Synergetic effect of ferrocene and MoS2 in polystyrene composites with enhanced thermal stability, flame retardant and smoke suppression properties
Keqing Zhou,
Qiangjun Zhang,
Jiajia Liu,
Biao Wang,
Saihua Jiang,
Yongqian Shi,
Yuan Hu* and
Zhou Gui*
State Key Laboratory of Fire Science, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, People's Republic of China. E-mail: yuanhu@ustc.edu.cn; zgui@ustc.edu.cn; Fax: +86-551-3601664; Fax: +86-551-3601669; Tel: +86-551-63601664 Tel: +86-551-63601288
First published on 28th February 2014
Abstract
As a graphene-like layered nanomaterial, molybdenum disulfide (MoS2) has gained intensive attention from the materials community. In our research, MoS2 is firstly modified with ferrocene (Fe–MoS2) on a large scale and then is used as a nanofiller to prepare PS composites by a masterbatch-based melt blending method. The aim of our present study is to study the synergistic effect of ferrocene and MoS2 on the thermal stability, fire resistance and smoke suppression properties of the PS composites. It was found that the thermal stability of the PS composite was obviously enhanced upon the introduction of 3.0 wt% Fe–MoS2. The cone test results indicated that the PS/Fe–MoS2 composites exhibited superior flame retardance to PS/MoS2 and PS/ferrocene composites. Furthermore, the addition of Fe–MoS2 could improve the smoke suppression properties of PS composites, as evidenced by the reduction of the carbon monoxide production rate and smoke production rate (SPR). The total flammable gaseous products from the PS composites were decreased which further led to the inhibition of smoke. Such a significant improvement in thermal stability, fire resistance and smoke suppression properties was mainly attributed to good dispersion of the modified MoS2 nanosheets, synergistic effects between ferrocene and MoS2 nanosheets, physical barrier effects of MoS2 nanosheets and the presence of ferrocene and MoS2 can promote char formation simultaneously.
1. Introduction
Since its discovery in 2004, graphene, a two-dimensional (2D) monolayer of carbon atoms with unique properties and promising applications in many fields, has become one of the most studied nanomaterials.1 Recent progress in understanding 2D ordered crystals of graphene has attracted more and more researchers to the nanosheet field. Apart from graphene, various 2D inorganic layered nanomaterials such as transition metal dichalcogenides (TMDs, e.g. MoS2 and WS2), transition metal oxides (TMOs) and hexagonal boron nitride (h-BN) have been attracting intensive attention. Among these 2D layered materials, MoS2 is one of the most attractive and has a similar structure with graphite. The basic unit of MoS2 is composed of a molybdenum atom coordinated with six sulfur atoms. It is organized in two layers of sulfur atoms forming a sandwich structure, with a layer of molybdenum atoms in the middle. The bulk MoS2 is formed of these 2D layers held together by van der Waals forces. It is well known that MoS2 has been applied in many fields such as lithium ion batteries, photocatalysts, sensors and conductive fillers in polymer composites which due to their unmatched properties.2–8Polymer-based composites are one of the most promising applications of layered nanomaterials. As is well known, 2D layered nanomaterials such as clay, graphene and layered metallic phosphate usually obviously improve the properties of polymers, including strength, modulus, thermal stability and gas barrier effects.9,10 Due to the various advantages over conventional materials, such as low density, high mechanical properties and high chemical resistance, the need for synthetic polymers is increasingly urgent. However, the inherent flammability limits many potential applications for safety considerations. The development of new polymeric materials with enhanced thermal stability and fire resistance properties has been an active area of research to improve public safety.11
Fire retardation of polymers can be achieved through the use of conventional fire retardants, such as halogenated organic compounds, organophosphorous compounds, aluminium trihydrate, magnesium hydroxide or intumescent systems. But these fire retardant systems usually exhibit their own obvious drawbacks, a very high filler loading within the polymer matrix is needed for the application of aluminium trihydrate and magnesium hydroxide which usually have an adverse effect on the mechanical properties of the treated polymers, in addition, many of the conventional fire retardant additives trigger environmental and toxicity concerns. Therefore, it is rather crucial to explore nontoxic and environment friendly flame retardancy approaches for polymer materials. In recent years, polymer nanocomposites which could avoid the disadvantages associated with traditional fire retardant systems have been reported to significantly improve thermal and combustion properties of polymers only by adding low loading of nanoscale additives, offering an alternative to conventional flame retardants.11,12 Therefore, a feasible way to improve thermal stability and flame retardancy of polymer is to prepare polymer nanocomposites by incorporating some nanofillers. It has become a prominent area of current research and development. The motivation for such popularity mainly stems from the combination effect of nanofillers and polymer matrix which leads to significantly enhanced mechanical, optical, thermal, electronic, or magnetic properties.13,14 These performances are obtained, not only by using the inherent properties of the nanofiller, but more importantly by optimizing the dispersion, interface chemistry and nanoscale morphology to take advantage of the enormous surface area per unit volume that nanofillers have. One of the most important factors for polymer nanocomposites is attributed to the compatibility between the nanofiller and the polymeric matrix which determines the dispersion and interface interaction of constitutive phases and further influences the macroscopic property.15 However, uniform dispersion of filler particles (especially for nanoparticles) in polymer matrices is a challenge for the preparation of nanocomposites. Therefore, large efforts have been concentrated on achieving a homogeneous and well-dispersed system by developing either covalent or non-covalent functionalization of the filler surface. In the early ages of the polymer nanocomposites research, layered silicates and layered double hydroxides (LDHs) are the most widely investigated.16–21 However, the discovery of graphene with its combination of extraordinary physical properties and ability to be dispersed in various polymer matrices has created a new class of polymer nanocomposites.22,23
