Long term and electrochemical corrosion investigation of cold worked AISI 316L and 316LVM stainless steels in simulated body fluid
Mohd Talhaa,
C. K. Beheraa,
Sudershan Kumarb,
Om Palc,
Gurmeet Singhc and
O. P. Sinha*a
aCentre of Advanced Study, Department of Metallurgical Engineering, Indian Institute of Technology (Banaras Hindu University), Varanasi, 221005, U.P., India. E-mail: opsinha.met@itbhu.ac.in; Fax: +91-542-2369478; Tel: +91-9452825977
bDepartment of Chemistry, Hindu College, University of Delhi, Delhi, 110007, India
cDepartment of Chemistry, University of Delhi, Delhi, 110007, India
First published on 15th January 2014
Abstract
AISI 316L and 316LVM stainless steels in annealed (solution quenched from 1050 °C) and rolled (10% and 20% cold work) conditions were assessed for their long term and electrochemical corrosion behavior in simulated body fluid (SBF) at 37 °C. The techniques used for the characterization of their corrosion resistance were the weight loss method, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization. Scanning electron microscopy (SEM) was used to investigate the surface morphologies of the alloys after the polarization tests. Surface analysis of the films formed on the steels in SBF was carried out using X-ray photoelectron spectroscopy (XPS). The weight loss and corrosion rate decreased with increasing degree of cold working. The resistance of a passive film is directly related to the material's corrosion resistance and increases on cold working, indicating the formation of a larger protective oxide layer on the surface of cold worked samples. The corrosion current density (Icorr) decreased with increasing degree of cold working and, simultaneously, the corrosion potentials (Ecorr) became more positive. On observing the pit morphologies using SEM, shallower and smaller pits were associated with cold worked samples as compared to annealed samples. The XPS results indicated that the main elements in the passive oxide layer were Cr, Fe and Mo. The Cr-oxide (ox)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr-hydroxide (hy) ratio and the Fe-oxide (ox)
Cr-hydroxide (hy) ratio and the Fe-oxide (ox)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe-hydroxide (hy) ratio were observed to be higher for rolled materials than for annealed materials, indicating that the passive films on rolled materials are more protective and improve the corrosion resistance.
Fe-hydroxide (hy) ratio were observed to be higher for rolled materials than for annealed materials, indicating that the passive films on rolled materials are more protective and improve the corrosion resistance.
1. Introduction
Stainless steel (SS) is a representative of metallic biomaterials and is widely used in orthopedic implants, with the main advantages of easy processing, good mechanical properties, good corrosion resistance, adequate biocompatibility and very low cost.1–3 Pitting corrosion is one of the most severe types of localized attack on SSs, and can adversely affect both the biocompatibility and mechanical strength of the implant and limit the applications of SS in biosystems.4 The pitting corrosion resistance of SSs is significantly affected by metallurgical parameters,5–7 i.e. cold working, alloy composition, inclusions, heat treatment, grain size, sensitisation, and secondary precipitates. SSs are subjected to different levels of cold working during the final manufacturing stages of components. Cold working (c.w.) might affect the pitting corrosion resistance, due to deformed substructures such as planar dislocation arrays8,9 and deformation twinning10 that could be introduced. Several investigations carried out in the past have reported the uncertain role of cold working on the localized corrosion resistance of SSs.5,11–19 It has been reported that due to cold working, strain-induced martensite and residual stresses are significantly introduced onto the surface of SSs, which affects the localized corrosion resistance by increasing the number of active anodic sites.13,19 Both the thickness and composition of passive films on the surface of SS are likely to be modified in many ways by cold working.20–24 Barbucci et al.25 reported that the passive currents of 304SS in sulfate + chloride solutions significantly increased with the degree of cold working. In addition, the pitting corrosion resistance was observed to decrease with increasing cold working in a 3.5% NaCl solution.26 In a study of the effect of cold working on the pitting potentials of different austenitic SSs, 18Cr–10Ni, 25Cr–10Ni, 17Cr–10Ni–2.4Mo and 16Cr–14Ni–Mo, it was found that in 30% NaCl solution the pitting potentials were not greatly affected by cold working, but for the cold worked specimens more pits with smaller sizes were observed.18 The quasi-martensite produced in 18Cr–8Ni steels by cold working did not change the pitting potential values in chloride solutions, but increasing pit densities were observed with increasing cold working.19 Mudali et al.17 reported that the pitting and crevice corrosion resistances of a ferritic SS were not affected by cold working of up to 20% in 0.5 M NaCl solution, irrespective of the defect structure that contained deformation bands. The susceptibility of cold worked 18Cr–10Ni–2Mo steel to pitting corrosion in NaCl solution was investigated, with the number of pits generally increasing with increasing deformation, except at 15% deformation where a low pit count and large average pit size were reported.5 Phadnis et al.22 reported that rolled 304SS showed a lower passive current density and exhibited re-passivation, which was not observed in heat treated material.Cold working has not yet been related clearly to the corrosion resistance of austenitic SSs, especially for long term applications in simulated body fluid (SBF). In the present work, an attempt was made to study the effect of cold working on the long term and electrochemical corrosion resistance of 316L and 316LVM SS for biomedical applications. The techniques used in this study for the characterization of the corrosion resistance were the weight loss method, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization. The structural evolution of the passive films was also discussed with the help of EIS. Scanning electron microscopy (SEM) was used to investigate the surface morphologies of the alloys after polarization. X-ray photoelectron spectroscopy (XPS) was used to detect the chemical compositions of the passive films formed on the surfaces of the SSs after 1 h of immersion in Hank's solution. Optical microscopy was employed to study the microstructural changes produced after cold working. Macrohardness measurements were also carried out on the samples.
2. Results and discussion
2.1. Changes in microstructure and hardness caused by cold work
The microstructures of annealed and cold-rolled (20%) 316LVM SS are shown in Fig. 1, which shows only austenitic grains with annealing twins. After cold rolling, the initially equiaxed grains were elongated along the rolling direction. The width of the grains changed, with a percentage reduction in the thickness. It was also observed that the length of the annealing twins increased after cold working. There were no visible slip traces/bands in the SS samples after 20% cold working. Fig. 2 shows the variations in hardness with respect to the cold rolling level. The reported hardness values are averages of six readings taken from different regions of the samples. As shown, the hardness increased on increasing the percentage reduction in the thickness, which was due to the work-hardening effect. The increase in hardness could be attributed to increases in both the dislocation density and the grain boundary density caused by cold working. | ||
| Fig. 1 Optical micrographs of 316LVM at 100× magnification, showing the austenitic microstructures: (a) 0% c.w., (b) 20% c.w. | ||
2.2. Weight loss
The weight loss of the AISI 316L and 316LVM alloys during immersion tests of up to 24 weeks is presented in Fig. 3. For all tests, six measurements were performed and average values of the mass loss were calculated. It can be seen from the figure that weight loss increases with time for both solutions. The surfaces of all tested samples were examined using both optical microscopy and SEM. No sign of localized corrosion was observed on any specimen. Therefore, the weight loss data were used to calculate the corrosion rates using eqn (1) (ASTM G31-72 (ref. 27)):
 | (1) |
 | ||
| Fig. 3 Weight loss versus immersion time at 37 °C for the tested SSs: (a) in Hank's solution, (b) in 3.5 wt% NaCl solution; a = annealed, b = 10% c.w., c = 20% c.w. | ||
| Sample | Corrosion rate (μm y−1) | |
|---|---|---|
| Hank's solution | NaCl solution | |
| a a = annealed, b = 10% c.w., c = 20% c.w. | ||
| 316L(a) | 2.919 | 4.590 |
| 316L(b) | 2.035 | 2.577 |
| 316L(c) | 1.752 | 2.384 |
| 316LVM(a) | 2.781 | 4.283 |
| 316LVM(b) | 1.808 | 2.479 |
| 316LVM(c) | 1.552 | 2.107 |
2.3. Electrochemical impedance spectroscopy (EIS)
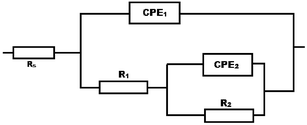
To investigate the relative stabilities of the passive films formed on the surface of different SSs, impedance spectra at the passive potential were recorded after passive film generation. Representative Bode plots (phase and modulus) and Nyquist plots of the recorded EIS spectra are shown in Fig. 4 for annealed and rolled samples at 37 °C in Hank's solution. All spectra were analyzed in terms of their equivalent electric circuits (EEC) presented in Fig. 5. When only one time constant was used in the modeling of the EIS spectra, large discrepancies between the experimental and modeled data were obtained during the fitting procedure. However, when the EEC presented in Fig. 5 was used, the agreement between the experimental (shown by point symbols) and modeled (shown by solid lines) data was admirable. Because the impedance data for a solid–electrolyte interface often reveal a frequency dispersion that is attributed to a “capacitive dispersion”, the capacitance is expressed in terms of the Constant-Phase Element (CPE).28 The impedance of the CPE is given by:
 | (2) |
The values of the electrochemical impedance parameters obtained from the fitting procedure are listed in Table 2. The EEC used describes the behavior of a process characterized by two time constants, namely the high-frequency (HF) time constant (CPE1 – R1) and the low-frequency (LF) time constant (CPE2 – R2). Based on the EEC parameter values obtained by fitting the experimental data and also based on the available literature,29–31 the physical meaning of the HF time constant can be associated with faster charging/discharging and charge-transfer processes, represented by the parallel combination of the double layer/space charge capacitance (CPE1) and the charge transfer resistance (R1) respectively. On the other hand, the LF time constant (CPE2 – R2) can be related to slower mass-transport processes in the oxide phase, namely diffusion of electro-active species through the formed passive film. Therefore, the HF time constant can be related to the properties of the electrode–electrolyte interface, while the LF time constant reflects the mass-transport processes through the formed passive oxide film.32 The CPE is a combination of properties related to both the surface and the electro-active species, and is independent of frequency. Use of the CPE is required due to distribution of relaxation times as a result of inhomogeneities present at the micro or nano (atomic/molecular) level, such as surface roughness/porosity, adsorption and diffusion.33
| Stainless steels | Rs (Ω cm2) | CPE1 (F cm−2) | n1 | R1 (Ω cm2) | CPE2 (F cm−2) | n2 | R2 (Ω cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|
| a a = annealed, b = 10% c.w., c = 20% c.w. | ||||||||
| 316L(a) | 3.20 | 2.93 × 10−6 | 0.87 | 106.3 | 2.58 × 10−5 | 0.81 | 3.29 × 104 | 0.0860 |
| 316L(b) | 4.39 | 1.37 × 10−6 | 0.81 | 128.6 | 2.69 × 10−5 | 0.87 | 3.99 × 104 | 0.0700 |
| 316L(c) | 4.11 | 3.33 × 10−6 | 0.95 | 132.3 | 3.33 × 10−5 | 0.89 | 4.57 × 104 | 0.0107 |
| 316LVM(a) | 3.73 | 3.58 × 10−6 | 0.98 | 109.1 | 3.24 × 10−5 | 0.76 | 3.66 × 104 | 0.0041 |
| 316LVM(b) | 2.76 | 4.30 × 10−6 | 0.82 | 164.9 | 3.45 × 10−5 | 0.70 | 4.54 × 104 | 0.0032 |
| 316LVM(c) | 4.28 | 4.88 × 10−6 | 0.87 | 182.2 | 4.30 × 10−5 | 0.84 | 6.35 × 104 | 0.0131 |
Depending on the value of exponent n, the physical meaning of the CPE can be related to pure capacitance (n = 1), pure resistance (n = 0), pure inductance (n = −1), or mass-transport related impedance, i.e. Warburg impedance (n = 0.25–0.5). The deviation of n from these theoretical values related to pure EEC elements specifies a deviation from the ideal behavior of the system. Since the value of n1 is very close to unity, the physical meaning of CPE1 as capacitance is thus justified. On the other hand, the value of n2 is in the region that characterizes mass-transport processes, and R2 can thus be associated with the mass-transport resistance. The mass-transport processes take place inside the passive film rather than in solution,29,30 and are related to the diffusion of metal and oxygen in the passive film. Hence, taking into account the physical meaning of the EEC, the sum of the two resistances is related to the total resistance of a surface to general corrosion. The results of the fitting procedure indicate that impedance measurements can detect the duplex nature of the oxide layer formed on these types of steels, composed of an inner chromium-rich film, which behaves as a p-type semiconductor, and an outer iron-rich film, which behaves as an n-type semiconductor.34–38 The values of impedance for 316LVM were higher than those for 316L, and for both samples they increased with increasing cold working; meanwhile the phase angle shifted toward higher frequencies. R2 represents the film resistance and R1 corresponds to the resistance inside the film pores. A comparison of the data in Table 2 shows that for the tested steels, the corrosion resistance (R1 and R2) of the oxide layers associated with the annealed samples was lower and increased with the degree of cold working. The total resistance of the surface to general corrosion was higher for cold worked samples. The resistance inside the film pores (R1) was smaller than the film resistance (R2). This may be explained by the fact that the electrolyte solution in the micropores or interstitials was partly short-circuited.39
2.4. Potentiodynamic polarization study
Fig. 6 shows the polarization curves for different SSs in Hank's solution at 37 °C. The figure shows that the polarization current was very low before pitting occurred, and then the current increased to a high level. Relevant polarization parameters were determined from the Tafel extrapolation of the polarization curves for the different steels, and their values are recorded in Table 3. It was observed that the values of Icorr decreased with increasing cold working, indicative of greater protection of the passive films as a result of cold working. Since the Icorr values are directly proportional to the corrosion rate i.e. the corrosion rate increases with Icorr. The Ecorr was more positive for 316LVM, and it increased with increasing cold working for both samples. Another important requirement for selecting a material for biomedical application is to determine the breakdown potential (Ebd), i.e., the potential at which the passive film breaks and the anodic current significantly increases. In fact, in the presence of chloride ions, the passive layer becomes unstable at this potential and it produces a localized rapid attack on the metal with the formation of pitting. In this study, the minimum breakdown potential was determined for annealed 316L and increased with the degree of cold working. These results are in close agreement with the results obtained from the weight loss and EIS studies. | ||
| Fig. 6 Polarization curves for stainless steels in Hank's solution at 37 °C: (a) for 316L, (b) for 316LVM; a = annealed, b = 10% c.w., c = 20% c.w. | ||
| Stainless steels | Ecorr (V vs. SCE) | Icorr (μA cm−2) | βc (V dec−1) | βa (V dec−1) | Ebd (V vs. SCE) |
|---|---|---|---|---|---|
| a a = annealed, b = 10% c.w., c = 20% c.w. | |||||
| 316L(a) | −0.654 | 5.187 | 5.546 | 3.202 | 0.394 |
| 316L(b) | −0.558 | 3.664 | 6.366 | 4.282 | 0.404 |
| 316L(c) | −0.526 | 3.098 | 5.909 | 4.336 | 0.439 |
| 316LVM(a) | −0.573 | 3.989 | 5.626 | 4.172 | 0.440 |
| 316LVM(b) | −0.512 | 2.673 | 5.551 | 4.414 | 0.506 |
| 316LVM(c) | −0.433 | 2.364 | 5.597 | 4.559 | 0.537 |
2.5. Scanning electron microscopy (SEM) analysis
At the end of the polarization tests, the surfaces of all tested samples were observed by SEM using an FEI Quanta 200FEG microscope in order to analyze the pitting corrosion on the surfaces of the SSs. Fig. 7 shows the SEM micrographs of the different corroded surfaces after the polarization tests in Hank's solution. The SEM micrographs confirmed the pitting corrosion on the surfaces of the SSs. Pits were in fact found on all the steel sample surfaces, but shallower and smaller pits were associated with cold worked samples as compared to annealed samples. Thus, it is evident that the SEM results are in good agreement with the weight loss, EIS and potentiodynamic polarization results.2.6. X-Ray photoelectron spectroscopy (XPS) analysis
The results of surface analysis by XPS of the passive films of annealed and 20% cold worked 316LVM SS are shown in Fig. 8. Iron, chromium, molybdenum, oxygen and nitrogen were the species detected in the survey scan, while nickel and manganese were absent. The O 1s peak resolved into oxide (ox) and hydroxide (hy) at binding energy values of 530.3 and 531.8 eV respectively. The Fe 2p3/2 peak resolved into its oxide and hydroxide forms with binding energies 711.04 and 712.9 eV respectively, and the Cr 2p3/2 peak also resolved into oxide and hydroxide forms with binding energies 576.7 and 578.3 eV respectively. The film of Cr and Fe is made up of two layers—an oxide and a hydroxide. A bilayer passive film composed of oxide and hydroxide was also proposed by Raja et al.40 Elemental peaks were not detected for Fe and Cr. Nickel and manganese were absent from both samples. The wt% of Mn is much less in these steels, but Ni is present in sufficient amounts; however, Ni was not obtained in the passive films. The absence of nickel from the films agrees with the work of Halada et al.41 and Phadnis et al.22 on SSs. A study by Chen et al.42 on SS304 showed that the film contained about 2–3% nickel. The films analyzed here showed no nickel peaks for annealed as well as 20% cold worked samples. It is assumed that nickel is selectively leached out of the film43 or is enriched in the substrate.41 Phadnis et al.22 reported that nickel does not leach out of the film, but chromium and iron diffuse out preferentially to form the passive film due to their high oxygen affinity, while nickel is enriched in the substrate. Mo4+ and Mo6+ oxide peaks were obtained for both samples along with elemental peaks. An additional elemental peak, Mo 3d3/2, was also obtained for the 20% cold worked sample, indicating a slightly higher percentage of Mo on the cold worked surface. The oxide peaks were stronger for the 20% cold worked specimen in comparison with the annealed one. For the 20% cold worked specimen: ICr-ox/ICr-hy = 1.35, IFe-ox/IFe-hy = 1.29; for the annealed sample: ICr-ox/ICr-hy = 1.23, IFe-ox/IFe-hy = 1.25. The lower protection of the passive film for the annealed specimen might be due to the presence of more hydroxides, which can lead to impairment of the localized corrosion resistance.44 Similar results were also obtained by Yao Fu et al.45 for high nitrogen steels in 3.5 wt% NaCl and 0.5 M H2SO4 + 0.5 M NaCl solutions. The more stable and protective passive film thus increases the resistance, resulting in improved corrosion resistance for cold worked samples in Hank's solution. | ||
| Fig. 8 The X-ray photoelectron spectra recorded for the passive film of 316LVM SS, (a) annealed and (b) 20% cold worked. | ||
Pit initiation is closely related to passive film stability and to non-metallic inclusions acting as triggers for future pits. In general, spontaneous passivation combined with minimum dissolution occurs for SSs in a neutral chloride medium, in contrast to their active passive behavior in an acidic chloride medium. It is well known that rolling results in a preferential texture of high index low density planes along the rolling direction of austenitic SS. The effect of this texture is a reduced binding energy of the atoms in this less close packed plane, which can result in enhanced dissolution in an acidic chloride medium, where the metal dissolution step plays a dominant role in the rate control mechanism. This is not the case in a neutral chloride solution, where oxygen diffusion is the rate-controlling step. This difference could be partially due to differences in the materials and solution systems investigated. Enhanced surface diffusion due to increased density of defects could have contributed to the stable passive film formation at 20% cold working, leading to improvements in pitting resistance.46 Moreover, it was observed by some researchers that the chromium content in the passive film formed on a deformed austenitic SS surface was richer than that formed on heat treated samples, and the higher chromium content improved the corrosion resistance of passive films.22 Hamdy et al.47 demonstrated that the surface of 23% cold deformed austenitic SS had a smooth appearance and a uniform distribution of the surface oxide layer. The presence of a uniformly distributed surface oxide layer decreases the surface defects at which active anodic sites can be formed, which is the main source of localized corrosion. 316LVM was observed to be more corrosion resistant than 316L in annealed condition. Similar results were obtained in our previous study.48 Inclusions on the surface of SSs accelerate pitting. 316LVM is a vacuum melted alloy (VM = vacuum melted), and so has less inclusions compared to 316L. Hence, the amounts of elemental impurities such as S, P and oxides are lower in 316LVM steels, and thus the nucleation sites for pitting, such as MnS inclusions, are probably reduced, which decreases the susceptibility to pitting attacks.4,49
3. Experimental
3.1. Material
The SSs selected for study were obtained from Mishra Dhatu Nigam Ltd. (MIDHANI), Hyderabad, India. These steels were received in hot rolled and annealed conditions. The chemical compositions of the steels are given in Table 4.| Sample | C | Cr | Mn | Ni | Si | Mo | S | P | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 316L | 0.021 | 17.19 | 1.56 | 14.0 | 0.71 | 2.77 | 0.004 | 0.015 | 0.060 | Balance |
| 316LVM | 0.021 | 17.24 | 1.68 | 14.42 | 0.24 | 2.83 | 0.004 | 0.007 | 0.07 | Balance |
3.2. Cold working and microstructural observations
Annealed specimens of 80 mm × 12 mm × 2 mm were cut with the help of a power saw machine using water as a coolant, and cold rolled to 10% and 20% reductions in thickness at room temperature using a simple rolling mill. Specimens of size 10 mm × 10 mm were cut from them, polished successively with emery papers of 600, 800, 1000 and 1200 grades, mirror polished using 0.3 μ alumina and etched in aqua regia until the grain boundaries were revealed for observing the changes in the microstructure due to cold working. Hardness values for both the annealed and cold worked (c.w.) specimens were measured using a Vickers hardness tester (LECOR Vickers Hardness Tester LV700 AT) at 10 kg load.3.3. Immersion tests
For the immersion tests, specimens of 20 mm × 10 mm with their original thickness were cut from annealed and rolled strips with a 1.5 mm-diameter hole at one end, polished successively with emery papers of 600, 800, 1000 and 1200 grades, washed with deionized water followed by acetone and dried. Each coupon was weighed on a Mettler Toledo JB1603 balance with a precision of 0.001 mg just before immersion in the solution. After removing the samples from the test solutions, they were rinsed with deionized water and ethanol, and dried in air, and then the final weight loss was measured using the same scales.The beakers used for the immersion tests were soaked for one day in a 5 vol% concentrated HNO3 solution. They were then rinsed with deionized water until the surface was no longer slippery and immersed in deionized water for another 24 h. Finally, the beakers were rinsed five times and placed in a drying oven at 180 °C for 3 h to remove any possible bacteria. This procedure was followed by ASTM standard D5245-92.50
Each coupon of SS was hung by fluorocarbon plastic strings in a separate beaker and fully immersed in 120 ml of solution. Two types of solution were used for the immersion tests, SBF and 3.5 wt% NaCl solutions, both prepared in deionized water. The SBF used in this study was Hank's solution of pH 7.4 ± 0.2.51 The chemicals used to prepare the solutions were of analytical reagent grade and were used without further purification. All the beakers, covered with perforated rubber cork, were placed inside a thermostat for 12 to 24 weeks at 37 °C, which is equivalent to human body temperature.52 The water levels in the beakers were checked every three days and deionized water was added if the water levels decreased.
3.4. Electrochemical corrosion tests
Potentiodynamic polarization curves were obtained by changing the electrode potential automatically from −1.2 to 2 V versus EOC at a scan rate of 0.01 V s−1. A constant distance of approximately 1–2 mm between the tip of the luggin capillary and the working electrode surface was maintained throughout the experiment. The current was passed through the cell at different predetermined values through the counter electrode, and the potential between the working and reference electrodes was measured. Potential values at a given current were found to be generally constant for 1 min. The corrosion potential (Ecorr) and corrosion current density (Icorr) as well as other parameters were automatically extracted from the polarization curves using the Tafel extrapolation. All the measurements were repeated at least three times and a fresh solution was used for each new test. Finally, SEM was used to investigate the surface morphology of the stainless steels after the polarization studies.
3.5. X-Ray photoelectron spectroscopy (XPS) analysis
XPS (AMICUS, Kratos Analytical, Shimadzu, U.K.) was used to detect the chemical compositions of the passive films formed on the working electrodes after 1 h of immersion in Hank's solution. The Mg Kα line was used as the X-ray source. Survey spectra, together with high resolution Fe 2p, Cr 2p, Mo 3d, O 1s, N 1s and C 1s regions, were recorded. The C 1s peak was assumed to be at 285.4 eV and was used as an internal standard to determine the binding energies of the other photoelectron peaks. Linear background subtraction was carried out to obtain the XPS signal intensities.4. Conclusions
The effects of prior cold work on the long term and electrochemical corrosion of SSs in SBF were investigated. The following conclusions were drawn:• The macrohardness of the SSs increases with the degree of deformation applied in the process.
• The weight loss and corrosion rate were higher for 3.5 wt% NaCl solution and decreased with increasing degree of cold working for both solutions, i.e. 3.5 wt% NaCl and SBF.
• The EIS results indicated the formation of a larger protective oxide layer on the surface of the cold worked samples, which was demonstrated by the higher values of R1 and R2.
• As the cold working of the alloys increased, improvements in the pitting resistance were observed. The rolled materials showed lower passive current densities, while the annealed materials showed passive current densities more than one order of magnitude higher. The corrosion potentials of the cold worked SSs were more positive.
• As for the pit morphologies, shallower and smaller pits were associated with cold worked samples as compared to annealed samples.
• The XPS results showed that the main elements in the oxide layers on steels are Cr, Fe and Mo. The Cr-ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr-hy ratio and the Fe-ox
Cr-hy ratio and the Fe-ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe-hy ratio were observed to be higher for the rolled materials than for the annealed materials, indicating that the passive films on rolled materials are more stable and protective, which improves the corrosion resistance.
Fe-hy ratio were observed to be higher for the rolled materials than for the annealed materials, indicating that the passive films on rolled materials are more stable and protective, which improves the corrosion resistance.
Acknowledgements
One of the authors, Mohd Talha is pleased to acknowledge the financial support of a Senior Research Fellowship provided by the University Grant Commission (UGC), New Delhi, India.References
- K. Hayashi, in Biomaterial Science, ed. Ohmu-sha, JSME, Tokyo, 1993, p. 1 Search PubMed.
- M. Sumita, Orthop. Surg., 1997, 48, 927 Search PubMed.
- A. Yamamoto, Y. Kohyama, D. Kuroda and T. Hanawa, Mater. Sci. Eng., C, 2004, 24, 737–743 CrossRef PubMed.
- A. Shahryari, S. Omanovic and J. A. Szpunar, Mater. Sci. Eng., C, 2008, 28, 94–106 CrossRef CAS PubMed.
- Z. Szklarska-Smialowska, Corrosion, 1971, 27, 223 CrossRef CAS.
- A. J. Sedriks, Corrosion of Stainless Steels, John-Wiley and Sons, New York, 1979 Search PubMed.
- A. J. Sedriks, in Proceedings of the International Conference on Stainless Steels 85, The Institute of Metals, London, 1985, p. 125 Search PubMed.
- J. W. Simmons, Mater. Sci. Eng., A, 1996, 207, 159 CrossRef.
- Y. J. Oh and J. H. Hong, J. Nucl. Mater., 2000, 278, 242 CrossRef CAS.
- T. H. Lee, C. S. Oh, S. J. Kim and S. Takaki, Acta Mater., 2007, 55, 3649 CrossRef CAS PubMed.
- D. C. Cook, Metall. Trans. A, 1987, 18, 201 CrossRef.
- R. Stefec and F. Franz, Corros. Sci., 1978, 18, 161 CrossRef CAS.
- K. Elayaperumal, P. K. De and J. Balachandra, Corrosion, 1972, 28, 269 CrossRef CAS.
- R. K. Dayal, N. Parvathavarthini and J. B. Gnanamoorthy, in Proceedings of the Ninth International Congress on Metallic Corrosion, National Research Council, Canada, Toronto, 1984, vol. 3, p. 141 Search PubMed.
- G. Salvago, G. Fumagalli and D. Sinigaglia, Corros. Sci., 1983, 23, 515 CrossRef CAS.
- B. Mazza, et al., Corros. Sci., 1979, 19, 907 CrossRef CAS.
- U. K. Mudali, N. Parvathavarthini, R. K. Dayal and J. B. Gnanamoorthy, Trans. Indian Inst. Met., 1988, 41, 35 Search PubMed.
- P. Forchhammer and H. J. Engell, Werkst. Korros., 1969, 20, 1 CrossRef CAS.
- A. Randak and F. W. Trautes, Werkst. Korros., 1970, 21, 97 CrossRef CAS.
- C. O. A. Olsson and D. Landolt, Electrochim. Acta, 2003, 48, 1093–1104 CrossRef CAS.
- I. Milosev and H. H. Strehblow, J. Biomed. Mater. Res., 2000, 52, 404–412 CrossRef CAS.
- S. V. Phadnis, A. K. Satpati, K. P. Muthe, J. C. Vyas and R. I. Sudaresan, Corros. Sci., 2003, 45, 2467 CrossRef CAS.
- F. Navai and O. Debbouz, J. Mater. Sci., 1999, 34, 1073 CrossRef CAS.
- F. Navai, J. Mater. Sci., 1995, 30, 1166 CrossRef CAS.
- A. Barbucci, G. Cerisola and P. L. Cabot, J. Electrochem. Soc., 2002, 149, B534 CrossRef CAS PubMed.
- Y. Fu, X. Q. Wu, E. H. Han, W. Ke, K. Yang and Z. H. Jiang, J. Electrochem. Soc., 2008, 155, C455 CrossRef CAS PubMed.
- ASTM Standard G31, 2004, Standard Practice for Laboratory Immersion Corrosion Testing of Metals, ASTM International, West Conshohocken, PA, 1972, DOI:10.1520/G0031-72R04, http://www.astm.org.
- J. B. Jorcin, M. E. Orazem, N. Pebere and B. Tribollet, CPE analysis by local electrochemical impedance spectroscopy, Electrochim. Acta, 2006, 51, 1473–1479 CrossRef CAS PubMed.
- S. Omanovic and S. G. Roscoe, Langmuir, 1999, 15, 8315 CrossRef CAS.
- S. Omanovic and S. G. Roscoe, J. Colloid Interface Sci., 2000, 227, 452 CrossRef CAS PubMed.
- C. M. Abreu, M. J. Cristobal, R. Losada, X. R. Novoa, G. Pena and M. C. Perez, J. Electroanal. Chem., 2004, 572, 335 CrossRef CAS PubMed.
- Z. B. Saleh, A. Shahryari and S. Omanovic, Thin Solid Films, 2007, 515, 4727–4737 CrossRef PubMed.
- E. N. Flores, Z. Chong and S. Omanovic, J. Mol. Catal. A: Chem., 2005, 226, 179 CrossRef PubMed.
- K. Azumi, T. Ohtsuka and N. Sato, Trans. Jpn. Inst. Met., 1986, 27, 382 CAS.
- H. Ge, G. Zhou and W. Wu, Appl. Surf. Sci., 2003, 211, 321 CrossRef CAS.
- H. Y. Ha, H. Jang, H. S. Kwon and S. J. Kim, Effects of nitrogen on the passivity of Fe–20Cr alloy, Corros. Sci., 2009, 51, 48–53 CrossRef CAS PubMed.
- T. L. Sudesh, L. Wijesinghe and D. J. Blackwood, Photocurrent and capacitance investigations into the nature of the passive films on austenitic stainless steel, Corros. Sci., 2008, 50, 23–34 CrossRef PubMed.
- Y. X. Qiao, Y. G. Zheng, W. Ke and P. C. Okafor, Electrochemical behaviour of high nitrogen stainless steel in acidic solutions, Corros. Sci., 2009, 51, 979–986 CrossRef CAS PubMed.
- Z. Grubac and M. Metikos-Hukovic, J. Electroanal. Chem., 2004, 565, 85 CrossRef CAS PubMed.
- V. S. Raja, P. Veluchamy and H. Minoura, Corrosion, 1999, 55, 1119 CrossRef CAS.
- G. P. Halada, D. Kim and C. R. Clayton, Corrosion, 1996, 52, 36 CrossRef CAS.
- G. Chen, S. V. Kagwade, G. E. French, T. E. Ford, R. Mitchel and C. R. Clayton, Corrosion, 1996, 52, 891 CrossRef CAS.
- S. Jin and A. Atrens, Appl. Phys. A: Solids Surf., 1988, 46, 51 CrossRef.
- C. R. Clayton and Y. C. Lu, J. Electrochem. Soc., 1986, 133, 2465 CrossRef CAS PubMed.
- Y. Fu, X. Wu, E. H. Han, W. Ke, K. Yang and Z. Jiang, Electrochim. Acta, 2009, 54, 1618–1629 CrossRef CAS PubMed.
- U. K. Mudali, P. Shankar, S. Ningshen, R. K. Dayal, H. S. Khatak and B. Raj, Corros. Sci., 2002, 44, 2183–2198 CrossRef.
- A. S. Hamdy, E. El-Shenawy and T. El-Bitar, Mater. Lett., 2007, 61, 2827–2832 CrossRef CAS PubMed.
- M. Talha, C. K. Behera and O. P. Sinha, J. Chem. Pharm. Res., 2012, 4, 203–208 CAS.
- Z. S. Yu, Applications of Rare Earths in Iron and Steel, Metallurgical Industry Press, Beijing, 1987, p. 282 Search PubMed.
- ASTM Standard D5245, 2005, Standard Practice for Cleaning Laboratory Glassware, Plasticware, and Equipment used in Microbiological analyses, ASTM International, West Conshohocken, PA, 1992, DOI:10.1520/D5245-92R05, http://www.astm.org.
- I. Gurappa, Mater. Charact., 2002, 49, 73–79 CrossRef CAS.
- H. Yang, K. Yang and B. Zhang, Mater. Lett., 2007, 61, 1154–1157 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |