Novel biodegradable heparin-coated nanocomposite system for targeted drug delivery
Amaneh Javida,
Shahin Ahmadian*ab,
Ali Akbar Sabourya,
Seyed Mehdi Kalantarc and
Saeed Rezaei-Zarchid
aInstitute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran. E-mail: ahmadian@ibb.ut.ac.ir; Tel: +98 21 66956983
bCenter of Excellence of Nano-Biomedicine, Nano-Science and Nano-Technology Research Center, University of Tehran, Tehran, Iran
cResearch and Clinical Center of Infertility, Shahid Sadoughi University Medical Sciences, Yazd, Iran
dDepartment of Biology, Payame Noor University, Yazd, Iran
First published on 27th January 2014
Abstract
Bare (∼10 nm) and heparin (HP)-coated superparamagnetic iron oxide nanoparticles (SPIO NPs; 42 nm) were formulated by a co-precipitation technique. The bare and HP–SPIO NPs had saturation magnetization of 50–55 emu g−1 at 300 K. The anticancer drugs, doxorubicin (DOX) and paclitaxel (PTX), were separately partitioned in the SPIO core to compare their anti-neoplastic effects on the proliferation of A2780 and OVCAR-3 human ovarian cancer cells. The results revealed that the DOX–HP–SPIO NPs (85 nm) and PTX–HP–SPIO NPs (71 nm) showed sustained and pH-sensitive release of DOX (87%) and PTX (75%), respectively, at pH 6.0, even up to two weeks. Meanwhile, 10 μg ml−1 DOX–HP–SPIO NPs and PTX–HP–SPIO NP caused 95 and 84%, and 85 and 77% apoptosis in A2780 and OVCAR-3 cells, respectively, with a sharp decrease in the level of bcl-2 and survivin proteins and increased expression of proapoptotic proteins, like bax and NF-κB. So, the presently formulated nanocomposite-based drug delivery system was readily internalized into tumor cells and induced a higher apoptosis rate.
1. Introduction
Amongst the broad spectrum of nanoscale materials being investigated for biomedical use, the superparamagnetic iron oxide nanoparticles (SPIO NPs) have created significant interest due to their intrinsic magnetic properties for the guided delivery of drugs.1,2 Freeman et al. were the first to introduce the concept of use of magnetism in medicine in the 1970s.3 Since then, much research has been done in this area, leading to the design of various magnetic particles and vectors. In order to fully exploit the potential of SPIO NPs for image-guided drug delivery upon systemic administration, they should be biocompatible, stable in the circulation, and have the potential for prolonged circulation in the bloodstream. This can be achieved by coating the SPIO NPs with hydrophilic polymers that give them ‘stealth’ properties. Coating particles with hydrophilic polymers was carried out to improve the particles’ colloidal stability and also prolong their circulation kinetics.4,5However, the coated SPIO NPs have limited drug loading capacity and rapid dissociation after administration for drug delivery purposes. The main objective of today's leading research is to optimize the properties of these magnetic particles to: provide an increase in magnetic nanoparticle concentration in the blood vessels; reduce early clearance from the body; minimize nonspecific cell interactions, thus minimizing side effects; and increase their internalization efficiency within target cells, thus reducing the total dose required.6,7
The rationale behind the association of drugs with colloidal carriers such as NPs or liposomes is due to the limited success of conventional drug therapy. Systemically administering bolus doses of powerful chemotherapeutics often results in intense side effects due to the action of the drugs on sites other than the intended target sites.8 With such nonspecific drug action, the concentration of the drug administered to the patient is a vicious predicament between choosing a near-toxic effective dose and a comfortable ineffective dose.9
Carbohydrate moieties and hydrophilic synthetic polymers are widely utilized for these purposes. Heparin and heparan sulfates are a family of very heterogeneous and highly charged (sulfated and anionic) glycosaminoglycans, which naturally cover the surface of all eukaryotic cells.10 These sugars possess various biological activities, particularly seen in heparin, which has been used as an anticoagulant drug since 1930.11,12 Heparin is non-cytotoxic, biodegradable, and water-soluble natural polysaccharide, coupled with a variety of biological activities including anti-coagulation, anti-inflammation, anti-angiogenesis and anti-tumor cell proliferation, and has attracted intense attention. Many heparin–drug conjugates have been developed for cancer chemotherapy as macromolecular prodrugs. These heparin conjugates, containing anticancer agents such as paclitaxel (PTX), exhibited enhanced targeting ability to the tumor and higher therapeutic efficacy compared to free drugs.13,14
Taking into account the advantage of the excellent properties of HP and polymer–drug conjugates, we have successfully synthesized HP-based SPIO NP drug conjugates, carrying two different anticancer drugs, doxorubicin (DOX) and PTX, for intra-tumoral drug delivery. The cytotoxicity response of the drug loaded HP–SPIO NPs was determined by the biochemical parameters and survival of human ovarian cancer cell lines of OVCAR-3 and A2780.
2. Materials and methods
2.1. Chemicals
The ovarian cancer cell lines, A2780 (NCBI code, C461) and OVCAR-3 (NCBI code, C430) were purchased from Pasteur Institute, Tehran, Iran. RPMI-1640 medium and all of the additives were purchased from GIBCO Co. (Grand Island, NY, USA). DOX, PTX and heparin were purchased from Sigma-Aldrich Chemical Co. (St. Louis. Missouri, USA). All other chemicals used were of the highest purity and biological grade available from commercial sources.2.2. Synthesis of bare and HP–SPIO NPs
Bare SPIO NPs were prepared by a co-precipitation technique, with some modifications to the previously reported method.15–17 Firstly, 5.41 g of FeCl3·6H2O (99% purity) and 1.99 g FeCl2·4H2O (99% purity) were dissolved in 100 ml of distilled water (DW) in a triple-necked flask. The pH of the mixture (100 ml) was maintained at pH 6.9 with slow addition of 25 ml of NH4OH (25–28%, w/w) while stirring constantly under the protection of dry nitrogen at 60 °C. During this time, the solution color changed from yellow to black, indicating the formation of iron oxide particles.After 1 h of stirring, the prepared SPIO NPs were rinsed with DW and subsequently mixed with unfractionated heparin (UF-HP; 5.47 mg ml−1) for 2 h. The resultant product was then sonicated for 1 h, centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min. and vigorously stirred for 1 h at 90 °C under a nitrogen atmosphere. An external magnetic field, Mext, was applied for 15–20 s to obtain aggregates of modified particles. Centrifugation (4000 × g; 10 min) was carried out to completely separate the particles remaining in the supernatant. The supernatant was concentrated through an ultrafiltration system (Model 8200; Amicon Corp., Danvers, MA) using ultrafiltration Amicon YM membrane (100K MWCO, Millipore, Billerica, MA). The resulting HP–SPIO NPs were stored at 4 °C until further use.
000 × g for 30 min. and vigorously stirred for 1 h at 90 °C under a nitrogen atmosphere. An external magnetic field, Mext, was applied for 15–20 s to obtain aggregates of modified particles. Centrifugation (4000 × g; 10 min) was carried out to completely separate the particles remaining in the supernatant. The supernatant was concentrated through an ultrafiltration system (Model 8200; Amicon Corp., Danvers, MA) using ultrafiltration Amicon YM membrane (100K MWCO, Millipore, Billerica, MA). The resulting HP–SPIO NPs were stored at 4 °C until further use.
2.3. Drug loading into the HP–SPIO NPs
HP–SPIO NPs were synthesized to prepare two different drug formulations using DOX and PTX in order to compare their effects on two human epithelial ovarian cancer cell lines. Drug loading onto HP–SPIO NPs was carried out using an oil-in-water single emulsion evaporation method. Briefly, 0.1–10 μg ml−1 (0.1–250 μM) of either of the drugs (10% w/w) were separately dissolved in 1 ml of acetonitrile. The drugs were added drop-wise to the HP–SPIO NP suspension and stirred overnight to allow the drug-partitioning into NPs to prepare separate formulations of DOX–HP–SPIO NPs and PTX–HP–SPIO NPs. The unbound drugs were separated by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 10 °C. Three washes were performed to remove unbound drugs and the resulting pellets were lyophilized to get powdered DOX–HP–SPIO NPs and PTX–HP–SPIO NPs. DOX and PTX loading onto bare SPIO NPs was also carried out with same technique to evaluate the significance of the HP coating in drug delivery.
000 rpm for 10 min at 10 °C. Three washes were performed to remove unbound drugs and the resulting pellets were lyophilized to get powdered DOX–HP–SPIO NPs and PTX–HP–SPIO NPs. DOX and PTX loading onto bare SPIO NPs was also carried out with same technique to evaluate the significance of the HP coating in drug delivery.
2.4. Loading-efficiency of incorporated drug
The quantification and release kinetics of drug-loading onto the modified nanocomposites was carried out through UV/Visible double-beam spectrophotometer (Hitachi U-2000) at 200–800 nm. Drug incorporation efficiency was observed both as drug loading (% w/w) and drug entrapment (%), by the below-stated eqn (1) and (2), respectively.
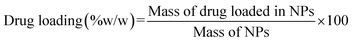 | (1) |
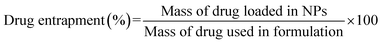 | (2) |
2.5. Physicochemical properties of drug-loaded HP–SPIO NPs
The AFM topographic images were collected in contact mode using silicon nitride cantilevers (PSIA, Korea, spring constant 0.6 N m−1; tip radius <10 nm) using an XE100 (PSIA, Korea) in air. For AFM analysis, the HP–SPIO NPs were diluted with DW and a drop of the diluted material was placed on a glass plate and analyzed with AFM equipment.
2.6. In vitro drug release profile
Five milligrams of DOX–HP–SPIO NPs and PTX–HP–SPIO NPs were suspended in 10 ml of RPMI medium under different pH conditions (pH 1.5, 6.0 and 7.0) and sonicated to produce a clear solution. The solutions containing bare SPIO NPs, HP–SPIO NPs, DOX–HP–SPIO and PTX–HP–SPIO NPs were placed into dialysis bags (molecular weight cut-off 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) containing 35 ml of PBS and incubated in a water bath at 37 °C with gentle shaking at 50 rpm. At predetermined intervals, buffered solutions were collected and replaced with an equivalent volume of fresh PBS. The amount of drug released was determined by HPLC and the drug content was determined spectrophotometrically at 200–800 nm.
000 g mol−1) containing 35 ml of PBS and incubated in a water bath at 37 °C with gentle shaking at 50 rpm. At predetermined intervals, buffered solutions were collected and replaced with an equivalent volume of fresh PBS. The amount of drug released was determined by HPLC and the drug content was determined spectrophotometrically at 200–800 nm.
2.7. Human ovarian cancer cell culture and in vitro cytotoxicity detection
The A2780 and OVCAR-3 cells were maintained in the culture conditions stated in the Materials and methods section for 48 hours, at a density of 1 × 105 cells per well, in a humidified atmosphere of 5% CO2 in air at 37 °C. The cells were then incubated with bare, HP-coated, DOX–HP–SPIO and PTX–HP–SPIO NPs, at a fixed concentration of 5 μg ml−1, for 48 h. After the incubation completion, 100 μl of the medium containing 20 μl MTT solution was added to each well, and the plates were incubated for an additional 4 h, followed by the addition of 100 μl MTT solubilization solution, containing 10% Triton X-100 + 0.1 N HCl in anhydrous isopropanol, to each well. The solution was gently mixed to dissolve MTT formazan crystals, and the absorbance of each well was measured with a microplate reader at a wavelength of 570 nm. The background absorbance of the wells was measured at 690 nm and subtracted from the results taken from the 570 nm experiment. Untreated cells were used as the control. Cell viability was expressed as a percentage of a control that had not been treated with drug-loaded nanoparticles, using the following equation:
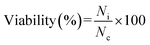 | (3) |
2.8. Apoptotic detection by flow cytometry
A2780 and OVCAR-3 cells were plated at 5 × 105 cells per well in 2 ml RPMI-1640 in six-welled plates and incubated for 24 h. The medium was then replaced with 10 μg ml−1 of each of the bare SPIO, HP–SPIO, DOX–HP–SPIO and PTX–HP–SPIO NPs, in separate plates and incubated again for 48 hours. Apoptotic cells were identified with fluorescein isothiocyanate-labeled Annexin V (Annexin V-FITC). Propidium iodide (PI) (BioVision, Mountain View, CA) was used, according to the manufacturer's protocol, to mark the dead cells. All the test materials were diluted with RPMI-1640 medium. The treated cells were harvested, trypsinized, washed with PBS, incubated with Annexin V-FITC and PI for 15 minutes at room temperature in the dark, and analyzed via the FACS-Calibur flow cytometer (Becton, Dickinson & Company, Mountain View, CA) with data acquisition software (CellQuest; Becton, Dickinson and Company).2.9. Immuno-blot analysis and investigation of gene expression
A2780 and OVCAR-3 cells were lysed in a buffer containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.2% (v/v) Nonidet P40 and protease inhibitor cocktail on ice for a period of 10 minutes. Cell debris was pelleted by centrifugation, and the total protein concentration of the soluble extracts was determined by the Bradford assay, according to the manufacturer's instructions. Total soluble protein extract (30 μg) for each sample was resolved by SDS PAGE (12% gels). After electrophoresis, the survivin, Bcl-2, bax and NF-κB proteins were transferred to nitrocellulose, and the blot was blocked for 1 hour at room temperature with a solution of 5% dried milk in PBST (0.1% (v/v) Tween 20 in PBS). The blot was incubated overnight at 4 °C with either of the anti-Bcl-2 monoclonal antibody, anti-bax, anti-NF-κB and anti-survivin polyclonal antibodies to evaluate the levels of protein expression. Primary antibodies were detected using an HRP conjugated secondary antibody and enhanced chemiluminescence (ECL) was measured as described by the manufacturer.2.10. Statistical analysis
All data were analyzed with SPSS software (version 14.0; SPSS Inc, Chicago, IL). Results were presented as mean ± standard deviation (SD). The two-way analysis of variance (ANOVA) and Student's t-test were used to compare data from different treatment groups, and differences were considered significant at p < 0.05.3. Results and discussion
3.1. Characterization of the bare, HP-coated and drug loaded SPIO NPs
The zeta potential is a bulk property, the magnitude of which gives an indication of the potential stability of colloidal system. A large negative or positive zeta potential value in the suspension diminishes the aggregation behavior of particles.21 The high positive-zeta potential of the HP–SPIO NPs did not change significantly with the increase of HP coating or after drug loading, describing their colloidal stability in the aqueous solution that could escape the reticuloendothelial system, preventing their uptake by macrophages.22
The formation of magnetic beads in HP-coated NPs was confirmed by a change in saturation magnetization amounts. According to VSM analysis, the SPIO NPs retained their superparamagnetic behavior even after HP coating. Fig. 3B demonstrates typical hysteresis curves at 10, 150 and 300 K for the optimized HP–SPIO NPs. The hysteresis loop had negligible coercivity at room temperature, and the saturation magnetization (Ms) value at 1.0 T (after subtracting the diamagnetic background) was between 50 and 55 emu g−1 at 300 K, which is lower than the bulk value, 90 emu g−1.22 The nanoparticles were not supermagnetic at 10 K. Ideal superparamagnetic materials should have zero coercivity and zero remanence.23 From the magnetization values, it can be measured that 100% w/w HP–SPIO NPs had in fact only about 15% w/w HP coating on their surface.
The inset of Fig. 3B shows a photograph of HP–SPIO NPs dispersed in solution (left). The application of an external magnetic field (Mext) to the container caused the attraction of NPs towards it and their attachment to the wall of the container in close proximity to the magnet and the dispersion became clear (right). Removal of Mext and shaking led to the complete recovery of dispersion, confirming that the prepared HP–SPIO NPs were sensitive to Mext and showed superparamagnetic properties, which shows that the magnetite (Fe3O4) load in the presently synthesized formulation is quite sufficient to respond to Mext.
3.2. Drug loading profile of HP–SPIO NPs
Drug loading onto the HP–SPIO NPs was carried out using an oil-in-water single emulsion evaporation method. The surface morphology of drug-loaded nanocomposites was evaluated by SEM. Fig. 4 shows the SEM micrographs of the bare (a), HP–SPIO (b), DOX–HP–SPIO (c) and PTX–HP–SPIO NPs (d). The micrographs revealed that the HP–NP complex was largely composed of globular structures of various sizes, dispersed throughout the matrix (Fig. 4B). The mean diameter of the globular structures was estimated to be about 42 nm. After drug loading, the dispersed and spherical structures had increased particle size (85 and 71 nm) for the DOX– and PTX–HP–SPIO NPs, respectively (Fig. 4C and D).Fig. 4E shows that 10 μg ml−1 DOX–HP–SPIO NPs and PTX–HP–SPIO NPs showed 66 and 57% loading efficiencies at physiological pH.
Fig. 5A demonstrates the schematic cutaway of drug-loaded (DOX or PTX) HP-coated Fe3O4 nanoparticles. Fig. 5B demonstrates the UV-Vis spectra of bare (492 and 529 nm), HP–SPIO (223 nm), pure DOX (486 nm), DOX–HP–SPIO NPs (320 and 507 nm), pure PTX (234 nm) and PTX–HP–SPIO-NPs (266 and 492 nm).
To confirm drug loading onto HP–SPIO NPs, FT-IR spectra of (a) bare SPIO NPs, (b) pure HP, (c) free DOX, (d) DOX–HP–SPIO, (e) free PTX and (f) PTX–HP–SPIO NPs were obtained (Fig. 5C). The SPIO NP spectrum showed vibration at 560 cm−1 for Fe–O. The HP spectrum showed vibrations at 3510 cm−1 (–COO−), 1774 and 1315 cm−1 (–SO4−). The DOX spectrum showed bands at 3340 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 2870 cm−1 (asymmetric methylene group stretching), 1730 cm−1 (C
O stretching), 2870 cm−1 (asymmetric methylene group stretching), 1730 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1690, 990 and 760 cm−1 (O–H out-of-plane bending). Meanwhile, the DOX–HP–SPIO NPs spectrum showed bands at 3650 cm−1 (O–H stretching), 2859 cm−1 (asymmetric methylene group stretching), 1610 cm−1 (C
O stretching), 1690, 990 and 760 cm−1 (O–H out-of-plane bending). Meanwhile, the DOX–HP–SPIO NPs spectrum showed bands at 3650 cm−1 (O–H stretching), 2859 cm−1 (asymmetric methylene group stretching), 1610 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1596 cm−1 (O–H bending) and 790 cm−1 (Fe–O stretching). The PTX spectrum had bands at 1350, 1087 and 710 cm−1 related to CH3 deformation, C–O stretching and C–C
O stretching), 1596 cm−1 (O–H bending) and 790 cm−1 (Fe–O stretching). The PTX spectrum had bands at 1350, 1087 and 710 cm−1 related to CH3 deformation, C–O stretching and C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O deformation. The band at 3567 cm−1 in the PTX–HP–SPIO NPs spectrum was related to N–H/O–H stretching, 1495 cm−1 for CH3 deformation, 890 cm−1 for C–H in-plane deformation and 450 cm−1 for Fe–O stretching. The results show that the hydrophobic drugs like PTX and DOX were partitioned in the iron oxide core of HP–SPIO NPs. This method has the advantage of offering greater flexibility for loading the hydrophobic drugs.
O deformation. The band at 3567 cm−1 in the PTX–HP–SPIO NPs spectrum was related to N–H/O–H stretching, 1495 cm−1 for CH3 deformation, 890 cm−1 for C–H in-plane deformation and 450 cm−1 for Fe–O stretching. The results show that the hydrophobic drugs like PTX and DOX were partitioned in the iron oxide core of HP–SPIO NPs. This method has the advantage of offering greater flexibility for loading the hydrophobic drugs.
3.3. Drug release profile
The release study was carried out to estimate the amount of drug released from the HP–SPIO NPs under in vitro conditions. According to Fig. 6, the release of free and combined DOX and PTX was 27, 87, 24 and 75%, respectively, in the pH range of 4.0–6.0. As acidic pH prevails in the tumor microenvironment, it is necessary that the antitumoral drugs be released at acidic pH, instead of pH 7.4. Such release behavior can diminish the side effects of chemotherapeutic agents. Both DOX–HP–SPIO and PTX–HP–SPIO formulations showed sustained drug release for about 12 days. The diffusion of anticancer drugs loaded into the polymeric nanoshell could be due to the influence of concentration gradient, which is similar to the observations in oleic acid coated iron oxide nanoparticles.19 Thus, our formulation offered sustained release of anticancer drugs from the HP–SPIO NPs, which is an essential requirement of cancer therapy. | ||
| Fig. 6 In vitro release profiles of DOX and PTX from bare SPIO and HP–SPIO NPs at pH 1.5–7.0, at 37 °C (n = 3). | ||
Fig. 6 shows the drug release profiles of the free and conjugated DOX and PTX over the pH range of 1.5–7.0. At pH 7.0, a small amount of the drug release was observed after the incubation period of 48 hours. This is a desirable characteristic as pH 7.4 is an undesired pH for the proper release of drugs from nano-conjugated drug carrier. This will also prevent the premature release of the drugs before the nano-conjugates reach the cancer cells. Fig. 6 shows that pH 6.0 provided desirable conditions for the proper drug release. The first 10 hours represent the period of initial rapid release, followed by a steady state. This pH-dependent drug release behavior is favorable for the chemotherapeutic process as it can significantly reduce the pre-term drug release at the body pH level (pH 7.4) and maximize the amount of drug reaching the target tumor cells once the drug-loaded nano-composites internalize and enter the tumor by endocytosis (pH 4.5–6.5).
3.4. Cellular cytotoxicity followed by uptake of drug loaded HP–SPIO NPs
For the MTT assay, A2780 and OVCAR-3 cells were separately incubated with 0.1–10 μg ml−1 DOX and PTX for 120 h. Fig. 7A demonstrates the MTT results for the antineoplastic effects of free and nanocomposite-based DOX and PTX against the growth of A2780 cells. According to Fig. 7A, 10 μg ml−1 concentrations of both DOX–HP–SPIO and PTX–HP–SPIO were the most effective against A2780 cells during the incubation period of 120 h (5 and 16% survival; p < 0.001). Meanwhile, 60 and 55% survival was recorded in A2780 cells incubated with 10 μg ml−1 free DOX and PTX, respectively. Additionally, 10 μg ml−1 DOX and PTX loaded in SPIO NPs caused 50% growth inhibition.Fig. 7B demonstrates the MTT results for the antineoplastic effects of free and nanocomposite-based DOX and PTX against the growth of OVCAR-3 cells. As shown in Fig. 7B, 15 and 23% survival was recorded in OVCAR-3 cells incubated with DOX–HP–SPIO NPs and PTX–HP–SPIO NPs for 120 h, respectively (p < 0.001). Meanwhile, 69 and 57% survival was recorded in OVCAR-3 cells, incubated with 10 μg ml−1 free DOX and PTX, respectively. Additionally, 10 μg ml−1 of DOX and PTX, loaded in SPIO NPs, caused 50% growth inhibition, which is identical to the effect of SPIO-loaded DOX and PTX against A2780 cells.
As shown by the (#) marks in the plots of Fig. 7A and B, the IC50 analysis revealed that 1 μg ml−1 concentration of the DOX and PTX drugs loaded in HP–SPIO NPs was the dose at which 50% of the A2780-cell growth was inhibited. The same pattern was seen in the OVCAR-3 cells, treated with DOX–HP–SPIO NPs. Meanwhile, 5 μg ml−1 concentration of PTX loaded in HP–SPIO was the IC50 against OVCAR-3 cell growth (p ≤ 0.5). In addition, 10 μg ml−1 concentration of the DOX and PTX loaded in bare SPIO NPs was recorded to be the IC50 against both cell lines, as compared to the control group (data not shown because of being non-significant) (p < 0.01). This drug concentration was 10 times more than that delivered by the HP–SPIO NPs (p < 0.01). It is important to state that the free drugs could not reach the IC50 values at the experimental concentrations of 0.1–10 μg ml−1. These results prove the significance of the heparin coating on the SPIO NPs and targeting the A2780 and OVCAR-3 cells with much lower concentrations of DOX and PTX loaded in these novel nanocomposites.
Fig. 7C–F show the confocal fluorescence micrographs of A2780 (C & D) and OVCAR-3 cells (E & F), incubated for 3 h with DOX–HP–SPIO NPs and PTX–HP–SPIO NPs, labeled with FITC. In addition, about 50% survival was observed in A2780 and OVCAR-3 cells when treated with DOX–SPIO NPs and PTX–SPIO NPs, respectively (p < 0.05). While, 43% survival was seen in A2780 cells when administered with PTX–SPIO NPs.
3.5. Apoptotic detection
To compare the apoptotic activity of bare and drug loaded SPIO NPs, A2780 and OVCAR-3 cells were separately treated with fresh medium containing 10 μg ml−1 bare, HP–SPIO, DOX–HP–SPIO and PTX–HP–SPIO NPs for 48 hours. As shown in Fig. 8A, A2780 and OVCAR-3 cells underwent 93 and 87.1% apoptosis when administered with 5 μg ml−1 PTX–HP–SPIO NPs (p < 0.05), as compared to the drug itself (69.5 and 67.3%, respectively). According to Fig. 8B, DOX–HP–SPIO NPs caused 96.5 and 91.3% cell death in A2780 and OVCAR-3 cells, respectively, as compared to DOX itself (70.9 and 62.7%; p < 0.05).3.6. Western blot analysis for gene expression
During the present study, the expression of bax, bcl-2, NF-κB and survivin proteins were evaluated in A2780 and OVCAR-3 cells, treated with DOX, PTX, DOX–HP–SPIO NPs and PTX–HP–SPIO NPs, respectively. According to Fig. 8C, the level of bcl-2 and survivin proteins had a sharp decrease while NF-κB was also regulated in the cultures treated with DOX–HP–SPIO NPs in A2780 and OVCAR-3 cells. This represents the activation of apoptotic mechanism in cancer cells after the nanoparticle-based drug delivery in vitro (p < 0.05).3.7. Assessment of cell morphology
Fig. 8D shows the bright-field phase contrast photographs of A2780 and OVCAR-3 cells, seeded with (10 μg ml−1 drug) free PTX, free DOX, DOX–HP–SPIO and PTX–HP–SPIO NPs for 48 hours. The incubation of the cells with free drugs did not alter the morphology of A2780 and OVCAR-3 cell types. The viability of the cells after each period of incubation with the free DOX and PTX were close to that of the control cells and varied in the range from 92% to 96%, whereas DOX–HP–SPIO as well as PTX–HP–SPIO NPs were reported to have in vitro toxicity both cell lines, as shown by arrows. Both the particle size and material composition might play an important role in enhancing the cytotoxicity of nanocomposite-based drugs.The intra-tumoral administration of anticancer drugs represents a growing trend for maximizing local tumor control with minimal systemic toxicity. However, it requires a novel drug delivery system for treatment efficacy and ease of administration. SPIO NPs have been widely used in the delivery of chemotherapeutics, achieving promising results.1 The authors propose that combining intra-tumoral administration with a magnetic nanocarrier in chemotherapy provides opportunities for treating cancers in a safe and effective manner. We fabricated a SPIO-NP drug delivery system for intra-tumoral administration that was comprised of a magnetic Fe3O4 core and a shell of a biocompatible material, heparin, coated onto the nanoparticles by a single emulsion evaporation method. The DOX–HP–SPIO NPs showed high loading content and encapsulation efficiency, and they supported a sustained and steady drug release.2 In vitro, the DOX–HP–SPIO NPs were easily internalized into the tumor cells and induced significant amount of apoptosis.
Systemic chemotherapy against cancers such as ovarian, breast, prostate, lung, and gastrointestinal cancers can cause severe side effects because of the toxicity caused by the anticancer drugs on normal tissues. Moreover, the efficacy of anticancer drugs can be diminished because of rapid clearance from circulation and poor distribution to the target tumor.24,25 Intra-tumoral injection of chemotherapeutic agents is potentially a more effective alternative to systemic administration because direct delivery of anticancer drug to the target may improve the stability and efficacy of anticancer drugs. Such targeted delivery would be expected to provide a high local concentration of agents, reducing systemic drug levels and thereby decreasing the incidence of side effects compared with traditional treatments.26
The size of the nanoparticles is a key parameter that determines their properties, application and fate. First, given that the smallest capillaries in the body are about 4 μm, particles larger than 4 μm will most likely become trapped in the lungs.27 Particles smaller than that will usually be eliminated by reticuloendothelial system (RES). After intravenous administration, particles larger than 200 nm are usually sequestered by the spleen, as a result of mechanical filtration.28 These particles are eventually removed by phagocytes, resulting in decreased blood circulation times. On the other hand, particles smaller than 10 nm are rapidly removed through extravasations and renal clearance.29 Particles ranging from 10 to 100 nm are optimal for systemic administration and demonstrate the most prolonged blood circulation times. The particles in this size range are small enough to both evade the RES and penetrate the very small capillaries within the body tissues, and therefore they may offer the most effective distribution in certain tissues. However, complete evasion of the RES does not seem feasible and unwanted migration to normal tissues in the body could cause toxic side effects.30
In this study, we synthesized SPIO NPs coated with unfractionated heparin (UF-HP) with a mean diameter of 42 nm and investigated the effect of the biodegradable nanocomposite coating on the targeted and efficient delivery of DOX and PTX to A2780 and OVCAR-3 cells. After drug loading, these nanocomposites became about 85 nm (DOX–HP–SPIO NPs) and 71 nm (PTX–HP–SPIO NPs) in size. In particular, we compared the uptake efficiency of nano-conjugated drugs as compared to the bare drugs.31 The uptake of nano-conjugated drugs is known to be mediated by endocytosis or phagocytosis. Therefore, incubation of drug loaded HP–SPIO NPs is required, for longer than several hours, to improve drug-release efficiency. Previous studies have reported that the iron content taken in by cells has a broad range dependence on the cell type, exposure time and culture methods (10–120 pg Fe per cell, depending on the tissue and tumor type).32,33 During the present study, targeting the A2780 and OVCAR-3 cells required a relatively long incubation time (∼120 h) but lower drug and nanocomposite concentrations (10 μg ml−1), which may be attributed to the target-specificity and natural polymer (HP) coating.
Heparin has also been found to be an inhibitor of the replication of human immunodeficiency virus, and also an inhibitor of angiogenesis and tumor growth.34 Previous studies showed that the coating of HP on the surface of biomaterials increased hydrophilicity, which resulted in facilitated cell attachment to the biomaterial surface. Consistently, heparin coating of SPIO increased hydrophilicity, which may have caused enhanced cellular uptake.15 Heparin is a highly sulfated natural glycosaminoglycan and recent studies have revealed that HP strongly binds to various growth factors such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) through the interaction with its sulfate group.31 Tumor cells hyper-express the growth factor receptors. So, we can assume that the increased internalization of SPIO NPs can be increased by its attachment with HP, which interacts with the growth hormones, attached to their receptors on the tumor cell membrane, rendering an increased uptake of drug-loaded nanoparticles.35
In the present study, the drug release rate from HP–SPIO NPs was higher at acidic pH (pH 6.0) than at neutral pH (pH 7.4), which may lead to increased accumulation of DOX and PTX in tumor cells and thereby add therapeutic efficiency to the delivery system.36 After delivery to the tumor site, the next important step is internalization into the tumor cell. This is directly related to the cytotoxicity of the drug, because DOX and PTX only show their antitumor efficiency when they bind to DNA or inhibit microtubule disassembly. In this study, the authors found the DOX–HP–SPIO NPs and PTX–HP–SPIO NPs were readily taken up by A2780 and OVCAR-3 cells, with a higher rate of cellular uptake and of larger amount than the free drugs. The drug-loaded SPIO NPs can be transported into tumor cells by a process called endocytosis or phagocytosis, through either specific or nonspecific cellular uptake, depending on the surface properties of the SPIO NPs.37 However, the exact mechanism of cellular uptake may be far more complicated than the current understanding, and further studies are clearly needed.
In vitro, the nanocomposite-based DOX and PTX were found to show a higher apoptosis-inducing effect in both cell lines than free drugs. These results are in agreement with previous reports. Kohler et al.reported that the methotrexate-immobilized poly(ethylene glycol) SPIO NPs induce higher cytotoxicity in glioma cells than free methotrexate, depending on higher uptake and retaining its crystal structure in the cell cytoplasm.38 Chen et al. reported that the application of 5-bromotetrandrine and SPIO-NPs inhibited the expression of bcl-2 protein and upregulated the expression of bax and caspase-3 proteins in human leukemia K562 cells.39,40 These results are in line with our western blot and apoptosis analyses. The present results reveal that the SPIO NPs may suppress tumor cell proliferation and induce apoptosis by blocking multiple pathways.
4. Conclusions
In summary, we constructed drug-loaded HP–SPIO NPs by a co-precipitation technique for intratumoral drug delivery. The nanoparticles supported sustained and steady release of DOX and PTX. Moreover, drug release from the HP–SPIO NPs was pH sensitive, with a faster release rate in an acidic environment than in a neutral environment. In vitro, the DOX–HP–SPIO NPs were readily internalized into tumor cells, and they induced a higher apoptosis rate. The heparin coating on nanoparticle surface and its use in targeted drug delivery was demonstrated to increase the amount of NP uptake into ovarian cancer cells along with dramatic anti-neoplastic activity, in comparison to free drugs. This suggests that the modification of SPIO NPs with HP could be used to formulate a better drug delivery vehicle and simultaneously facilitate the drug uptake to specific cancer cells for successful cancer therapy. This work provides an exciting new modality for developing an effective drug delivery system.Acknowledgements
The authors are thankful to the Research Council of the University of Tehran, Tehran, Iran, for its financial support of the present work.References
- F. Dilnawaz, A. Singh, C. Mohanty and S. K. Sahoo, Biomaterials, 2010, 31, 3694 CrossRef CAS PubMed.
- C. S. S. R. Kumar and F. Mohammad, Adv. Drug Delivery Rev., 2011, 63, 789 CrossRef CAS PubMed.
- M. W. Freeman, A. Arrott and J. H. L. Watson, J. Appl. Phys., 1960, 31, S404 CrossRef PubMed.
- H. Basti, L. B. Tahar, L. S. Smiri, F. Herbst, M. Vaulay, F. Chau, S. Ammara and S. Benderbous, J. Colloid Interface Sci., 2010, 341, 248 CrossRef CAS PubMed.
- D. H. Kim, K. N. Kim, K. M. Kim and Y. K. Lee, J. Biomed. Mater. Res., Part A, 2009, 88, 1 CrossRef PubMed.
- M. Mahmoudi, S. Sant, B. Wang, S. Laurent and T. Sen, Adv. Drug Delivery Rev., 2011, 63, 24 CrossRef CAS PubMed.
- L. Douziech-Eyrolles, H. Marchais and K. Herve, Int. J. Nanomed., 2007, 2, 541 Search PubMed.
- X. Wang, Y. Wang, Z. G. Chen and D. M. Shin, Cancer Research and Treatment, 2009, 41, 1 CrossRef CAS PubMed.
- S. Acharya and S. K. Sahoo, Adv. Drug Delivery Rev., 2011, 63, 170 CrossRef CAS PubMed.
- M. M. Kemp and R. J. Linhardt, WIREs Nanomedicine and Nanobiotechnology, 2010, 2, 77 CrossRef CAS PubMed.
- N. Meng, S. Zhang, N. Zhou and J. Shen, Nanotechnology, 2010, 21, 185 Search PubMed.
- A. Villanueva, M. Cañete, A. G. Roca, M. Calero, S. Veintemillas-Verdaguer and C. J. Serna, Nanotechnology, 2009, 20, 1 CrossRef PubMed.
- S. J. Kyoung, D. P. Hyung, D. P. Ki, H. K. Young and S. Jung-Wong, Biomacromolecules, 2004, 5, 1877 CrossRef PubMed.
- K. K. Sang, V. Bagalkot, L. Eunhye, L. Seulki, L. Yong-kyu, S. K. Tadiparthi, T. M. Hyun and Y. Byun, Thromb. Res., 2006, 117, 419 CrossRef PubMed.
- M. J. Jung, S. S. Lee, Y. H. Hwang, H. S. Jung, J. W. Hwang, M. J. Kim, S. Yoon and D. Y. Lee, Biomaterials, 2011, 32, 9391 CrossRef CAS PubMed.
- S. Mohammadi-Samani, R. Miri, M. Salmanpour, N. Khalighian, S. Sotoudeh and N. Erfani, Res. Pharm. Sci., 2013, 8, 25 CAS.
- N. Saifuddin and S. Dinara, Adv. Nat. Appl. Sci., 2012, 6, 249 CAS.
- X. Liu, M. D. Kaminski, Y. Guan, H. Chen, H. Liu and A. J. Rosengart, J. Magn. Magn. Mater., 2006, 306, 248 CrossRef CAS PubMed.
- E. Strable, J. W. M. Bulte, B. Moskowitz, K. Vivekanandan, M. Allen and T. Douglas, Chem. Mater., 2001, 13, 2201 CrossRef CAS.
- T. K. Jain, M. A. Morales, S. K. Sahoo, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharmacol., 2005, 2, 194 CrossRef CAS PubMed.
- M. Vandana and S. Sahoo, Nanomedicine, 2009, 4, 773 CrossRef CAS PubMed.
- S. K. Sahoo and V. Labhasetwar, Drug Discovery Today, 2003, 8, 1112 CrossRef CAS.
- M. Ozmena, K. Can, G. Arslan, A. Tor, Y. Cengeloglu and M. Ersoz, Desalination, 2010, 254, 162 CrossRef PubMed.
- R. V. Chari, Acc. Chem. Res., 2008, 41, 98 CrossRef CAS PubMed.
- T. Murakami, K. Tsuchida, M. Hashida and H. Imahori, Mol. BioSyst., 2010, 6, 789 RSC.
- C. M. Springate, J. K. Jackson, M. E. Gleave and H. M. Burt, Int. J. Pharmacol., 2008, 350, 53 CrossRef CAS PubMed.
- T. Neuberger, B. Schopf, H. Hofmann, M. Hofmann and B. von Rechenberg, J. Magn. Magn. Mater., 2005, 293, 48 CrossRef PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995 CrossRef CAS PubMed.
- H. S. Choi, W. Liu and P. Misra, Nat. Biotechnol., 2007, 25, 1165 CrossRef CAS PubMed.
- C. Chouly, D. Pouliquen, I. Lucet, J. J. Jeune and P. Jallet, J. Microencapsulation, 1996, 13, 245 CrossRef CAS PubMed.
- J. Lee, M. Jin Jung, Y. H. Hwang, Y. J. Lee, D. S. Lee, D. Y. Lee and H. Shin, Biomaterials, 2012, 33, 4861 CrossRef CAS PubMed.
- A. S. Arbab, G. T. Yocum, H. Kalish, E. K. Jordan, S. A. Anderson and A. Y. Khakoo, Blood, 2004, 104, 1217 CrossRef CAS PubMed.
- A. Javid, S. Ahmadian, A. A. Saboury, S. M. Kalantar and S. Rezaei-Zarchi, Chem. Biol. Drug Des., 2013, 82, 296 CAS.
- A. M. C. G. Dias, A. Hussain, A. S. Marcos and A. S. A. Roque, Biotechnol. Adv., 2011, 29, 142 CrossRef CAS PubMed.
- D. Lee, Macromol. Res., 2011, 19, 843 CrossRef CAS PubMed.
- L. Li, J. K. Kim, K. M. Huh, Y. Lee and S. Y. Kim, Carbohydr. Polym., 2012, 87, 2120 CrossRef CAS PubMed.
- X. Huang, X. Teng, D. Chen, F. Tang and J. He, Biomaterials, 2010, 31, 438 CrossRef CAS PubMed.
- N. Kohler, C. Sun, A. Fichtenholtz, J. Gunn, C. Fang and M. Zhang, Small, 2006, 2, 785 CrossRef CAS PubMed.
- B. Chen, J. Cheng and Y. Wu, Int. J. Nanomed., 2009, 4, 73 CrossRef CAS.
- S. K. Sahu, S. Maiti, A. Pramanik, S. K. Ghosh and P. Pramanik, Carbohydr. Polym., 2012, 87, 2593 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |







