Porphyrins with β-acetylene-bridged functional groups for efficient dye-sensitized solar cells†
Tomoaki Sakurada,
Yonbon Arai* and
Hiroshi Segawa*
Research Center for Advanced Science and Technology, The University of Tokyo, 4-6-1, Komaba, Meguro-ku, Tokyo 153-8904, Japan. E-mail: arai@dsc.rcast.u-tokyo.ac.jp; csegawa@mail.ecc.u-tokyo.ac.jp; Fax: +81-3-5452-5299; Tel: +81-3-5452-5140
First published on 6th March 2014
Abstract
A series of porphyrins with mono or double acetylene-bridged functional groups at pyrrolic β-positions were synthesized and a systematic study on the β-substituents in porphyrin-sensitized solar cells demonstrates the superiority of the double-phenylcarboxyl anchor, leading to over 90% IPCE and an overall efficiency of 5.7%.
Dye-sensitized solar cells (DSSCs) have attracted a great deal of attention since the breakthrough by O'regan and Grätzel.1–7 Developing dye molecules with suitable properties and anchor moieties is a central task to achieve better DSSC performance. Appropriate selection of π-conjugated bridges between the dye chromophore and anchoring groups is important to improve light harvesting and photon-to-electron conversion efficiencies. Also, the type of anchoring groups including electron-acceptor moiety has significant influence on electron injection efficiency, where carboxylic acid and pyridine based-groups are usually more efficient.5 Since a suitable combination of π-conjugated bridges and acceptor/anchor groups depend on dye structure, systematic studies on these moieties in DSSCs are essential.
Porphyrins are one of the most promising dyes for the DSSC sensitizers6–8 since a zinc porphyrin derivative (YD2-o-C8) yielded the highest overall efficiency of DSSCs.8 To date, a great variety of carboxylic acid based acceptor/anchor groups with π-conjugated bridges including unsaturated alkyl groups and aromatic rings were introduced at meso8–10,12,13 and pyrrolic β-positions.11–13 Among others, porphyrin derivatives with an acetylene-bridged phenylcarboxyl group at a meso position achieved a distinctively high overall efficiencies.8,9 In spite of such a superior feature, the introduction of acetylene-bridged acceptor/anchors at β-positions into porphyrin-sensitized solar cells remains to be only a few examples, yielding less than 2.7%.13
Herein, we performed a systematic study on DSSCs with zinc porphyrins with acetylene-bridged functional groups at β-positions regarding the type and number of acceptor/anchor groups. The interested functional groups consist of phenylcarboxyl, fluorophenylcarboxyl, thienylcyanoacryl and pyridinyl groups (Chart 1). Other than P-PhCOOH and P-Py,14 the porphyrin derivatives have been first prepared in this study. DSSCs were constructed using porphyrin-sensitized nanoporous TiO2 films, an I3−/I− electrolyte, and a Pt counter electrode. Detailed procedures of the porphyrin synthesis and devise fabrication are shown in ESI.†
 | ||
| Chart 1 Molecular structures of zinc porphyrins with acetylene-bridged functional groups at β-positions. | ||
Density functional theory (DFT) calculations exhibited that all the porphyrins have electron density of LUMO at their anchoring groups (Fig. S1†), which would be desirable for efficient electron injection to TiO2 conduction band. UV-vis spectra show that absorption bands of P-(PhCOOH)2 significantly red-shifts compared with P-PhCOOH (Fig. 1 and Table 1), which is ascribed to the extension of π-conjugated system (Fig. S1†). While P-PhCOOH, P-FPhCOOH, and P-Py have similar spectra, P-ThCNCOOH exhibits a clear shoulder peak at longer wavelengths of the Soret band. This can be attributed to a significant lowering of the LUMO+2 level of P-ThCNCOOH (Fig. S1†).14
| Dye | λmaxa/nm | λmaxb/nm | E0–0c/V | EOxd/V | Eox*e/V |
|---|---|---|---|---|---|
| a Absorption wavelengths at Soret and Q band maxima in THF.b Fluorescence wavelengths at emission maxima in THF by exciting at Soret band.c E0–0 was determined from the interception of absorption and emission spectra (vs. SCE).d First oxidation potentials determined by using differential pulse voltammetry in THF containing n-Bu4NPF6 as a supporting electrolyte (vs. SCE).e Excited state oxidation potentials approximated from E0–0 and EOx (vs. SCE). | |||||
| P-PhCOOH | 436, 565, 603 | 619 | 2.00 | 1.00 | −1.03 |
| P-FPhCOOH | 433, 563, 602 | 618 | 2.01 | 1.03 | −1.00 |
| P-ThCNCOOH | 437, 475(sh), 568, 606 | 624 | 1.99 | 1.01 | −1.01 |
| P-Py | 435, 565, 603 | 620 | 2.00 | 1.03 | −1.00 |
| P-(PhCOOH)2 | 455, 576, 618 | 649 | 1.91 | 1.02 | −0.95 |
To examine energy level matching, fluorescence spectra and differential pulse voltammograms of the porphyrins were recorded (Fig. S2 and S3†). Obtained oxidation potentials of the HOMOs and the exited states (Table 1) indicate that electron transfer reactions of the porphyrins with TiO2 (∼−0.7 V) and I3−/I− (∼0.2 V) are thermodynamically feasible.
All DSSC characterizations were performed using a typical electrolyte condition (0.1 M LiI, 0.6 M 1,2-dimethyl-3-propylimidazolium iodide, 0.025 M I2 and 0.5 M 4-tert-butyl pyridine in acetonitrile). Since porphyrins often form aggregates reducing photovoltaic performance, deoxycholic acid (DCA) was used as a coadsorbent and the effect of the mixing ratios of DCA to porphyrin on incident-photon-to-current conversion efficiency (IPCE) was investigated. With increasing DCA concentration, increased IPCE values were observed for all the porphyrins (Fig. 2 and S4†). Without DCA, P-(PhCOOH)2 yields the lowest IPCE maximum of 51% among the porphyrins. Interestingly, the DCA addition substantially increases the IPCE maximum of P-(PhCOOH)2 up to 92%, which is higher than those of the other porphyrins (76–80%). Such a larger IPCE change of the double-substituted porphyrin than the mono-substituted ones indicates its stronger aggregation tendency, which could be caused by increased π–π interaction. Investigation of absorption spectral changes of P-(PhCOOH)2 on TiO2 with increasing DCA concentration showed sharpening and red-shift of the absorption band, indicating that the porphyrin tends to form H-type aggregates (Fig. S5†).15 Assuming that unfavorable aggregates of all the porphyrins were sufficiently suppressed by the DCA addition, the internal quantum efficiency of P-(PhCOOH)2 is estimated to be higher than the other porphyrins considering sufficient light harvesting at the regions of IPCE maxima (Fig. S6†). Furthermore, taking into consideration comparable fluorescence yields (Fig. S2†) and Eox* values (Table 1) for all the porphyrins, it is suggested that the double-acetylene bridged anchor is a more efficient mediator of electron transfer from excited porphyrin to TiO2 than the mono ones.
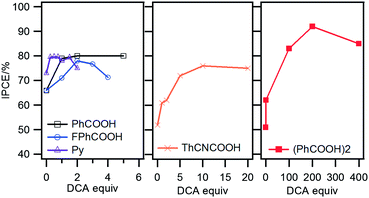 | ||
| Fig. 2 Plots of IPCE maxima of the porphyrin-sensitized solar cells as a function of the mixing ratio of DCA to porphyrin. | ||
IPCE spectra of the DSSCs at optimized DCA conditions show that P-(PhCOOH)2 yields extended photocurrent at longer wavelengths than the other porphyrins (Fig. 3), which corresponds to the absorption spectra (Fig. 1). Photovoltaic parameters obtained from photocurrent–voltage curves at the optimized DCA conditions (Fig. S7†) show a distinctly higher overall efficiency of 5.7% for P-(PhCOOH)2 than the other porphyrins (Table 2). This could originate from the improvements in electron injection and light harvesting abilities. It is noted that P-PhCOOH and P-FPhCOOH give comparable η values, which is distinct from the case of meso-substitution of a porphyrin derivative by the same acetylene-bridged anchors.10h,i More detailed roles of the acceptor/anchor types in the porphyrin-sensitized solar cells are under investigation.
| Dye | JSC/mA cm−2 | VOC/mV | FF | η/% |
|---|---|---|---|---|
| a Under AM1.5 illumination (100 mW cm−2). | ||||
| P-PhCOOH | 10.6 | 632 | 0.74 | 4.9 |
| P-FPhCOOH | 9.62 | 653 | 0.73 | 4.5 |
| P-ThCNCOOH | 9.67 | 646 | 0.74 | 4.6 |
| P-Py | 8.62 | 629 | 0.74 | 4.0 |
| P-(PhCOOH)2 | 12.8 | 628 | 0.73 | 5.7 |
To compare the adsorption strength of the porphyrins on TiO2, the desorption times of porphyrin-adsorbed TiO2 films in soluble basic solution (THF with 0.01 M NaOH) were estimated. Importantly, P-(PhCOOH)2 resulted in no desorption after 5 h, whereas all the other porphyrins desorbed completely within the time (Fig. S8†). Such a stronger adsorption property of P-(PhCOOH)2 may be beneficial for longer durability of DSSCs.
In conclusion, some substitution effects of acetylene-bridged acceptor/anchor groups at β-positions in porphyrin-sensitized solar cells were revealed. While a double substitution at a pyrrole ring leads to a poorer IPCE than mono substitutions, the presence of a coadsorbent makes the double substitution lead to near unity IPCE, surpassing IPCE maxima by the mono substitutions. Also, the double substitution leads to longer wavelength photocurrent and higher overall efficiency than the mono substitutions. In addition, the double substitution makes distinctively stronger adsorption state on TiO2 than the mono substitutions. Therefore, the introduction of a double acetylene-bridged acceptor/anchor at β-positions of a pyrrole ring would be a promising approach for creating highly efficient porphyrin sensitizers. Further efficiency improvement could be plausible by proper modifications of the other meso- and/or β-positions with electron-releasing and/or bulky groups.8
We thank Prof. S. Uchida and Prof. J. Nakazaki for technical advises. This work was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST) “Development of Organic Photovoltaics toward a Low-Carbon Society” from the Cabinet Office of Japan.
Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- L. Giribabu, R. K. Kanaparthi and V. Velkannan, Chem. Rec., 2012, 12, 306 CrossRef CAS PubMed.
- Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032 CAS.
- M. J. Griffith, K. Sunahara, P. Wagner, K. Wagner, G. G. Wallace, D. L. Officer, A. Furube, R. Katoh, S. Mori and A. J. Mozer, Chem. Commun., 2012, 48, 4145 CAS.
- L.-L. Li and E. W.-G. Diau, Chem. Soc. Rev., 2013, 42, 291 CAS.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CAS.
- (a) T. Bessho, S. M. Zakeeruddin, C. Y. Yeh, E. W.-G. Diau and M. Grätzel, Angew. Chem., Int. Ed., 2010, 49, 6646 CrossRef CAS PubMed; (b) C.-L. Wang, Y.-C. Chang, C.-M. Lan, C.-F. Lo, E. W.-G. Diau and C.-Y. Lin, Energy Environ. Sci., 2011, 4, 1788 RSC; (c) Y. C. Chang, C. L. Wang, T. Y. Pan, S. H. Hong, C. M. Lan, H. H. Kuo, C. F. Lo, H. Y. Hsu, C. Y. Lin and E. W.-G. Diau, Chem. Commun., 2011, 47, 8910 CAS; (d) C. H. Wu, T. Y. Pan, S. H. Hong, C. L. Wang, H. H. Kuo, Y. Y. Chu, E. W.-G. Diau and C. Y. Lin, Chem. Commun., 2012, 48, 4329 CAS; (e) C.-L. Wang, C.-M. Lan, S.-H. Hong, Y.-F. Wang, T.-Y. Pan, C.-W. Chang, H.-H. Kuo, M.-Y. Kuo, E. W.-G. Diau and C.-Y. Lin, Energy Environ. Sci., 2012, 5, 6933 RSC.
- (a) J. Rochford, D. Chu, A. Hagfeldt and E. Galoppini, J. Am. Chem. Soc., 2007, 129, 4655 CrossRef CAS PubMed; (b) S. Eu, S. Hayashi, T. Umeyama, A. Oguro, M. Kawasaki, N. Kadota, Y. Matano and H. Imahori, J. Phys. Chem. C, 2007, 111, 3528 CAS; (c) M. Tanaka, S. Hayashi, S. Eu, T. Umeyama, Y. Matano and H. Imahori, Chem. Commun., 2007, 2069 CAS; (d) S. Eu, S. Hayashi, T. Umeyama, Y. Matano, Y. Araki and H. Imahori, J. Phys. Chem. C, 2008, 112, 4396 CrossRef CAS; (e) C.-Y. Lin, C.-F. Lo, L. Luo, H.-P. Lu, C.-S. Hung and E. W.-G. Diau, J. Phys. Chem. C, 2009, 113, 755 CrossRef CAS; (f) H. P. Lu, C. L. Mai, C. Y. Tsia, S. J. Hsu, C. P. Hsieh, C. L. Chiu, C. Y. Yeh and E. W.-G. Diau, Phys. Chem. Chem. Phys., 2009, 11, 10270 RSC; (g) H. Imahori, S. Hayashi, H. Hayashi, A. Oguro, S. Eu, T. Umeyama and Y. Matano, J. Phys. Chem. C, 2009, 113, 18406 CrossRef CAS; (h) S. Mathew, H. Iijima, Y. Toude, T. Umeyama, Y. Matano, S. Ito, N. V. Tkachenko, H. Lemmetyinen and H. Imahori, J. Phys. Chem. C, 2011, 115, 14415 CAS; (i) A. O. Biroli, F. Tessore and M. Pizzotti, et al., J. Phys. Chem. C, 2011, 115, 23170 Search PubMed; (j) Y. Liu, H. Lin, J. T. Dy, K. Tamaki, J. Nakazaki, D. Nakayama, S. Uchida, T. Kubo and H. Segawa, Chem. Commun., 2011, 47, 4010 CAS; (k) Y. Liu, N. Xiang, X. Feng, P. Shen, W. Zhou, C. Weng, B. Zhao and S. Tan, Chem. Commun., 2009, 2499 RSC; (l) W. Zhou, B. Zhao, P. Shen, S. Jiang, H. Huang, L. Deng and S. Tan, Dyes Pigm., 2011, 91, 404 CAS; (m) C.-W. Lee, H.-P. Lu, N. M. Reddy, H.-W. Lee, E. W.-G. Diau and C.-Y. Yeh, Dyes Pigm., 2011, 91, 317 CAS; (n) N. Xiang, X. Huang, X. Feng, Y. Liu, B. Zhao, L. Deng, P. Shen, J. Fei and S. T. Tan, Dyes Pigm., 2011, 88, 75 CAS; (o) W. Zhou, Z. Cao, S. Jiang, H. Huang, L. Deng, Y. Liu, P. Shen, B. Zhao, S. Tan and X. Zhang, Org. Electron., 2012, 13, 560 CAS; (p) C. Jiao, N. Zu, K.-W. Huang, P. Wang and J. Wu, Org. Lett., 2011, 13, 3652 CAS; (q) E. M. Barea, R. Caballero, L. López-Arroyo, A. Guerrero, P. de la Cruz, F. Langa and J. Bisquert, ChemPhysChem, 2011, 12, 961 CrossRef CAS PubMed; (r) K. K. Pasunooti, J.-L. Song, H. Chai, P. Amaladass, W.-Q. Deng and X.-W. Liu, J. Photochem. Photobiol., A, 2011, 218, 219 CrossRef CAS PubMed; (s) J. Warnan, L. Favereau, F. Meslin, M. Severac, E. Blart, Y. Pellegrin, D. Jacquemin and F. Odobel, ChemSusChem, 2012, 5, 1568 CrossRef CAS PubMed; (t) B. Pelado, P. de la Cruz, V. González-Pedro, E. M. Barea and F. Langa, Tetrahedron Lett., 2012, 53, 6665 CAS; (u) H. He, A. Gurung, L. Si and A. G. Sykes, Chem. Commun., 2012, 48, 7619 CAS; (v) C. Y. Lee, C. X. She, N. C. Jeong and J. T. Hupp, Chem. Commun., 2010, 46, 6090 CAS.
- (a) Q. Wang, W. M. Carnpbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397 CrossRef CAS PubMed; (b) L. Schmidt-Mende, W. M. Campbell, Q. Wang, K. W. Jolley, D. L. Officer, M. K. Nazeeruddin and M. Grätzel, ChemPhysChem, 2005, 6, 1253 CrossRef PubMed; (c) W. M. Campbell, K. W. Jolley, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. Schmidt-Mende, M. K. Nazeeruddin, Q. Wang, M. Grätzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 1760 CrossRef; (d) J. K. Park, H. R. Lee, J. P. Chen, H. Shinokubo, A. Osuka and D. Kim, J. Phys. Chem. C, 2008, 112, 16691 CAS; (e) M. Ishida, S. W. Park, D. Hwang, Y. B. Koo, J. L. Sessler, D. Y. Kim and D. Kim, J. Phys. Chem. C, 2011, 115, 19343 CrossRef CAS.
- R. Deshpande, B. Wang, L. Dai, L. Jiang, C. Scott Hartley, S. Zou, H. Wang and L. Kerr, Chem. – Asian J., 2012, 7, 2662 CrossRef CAS PubMed.
- C. W. Lee, H. P. Lu, C. M. Lan, Y. L. Huang, Y. R. Liang, W. N. Yen, Y. C. Liu, Y. S. Lin, E. W.-G. Diau and C. Y. Yeh, Chem.–Eur. J., 2009, 15, 1403 CrossRef CAS PubMed.
- J. C. Earles, K. C. Gordon, A. W. I. Stephenson, A. C. Partridge and D. L. Officer, Phys. Chem. Chem. Phys., 2011, 13, 1597 RSC.
- (a) M. Kasha, Radiat. Res., 1963, 20, 55 CAS; (b) C.-F. Lo, L.-Y. Luo, E. W.-G. Diau, I.-J. Chang and C.-Y. Lin, Chem. Commun., 2006, 1430 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra41317a |
| This journal is © The Royal Society of Chemistry 2014 |


