 CrossRef CAS PubMed.
- A. V. Vorontsov, Y. C. Chen and P. G. Smirniotis, J. Hazard. Mater., 2004, 113, 89 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |