Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conceptual, self-assembling graphene nanocontainers
Simon
Boothroyd
and
Jamshed
Anwar
*
Chemical Theory and Computation, Department of Chemistry, Lancaster University, Lancaster LA1 4YB, UK. E-mail: j.anwar@lancaster.ac.uk
First published on 15th June 2015
Abstract
We show that graphene nano-sheets, when appropriately functionalised, can form self-assembling nanocontainers which may be opened or closed using a chemical trigger such as pH or polarity of solvent. Conceptual design rules are presented for different container structures, whose ability to form and encapsulate guest molecules is verified by molecular dynamics simulations. The structural simplicity of the graphene nanocontainers offers considerable scope for scaling the capacity, modulating the nature of the internal environment, and defining the trigger for encapsulation or release of the guest molecule(s). This design study will serve to provide additional impetus to developing synthetic approaches for selective functionalisation of graphene.
Introduction
Molecular and nanoscale containers have (and promise) numerous applications that include molecular detectors, nano-scale reaction vessels, storage vessels for unstable molecules, catalysis through spatial constraint, and drug delivery systems.1–10 Consequently, there is much interest in their design and synthesis. Of a particular interest are molecular capsules that utilize non-covalent interactions to self-assemble and hence offer the possibility of reversible encapsulation.11The design of molecular containers has advanced considerably since the pioneering work by Collet, Cram and their co-workers1,12 on carcerands and hemicarcerands. These molecular containers tended to comprise covalently-linked structures, with the consequence that guest molecule(s) were entrapped permanently. Subsequent focus has been on the development of approaches to control the opening of the containers to enable insertion or release of the guest molecule,13 and the design and creation of molecular containers (often termed capsules) that reversibly self-assemble.14–16 The self-assembly approach is based on molecular component subunits that either have complementary non-covalent interactions which come together to form a single capsule14 or employ metal ions to link the ligands.16 A more recent development is the design of containers that utilise hydrophobic interactions to assemble and function in an aqueous environment.17
A related development has been the discovery of fullerenes and carbon nanotubes, both of which can serve as molecular containers.18–21 The nanotubes offer more scope in terms of capacity as their inner diameter can vary from about 1 nm upwards to 20 nm or more for multi-walled tubes.20 In contrast, the capacity of the fullerenes is relatively fixed and limited given the constraints of their structure. Open-cage fullerenes (fullerenes with an orifice) can be prepared by chemical approaches but the procedure is involved.18 As for carbon nanotubes, filling of the open tubes can be carried out at low temperature but closing filled tubes is still an issue for delicate guest molecules e.g. organics.21
Here we present conceptual design rules for creating self-assembling, graphene-based nanocontainers, which at first would appear counter intuitive given that graphene22 is a 2-dimensional material. We employ principles of molecular self-assembly based on non-covalent interactions to induce graphene nano-sheets, whose edges have been appropriately functionalised, to form nanocontainers. We consider both strong electrostatic interactions (in both polar and non-polar solvents) and a combination of hydrophobic and polar interactions in a polar solvent such as water, where the hydrophobic effect (the strong affinity of water molecules for themselves causing exclusion of hydrophobic molecules) is exploited akin to formation of lipid membranes in biological systems. The selective edge-functionalization of graphene as proposed is currently outside the scope of experimental capability but edge functionalization of graphene is of considerable interest and there have been some recent exciting advances.23–25
The primary design approaches that were considered are illustrated in Fig. 1. Thus we attempted to design nano-sheets by way of edge functionalization that would self-assemble to yield: (i) cubes; (ii) pyramids; (iii) opened-ended (triangular prism) tubes involving three sheets; and (iv) tubes resulting from the rolling up of a single nano-sheet. For cube formation the nano-sheets were squares, whilst for the pyramid design the sheets were triangular. The nano-sheet edges were functionalised using full charges of +1.0 e and −1.0 e (a starting point for developing a conceptual understanding of the design rules) with the charges being placed on the protruding carbon atoms of the zigzag and chair edges, while the corner atoms of the sheet remained neutral. For the cube, two types of functionalised nano-sheets are needed: Type 1 for which three of the edges have negative charges with the other edge being positive – these form the sides of the cube and hence four of these are required; and Type 2 for which all the edges are positively charged – these form the top and the bottom of the cube and therefore two of these are required. Likewise for the pyramid: four of the triangular sheets had two negatively charged edges and one positively charged edge; the edges of the fifth sheet were all positively charged. The triangular prism tube container required three functionalised nano-sheets of a single type, each with its two opposite edges functionalized with opposite charges. For the single-sheet cylindrical tube, the edges that come together had opposite charges. Given that the tube designs had open ends, the edges exposed at the open ends were functionalised by a variety of functional groups selected to open and close the open ends in response to a chemical trigger.
To identify the success or otherwise of the various approaches, we carried out molecular dynamics simulations of the designer graphene nano-sheets either in vacuum (which approximates a non-polar solvent) or in water. The simulations track how the interacting molecular structures evolve towards low free energy configurations as a function of time.
Results and discussion
Of the 4 approaches to designing graphene nanocontainers which were investigated, only the two tube-approaches i.e. triangular prism tube and the single sheet rolled-up tube, showed promise in terms of forming nanocontainers. Simulations of the multi-sheet designs to yield either cubes or pyramids were carried out in both vacuum (to represent a non-polar solvent) and in water. In all cases the nano-sheets did not self-assemble to form any kind of molecular container. An expectation was that such systems might form extended structures, which they do but not of the container kind. It appears that the force required for two sheets linked at an edge to align perpendicularly to each other is missing, that is, there is no corner L-bracket. In principle, one could introduce this L-bracket by using rigid molecular moieties for the edge interactions.In contrast, the component nano-sheets of the triangular prism container readily self-assembled to form a 3-sheet nanotube with open ends (see Fig. 2). The self-assembly was characterised by three of the charged sheets positioning themselves so that each negative edge was adjacent to a positive edge, while the alkyl chains aggregated to form the cap. The formation of these prism tubes was observed both in the small and the large system simulations. In the larger system containing 36 nano-sheets, we observed the formation of 3 triangular prism containers from the maximum possible of 12 – a 25% yield. The formation of non-container structures was also observed with some nano-sheets forming extended chains while others wrapped around the outside of the formed prism containers. Containers of greater than three sides formed but underwent a collapse into simpler structures. The alkyl chains designed to cap the open ends appeared to play a positive role in the formation of the prism tubes providing steric hindrance to stop the sheets from intercalating inappropriately, and in favour of forming the triangular prism structures instead. Further, the alkyl chains formed effective caps as designed, being forced by the hydrophobic effect to cover significant portions of the openings (Fig. 2b).
For the single nano-sheet approach, the design objective was for the sheet to roll up of its own accord with the opposing edges making contact to form a tube and then to have organic functional groups to effectively cap the ends. Self-assembly of a 2D inorganic material has been reported earlier, though resulting from strain rather than edge functionalization.26 The use of two graphene disc sheets to cap the tube whilst conceptually elegant requires a more complex design that would challenge the self-assembly process, resulting in a low probability of a successful outcome. To induce contact between the opposing edges, we first considered hydrophobic interactions at the edges as a means to bring the two edges together in water; the hydrophobicity of the carbons along the two edges was increased substantially by decreasing the interaction potential (Lennard Jones epsilon parameter from ε = 0.29 kJ mol−1 to ε = 0.029 kJ mol−1). The graphene sheets failed to roll up, presumably because there was not enough distinction in terms of hydrophobicity (from the perspective of the water molecules) between the functionalised hydrophobic edges and the pristine edges of graphene. We then explored the use of strong opposite charges at the edges and examined the behaviour of the functionalised sheets in both water and in vacuum (the latter representing a non-polar solvent). In water, the sheets failed to roll up to form tubes because of the strong interaction of the edges with water, favouring instead the 3-sheet prism tubes. In contrast, in vacuum (representing a non-polar solvent) the nano-sheets functionalised with the strong charges on opposing edges rapidly rolled up to form a tube (Fig. 3). In order to cap the open ends we employed polar –COOH moieties, expecting them to hydrogen bond across the tube opening and hence shield it, and in this they proved to be effective though there is room for improvement (Fig. 3b). We appreciate now that reducing the density of the –COOH moieties along the edges would be beneficial: this will reduce the interaction between adjacent –COOH groups in favour of an interaction with groups across the entrance.
For the nanotube containers (both the 3-sheet prism tube and the single-sheet rolled up tube), simulations were also carried out to investigate their container capability for two small guest molecules, a polar peptide (aspartic acid-valine-serine) and the non-polar trans-stilbene molecule. For the small polar peptide, the triangular prism container was observed to retain a previously encapsulated molecule for the entire simulation run extending to 2 ns. The molecule showed considerable motion within the capsule but was prevented from breaking free.
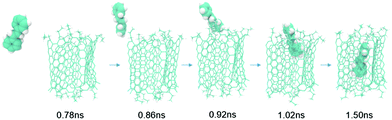
For the non-polar stilbene molecule, we were able to observe self-loading: one of the stilbene molecules was observed to translocate from the bulk polar solvent into the cavity of the triangular prism over a period of about 0.7 ns, due to its preference for a hydrophobic environment. The stilbene molecule was retained by the container for a further 500ps until the end of the simulation (Fig. 4). We note that pristine graphene nano-sheets can also attract hydrophobic molecules and serve as carrier particles,27,28 but such behaviour would not provide the full advantages of a molecular container.
We have by choice employed a physics-type approach (very simple models) in terms of modelling to access the generic features, rather than getting bogged down in specific chemistry. The next step, once selective functionalization of the graphene edges has been experimentally mastered (see below), is to underpin the conceptual designs by elegant and specific chemistry that is able to guide synthesis. So in principle it would be possible by means of molecular simulation to identify the exact functional groups required for the self-assembly of the designer graphene containers and for their entry points, such that a switch in solvent, pH or temperature could cause the formation or total collapse of the container, or induce opening or closure of its entry points. Equally, the required functionalisation of the interior/exterior surface of the container for a particular purpose (e.g. for stability or for altering the character of the cavity) could also be identified.
The graphene-based molecular capsules exhibit a certain structural simplicity relative to the general chemical complexity associated with the more conventional molecular containers e.g. the self-assembling capsules developed by Rebek and co-workers.14 In particular, the graphene-based molecular containers offer ready scalability in terms of design to accommodate different-sized guest molecules. The primary parameter here is the dimensions of the starting nano-sheets. Further, the other essential design features including the strength of the edge interactions that hold the containers together, the chemical nature of the cavity environment, and how the opening and closure of the nanotube ends is triggered, are all based on simple chemistry, assuming selective edge-functionalization of graphene is mastered. There is therefore considerable scope in developing graphene-based molecular containers that have a specific sized cavity, are highly selective, and encapsulate or release their content to specific chemical or thermal triggers.
This conceptual design study gives strong direction as to the approach that is likely to lead to success in developing graphene-based nanocontainers. In translating these concepts and synthesising the capsules, there remain some experimental challenges but which no doubt will be surmounted in due time. A particular challenge is how might one selectively functionalise the edges of a graphene sheet, not just relative to the surface but also between the different edges.
Summarising, we have presented creative design rules for self-assembling graphene nanocontainers using molecular simulation. The graphene-based nanocontainers exhibit a certain structural simplicity and offer considerable scope for scaling the container capacity, modulating the nature of the internal environment, and defining the trigger for encapsulation or release of the guest molecule(s). Our conceptual design approach truly leads synthesis and fabrication and is set to give strong direction to graphene research towards selective functionalisation of graphene edges.
Computational methods
The molecular dynamics simulations were carried out either in the constant temperature-constant volume (NVT) ensemble (vacuum studies) or in the isothermal-isobaric (NPT) ensemble. The graphene nano-sheets and functional groups were modelled using parameters from the all-atom optimized potentials for liquid simulations (AA-OPLS) force field.29 The water model was SPC. Initial systems investigated employed nano-sheets of a relatively small size of about 1.94 × 1.90 nm to gain a broader idea of how the designer nano-sheets behave. The sheet size was then increased to 3.41 × 3.60 nm and the number of sheets per system increased to a maximum of 36. The number of water molecules ranged from 2000 to 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules. The simulations were carried out using GROMACS 4.6.5.30 The simulation temperature was 310 K and external pressure 1 bar (for the isothermal isobaric simulation). The interaction cut-off for VDW was 1.4 nm. Electrostatic interactions were also truncated at 1.4 nm but using a shifted potential, as long-range interactions and correlation effects were considered to be unimportant. A time step of 0.001 ps was chosen. The systems were simulated for up to 7 nanoseconds.
000 molecules. The simulations were carried out using GROMACS 4.6.5.30 The simulation temperature was 310 K and external pressure 1 bar (for the isothermal isobaric simulation). The interaction cut-off for VDW was 1.4 nm. Electrostatic interactions were also truncated at 1.4 nm but using a shifted potential, as long-range interactions and correlation effects were considered to be unimportant. A time step of 0.001 ps was chosen. The systems were simulated for up to 7 nanoseconds.
Notes and references
- D. J. Cram and J. M. Cram, in Container molecules and their guests, Royal Society of Chemistry, Cambridge, 1994 Search PubMed.
- D. Turner, A. Pastor, M. Alajarin and J. Steed, Design Approaches and Applications, in Supramolecular Assembly via Hydrogen Bonds I, ed. D. M. Mingos, Springer-Verlag, Berlin, Heidelberg, 2004, pp. 97–168 Search PubMed.
- G. Zhang and M. Mastalerz, Organic cage compounds – from shape-persistency to function, Chem. Soc. Rev., 2014, 43, 1934–1947 RSC.
- J. Ma, S. Wang, C. Zhao, H. Wang and Y. Dong, Cu(II)4L4 coordination-driven molecular container: a reusable visual colorimetric sensor for Ag(I) ions, Chem. Commun., 2014, 50, 4721–4724 RSC.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed.
- J. Kang and J. Rebek Jr., Acceleration of a Diels–Alder reaction by a self-assembled molecular capsule, Nature, 1997, 385, 50–52 CrossRef CAS PubMed.
- M. Yoshizawa, T. Kusukawa, M. Fujita and K. Yamaguchi, Ship-in-a-bottle synthesis of otherwise labile cyclic trimers of siloxanes in a self-assembled coordination cage, J. Am. Chem. Soc., 2000, 122, 6311–6312 CrossRef CAS.
- G. Hettiarachchi, D. Nguyen, J. Wu, D. Lucas, D. Ma, L. Isaacs and V. Briken, Toxicology and drug delivery by cucurbit[n]uril type molecular containers, PLoS One, 2010, 5, e10514 Search PubMed.
- D. Ma, G. Hettiarachchi, D. Nguyan, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken and L. Isaacs, Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals, Nat. Chem., 2012, 4, 503–510 CrossRef CAS PubMed.
- Y. Lai, D. Cascio and T. O. Yeates, Structure of a 16-nm cage designed by using protein oligomers, Science, 2012, 336, 1129 CrossRef CAS PubMed.
- M. M. Conn and J. Rebek Jr., Self-assembling capsules, Chem. Rev., 1997, 97, 1647–1668 CrossRef CAS PubMed.
- J. Canceill, L. Lacombe and A. Collet, A new cryptophane forming unusually stable inclusion complexes with neutral guests in a lipophilic solvent, J. Am. Chem. Soc., 1986, 108, 4230–4232 CrossRef CAS.
- E. L. Piatnitski and K. D. Deshayes, Hemicarceplexes that release guests upon irradiation, Angew. Chem., Int. Ed., 1998, 37, 970–972 CrossRef CAS.
- F. Hof, S. L. Craig, C. Nuckolls and J. Rebek Jr., Molecular encapsulation, Angew. Chem., Int. Ed., 2002, 41, 1488–1508 CrossRef CAS.
- B. C. Hamman, K. D. Shimizu and J. Rebek Jr., Reversible encapsulation of guest molecules in a calixarene dimer, Angew. Chem., Int. Ed. Engl., 1996, 35, 1326–1326 CrossRef PubMed.
- T. Kusukawa and M. Fujita, Self-assembled M6L4-type coordination nanocage with 2,2′-bipyridine ancillary ligands. facile crystallization and X-ray analysis of shape-selective enclathration of neutral guests in the cage, J. Am. Chem. Soc., 2002, 124, 13576–13582 CrossRef CAS PubMed.
- C. L. D. Gibb and B. C. Gibb, Well-DefinedOrganic nanoenvironments in water: the hydrophobic effect drives a capsular assembly, J. Am. Chem. Soc., 2004, 126, 11408–11409 CrossRef CAS PubMed.
- G. C. Vougioukalakis, M. M. Roubelakis and M. Orfanopoulos, Open-cage fullerenes: towards the construction of nanosized molecular containers, Chem. Soc. Rev., 2010, 39, 817–844 RSC.
- S. C. Tsang, Y. K. Chen, P. J. F. Harris and M. L. H. Green, A simple chemical method of opening and filling carbon nanotubes, Nature, 1994, 372, 159–162 CrossRef CAS PubMed.
- S. Hampel, D. Kunze, D. Haase, K. Kramer, M. Rauschenbach, M. Ritschel, A. Leonhardt, J. Thomas, S. Oswald and V. Hoffmann, et al., Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumour cell growth, Nanomedicine, 2008, 3, 175–182 CrossRef CAS PubMed.
- S. Y. Hong, G. Gerard Tobias, K. T. Al-Jamal, B. Ballesteros, H. Ali-Boucetta, S. Lozano-Perez, P. D. Nellist, R. B. Sim, C. Finucane, S. J. Mather, M. L. H. Green, K. Kostarelos and B. G. Davis, Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging, Nat. Mater., 2010, 9, 485–490 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- M. Quintana, A. Montellano, A. E. d. R. Castillo, G. V. Tendeloo, C. Bittencourt and M. Prato, Selective organic functionalization of graphene bulk or graphene edges, Chem. Commun., 2011, 47, 9330–9332 RSC.
- I.-Y. Jeona, Y.-R. Shin, G.-J. Sohn, H.-J. Choi, S.-Y. Bae, J. Mahmood, S.-M. Jung, J.-M. Seo, M.-J. Kim, D. W. Chang, L. Dai and J.-B. Baek, Edge-carboxylated graphene nanosheets via ball milling, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5588–5593 CrossRef PubMed.
- I. Y. Jeon, H. J. Choi, S. M. Jung, J. M. Seo, M. J. Kim, L. Dai and J. B. Baek, Large-scale production of edge-selectively functionalized graphene nanoplatelets via ball milling and their use as metal-free electrocatalysts for oxygen reduction reaction, J. Am. Chem. Soc., 2013, 135, 1386–1393 CrossRef CAS PubMed.
- M. Remškar and A. Mrzel, Inorganic nanotubes: self-assembly and geometrical stabilisation of new compounds, Vacuum, 2003, 71, 177–183 CrossRef.
- P. Lazar, F. Karlický, P. Jurečka, M. Kocman, E. Otyepková, K. Šafářová and M. Otyepka, Adsorption of small organic molecules on graphene, J. Am. Chem. Soc., 2013, 135, 6372–6377 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS PubMed.
- W. L. Jorgensen and J. Tirado-Rives, The OPLS (optimized potentials for liquid simulations) potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin, J. Am. Chem. Soc., 1988, 110, 1657–1666 CrossRef CAS.
- B. Hess, C. Kutzner, D. van der Spoel and E. Lindahl, GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation, J. Chem. Theory Comput., 2008, 4, 435–447 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |