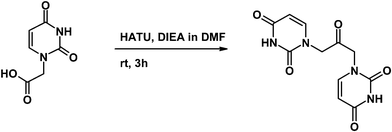
Synthesis and supramolecular assembly of 1,3-bis(1′-uracilyl)-2-propanone†
Giovanni N. Roviello*a,
Giuseppina Roviellob,
Domenica Musumecic,
Domenica Capassod,
Sonia Di Gaetanoa,
Michele Costanzod and
Carlo Pedoned
aIstituto di Biostrutture e Bioimmagini – CNR, Via Mezzocannone 16, 80134 Napoli, Italy. E-mail: giovanni.roviello@cnr.it; Fax: +39 (0)81-2534574; Tel: +39 (0)81-2534585
bDepartment of Science and Technology, University of Naples “Parthenope”, Centro Direzionale, 80143 Napoli, Italy
cDepartment of Chemical Science, University of Naples “Federico II”, Via Cinthia, 80126 Napoli, Italy
dDepartment of Pharmacy, University of Naples “Federico II”, Via Mezzocannone 16, 80134, Napoli, Italy
First published on 17th June 2014
Abstract
In this research work, we investigated the properties of a novel heterocyclic molecule, named by us U2CO, whose structure consists of two uracil rings connected by a 2-propanone moiety. We studied by UV the spectroscopic characteristics of U2CO as a function of temperature and ionic strength of the solution. Furthermore, dynamic light scattering (DLS) studies conducted on samples containing the uracil-containing derivative indicated the formation of non-covalent supramolecular networks based on multiple U2CO units. These aggregates contained hydrophobic cavities able to encapsulate drugs such as doxorubicin, as evidenced by fluorescence studies. This property, together with the good stability in human serum of U2CO, determined by HPLC analysis, could be an interesting feature in developing new drug delivery systems. Interestingly, UV studies suggested that U2CO is able to form complexes with copper(II) cations, which is a useful characteristic in view of potential anti-oxidant approaches.
Introduction
Aromatic and heteroaromatic compounds, of both natural and synthetic origin, have a great importance in biology and medicine due to their ability to interact with natural targets or form supramolecular structures. For this reason, great efforts have therefore been focused on the synthesis of aromatic compounds with remarkable biological activity of potential application in biomedicine. Interestingly, some heteroaromatic compounds are able to form supramolecular structures or nano-aggregates which have been widely investigated, as in the case of several molecular systems based on pyrimidine heterocycles, such as uracil and its derivatives,1,2 with the aim of developing new nanomaterials of interest in drug delivery applications.3 Furthermore, some uracil-based compounds showed promising results regarding their interaction with biomacromolecules.4,5In this context, we realized a novel heteroaromatic molecule, named by us U2CO, containing two uracil rings anchored to a 2-propanone subunit. This compound, whose chemical and biological properties are object of the present study, is structurally related to biologically relevant molecules, such as: (1) the natural alkaloid (−)-anaferine, i.e. 1,3-bis(2-piperidyl)-2-propanone, present in vegetal extracts of Withania somnifera,6 an asiatic solonacea plant used in traditional medicine for its sedative and hypnotic properties; (2) several antimuscarinic drugs based on a 1,3-disubstituted 2-propanone backbone with two 6-term rings (phenyl and piperazine moieties), as represented in Fig. 1.7
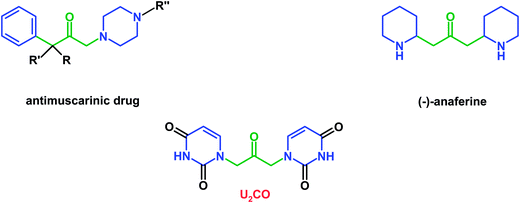 | ||
| Fig. 1 Molecular structures of disubstituted 2-propanone derivatives with biological activity in comparison with U2CO. | ||
The application of the Dakin–West reaction8–11 to the synthesis of the target compound, the study of its ability to form non-covalent supramolecular networks, able to host drugs, as well as the binding to copper(II) cations are some of the issues investigated in the present work.
Results and discussion
The uracil-based compound U2CO was synthesized starting from uracilyl acetic acid (UCH2COOH, Scheme 1), following a procedure similar to the ones previously described for several 1,3-disubstituted-2-propanone derivatives.8–11The identity of the desired product was confirmed by X-ray characterization (Fig. 2a and b) as well as by NMR (1H-NMR in Fig. 2c) and mass spectrometry.
The molecule crystallizes in monoclinic system (space group P2/c). In the asymmetric unit cell, two independent half molecules are present. By applying the operators of symmetry, two molecules with different orientations of the uracil groups are generated: one molecule presents a torsion angle C1–N2–C5–C6 equal to 80.22°; for the second one the corresponding angle is equal to −104.63°. Both molecules exhibit an endo–endo conformational arrangement. In fact, the two arms of the uracil rings present the same orientation with respect to the carbonyl group.12 It is worth noting that the heteroatoms of the uracil rings point towards opposite sides with respect to the plane containing the ketone group. Finally, the NH and CO groups of different uracil rings, symmetrically related to each other, are strictly connected through a close net of hydrogen bonds.
Spectroscopic studies
The spectroscopic properties of U2CO have been analyzed by evaluating the effects of temperature and ionic strength on its UV spectra. In particular, the UV spectrum relative to a 15 μM solution of U2CO in H2O-0.1% DMSO (pH 7.0) has been recorded in the 222–320 nm wavelength range at different temperatures ranging from 10 to 80 °C. As evident in Fig. 3, a decrease of absorbance at 260 nm is observed upon heating. A similar trend has been also detected by UV melting experiments, which revealed a quasi-sigmoidal behaviour, with a Tm of about 50 °C.Subsequently, the effect of ionic strength on the spectroscopic properties of U2CO has been evaluated recording the UV spectrum in the 250–320 nm wavelength range relative to a 38 μM solution of U2CO in H2O-0.2% DMSO (pH 7.0) at different NaCl concentrations (20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 mM). As shown in Fig. 4, a decrease in the absorbance of the band centred at about 260 nm is observed upon increasing the NaCl concentration. These data suggest that, upon heating, the U2CO molecule is able to explore multiple conformations, unfavoured at lower temperatures but favoured in correspondence of high ionic strength values. The hypochromic effect could be attributed to ion–dipole interactions between the π electronic clouds of the two heteroaromatic rings and ions in solution.
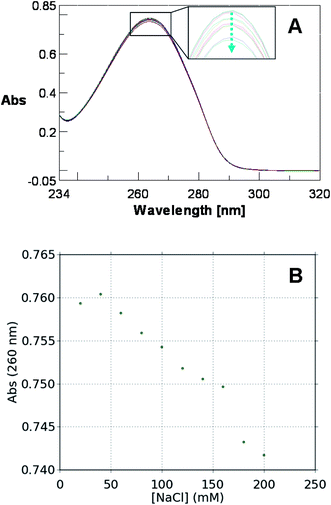 | ||
| Fig. 4 Variation of the UV spectrum (A) and of the absorption value recorded at 260 nm (B) for a 38 μM solution of U2CO in H2O-0.2% DMSO as a function of NaCl concentration. | ||
Next we have investigated the interaction of U2CO with transition metal ions such as Ni2+ and Cu2+, monitored by UV experiments. This study has been performed by titrating a 4 μM solution of U2CO in H2O-0.02% DMSO (pH 7) with two aqueous solutions of NiCl2 hexahydrate and CuCl2 dihydrate, respectively.
No significant variation in the UV profile of U2CO has been detected on increasing the Ni2+ concentration (Fig. 5a), thus indicating that 1,3-bis(1-uracilyl)-2-propanone does not significantly interact with this bivalent cation. On the contrary, U2CO is able to sensibly interact with Cu2+, as evidenced by the relevant variation in the UV spectrum of the heteroaromatic compound on increasing the cation concentration (Fig. 5b). The ability of U2CO to bind rameic cations is interesting because the complexation of this metal cation is crucial in many innovative biomedical approaches.13–19 Interestingly, U2CO was found unable to bind nucleic acids as determined by UV and CD experiments with different DNA and RNA strands (and their complexes) at 10 °C, pH 7.5. No significant variation in UV and CD profiles of the examined nucleic acids has been indeed observed in the presence of U2CO at various nucleic acid: U2CO ratios. These preliminary findings suggest that the use of U2CO in cell apparently would not cause any conformational variation in DNA and RNA, nor interfere with biological processes mediated by these biomolecules. Analogously, U2CO does not seem able to interact with serum proteins, and thus to interfere with the associated biological mechanisms; in fact CD experiments performed on Bovine Serum Albumin – here used as model protein – did not result in any appreciable variation of CD bands upon addition of different amounts of U2CO (data not shown).
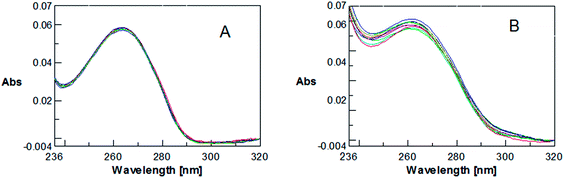 | ||
| Fig. 5 UV spectra relative to a 4 μM solution of U2CO in H2O-0.02% DMSO in the presence of Ni2+ (A) and Cu2+ (B) in the 0–40 equivalents range (experiments performed at room temperature). | ||
Dynamic light scattering studies
Subsequently, the formation of supramolecular networks, formed by multiple units of U2CO held together by weak interactions, has been investigated by taking into account previous literature examples of supramolecular architectures based on both oligonucleotide and nucleoside systems.20–23 More in detail, dynamic light scattering studies have been carried out in order to get information on the self-assembly of the heteroaromatic molecule investigated in the present work by analyzing a 129 μM solution of U2CO in H2O-0.3% DMSO (pH 7.0) at 25 °C. These results showed the networking ability of U2CO, forming aggregates with an average hydrodynamic diameter of about 190 nm (Fig. 6a).A kinetic study has been also performed on the same sample to monitor the size of the U2CO-based aggregates as a function of time, by recording DLS spectra at 0, 2 and 24 h. As shown in Fig. 6b, the distribution of the hydrodynamic diameters appear to be less polydisperse with time. Furthermore, since DLS spectra of U2CO at 2 and 24 h are almost superimposable, we hypothesize that the supramolecular organization of the system results substantially stable already after 2 h.
In order to evaluate the thermal stability of the supramolecular networks, the variation of the hydrodynamic diameter values as a function of temperature has been examined, and to this purpose DLS spectra have been recorded at 25, 30, 35, 40, 45 and 50 °C. Interestingly, no appreciable change in hydrodynamic diameters has been detected in the range 25–45 °C. On the other hand, at temperature values ≥50 °C no appreciable supramolecular aggregation has been observed, thus suggesting that the system under examination is stable in the 25–45 °C temperature range, but is disrupted at higher temperature. The effect of the dilution on the stability of the supramolecular networks has been also investigated: DLS experiments were conducted also investigating the effects of dilution on solutions of U2CO. In all cases, we found that the average size of the networks based on the studied compound did not vary significantly with concentration.
Fluorescence studies
The formation of polymeric networks has been also confirmed by fluorescence spectroscopy. In particular, the fluorescence spectrum relative to a 51 μM solution of doxorubicin (dox), a well-known antibiotic and antineoplastic drug belonging to the anthracycline family, whose action is due to its ability to intercalate DNA nucleobases,24 has been measured at λex = 480 nm. Increasing aliquots of the uracil derivative have been, thus, added to the dox solution in the same experimental conditions in which the formation of supramolecular networks has been previously proven, and the corresponding fluorescence spectra have been recorded.As it can be observed in Fig. 7, the fluorescence intensity of doxorubicin increases upon addition of increasing amounts of U2CO, suggesting that the anticancer drug is encapsulated into the hydrophobic cavities of a supramolecular network formed by U2CO. Nevertheless, it is also worth to underline that the fluorescence signal is stabilized when the concentration of the uracilyl compound reaches the 130 μM value, i.e. at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 U2CO/dox ratio.
1 U2CO/dox ratio.
Biological studies
In view of possible biomedical applications of U2CO, we also investigated its cytotoxicity. This evaluation has been performed on two human tumour cell lines, HeLa and A431 and on human primary culture. Cell viability has been assessed by the crystal violet assay,25 and in no case relevant cytotoxic effects have been observed.Enzymatic stability of the uracil derivative has been also investigated by incubating 0.1 mM U2CO in 99% human serum at 37 °C and analyzing by RP-HPLC the samples withdrawn at different times (0, 1, 2, 3, 4, 5, 6 and 30 h). From the results of this assay (Fig. 8), it can be deduced that U2CO is stable in human serum. However, the variation of the HPLC profile observed after 30 h indicates that some enzymatic degradation of the heteroaromatic compound occurs, a desirable feature for a biomedical device in order to avoid its accumulation in cell.
Experimental section
Chemicals
HATU was purchased from ABI. DMF, DMSO, DIEA and solvents for HPLC were Romil. UCH2COOH was purchased from ABCR GmbH & Co. DMF-d7, CuCl2·2H2O and NiCl2·6H2O were from Sigma.Synthesis of U2CO
U2CO was synthesized starting from uracilyl acetic acid (Scheme 1). UCH2COOH (0.35 g, 2.1 mmol) was activated by treatment with HATU (0.9 equiv., 0.67 g, 1.8 mmol) and DIEA (2.3 equiv., 845 μL, 4.8 mmol) in DMF (1.75 mL). The mixture, kept under argon atmosphere, was stirred at room temperature and after 3 h was poured onto 500 mL of water. After freezing the sample was lyophilized and the solid obtained was treated with water in order to allow the desired product to precipitate. After crystallization from water and acetic acid, pure U2CO was obtained with a 35% yield. The compound was ≥95% pure by HPLC analysis.Characterization of U2CO
NMR δH (400 MHz, DMF-d7): 11.36 (2H, s, CONHCO), 7.59 (2H, d, C5–H), 5.63 (2H, d, C6–H), 4.94 (4H, s, NCH2CO); δC (50 MHz, DMF-d7): 201.9, 166.7, 148.6, 154.1, 104.1, 56.8. HPLC: analytical RP-HPLC was performed on a C18 column (Phenomenex Jupiter C18 300 Å, 5 μm, 4.6 × 250 mm) with a 4 mL min−1 flow rate and a linear gradient from 2% (for 5 min) to 80% B in A over 20 min: tR = 3.4 min (A = 0.1% TFA in water; B = 0.1% TFA in acetonitrile). LC-ESI-MS (Fig. S1†): m/z 279.14 (found), 279.24 (expected for [C11H10N4O5 + H]+). UV: UV profile for the uracilyl derivative is reported in Fig. S1† and shows two absorbance maxima at 218 and 266 nm, respectively.Single crystal X-ray crystallography
Single white crystals of U2CO suitable for X-ray analysis were obtained at 173 K from a water–acetic acid solution. Data collection was performed at 173 K on a Bruker-Nonius kappa CCD diffractometer (MoKα radiation, CCD rotation images, thick slices, φ scans + ω scans to fill the asymmetric unit). Cell parameters were determined from 160 reflections in the range 3.02° ≤ θ ≤ 19.21. Semiempirical absorption corrections (multi-scan SADABS) were applied. The structure was solved by direct methods (SIR 97 package) and refined by the full matrix least-squares method (SHELXL program of SHELX97 package) on F against all independent measured reflections, using anisotropic thermal parameters for all non-hydrogen atoms. All H atoms were positioned geometrically. 185 refined parameters, R1 = 0.0710; wR2 = 0.2209 (on reflections with I > 2σ(I)) and R1 = 0.1173, wR2 = 0.2947 on all reflections. Max. and min. residual electron density (e Å−3): +0.426 and −0.539. Crystal data and details of the data collection are reported in Table S1.†UV and CD studies
UV spectra were recorded on a UV-Vis Jasco model V-550 spectrophotometer equipped with a Peltier ETC-505T temperature controller by using Hellma quartz Suprasil cells, with a light path of 1 cm. Circular dichroism (CD) spectra were obtained on a Jasco J-715 spectropolarimeter, equipped with a Peltier PTC-423S/15 temperature controller, using a Hellma quartz cell with a light path of 1 cm and a Hellma Tandem quartz cell 2 × 0.4375 cm.Dynamic light scattering
DLS studies were conducted on a Zetasizer Nano ZS (Malvern Instruments Ltd). A stock solution of U2CO was filtered through a 0.2 μm Millex syringe driven filter unit (Millipore, Bedford, MA). All measurements were performed in triplicates for a 2 min acquisition time. The hydrodynamic diameter of the scattering molecules was derived using the Malvern software from the diffusion coefficient by the Einstein–Stokes equation.Human serum stability assay
The serum stability assay was performed following a procedure we have previously reported.26Cell lines and culture conditions
Human umbilical vein endothelial cells (HuVec) were grown in Endothelial Basal Medium (EBM-2) supplemented with 2% FCS, VEGF 0.5 ng mL−1, eparin 22.5 μg mL−1, EGF 0.1 ng mL−1, bFGF 1 ng mL−1, hydrocortisone 1 μg mL−1, amphotericin B 50 ng mL−1, gentamycin 50 μg mL−1 (EGM-2) (Lonza, Milan, Italy). Cells grown in T 25 primary flash (Beckton Dickinson) were maintained in humidified air containing 5% CO2 at 37 °C. Cells from passage 3–8 were used for the experiments. Human adenocarcinoma cell line (HeLa) and epidermoid carcinoma cell line (A431) (ATCC U.S.) were grown in DMEM supplemented with 10% fetal bovin serum (FBS), 1% glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Euroclone, Milano, Italy) at 37 °C in humidified air containing 5% CO2.Cytotoxicity assay
Cells were seeded in 96-well plates at a density of 4 × 103 cells per well and treated with increasing concentrations of U2CO (0.5, 5, 50 μM). After 72 h incubation, cell viability was determined by the crystal violet (Sigma-Aldrich) assay, which correlates optical density with cell number. Briefly, cells were washed with PBS and fixed by adding 10% formalin solution. After 15 minutes cells were washed with deionized water and stained with 100 μL of 0.1% crystal violet solution in water for 30 minutes. The excess dye was removed by washing with deionized water and the plates were air-dried prior to bound dye solubilization in 200 μL of 10% acetic acid. The optical density of dye extracts was measured directly in plates at 595 nm by using a BioRad microplate reader model 680. All experiments were performed in triplicate.Conclusions
In this work we have synthesized and studied the heteroaromatic compound U2CO, characterized by two uracil rings linked through a 2-propanone backbone. UV spectroscopic studies performed on varying the temperature and ionic strength suggest that upon heating U2CO adopts conformations otherwise unfavoured. In our experiments these conformations occur also at high ionic strength, probably allowed by the ion–dipole interactions in which the π electronic clouds of the two uracil rings mutually interact, causing a slight hypochromic effect. Furthermore, DLS studies show the formation of non-covalent aggregate systems with an average hydrodynamic diameter of 190 nm, due to multiple U2CO molecules interacting by weak bonds. These molecular networks are stable on increasing the temperature in the range between 25 and 45 °C and with dilution. Preliminary spectroscopic studies indicate that U2CO is not able to bind nucleic acids nor to interact with proteins. This feature is of clear interest for future applications of the here studied heteroaromatic compound, being apparently unable to interfere with the biomolecular processes mediated by DNA, RNA and serum proteins. UV titration experiments showed the ability of U2CO to specifically bind copper(II) cations: this property could be useful in the development of new chelators able to contrast pro-oxidative effects of copper(II) metal ion in cell. Moreover, fluorescence spectroscopic studies indicated the presence of hydrophobic cavities in the interior of the U2CO-based supramolecular networks able to host molecules endowed with therapeutic activity such as doxorubicin. Finally, the good stability of the heteroaromatic compound in human serum and the absence of cytotoxicity on different human cell lines further support the potential use of U2CO in novel drug delivery strategies.Acknowledgements
We thank Prof. Antonio Roviello, Dr Valentina Roviello, Dr Oreste Tarallo and Mr Leopoldo Zona for their precious suggestions and invaluable technical assistance. We are also grateful to the institutions that supported our laboratory (Consiglio Nazionale delle Ricerche and Università degli Studi di Napoli ‘Federico II’).References
- T. Marangoni and D. Bonifazi, Nano- and microstructuration of supramolecular materials driven by H-bonded uracil 2,6-diamidopyridine complexes, Nanoscale, 2013, 5, 8837–8851 RSC.
- J.-H. Wang, O. Altukhov, C.-C. Cheng, F.-C. Chang and S.-W. Kuo, Supramolecular structures of uracil-functionalized PEG with multi-diamidopyridine POSS through complementary hydrogen bonding interactions, Soft Matter, 2013, 9, 5196–5206 RSC.
- U. Manna, S. Bharani and S. Patil, Layer-by-layer self-assembly of modified hyaluronic acid/chitosan based on hydrogen bonding, Biomacromolecules, 2009, 10, 2632–2639 CrossRef CAS PubMed.
- A. V. Solomonov, E. V. Rumyantsev, S. P. Ivanov, B. A. Kochergin and E. V. Antina, Spectroscopic studies of the supramolecular interactions between uracil and 5-hydroxy-6-methyluracil with bovine serum albumin and its bilirubin complex, Protein J., 2013, 32, 343–355 CrossRef CAS PubMed.
- V. Nair, G. Chi, Q. Shu, J. Julander and D. F. Smee, A heterocyclic molecule with significant activity against Dengue virus, Bioorg. Med. Chem. Lett., 2009, 1425–1427 CrossRef CAS PubMed.
- A. E. Schwarting, J. M. Bobbitt, A. Rother, C. K. Atal, K. L. Khanna, J. D. Laery and W. G. Walter, The alkaloids of W. somnifera, Lloydia, 1963, 26, 258–273 CAS.
- C. Kaiser, V. H. Audia, J. P. Carter, D. W. McPherson, P. P. Waid, V. C. Lowe and L. Noronha-Blob, Synthesis and antimuscarinic activity of some 1-cycloalkyl-1-hydroxy-1-phenyl-3-(4-substituted piperazinyl)-2-propanones and related compounds, J. Med. Chem., 1993, 36, 610–616 CrossRef CAS.
- H. D. Dakin and R. A. West, A general reaction of amino acids, J. Biol. Chem., 1928, 78, 91–105 CAS.
- T. T. Curran, Implementation of the Dakin–West reaction for the preparation of an α-amino-pentafluoroethyl ketone, J. Fluorine Chem., 1995, 74, 107–112 CrossRef CAS.
- K. Tran and D. Bickar, Dakin–West synthesis of β-aryl ketones, J. Org. Chem., 2006, 71, 6640–6643 CrossRef CAS PubMed.
- G. N. Roviello, G. Roviello, D. Musumeci, E. M. Bucci and C. Pedone, Dakin–West reaction on 1-thyminyl acetic acid for the synthesis of 1,3-bis(1-thyminyl)-2-propanone, a heteroaromatic compound with nucleopeptide-binding properties, Amino Acids, 2012, 43, 1615–1623 CrossRef CAS PubMed.
- S. Varughese and S. M. Draper, Solid state conformational preferences of a flexible molecular backbone derived from acetone: dependence on electron donating/withdrawing ability of substitutions, Cryst. Growth Des., 2010, 10, 2298–2305 CAS.
- V. Antoniades, A. Sioga, E. M. Dietrich, S. Meditskou, L. Ekonomou and K. Antoniades, Is copper chelation an effective anti-angiogenic strategy for cancer treatment?, Med. Hypotheses, 2013, 81, 1159–1163 CrossRef CAS PubMed.
- Z. D. Liang, Y. Long, W. B. Tsai, S. Fu, R. Kurzrock, M. Gagea-Iurascu, F. Zhang, H. H. Chen, B. T. Hennessy, G. B. Mills, N. Savaraj and M. T. Kuo, Mechanistic basis for overcoming platinum resistance using copper chelating agents, Mol. Cancer Ther., 2012, 11, 2483–2494 CrossRef CAS PubMed.
- H. Wei, W. J. Zhang, T. S. McMillen, R. C. Leboeuf and B. Frei, Copper chelation by tetrathiomolybdate inhibits vascular inflammation and atherosclerotic lesion development in apolipoprotein E-deficient mice, Atherosclerosis, 2012, 223, 306–313 CrossRef CAS PubMed.
- J. Y. Yoo, J. Pradarelli, A. Haseley, J. Wojton, A. Kaka, A. Bratasz, C. A. Alvarez-Breckenridge, J. G. Yu, K. Powell, A. P. Mazar, T. N. Teknos, E. A. Chiocca, J. C. Glorioso, M. Old and B. Kaur, Copper chelation enhances antitumor efficacy and systemic delivery of oncolytic HSV, Clin. Cancer Res., 2012, 18, 4931–4941 CrossRef CAS PubMed.
- G. J. Cooper, Selective divalent copper chelation for the treatment of diabetes mellitus, Curr. Med. Chem., 2012, 19, 2828–2860 CrossRef CAS.
- G. J. Cooper, Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes and Alzheimer's disease, Drugs, 2011, 71, 1281–1320 CrossRef CAS PubMed.
- S. A. Lowndes, H. V. Sheldon, S. Cai, J. M. Taylor and A. L. Harris, Copper chelator ATN-224 inhibits endothelial function by multiple mechanisms, Microvasc. Res., 2009, 77, 314–326 CrossRef CAS PubMed.
- G. N. Roviello, D. Musumeci, E. M. Bucci and C. Pedone, Evidences for supramolecular organization of nucleopeptides: synthesis, spectroscopic and biological studies of a novel dithymine L-serine tetrapeptide, Mol. BioSyst., 2011, 7, 1073–1080 RSC.
- G. N. Roviello, A. Mottola, D. Musumeci, E. M. Bucci and C. Pedone, Synthesis and aggregation properties of a novel enzymatically resistant nucleoamino acid, Amino Acids, 2012, 43, 1465–1470 CrossRef CAS PubMed.
- L. Simeone, C. Irace, A. Di Pascale, D. Ciccarelli, G. D'Errico and D. Montesarchio, Synthesis, self-aggregation and bioactivity properties of a cationic aminoacyl surfactant, based on a new class of highly functionalized nucleolipids, Eur. J. Med. Chem., 2012, 57, 429–440 CrossRef CAS PubMed.
- L. Simeone, D. Milano, L. De Napoli, C. Irace, A. Di Pascale, M. Boccalon, P. Tecilla and D. Montesarchio, Design, synthesis and characterisation of guanosine-based amphiphiles, Chem.–Eur. J., 2011, 17, 13854–13865 CrossRef CAS PubMed.
- G. R. Chamberlain, D. V. Tulumello and S. O. Kelley, Targeted delivery of doxorubicin to mitochondria, ACS Chem. Biol., 2013, 8, 1389–1395 CrossRef CAS PubMed.
- R. G. Gillies, N. Didier and M. Denton, Determination of cell number in monolayer cultures, Anal. Biochem., 1986, 159, 109–113 CrossRef CAS.
- G. N. Roviello, D. Musumeci, M. Castiglione, E. M. Bucci, C. Pedone and E. Benedetti, Solid phase synthesis and RNA-binding studies of a serum-resistant nucleo-ε-peptide, J. Pept. Sci., 2009, 15, 155–160 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mechanicistic considerations and characterizations of the heteroaromatic compound. CCDC 932679. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra03713h |
| This journal is © The Royal Society of Chemistry 2014 |