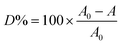Biogenic synthesis of photocatalytically active ZnS/ESM composites
Guanghui Zhang,
Caihong Li*,
Xuedong Zhang,
Xing Guo,
Yuchen Liu,
Weiyan He,
Jinrong Liu,
Hong Wang and
Yanfang Gao
School of Chemical Engineering, Inner Mongolia University of Technology, Hohhot, 010051, People's Republic of China. E-mail: licaihong616lch@163.com; Fax: +86-471-6575722; Tel: +86-471-6575722
First published on 4th March 2014
Abstract
The ZnS/ESM composites have been successfully fabricated using ESM (eggshell membrane), sodium sulfide and zinc sulfate as the main raw materials through an environmental and economical liquid impregnation method. The obtained samples were characterized via X-ray diffraction, scanning electron microscopy, energy dispersive spectroscopy, UV-vis diffuse reflectance spectra and Fourier-transform infrared spectra. The results demonstrated that the formed ZnS nanoparticles uniformly distributed on the surface of the egg membrane fibers, and the crystallinity and the amount of the ZnS loaded on the ESM fibers could be adjusted by changing the cycle indices of the impregnation. Furthermore, we tested the photocatalytic effects and analyzed the mechanism of the resulting ZnS/ESM composites as photocatalysts to degrade the harmful organic pollutant methyl orange under UV light irradiation. The results showed that the ESM support made it easy to recover the photocatalysts and enhanced the intensity of light absorption and the stability of the ZnS/ESM composites as photocatalysts. Based on the high photocatalytic activity and good stability, the ZnS/ESM composites with 4 cycle indices of the impregnation can be expected to be a practical photocatalyst.
Introduction
Since the discovery of the “Honda–Fujishima effect”,1 semiconductor photocatalysts have attracted much interest as a key material for the degradation of harmful organic pollutants.2 Various novel semiconductor catalysts for photocatalysis have been synthesized and studied in the past few decades, of which TiO2 has been generally used as a catalyst in photochemistry and environmental protection.3,4 Some studies recently indicated that transition metal sulfides, e.g. ZnS, showed more potential in the field of photocatalytic degradation organic pollutants than that of TiO2.5,6 ZnS is an important transition metal sulfide with wide direct band gap energy of ∼3.68 eV7,8 and can offer the potential for complete elimination of organic or toxic water pollutants owing to the rapid generation of electron–hole pairs and the highly negative redox potentials of excited electrons as semiconductor photocatalyst.6,9,10 Furthermore, it is not toxic, water insoluble and comparatively inexpensive.11 However, the practical applications of ZnS nanomaterials in photocatalysis field are seriously limited because of the poor stability and the aggregation of ZnS nanoparticles,12 coupled with the tremendous difficulties in separation and recycling of the photocatalyst. In order to synthesis ZnS nanoparticles with well dispersion and stability, a great deal of work has been carried out recently. On one hand, in some preparation methods of ZnS nanoparticles reported surfactant stabilizers or supporters were used to prevent the particles agglomeration and modify the surface of nanoparticles, such as mercaptoethanol,13 cetyltrimethylammonium,14–16 polyvinylpyrrolidone, polystyrene and so on.17–19 However, the use of abundant surfactants will damage the environment and increase the economic cost for the preparation of the photocatalytic materials. Therefore, it is highly desirable to develop a facile method to fabricate ZnS nanoparticles with well dispersion and stability. On the other hand, in order to solve the above problems, recently, ZnS composites were prepared and studied extensively as photocatalysts for the degradation of organic or toxic water pollutants, such as ZnS–montmorillonite nanocomposites and ZnS–polymer nanocomposite thin films.15,17ESM is the innermost portion of eggshell which can be readily obtained as waste from kitchens and industry.20 ESM is mainly composed of a thin inner and a thick outer membrane including proteins such as collagen (types I, V, and X), sialoprotein and osteopontin, and a small amount of saccharides with an interwoven fibrous structure.21–23 A few applications of ESM including matrices for adsorption of heavy metal ions and dyes,24–26 platforms for enzyme immobilization,27,28 and biological template for PbS nanoclusters synthesis have been reported,29 which suggest that ESM could be used as a convenient catalyst carrier.
In this work, the ZnS/ESM were prepared and characterized as an efficient photocatalyst for degrading the harmful organic pollutants under UV light radiation. We prepared the ZnS/ESM nanocomposites through a simple bioinspired process with ESM fibers as the reactive substrate and the directing template. With the complicated electrostatic cooperating effects and the interaction of carboxyl groups and some amino acids, Zn2+ could be anchored on the ESM and in situ reacted with S2− successively to generate ZnS nanoparticles. We tested the photocatalysis effects and analyzed the mechanism of the resulting ZnS/ESM composites as photocatalysts to degrade the harmful organic pollutants methyl orange.
Experimental section
Preparation of the ZnS/ESM composites
Fresh eggshells were washed with deionized water, and then were immersed in 4 M acetic acid solution for 30 minutes. The eggshell membranes were easily removed from the egg-shell by hand. After the eggshell membranes removed were washed with deionized water, they were dried in electric thermostatic drying oven at 25 °C. All the other chemicals used in the present experiments were of analytical grade and were used without further purification. Zn-precursor was 0.1 M ZnSO4 solution and S-precursor was 0.1 M Na2S solution. ZnS/ESM composites were prepared by the liquid impregnation method. Firstly, 0.6 g ESM was immersed into Zn-precursor solution for 4 h at room temperature, taken out and rinsed with deionized water. Subsequently, the ESM was dipped into S-precursor solution for 4 h, taken out and rinsed with deionized water. We marked the whole process above mentioned as a circulation. To investigate the influence of circulation times on the ZnS/ESM composites, controlled experiments were carried out by varying the cycle indexes denoted as CI1, CI2, CI3, and CI4 respectively while keeping other experimental conditions unchanged. After treated by the above processes, the ESM became gradually milk white from initial light pink. Samples were fished out of the solutions and dried in electric thermostatic drying oven at 25 °C for further characterization and photocatalytic degradation tests.Photocatalytic measurement method
The solution of methyl orange was used as a model contaminant for studying photoactivity of the prepared samples. Photodegradation experiments were performed with a photocatalytic reactor system (BL-GHX-V, Bilon Co., China). A 300 W Hg lamp was used as the light source, providing UV-light irradiation for the photodegradation process. The photocatalyst (0.3 g) was mixed with 300 ml of 10 mg l−1 methyl orange solution and the distance from lamp to the surface of the methyl orange solution was set to 25 cm, under magnetic stirring in the absence of light for 20 min to attain adsorption equilibrium. Then, the Hg lamp was opened and the samples were withdrawn at regular time intervals. The concentration of methyl orange in each degraded sample was determined spectrophotometrically at λ = 464 nm using calibration curve. The degradation efficiency (D%) was determined according to the following formula:here A0 is the initial concentration of methyl orange and A is the concentration of methyl orange after an irradiation time.
Characterization methods
The structural properties of the formed ZnS/ESM composites were investigated via XRD (D8-Advanced, Bruker Co., Germany) with a Cu kα radiation (λ = 0.15418 nm) source at 40 kV and 40 mA. The range of the diffraction angle (2θ) was 20° to 80°. The morphology, particle size and particle size distribution of the ZnS particles loaded on the ESM were analyzed by SEM (S-4800, Hitachi, Ltd, Japan and Quanta FEG-650, FEI Co., America). The energy dispersive spectroscopy (EDS) attached to SEM was used to analyze the composition of the composites. The absorption spectra of the composites was determined using the UV-vis diffuse reflectance spectra (UV-3600, Shimadzu, Co., Japan). The used biomaterial substrate and the prepared composites were examined by the Fourier-transform infrared (FTIR) spectra (Nexus-670, Nicolet, Co., America).Results and discussion
Fig. 1 shows the XRD patterns of the original ESM and the ZnS/ESM composites, respectively. The results of XRD showed that the natural ESM (a) was almost amorphous due to its ingredients containing plenty of amines, amides, and carboxylic groups. It can be seen that samples (c, d and e) exhibit several diffraction peaks at 28.56°, 47.52° and 56.29° corresponding respectively to (111), (220) and (311) planes of polycrystalline cubic structure ZnS (JCPDS no. 05-0566). It was clear that the peak intensities increased and the full width at half maxima narrowed with the increase of cycle indexes, indicating the higher crystallinity and the more ZnS nanocrystallites loaded on the ESM, and the average crystallite size of the sample (e) was calculated to be 13.5 nm by the Scherrer formula. The sample (b), however, had no diffraction peaks at the location of the corresponding. It was probably because that the amount of ZnS loaded on the ESM was too little. | ||
| Fig. 1 XRD patterns of the natural ESM (a) and the ZnS/ESM composites as prepared via CI1 (b), CI2 (c), CI3 (d) and CI4 (e), respectively. | ||
To get the microstructure information of the ESM and the ZnS/ESM composites, the samples were further investigated by scanning electron microscope (SEM). Fig. 2(a) exhibits an overview of the natural ESM. An intricate network-like structure is clearly observed in Fig. 2(a), and the diameter of the protein fibers is about 0.5–1 μm, and with some spheroid-like particles on the fibers. Fig. 2(b) shows the image of ZnS/ESM composites. The ESM fibers are almost “coated” with spherical ZnS particles, which are nearly absent in the space between fibers and the ZnS particles with the average size about 40 nm uniformly distribute on the surface of the ESM fibers. The average crystallite size was calculated to be 13.5 nm by the Scherrer formula. It indicated that small-sized ZnS nanocrystallites were actually assembled into spheroids distributing uniformly on the ESM fibers. It was noteworthy that these spheroids composed of ZnS nanocrystallites were bound so closely to the ESM fibers to achieve ZnS/ESM hybrid composites, which even treated ultrasonically in ethanol medium for about 10 hours, only a few ZnS nanoparticles were shaken off and into the suspension. Fig. 2(c) shows the image of ZnS which prepared by direct precipitation method. It is clear that the samples present spherical morphology with wide particle size distribution and serious aggregation phenomenon.
 | ||
| Fig. 2 SEM images of the natural ESM (a) and (b) the ZnS/ESM composites as prepared via CI4, (c) the ZnS prepared by direct precipitation method. | ||
The EDS spectrum of the ZnS/ESM composites is illustrated in Fig. 3. The results reported by Tsai showed that the natural ESM was composed of C, N, O and S elements,30 while the sample of the ZnS/ESM composite has extra Zn element (atomic ratio of Zn and S is 19.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.17). Combined with the results of XRD and SEM for the ZnS/ESM composites we can confirm that ZnS nanocrystallites were exactly fabricated on the ESM fibers.
23.17). Combined with the results of XRD and SEM for the ZnS/ESM composites we can confirm that ZnS nanocrystallites were exactly fabricated on the ESM fibers.
Fig. 4 displays the typical FTIR spectra of the sample dipped ESM in Zn-precursor (Zn2+/ESM) and the final sample ZnS/ESM compared with the natural ESM, respectively. In Fig. 4(a), the peaks at around 1662, 1531, and 1240 cm−1 are assigned as amide I, amide II, and amide III bands of type I collagen of the original ESM fibers, respectively.31 The peaks at 1452 cm−1 and 3279 cm−1 are attributed to the vs (COO) stretching vibration and the vs (OH), respectively.32 Comparing Fig. 4(b) with (a), the red shifts of amide I, amide II, amide III, and –OH for the sample dipped into Zn-precursor solution, suggested that there occurred environmental changed around the imino groups and C–N bond.33 These shifts might be due to the interaction of Zn2+ with carboxyl groups and imido residues of ESM proteins. Comparing Fig. 4(c) with (b), all the absorption bands in Fig. 4(c) become weaker, especially at 3270, 1650 cm−1. These changes could be attributed to the ZnS formation on the ESM.
 | ||
| Fig. 4 DRIFTS spectra of the natural ESM, Zn2+/ESM and the ZnS/ESM composite as prepared via CI4, respectively. | ||
Combined with the above analyses, it was clear that the biomaterial ESM participated in the synthesis reactions, and played key roles in the formation and assembly of ZnS nanocrystallites. The ESM fiber is composed of collagenous core and glycoprotein mantle. The collagenous core is made up of collagen I, V, and X, as well as osteopontin proteins.34 The glycoprotein mantle contains alkaline amino acids, like lysine acid (2.98 wt%), arginine acid (5.93 wt%), having massive negative functional groups such as amido and imido residues.35 Concretely, there are complicated electrostatic cooperating effects and interaction of carboxyl groups from Zn2+ with some amino acids containing negatively charged or neutral groups from glycoprotein mantle of ESM fibers. As a result, when the ESM was immersed into Zn-precursor solution, Zn2+ could be tightly adsorbed by negative function groups on the ESM fibers and generated Zn2+/ESM. By sequential soakage treatments of the ESM into Na2S solution, S2− would be grasped toward the active positions to achieve in situ nucleation of the ZnS nanocrystallites on the ESM fiber mantle. With the increase of the cycle indexes, the ZnS nanoparticles self-assembled spheroid-like nanocrystallites on the ESM fibers. The acidic amino acids and some other functional residues on the ESM mantle not only might induce the in situ formation of ZnS nanocrystallites, but also act as the surfactants for well dispersed ZnS nanocrystallites. The whole synthesis process of ZnS nanocrystallites can be illustrated in Fig. 5.
Fig. 6(a) displays the UV-vis diffuse reflectance spectra (UV-DRS) of the ZnS/ESM composites prepared with different cycle indexes (CI1, CI2, CI3 and CI4). It is clear that a red shift in the absorption edge is occurred and the absorption intensity both in UV and visible light regions is enhanced with the increase of cycle indexes, which agreed with the results of XRD shown in Fig. 1. These phenomena could be ascribed to the higher crystallinity and the more ZnS nanoparticles loaded on the ESM with the increase of the cycle indexes. Fig. 6(b) separately shows the optical absorption spectra for the natural ESM, the ZnS/ESM composites (CI4) and the ZnS prepared by the direct precipitation method. As can be seen clearly, the natural ESM has the ability of the light absorption in UV light regions; the ZnS/ESM (CI4) composites have higher light absorption intensity in the visible light regions, compared to the ZnS prepared by the direct precipitation, which might provide a chance for the ZnS/ESM composites as photocatalysts in visible light regions. It was noteworthy that the diameter of the ZnS/ESM particles (about 40 nm) was smaller than that of the ZnS (about 500 nm) prepared by the direct precipitation method from the results of Fig. 2. According to the previous work,36 the ZnS nanomaterials showed a blue shift compared to bulk materials. Therefore, the results of Fig. 6(b) could not be attributed to the decrease of the particle size. While it might be due to the favorable dispersibility of the ZnS nanoparticles on the ESM and the light absorption of the ESM supporter. In addition, the surfaces of the ZnS nanoparticles were modified by the biomacromolecules on the ESM which leaded to the decrease of the surface defects.
To investigate the photocatalytic activity of the present ZnS/ESM composites, the photocatalytic degradation of the model organic pollutant MO dye was examined in the presence of the obtained ZnS/ESM composites under the UV irradiation, and the blank experiment was also investigated for comparison. The characteristic absorption of MO at 464 nm was chosen as the monitored parameter for the photocatalytic degradation process. Fig. 7 shows the photocatalytic degradation of MO in the presence of the ZnS/ESM composites with different cycle indexes and the blank test under UV irradiation. The results showed that the amount of organic dye adsorption across the catalysts in the dark was almost 3% and negligible compared to the degradation amount under the UV irradiation. It could be seen that the degradation rate of MO increased from 57% to 97% with the increase of cycle indexes from 1 to 4, indicating that the photocatalytic activity of the ZnS/ESM composites showed great improvement. This trend can be attributed to that the ZnS crystallinity and its amount loaded on the ESM were increased with the increase of the cycle indexes, which agreed with the results of XRD shown in Fig. 1 and the UV-DRS measurement shown in Fig. 6(a). It can be seen from Fig. 7, compared with commonly utilized photocatalyst P25, the ZnS/ESM composites showed approximate photodegradation activity. While compared with the pure ZnS prepared by the direct precipitation method, the ZnS/ESM composites showed apparently higher photodegradation activity, which can be ascribed to the smaller size and the well dispersity of the ZnS nanoparticles fabricated on the ESM. Moreover, the addition of ESM broadened the light absorption regions of the ZnS/ESM composites from the results of the UV-DRS measurement in Fig. 6(b). In addition, the photocatalytic activity of the present ZnS/ESM composites (CI4) under the visible light illumination was also investigated. A 500 W Xe lamp was used as the light source and the other conditions were the same with that of the conditions under the UV irradiation. The results showed that the degradation rate of MO was 28%, which might be attributed to that the intensity of the light absorption of the ZnS/ESM composites (CI4) in the visible light regions was weak.
Fig. 8 shows the typical time-dependent UV-vis absorption spectra of the MO solution during the photodegradation in the presence of ZnS/ESM composites (CI4). It can be seen that the absorption peak at 464 nm, corresponding to the MO molecule, weaken gradually as the exposure time increase and nearly disappear after 160 min, indicating that a strong oxidation of MO had occurred in the presence of the ZnS/ESM composites (CI4) under the UV light irradiation.
 | ||
| Fig. 8 UV-vis absorption spectra changes of MO solution in the presence of the ZnS/ESM composites CI4 at different time intervals. | ||
For the application as a practical photocatalyst, the photocatalytic stability and the reuse of the photocatalyst are very important. Hence, the durability of the ZnS/ESM composites was evaluated through repeated use. The catalysts of the ZnS/ESM composites (CI4) were used in the degradation of MO according to the standard procedure, after 160 min, filtered from the reaction mixture and the recovery rate attained to 98%. The recovered photocatalysts were dried in the electric thermostatic drying oven at 25 °C, and then were reused to degrade MO in a fresh solution keeping the constant amount of catalyst. Fig. 9 shows the results of the 4 cycling runs in photodegradation of MO catalyzed by the ZnS/ESM composites (CI4), and the degradation rates after 160 min were 97%, 95%, 94%, 92%, respectively, for the consecutive 4 cycling runs. The slightly decrease in photocatalyst activity was partly caused by the inevitable loss of the amount of the photocatalyst during the washing and centrifugation process. The well stability of the prepared photocatalyst indicated that the ZnS combined steadily with the ESM, and the ZnS/ESM composites can be used repeatedly as a useful photocatalyst for degrading organic pollutant MO.
 | ||
| Fig. 9 Results of the cycling runs in the photocatalytic degradation of MO solution with the ZnS/ESM composites prepared via CI4 as photocatalyst under the UV light irradiation at room temperature. | ||
Some papers had reported that the photocatalytic activity of photocatalyst can be strongly influenced by the particle size and particle size distribution.37 Moreover, the recombination of the electron–hole pairs within the semiconductor particle was drastically reduced as the particle size decreased.38 In our experiments, the ZnS/ESM composites showed excellent photocatalytic activity, which might attribute to the reasons as follows. The ZnS nanoparticles we prepared were about 40 nm in diameter and had uniform size distribution when the ESM was introduced as template. Moreover, the amount of photogenerated charge carriers greatly depends on the light absorption of photocatalyst. In our experiments, both the ESM and the ZnS had the light absorption in UV light regions, therefore, the introduction of the ESM can contribute to the photogenerated charge carriers. The surface of ZnS nanoparticles could be modified by the biomacromolecules and amino acid residues on the ESM fibers, so that the ZnS/ESM composites had higher stability than ZnS as photocatalyst. Finally, the ESM had good adsorption ability to the water-soluble dye,26 which could accelerate the process of photocatalytic reaction. In a word, the ZnS/ESM composites prepared with the ESM as supporter and template had a more excellent photocatalytic performance than pure ZnS.
Fig. 10 shows schematically the photocatalytic degradation process of MO in the presence of ZnS/ESM composites (CI4). The MO was first adsorbed on the surface of the ZnS/ESM composites because of the adsorption ability of the ESM to the water-soluble dye. Then, under the UV-light irradiation condition, the photons absorbed onto the surface of ZnS/ESM composites would excite the electrons transition from the valance band of ZnS nanoparticles on the ESM to the conduction band of them, generating positive holes (hvb+) at the valence band edge and electron (ecb−) in the conduction band. Subsequently, the hvb+ and ecb− migrated to the surface of ZnS nanoparticles, then reacted with H2O or O2 which was adsorbed on the surface of catalysts and generated ˙OH or ˙O2. Finally, ˙OH and ˙O2 reacted with the MO molecules which adsorbed on the surface of ZnS/ESM composites, as a result, the MO molecules were degraded.
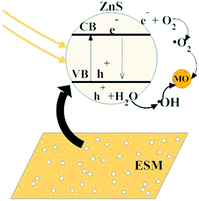 | ||
| Fig. 10 Schematic diagram for the ZnS/ESM composite prepared via CI4 degrading MO solution under the UV light irradiation. | ||
Conclusions
The ZnS/ESM composites were prepared by a simple, environmental liquid impregnation method. The photocatalytic activity of the ZnS/ESM composites prepared was studied as photocatalysts for the degradation of methyl orange under UV light irradiation. The formed ZnS uniformly distributed on the ESM fibers, and the crystallinity and the amount of the ZnS loaded on the ESM were increased with the increase of the cycle indexes. The ESM supporters added broadened the light absorption region of the ZnS/ESM composites which might make it possibly for the ZnS/ESM composites as photocatalysts in the visible light regions, and it was convenient for the photocatalysts prepared to recover for reuse. The results on photodegradation activity for the methyl orange and the stability tests of cycling runs of the photocatalyst indicate that the as-prepared ZnS/ESM composites can be applied as a practical photocatalyst for degradation of organic dyes. Moreover, the present synthesis procedure is a sort of green, moderate, and effective strategy for the preparation of other series of functional composites with ESM and other biomaterials as supports and templates.Acknowledgements
The authors are grateful to Ms Hou and Ms Song for their assistances with analysis of electron microscopy and Mr Cao for his assistance with measurement of X-ray diffraction.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- Y. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, ACS Nano, 2012, 6, 9777–9786 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- M. Liu, L. Piao, L. Zhao, S. Ju, Z. Yan, T. He, C. Zhou and W. Wang, Chem. Commun., 2010, 46, 1664–1666 RSC.
- S. Xiong, B. Xi, C. Wang, D. Xu, X. Feng, Z. Zhu and Y. Qian, Adv. Funct. Mater., 2007, 17, 2728–2738 CrossRef CAS.
- J. S. Hu, L. L. Ren, Y. G. Guo, H. P. Liang, A. M. Cao, L. J. Wan and C. L. Bai, Angew. Chem., Int. Ed., 2005, 117, 1295–1299 CrossRef.
- R. Xing, Y. Xue, X. Liu, B. Liu, B. Miao, W. Kang and S. Liu, CrystEngComm, 2012, 14, 8044–8045 RSC.
- S. Xiong, B. Xi, C. Wang, D. Xu, X. Feng, Z. Zhu and Y. Qian, Adv. Funct. Mater., 2007, 17, 2278–2737 CrossRef.
- M. Sharma, T. Jain, S. Singh and O. P. Pandey, Sol. Energy, 2012, 86, 626–633 CrossRef CAS PubMed.
- M. Y. Lu, M. P. Lu, Y. A. Chung, M. J. Chen, Z. L. Wang and L. J. Chen, J. Phys. Chem. C, 2009, 113, 12878–12881 CAS.
- H. Zhang, B. Wei, L. Zhu, J. Yu, W. Sun and L. Xu, Appl. Surf. Sci., 2013, 270, 133–138 CrossRef CAS PubMed.
- Y. Zhang, X. Liang and L. Li, Mater. Lett., 2010, 64, 1521–1523 CrossRef CAS PubMed.
- A. Chatterjee, A. Priyam, S. C. Bhattacharya and A. Saha, Colloids Surf., A, 2007, 297, 258–266 CrossRef CAS PubMed.
- A. Murugadoss and A. Chattopadhyay, Bull. Mater. Sci., 2008, 31, 533–539 CrossRef CAS PubMed.
- S. Miao, Z. Liu, B. Han, H. Yang, Z. Miao and Z. Sun, J. Colloid Interface Sci., 2006, 301, 116–122 CrossRef CAS PubMed.
- S. K. Mehta, S. Kumar, S. Chaudhary, K. K. Bhasin and M. Gradzielski, Nanoscale Res. Lett., 2009, 4, 17–28 CrossRef CAS PubMed.
- M. H. Ullah, J. H. Kim and C. S. Ha, Mater. Lett., 2008, 62, 2249–2252 CrossRef CAS PubMed.
- Y. Xie, C. Zhang, S. Miao, Z. Liu, K. Ding, Z. Miao, G. An and Z. Yang, J. Colloid Interface Sci., 2008, 318, 110–115 CrossRef CAS PubMed.
- H. Song and S. Lee, Nanotechnology, 2007, 18, 1–5 Search PubMed.
- D. A. Carrino, J. E. Dennis, T. M. Wu, J. L. Arias, M. S. Fernandez, J. P. Rodriguez, D. J. Fink, A. H. Heur and A. I. Caplan, Connect. Tissue Res., 1996, 35, 325–328 CrossRef CAS.
- F. Yi, Z. Guo, L. Zhang, J. Yu and Q. Li, Biomaterials, 2004, 25, 4591–4593 CrossRef CAS PubMed.
- M. S. Fernandez, A. Moya, L. Lopez and J. L. Arias, Matrix Biol., 2001, 19, 793–803 CrossRef CAS.
- K. Suyama, Y. Fukazawa and Y. Umetsu, Appl. Biochem. Biotechnol., 1994, 45–46, 871–879 CrossRef CAS.
- B. Koumanova, P. Peeva, S. J. Allen, K. A. Gallagher and M. G. Healy, J. Chem. Technol. Biotechnol., 2002, 77, 539–544 CrossRef CAS.
- M. Arami, N. Y. Limaee, N. M. Mahmoodi and N. S. Tabrizi, J. Hazard. Mater., 2006, 135, 171–174 CrossRef CAS PubMed.
- G. Annadurai, L. Y. Ling and J. F. Lee, J. Hazard. Mater., 2008, 152, 337–346 CrossRef CAS PubMed.
- B. Wu, G. Zhang, S. Shuang and M. M. F. Choi, Talanta, 2004, 64, 546–552 CrossRef CAS PubMed.
- Y. Zhang, G. Wen, Y. Zhou, S. Shuang, C. Dong and M. M. F. Choi, Biosens. Bioelectron., 2007, 22, 1791–1796 CrossRef CAS PubMed.
- H. Su, J. Han, N. Wang, Q. Dong, D. Zhang and C. Zhang, Smart Mater. Struct., 2008, 17, 1–4 CrossRef.
- W. T. Tsai, J. M. Yang, C. W. Lai, Y. H. Cheng, C. C. Lin and C. W. Yeh, Bioresour. Technol., 2006, 97, 488–493 CrossRef CAS PubMed.
- Z. Xu, K. G. Neoh and A. Kishen, Mater. Sci. Eng., C, 2010, 30, 823–824 Search PubMed.
- H. Su, J. Xu, J. Chen, W. J. Moon and D. Zhang, Appl. Phys. A, 2012, 106, 94–95 Search PubMed.
- A. Sellinger, P. M. Weiss, A. Nguyen, Y. F. Lu, R. A. Assink, W. L. Gong and C. J. Brinker, Nature, 1998, 394, 256–260 CrossRef CAS.
- J. Zhan, X. Yang, D. Wang, S. Li, Y. Xie, Y. Xia and Y. Qian, Adv. Mater., 2000, 12, 1348–1351 CrossRef CAS.
- P. K. Ajikumar, R. Lakshminarayanan, B. T. Ong, S. Valiyaveettil and R. M. Kini, Biomacromolecules, 2003, 4, 1321–1326 CrossRef CAS PubMed.
- X. Fang, T. Zhai, U. K. Gautam, L. Li, L. Wu, Y. Bando and D. Golberg, Prog. Mater. Sci., 2011, 56, 234–235 CrossRef PubMed.
- Y. Li, X. He and M. Cao, Mater. Res. Bull., 2008, 43, 3100–3109 CrossRef CAS PubMed.
- H. R. Pouretedal and A. Norozi, J. Hazard. Mater., 2009, 162, 674–680 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |