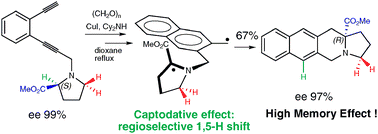
Shovan Mondal, Malek Nechab, Nicolas Vanthuyne and Michèle P. Bertrand
Chem. Commun., 2012,48, 2549-2551
DOI:
10.1039/C2CC17830C,
Communication
The tandem Crabbé homologation-radical rearrangement of terminal