MoS2 had been incorporated into many polymer matrixes such as polyaniline, polythiophene, polypyrrole and poly[oxymethylene-(oxyethylene)] to prepared polymer nanocomposites.7,24–26 As far as we know, these literatures are mainly concentrated on their conductive performance. Few of them have dealed with the thermal, mechanical, fire safety and smoke suppression properties of the composites. As a typical layered inorganic material, MoS2 is expected to disperse and exfoliate in polymers and results in the physical barrier effects which inhibit the diffusion of heat and the decomposition products of the polymer and also the transition metal element Mo promotes the formation of the charred layer acting as a physical barrier which could slow down heat and mass transfer during the burning. So it is reasonable that MoS2 may improve the thermal stability and fire resistance of polymer-based composites just like montmorillonite (MMT), LDHs and graphene. In our previous work, MoS2 had been incorporated into poly (vinyl alcohol) by solution blending method and the thermal stability, fire resistance and mechanical properties are improved apparently comparing with pure PVA.27 Wilkie et al. added MoS2 nanoparticles into PS and PMMA matrix to prepare polymer nanocomposites and the thermal stability, fire resistance and mechanical properties are also improved apparently comparing with pure polymer.28 The thermal stability, flame retardancy and smoke suppression properties of polystyrene composites containing molybdenum disulfide and graphene have been compared in our previous work, it indicates that the thermal stability and smoke suppression properties of the PS/MoS2 composites are higher than PS/GNS composites, which are mainly ascribe to the physical barrier effect, the strong charring effect and inbibitional effect of MoS2 in PS matrix during combustion.29
Polystyrene is a general thermoplastic manufactured on a very large scale due to its outstanding properties, such as low density, excellent mechanical durability, thermal resistance, and convenience of processing and molding. However, its high flammability, severe melt-dripping and release a large amount of smoke during combustion greatly limit its application in some areas. In addition, it is well known that in most cases the real killer in fires is not the heat of the fire itself but the smoke and volatiles produced. The smoke, smoke particulates and some toxic compounds (especially carbon monoxide) produced during the course of a real fire are known to cause more than 70% of the fatality. Therefore, reducing the amount of smoke formed during burning will be significant for saving lives in real fires. One of the most important factors determining the tendency of a hydrocarbon-oxygen diffusion flame to release smoke is the molecular structure of the fuel. In general, the extent of smoke formation increases with increasing carbon–hydrogen ratio of the fuel, so that aromatic hydrocarbons produce copious quantities of smoke. In accordance with this, polymers such as polystyrene with aliphatic chains and aromatic side groups, which decompose to yield predominantly aromatic hydrocarbons, produce very large amounts of smoke. In order to reduce the release volume of smoke during combustion, various alloys, organic substances, and inorganic compounds, including compounds of antimony, zinc, copper, especially iron and molybdenum, have been widely used to act as flame retardants and smoke suppressants for organic polymers. Skinner reported that some of the very promising results that have been obtained using molybdenum compounds as flame-retardants and smoke-suppressants in halogenated polymers.30 Ferrocene and ferrocene derivatives had been synthesized and incorporated into PVC to improve the flame-retardancy and smoke-suppression properties of the composites.31,32 The metallocenes such as chromocene, manganocene, ferrocene, cobaltcene and nickelocene are proved to be an excellent flame suppressant.33 Ferrocene and ferrocenium-containing salts were employed to modify montmorillonite and then prepared nanocomposites of polystyrene and ethylene vinyl acetate copolymers which can improve the thermal stability and fire resistance properties.34
According to a report in literatures, non-electron donors such as ferrocene having a high ionization potential (6.88 eV), were shown to be intercalated into MoS2 by restacking of exfoliating MoS2.35 Therefore, we are interested in inclusion of ferrocene into MoS2 get new intercalation compounds having widely ranging characteristics. Inspired by the effectiveness of ferrocene and molybdenum in flame retardant and smoke suppression of the polymer, a novel intercalation compounds containing ferrocene and MoS2 (Fe–MoS2) were prepared and used as flame retardant and smoke suppression agents to improve the fire resistance and smoke suppression properties of the PS composites. To the best of our knowledge, there are no reports about using Fe–MoS2 to improve the thermal stability, fire resistance and smoke suppression properties of the polymer composites.
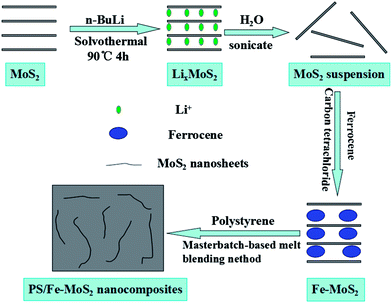
In the present work, the ferrocene were firstly intercalated into MoS2 layers to prepare Fe–MoS2 by a method of exfoliation followed by its restacking and then the Fe–MoS2 were used to prepare PS/MoS2 composites by a masterbatch-based melt blending method. The synthetic route of the PS/MoS2 nanocomposites is shown in Scheme 1. The thermal stability, fire resistance and smoke suppression properties of the resulting materials are investigated. This work aims to study the synergetic effect of ferrocene and MoS2, on the thermal stability, fire retardation behavior and smoke suppression properties of PS composites which are evaluated by thermogravimetric analysis, cone calorimetry, and thermogravimetric analysis/infrared spectrometry (TG-IR). Additionally, mechanisms of the improved properties are discussed.
2. Materials and experimental method
2.1 Materials
PS (158 K) was obtained from BASF-YPC Co., Ltd. (China), molybdenum disulfide (MoS2, AP), ferrocene, carbon tetrachloride and n-hexane (AP) removing the moisture with the coronizing molecular sieve were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). The n-butyl lithium (2.2 M in hexane) was purchased from Alfa Aesar without further purified. The distilled water was produced in our laboratory.2.2 Preparation of LixMoS2
LixMoS2 was prepared by the solvothermal methodology, similar to our previous work.27 In a typical experiment, 1.0 g MoS2 was put into the autoclave and 36 mL 0.5 M solution of n-butyl lithium in hexane was then added. The autoclave was tightly sealed and heated at 100 °C for 4 h. The product was filtered, washed with anhydrous hexane, and then dried at 60 °C in vacuum oven.2.3 Preparation of Fe–MoS2
Exfoliation of MoS2 was achieved via the rapid hydrolysis and ultrasonication of LixMoS2. In a typical reaction, 0.5 g LixMoS2 was hydrolysed in 1 L water, and ultrasonicated at ambient temperature for 4 h to produce a colloidal suspension of single or few MoS2 layers. Ferrocene were dissolved in carbon tetrachloride to form saturated solution and then react with the prepared MoS2 suspension by vigorous stirring 24 h.35 The Fe–MoS2 were collected after being vacuum filtration, washed with carbon tetrachloride for several times and then dried in vacuum at 60 °C for 24 h.2.4 Preparation of PS composites
PS/Fe–MoS2 composites containing 3% Fe–MoS2 were prepared by a masterbatch-based melt blending method. The masterbatch was prepared by solvent blending, described in our earlier work.29 Scheme 1 shows the synthesis strategy. All of the materials were dried in an oven at 80 °C for 12 h before use. They were melted and mixed in a twin-roller mixer (Kechuang Machinery Co., Ltd., Shanghai, China) for 10 min. The temperature of the mixer was maintained at 190 °C, and the roller speed was 100 rpm. The composites were pressed into sheets in a 1.00 MN semiautomatic molding press (HPC-100D, Xima Weida Machinery Co., Ltd., Shanghai, China). The resulting composites were hot-pressed into sheets of suitable thickness and size for characterization. The PS/ferrocene and PS/MoS2 composites were prepared in a similar procedure.2.5 Characterization
X-ray diffraction (XRD) was performed using a Japan Rigaku D/Max-Ra rotating-anode X-ray diffractometer equipped with a Cu Kα tube and a Ni filter (λ = 0.1542 nm). Transmission electron microscopy (TEM, JEM-2100F, Japan Electron Optics Laboratory Co., Ltd.) was employed to investigate the micro-morphology of the PS nanocomposites. Before observation, PS nanocomposites were cut into ultrathin sections using a CM1900 microtome (Leica, Wetzlar, Germany). The ultrathin sections were transferred from liquid nitrogen to carbon-coated copper grids and then observed by TEM. Thermogravimetric analysis (TGA) was carried out using a Q5000 thermoanalyzer instrument (TA Instruments Inc., New Castle, DE) under air flow of 25 mL min−1. In each case, the samples were heated from room temperature to 800 °C at a linear heating rate 20 °C min−1. Samples were measured in an alumina crucible with a mass of about 5.0 mg. The samples were run in triplicate; the temperature reproducibility of the instrument is ±1 °C, while the mass reproducibility is ±0.2%. Flammability of the samples was characterized using a cone calorimeter (Fire Testing Technology, UK) according to ISO 5660. Square specimens (100 × 100 × 3 mm3) were irradiated at a heat flux of 35 kw m−2, corresponding to a mild fire scenario. All the measurements were repeated three times and the results were averaged. The thermogravimetric analysis/infrared spectrometry (TG-IR) of the samples was performed using a TGA Q5000 IR thermogravimetric analyzer that was interfaced to the Nicolet 6700 FTIR spectrophotometer through a Thermo-Nicolet TGA special connector. The stainless steel transfer pipe and gas cell were heated at 200 °C, to avoid the condensation of volatile compounds. Each sample specimen (about 5 mg) was carried out at a heating rate of 20 °C min−1 from room temperature to 700 °C under a nitrogen flow at a flow rate of 35 mL min−1.3. Results and discussion
3.1 Characterization of Fe–MoS2
To confirm the successful intercalation of ferrocene into the MoS2, XRD analysis was conducted on the pristine and Fe–MoS2 nanosheets. XRD patterns of MoS2 and Fe–MoS2 are shown in Fig. 1. An intense reflection at 2θ = 14.48° (corresponding interlayer distance was 0.62 nm) was observed for unmodified MoS2, which was attributed to the (002) plane of MoS2. In comparison with bulk MoS2, it can be noted that the (001) peak of Fe–MoS2 has shifted to 2θ = 7.43°, which corresponds to d-spacing of 1.18 nm. It has been generally accepted that the expansion of the interlayer space is due to successful intercalation ferrocene molecules into MoS2.35In addition, TGA was carried out to provide further evidence for successful intercalation of ferrocene into MoS2. Fig. 2 shows the TG and DTG curves of the bulk MoS2 and Fe–MoS2 under air atmosphere at a heating rate of 20 °C min−1. The total weight loss of MoS2 is 11 wt% when it is heated to 700 °C. This value is close to the weight loss of the sulfur dioxide molecules (10 wt%), it means that the MoS2 transforms to molybdenum oxide and sulfur oxide under air atmosphere in heating process and reaches the maximum rate at about 510 °C.27 Comparing the TG curve of MoS2 with that of ferrocene modified MoS2 samples, it can be observed that the resulting Fe–MoS2 hybrid exhibits a similar thermogravimetric profile and the mass loss of the modification MoS2 sample in the temperature range of 100–700 °C are much larger than that of bulk MoS2. The temperature of the maximum mass loss rate is in advance to 450 °C and the total weight loss reaches 23 wt% at 700 °C. The mass loss of the ferrocene modified MoS2 in this temperature range should be attributed to the evaporation/decomposition of the loaded ferrocene and the MoS2 transforms to molybdenum oxide and sulfur oxide under air atmosphere in heating process. Based on the information above, it is reasonable that ferrocene have been successfully intercalated into MoS2.
3.2 Morphology and structure
The morphology and structure of polymer/layered inorganic nanocomposites are generally characterized by XRD and TEM analysis. The XRD technique was used to characterize the crystallographic structure of the MoS2 nanosheets and their dispersed homogeneity in the PS. Fig. 3 shows the XRD patterns of the virgin PS, PS/ferrocene, PS/MoS2, and PS/Fe–MoS2 composites. The virgin PS exhibits a broad crystalline peak at around 10° and 20°. For the PS/ferrocene system, it displays similar characteristics diffraction peaks to PS matrix without other diffraction peaks. In contrast, the strongest diffraction peak (002) of the MoS2 is still observed in the XRD patterns of PS/MoS2 composite except for the characteristics diffraction peaks of the PS matrix, indicating the presence of stacked structure of MoS2. But for the PS/Fe–MoS2 system, the characteristic peak of pure PS can also be observed. Moreover, almost all the peaks of the MoS2 in PS matrix disappeared comparing with the original inorganic sample except for the (002) peak. The intensity of the (002) peak is still seen in PS/Fe–MoS2 composites, and the peak position does not change. However, the intensity is obviously decreased. It indicates that some of the Fe–MoS2 in the PS matrix is few-layer platelets which are not exfoliated. It can be concluded that a good dispersion of Fe–MoS2 in PS matrix has been obtained in PS3, but the MoS2 layers are not fully exfoliated.27 However, these conjectures still need to be supported by TEM analysis.TEM analysis was employed to provide supporting information in order to verify the results obtained from the XRD patterns as well as to observe the degree of dispersion of MoS2 within the polymer matrix. Fig. 4 presents TEM images of PS/MoS2 and PS/Fe–MoS2 nanocomposites, showing two representative structures. It is clearly visible that the Fe–MoS2 sheets are dispersed well in the PS matrix with mild agglomerate structure which may be the reason for the small (002) peak appeared in the XRD pattern of the PS/Fe–MoS2 composites. On the contrary, the massive MoS2 nanosheets agglomerates are dispersed throughout the polymer matrix in the PS/MoS2 composites. It is difficult to break the physical and chemical interactions of the MoS2 nanosheets just using the mixing process. The relative good dispersion of Fe–MoS2 nanosheets in the composites can be attributed to: (1) the cyclopentadiene ring structure in ferrocene molecules improved the compatibility and interfacial interaction between Fe–MoS2 nanosheets and PS matrix; and (2) the expansion of the interlayer space of the Fe–MoS2 nanosheets are convenient for the entrance of PS chains and the high matrix viscosity in the melt-compounding step combines tearing and exfoliating MoS2.36
 | ||
| Fig. 4 TEM observations of the ultrathin sections obtained from PS/Fe–MoS2 (a) and PS/MoS2 and (b) composites. | ||
3.3 Thermal behavior of the nanocomposites
As is known to all, incorporation of 2D layered nanofillers usually increase the thermal stability of a polymer matrix due to the physical barrier effect which retards the diffusion of degradation products, gases and heat. In the temperature range of 300–430 °C where PS is mainly degraded, MoS2 (99% residue) is only slightly decomposed, so MoS2 nanosheets must present better physical barrier effects, which may increase the thermal stability of PS. TGA is one of the most widely used techniques for rapid evaluation of the thermal stability for various polymers. In this work, TGA is employed to evaluate the thermal stability of the PS, PS/ferrocene, PS/MoS2 and PS/Fe–MoS2 composites. TG profiles of pure PS and PS composites under air atmosphere are presented in Fig. 5, and the corresponding data are listed in Table 1. It can be observed that the PS composites exhibit similar thermal decomposition process to the pure PS, they all only consist of one stage which is mainly attributed to the decomposition of the macromolecular chains.37 However, the thermal stability of the PS composites is all enhanced comparing with that of the neat PS, which can be ascribed to the barrier effect of MoS2 nanosheets on the diffusion of volatile products throughout the composite materials and the presence of ferrocene can promote char formation in the PS/ferrocene system and the dense char layer will provide a good barrier to prevent the transfer of heat and volatiles, resulting in significant improvement of the thermal stability. | ||
| Fig. 5 TGA thermograms of pure PS, PS/ferrocene, PS/MoS2 and PS/Fe–MoS2 composites in air atmosphere. | ||
The temperatures corresponding to 5 and 10 wt% weight loss (T−5% and T−10%) which are used to evaluate the decomposition of PS on the onset stage, and 50 wt% weight loss (T−50%) are also reported in Table 1. From Table 1, it is summarized that the T−5% and T−10% of the PS/MoS2 composites are higher than that of pure PS. When adding 3 wt% MoS2, T−5% and T−10% of the samples is increased to 322 and 343 °C, which are 3 and 11 °C higher than that of pure PS. For T−50% of the samples, after adding MoS2, T−50% of the composites is enhanced to 399 °C which is 23 °C higher than that of pure PS. The addition of ferrocene can also enhance the thermal stability of the PS/ferrocene composites. However, for PS/Fe–MoS2 composites, the T−5%, T−10% and T−50% of the composites are all higher than that of pure PS, PS/ferrocene and PS/MoS2 composites. When adding 3 wt% Fe–MoS2, T−5% and T−10% of the samples is increased to 389 and 399 °C, which are 67 and 56 °C higher than that of PS/MoS2 composites. For T−50% of the samples, after adding Fe–MoS2, T−50% of the composites is enhanced to 423 °C which is 47 °C higher than that of virgin PS. It indicates that the presence of ferrocene and MoS2 can defer the thermal degradation of PS. The PS/Fe–MoS2 composites have higher thermal stability than pure PS, PS/ferrocene and PS/MoS2 composites. It means the presence of ferrocene and MoS2 in the PS composites has significant synergistic effect on improving the thermal stability of the PS composites. Moreover, it is well known that the char formed during thermal degradation and combustion is also important for fire safety applications. The char yield of pure PS is only 0.27% at 800 °C, and the char yield of the PS/ferrocene, PS/MoS2 and PS/Fe–MoS2 composites is increased to 1.45%, 3.00% and 3.25%, respectively. It is clear that the presence of ferrocene and MoS2 can promote char formation in the PS composites and the dense char layer will provide a good barrier to prevent the transfer of heat and volatiles, resulting in significant improvement of the thermal stability PS composites during a fire. All in all, these results suggest that incorporating ferrocene and MoS2 nanosheets into PS would retard the thermal degradation of PS molecular chains, the significantly enhanced thermal stability of the PS/Fe–MoS2 composites can be attributed to the synergistic effect between Fe–MoS2 nanosheets and PS, the physical barrier effect of the MoS2 nanosheets and the presence of ferrocene and MoS2 can promote char formation simultaneously.
3.4 Fire behavior of the PS composites
Fire performance tests were performed using cone calorimeter, which is one of the most effective methods for the laboratory evaluation of the fire properties of polymers. Several key parameters like heat release rate (HRR), total heat release (THR), peak HRR (PHRR), time to ignition (TTI), time to peak HRR, average mass loss rate (AMLR), smoke production rate (SPR) and average smoke extinction area (ASEA) which could be employed to evaluate the developing, spreading, and intensity of fires can be obtained from cone calorimeter.38The HRR, in particular the PHRR value, proves to be the most important parameter to evaluate fire safety. The reduction in PHRR is important for fire safety, as PHRR represents the point in a fire where heat is likely to propagate further, or ignite adjacent objects.39 The HRR plots for pure PS and its composites are shown in Fig. 6. It is very interesting to find that the PS/ferrocene composites have a higher PHRR values than the pure PS, it may be attributed to the combustion-supporting effect of the ferrocene which had been usually used for rocket fuel additives. As for the PS/MoS2 composites, the PHRR value of PS/MoS2 composites are 13.0% lower than that of virgin PS. But for the PS/Fe–MoS2 composites, the PHRR value decreased 21% comparing with the virgin PS, it is evident that the addition of Fe–MoS2 can improve the fire resistance of the composites more obvious than PS/MoS2 and PS/ferrocene composites, it means the presence of ferrocene and MoS2 also has significant synergistic effect in improving the flame retardancy of the PS composites. The reduction of HRR values was accompanied by a pronounced prolongation of burning time with a flat curve, while it presents a relative sharp and short HRR curve for pure PS as shown in Fig. 6, which can be ascribed to the degradation as well as the stabilization of the char formation.40 The better flame retardancy of the PS/Fe–MoS2 composites is mainly due to the better dispersion of Fe–MoS2 nanosheets in polymer matrix which can improve the physical barrier effect of the layered nanosheets. In addition, the fire resistance of the composites is also strongly dependent on the formation of carbonaceous char during the thermal degradation which reduces the diffusion of volatile combustible fragments generated by polymer degradation which diffuse towards the surface of the burning polymer to evaporate to feed the flame. The TGA results have proved that the presence of ferrocene and MoS2 in PS matrix all can promote char formation. Therefore, the better flame retardancy of the PS/Fe–MoS2 composites is due to the physical barrier effect of the MoS2 nanosheets and charring effect of the ferrocene and MoS2.
3.5 Smoke suppression properties
It is well known that the smoke, smoke particulates and some toxic compounds (especially carbon monoxide) produced during the course of a real fire is the main culprit for the fatality. Therefore, reducing the amount of smoke and carbon monoxide formed during burning will be significant for saving lives in real fires. The curves of the amount of CO release as a function of time for the PS, PS/ferrocene, PS/MoS2 and PS/Fe–MoS2 composites which are obtained from cone test are presented in Fig. 7(a). It can be observed that the addition of the ferrocene almost have no influence on the CO release. In contrary, CO production for the samples with MoS2 is much less than that of pure PS resins, especially for the PS/Fe–MoS2 composites. The above phenomena can be illustrated in the following. The presence of MoS2 can promote char formation in the PS/MoS2 composites obviously. That implies there is more compact char residue formed on the surface of the sample with MoS2. The compact char residue and physical barrier effect of MoS2 nanosheets can restrain combustible gases, so the released flammable gases can be complete combustion, which leads to the little carbon monoxide production rate.Smoke production rate (SPR) is defined as the rate at which smoke is produced per unit time. The SPR curves of the samples which can be obtained from the cone test are shown in Fig. 7(b). There is a distinction of the smoke release behavior between PS and its composites. It can be seen clearly that the addition of the ferrocene has significant effect on reduction of the SPR which is in accordance with the reported results.33 For the PS/MoS2 composites, the presence of the MoS2 almost has no influence on the reduction of the SPR. However, the SPR value of the PS/Fe–MoS2 composites decreased by 20% comparing with pure PS and PS/MoS2 composites. It also means that the presence of ferrocene and MoS2 in the PS composites has significant synergistic effect in improving the smoke suppression properties of the PS composites.
The TG-FTIR technique is a useful tool in dynamical analysis as it monitors continuously both the time-dependant evolution of the gases and the weight of the residue. It has been widely used in polymer thermal degradation, which can make a great contribution to the understanding of thermal degradation mechanism. Recently, TG-FTIR technique also had been used to investigate the smoke suppression properties of the polymer nanocomposites in our previous work.41 To investigate the influence of Fe–MoS2 on the evolved gaseous volatiles during pyrolysis, the volatile components of PS and PS/Fe–MoS2 are investigated by TG-FTIR technique. Fig. 8 shows the gas phase 3D TG-FTIR spectra of virgin PS and PS/Fe–MoS2 composites at a heating rate of 20 °C min−1 in nitrogen. It shows that the typical thermal degradation process of the composites is similar to pure PS. To further investigate the composition evolved in the gas phase of the PS and PS/Fe–MoS2 composites, the FTIR spectrum of PS and PS composites selected at their maximum weight loss rates was plotted in Fig. 9. It also shows that the typical thermal degradation process of the PS/Fe–MoS2 composites is similar to pure PS. According to a recent investigation, the decomposed products of PS under nitrogen are mainly monomer, dimer, and trimer of phenyl alkenyl. The absorption bands at 3073, 1496, 773, and 698 cm−1 are assigned to aromatic compounds, and the absorption band observed at 1597 cm−1 is attributed to alkenyl units.29,42
 | ||
| Fig. 8 The 3D diagrams of the gaseous volatiles during combustion process of (a) pure PS and (b) PS/Fe–MoS2 nanocomposites. | ||
 | ||
| Fig. 9 IR spectra of gasified pyrolysis products for pure PS and PS/Fe–MoS2 composites at their maximum weight loss rates. | ||
In order to further study the differences between PS and PS/Fe–MoS2 composites, the Gram–Schmidt (GS) curves as well as pyrolysis products vs. time are shown in Fig. 10 and 11. The Gram–Schmidt curves,43 based on vector analysis, reconstruct the acquired interferograms, allowing the plots of the total evolved gases detected by the spectrometer to be obtained (Fig. 10). For comparison purpose, the absorbance values were normalized by the total weight loss in the TGA. The absorbance intensity of the decomposition products from PS/Fe–MoS2 is much lower than that from virgin PS. Three explanations are presented for the phenomena. Firstly, layered MoS2 nanosheets can act as a barrier, which could decrease the thermal decomposition rate and limit gas diffusion. Secondly, the presence of ferrocene and MoS2 can promote char formation, which results in the increase in char yield in the condensed phase and thus makes combustion become difficult. Thirdly, it is probably because of that molybdenum suppresses formation of volatile aromatic compounds operating in the condensed phase.44
 | ||
| Fig. 11 Intensity of characteristic peaks for pyrolysis products of PS and the PS/Fe–MoS2 composites. | ||
In order to further understand the change of the pyrolysis products, the absorbance of pyrolysis products for PS and PS/Fe–MoS2 composites versus time is represented in Fig. 11. For comparison purpose, the absorbance values were normalized by the total weight loss in the TGA. It can be seen that the absorbance intensity of typical gaseous organic volatiles (aromatic compounds) for PS is much higher than that of PS/Fe–MoS2 composites. It can be obviously observed that the addition of Fe–MoS2 decreased the evolution of all aromatics compounds significantly. The maximum intensity of the evolved products has reduced to 55% of those evolved by pure PS, which implies that the amount of the volatiles released from the PS/Fe–MoS2 composites is much less than that from the pure PS. The reduced amount of the organic volatiles further leads to the inhibition of smoke, because the organic volatiles may crack into smaller hydrocarbon molecules and smoke particles. The gaseous hydrocarbons are condensed and the smoke particles are aggregated to form smoke.41 Therefore, there is no doubt that the presence of the Fe–MoS2 can enhance the smoke suppression properties significantly.
Comparing the results of thermal stability, flammability and smoke suppression properties of the materials in this study, we can conclude that the thermal stability, fire resistance and smoke suppression properties of PS/MoS2 composites are strongly affected by the dispersion of MoS2 nanosheets, physical barrier effect and charring effect. The results of the investigation of residues of the composites after combustion provide other useful information about the flame retardation mechanism of the above composites. Fig. 12 shows macro-morphologies of the final chars after cone calorimeter tests of PS and PS composites by using digital camera. After burning, neat PS does not form char while the PS/MoS2 composites form visible char, and the char is increased when the Fe–MoS2 is added (Fig. 12(d)). These results are good in agreement with the TG results. For PS/MoS2 composites, a fragile and cracked crust has been recovered at the end of the test, as shown in Fig. 12(c). For PS/Fe–MoS2 samples, it gives rise to the formation of a cohesive and uniform carbonaceous residue (Fig. 12(d)). This better cohesion of the combustion residue of the composites based on Fe–MoS2 could be explained by the well dispersed modified nanosheets forming a compact char layer. The continuous and compact char surface is good barriers to protect the underlying polymers and inhibit the exchange of degradation products, combustible gases and oxygen. Therefore, the strong synergistic effect, physical barrier effect of nanosheets and the promoted charring effect should be the main reasons for the excellent thermal stability, flame retardant and smoke suppression properties of the PS/Fe–MoS2 nanocomposites.
 | ||
| Fig. 12 Digital photos of the final chars after cone calorimeter tests of PS and PS composites: (a) pure PS; (b) PS/ferrocene; (c) PS/MoS2 and (d) PS/Fe–MoS2. | ||
4. Conclusion
In this paper, we have developed a facile, cost-effective approach for modifying MoS2 nanosheets with ferrocene in a large scale. The modified MoS2 nanosheets were used as nanofillers to prepare PS composites by a masterbatch-based melt blending method. The TGA, cone test and TG-IR results indicated that the thermal stability, fire resistance and smoke suppression properties of the PS/Fe–MoS2 composites are higher than that of the pure PS, PS/ferrocene and PS/MoS2 composites. When adding 3 wt% Fe–MoS2, T−5%, T−10% and T−50% of the composites is increased to 389, 399 and 423 °C, which are 67, 56 and 24 °C higher than that of PS/MoS2 composites. The cone test results indicated the PS/Fe–MoS2 composites exhibited superior flame retardance to PS/MoS2 and PS/ferrocene composites. TG-IR results showed that the main decomposition products of PS/Fe–MoS2 composites were aromatic compounds and alkenyl units which were similar to those of pure PS. However, much less flammable gas products were released relative to pure PS which further leads to the inhibition of smoke. It is obvious that the ferrocene and MoS2 have synergistic effect on improving the thermal stability, fire resistance and smoke suppression properties of the PS composites. The improvements of the PS/Fe–MoS2 composites are mainly attributed to good dispersion of modified MoS2 nanosheets, synergistic effect between ferrocene and MoS2 nanosheets, physical barrier effects of MoS2 nanosheets and the presence of ferrocene and MoS2 can promote char formation.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (2012CB719701), National Basic Research Program of China (973 Program) (no. 2012CB922002) and National Key Technology R&D Program (2013BAJ01B05).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 22, 666 CrossRef PubMed.
- L. C. Yang, S. N. Wang, J. J. Mao, J. W. Deng, Q. S. Gao, Y. Tang and O. G. Schmidt, Adv. Mater., 2013, 25, 1180 CrossRef CAS PubMed.
- K. Bindumadhavan, S. K. Srivastava and S. Mahanty, Chem. Commun., 2013, 49, 1823 RSC.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140 CrossRef CAS PubMed.
- S. X. Wu, Z. Y. Zeng, Q. Y. He, Z. J. Wang, S. J. Wang, Y. P. Du, Z. Y. Yin, X. P. Sun, W. Chen and H. Zhang, Small, 2012, 8, 2264 CrossRef CAS PubMed.
- Q. Y. He, Z. Y. Zeng, Z. Y. Yin, H. Li, S. X. Wu, X. Huang and H. Zhang, Small, 2012, 8, 2994 CrossRef CAS PubMed.
- R. Bissessur and W. White, Mater. Chem. Phys., 2006, 99, 214 CrossRef CAS PubMed.
- G. F. Ma, H. Peng, J. J. Mu, H. H. Huang, X. Z. Zhou and Z. Q. Lei, J. Power Sources, 2013, 229, 72 CrossRef CAS PubMed.
- C. L. Bao, Y. Q. Guo, L. Song, H. D. Lu, B. H. Yuan and Y. Hu, Ind. Eng. Chem. Res., 2011, 50, 11109 CrossRef CAS.
- C. L. Bao, Y. Q. Guo, L. Song and Y. Hu, J. Mater. Chem., 2011, 21, 13942 RSC.
- T. Kashiwagi, F. M. Du, J. F. Douglas, K. I. Winey, R. H. Harris and J. R. Shields, Nat. Mater., 2005, 4, 928 CrossRef CAS PubMed.
- F. Laoutid, L. Bonnaud, M. Alexandre, J. M. Lopez-Cuesta and P. H. Dubois, Mater. Sci. Eng., R, 2009, 63, 100 CrossRef PubMed.
- D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. M. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587–1595 CrossRef CAS PubMed.
- S. K. Kumar and R. Krishnamoorti, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 37 CrossRef CAS PubMed.
- C. L. Bao, Y. Q. Guo, L. Song, Y. C. Kan, X. D. Qian and Y. Hu, J. Mater. Chem., 2011, 21, 13290 RSC.
- Z. Matusinovic and C. A. Wilkie, J. Mater. Chem., 2012, 22, 18701 RSC.
- P. Ding, W. Chen and B. J. Qu, Prog. Nat. Sci., 2006, 16, 573 CrossRef CAS.
- M. Alexandre and P. Dubois, Mater. Sci. Eng., R, 2000, 28, 1 CrossRef.
- S. R. Suprakas and M. Okamoto, Prog. Polym. Sci., 2003, 28, 1539 CrossRef PubMed.
- B. M. Alexander, Polym. Adv. Technol., 2006, 17, 206 CrossRef.
- S. Pavlidou and C. D. Papaspyrides, Prog. Polym. Sci., 2008, 33, 1119 CrossRef CAS PubMed.
- R. Verdejo, M. M. Bernal, L. J. Romasanta and M. A. Lopez-Manchado, J. Mater. Chem., 2011, 21, 3301 RSC.
- R. P. Jeffrey, R. D. Daniel, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5 CrossRef PubMed.
- R. Bissessur, D. Gallant and R. Brüning, Mater. Chem. Phys., 2003, 82, 316 CrossRef CAS.
- B. Z. Lin, C. Ding, B. H. Xu, Z. J. Chen and Y. L. Chen, Mater. Res. Bull., 2009, 44, 719 CrossRef CAS PubMed.
- T. M. Wang, W. M. Liu, J. Tian, X. Shao and D. C. Sun, Polym. Compos., 2004, 25, 111 CrossRef CAS.
- K. Q. Zhou, S. H. Jiang, C. L. Bao, L. Song, B. B. Wang, G. Tang, Y. Hu and Z. Gui, RSC Adv., 2012, 2, 11695 RSC.
- Z. Matusinovic, R. Shukla, E. Manias, C. G. Hogshead and C. A. Wilkie, Polym. Degrad. Stab., 2012, 97, 2481 CrossRef CAS PubMed.
- K. Q. Zhou, W. Yang, G. Tang, B. B. Wang, S. H. Jiang, Y. Hu and Z. Gui, RSC Adv., 2013, 3, 25030 RSC.
- G. A. Skinner and P. J. Haines, Fire Mater., 1986, 10, 63 CrossRef CAS.
- P. Carty, J. Grantt and E. Metcalfets, Appl. Organomet. Chem., 1996, 10, 101 CrossRef CAS.
- P. Carty, T. J. Grant and A. Simpson, Appl. Organomet. Chem., 1988, 2, 277 CrossRef CAS.
- Y. Koshiba, Y. Takahashi and H. Ohtani, Fire Saf. J., 2012, 51, 10 CrossRef CAS PubMed.
- C. Manzi-Nshuti and C. A. Wilkie, Polym. Degrad. Stab., 2007, 92, 1803 CrossRef CAS PubMed.
- W. M. R. Divigalpitiya, R. F. Frindt and S. R. Morrison, Science, 1989, 246, 369 CAS.
- C. L. Bao, L. Song, C. A. Wilkie, B. H. Yuan, Y. Q. Guo, Y. Hu and X. L. Gong, J. Mater. Chem., 2012, 22, 16399 RSC.
- C. L. Bao, Y. Q. Guo, B. H. Yuan, Y. Hu and L. Song, J. Mater. Chem., 2012, 22, 23057 RSC.
- K. Q. Zhou, B. B. Wang, S. H. Jiang, H. Y. Yuan, L. Song and Y. Hu, Ind. Eng. Chem. Res., 2013, 52, 6303 CrossRef CAS.
- C. Manzi-Nshuti, D. Chen, S. Su and C. A. Wilkie, Polym. Degrad. Stab., 2009, 94, 1290 CrossRef CAS PubMed.
- J. Q. Wang and Z. D. Han, Polym. Adv. Technol., 2006, 7, 335 CrossRef.
- Y. Y. Dong, Z. Gui, Y. Hu, Y. Wu and S. H. Jiang, J. Hazard. Mater., 2012, 209, 34 CrossRef PubMed.
- B. N. Jang and C. A. Wilkie, Polymer, 2005, 46, 2933 CrossRef CAS PubMed.
- A. Marcilla, A. Gómez and S. Menargues, J. Anal. Appl. Pyrolysis, 2005, 74, 224 CrossRef CAS PubMed.
- S. V. Levchik and E. D. Weil, Polym. Adv. Technol., 2005, 16, 707 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |