A review on framework (MOF/COF/POP)-based materials for efficient conversion of CO2 to bio-active oxazolidinones
Pooja
Rani
,
Rajesh
Das†
 and
C. M.
Nagaraja
and
C. M.
Nagaraja
 *
*
Department of Chemistry, Indian Institute of Technology Ropar, Rupnagar-140001, Punjab, India. E-mail: cmnraja@iitrpr.ac.in; Tel: +91-1881-232057
First published on 29th November 2024
Abstract
Excessive reliance on fossil fuels has increased atmospheric CO2 emissions, resulting in the greenhouse effect that endangers global climate stability and human well-being. Consequently, the storage and chemical conversion of CO2 into sustainable products can play a vital role in reducing anthropogenic emissions. Hence, there is an upsurge in research on selective carbon capture, sequestration and utilization (CCSU) to mitigate the rising atmospheric CO2 concentration. Carbon capture and utilization (CCU), in particular, has attracted considerable interest because it enables the utilization of CO2 as a C1 feedstock for generating commodity chemicals and fuels such as cyclic or polycarbonates, cyclic carbamates, oxazolidinones, formamides, methane, methanol and so on. Among these products, oxazolidinones are essential five-membered heterocyclic compounds found in several important pharmaceuticals. Oxazolidinones also function as versatile intermediates and chiral agents in organic synthesis. Thus, developing highly efficient heterogeneous catalysts containing dense basic and catalytic sites is potentially significant for effectively capturing and transforming CO2 into 2-oxazolidinones under ambient conditions. In this regard, porous framework-based materials viz metal–organic frameworks (MOFs), covalent organic frameworks (COFs) and porous organic polymers (POPs) are excellent candidates owing to their fascinating attributes, like ample active sites, intrinsic porosity and accessible functionalities. These framework-based materials have been exploited as recyclable catalysts in efficient cyclization of CO2 with aziridines, propargylic amines and alcohols coupled with amines/epoxides to produce oxazolidinones. This review provides a detailed analysis of recent advancements in developing porous framework-based recyclable catalysts for environmentally friendly conversion of CO2 to oxazolidinones. Furthermore, future considerations and challenges for fabricating efficient framework-based catalysts in transforming CO2 into value-added oxazolidinones are also discussed.
1. Introduction
The escalating energy demand driven by excessive population progression is primarily fulfilled through the widespread utilization of fossil fuels like natural gas, petroleum and coal combustion, leading to extensive release of greenhouse gases like CO2, nitrogen oxide and fluorinated gases, etc. (Fig. 1).1–3 As a result, CO2 concentration in the atmosphere is increasing with time, from 280 ppm (early 1800s) to 421 ppm (2024) and it continues to rise.4,5 This rapid escalation in atmospheric CO2 has resulted in several detrimental environmental issues, such as ocean acidification, climate change, global warming and other related challenges.6–8 Therefore, it is crucial to establish sustainable procedures to alleviate the increasing CO2 concentration in the atmosphere. In this context, CO2 capture and storage/sequestration (CCS) has been employed to mitigate CO2 emissions from industries and power plants.9,10 However, the current CCS technology necessitates significant energy for separation, compression, transportation and storage.11–13 | ||
| Fig. 1 (a) Global greenhouse gas emission from various processes. (b) Global greenhouse gas emission by economic sector.1–3 | ||
An alternative value-added approach is carbon capture and utilization (CCU), where CO2 serves as a C1 feedstock for producing value-added compounds,14–16 such as cyclic17,18 or polycarbonates,19 high-value formamides,20 cyclic carbamates,21 oxazolidinones22,23 and so on. Moreover, the generation of C1 (CO, CH4, HCHO, HCOO−, CH3OH) and C2 (C2H5OH) products can be accomplished by the reduction of CO2 with the application of appropriate catalysts (Fig. 2).24,25
Notably, oxazolidinones represent a significant class of crucial five-membered heterocyclic compounds that are vital in various active pharmaceutical agents. These compounds are noteworthy for their development as novel synthetic antibiotics, which have demonstrated remarkable efficacy in the treatment of multiple drug-resistant Gram-positive bacterial infections.26 For instance, tedizolid phosphate is a highly effective medication against Gram-positive bacteria, known for its low incidence of drug resistance and minimal adverse reactions, making it particularly suitable for elderly individuals and children.27 Similarly, linezolid effectively inhibits bacterial protein synthesis and is compatible with other medications, making it a widely utilized treatment for bacterial skin infections (Fig. 3).28 Beyond their applications in medicine, oxazolidinones also hold promise in organic synthesis serving as versatile intermediates and chiral agents in asymmetric synthesis, which is essential for producing a wide range of pharmaceuticals and fine chemicals.
 | ||
| Fig. 3 Important value-added antibiotics and drugs comprising different 2-oxazolidinone derivatives. | ||
This versatility contributes significantly to their economic and medical value on a global scale, as they facilitate the development of new compounds that can lead to more effective therapies and innovations in chemical manufacturing.29 Moreover, the demand for oxazolidinone compounds extends beyond the pharmaceutical sector. They are increasingly sought after in various industries, including agriculture, where they are used in the formulation of pesticides, insecticides30 and dye industry, further broadening their utility.31 Given the diverse applications of oxazolidinones and the growing need for sustainable practices in chemical synthesis, developing green and efficient synthesis methods for these compounds has become increasingly important. Therefore, the exploration of oxazolidinones is not only significant for advancing medical treatments but also for fostering economic growth and sustainability in various industrial applications.
Several methods have been developed for synthesizing oxazolidinones, with the CO2 cycloaddition reaction standing out as particularly challenging yet promising in organic chemistry. Recently, notable advances have been made in this area. CO2 can be used as a safer alternative to toxic reagents like phosgene to produce cyclic carbamates through coupling reactions with amines. Several efficient synthetic routes have been designed to incorporate CO2 into cyclic carbamates for the production of oxazolidinones.32 Thus, it is indispensable to develop effective catalytic systems for sustainable synthesis of oxazolidinones by utilization of the greenhouse gas CO2 under mild conditions. However, the inert nature of CO2 presents a considerable obstacle to its functionalization under ambient conditions.33 Consequently, researchers worldwide are dedicating significant efforts in developing high-performance catalytic systems for efficient CCU to produce valuable oxazolidinones.34,35 To accomplish this goal, the catalyst employed must demonstrate strong CO2 affinity, excellent catalytic activity and exceptional moisture stability. Moreover, to efficiently utilize CO2 from direct air or flue gas of industries, the catalyst should possess high CO2-philic sites for effective capture from low concentrations and catalytic active sites for promising conversion to oxazolidinones.36 In this context, researchers worldwide are investigating the application of various catalysts, such as homogeneous metal complexes,37,38 inorganic semiconductors,39 ionic liquids40 and carbonaceous materials,41 in CO2 capture and conversion. However, the exact structure–activity relationship of these catalysts is not well understood. Among various heterogeneous catalytic systems explored for CCU, framework-based materials, particularly MOFs and COFs/POPs, present distinct advantages owing to their modular design featuring customized pore size and functionality.42 Furthermore, MOFs43 and COFs44 provide versatility in combining various features like introducing high density of CO2-philic and catalytic sites, which further empowers them with immense potential in selectively capturing and converting CO2 into oxazolidinones.
For the past two decades, there has been a notable increase in the utilization of MOFs within the realm of CCU.45,46 Owing to the unique combination of inorganic and organic building blocks, MOFs/PCPs exhibit distinctive characteristics like tunable pore size, exceptionally high surface area and functionality, making them ideal candidates for a range of applications, including selective gas storage,47 separation,48 catalysis,49–52 sensing,53–55 drug delivery,56 and so on. MOFs are assembled by connecting metal ions (nodes) or clusters (SBUs) with multidentate organic spacers, resulting in frameworks exhibiting various structural architectures. The network topology and functionality of MOFs/PCPs can be customized by strategically selecting organic spacers to generate infinite 1D, 2D, or 3D network structures.57
On the other hand, COFs are a novel class of organic polymers characterized by permanent porosity, high crystallinity and structured architectures akin to MOFs. Moreover, COFs have customizable chemical and physical properties, making them ideal applicants for diverse applications, including gas storage,58 separation,59 photoelectricity60 and catalysis, particularly in CO2 capture and conversion.61 Highly effective COFs/POPs can be rationally designed by utilizing organic linkers containing basic sites like azine (C–N–N–C), azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), imine (C
N), imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and triazine moieties to proficiently capture CO2 and transform it into valuable products under mild conditions.62 Thus, the strategic development of framework-based materials has seen a rapid increase in establishing an ideal platform for selectively capturing and utilizing CO2 under mild conditions. This is apparent from the rise in the number of publications in this area over the past decade (Fig. 4).
N) and triazine moieties to proficiently capture CO2 and transform it into valuable products under mild conditions.62 Thus, the strategic development of framework-based materials has seen a rapid increase in establishing an ideal platform for selectively capturing and utilizing CO2 under mild conditions. This is apparent from the rise in the number of publications in this area over the past decade (Fig. 4).
The current research on CO2 transformation to value-added products catalyzed by porous framework-based materials is highly advanced. Some excellent reviews focussed on the necessity of CCU and its applications are reported.63–65 Based on the current knowledge, several high-quality reviews have been published in the scientific literature that highlights the advancements in the progress of MOFs and COFs supported catalysts for CO2 conversion to cyclic carbonates66–69 and fuels.70 For instance, He and co-workers systematically discussed CO2 transformations over nanomaterials.71 Singh and co-workers comprehensively reviewed advancements in porous materials for CO2 capture and utilization.72 Cao and co-workers comprehensively discussed CO2 transformation to useful products over porous MOFs and COFs via thermo-, electro- and photocatalytic processes.73 However, no comprehensive review has addressed porous framework-based materials for CO2 transformation to oxazolidinones. Thus, it is advantageous to review the recent advancements in CO2 conversion using MOFs and COFs/POPs-based catalysts and examine approaches for enhancing the activity of these catalysts towards sustainable synthesis of oxazolidinones.
The present review includes a comprehensive exploration of the progress made in developing recyclable catalysts derived from MOFs and COFs/POPs for environmentally sustainable conversion of CO2 into oxazolidinones. Various methods of synthesizing oxazolidinones from CO2 are thoroughly discussed, displaying the advancements in MOF/COF structures on carbon dioxide conversion to oxazolidinones. Also, the underlying mechanism for CO2 conversion to oxazolidinones has been described. Furthermore, we offer insights into the challenges that must be tackled and potential avenues for future research. We anticipate that the present review will promote a comprehensive understanding of CO2 transformation reactions to oxazolidinone synthesis over framework-based materials, serving as a treasured resource for researchers working in this domain and will be pivotal for further investigations into effective CO2 utilization.
2. Rational design of MOFs and COFs/POPs for CO2 utilization
2.1. Design of MOFs for CO2 utilization
Over the last two decades, MOFs have become a significant part of porous crystalline reticular frameworks owing to their multi-functional applications. MOFs are constructed by rationally assembling metal nodes and organic linkers (Scheme 1) with desired structural and functional properties. This reticular synthesis allows for the design of frameworks with tailored properties. MOFs feature open metal sites (OMS), Lewis basic sites (LBS), polar functional groups, tunable pore sizes and hydrophobic stability, making them ideal for CO2 adsorption and conversion reactions. The following discussion includes a brief overview of the rational design and synthesis of CO2-philic MOFs for efficient transformation of carbon dioxide to value-added oxazolidinones.From the aforementioned discussion, it is apparent that for effective capture and conversion of CO2 into value-added chemicals under mild conditions, it is essential to rationally design frameworks by incorporating basic and active sites. In this context, Liu et al. rationally designed a linker (Scheme 1, H2L8) having dual functional groups (carboxyl and triazole) wherein the –COOH group forms classical paddlewheel CuII2 clusters and the triazole group supports stabilizing CuI clusters having high activity. The synergistic effects of CuI and CuII clusters boosted the CO2 conversion efficiency. Further, the application of aminotriazoles as building blocks has resulted in frameworks with abundant LB sites exhibiting high adsorption capacity of CO2 along with conversion efficiency.88
Thus, the rational choice of the linkers facilitates the successful preparation of MOFs with optimal properties of CO2-philicity and catalytic activity for utilization of carbon dioxide to form oxazolidinones. Various organic linkers employed in constructing MOFs with high-density Lewis basic/polar functional groups and catalytic sites to promote effective capture and transformation of CO2 to oxazolidinones are shown in Scheme 1.
2.2. Design of COFs for CO2 utilization
COFs are an emerging class of crystalline porous organic polymers constructed from organic linkers composed of light elements (B, C, N, O, S, and F) through covalent bonding to form extended 2D or 3D framework structures.89–92 In COFs, the organic building units are generally bonded together by strong covalent bonds. The strong covalent connections within the building units are crucial in forming extended crystalline networks. The distinctive properties of COFs include large surface area, crystallinity, thermal/chemical stability and tailored properties making them ideal catalysts for various applications.93,94 These applications encompass gas adsorption/separation, sensing, proton conduction, catalysis, energy storage, etc.95–99 COFs are synthesized using various organic linkers to construct frameworks with diverse architectures. The pore dimensions and functionality can be finely modified by using appropriate linkers, rendering the formation of COFs with 1D, 2D, or 3D structures, as illustrated in Scheme 2a. The 1D COFs are linear or rod-like structures where the covalent connectivity extends primarily in one dimension (Scheme 2).100 The 2D COFs are planar frameworks with covalent bonds extending in two distinct directions, generating sheet-like networks.101 These layers stack on top of one another through weak van der Waals interactions or π–π stacking. On the other hand, 3D COFs represent the most complex and fully interconnected networks, extending in three spatial dimensions.102 These 3D covalent linkers render rigid, highly porous structures featuring exceptional stability and large surface area.The synthesis of 1D, 2D or 3D COFs can be achieved by rationally choosing organic building blocks. For instance, a C2 + C2 amalgamation of organic building units along a direction results in 1D COF. Whereas a combination of C3 + C2 or C3 + C3 linkers results in hexagonal COFs and a C4 + C2 combination affords tetragonal COFs with an extended 2D structure.89 It is important to highlight that the formation of 3D COFs requires a minimum of one tetrahedral (Td) organic unit.92 For example, combining a tetrahedral node with a C2 or C3 organic building block renders 3D COFs, as depicted in Scheme 2. Developing COFs for specific purposes presents significant challenges. However, altering COF structures to integrate specific active sites within the framework represents an intriguing approach to address this challenge. Therefore, COF backbone design and PSM processes are commonly employed to tailor the structure and functionality of COFs. Further, the catalytic efficiency for converting CO2 into value-added oxazolidinones can be greatly improved by strategically integrating Lewis acidic metallic active sites into COFs using PSM. The alkynophilic metals like Cu and Ag can be precisely integrated into the framework at free bipyridine or Lewis basic sites to promote high efficacy in catalytic conversions of CO2 to oxazolidinones.103,104 Thus, rational design and synthesis of COFs having diverse active sites could provide great opportunities to realize CO2 conversion to value-added oxazolidinones. Scheme 2 comprises the amines and aldehydes that can be utilized to prepare COFs, which can promote the effective utilization of CO2 to form oxazolidinones with high yield and selectivity. The detailed literature related to the prepared COFs is covered in the later sections of the review article.
3. Versatile synthetic routes to oxazolidinones employing CO2 as a C1 building block
Typically, the synthesis of oxazolidinones utilizing CO2 has been accomplished by following three different strategies: (i) cycloaddition of CO2 to aziridines, (ii) carboxylative cyclization of CO2 to propargyl amines and (iii) three-component reaction of CO2 with propargyl alcohols and primary amines or with primary amines and epoxides (Fig. 5). The following section describes these transformations and provides the in-sight mechanistic details for synthesizing oxazolidinones utilizing framework-based heterogeneous catalysts.105–1073.1. Cycloaddition of CO2 with aziridines
Aziridine, an N-containing analog of epoxide, is a crucial heterocycle present in numerous naturally occurring compounds and pharmaceuticals having biological activity.108 It is also recognized as a versatile and widely used intermediate for developing many N-containing compounds. An exciting and promising practical use of aziridines is their involvement in preparing 2-oxazolidinone derivatives through coupling with CO2.The effective generation of 2-oxazolidinones from aziridines by their activation and further coupling with CO2 necessitates Lewis/Brønsted acidic sites and a nucleophilic co-catalyst.109 Literature studies have demonstrated that catalysts incorporating Lewis acidic sites like Zn2+ and Cu2+ can facilitate the activation of aziridine ring.110 Additionally, nucleophilic halide ions are essential to promote aziridine ring opening.111
The general catalytic cycle involves the following steps: the initiation of the catalysis takes place through the interaction of substrate (aziridines) and CO2 at the acidic and basic sites of the catalyst, respectively. Then, the substrate/aziridine undergoes activation via interaction at the unsaturated metal site. In the second step, aziridine ring-opening occurs by nucleophilic attack of halide (Br−), resulting in two distinct pathways labeled as path 1 and 2. Notably, path 1 leads to a more stable carbamate salt intermediate by opening the aziridine ring at the highly substituted carbon, leading to the major product formation. Then, CO2 insertion at the ‘N’ site of the intermediate results in carbamate salt, which undergoes intramolecular ring closure to yield the corresponding oxazolidinone (Fig. 6). Consequently, an effective catalyst is necessary to promote efficient coupling of CO2 with aziridine to yield desired oxazolidinone product with excellent selectivity and yield.112
 | ||
| Fig. 6 General mechanistic route for the MOF/TBAB-catalyzed carboxylative cycloaddition of CO2 with aziridine. | ||
The instability of certain MOFs in wet flue gas conditions is a persistent issue and necessitates pre-drying of flue gas. In this context, Bio-MOFs derived from amino acids hold promise as a potential solution for bridging the gap between CO2 capture and transformation.116 Thus, amino acid linker-based MOFs (AA-MOFs) can alleviate the reaction parameters by combining Lewis acidic metals, basic sites and H-bonding groups. Further, Zn-glutamate units are recognized for their role as active sites in biological systems, exemplified by the enzyme carboxypeptidases, which facilitate the degradation of peptides. In this direction, Park and co-workers reported the first example of amino acid-based Zn-glutamate MOF, {[Zn(H2O)(C5H7NO4)]·H2O}n (ZnGlu) having Lewis acidic Zn2+ sites and basic –NH sites which are employed for synthesis of oxazolidinones from aziridine in presence of TBAB with 90% yield at RT under 1 MPa CO2 (Scheme 1 and Fig. 7a).117 Notably, enhanced CO2 cycloaddition was observed using water as a solvent, which supports the feasibility of CO2 conversion from wet flue gas. Mechanistic investigation revealed that ZnGlu offered both Lewis acid and basic sites. Removal of water from the catalyst opens the pores, facilitating access and interaction with the substrates. Additionally, further removal of water can generate acidic open metal sites. When there is an adequate amount of water, opportunistic catalysis is expected to happen, akin to specific zeolitic imidazolate frameworks, where CO2 inserts at the labile Zn–OH2 bonds.118 Zhao and co-workers prepared a novel Cu-MOF {[Cu2(BCP)(H2O)2]·3DMF}n possessing nano-sized censer-like [Cu30] cages (Fig. 7b). The [Cu30] cage is sectioned into two sub-cages, the larger one is labeled A and the smaller one as cage B (Fig. 7c).79 Three Cu2(O2CR)4 paddlewheel units occupied cage A's top and bottom, each with a window size of 8.1 Å and 16 Å (Fig. 7c). Because of Lewis acidic open Cu2+ sites in Cu-MOF, it showed promising results in the reaction of CO2 with 1-ethyl-2-phenylaziridine resulting in 3-ethyl-5-phenyloxazolidin-2-one (major product) with 99% yield and excellent regioselectivity (98%) at 100 °C under 2 MPa CO2 with recyclability up to ten cycles (Table 1 and Fig. 7d).
 | ||
| Fig. 7 (a) 2D structure of ZnGlu showing 1D pore channels and its application towards CO2 fixation.117 Copyright 2016, the Royal Society of Chemistry. (b) The 3D framework of Cu-MOF. (c) Nano-sized [Cu30] cage. (d) Carboxylative cyclization of aziridines catalyzed by Cu-MOF.79 Copyright 2016, WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Hexagonal channels of MMPF-10. (f) Pentagonal windows of MMPF-10. (g) Carboxylative cyclization of CO2 to aziridines using MMPF-10 as a catalyst.120 Copyright 2018, the Royal Society of Chemistry. | ||
| MOF/COF/POP | Co-catalyst | Catalyst loading (mol%) | Substrate | Product | Reaction conditions (Temp. (°C)/Pressure (MPa)/Time (h)) | Yield (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| MOF-based catalysts for cycloaddition of CO 2 with aziridines | |||||||||
| {[Zn(H2O)(C5H7NO4)]·H2O}n (ZnGlu) | TBAB | 0.8 |

|
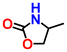
|
25 | 1 | 24 | 94 | 117 |
| {[Cu2(BCP)(H2O)2]·3DMF}n Cu-MOF | TBAB | 10 |

|
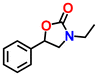
|
100 | 2 | 12 | >99 | 79 |
| [Cu4(CuTBCPPP)(H2O)4] MMPF-10 | TBAB | 0.625 |

|

|
100 | 2 | 10 | >99 | 120 |
| Zn-MOF | TBAB | 2.8 |
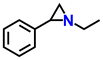
|

|
70 | 2 | 12 | >99 | 122 |
| PCN-222(Co) | TBAB | 1.0 |

|

|
40 | 0.1 | 18 | 82 | 123 |
| {[M2(XN)2(IPA)2]·2H2O}n (M = Co, Mn, Ni) | TBAB | 3.63 |

|

|
30 | 1 | 10 | 89 | 124 |
| {Na[LnCo(DATP)2(Ac)(H2O)](NO3)·DMA·11H2O}n (Ln = Er and Yb) | TBAB | 0.68 |

|

|
70 | 1 | 10 | 94 | 125 |
| {[NH2(CH3)2][In(CPT)2]·3CH3CN·3DMA}n(In-MOF) | TBAB | 1.7 |

|

|
30 | 1 | 10 | >99 | 126 |
| {[Ni(DCTP)]·6.5DMF}n (Ni-MOF) | NA | 2.4 |

|

|
70 | 2 | 10 | 95 | 127 |
| {[K1.2Na2.8ZnI8(HL1)12]·4H2O}n (Zn-MOF) | TBAB | 5.3 |

|

|
70 | 2 | 12 | 99 | 129 |
| {[H2N(CH3)2]3[Zn3(BTB)2(5-atz)3]· 3EtOH·3H2O·3DMF}n (Zn-MOF) | TBAB | 3.2 |

|

|
70 | 1 | 10 | 94 | 130 |
| {(NH2Me2)[Co3(μ3-OH)(BTB)2(H2O)]·9H2O·5DMF}n (Co-MOF) | TBAB | 1.8 |

|

|
25 | 1 | 10 | 99 | 159 |
| {[Cu2((L2)4−)(H2O)2]·3DMF·2H2O}n (Cu-MOF) | TBAB | 0.95 |

|

|
60 | 0.5 | 12 | 98 | 131 |
| [Ce2(DCTP)2(DMA)2(OAc)2]n (Ce-MOF) | TBAB | 1.4 |

|

|
70 | 0.5 | 10 | 97 | 132 |
| [Eu2Cu2I2(IN)6(DMF)4]·4DMF (Eu-MOF) | TBAB | 1.0 |

|

|
60 | 1 | 12 | 92 | 133 |
| COF-based catalysts for cycloaddition of CO 2 with aziridines | |||||||||
| 2,3-DhaTph and 2,3-DmaTph | TBAI | 0.02 mmol |

|

|
50 | 2 | 3 | 93 | 136 |
| MOF-catalyzed cyclic carboxylation of propargyl amines with CO 2 | |||||||||
| [Cd3(L3)2(BDC)3]2·16DMF (Cd-MOF) | NA | 0.4 |

|

|
60 | 0.5 | 24 | 99 | 84 |
| TNS-Ag8 | DBU | 1.0 |

|

|
25 | 0.1 | 24 | 99 | 148 |
| TOS-Ag4 | 99 | ||||||||
| TMOF-3-Ag | DBU | 0.1 |

|

|
25 | 0.1 | 6 | >99 | 196 |
| NiBDP-AgS | DBU | 0.5 |

|

|
60 | 0.1 | 4 | 90 | 85 |
| Ag-MOF-1 | DBU | 20 mg |

|

|
25 | 0.1 | 24 | 55 | 153 |
| Ag27-MOF | DBU | 1.0 |

|

|
25 | 0.1 | 6 | 97 | 154 |
| Ag-MOF | DBU | 2.0 |

|

|
25 | 0.1 | 5 | 98 | 155 |
| [Zn22(Trz)8(OH)12(H2O)9.8H2O]n (Zn-MOF) | TBD | 0.27 |

|

|
70 | 0.1 | 12 | 99 | 156 |
| {[(CuI6I5)CuII3(L6)6(DMA)3](NO3)·9DMA} (Cu-MOF) | TEA | 5.7 |

|

|
25 | 0.1 | 1 | 93 | 157 |
| [Mg3Cu2I2(IN)4(HCOO)2(DEF)4]n(Mg-Cu-MOF) | TEA | 10 mg |

|

|
25 | 0.1 | 6 | 93 | 158 |
| [CuII2CuI4I4L8] (Cu-MOF) | NA | 0.7 |

|

|
60 | 0.1 | 1 | 79.7 | 88 |
| (Co-BTB) | TBD | 1.8 |

|

|
70 | 2 | 5 | 32 | 159 |
| (Co-XN) | 76 | ||||||||
| Cu-TSP | DBU | 2.0 |

|

|
50 | 0.1 | 24 | 99 | 160 |
| WYU-11 | TMG | 1.0 |

|

|
60 | 0.1 | 24 | 99 | 161 |
| (Cu-TCPP(Fe)) | DBU | 1.0 |

|

|
50 | 0.1 | 24 | 91 | 162 |
| [Eu2Cu2I2(IN)6(DMF)4]·4DMF (Eu-MOF) | TBD | 0.8 |

|

|
70 | 0.1 | 12 | 98 | 132 |
| Th-MOF | TEA | 10 mg |

|

|
25 | 0.1 | 6 | 94 | 163 |
| 10.1–20.4–30.5-JNM | DBU | 3.0 |

|

|
25 | 0.1 | 3 | 94 | 164 |
| Cu2O@ZIF-8 | DBU | 5.0 |

|

|
40 | 0.1 | 6 | 99 | 167 |
| Cu2O@MIL-101(Cr)-DABCO | DABCO | 5.0 |

|

|
25 | 0.1 | 12 | 97.5 | 168 |
| CuBr@NH2-MIL-101 | DBU | 5.0 |

|

|
25 | 0.1 | 8 | 97 | 169 |
| Cu(I)-GSH/ZIF-8 | DBU | 5.0 |

|
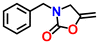
|
60 | 0.1 | 6 | 98 | 170 |
| COFs catalyzed cyclic carboxylation of propargyl amines with CO 2 | |||||||||
| Ag@TpPa-1 | N-Iodosuccinimide | 40 mg |

|

|
25 | 0.1 | 16 | 78 | 178 |
| Ag@TpTta | 88 | ||||||||
| Cu-NPs@COF | DBU | 30 mg |

|

|
50 | 0.1 | 12 | 95 | 179 |
| Pd(II)@TFR-OT | NA | 15 mg |

|

|
25 | 0.1 | 10 | 92 | 180 |
| Ag@2,6-FPP-TAPT | DBU | 0.052 |

|

|
50 | 0.1 | 2 | 99 | 181 |
| Ag@Pybpy-COF | DBU | 0.2 |

|

|
50 | 0.1 | 0.5 | 99 | 182 |
| Cd-Bpy-COF | DBU | 10 mg |

|

|
60 | 0.1 | 12 | 99.9 | 183 |
| CuITpBD-COF | DBU | 5 mg |
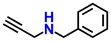
|

|
80 | 0.1 | 6 | 95 | 184 |
| POPs catalyzed cyclic carboxylation of propargyl amines with CO 2 | |||||||||
| Pd@BBA-2 | NA | 1.12 |

|
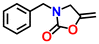
|
60 | 0.1 | 10 | 95 | 188 |
| AgN@COF | DBU | 20 mg |

|

|
55 | 0.1 | 10 | 94 | 189 |
| Ag@BT-COP | DBU | 1.08 |

|

|
60 | 0.1 | 14 | 99.9 | 190 |
| Ag@NPOPs-1 | DBU | 1 mg |

|

|
50 | 0.1 | 2 | 97 | 191 |
| Ag@NPOPs-1 | 93 | ||||||||
| Cu@NHC-1 | NA | 1.3 |

|

|
50 | 0.1 | 7.5 | 93 | 192 |
| MOF catalyzed synthesis of oxazolidinones by one-pot three-component reaction | |||||||||
| TMOF-3-Ag(I) | PPh3 | NA |

|

|
50 | 0.1 | 12 | >99 | 196 |
| MOF-SO3Ag | DBU | 0.15 |

|

|
25 | 0.1 | 26 | 99 | 197 |
| UiO-66–40 | NA | 70 mg |

|

|
85 | 0.1 | 12 | 87 | 198 |
| Ni-MOF | TBAI | 60 mg |

|

|
90 | 0.1 | 12 | 80 | 202 |
| Cu(I)@NHC–MOF | DBU | 0.5 |

|

|
25 | 0.1 | 12 | 99 | 209 |
| Ag(I)@MOF-NHC | DBU | 1.0 |

|
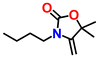
|
25 | 0.1 | 12 | 99 | 212 |
| CMOF-801(ASP) | NA | 50 mg |

|

|
90 | 0.1 | 12 | 90 | 213 |
| {[Ni2(tpxn)(oxdz)2(H2O)2]·13H2O}n Ni-MOF | TBAB | 2.5 |

|

|
80 | 0.1 | 12 | 85.5 | 214 |
| MOF catalyzed synthesis of oxazolidinones by one-pot four-component reaction | |||||||||
| PCN-(BPY-CuI)-(TPDC-F7) | NA | 5.0 |

|

|
75 | 0.1 | 24 | 84 | 80 |
| Cu@UiO-66-NH2 | NA | 0.6 mmol |

|

|
75 | 0.2 | 24 | 215 | |
| COF-catalyzed synthesis of oxazolidinones by three-component reaction | |||||||||
| Zn@RIO-1 | NA | 15 mg |

|

|
80 | 0.1 | 12 | 94 | 219 |
| TpMA(MC)@Ag | DBU | 15 mg |

|

|
25 | 0.1 | 6 | 95 | 220 |
| Ag@TFPNDA-COF | NA | 15 mg |

|

|
25 | 0.1 | 4 | 92 | 221 |
The Cu-MOF catalyzed the cycloaddition reaction with a wide range of aziridines containing various substituents (R1) at the N-atom and on the phenyl ring (R2) (Fig. 7d). Mechanistic investigations revealed that 1D channels and nanoscopic [Cu30] cages of the Cu-MOF effectively trapped CO2 and aziridines and the confined pore environment within nanoscopic [Cu30] cages boosted reactivity between aziridines and CO2. The Cu2(O2CR)4 paddlewheel unit showed steric crowding near Cu2+ ions, effectively hindering the coordination of Br− ions to the metal center. This arrangement promoted a more efficient nucleophilic attack of Br− ions on aziridine, facilitating its activation for coupling with CO2. The detailed catalytic mechanism is discussed in Section 3.1.
Metal–metalloporphyrin frameworks (MMPFs) represent a significant subset of MOFs that feature mono-, bi- or multi-metallic systems owing to the unique features of porphyrin scaffold supporting the anchoring of diverse metal ions at the pyrrole ring via post-synthetic modification.119 Porphyrin-based linkers are chemically adaptable, facilitating tunable structure and functionality by modification at the meso-positions. In this direction, Ma and co-workers reported a novel metal–metalloporphyrin framework [Cu4(CuTBCPPP)(H2O)4] (MMPF-10) composed of elongated hexagonal channels (25.6 Å × 15.6 Å) running parallel to c-axis (Fig. 7e), generated from four Cu-paddlewheels with a ring of tetra metallated porphyrin ligands.120 This arrangement resulted in pentagonal windows with a cavity diameter of 11 Å (Fig. 7f). Owing to the Lewis acidity of Cu(II), MMPF-10 displayed good catalytic presentation for the fixation of CO2 to oxazolidinones at 100 °C under 2 MPa CO2 (Table 1 and Fig. 7g). Notably, increasing the molecular size of the substrates did not affect the products yield significantly. An yield of 71% was achieved for isopropyl-substituted aziridine, which is understandable considering the increased steric hindrance caused by the isopropyl group linked to the N-atom. The MMPF designed here from a larger porphyrin linker holds larger pores, facilitating diffusion of substrates to the catalytic sites exposed in the pore channels of the MOF, thereby, resulting in enhanced catalytic activity.121 This work constitutes the first example of an MMPF-based catalyst that efficiently converts CO2 to oxazolidinones. In another instance, the development of a multifunctional nanocage-based Zn-MOF containing 24-nuclear Zn-nanocages having pores (5.5 Å) with a pore volume of 0.65 cm3 g−1 has been reported by Zhao's group (Fig. 8a).122 The MOF displayed promising CO2 adsorption capacity (35.6 wt%) at 273 K with a BET surface area of 1151 m2 g−1. Owing to the potential adsorbing capacity of CO2 and plenitude Lewis acidic Zn-active sites in the MOF, it was explored for fixation of CO2 to form oxazolidinones with TBAB as a co-catalyst at 70 °C under 2.0 MPa CO2 with up to ten catalytic cycles (Fig. 8b). Aziridines with various substituent groups were examined and the ethyl group substituted substrate exhibited the highest conversion (99%) and selectivity over propyl (91%) and butyl (93%) group containing substrates. This observation has been correlated to reduced steric hindrance of ethyl over propyl and butyl substituents. Besides, the effect of substituent groups (–Cl and –CH3) on the phenyl ring was also studied to obtain the corresponding aziridines, 1-ethyl-2-(4-chlorophenyl)-aziridine (92%) and 1-ethyl-2-(4-methyl)-aziridine (99%). The lower yield of 1-ethyl-2-(4-chlorophenyl)-aziridine was attributed to the electron-withdrawing effect of the Cl-group (Fig. 8b). The cooperative catalytic effect of abundant Zn-Lewis acidic sites and TBAB, facilitated an effective cyclization reaction between aziridines and CO2 at atmospheric conditions.
 | ||
| Fig. 8 (a) 3D structural view of Zn-MOF. (b) Cycloaddition of CO2 with aziridines catalyzed by Zn-MOF.122 Copyright 2018, the Royal Society of Chemistry. (c) 3D structure of PCN-222(Co). (d) Synthesis of oxazolidinones using PCN-222(Co) as catalyst.123 Copyright 2019, American Chemical Society. | ||
It is worth noting that, in porphyrin-based frameworks, such as PCN family, the Lewis acidity arises from the presence of Zr6 cluster nodes and metalloporphyrin within the polymeric matrix rendering them promising catalysts for CO2 cycloaddition reactions. Expansive channels and pores significantly enhance interactions between the substrate and catalyst, resulting in increased catalytic efficiency. Thus, porphyrin-based materials are utilized to couple CO2 with aziridines at ambient pressure. In this regard, Martín-Matute and co-workers reported a one-pot, microwave-assisted preparation of pristine PCN-222 and metallated PCN-222(M) (M = Co, Ni, Cu, or Zn) frameworks (Fig. 8c) and studied their catalytic activity.123 Indeed, PCN-222(Co) demonstrated significant catalytic activity, yielding a diverse range of oxazolidinones under mild conditions (Fig. 8d). The yield of the products decreased with an increase in the chain length of the alkyl substituent on the N-atom due to steric hindrance (Fig. 8d). Moreover, the material maintained its catalytic activity and crystallinity across four recycling runs. Kang et al. reported three new isomorphous MOFs {[M2(XN)2(IPA)2]·2H2O}n (M = Co, Mn and Ni) possessing 1D pores of 3.74 × 9.90 Å along b-axis (Scheme 1).124 Taking into account the presence of unsaturated Lewis-acid active sites on the surface of the MOFs, they were employed as promising catalysts for converting CO2 into valuable oxazolidinones at 30 °C under 1 MPa CO2 with TBAB as co-catalyst (Table 1 and Fig. 9a). The MOF exhibited good recyclability for five cycles. Aziridine with an ethyl substitution at the N-atom rendered a higher yield of products with superior selectivity compared to those with propyl and butyl substitutions, owing to the reduced steric hindrance of the ethyl group. Similarly, the yield of 3-ethyl-5-(4-chlorophenyl)oxazolidin-2-one was found to be lower than that of 3-ethyl-5-p-tolyloxazolidin-2-one, which has been attributed to electron-withdrawing effect of –Cl group (Fig. 9a). Mechanistic investigation revealed that stable Co2(COO)4 unit's electric neutrality and steric hindrance significantly impede the coordination interaction between Br− and Co2+, thereby increasing the chances for Br− to nucleophilically attack aziridines. Thus, the combined effect of Br− from TBAB and the unsaturated Lewis-acid Co2+ active sites in Co-MOF was crucial for the CO2 coupling reaction.
 | ||
| Fig. 9 (a) Fixation of CO2 to aziridines catalyzed by Co-MOF.124 (b) Synthesis of oxazolidinones catalyzed by Er-MOF.125 (c) Rhombic pores (16 Å × 18 Å) in In-MOF. (d) 1D circular channels in In-MOF along the b-axis. (e) Preparation of oxazolidinones catalyzed by In-MOF.126 Copyright 2021, American Chemical Society. | ||
Zhao's group reported two isostructural heterometallic MOFs, {Na[LnCo(DATP)2(Ac)(H2O)](NO3)·DMA·11H2O}n (Ln = Er and Yb). Er-MOF displayed diverse building blocks [Er(COO)4(Ac)(H2O)] and [Co(DATP)2], which are interconnected through H2DATP ligands to form a 2D layer architecture.125 Given the unsaturated Ln3+ Lewis acid sites in Er-MOF and Yb-MOF, they served as promising catalysts for the CO2 fixation reaction to form oxazolidinones at 70 °C under 1 MPa CO2 with up to ten catalytic cycles (Table 1 and Fig. 9b). The seven-coordinated Ln3+ ion, which is unsaturated and has more Lewis acid sites, effectively activated the substrates and CO2, leading to faster catalytic reactions. It is worth noting that the application of main-group metal-based MOFs for the cycloaddition of CO2 to aziridines was rarely studied. In this context, in 2021, Zhao and co-workers reported a quadruplex-interpenetrated porous In-MOF, {[NH2(CH3)2][In(CPT)2]·3CH3CN·3DMA}n exhibiting two types of pores, viz a rhombic (16 Å × 18 Å) and circular (10 Å) along a- and b-axis, respectively (Fig. 9c and d).126 Notably, despite the quadruplex-interpenetrated structure, the In-MOF possesses a total solvent-accessible volume of 70.2%. Due to its high porosity and Lewis acidic In3+ sites, In-MOF was employed as a heterogeneous catalyst for CO2 coupling with aziridines using TBAB at 30 °C under 1 MPa CO2 pressure (Table 1 and Fig. 9e). Further, In-MOF displayed a high level of catalytic versatility across a range of aziridines with recyclability up to five cycles. Under mild conditions, the compound 3-ethyl-5-phenyloxazolidin-2-one was obtained with a high yield of 99%. Mechanistic studies revealed that the porous structure of In-MOF efficiently enriches and captures CO2 molecules, with an isosteric heat of adsorption (Qst) of approximately 36 kJ mol−1, indicating a notable affinity for CO2.
It is worth mentioning that, most of the literature discussed in the aforementioned sections involves the requirement of co-catalyst, TBAB for the opening of the aziridine ring. To overcome this issue, Shi et al. reported a 3D Ni-MOF, {[Ni(DCTP)]·6.5DMF}n exhibiting two distinct 1D channels along the a-axis, with openings measuring 11.2 Å × 10.4 Å (Channel A) and 7.8 Å × 5.5 Å (Channel B), respectively (Fig. 10a and b).127 The spacious pores offered a promising opportunity for CO2 adsorption and catalytic processes. As a result, Ni-MOF efficiently catalyzed the transformation of CO2 to oxazolidinones without a co-catalyst at 70 °C and 2 MPa CO2 in 10 h, with recyclability up to five cycles (Table 1 and Fig. 10c). Furthermore, Ni-MOF also showed substantial catalytic activity for the large-scale demonstration. The good catalytic performance demonstrated by Ni-MOF could be attributed to the extensive porous structure formed by DCTP and Ni2+ effectively capturing and enriching CO2 molecules on the surface. The combined catalytic effect of Ni2+ ions, acting as Lewis acidic centers and pyridyl-N sites from the DCPT ligand significantly facilitated the cycloaddition reaction between CO2 and aziridines.
 | ||
| Fig. 10 (a and b) The 3D channels A and B in Ni-MOF. (c) Cycloaddition of CO2 with aziridines catalyzed by Ni-MOF.127 Copyright 2021, Springer Nature. (d) 3D view of Zn-MOF. (e) Preparation of oxazolidinones using CO2 and aziridines catalyzed by Zn-MOF.129 Copyright 2021, the Royal Society of Chemistry. | ||
Notably, polynuclear metal cluster-based MOFs can greatly enhance catalytic efficiency owing to the availability of high-density catalytic sites.128 Most of the cluster-based MOFs exhibit common oxidation states. However, those containing uncommon low oxidation sites are rare and offer significant opportunities in catalysis. In this regard, Zhao and co-workers reported a unique cluster-based MOF with unusual multi-centered ZnI–ZnI bonds, {[K1.2Na2.8ZnI8(HL1)12]·4H2O}n (Zn-MOF) (Fig. 10d).129 A distinctive Zn8 cubic cluster is formed by assembling eight zinc ions, where the distance between adjacent zinc ions (ZnI–ZnI) measures 2.357 Å. Given the numerous Lewis acidic active sites in Zn-MOF, it was exploited for cycloaddition of CO2 with aziridines to form oxazolidinones at 70 °C under 2 MPa of CO2 pressure with recyclability for up to five cycles with broad substrate universality (Table 1 and Fig. 10e). Indeed, this is the first multi-center metal–metal bonded cluster-based MOF utilized as a catalyst for carbon dioxide fixation. In another example of Zn-MOF, Kang and Liu group reported a Zn-MOF, {[H2N(CH3)2]3[Zn3(BTB)2(5-atz)3]·3EtOH·3H2O·3DMF}n featuring a continuous 1D chain along the a-axis, expanding into a 3D pillar-chain framework by inclusion of BTB3− ligands (Scheme 1 and Fig. 11a).130 An isosceles triangular channel with an approximate diameter of 13.4 Å can be observed in the Zn-MOF (Fig. 11a). Due to the potent Lewis acidity of unsaturated Zn sites and the Lewis basicity of uncoordinated –NH2, Zn-MOF demonstrated effective catalysis in transforming CO2 into oxazolidinones at 70 °C under 1 MPa CO2 pressure with reusability for at least three cycles (Table 1 and Fig. 11b). The impact of steric hindrance from ethyl, propyl, butyl, or benzyl groups on N-atoms was investigated, revealing a decrease in yield and selectivity with an increase in the size of substituent groups. In contrast, when the R2 group was substituted by –Cl, –Br, or –CH3 groups, the yield of the corresponding oxazolidinones followed the trend –Cl(53%), <–Br(79%) < –CH3 (82%) suggesting that electron-donating groups promote efficient coupling of aziridines with CO2 (Fig. 11b). The comprehensive mechanistic investigation demonstrated that the unsaturated four-coordinated Zn2+, characterized by its strong Lewis acidity, effectively activates the N-atom of aziridine intermediate, thereby accelerating the ring-opening process.
 | ||
| Fig. 11 (a) 3D view of Zn-MOF. (b) Fixation of CO2 to oxazolidinone catalyzed by Zn-MOF.130 Copyright 2022, the Royal Society of Chemistry. (c) The 3D framework of Cu-MOF.131 Copyright 2023, American Chemical Society. (d) 2D Eu-MOF.133 Copyright 2024, Elsevier. | ||
The application of a Cu-organic framework, {[Cu2(L2)4−(H2O)2]·3DMF·2H2O}n (Cu-MOF) having Cu2(CO2)4 units linked together through the (L2)4− ligand possessing nanocage structure with 1.1 nm wide inner cavity has been reported (Fig. 11c).131 Additionally, the nanocages are interconnected to create a 3D framework characterized by two types of 1D channels (0.70 nm and 0.32 nm) along the c-axis (Fig. 11c). The Cu-MOF exhibited significant selectivity for CO2 due to its strong interactions with exposed Cu(II) sites and ligands within the framework. The MOF efficiently catalyzed the conversion of CO2 to oxazolidinones at 60 °C under 0.5 MPa CO2 pressure with good substrate scope (Table 1). DFT calculations revealed that the adsorbed CO2 molecules are predominantly situated near the unsaturated dinuclear Cu(II) site, F-site and the adjacent H-site within the benzene ring of the ligand L2. This work highlights the importance of nanocage-based MOFs for CO2 fixation and conversion reactions. In another instance, Liu et al. reported Ce-based lanthanide MOF, [Ce2(DCTP)2(DMA)2(OAc)2]n, which was further employed as a heterogeneous catalyst for facilitating the cycloaddition reaction between aziridines and CO2, resulting in value-added oxazolidinones in 10 h, at 70 °C under 0.5 MPa CO2 (Table 1).132 The Ce-MOF demonstrated effective catalytic activity with various substituent groups under optimal conditions. Mechanistic analysis disclosed that the combination of abundant Ce centers and uncoordinated pyridine ligands within Ce-MOF works synergistically to catalyze the reaction effectively.
Recently, 2D MOFs are gaining significant interest owing to their layered structure with highly exposed catalytic sites. In this regard, Cao and co-workers reported a novel 2D-MOF, [Eu2Cu2I2(IN)6(DMF)4]·4DMF (Eu-MOF) composed of Eu3+ ions and Cu2I2 clusters, exhibiting sql topology (Fig. 11d).133 Through catalytic investigation, it was discovered that Eu-MOF (1 mol%) effectively catalyzed cyclization of CO2 with aziridines under 1 MPa CO2 pressure at 60 °C in 12 h (Table 1). The previous discussion displayed that MOF-based catalysts possessing Lewis acidic metal sites and basic (–NH/–NH2) sites show promising activity for efficiently transforming aziridines into oxazolidinones by utilizing CO2 under environmentally friendly conditions. It is worth highlighting that, presence of open metal sites (OMSs) within MOFs significantly enhances the activation of CO2. These OMSs function as Lewis acidic sites, effectively promoting the functionalization of CO2 with aziridines. In addition to OMSs, the pore size of the MOF plays a critical role in CO2 activation. Given the small size of CO2 molecule, microporosity is particularly advantageous. The finer pores allow for better interaction and accommodation of CO2, leading to more efficient capture and conversion processes.
 | ||
| Fig. 12 (a) Structures of 2,3-DhaTph and 2,3-DmaTph COF. (b) Synthesis of oxazolidinones. (c) Recyclability study of COFs.136 Copyright 2016, the Royal Society of Chemistry. | ||
3.2. Carboxylative cyclization of CO2 with propargyl amines
The utilization of aziridines for the cycloaddition reaction with CO2 proves beneficial for generating oxazolidinones. However, the synthesis of these aziridine substrates necessitates the use of toxic bromine. Furthermore, aziridines are inherently unstable in atmospheric conditions and susceptible to oxidation or hydrolysis upon exposure to humidity or oxygen.137 Another promising approach for synthesizing oxazolidinones is through carboxylative cyclization of propargyl amines with CO2. N-Propargyl amines represent a highly versatile and distinctive category of heteroatom-containing alkynes with a wide range of reaction patterns.138 They are commonly utilized as fundamental building units for the formation of various N-heterocycles, including pyrroles, pyrazines, pyridines, imidazoles, quinolines, lactams and other compounds.139 Recently, considerable efforts have been engaged toward transforming these commodity chemicals into value-added oxazolidinones through carboxylative cyclization with CO2. For an efficient conversion process of propargyl amines to oxazolidinones using CO2, the catalyst should possess Lewis acidic sites for activating the alkyne group. Additionally, Lewis basic sites are essential for the deprotonation of the amines, thereby enhancing the overall conversion efficiency.The general catalytic mechanism involves the activation of the alkyne group of propargylic amine by the metal center. This activation is followed by deprotonation of propargylic amine using DBU as a base. Subsequently, the insertion of CO2 takes place to form a carbamate. The following step involves an intramolecular cyclization process, wherein the carbamate intermediate undergoes rearrangement to form 2-oxazolidinones. This cyclization step is crucial for the synthesis of the desired product. Finally, the formed 2-oxazolidinones are separated from the metal center, regenerating the catalyst and enabling its participation in subsequent catalytic cycles (Fig. 13).140
 | ||
| Fig. 13 General mechanistic route for the MOF-catalyzed carboxylative cyclization of CO2 to propargyl amines. | ||
Functionalized flexible MOFs are ideal candidates for advancing artificial switchable catalysts owing to their natural cavities, dynamic characteristics and customizable functional groups within the framework channels.143,144 The dynamic nature of flexible MOFs enables tailoring of the pore channels through external stimuli for desired applications. This ability to fine-tune the porous structure in situ can significantly influence the catalytic performance of heterogeneous catalysts based on flexible MOFs. However, the potential of flexible MOFs for switchable catalysis remains largely untapped.145,146 In contrast to other catalysts, the dynamic responses of functionalized flexible MOFs to guest molecules can trigger conformational shifts that contribute to substrate selectivity, similar to complex biological systems.147 Keeping this in mind, in 2017, Sun and co-workers reported a dynamic, functional Cd-MOF, [Cd3(L3)2(BDC)3]2·16DMF decorated with –NH2 groups from tripodal imidazole linker. Cd-MOF displayed a dynamic five-fold interpenetrating structure (Fig. 14a).84 The dynamic structure of Cd-MOF was switched on and off through reversible structural transformations. The –NH2 groups decorated on the channel surfaces boosted CO2 interaction with the MOF. Thus, the MOF was exploited for carboxylative cyclization of carbon dioxide with propargyl amines at 60 °C under 0.5 MPa CO2 pressure with good substrate scope and recyclability (Table 1 and Fig. 14b). The promising activity demonstrated by MOF to form oxazolidinones using CO2 and terminal propargyl amines indicated that small-sized substrates entered the channels and displayed catalytic reactions smoothly. The catalytic mechanism was validated by collecting FT-IR spectra of Cd-MOF after it was immersed in N-methylpropargylamine. Indeed, the FT-IR studies validated the interaction of the amine substrate with the MOF (Fig. 14c). Thus, the free –NH2 groups in Cd-MOF play a crucial role in the catalytic reaction. These experimental findings demonstrated that Cd-MOF functioned as a flexible and switchable catalytic system with selective properties for substrates, akin to advanced biological systems.
 | ||
| Fig. 14 (a) 3D view of Cd-MOF. (b) Cyclization reaction of CO2 with propargyl amines catalyzed by Cd-MOF. (c) FTIR spectra of Cd-MOF, MOF after immersed in N-methylpropargylamine and N-methylpropargylamine.84 Copyright 2017, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Considering the alkynophilic character of Ag–metal, Duan and co-workers utilized thiourea functionalized MOFs and then embedded Ag nanoclusters to obtain TNS-Ag8 and TOS-Ag4 which possess 1D channels of dimension (9.0 × 9.0 Å2) and (10.0 × 10.0 Å2), respectively (Fig. 15a).148 The surface of the Ag clusters is decorated with H-bond donor (–NH2 and –NH–) groups and H-bond acceptor (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and S) groups, offering potential H-bonding interactions with substrates. These interactions facilitate synergistic activation and fixation during catalytic transformations.149 Further, TNS-Ag8 and TOS-Ag4 showed high-density catalytic sites with uniformly distributed pores, facilitating them as efficient π-activators for coupling of propargylamine with CO2 to yield oxazolidinones (99%) at RT under 0.1 MPa CO2 (Table 1 and Fig. 15b). The combined effect of π-activation and H-bonding interactions promoted trapping of substrates around the catalytic sites, facilitating chemical transformations under mild reaction conditions (Fig. 15c).
N– and S) groups, offering potential H-bonding interactions with substrates. These interactions facilitate synergistic activation and fixation during catalytic transformations.149 Further, TNS-Ag8 and TOS-Ag4 showed high-density catalytic sites with uniformly distributed pores, facilitating them as efficient π-activators for coupling of propargylamine with CO2 to yield oxazolidinones (99%) at RT under 0.1 MPa CO2 (Table 1 and Fig. 15b). The combined effect of π-activation and H-bonding interactions promoted trapping of substrates around the catalytic sites, facilitating chemical transformations under mild reaction conditions (Fig. 15c).
 | ||
| Fig. 15 (a) Structure of TOS-Ag4. (b) Cycloaddition of propargylamine derivatives with CO2 catalyzed by TOS-Ag4. (c) Propargylamine-impregnated crystals of 1a@TOS-Ag4.148 Copyright 2018, American Chemical Society. (d) Scheme for NiBDP-AgS synthesis.85 Copyright 2018, the Royal Society of Chemistry. | ||
From a mechanistic perspective, the substrate molecules are effectively confined within the pores of the framework through H-bonding interactions with the –NH groups. This confinement facilitates the alignment of the trapped molecules in optimal orientations, enabling the Ag atoms to engage with the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– bond via π coordination, thereby enhancing substrate activation. Simultaneously, CO2 molecules interact with the –NH groups, promoting a conducive environment for the activated substrates to undergo a nucleophilic attack. This interaction results in the conversion of substrates into products. The synergistic interplay between π-activation and H-bonding interactions significantly accelerates the capture of substrates around the catalytic sites. This dual mechanism not only enhances substrate availability but also catalyzes the chemical transformation under mild reaction conditions, making it an efficient process. The ability to be recycled and achieve high turnover numbers showcases the extensive potential applicability of these engineered materials as catalysts for π activation, making them suitable for real-world applications within the chemical industry. In this regard, pyrazolate-based MOFs have shown significantly improved alkaline stability compared to their carboxylate-based frameworks150 which are attributed to the high pKa of N–H bond in pyrazole, resulting in robust M–N coordination.151 Therefore, pyrazolate-based MOFs represent one of the earliest examples of frameworks exhibiting high stability in a concentrated alkaline solution, such as NaOH.152 In light of this, Fei and co-workers reported an alkali-resistant Ag(I)-anchored MOF (NiBDP-AgS), which was prepared via PSM of a pyrazolate-MOF via thiol functionalization (Fig. 15d).85 Owing to its high robustness and strong interaction ability with CO2 and alkyne-comprised molecules, NiBDP-AgS displayed promising catalytic activity for cyclic carboxylation of propargylic amines to oxazolidinones (Table 1). Further, Duan's group recently reported a new Ag–S-based porous MOF (Ag-MOF-1), having unique structural features that enable efficient cycloaddition of CO2 with propargyl amines (Fig. 16a and b).153 The novel Ag-MOF-1 catalyst, with its distinctive double-helical Ag–S rods and thiosemicarbazide ligands, demonstrated promising activity for oxazolidinones synthesis.
C– bond via π coordination, thereby enhancing substrate activation. Simultaneously, CO2 molecules interact with the –NH groups, promoting a conducive environment for the activated substrates to undergo a nucleophilic attack. This interaction results in the conversion of substrates into products. The synergistic interplay between π-activation and H-bonding interactions significantly accelerates the capture of substrates around the catalytic sites. This dual mechanism not only enhances substrate availability but also catalyzes the chemical transformation under mild reaction conditions, making it an efficient process. The ability to be recycled and achieve high turnover numbers showcases the extensive potential applicability of these engineered materials as catalysts for π activation, making them suitable for real-world applications within the chemical industry. In this regard, pyrazolate-based MOFs have shown significantly improved alkaline stability compared to their carboxylate-based frameworks150 which are attributed to the high pKa of N–H bond in pyrazole, resulting in robust M–N coordination.151 Therefore, pyrazolate-based MOFs represent one of the earliest examples of frameworks exhibiting high stability in a concentrated alkaline solution, such as NaOH.152 In light of this, Fei and co-workers reported an alkali-resistant Ag(I)-anchored MOF (NiBDP-AgS), which was prepared via PSM of a pyrazolate-MOF via thiol functionalization (Fig. 15d).85 Owing to its high robustness and strong interaction ability with CO2 and alkyne-comprised molecules, NiBDP-AgS displayed promising catalytic activity for cyclic carboxylation of propargylic amines to oxazolidinones (Table 1). Further, Duan's group recently reported a new Ag–S-based porous MOF (Ag-MOF-1), having unique structural features that enable efficient cycloaddition of CO2 with propargyl amines (Fig. 16a and b).153 The novel Ag-MOF-1 catalyst, with its distinctive double-helical Ag–S rods and thiosemicarbazide ligands, demonstrated promising activity for oxazolidinones synthesis.
 | ||
| Fig. 16 (a) Structure of Ag-MOF-1. (b) CO2 conversion to oxazolidinones catalyzed by Ag-MOF-1.153 Copyright 2019, American Chemical Society. (c) 2D structure of Ag27-MOF with topology. (d) Preparation of oxazolidinones catalyzed by Ag27-MOF.154 Copyright 2020, Wiley–VCH GmbH. | ||
The strong Ag–S bonds and inhibition of non-porous Ag NPs formation contribute to the catalyst's efficiency and stability during catalysis. As the size of the aryl substituents increased, the conversion decreased due to factors such as steric hindrance and reduced accessibility of catalytic sites. In a similar approach, Sun and co-workers reported a robust 2D MOF (Ag27-MOF) using pyridyl functionalized porphyrin (TPyP-H2) ligand, which functioned as a promising catalyst for oxazolidinones synthesis using propargyl amines and CO2 under atmospheric pressure (Fig. 16c and d).154 The metallic node in Ag27-MOF is saddle-shaped, offering an accessible platform where densely packed Ag atoms act as π-Lewis acid sites to activate the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– bond of propargyl amines. Consequently, various sterically restricted alkyne substrates were efficiently activated via π-interactions with cationic Ag centers. This study demonstrated that incorporating high-density Ag centers into a 2D framework as π-Lewis acid catalytic sites not only improves the stability of the 2D MOF but also enhances the activation of organic alkyne substrates, particularly bulky ones, thereby greatly boosting catalytic performance. Thus, 2D MOFs composed of catalytic metal clusters could be convincing catalysts for CO2 transformation reactions. In another example, Sun and co-workers reported an Ag-MOF and applied it towards the fixation of CO2 to propargyl amines, resulting in oxazolidinones.155 The Ag-MOF demonstrated significant flexibility in terms of the range of substrates, its ability to selectively target different sizes, and its ease of recycling as a heterogeneous catalyst at RT and atmospheric pressure conditions.
C– bond of propargyl amines. Consequently, various sterically restricted alkyne substrates were efficiently activated via π-interactions with cationic Ag centers. This study demonstrated that incorporating high-density Ag centers into a 2D framework as π-Lewis acid catalytic sites not only improves the stability of the 2D MOF but also enhances the activation of organic alkyne substrates, particularly bulky ones, thereby greatly boosting catalytic performance. Thus, 2D MOFs composed of catalytic metal clusters could be convincing catalysts for CO2 transformation reactions. In another example, Sun and co-workers reported an Ag-MOF and applied it towards the fixation of CO2 to propargyl amines, resulting in oxazolidinones.155 The Ag-MOF demonstrated significant flexibility in terms of the range of substrates, its ability to selectively target different sizes, and its ease of recycling as a heterogeneous catalyst at RT and atmospheric pressure conditions.
As discussed in the previous sections, normally noble metal-based catalysts are employed for the coupling of the alkynes with CO2. However, Zhao and co-workers reported a [Zn116] nanocage-based lantern-like 3D architecture, [Zn22(Trz)8(OH)12(H2O)9.8H2O]n (Zn-MOF) (Scheme 1), exhibiting large nanocage, assembled by Zn-clusters and Trz (C4N12O)4− linkers as building blocks.156 The MOF is comprised of two types of clusters: six [Zn14O21] clusters and eight [Zn4O4] clusters (Fig. 17a). The distinctive cage features a spacious interior cavity (0.81 × 1.03 nm) with an external edge of about 2.37 × 3.65 nm. This Zn-MOF demonstrated promising catalytic activity for the synthesis of 2-oxazolidinones utilizing CO2 and propargyl amines under ambient pressure at 70 °C within 12 h with recyclability up to ten cycles (Table 1 and Fig. 17b). Remarkably, the scope of catalysis was extended to various propargylic amine derivatives to obtain high yields of the desired products by transforming terminal propargylic amines with n-butyl or cyclohexyl substituents at the N-position (Fig. 17b). This MOF catalyst, devoid of noble metals, marked a significant step towards the environmentally friendly transformation of CO2 into oxazolidinones. In another demonstration of noble-metal free alkyne activation, Zhao's group reported a novel CuI/CuII mixed valence MOF {[(CuI6I5)CuII3(L6)6(DMA)3](NO3)·9DMA} having stp-type topology (Fig. 17c).157 Thanks to its exceptional chemical and thermal stability, CuI/CuII active centers and easily accessible free –NH2 groups exposed in the 1D channels of Cu-MOF rendered effective catalytic performance for cyclization of propargylamine with CO2 at 30 °C under 1 atm CO2 pressure within 1 hour, without the need of any co-catalyst or solvent (Table 1 and Fig. 17d). Different propargyl amines with various substituent groups were utilized, resulting in high yields of the corresponding 2-oxazolidinone products, ranging from 86% to 99% yield (Fig. 17d). Further, the abundance of free –NH2 groups within the spacious channels of Cu-MOF contributed to the alkalinity of the catalytic system, thus, facilitating the ring closure process to form oxazolidinones. The synergistic catalytic effect was enhanced by the ternary active sites comprising [CuI6I5] nodes, CuII paddle wheel nodes, and uncoordinated –NH2 groups within the MOFs, improving overall catalytic efficiency. Importantly, Cu-MOF effectively catalyzed the synthesis of oxazolidinones from propargylamine reaction with simulated flue gas. This work offered a promising route for directly using waste gases in industrial processes.
 | ||
| Fig. 17 (a) 3D framework of Zn-MOF. (b) The cyclization reaction of CO2 with propargylic amines catalyzed by Zn-MOF.156 Copyright 2020, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. (c) 3D view of Cu-MOF. (d) Cycloaddition of CO2 with propargylic amines catalyzed by Cu-MOF.157 Copyright 2021, Wiley–VCH GmbH. (e) 3D view of Mg-Cu-MOF. (f) Preparation of oxazolidinones using Mg-Cu-MOF.158 Copyright 2021, American Chemical Society. | ||
In a similar demonstration, Wu and co-workers reported noble-metal free 3D framework, [Mg3Cu2I2(IN)4(HCOO)2(DEF)4]n (Mg-Cu-MOF) (Fig. 17e) exhibiting outstanding stability and well-dispersed active metal sites contributing to its high catalytic activity.158 Under mild conditions, Mg-Cu-MOF effectively catalyzed the transformation of CO2 to produce 2-oxazolidinones from propargyl amines using TEA as a base (Table 1 and Fig. 17f), demonstrating recyclability for up to five cycles. The desired products were obtained in high yields using various N-aryl- or N-alkyl-substituted terminal propargylic amines. However, N-phenylprop-2-yn-1-amine exhibited low reactivity, with only trace amounts of the product. This unfavorable outcome may be attributed to weak N-nucleophilicity, which likely impedes the nucleophilic attack on CO2, preventing the formation of the carbamate intermediate. Li and co-workers in 2022, reported a Cu-MOF with mixed valency, [CuII2CuI4I4L8], consisting of two distinct, paddle–wheel Cu(II) clusters and Cu4I4 nodes which acted as a pillar-linker to form a 3D framework having 60.9% solvent-accessible volume.88 The pore configuration of Cu-MOF displayed a 3D architecture and retained a larger 1D pore structure (12.1 Å). The walls of the 1D pore channels are adorned with a significant quantity of –NH2 groups. The substantial size of the pores, along with the abundance of Lewis acidic and basic sites, rendered efficient catalytic activity for converting propargylic amines into oxazolidinones by utilizing CO2 under ambient pressure without any co-catalyst at 60 °C within 1 h with excellent substrate scope. The mechanism involves the synergistic effect of the –NH2 groups exposed in the pores and cuprous ions trigger the dehydrogenation of alkynyl amine, leading to a transition state and formation of a protonated amino group. At the same time, CO2 is activated at the active sites of both divalent and monovalent Cu, resulting in the formation of a carbamate anion which is further converted into an oxazolidinone product.
In an important demonstration of tailoring the Lewis acid–base sites to achieve turn on/off catalysis, Tian et al. reported a Co-MOF, {(NH2Me2)[Co3(μ3-OH)(BTB)2(H2O)]·9H2O·5DMF}n (Co-BTB) featuring a rhombic window (13.7 Å × 16.5 Å) along the a-axis (Fig. 18a–d).159 It was noted that Co-BTB facilitated the fixation of CO2 to aziridines with a yield of 99%. However, the fixation of CO2 to propargyl amines resulted in only 32% oxazolidinone, attributed to the absence of Lewis base sites (Fig. 18e). Notably, the catalytic performance of Co-BTB was improved by incorporating ligand, XN (4′-(4′′-pyridyl)2,4′:6′,4′′-terpyridine) with Lewis basic sites into Co-BTB which resulted in MOF, ({(NH2Me2)[Co3(μ3-OH)(NHMe2)(BTB)2(XN)]·8H2O·4DMF}n) named as Co-XN having less Lewis acid sites and more basic sites (Fig. 18c and d). Co-XN featured a 3D framework structure composed of rhombus channels (10 Å × 10 Å) (Fig. 18d). The incorporation of Lewis basic sites resulted in 2.4 times rise in the yield of oxazolidinones (Fig. 18e). Moreover, the reaction mechanism validated that Lewis acid sites efficiently facilitated the ring-opening and cycloaddition of aziridines, while the presence of Lewis basic sites accelerated the dehydrogenation of propargyl amines. Thus, controlling Lewis acid–base sites within MOFs on a molecular scale can facilitate achieving tailored catalytic activity for effective CO2 transformation to desired products. Jing and co-workers reported a 3D Cu-framework, Cu-TSP for coupling propargylic amines with CO2.160 Owing to the high porosity and suitable arrangement of Cu centers, Cu-TSP efficiently catalyzed the synthesis of 2-oxazolidinones from CO2 using DBU as the base at 50 °C under 0.1 MPa CO2, with recyclability up to five cycles (Table 1) with broad substrate scope.
 | ||
| Fig. 18 (a–d) Synthesis of Co-BTB and Co-XN. (e) Effect of Lewis acid–base sites on the catalytic activity of Co-BTB and Co-XN.159 Copyright 2022, Wiley–VCH GmbH. | ||
In 2023, Liu and co-workers reported a pyrene-based MOF, [Cd2(PTTB)(H2O)2] (WYU-11), (Fig. 19a), exhibiting remarkable catalytic efficacy for the preparation of 2-oxazolidinones by utilizing CO2, under mild conditions (60 °C, atmospheric CO2) with a wide range of propargylamine substrates.161 The steric hindrance effect can be observed from the low yield obtained from the introduction of the –CH3 group for the N-aryl-substituted group (Fig. 19b). Further, with N-phenylprop-2-yn-1-amine, only a trace amount of the product was formed owing to the weak N-nucleophilicity prohibiting the nucleophilic attack of CO2. The combined action of WYU-11 and 1,1,3,3-tetramethylguanidine (TMG) synergistically activated propargylic amine substrates and drove the reaction forward (Fig. 19b). The application of a mixed metal Cu–Fe porphyrin-based porous framework (Cu-TCPP(Fe)) composed of Cu-carboxylate layers has been reported recently (Fig. 19c).162 These layers result in a rhombic-shaped 2D lattice in the ab plane pillared by Fe-TCPP ligands. The large porous structure and appropriate coordination of the Cu centers in the Cu-TCPP(Fe) catalyst facilitated an efficient catalytic transformation of propargylic amines to 2-oxazolidinones using CO2 under mild reaction conditions (Table 1 and Fig. 19d). Moreover, the numerous H-bonding sites on the ligands strengthened the interaction between the framework and reactants. Additionally, the spacious pores and optimal coordination of the Cu centers facilitated an effective catalytic transformation under mild conditions.
 | ||
| Fig. 19 (a) Structure of WYU-11. (b) CO2 conversion to oxazolidinones catalyzed by WYU-11.161 Copyright 2023, American Chemical Society. (c) Structure of Cu-TCPP(Fe). (d) CO2 conversion to oxazolidinones catalyzed by Cu-TCPP(Fe).162 Copyright 2024, the Royal Society of Chemistry. | ||
The synthesis of a Cu(I)-based Th-MOF, {[Cu5I6Th6(μ3-O)4(μ3-OH)4(H2O)10(L)10]·OH·4DMF·H2O}n constituted by [Th6] clusters and [CuxIy] subunits has been reported by Wei and co-workers (Fig. 20a).163 Owing to the excellent chemical stability and exposed CuI sites, it was exploited for the utilization of CO2 along with propargylic amines to form oxazolidinones at RT using TEA as a base. Moreover, the gram scale experiment resulted in an 85% yield of oxazolidinones has been achieved (Fig. 20b).
 | ||
| Fig. 20 (a) Structure of Th-MOF. (b) CO2 conversion to oxazolidinones catalyzed by Th-MOF.163 Copyright 2024, American Chemical Society. (c) Structures of 1X%–20.5−X%–30.5-JNM (X = 0, 10, 25, 50). (d) CO2 conversion to oxazolidinones catalyzed by 10.1–20.4–30.5-JNM.164 Copyright 2024, American Chemical Society. | ||
The bioactive macromolecule dehydroabietylamine derivative was successfully transformed to the corresponding 2-oxazolidinone with a 91% yield. The high yield was achieved due to the large pore aperture and excellent catalytic activity of Th-MOF. It is worth mentioning that, such transformations of macromolecular substrates efficiently are rarely achieved using MOF-based catalysts. These results highlight the potential of Th-MOF in the synthesis of biological compounds. The widely distributed [CuxIy] subunits within the channels of Th-MOF enabled it to serve as an efficient heterogeneous catalyst for the synthesis of oxazolidinones. Chen et al. recently reported isostructural series of 2D MTV-MOFs, 1X%–20.5−X%–30.5-JNM (X = 0, 10, 25, 50), comprising Cu- and Ag-based cyclic trinuclear clusters by varying the ratio of Cu and Ag (Fig. 20c).164 It was found that when 10% Ag was incorporated into the framework, JNM showed highest catalytic activity for the conversion of propargyl amines to oxazolidinones using CO2 and DBU as a base. Under solvent-free conditions and atmospheric pressure, a TOF of 243 h−1 was achieved using 10.1–20.4–30.5-JNM as the catalyst (Fig. 20d). This TOF is about 20 times higher than 20.5–30.5-JNM (10.8 h−1). Further, the catalyst 10.1–20.4–30.5-JNM maintained its activity for the simulated flue gas, resulting in isolated yields of up to 76%. This study offers novel insights for the rational design of synergistic MTV-MOFs as catalysts at the molecular level, aiming to achieve efficient and environmentally friendly chemical conversion of CO2.
As discussed previously, it is apparent that MOF-based catalysts composed of alkynophilic Ag(0)/Ag(I) and Cu(0)/Cu(I) metals are promising catalytic materials for effective coupling of CO2 with propargyl amines as starting materials, to form oxazolidinones under ambient conditions. The catalytic sites within MOFs are widely distributed and easily available, promoting the complete utilization of the active sites. Metal nodes within MOFs are interchangeable with other metals and functional groups can be grafted on the organic linkers. This allows for carefully regulating MOF activities tailored to different reactions.165 A single MOF catalyst can readily incorporate multiple active sites, potentially leading to a synergistic effect that enhances catalytic efficiency.166 Typically, the interactions between MOF and CO2 molecules are crucial, as strengthening these interactions enhances the material's ability to capture CO2, particularly at low-pressure conditions. Achieving this necessitates modification of the inherent characteristics of MOFs through careful design and synthesis using de novo methods or PSM.
The goal is to leverage the chemical compatibility and tunability of the MOFs to increase their affinity for CO2 molecules. In this regard, Gu et al., prepared Cu2O@ZIF-8 catalyst by incorporating Cu2O NPs into robust ZIF-8 with BET surface area of 1343 m2 g−1, comparatively lower than the original ZIF-8 (1974 m2 g−1) due to substantial encapsulation of Cu2O NPs (Fig. 21a).167 Taking advantage of the outstanding CO2 adsorption capability of robust ZIF-8 and highly active Cu2O sites, the Cu2O@ZIF-8 composite efficiently facilitated the conversion of CO2 to valuable oxazolidinones at 40 °C under atmospheric pressure of CO2 using CH3CN as a solvent and DBU as a base with good substrate scope (Table 1 and Fig. 21b). Notably, the conversion of N-phenylprop-2-yn-1-amine was negligible (2%) due to its weak N-nucleophilicity hindering its nucleophilic attack with CO2, thereby, preventing formation of carbamate intermediate (Fig. 21b). It can be seen that the internal propargylamine substrate produced only a moderate yield of the product (48%) over 12 h (Fig. 21b). The Cu2O/DBU binary system efficiently promoted the CO2 fixation, resulting in oxazolidinones through a mechanism that includes H+ migration from propargylic amine to base followed by intramolecular cyclization and H-demetalation steps. Similarly, Wu and co-workers reported Cu2O@MIL-101(Cr)-DABCO, which was meticulously engineered by sequentially incorporating DABCO and Cu2O into the MIL-101(Cr) MOF using a stepwise assembly approach.168 Due to the significant confinement of Cu2O NPs within the MOF and the robust coordination interaction between DABCO and the MOF, Cu2O@MIL-101(Cr)-DABCO efficiently facilitated the cyclization of CO2 to propargylic amines with a wide range of substrate scope. This catalyst exhibited good reusability without the need for a co-catalyst or solvent, operating at RT under atmospheric pressure conditions.
 | ||
| Fig. 21 (a) Synthesis of Cu2O@ZIF-8. (b) Preparation of oxazolidinones catalyzed by Cu2O@ZIF-8.167 Copyright 2022, Wiley–VCH GmbH. (c) Synthesis of CuBr@NH2-MIL-101. (d) Reaction of propargylic amines with CO2 yielding oxazolidinones catalyzed by CuBr@NH2-MIL-101.169 Copyright 2023, Elsevier. | ||
The application of CuBr anchored MOF composite, CuBr@NH2-MIL-101 (Fig. 21c) for selective CO2 adsorption has been reported by Hu and co-workers.169 CuBr@NH2-MIL-101 displayed excellent results for carboxylative fixation of CO2 to propargylic amines under ambient conditions (25 °C, DBU, CH3CN, 0.1 MPa CO2) (Table 1 and Fig. 21d) with good substrate universality. Recently, Astruc and co-workers reported synthesis of a Cu(I)-GSH/ZIF-8 composite using a biologically essential Cu-glutathione (GSH) redox system.170 The BET surface and pore volume of ZIF-8 remain the same after the introduction of 0.3 wt% Cu(I)-GSH and 0.45 wt% Cu(I)-GSH, displaying Cu(I)-GSH polymer loading on ZIF-8 surface, not in the pores. Cu(I)-GSH/ZIF-8 was exploited as a heterogeneous catalyst for the carboxylative cyclization of propargyl amines with CO2 with high yields under atmospheric pressure. Notably, Cu(I)-GSH/ZIF-8 displayed a good substrate tolerance to electron-withdrawing or electron-donating groups on the N-benzyl unit of propargylic amines.
The aforementioned literature studies have shown that PSM of frameworks is a useful tool to integrate CO2-philic and catalytic sites to achieve catalytic transformations with high yield and selectivity.171 The reaction of propargyl amines with CO2 to produce 2-oxazolidinone is a vital industrial reaction. However, it typically requires noble-metal catalysts with organic bases under severe conditions. Notably, Cu-containing MOFs exhibit significant catalytic activity in converting CO2 to oxazolidinones using propargylamine substrates.169–172 Thus, being an affordable metal, Cu-based catalysts can replace noble metals like Ag, Pt and Au without compromising catalytic proficiency and reducing catalysis costs.
To date, certain metal-incorporated COFs have been effectively employed in transforming CO2 into a variety of oxazolidinones. For instance, the application of Ag-NPs anchored Tp-based COFs (TpPa-1 and TpTta) for carboxylation of propargylic amines has been reported by Islam and co-workers.178 The Ag-decorated COFs exhibited remarkable catalytic capabilities in incorporating atmospheric CO2 into unsaturated amines under neat conditions, producing industrially significant cyclic carbamates and oxazolidinones. Especially, Ag@TpTta facilitated efficient, atom-economical and high-yield synthesis of alkylidene-oxazolidinones under solvent-free conditions utilizing 1 atm CO2 pressure and temperatures ranging from 40 to 80 °C (Table 1). Later, the same group reported incorporation of Cu-NPs in the 1D pores of triazole-based COF (Cu-NPs@COF).179 The Cu-NP-embedded COF showed promising catalytic activity for producing 2-oxazolidinones via cycloaddition reaction of CO2 with propargyl amines under mild conditions. The reaction involving CO2 and propargylic amines offered the production of highly valuable chemicals. These studies were extended to the incorporation of Pd(II) in the COF to obtain (Pd(II)@TFR-OT COF) and its application for synthesis of oxazolidinone via chemical fixation of CO2 was demonstrated under sunlight.180
The encapsulation of Ag NPs in porous pyridine-based COFs has been demonstrated by Bai and co-workers.181 The presence of pyridyl-N in the framework effectively incorporated Ag metal within the pores, resulting in Ag@2,6-FPP-TAPT and Ag@3,5-FPP-TAPT COFs (Scheme 2 and Fig. 22a). The orientation of pyridyl-N determined the placement of Ag sites in diverse pores and constrained them to specific dimensions, greatly influencing their catalytic activity and stability. These materials were utilized as eco-friendly effective catalysts for synthesizing various oxazolidinones using propargylic amines and CO2 under ambient conditions (50 °C and 1 atm pressure) (Table 1 and Fig. 22b). Detailed investigations revealed that propargylamine substrates could be trapped and interact with extremely small, well-dispersed Ag sites within the pores. Additionally, 2,6-FPP-TAPT could absorb CO2, enhancing the reaction efficiency by facilitating the rapid conversion of CO2. In a similar approach, our group recently reported a pyrene-based COF, Pybpy-COF designed to securely anchor catalytic Ag(0) NPs (Fig. 23a) and applied it to synthesize commodity chemicals using CO2 under standard environmental conditions.182 Ag@Pybpy-COF catalyzed CO2 conversion to 2-oxazolidinones using diverse propargylic amines under mild conditions (Table 1 and Fig. 23b). The remarkable catalytic efficacy of Ag@Pybpy-COF is attributed to the abundance of exposed, alkynophilic Ag(0) catalytic sites strategically distributed on the pore surface (787 m2 g−1) of Pybpy-COF. This study showcased the potential use of Pybpy-COF as a reliable platform for anchoring Ag NPs and its application for transforming CO2 into valuable oxazolidinones. Lan and co-workers embedded Cd single-atom sites in a 2,2′-bipyridine based COF (Cd-Bpy-COF).183 The strategic placement of bipyridine coordination units within the framework anchored Cd single sites with high loading, exposed substantial active sites to boost catalytic activity (Fig. 24a). The resulting Cd-Bpy-COF demonstrated exceptional performance and remarkable stability in catalyzing the CO2 conversion to oxazolidinones using propargyl amines under ambient reaction conditions. The fusion of COFs with Cd sites significantly enhanced CO2 adsorption and its activation, thereby facilitating subsequent cyclization reactions (Fig. 24a).
 | ||
| Fig. 22 (a) Synthetic routes for the Ag@2,6-FPP-TAPT and Ag@3,5-FPP-TAPT catalysts. (b) Preparation of oxazolidinones catalyzed by Ag@2,6-FPP-TAPT.181 Copyright 2022, the Royal Society of Chemistry. | ||
 | ||
| Fig. 23 (a) Preparation of Pybpy-COF and Ag@Pybpy-COF. (b) Synthesis of oxazolidinones using propargylic amines and CO2 catalyzed by Ag@Pybpy-COF.182 Copyright 2024, American Chemical Society. | ||
 | ||
| Fig. 24 (a) Preparation of oxazolidinones using propargyl amines and CO2 catalyzed by Cd-Bpy-COF.183 Copyright 2023, the Royal Society of Chemistry. (b) Synthesis of CuI-TpBD-COF. (c) Preparation of oxazolidinones catalyzed by CuITpBD-COF.184 Copyright 2024, the Royal Society of Chemistry. | ||
A proposed mechanism for Cd-Bpy-COF is illustrated in Fig. 24a. The process begins with the activation of propargylic amine at the Cd sites through interactions with its N–H bond and is subsequently activated by DBU. This activation allows for an electrophilic attack by CO2, resulting in the formation of a carbamate intermediate. Following this, the negatively charged oxygen atom of the carbamate attacks the Cu–C bond, facilitated by activation from Cd, which leads to intramolecular cyclization. Finally, a proton from DBUH+ is transferred to the triple bond, yielding the desired product. On the other hand, the construction of CuI-anchored β-ketoamine COF (CuITpBD-COF) and its utilization for carboxylative cycloaddition of CO2 with propargylic amines has been reported (Fig. 24b and c).184 CuI@TpBD-COF displayed proficient catalytic results when simulated flue gas was utilized as a CO2 source.
The previous discussions signified the application of COF-based frameworks for catalyzing the synthesis of oxazolidinones using propargylamine as substrates. While significant progress has been made in synthesizing oxazolidinones using COF catalysts, most literature reports utilize noble metal (Ag, Pd) incorporated COFs for the effective activation of propargylic hydrogen because of the excellent alkynophilicity of these metals. Thus, research on developing noble-metal-free catalysts is still in its early stages. Meeting industrial demand requires noble-metal-free catalysts, which have garnered significant attention from scientists dedicated to advancing this research. Therefore, creating efficient COF catalysts using non-noble metals is crucial for developing affordable and sustainable methods for environmentally friendly CO2 conversion into oxazolidinones. However, greater emphasis should be placed on using eco-friendly and economically viable metals for CO2 conversion reactions.
The synthesis of two microporous POPs, BBA-1 and modified BBA-2 has been reported by Ghosh et al.188 They decorated Pd NPs on these POPs to produce Pd@BBA-1 and modified Pd@BBA-2 nanomaterials. The presence of Pd NPs on the surface facilitated efficient cyclic carboxylation of a diverse range of propargylamine, resulting in corresponding 2-oxazolidinones under 0.1 MPa CO2 with DMSO solvent at temperatures ranging from 40 to 80 °C. Remarkably, Pd@BBA-2 exhibited good recyclability for generating oxazolidinones. Similarly, the application of Ag NP anchored COF (AgN@COF) for coupling of CO2 with terminal propargylic amines and propargylic alcohols, rendering 2-oxazolidinones at 55 °C under 1 atm of CO2 has also been reported.189
The application of a thiadiazole-based COP (BT-COP) for anchoring Ag NPs and its catalytic investigation has been reported by Lan and co-workers.190 Here, the S-atom within the thiadiazole unit served as an anchoring site for Ag NPs growth. The resulting Ag@BT-COP exhibited notable CO2 adsorption capabilities, along with commendable catalytic performance and reusability for efficiently converting CO2 and propargylic amines to valuable 2-oxazolidinones at 60 °C under standard 1 atm CO2 pressure (Table 1). This research work highlighted the potential utility of functionalized COPs in CO2 fixation, shedding light on the advancement of efficient catalysts for converting CO2 into appreciated chemicals. In another example, Zhang and co-workers introduced two nitrogen-rich porous organic polymers (NPOPs) comprised of covalent triazine and triazole N-heterocycles. Ag NPs were effectively incorporated into the POPs, resulting in Ag@NPOPs characterized by excellent distribution and small particle size.191 These Ag@NPOPs were then employed in potential conversion reactions involving CO2, terminal alkynes and propargylic amines under mild reaction conditions (50 °C, 1 atm CO2). Ag@NPOP-1 demonstrated exceptional catalytic stability and durability, being successfully reproduced and reused five times. This research offered valuable insights for designing and fabricating novel multifunctional catalysts. The design of a noble metal-free Cu-based NHC POP catalyst (Cu@NHC-1) by copolymerization technique, followed by complexation with Cu(OAc)2, and its utilization for converting low-concentration CO2 into oxazolidinones has been reported.192 Owing to its porous structure, N-activation sites and catalytic Cu center working together synergistically, Cu@NHC-1 exhibited exceptional efficiency and selectivity in adsorbing, activating, and converting low-concentration CO2 (30 vol%) to oxazolidinones. The practical potential of this catalyst is evidenced by its capability to effectively convert CO2 from lime kiln waste gas into oxazolidinones with satisfactory yields under mild conditions. Moreover, the gram-scale reaction yields the desired product in high quantities, showcasing the catalyst's significant potential for industrial CO2 conversion. Recently, our research group reported the strategic incorporation of catalytically active Pd(II) into a porous covalent triazine framework (CTF) composed of bipyridine sites (bpy-CTF) via PSM.193 The resulting Pd(II)@bpy-CTF catalyst demonstrated exceptional catalytic performance in the cyclization of CO2 with propargylic amines, producing high-value oxazolidinones under mild conditions. The exceptional catalytic behavior of Pd(II)@bpy-CTF is attributed to a high abundance of N-rich triazine units within the framework and exposed catalytic Pd(II) sites within the 1D channels of the CTF.
Based on the literature studies, it can be concluded that POPs remain highly regarded for their role in the selective capture and utilization of CO2 into valuable chemicals like oxazolidinones, contributing significantly to combat global warming. The engineering of their pore environments enhances their affinity for CO2, enabling the development of highly microporous networks rich in heteroatoms that provide abundant catalytic sites. This structural versatility allows POPs to effectively facilitate reactions such as the utilization of CO2 to prepare oxazolidinones. Their relatively low cost and ease of synthesis compared to other porous materials further underscore their potential in sustainable carbon management strategies.
3.3. Synthesis of oxazolidinones by a one-pot multi-component reaction involving CO2
As discussed before, the three-component reactions involving CO2, propargyl alcohol and primary amine represent a highly promising and innovative strategy for synthesizing 3-substituted-2-oxazolidinones. An alternative three-component approach involves epoxides, primary amines and CO2 resulting in the preparation of oxazolidinones. Literature studies revealed that efficient formation of oxazolidinones through a three-component coupling of alkynes/amines with CO2 necessitates (Cu(0/I) or Ag(0/I)) metal sites due to the alkynophilicity exhibited by these metals. Here, the reaction begins with the polarization of the alkyne –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– bond at the catalytic sites of Cu/Ag. Then, CO2 is inserted into the deprotonated alkyne, followed by a ring-closure step, rendering α-alkylidene cyclic carbonate, which reacts with propargylic amines to produce oxazolidinones (Fig. 25b).194
C– bond at the catalytic sites of Cu/Ag. Then, CO2 is inserted into the deprotonated alkyne, followed by a ring-closure step, rendering α-alkylidene cyclic carbonate, which reacts with propargylic amines to produce oxazolidinones (Fig. 25b).194
 | ||
| Fig. 25 (a) Three-component synthesis of oxazolidinones catalyzed by TMOF-3-Ag. (b) Catalytic mechanism of the three-component reaction.196 Copyright 2018, American Chemical Society. | ||
In another example, the utilization of SO3H-functionalized MOF (MOF-SO3H) comprising polar sulfonate group for anchoring alkynophilic Ag(I) sites has been studied (Fig. 26a).197 The reduction in the BET surface area from 1191 m2 g−1 (pristine MOF-SO3H) to 851 m2 g−1 (MOF-SO3Ag) validates the incorporation of Ag(I) at the –SO3H groups exposed in the 1D channels of MOF. MOF-SO3H exhibited a high heat of interaction value of 37.8 kJ mol−1, which enhanced its selective CO2 adsorption capabilities over other gases, attributed to the presence of polar sulfonate groups exposed in the pores. The combined influence of polar sulfonate groups and catalytically active Ag(I) sites created a favorable environment for catalyzing the three-component reaction of propargylic alcohols and primary amines under 1 atm CO2 at RT (Table 1 and Fig. 26b). More importantly, the absence of leaching of the catalytic active site, Ag(I) was established with catalytic recyclability for up to five cycles (Fig. 26c).
 | ||
| Fig. 26 (a) Synthesis of MOF-SO3Ag. (b) Synthesis of oxazolidinones by a three-component reaction catalyzed by MOF-SO3Ag. (c) Recyclability test of MOF-SO3Ag.197 Copyright 2020, American Chemical Society. | ||
Towards the development of noble-metal-free MOF for one-pot synthesis of oxazolidinones, Yamani and co-workers198 reported a series of UiO-66199–201 frameworks possessing linker-induced defects. Here, the application of linker-induced defects-rich UiO-66 MOF has demonstrated improved catalytic activity for the reaction of aromatic amines, epoxides and CO2 over the pristine MOF. A wide range of biologically relevant oxazolidinones were obtained in yields exceeding 90% while maintaining structural rigidity and recyclability for up to 5 cycles. This work opened new doors for linker-induced defects-based MOFs, resulting in the fixation of CO2 into value-added products. An example of Ni-based MOF prepared from trismic acid linker has been employed as a noble-metal-free catalyst for the synthesis of oxazolidinones (Fig. 27a).202 The MOF displayed a CO2 uptake of 37 cc g−1 and on thermal activation, it generates open metal sites that acted as Lewis acidic sites in the CO2 utilization reaction involving aromatic amines and epoxides. This resulted in the production of oxazolidinone at 90 °C and 1 bar pressure using TBAI as a co-catalyst (Table 1 and Fig. 27b).
 | ||
| Fig. 27 (a) Synthesis of Ni-MOF. (b) Preparation of oxazolidinones catalyzed by Ni-MOF.202 Copyright 2021, Elsevier. (c) Synthesis of Cu(I)@NHC-MOF. (d) The three-component CO2 fixation catalyzed by Cu(I)@NHC-MOF.209 Copyright 2021, the Royal Society of Chemistry. | ||
The detailed literature study indicated that the majority of catalysts used for the transformation of CO2 using propargylic alcohols to form oxazolidinones incorporate noble metals.203–206 The application of non-noble metal-based catalysts like Cu(I) for transforming CO2 into oxazolidinones is important from a green and sustainable chemistry perspective. In this regard, N-heterocyclic carbene (NHC) based linkers provide an appropriate platform for boosting CO2-philicity and anchoring catalytic metal ions at the carbene carbon center.207,208 Keeping this in mind, our group209 post-synthetically modified NHC-based MOF210 by Cu(I) ions to generate Cu(I)@NHC-MOF211 as shown in (Fig. 27c). The cooperative involvement of CO2-attracting NHC and catalytically active Cu(I) sites created a promising platform for transforming CO2 into oxazolidinones, specifically at RT and atmospheric CO2 pressure (Table 1 and Fig. 27d). The synthesized hybrid material showed high recyclability for up to ten cycles. Further, PSM of NHC-MOF by incorporation of Ag(I) ions to form Ag(I)@MOF-NHC and its catalytic investigation has also been reported.212 The Ag(I)@MOF-NHC displayed significant catalytic efficacy in a one-pot reaction involving primary amines, CO2 and propargylic alcohol, leading to the production of various oxazolidinones under environmentally friendly conditions, specifically at RT and atmospheric pressure with simulated flue gas.
The construction of a chiral framework, CMOF-801(ASP) and its application for the synthesis of chiral oxazolidinones by asymmetric CO2 utilization has been reported by Morsali and co-workers. The chiral MOF was generated by substituting fumaric acid with L-aspartic acid (Scheme 1) in MOF-801.213 The CMOF-801(ASP) facilitated asymmetric catalysis for producing valuable products with optimal efficiency in a short time by simultaneously incorporating Zr sites as Lewis acidic sites, –OH and NH3+ sites as Brønsted acid sites and –NH2 as Lewis base sites. The enhanced catalytic performance of CMOF-801(ASP) stemmed from its ample active sites, created using the missing-cluster defect strategy, which notably eased substrate diffusion and activation. Chiral oxazolidinones with 90% conversion and 98% ee were obtained using the catalyst having three functional catalytic sites without a co-catalyst under solvent-free conditions (90 °C, 12 h, 1 bar CO2) (Table 1). This research showcased that introducing cluster defects alongside Lewis basic chiral linkers can generate numerous catalytic sites within MOFs. This setup allows for swift and eco-friendly asymmetric catalysis to occur under mild conditions. Recently, Mandal and co-workers reported a Ni-MOF with remarkable polarizing properties, showing significant uptake of CO2 and water vapor, reaching up to 10.53 cm3 g−1 and 290 cm3 g−1, respectively, at 298 K.214 Leveraging its CO2-philic and catalytic features, Ni-MOF was employed as an excellent heterogeneous catalyst (2.5 mol%) along with a very low concentration of TBAB (0.02 mmol) as a co-catalyst for the preparation of oxazolidinones via a one-pot reaction involving an epoxide, a substituted aniline and CO2, without use of any solvent.
The literature studies demonstrated that Lewis acidic sites increase the catalytic efficiency of the MOF, resulting in a high yield of oxazolidinones. In addition, Lewis basic sites and CO2-philic groups enhance the easy activation of carbon dioxide, resulting in efficient catalytic results. It is important to highlight that the interaction between the framework and CO2 molecules plays a crucial role in CO2 capture and conversion by the MOF. Strengthening this interaction can significantly increase the CO2 uptake and conversion capacity of the MOF material, particularly at low loading pressures.
 | ||
| Fig. 28 (a) Four-component oxazolidinone synthesis from PCN-(BPY-CuI)-(TPDC-F7). (b and c) Probable mechanism for the one-pot four-component reaction.80 Copyright 2023, Wiley–VCH GmbH. | ||
Mechanistic studies indicated that the MOF effectively enriched the substrates along its wall, with the adsorbed amine species playing a crucial role as external binding sites for dilute CO2 by favoring carbamate acid formation (Fig. 28b and c). This study presents an encouraging approach for the straightforward synthesis of 2-oxazolidinones, utilizing noble metal-free catalysts utilizing flue gas as a CO2 source and affordable industrial bulk raw materials, all under mild conditions. In a similar approach, our group has recently reported the application of non-noble metal copper nanoparticles (Cu NPs) grafted porous, CO2-philic MOF for one-pot synthesis of 2-oxazolidinones (Fig. 29a).215
 | ||
| Fig. 29 (a) Structure of Cu@UiO-66-NH2. (b) Four-component oxazolidinone synthesis from Cu@UiO-66-NH2.215 Copyright 2024, American Chemical Society. | ||
This approach enables the straightforward one-pot synthesis of 2-oxazolidinones through a four-component reaction involving phenylacetylene, CO2, acetone and aliphatic amines. Notably, the embedding of small-size Cu-NPs with an average diameter of 10 nm exhibiting high catalytic activity was achieved. The MOF with anchored Cu NPs demonstrated remarkable catalytic activity for converting CO2 into valuable 2-oxazolidinones by a one-pot, four-component reaction through C–H bond functionalization of alkynes under ambient conditions (Table 1 and Fig. 29b).
Based on the previous discussions, it is evident that COF-based materials possess extensive surface areas and consistent pores, enabling numerous approachable catalytic sites and rapid mass transfer of reactants and products.222 The structural framework and porous surroundings of COFs can be precisely designed and controlled, offering an effective strategy to improve catalytic performance, including selectivity, reaction kinetics and other factors.223 Additionally, the plentiful pores within COFs provide an ideal platform for hosting and enclosing diverse functional species, including metal NPs, organic molecules, fullerenes and ionic liquids. Metal-incorporated COFs and metal NPs incorporated COFs hold significant potential for applications in CO2 utilization owing to their high density of catalytic metal sites, thus enhancing the catalytic process.
4. Conclusions and future outlook
Recent advancements in framework-based materials have led to the development of novel and superior catalytic materials for CO2 capture and conversion to oxazolidinones. The key to their effectiveness lies in the distinctive chemistry of the framework architectures. By customizing the frameworks, it is possible to fine-tune the interactions between CO2 molecules and the internal structure of the MOFs/COFs/POPs, which include active functional groups, open metal sites and microscopic pores.224–227 This review systematically examines the evolution of framework-based materials as heterogeneous catalysts for the chemical transformation of CO2 to value-added oxazolidinones, covering literature developments from the earliest examples to the latest research. It is worth emphasizing that framework (MOF/COF/POP) materials provide an exceptional platform for in-depth investigation into the relationship between catalytic performance and the local reaction environment. By analyzing these interactions, researchers can gain critical insights that are essential for optimizing the design of next-generation efficient framework materials for industrial applications.228,229 Despite significant advancements, substantial challenges remain in developing framework-based catalysts that catalyze CO2 transformations from flue gas containing competing gases like nitrogen oxides (NOx) and sulfur oxides (SOx).230,231 Also, achieving catalytic reactions under mild conditions is crucial—specifically, at low temperatures and atmospheric pressure conditions facilitating CO2 transformation reactions from dilute gas or direct air.232–234 To enhance the efficiency of these reactions, it is crucial to improve the CO2 uptake capacity of framework materials.235,236 Consequently, incorporating a high density of CO2-philic sites leads to an increase in the local concentration of carbon dioxide around active sites, thereby promoting higher reaction yields.237,238Thus, a range of frameworks (MOFs/COFs/POPs) and their composites have been developed with diverse structural characteristics, such as Lewis acidic/basic and nucleophilic sites, to facilitate effective preparation of oxazolidinones under ambient conditions.239,240 Further, the incorporation of alkynophilic metal NPs with the frameworks having heteroatoms has greatly enhanced both the adsorption of CO2 and its conversion to oxazolidinones.241,242 However, there is considerable potential for developing noble metal-free framework-based catalysts aimed at efficiently converting CO2 into high-value oxazolidinone derivatives. In conclusion, framework-based materials hold a bright future as heterogeneous catalysts for CO2 conversion to oxazolidinones and other value-added chemicals (Fig. 30). The present review provides valuable information for the future design of novel framework-based materials for effective capture and chemical fixation of CO2 into high-value chemicals.
Abbreviations
| AA-MOFs | Amino acid linker-based MOFs |
| 4-AMBA | 4-Aminonethyl benzoic acid |
| ASP | Aspartic acid |
| 5-atz | 5-Amino-1H-tetrazole |
| BBA | Benzene-benzylamine |
| H4BCP | 5-(2,6-Bis(4-carboxyphenyl)pyridin-4-yl)isophthalic acid |
| BDC | 1,4-Benzenedicarboxylic acid |
| H2BDP-NH2 | 2-Amino-[1,4-bis(1H-pyrazol-4-yl)benzene] |
| BD | Biphenylamine |
| BPY | 2,2′-Bipyridine-5,5′-dicarboxylate |
| Bpy | 4,4′-Bipyridine |
| Bpy | 2,2′-Bipyridien-4,4′-CHO |
| H3BTB | 1,3,5-Tris(4-carboxyphenyl)benzene |
| 1,2,4,5-BTMS | 1,2,4,5-Benzenetetramethanesulfonate |
| H2btz | 1,5-Bis(5-tetrazolo)-3-oxapentane |
| H3-BTC | 1,3,5-Benzenetricarboxylic acid |
| CCSU | Carbon capture, sequestration and utilization |
| CCU | Carbon capture and utilization |
| CCS | CO2 capture and storage/sequestration |
| COP | Conjugated organic polymer |
| COFs | Covalent organic frameworks |
| CPT | 3,5-Bis(4′-carboxyphenyl)-1,2,4-triazole |
| CTF | Covalent triazine framework |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| H2DATP | 4′-(3,5-Dicarboxyphenyl)-2,2′:6′,2′′′-terpyridine |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| H2DCTP | 4′-(3,5-Dicarboxyphenyl)-4,2′:6′,4′′-terpyridine |
| DEF | N,N-Diethylformamide |
| DFT | Density functional theory |
| 2,3-Dha | 2,3-Dihydroxyterephthalaldehyde |
| 2,3-Dma | 2,3-Dimethoxyterephthalaldehyde |
| DMA | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| 2,6-FPP | 2,6-(4-Formylphenyl)pyridine |
| 3,5-FPP | 3,5-(4-Formylphenyl)pyridine |
| Glu | Glutamic acid |
| HBD | Hydrogen bond donor |
| HIN | Isonicotinic acid |
| IPA | Isophthalic acid |
| HL1 | Tetrazole monoanion |
| H4L2 | 2′-Fluoro-[1,1′:4′,1′′-terphenyl]-3,3′′,5,5′′-tetracarboxylic acid |
| L3 | N 1-(4-(1H-Imidazol-1-yl)benzyl)-N1-(2-aminoethyl)-ethane-1,2-diamine |
| H3L4 | (2Z,2′Z,2′′Z)-2,2′,2′′-((((1,3,5-Triazine-2,4,6-triyl)tris(azanediyl))tris(benzene-4,1-diyl))tris(ethan-1-yl-1-ylidene))tris(hydrazine-1-carbothioamide) |
| H3L5 | (2Z,2′Z,2′′Z)-2,2′,2′′-((((1,3,5-Triazine-2,4,6-triyl)tris(oxy))tris(benzene-4,1-diyl))tris(methanylylidene))tris(hydrazine-1-carbothioamide) |
| HL6 | 5-Aminonicotinic acid |
| L7 | 5-Hydroxynicotinic acid |
| H2L8 | 4,4′-(4-Amino-4H-1,2,4-triazole-3,5-diyl)dibenzoic acid |
| H2L9 | 4,4′-((1E,1′E)-(2-Oxocyclohexane-1,3-diylidene)bis(methanylylidene))dibenzoic acid |
| MMPF | Metal–metalloporphyrin framework |
| MOFs | Metal–organic frameworks |
| NA | Not applicable |
| NHC | N-heterocyclic carbene |
| NPs | Nanoparticles |
| PD | p-Phenylenediamine |
| PCPs | Porous coordination polymers |
| PCN | Porous coordination network |
| POPs | Porous organic polymers |
| ppm | Parts per million |
| Ppa | p-Phenylenediamine |
| H4PTTB | 1,3,6,8-Tetrakis(3-carboxyphenyl)pyrene |
| Py | Pyrene-NH2 |
| RT | Room temperature |
| SBUs | Secondary building units |
| TAPT | Tris(4-aminophenyl)triazine |
| TBAB | Tetrabutylammonium bromide |
| TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene |
| H10TBCPPP | Tetrakis-3,5-bis[(4-carboxy)phenyl]phenyl porphine |
| H2TCPP | Tetrakis(4-carboxyphenyl)porphyrin |
| TEA | Triethylamine |
| TFR-OT | Triformylresorsinol-O-tolidine |
| TFP | 2,4,6-Trihydroxybenzene-1,3,5-tricarbaldehyde |
| Tta | 4,4′,4′′-(1,3,5-Triazine-2,4,6-triyl)trianiline |
| TMG | 1,1,3,3-Tetramethylguanidine |
| TON | Turnover number |
| TPyP-H2 | 5,10,15,20-Tetra(4-pyridyl)porphyrin |
| TPDC-NH2 | 2′-Amino-[1,1′:4′,1′′-terphenyl]-4,4′′-dicarboxylic acid |
| Tp | Triformylphenol |
| Tph | 5,10,15,20-Tetrakis(4-aminophenyl)-21H,23H-porphine |
| H4Trz | Tri(1H-tetrazol-5-yl)methanol |
| TSP | 2,2′,2′′-(Benzene-1,3,5-triyltris(ethan-1-yl-1-ylidene))tris(hydrazine-1-carbothioamide) |
| Tta | 4,4′,4′′-(1,3,5-Triazine-2,4,6-triyl)-trianiline |
| XN | 4′-(4-Pyridine)4,2′:2′,4′′-terpyridine |
| TABH | (E)-2-(4-((E)-(Thioureidomethylene) amino)benzylidene)hydrazinecarbothioamide |
| Oxdz | 4,4′-(1,3,4-Oxadiazole-2,5-diyl)dibenzoate |
| Tpxn | N,N′,N′′,N′′′-tetrakis(2-pyridylmethyl)-1,4-diaminooxylylene |
Data availability
Data will be available on request.Conflicts of interest
The authors declare that they have no competing financial interests.Acknowledgements
C. M. N. acknowledges DST-SERB (CRG/2018/001176) for financial support. PR thanks DST-SERB (PDF/2023/000057) for funding in the form of NPDF.References
- S.-L. Hou, J. Dong, X.-Y. Zhao, X.-S. Li, F.-Y. Ren, J. Zhao and B. Zhao, Thermocatalytic Conversion of CO2 to Valuable Products Activated by Noble-Metal-Free Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2023, 62, e202305213 CrossRef CAS PubMed.
- M. Aresta, A. Dibenedetto and A. Angelini, Catalysis for the Valorization of Exhaust Carbon: from CO2 to Chemicals, Materials, and Fuels, Technological Use of CO2, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- R. Mansoor and M. Tahir, Recent developments in natural gas flaring reduction and reformation to energy-efficient fuels: a review, Energy Fuels, 2021, 35, 3675–3714 CrossRef CAS.
- A. Glikson, The lungs of the Earth: review of the carbon cycle and mass extinction of species, Energy Procedia, 2018, 146, 3–11 CrossRef CAS.
- IPCC, 2014: Climate Change 2014: Mitigation of Climate Change, in Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. O. Edenhofer, R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlömer, C. von Stechow, T. Zwickel and J. C. Minx, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014 Search PubMed.
- M. Z. Jacobson, Review of solutions to global warming, air pollution, and energy security, Energy Environ. Sci., 2009, 2, 148–173 RSC.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Müller, Worldwide innovations in the development of carbon capture technologies and the utilization of CO2, Energy Environ. Sci., 2012, 5, 7281–7305 RSC.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Direct Capture of CO2 from Ambient Air, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- J.-B. Lin, T. T. T. Nguyen, R. Vaidhyanathan, J. Burner, J. M. Taylor, H. Durekova, F. Akhtar, R. K. Mah, O. Ghaffari-Nik, S. Marx, N. Fylstra, S. S. Iremonger, K. W. Dawson, P. Sarkar, P. Hovington, A. Rajendran, T. K. Woo and G. K. H. Shimizu, A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture, Science, 2021, 374, 1464–1469 CrossRef CAS PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Carbon dioxide capture in metal–organic frameworks, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- C. Kolster, E. Mechleri, S. Krevor and N. Mac Dowell, The role of CO2 purification and transport networks in carbon capture and storage cost reduction, Int. J. Greenhouse Gas Control, 2017, 58, 127–141 CrossRef CAS.
- D. Wawrzyńczak, M. Panowski and I. Majchrzak-Kucęba, Possibilities of CO2 purification coming from oxy-combustion for enhanced oil recovery and storage purposes by adsorption method on activated carbon, Energy, 2019, 180, 787–796 CrossRef.
- G. Santori, C. Charalambous, M.-C. Ferrari and S. Brandani, Adsorption artificial tree for atmospheric carbon dioxide capture, purification and compression, Energy, 2018, 162, 1158–1168 CrossRef CAS.
- G. Singh, K. Prakash and C. M. Nagaraja, Fe(III)-Anchored Porphyrin-Based Nanoporous Covalent Organic Frameworks for Green Synthesis of Cyclic Carbonates from Olefins and CO2 under Atmospheric Pressure Conditions, Inorg. Chem., 2023, 62, 13058–13068 CrossRef CAS PubMed.
- G. Singh and C. M. Nagaraja, Rational design of Cu(I)-anchored porous covalent triazine framework (CTF) for simultaneous capture and conversion of CO2 at ambient conditions, J. CO2 Util., 2022, 63, 102132 CrossRef CAS.
- S. S. Dhankhar and C. M. Nagaraja, Construction of a 3D porous Co(II) metal–organic framework (MOF) with Lewis acidic metal sites exhibiting selective CO2 capture and conversion under mild conditions, New J. Chem., 2019, 43, 2163–2170 RSC.
- P. Rani, A. Husain, K. K. Bhasin and G. Kumar, Metal–Organic Framework-based selective molecular recognition of organic amines and fixation of CO2 into cyclic carbonates, Inorg. Chem., 2022, 61, 6977–6994 CrossRef CAS PubMed.
- R. Das, T. Ezhil and C. M. Nagaraja, Design of Bifunctional Zinc(II)–Organic Framework for Efficient Coupling of CO2 with Terminal/Internal Epoxides under Mild Conditions, Cryst. Growth Des., 2022, 22, 598–607 CrossRef CAS.
- R. Das, S. S. Manna, B. Pathak and C. M. Nagaraja, Strategic Design of Mg-Centered Porphyrin Metal–Organic Framework for Efficient Visible Light-Promoted Fixation of CO2 under Ambient Conditions: Combined Experimental and Theoretical Investigation, ACS Appl. Mater. Interfaces, 2022, 14, 33285–33296 CrossRef CAS PubMed.
- Z. Qin, L. Chen, Y. Li and K. Shen, Bifunctional Catalysts with Core–Shell Distributed ZrO2 and Co Nanoparticles Derived from MOF-on-MOF Heterostructures for Economical One-Pot Tandem CO2 Fixation, ACS Catal., 2023, 13, 8372–8383 CrossRef CAS.
- S.-F. Cai, H.-R. Li and L.-N. He, Bifunctionalization of unsaturated bonds via carboxylative cyclization with CO2: a sustainable access to heterocyclic compounds, Green Chem., 2021, 23, 9334–9347 RSC.
- V. Parihar, G. Singh, N. Duhan, S. Kumar, T. J. D. Kumar and C. M. Nagaraja, Non-Noble Metal Anchored 2D Covalent Organic Framework for Ambient CO2 Fixation to High-Value Compounds, ChemSusChem, 2024, e202401497, DOI:10.1002/cssc.202401497.
- J. Hu, J. Ma, Q. Zhu, Z. Zhang, C. Wu and B. Han, Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2−Oxazolidinones, Angew. Chem., Int. Ed., 2015, 54, 5399–5403 CrossRef CAS PubMed.
- Y. Zhou, Z. Wang, L. Huang, S. Zaman, K. Lei, T. Yue, Z. Li, B. You and B. Y. Xia, Engineering 2D photocatalysts toward carbon dioxide reduction, Adv. Energy Mater., 2021, 11, 2003159 CrossRef CAS.
- Y. Zhao, G. I. N. Waterhouse, G. Chen, X. Xiong, L.-Z. Wu, C.-H. Tunga and T. Zhang, Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks, Chem. Soc. Rev., 2019, 48, 1972–2010 RSC.
- M. Ampomah-Wireko, S. Chen, R. Li, C. Gao, M. Wang, Y. Qu, H. Kong, L. Nininahazwe and E. Zhang, Recent advances in the exploration of oxazolidinone scaffolds from compound development to antibacterial agents and other bioactivities, Eur. J. Med. Chem., 2024, 269, 116326 CrossRef CAS PubMed.
- G. G. Zhanel, R. Love, H. Adam, A. Golden, S. Zelenitsky, F. Schweizer, B. Gorityala, P. R. S. Lagacé-Wiens, E. Rubinstein, A. Walkty, A. S. Gin, M. Gilmour, D. J. Hoban, J. P. Lynch, 3rd and J. A. Karlowsky, Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens, Drugs, 2015, 75, 253–270 CrossRef CAS PubMed.
- S. J. Brickner, M. R. Barbachyn, D. K. Hutchinson and P. R. Manninen, Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious gram-positive infections, J. Med. Chem., 2008, 51, 1981–1990 CrossRef CAS PubMed.
- S. Arshadi, A. Banaei, S. Ebrahimiasl, A. Monfared and E. Vessally, Solvent-free incorporation of CO2 into 2-oxazolidinones: a review, RSC Adv., 2019, 9, 19465–19482 RSC.
- R. R. Roberts and L. A. D. Williams, Acaricidal and insecticidal activities of three synthetic oxazolidinones, Pestic. Sci., 1991, 33, 393–398 CrossRef CAS.
- X. Yang, L. Xu, Y. Zhu, S. Zhang, G. Jia and J. Du, Efficient fabrication of oxazolidinones for the carboxylative cyclization with carbon dioxide, J. CO2 Util., 2023, 74, 102531 CrossRef CAS.
- A. Dutta, S. Farooq, I. A. Karimi and S. A. Khan, Assessing the potential of CO2 utilization with an integrated framework for producing power and chemicals, J. CO2 Util., 2013, 2, 49–57 CrossRef.
- W.-G. Cui, G.-Y. Zhang, T.-L. Hu and X.-H. Bu, Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4, Coord. Chem. Rev., 2019, 387, 79–120 CrossRef CAS.
- S.-L. Hou, J. Dong and B. Zhao, Formation of C–X Bonds in CO2 Chemical Fixation Catalyzed by Metal−Organic Frameworks, Adv. Mater., 2020, 32, 1806163 CrossRef CAS PubMed.
- Y. Chen and T. Mu, Conversion of CO2 to value-added products mediated by ionic liquids, Green Chem., 2019, 21, 2544–2574 RSC.
- B. Ugale, S. Kumar, T. J. D. Kumar and C. M. Nagaraja, Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks, Inorg. Chem., 2019, 58, 3925–3936 CrossRef CAS PubMed.
- Z. Zhang, J.-H. Ye, D.-S. Wu, Y.-Q. Zhou and D.-G. Yu, Synthesis of Oxazolidin–2−ones from Unsaturated Amines with CO2 by Using Homogeneous Catalysis, Chem. – Asian J., 2018, 13, 2292–2306 CrossRef CAS PubMed.
- S. K. Meher, P. Nayak, S. Dhala, S. Tripathy and K. Venkatasubbaiah, Magnesium-porphyrin as an efficient photocatalyst for the transformation of CO2 to cyclic carbonates and oxazolidinones under ambient conditions, Catal. Sci. Technol., 2024, 14, 3125–3130 RSC.
- J. Huang, T. Wu, C. Dai, Y. Xie and C. Zeng, Improved Charge Separation and CO2 Affinity of In2O3 by K Doping with Accompanying Oxygen Vacancies for Boosted CO2 Photoreduction, Langmuir, 2024, 40, 3883–3891 CAS.
- Y. Zhou, W. Zhang, L. Ma, Y. Zhou and J. Wang, Amino Acid Anion Paired Mesoporous Poly(ionic liquids) as Metal-/Halogen-Free Heterogeneous Catalysts for Carbon Dioxide Fixation, ACS Sustainable Chem. Eng., 2019, 7, 9387–9398 CrossRef CAS.
- Z. Zhang, H. Gao, H. Wu, Y. Qian, L. Chen and J. Chen, Chemical Fixation of CO2 by Using Carbon Material-Grafted N-Heterocyclic Carbene Silver and Copper Complexes, ACS Appl. Nano Mater., 2018, 1, 6463–6476 CrossRef CAS.
- J. Liang, Q. Wu, Y.-B. Huang and R. Cao, Reticular frameworks and their derived materials for CO2 conversion by thermo–catalysis, EnergyChem, 2021, 3, 100064 CrossRef CAS.
- J. Yu, L.-H. Xie, J.-R. Li, Y. Ma, J. M. Seminario and P. B. Balbuena, CO2 Capture and Separations Using MOFs: Computational and Experimental Studies, Chem. Rev., 2017, 117, 9674–9754 CrossRef CAS PubMed.
- Y. Zeng, R. Zou and Y. Zhao, Covalent Organic Frameworks for CO2 Capture, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Carbon capture and conversion using metal–organic frameworks and MOF-based materials, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC.
- Y. Li, Y. Wang, W. Fan and D. Sun, Flexible metal–organic frameworks for gas storage and separation, Dalton Trans., 2022, 51, 4608–4618 RSC.
- F. Zhang, Y. Zhang, L. Ma, J. Yang and J. Li, Tailoring Lewis acid properties of Metal–Organic framework nodes via anion post-replacement for gas adsorption and separation, ACS Sustainable Chem. Eng., 2022, 10, 9359–9368 CrossRef CAS.
- P. Rani, A. Sharma, A. Husain, G. Kumar, H. Kaur, K. K. Bhasin and G. Kumar, Selective recognition of Fe3+ and CrO42− ions using a Zn(II) metallacycle and a Cd(II) coordination polymer and their heterogeneous catalytic application, CrystEngComm, 2019, 21, 7447–7459 RSC.
- P. Rani, A. Husain, K. K. Bhasin and G. Kumar, Coordination Polymers as a Functional Material for the Selective Molecular Recognition of Nitroaromatics and ipso-Hydroxylation of Arylboronic Acids, Chem. – Asian J., 2022, 17, e202101204 CrossRef CAS PubMed.
- P. Rani, Heena, N. Pundir, Gauri, A. Husain, K. K. Bhasin and G. Kumar, A Doubly Interpenetrated Cu(II)-based Metal-Organic Framework as Heterogeneous Catalyst for the ipso-Hydroxylation of Arylboronic Acids, Eur. J. Inorg. Chem., 2022, 26, e202200654 CrossRef.
- A. Husain, P. Rani, K. K. Nar, A. P. Singh, R. Kumar, K. K. Bhasin and G. Kumar, Tryptophan based copper(II) coordination polymer: Catalytic activity towards Suzuki-Miyaura cross-coupling reactions, CrystEngComm, 2021, 23, 7855–7864 RSC.
- P. Rani, A. Husain, K. K. Bhasin and G. Kumar, Zinc(II)-MOF: A Versatile Luminescent Sensor for Selective Molecular Recognition of Flame Retardants and Antibiotics, Inorg. Chem., 2024, 63, 3486–3498 CrossRef CAS PubMed.
- P. Rani, Gauri, A. Husain, K. K. Bhasin and G. Kumar, A Doubly Interpenetrated CuII Metal–Organic Framework for Selective Molecular Recognition of Nitroaromatics, Cryst. Growth Des., 2020, 20, 7141–7151 CrossRef CAS.
- P. Rani, N. Pundir, Heena, A. Husain, A. K. K. Bhasin, K. K. Bhasin and G. Kumar, Bifunctional metal-organic frameworks as selective turn-on fluorescence sensors for tryptophan and heterogeneous catalysts for Knoevenagel condensation reaction, Mater. Today Chem., 2023, 30, 101600 CrossRef CAS.
- H. D. Lawson, S. P. Walton and C. Chan, Metal–organic frameworks for drug delivery: a design perspective, ACS Appl. Mater. Interfaces, 2021, 13, 7004–7020 CrossRef CAS PubMed.
- O. I.-F. Chen, C.-H. Liu, K. Wang, E. Borrego-Marin, H. Li, A. H. Alawadhi, J. A. R. Navarro and O. M. Yaghi, Water-Enhanced Direct Air Capture of Carbon Dioxide in Metal–Organic Frameworks, J. Am. Chem. Soc., 2024, 146, 2835–2844 CrossRef CAS PubMed.
- R. E. Mow, G. A. Russell-Parks, G. E. B. Redwine, B. E. Petel, T. Gennett and W. A. Braunecker, Polymer-Coated Covalent Organic Frameworks as Porous Liquids for Gas Storage, Chem. Mater., 2024, 36, 1579–1590 CrossRef CAS PubMed.
- J. E. Lopez-Cazares, K. Kim, J. Y. S. Lin and K. Jin, Gas Permeation and Separation Characteristics of Microporous TpHz COF Membranes Synthesized by Substrate-Assisted Interfacial Polymerization, Ind. Eng. Chem. Res., 2024, 63, 3684–3694 CrossRef CAS.
- Q. Guo, Z. Lai, X. Zuo, W. Xian, S. Wu, L. Zheng, Z. Dai, S. Wang and Q. Sun, Photoelectric responsive ionic channel for sustainable energy harvesting, Nat. Commun., 2023, 14, 6702 CrossRef CAS PubMed.
- H. Li, A. Dilipkumar, S. Abubakar and D. Zhao, Covalent organic frameworks for CO2 capture: from laboratory curiosity to industry implementation, Chem. Soc. Rev., 2023, 52, 6294–6329 RSC.
- H. Lyu, H. Li, N. Hanikel, K. Wang and O. M. Yaghi, Covalent organic frameworks for carbon dioxide capture from air, J. Am. Chem. Soc., 2022, 144, 12989–12995 CrossRef CAS PubMed.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS.
- J. Gandara-Loe, L. Pastor-Perez, L. F. Bobadilla, J. A. Odriozola and T. R. Reina, Understanding the opportunities of metal–organic frameworks (MOFs) for CO2 capture and gas-phase CO2 conversion processes: a comprehensive overview, React. Chem. Eng., 2021, 6, 787–814 RSC.
- F. N. Al-Rowaili, U. Zahid, S. Onaizi, M. Khaled, A. Jamal and E. M. Al-Mutairi, A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products, J. CO2 Util., 2021, 53, 101715 CrossRef CAS.
- R. Luo, Y. Yang, K. Chen, X. Liu, M. Chen, W. Xu, B. Liu, H. Ji and Y. Fang, Tailored covalent organic frameworks for simultaneously capturing and converting CO2 into cyclic carbonates, J. Mater. Chem. A, 2021, 9, 20941–20956 RSC.
- F. Guo and X. Zhang, Metal–organic frameworks for the energy-related conversion of CO2 into cyclic carbonates, Dalton Trans., 2020, 49, 9935–9947 RSC.
- Q. Wang, P. Chen, X. Li, Y. Liang and Y. Pan, Chemical fixation of carbon dioxide into cyclic carbonates catalyzed by porous materials, Asian J. Org. Chem., 2023, 12, e202300308 CrossRef CAS.
- R. Luo, M. Chen, X. Liu, W. Xu, J. Li, B. Liu and Y. Fang, Recent advances in CO2 capture and simultaneous conversion into cyclic carbonates over porous organic polymers having accessible metal sites, J. Mater. Chem. A, 2020, 8, 18408–18424 RSC.
- R. Li, W. Zhang and K. Zhou, Metal–Organic–Framework–Based Catalysts for Photoreduction of CO2, Adv. Mater., 2018, 30, 1705512 CrossRef PubMed.
- Q.-W. Song, Z.-H. Zhou and L.-N. He, Efficient, selective and sustainable catalysis of carbon dioxide, Green Chem., 2017, 19, 3707–3728 RSC.
- G. Singh, J. Lee, A. Karakoti, R. Bahadur, J. Yi, D. Zhao, K. AlBahily and A. Vinu, Emerging trends in porous materials for CO2 capture and conversion, Chem. Soc. Rev., 2020, 49, 4360–4404 RSC.
- Q.-J. Wu, J. Liang, Y.-B. Huang and R. Cao, Thermo-, Electro-, and Photocatalytic CO2 Conversion to Value-Added Products over Porous Metal/Covalent Organic Frameworks, Acc. Chem. Res., 2022, 55, 2978–2997 CrossRef CAS PubMed.
- O. K. Farha, A. Özgür Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities, Nat. Chem., 2010, 2, 944–948 CrossRef CAS PubMed.
- D. Yuan, D. Zhao, D. Sun and H.-C. Zhou, An isoreticular series of metal–organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas uptake capacity, Angew. Chem., Int. Ed., 2010, 49, 5357–5361 CrossRef CAS PubMed.
- H. Li, C. E. Davis, T. L. Groy, D. G. Kelley and O. M. Yaghi, Coordinatively unsaturated metal centers in the extended porous framework of Zn3(BDC)3·6CH3OH (BDC = 1,4-benzenedicarboxylate), J. Am. Chem. Soc., 1998, 120, 2186–2187 CrossRef CAS.
- K. Sumida, S. Horike, S. S. Kaye, Z. R. Herm, W. L. Queen, C. M. Brown, F. Grandjean, G. J. Long, A. Dailly and J. R. Long, Hydrogen storage and carbon dioxide capture in an iron-based sodalite-type metal–organic framework (Fe-BTT) discovered via high-throughput methods, Chem. Sci., 2010, 1, 184–191 RSC.
- J.-R. Li, J. Yu, W. Lu, L.-B. Sun, J. Sculley, P. B. Balbuena and H.-C. Zhou, Porous materials with pre-designed single-molecule traps for CO2 selective adsorption, Nat. Commun., 2013, 4, 1538 CrossRef PubMed.
- H. Xu, X.-F. Liu, C.-S. Cao, B. Zhao, P. Cheng and L.-N. He, A Porous Metal–Organic Framework Assembled by [Cu30] Nanocages: Serving as Recyclable Catalysts for CO2 Fixation with Aziridines, Adv. Sci., 2016, 3, 1600048 CrossRef PubMed.
- F. Yang, J. Wang, Y. Wang, B. Yu, Y. Cao, J. Li, L. Wu, J. Huang and Y.-N. Liu, Perfluoroalkyl-Decorated Noble-Metal-Free MOFs for the Highly Efficient One-Pot Four-Component Coupling between Aldehydes, Amines, Alkynes, and Flue Gas CO2, Angew. Chem., Int. Ed., 2024, 63, e202318115 CrossRef CAS PubMed.
- A. R. Millward and O. M. Yaghi, Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed.
- B. Li, Z. Zhang, Y. Li, K. Yao, Y. Zhu, Z. Deng, F. Yang, X. Zhou, G. Li, H. Wu, N. Nijem, Y. J. Chabal, Z. Lai, Y. Han, Z. Shi, S. Feng and J. Li, Enhanced Binding Affinity, Remarkable Selectivity, and High Capacity of CO2 by Dual Functionalization of a rht-Type Metal–Organic Framework, Angew. Chem., Int. Ed., 2012, 51, 1412–1415 CrossRef CAS PubMed.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Direct Observation and Quantification of CO2 Binding Within an Amine-Functionalized Nanoporous Solid, Science, 2010, 330, 650–653 CrossRef CAS PubMed.
- D. Zhao, X.-H. Liu, C. Zhu, Y.-S. Kang, P. Wang, Z. Shi, Y. Lu and W.-Y. Sun, Efficient and reusable metal–organic framework catalysts for carboxylative cyclization of propargyl amines with carbon dioxide, ChemCatChem, 2017, 9, 4598–4606 CrossRef CAS.
- H. Yang, X. Zhang, G. Zhang and H. Fei, An alkaline-resistant Ag(I)-anchored pyrazolate-based metal–organic framework for chemical fixation of CO2, Chem. Commun., 2018, 54, 4469–4472 RSC.
- J. J. Gassensmith, H. Furukawa, R. A. Smaldone, R. S. Forgan, Y. Y. Botros, O. M. Yaghi and J. F. Stoddart, Strong and Reversible Binding of Carbon Dioxide in a Green Metal–Organic Framework, J. Am. Chem. Soc., 2011, 133, 15312–15315 CrossRef CAS PubMed.
- F. Debatin, A. Thomas, A. Kelling, N. Hedin, Z. Bacsik, I. Senkovska, S. Kaskel, M. Junginger, H. Müller, U. Schilde, C. Jäger, A. Friedrich and H.-J. Holdt, In Situ Synthesis of an Imidazolate-4-amide-5-imidate Ligand and Formation of a Microporous Zinc–Organic Framework with H2-and CO2-Storage Ability, Angew. Chem., Int. Ed., 2010, 49, 1258–1262 CrossRef CAS PubMed.
- A.-G. Liu, Y. Chen, P.-D. Liu, W. Qi and B. Li, Conversion of CO2 to epoxides or oxazolidinones enabled by a CuI/CuII-organic framework bearing a tri-functional linker, Inorg. Chem. Front., 2022, 9, 4425–4432 RSC.
- K. Geng, T. He, R. Liu, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- S.-Y. Ding and W. Wang, Covalent organic frameworks (COFs): from design to applications, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- M. S. Lohse and T. Bein, Covalent Organic Frameworks: Structures, Synthesis, and Applications, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef.
- B. Gui, G. Lin, H. Ding, C. Gao, A. Mal and C. Wang, Three-Dimensional Covalent Organic Frameworks: From Topology Design to Applications, Acc. Chem. Res., 2020, 53, 2225–2234 CrossRef CAS PubMed.
- X. Zhao, P. Pachfule and A. Thomas, Covalent organic frameworks (COFs) for electrochemical applications, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- Y.-N. Gong, X. Guan and H.-L. Jiang, Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance, Coord. Chem. Rev., 2023, 475, 214889 CrossRef CAS.
- J. Wang and S. Zhuang, Covalent organic frameworks (COFs) for environmental applications, Coord. Chem. Rev., 2019, 400, 213046 CrossRef CAS.
- X. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, H. Wang, H. Yi, B. Li, S. Liu, M. Zhang, R. Deng, Y. Fu, L. Li, W. Xue and S. Chen, Recent advances in covalent organic frameworks (COFs) as a smart sensing material, Chem. Soc. Rev., 2019, 48, 5266–5302 RSC.
- Q. Yang, M. Luo, K. Liu, H. Cao and H. Yan, Covalent organic frameworks for photocatalytic applications, Appl. Catal., B, 2020, 276, 11917 Search PubMed.
- T. Zhou, Y. Ma, H. Feng, Y. Lu, G. Che, C. Liu and Y. Lan, COFs-Based Metal-Free Heterojunctions for Solar-to-Chemical Energy Conversion, Adv. Funct. Mater., 2024, 2409396 CrossRef CAS.
- Z. Zhou, T. Ma, H. Zhang, S. Chheda, H. Li, K. Wang, S. Ehrling, R. Giovine, C. Li, A. H. Alawadhi, M. M. Abduljawad, M. O. Alawad, L. Gagliardi, J. Sauer and O. M. Yaghi, Carbon dioxide capture from open air using covalent organic frameworks, Nature, 2024, 635, 96–101 CrossRef CAS PubMed.
- Z. Chen, K. Wang, X. Hu, P. Shi, Z. Guo and H. Zhan, Novel One-Dimensional Covalent Organic Framework as a H+ Fluorescent Sensor in Acidic Aqueous Solution, ACS Appl. Mater. Interfaces, 2021, 13, 1145–1151 CrossRef CAS PubMed.
- T. Zhang, G. Zhang and L. Chen, 2D Conjugated Covalent Organic Frameworks: Defined Synthesis and Tailor-Made Functions, Acc. Chem. Res., 2022, 55, 795–808 CrossRef CAS PubMed.
- W. Huang, W. Zhang, S. Yang, L. Wang and G. Yu, 3D Covalent Organic Frameworks from Design, Synthesis to Applications in Optoelectronics, Small, 2024, 20, 2308019 CrossRef CAS PubMed.
- R. A. Maia, F. L. Oliveira, V. Ritleng, Q. Wang, B. Louis and P. M. Esteves, CO2 Capture by Hydroxylated Azine-Based Covalent Organic Frameworks, Chem. – Eur. J., 2021, 27, 8048–8055 CrossRef CAS PubMed.
- Y. Wang, C. Kang, Z. Zhang, A. K. Usadi, D. C. Calabro, L. S. Baugh, Y. D. Yuan and D. Zhao, Evaluation of Schiff-Base Covalent Organic Frameworks for CO2 Capture: Structure–Performance Relationships, Stability, and Performance under Wet Conditions, ACS Sustainable Chem. Eng., 2022, 10, 332–341 CrossRef CAS.
- Y. Xie, C. Lu, B. Zhao, Q. Wan and Y. Yao, Cycloaddition of Aziridine with CO2/CS2 Catalyzed by Amidato Divalent Lanthanide Complexes, J. Org. Chem., 2019, 84, 1951–1958 CrossRef CAS PubMed.
- M. B. Reddy and E. M. McGarrigle, Visible-light-induced bifunctionalisation of (homo) propargylic amines with CO2 and arylsulfinates, Chem. Commun., 2023, 59, 13711–13714 RSC.
- R. Pich, S. Mallet-Ladeira, P. Lavedan, J. F. Lahitte, J.-C. Remigy, D. Pla and M. Gómez, A Mechanistic Insight on CuI–Catalyzed Synthesis of Oxazolidinones through a Four–Component Reaction, Adv. Synth. Catal., 2024, 366, 822 CrossRef CAS.
- G. S. Singh, Synthetic aziridines in medicinal chemistry: a mini-review, Mini-Rev. Med. Chem., 2016, 16, 892–904 CrossRef CAS PubMed.
- R. Ma, L.-N. He and Y.-B. Zhou, An efficient and recyclable tetraoxo-coordinated zinc catalyst for the cycloaddition of epoxides with carbon dioxide at atmospheric pressure, Green Chem., 2016, 18, 226–231 RSC.
- X. Liu, S. Zhang, Q.-W. Song, X.-F. Liu, R. Ma and L.-N. He, Cooperative calcium-based catalysis with 1, 8-diazabicyclo [5.4. 0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure, Green Chem., 2016, 18, 2871–2876 RSC.
- L.-Q. Qiu, H.-R. Li and L.-N. He, Incorporating Catalytic Units into Nanomaterials: Rational Design of Multipurpose Catalysts for CO2 Valorization, Acc. Chem. Res., 2023, 56, 2225–2240 CrossRef CAS PubMed.
- T. K. Pal, D. De and P. K. Bharadwaj, Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS.
- H. Lin, Y. Yang, Y.-C. Hsu, J. Zhang, C. Welton, I. Afolabi, M. Loo and H.-C. Zhou, Metal–organic frameworks for water harvesting and concurrent carbon capture: a review for hygroscopic materials, Adv. Mater., 2024, 36, 2209073 CrossRef CAS PubMed.
- Z. Ji, H. Wang, S. Canossa, S. Wuttke and O. M. Yaghi, Pore chemistry of metal–organic frameworks, Adv. Funct. Mater., 2020, 30, 2000238 CrossRef CAS.
- Y. Lin, C. Kong, Q. Zhan and L. Chen, Metal–organic frameworks for carbon dioxide capture and methane storage, Adv. Energy Mater., 2017, 7, 1601296 CrossRef.
- M. H. Beyzavi, R. C. Klet, S. Tussupbayev, J. Borycz, N. A. Vermeulen, C. J. Cramer, J. F. Stoddart, J. T. Hupp and O. K. Farha, A Hafnium-Based Metal–Organic Framework as an Efficient and Multifunctional Catalyst for Facile CO2 Fixation and Regioselective and Enantioretentive Epoxide Activation, J. Am. Chem. Soc., 2014, 136, 15861–15864 CrossRef PubMed.
- A. C. Kathalikkattil, R. Roshan, J. Tharun, R. Babu, G.-S. Jeong, D.-W. Kim, S. J. Cho and D.-W. Park, A sustainable protocol for the facile synthesis of zinc-glutamate MOF: an efficient catalyst for room temperature CO2 fixation reactions under wet conditions, Chem. Commun., 2016, 52, 280–283 RSC.
- M. H. Beyzavi, C. J. Stephenson, Y. Liu, O. Karagiaridi, J. T. Hupp and O. K. Farha, Metal–organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates, Front. Energy Res., 2015, 2, 63 Search PubMed.
- D. Feng, Z. Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Zirconium–metalloporphyrin PCN–222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts, Angew. Chem., Int. Ed., 2012, 51, 10307 CrossRef CAS PubMed.
- X. Wang, W.-Y. Gao, Z. Niu, L. Wojtas, J. A. Perman, Y.-S. Chen, Z. Li, B. Aguila and S. Ma, A metal–metalloporphyrin framework based on an octatopic porphyrin ligand for chemical fixation of CO2 with aziridines, Chem. Commun., 2018, 54, 1170–1173 RSC.
- W. Y. Gao, C.-Y. Tsai, L. Wojtas, T. Thiounn, C.-C. Lin and S. Ma, Interpenetrating Metal–Metalloporphyrin Framework for Selective CO2 Uptake and Chemical Transformation of CO2, Inorg. Chem., 2016, 55, 7291 CrossRef CAS PubMed.
- C.-S. Cao, Y. Shi, H. Xu and B. Zhao, A multifunctional MOF as a recyclable catalyst for the fixation of CO2 with aziridines or epoxides and as a luminescent probe of Cr(VI), Dalton Trans., 2018, 47, 4545–4553 RSC.
- S. Carrasco, A. Sanz-Marco and B. Martín-Matute, Fast and Robust Synthesis of Metalated PCN-222 and Their Catalytic Performance in Cycloaddition Reactions with CO2, Organometallics, 2019, 38, 3429–3435 CrossRef CAS.
- X.-M. Kang, Y. Shi, C.-S. Cao and B. Zhao, Stable metal-organic frameworks with high catalytic performance in the cycloaddition of CO2 with aziridines, Sci. China: Chem., 2019, 62, 622–628 CrossRef CAS.
- X.-M. Kang, L.-H. Yao, Z.-H. Jiao and B. Zhao, Two Stable Heterometal–MOFs as Highly Efficient and Recyclable Catalysts in the CO2 Coupling Reaction with Aziridines, Chem. – Asian J., 2019, 14, 3668 CrossRef CAS PubMed.
- X.-R. Tian, Y. Shi, S.-L. Hou, Y. Ma and B. Zhao, Efficient Cycloaddition of CO2 and Aziridines Activated by a Quadruple-Interpenetrated Indium–Organic Framework as a Recyclable Catalyst, Inorg. Chem., 2021, 60, 15383–15389 CrossRef CAS PubMed.
- Y. Shi, J. Zhao, H. Xu, S.-L. Hou and B. Zhao, Eco-friendly co-catalyst-free cycloaddition of CO2 and aziridines activated by a porous MOF catalyst, Sci. China: Chem., 2021, 64, 1316–1322 CrossRef CAS.
- T. Zeng, X. Zhang, S. Wang, H. Niu and Y. Cai, Spatial Confinement of a Co3O4 Catalyst in Hollow Metal–Organic Frameworks as a Nanoreactor for Improved Degradation of Organic Pollutants, Environ. Sci. Technol., 2015, 49, 2350 CrossRef CAS PubMed.
- C.-S. Cao, Y. Shi, H. Xu and B. Zhao, An uncommon multicentered ZnI–ZnI bond-based MOF for CO2 fixation with aziridines/epoxides, Chem. Commun., 2021, 57, 7537–7540 RSC.
- X. Kang, Z. Jiao, X. Shi, Y. Tian and Z. Liu, Difunctional Zn-based metal–organic frameworks: chemical conversion of CO2 and luminescence recognition for secnidazole, J. Mater. Chem. C, 2022, 10, 16078–16087 RSC.
- C.-H. Zhang, Z.-L. Wu, R.-X. Bai, T.-D. Hu and B. Zhao, Highly Efficient Conversion of Aziridines and CO2 Catalyzed by Microporous [Cu12] Nanocages, ACS Appl. Mater. Interfaces, 2023, 15, 1879–1890 CrossRef CAS PubMed.
- T.-J. Liu, H. Xu, Y. Shi and B. Zhao, A highly stable cerium–organic framework: an efficient catalyst for the cycloaddition of CO2 and aziridines under mild-pressure, CrystEngComm, 2024, 26, 3687–3693 RSC.
- W.-M. Wang, N. Qiao, J.-L. Wang, Y. Chen, J.-C. Yan, C.-Y. Xu and C.-S. Cao, A [Cu2I2] cluster-based lanthanide metal–organic framework (MOF) catalyst for the highly efficient conversion of CO2 with propargylic alcohols and aziridines, Catal. Today, 2024, 425, 114359 CrossRef CAS.
- G. Lin, H. Ding, R. Chen, Z. Peng, B. Wang and C. Wang, 3D porphyrin-based covalent organic frameworks, J. Am. Chem. Soc., 2017, 139, 8705–8709 CrossRef CAS PubMed.
- Z. Liang, H.-Y. Wang, H. Zheng, W. Zhang and R. Cao, Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide, Chem. Soc. Rev., 2021, 50, 2540–2581 RSC.
- V. Saptal, D. B. Shinde, R. Banerjee and B. M. Bhanage, State-of-the-art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones, Catal. Sci. Technol., 2016, 6, 6152–6158 RSC.
- L. Degennaro, P. Trinchera and R. Luisi, Recent advances in the stereoselective synthesis of aziridines, Chem. Rev., 2014, 114, 7881–7929 CrossRef CAS PubMed.
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Synthesis and reactivity of propargylamines in organic chemistry, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS PubMed.
- E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, New page to access pyrazines and their ring fused analogues: Synthesis from N-propargylamines, Curr. Org. Synth., 2017, 14, 557–567 CrossRef CAS.
- Y. Zhao, J. Qiu, L. Tian, Z. Li, M. Fan and J. Wang, New Copper(I)/DBU Catalyst System for the Carboxylative Cyclization of Propargylic Amines with Atmospheric CO2: An Experimental and Theoretical Study, ACS Sustainable Chem. Eng., 2016, 4, 5553–5560 CrossRef CAS.
- P.-Z. Li, X.-J. Wang, J. Liu, J. S. Lim, R. Zou and Y. Zhao, A Triazole-Containing Metal–Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion, J. Am. Chem. Soc., 2016, 138, 2142–2145 CrossRef CAS PubMed.
- Y.-S. Wei, M. Zhang, R. Zou and Q. Xu, Metal–organic framework-based catalysts with single metal sites, Chem. Rev., 2020, 120, 12089–12174 CrossRef CAS PubMed.
- K.-Y. Wang, J. Zhang, Y.-C. Hsu, H. Lin, Z. Han, J. Pang, Z. Yang, R.-R. Liang, W. Shi and H.-C. Zhou, Bioinspired Framework Catalysts: From Enzyme Immobilization to Biomimetic Catalysis, Chem. Rev., 2023, 123, 5347–5420 CrossRef CAS PubMed.
- L. Sarkisov, R. L. Martin, M. Haranczyk and B. Smit, On the flexibility of metal–organic frameworks, J. Am. Chem. Soc., 2014, 136, 2228–2231 CrossRef CAS PubMed.
- S. Bureekaew, H. Sato, R. Matsuda, Y. Kubota, R. Hirose, J. Kim, K. Kato, M. Takata and S. Kitagawa, Control of interpenetration for tuning structural flexibility influences sorption properties, Angew. Chem., 2010, 122, 7826–7830 CrossRef.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. O. Yazaydın and J. T. Hupp, Metal–organic framework materials with ultrahigh surface areas: is the sky the limit?, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Flexible metal–organic frameworks, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- Z. Chang, X. Jing, C. He, X. Liu and C. Duan, Silver Clusters as Robust Nodes and π–Activation Sites for the Construction of Heterogeneous Catalysts for the Cycloaddition of Propargylamines, ACS Catal., 2018, 8, 1384–1391 CrossRef CAS.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocellà, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Cooperative insertion of CO2 in diamine-appended metal-organic frameworks, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, High thermal and chemical stability in pyrazolate-bridged metal–organic frameworks with exposed metal sites, Chem. Sci., 2011, 2, 1311–1319 RSC.
- V. Colombo, C. Montoro, A. Maspero, G. Palmisano, N. Masciocchi, S. Galli, E. Barea and J. A. R. Navarro, Tuning the adsorption properties of isoreticular pyrazolate-based metal–organic frameworks through ligand modification, J. Am. Chem. Soc., 2012, 134, 12830–12843 CrossRef CAS PubMed.
- K. Wang, X.-L. Lv, D. Feng, J. Li, S. Chen, J. Sun, L. Song, Y. Xie, J.-R. Li and H.-C. Zhou, Pyrazolate-based porphyrinic metal–organic framework with extraordinary base-resistance, J. Am. Chem. Soc., 2016, 138, 914–919 CrossRef CAS PubMed.
- X. Wang, Z. Chang, X. Jing, C. He and C. Duan, Double-Helical Ag−S Rod-Based Porous Coordination Polymers with Double Activation: σ-Active and π-Active Functions, ACS Omega, 2019, 4, 10828–10833 CrossRef CAS PubMed.
- M. Zhao, S. Huang, Q. Fu, W. Li, R. Guo, Q. Yao, F. Wang, P. Cui, C.-H. Tung and D. Sun, Ambient Chemical Fixation of CO2 Using a Robust Ag27 Cluster–Based Two–Dimensional Metal–Organic Framework, Angew. Chem., Int. Ed., 2020, 59, 20031–20036 CrossRef CAS PubMed.
- S. Lia, X. Tan, W. Sun, L. Zhang, H. Jia, L. Li, C. Zhao and X. Zhang, Design catalytic space engineering of Ag-Ag bond-based metal organic framework for carbon dioxide fixation reactions, Colloids Surf., A, 2021, 609, 125529 CrossRef.
- C.-S. Cao, S.-M. Xia, Z.-J. Song, H. Xu, Y. Shi, L.-N. He, P. Cheng and B. Zhao, Highly Efficient Conversion of Propargylic Amines and CO2 Catalyzed by Noble–Metal–Free [Zn116] Nanocages, Angew. Chem., Int. Ed., 2020, 59, 8586 CrossRef CAS PubMed.
- X.-L. Jiang, Y.-E. Jiao, S.-L. Hou, L.-C. Geng, H.-Z. Wang and B. Zhao, Green Conversion of CO2 and Propargylamines Triggered by Triply Synergistic Catalytic Effects in Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2021, 60, 20417 CrossRef CAS PubMed.
- A.-L. Gu, W.-T. Wang, X.-Y. Cheng, T.-D. Hu and Z.-L. Wu, Non-noble-metal metal–organic-framework-catalyzed carboxylative cyclization of propargylic amines with atmospheric carbon dioxide under ambient conditions, Inorg. Chem., 2021, 60, 13425–13433 CrossRef CAS PubMed.
- X.-R. Tian, X.-L. Jiang, S.-L. Hou, Z.-H. Jiao, J. Han and B. Zhao, Selectively Regulating Lewis Acid–Base Sites in Metal–Organic Frameworks for Achieving Turn–On/Off of the Catalytic Activity in Different CO2 Reactions, Angew. Chem., Int. Ed., 2022, 61, e202200123 CrossRef CAS PubMed.
- X. Xu, Z. Li, H. Huang, X. Jing and C. Duan, A novel copper metal–organic framework catalyst for the highly efficient conversion of CO2 with propargylic amines, Inorg. Chem. Front., 2022, 9, 3839–3844 RSC.
- X. Zhao, B.-B. Qin, T. He, H.-P. Wang and J. Liu, Stable Pyrene-Based Metal–Organic Framework for Cyclization of Propargylic Amines with CO2 and Detection of Antibiotics in Water, Inorg. Chem., 2023, 62, 18553–18562 CrossRef CAS PubMed.
- Z. Zhang, K. Shen, Q. Zhang, C. Duan and X. Jing, A novel porphyrin MOF catalyst for efficient conversion of CO2 with propargyl amines, Dalton Trans., 2024, 53, 10060–10064 RSC.
- Z.-L. Wu, Y.-T. Zhai, G.-G. Bian, L.-J. Guo, Y.-X. Zhang and H.-Y. Wei, Conversion of Propargylic Amines with CO2 Catalyzed by a Highly Stable Copper(I) Iodide Thorium-Based Heterometal−Organic Framework, Inorg. Chem., 2024, 63, 13450–13458 CrossRef CAS PubMed.
- X. Chen, J.-Y. Song, J. Zheng, Y.-M. Wang, J. Luo, P. Weng, B.-C. Cai, X.-C. Lin, G.-H. Ning and D. Li, Metal Variance in Multivariate Metal−Organic Frameworks for Boosting Catalytic Conversion of CO2, J. Am. Chem. Soc., 2024, 146, 19271–19278 CrossRef CAS PubMed.
- A. Corma, H. Garcia and F. X. Llabrés i Xamena, Engineering metal organic frameworks for heterogeneous catalysis, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- J. D. Evans, C. J. Sumby and C. J. Doonan, Post-synthetic metalation of metal–organic frameworks, Chem. Soc. Rev., 2014, 43, 5933–5951 RSC.
- A.-L. Gu, Y.-X. Zhang, Z.-L. Wu, H.-Y. Cui, T.-D. Hu and B. Zhao, Highly Efficient Conversion of Propargylic Alcohols and Propargylic Amines with CO2 Activated by Noble–Metal–Free Catalyst Cu2O@ZIF–8, Angew. Chem., Int. Ed., 2022, 61, e202114817 CrossRef CAS PubMed.
- C.-H. Zhang, T.-D. Hu, Y.-T. Zhai, Y.-X. Zhang and Z.-L. Wu, Stepwise engineering of the pore environment within metal–organic frameworks for green conversion of CO2 and propargylic amines, Green Chem., 2023, 25, 1938–1947 RSC.
- H.-Y. Cui, Y.-X. Zhang, C.-S. Cao, T.-D. Hu and Z.-L. Wu, Engineering noble-metal-free metal–organic framework composite catalyst for efficient CO2 conversion under ambient conditions, Chem. Eng. J., 2023, 451, 138764 CrossRef CAS.
- W. Wang, T. Wang, S. Chen, Y. Lv, L. Salmon, B. Espuche, S. Moya, O. Morozova, Y. Yun, D. D. Silvio, N. Daro, M. Berlande, P. Hapiot, J.-L. Pozzo, H. Yu, J.-R. Hamon and D. Astruc, Cu(I)-Glutathione Assembly Supported on ZIF-8 as Robust and Efficient Catalyst for Mild CO2 Conversions, Angew. Chem., Int. Ed., 2024, e202407430 CAS.
- Z. Wang and S. M. Cohen, Postsynthetic modification of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- S. Lin, C. S. Diercks, Y.-B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed.
- D. D. Zhu, J. L. Liu and S. Z. Qiao, Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide, Adv. Mater., 2016, 28, 3423–3452 CrossRef CAS PubMed.
- Z. Alsudairy, N. Brown, A. Campbell, A. Ambus, B. Brown, K. Smith-Petty and X. Li, Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective, Mater. Chem. Front., 2023, 7, 3298–3331 RSC.
- D.-H. Yang, Y. Tao, X. Ding and B.-H. Han, Porous organic polymers for electrocatalysis, Chem. Soc. Rev., 2022, 51, 761–791 RSC.
- T.-X. Wang, H.-P. Liang, D. A. Anito, X. Ding and B.-H. Han, Emerging applications of porous organic polymers in visible-light photocatalysis, J. Mater. Chem. A, 2020, 8, 7003–7034 RSC.
- J. Wu, F. Xu, S. Li, P. Ma, X. Zhang, Q. Liu, R. Fu and D. Wu, Porous polymers as multifunctional material platforms toward task–specific applications, Adv. Mater., 2019, 31, 1802922 CrossRef PubMed.
- S. Ghosh, T. S. Khan, A. Ghosh, A. H. Chowdhury, M. A. Haider, A. Khan and Sk. M. Islam, Utility of Silver Nanoparticles Embedded Covalent Organic Frameworks as Recyclable Catalysts for the Sustainable Synthesis of Cyclic Carbamates and 2-Oxazolidinones via Atmospheric Cyclizative CO2 Capture, ACS Sustainable Chem. Eng., 2020, 8, 5495–5513 CrossRef CAS.
- R. Khatun, S. Biswas, I. H. Biswas, Sk. Riyajuddin, N. Haque, K. Ghosh and Sk. M. Islam, Cu-NPs@ COF: A potential heterogeneous catalyst for CO2 fixation to produce 2-oxazolidinones as well as benzimidazoles under moderate reaction conditions, J. CO2 Util., 2020, 40, 101180 CrossRef CAS.
- P. Sarkar, A. H. Chowdhury, S. Biswas, A. Khan and Sk. M. Islam, 2D covalent organic framework: a photoactive heterogeneous catalyst for chemical fixation of CO2 over propargyl amines in water under sunlight, Mater. Today Chem., 2021, 21, 100509 CrossRef CAS.
- Y. Zhang, X. Lan, F. Yan, X. He, J. Wang, L. Ricardez-Sandoval, L. Chen and G. Bai, Controllable encapsulation of silver nanoparticles by porous pyridine-based covalent organic frameworks for efficient CO2 conversion using propargylic amines, Green Chem., 2022, 24, 930–940 RSC.
- G. Singh, N. Duhan, T. J. D. Kumar and C. M. Nagaraja, Pyrene-Based Nanoporous Covalent Organic Framework for Carboxylation of C–H Bonds with CO2 and Value-Added 2-Oxazolidinones Synthesis under Ambient Conditions, ACS Appl. Mater. Interfaces, 2024, 16, 5857–5868 CrossRef CAS PubMed.
- Y. Zhang, H. Li, X. He, A. Wang, G. Bai and X. Lan, Covalent organic frameworks embedding single cadmium sites for efficient carboxylative cyclization of CO2 with propargylic amines, Green Chem., 2023, 25, 5557–5565 RSC.
- J. Qiu, X. Qi, K. Zhu, Y. Zhao, H. Wang, Z. Li, H. Wang, Y. Zhao and J. Wang, CuI-anchored porous covalent organic frameworks for highly efficient conversion of propargylic amines with CO2 from flue gas, Green Chem., 2024, 26, 6172–6179 RSC.
- P. Kaur, J. T. Hupp and S. T. Nguyen, Porous organic polymers in catalysis: opportunities and challenges, ACS Catal., 2011, 1, 819–835 CrossRef CAS.
- S. Chongdar, S. Bhattacharjee, P. Bhanja and A. Bhaumik, Porous organic–inorganic hybrid materials for catalysis, energy and environmental applications, ChemComm, 2022, 58, 3429–3460 RSC.
- P. Bhanja, A. Modak and A. Bhaumik, Porous Organic Polymers for CO2 Storage and Conversion Reactions, ChemCatChem, 2019, 11, 244 CrossRef CAS.
- S. Ghosh, S. Riyajuddin, S. Sarkar, K. Ghosh and Sk. M. Islam, Pd NPs Decorated on POPs as Recyclable Catalysts for the Synthesis of 2−Oxazolidinones from Propargylic Amines via Atmospheric Cyclizative CO2 Incorporation, ChemNanoMat, 2020, 6, 160–172 CrossRef CAS.
- Sk. S. Islam, S. Biswas, R. A. Molla, N. Yasmin and Sk. M. Islam, Green Synthesized AgNPs Embedded in COF: An Efficient Catalyst for the Synthesis of 2−Oxazolidinones and α–Alkylidene Cyclic Carbonates via CO2 Fixation, ChemNanoMat, 2020, 6, 1386–1397 CrossRef CAS.
- X. Bai, Y. Zhang, F. Yan and X. Lan, Thiadiazol-based conjugated organic polymer anchoring Ag nanoparticles for efficient conversion of CO2 into oxazolidinones from propargylic amines, Appl. Surf. Sci., 2022, 604, 154566 CrossRef CAS.
- J. Wu, S. Ma, J. Cui, Z. Yang and J. Zhang, Nitrogen-Rich Porous Organic Polymers with Supported Ag Nanoparticles for Efficient CO2 Conversion, Nanomaterials, 2022, 12, 3088 CrossRef CAS PubMed.
- Z.-X. Rao, P.-B. Chen, J. Xu, Q. Wang, H.-T. Tang, Y. Liang and Y.-M. Pan, Direct Conversion of CO2 in Lime Kiln Waste Gas Catalyzed by a Copper–Based N–heterocyclic Carbene Porous Polymer, ChemSusChem, 2023, 16, e202300170 CrossRef CAS PubMed.
- R. Kishan, P. Rani, G. Singh and C. M. Nagaraja, Functionalized Covalent Triazine Framework (CTF) for Catalytic CO2 Fixation and Synthesis of Value-Added Chemicals, Cryst. Growth Des., 2024, 24, 7878–7887 CrossRef CAS.
- S. Arshadi, E. Vessally, A. Hosseinian, S. Soleimani-amiri and L. Edjlali, Three-component coupling of CO2, propargyl alcohols, and amines: An environmentally benign access to cyclic and acyclic carbamates (A Review), J. CO2 Util., 2017, 21, 108–118 CrossRef CAS.
- X. Liu, J. Li, N. Li, B. Li and X.-H. Bu, Recent advances on metal–organic frameworks in the conversion of carbon dioxide, Chin. J. Chem., 2021, 39, 440–462 CrossRef CAS.
- G. Zhang, H. Yang and H. Fei, Unusual Missing Linkers in an Organosulfonate-Based Primitive–Cubic (pcu)-Type Metal–Organic Framework for CO2 Capture and Conversion under Ambient Conditions, ACS Catal., 2018, 8, 2519–2525 CrossRef CAS.
- R. Das and C. M. Nagaraja, Highly Efficient Fixation of Carbon Dioxide at RT and Atmospheric Pressure Conditions: Influence of Polar Functionality on Selective Capture and Conversion of CO2, Inorg. Chem., 2020, 59, 9765–9773 CrossRef CAS PubMed.
- A. Helal, K. E. Cordova, M. E. Arafat, M. Usman and Z. H. Yaman, Defect-engineering a metal–organic framework for CO2 fixation in the synthesis of bioactive oxazolidinones, Inorg. Chem. Front., 2020, 7, 3571–3577 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- F. Vermoortele, B. Bueken, G. L. Bars, B. V. de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. V. Speybroeck, C. Kirschhock and D. E. De Vos, Synthesis modulation as a tool to increase the catalytic activity of metal–organic frameworks: the unique case of UiO-66 (Zr), J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- A. Helal, M. Fettouhi, M. E. Arafat, M. Y. Khan and M. A. Sanhoob, Nickel based metal-organic framework as catalyst for chemical fixation of CO2 in oxazolidinone synthesis, J. CO2 Util., 2021, 50, 101603 CrossRef CAS.
- D. Chakraborty, P. Shekhar, H. D. Singh, R. Kushwaha, C. P. Vinod and R. Vaidhyanathan, Ag Nanoparticles Supported on a Resorcinol–Phenylenediamine–Based Covalent Organic Framework for Chemical Fixation of CO2, Chem. – Asian J., 2019, 14, 4767–4773 CrossRef CAS PubMed.
- S. Dabral, B. Bayarmagnai, M. Hermsen, J. Schießl, V. Mormul, A. S. K. Hashmi and T. Schaub, Silver-Catalyzed Carboxylative Cyclization of Primary Propargyl Alcohols with CO2, Org. Lett., 2019, 21, 1422–1425 CrossRef CAS PubMed.
- Q.-W. Song, B. Yu, X.-D. Li, R. Ma, Z.-F. Diao, R.-G. Li, W. Li and L.-N. He, Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system, Green Chem., 2014, 16, 1633–1638 RSC.
- R. Karmakar and D. Lee, Reactions of arynes promoted by silver ions, Chem. Soc. Rev., 2016, 45, 4459–4470 RSC.
- Q.-W. Song, W.-Q. Chen, R. Ma, A. Yu, Q.-Y. Li, Y. Chang and L.-N. He, Bifunctional Silver(I) Complex–Catalyzed CO2 Conversion at Ambient Conditions: Synthesis of α–Methylene Cyclic Carbonates and Derivatives, ChemSusChem, 2015, 8, 821–827 CrossRef CAS PubMed.
- Q.-W. Song and L.-N. He, Robust silver(I) catalyst for the carboxylative cyclization of propargylic alcohols with carbon dioxide under ambient conditions, Adv. Synth. Catal., 2016, 358, 1251–1258 CrossRef CAS.
- R. Das and C. M. Nagaraja, Noble metal-free Cu(I)-anchored NHC-based MOF for highly recyclable fixation of CO2 under RT and atmospheric pressure conditions, Green Chem., 2021, 23, 5195–5204 RSC.
- C. Wu, F. Irshad, M. Luo, Y. Zhao, X. Ma and S. Wang, Ruthenium Complexes Immobilized on an Azolium Based Metal Organic Framework for Highly Efficient Conversion of CO2 into Formic Acid, ChemCatChem, 2019, 11, 1256–1263 CrossRef CAS.
- L. Zhang and Z. Hou, N-Heterocyclic carbene (NHC)–copper-catalysed transformations of carbon dioxide, Chem. Sci., 2013, 4, 3395–3403 RSC.
- R. Das, V. Parihar and C. M. Nagaraja, Strategic design of a bifunctional Ag(I)-grafted NHC-MOF for efficient chemical fixation of CO2 from a dilute gas under ambient conditions, Inorg. Chem. Front., 2022, 9, 2583–2593 RSC.
- Z. Sharifzadeh, S. A. A. Razavi and A. Morsali, Construction of hierarchically chiral metal–organic frameworks for fast and mild asymmetric catalysis, Green Chem., 2023, 25, 8661–8678 RSC.
- H. Bhambri and S. K. Mandal, A Ni-Based Metal-Organic Framework with Polarizing Traits for Efficient Heterogeneous Catalysis in the Sustainable Synthesis of Oxazolidinones, ChemCatChem, 2024, e202400429, DOI:10.1002/cctc.202400429.
- P. K. Giri, V. Parihar, S. Kumar and C. M. Nagaraja, Copper Nanoparticles Anchored on the Metal–Organic Framework as Recyclable Catalyst for CO2 Fixation to High-Value Compounds, ACS Appl. Nano Mater., 2024, 7, 15488–15497 CrossRef CAS.
- Y. Song, Q. Sun, B. Aguila and S. Ma, Opportunities of covalent organic frameworks for advanced applications, Adv. Sci., 2019, 6, 1801410 CrossRef PubMed.
- J. F. Kurisingal, H. Kim, J. H. Choe and C. S. Hong, Covalent organic framework-based catalysts for efficient CO2 utilization reactions, Coord. Chem. Rev., 2022, 473, 214835 CrossRef.
- R. Sani, T. K. Dey, M. Sarkar, P. Basu and Sk. M. Islam, A study of contemporary progress relating to COF materials for CO2 capture and fixation reactions, Mater. Adv., 2022, 3, 5575–5597 RSC.
- N. Haque, S. Biswas, S. Ghosh, A. H. Chowdhury, A. Khan and Sk. M. Islam, Zn(II)-Embedded Nanoporous Covalent Organic Frameworks for Catalytic Conversion of CO2 under Solvent-Free Conditions, ACS Appl. Nano Mater., 2021, 4, 7663–7674 CrossRef CAS.
- B. Banerjee, P. Chakrabortty, N. Haque, S. Ghosh, M. Sarkar, A. Khan and Sk. M. Islam, AgNPs Embedded in Porous Polymeric Framework: A Reusable Catalytic System for the Synthesis of α-Alkylidene Cyclic Carbonates and Oxazolidinones via Chemical Fixation of CO2, Catalysts, 2023, 13, 1467 CrossRef CAS.
- D. K. Nandi, N. Haque, S. Biswas, N. A. Siddiqui, A. Khan and Sk. M. Islam, Utility of silver nanoparticles embedded on a covalent organic framework as a highly active catalyst for carboxylative cyclization with CO2: a sustainable route for production of tetronic acids and oxazolidinones, New J. Chem., 2024, 48, 11982–11992 RSC.
- M. Xu, C. Lai, X. Liu, B. Li, M. Zhang, F. Xu, S. Liu, L. Li, L. Qin, H. Yi and Y. Fu, COF-confined catalysts: from nanoparticles and nanoclusters to single atoms, J. Mater. Chem. A, 2021, 9, 24148–24174 RSC.
- N. Huang, P. Wang and D. Jiang, Covalent organic frameworks: a materials platform for structural and functional designs, Nat. Rev. Mater., 2016, 1, 1–19 Search PubMed.
- S. S. Dhankhar, B. Ugale and C. M. Nagaraja, Co–Catalyst–Free Chemical Fixation of CO2 into Cyclic Carbonates by using Metal–Organic Frameworks as Efficient Heterogeneous Catalysts, Chem. – Asian J., 2020, 15, 2403–2427 CrossRef PubMed.
- I. H. Chowdhury, A. H. Chowdhury, P. Sarkar and Sk. M. Islam, Chemical fixation of carbon dioxide by heterogeneous porous catalysts, ChemNanoMat, 2021, 7, 580 CrossRef.
- M. Lu, M. Zhang, J. Liu, Y. Chen, J.-P. Liao, M.-Y. Yang, Y.-P. Cai, S.-L. Li and Y.-Q. Lan, Covalent Organic Framework Based Functional Materials: Important Catalysts for Efficient CO2 Utilization, Angew. Chem., Int. Ed., 2022, 61, e202200003 CrossRef CAS PubMed.
- X. Wang, J. Li, M. Kou, W. Dou, D. Bai, X. Tang, Y. Tang and W. Liu, Dual-Function Precious-Metal-Free Metal–Organic Framework for Photocatalytic Conversion and Chemical Fixation of Carbon Dioxide, Inorg. Chem., 2023, 62, 19015–19024 CrossRef CAS PubMed.
- K. O. Kirlikovali, S. L. Hanna, F. A. Son and O. K. Farha, Back to the basics: developing advanced metal–organic frameworks using fundamental chemistry concepts, ACS Nanosci. Au, 2022, 3, 37–45 CrossRef PubMed.
- M. Ding, X. Cai and H.-L. Jiang, Improving MOF stability: approaches and applications, Chem. Sci., 2019, 10, 10209–10230 RSC.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Stable metal–organic frameworks: design, synthesis, and applications, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- D. Muthukumar, C. M. Nagaraja, M. Badawi and R. S. Pillai, Computer modelling of trace SO2 and NO2 removal from flue gases by utilizing Zn(II) MOF catalysts, New J. Chem., 2023, 47, 18086–18095 RSC.
- M. Zanatta, Materials for Direct Air Capture and Integrated CO2 Conversion: Advancement, Challenges, and Prospects, ACS Mater. Au, 2023, 3, 576–583 CrossRef CAS PubMed.
- L.-P. Merkouri, T. R. Reina and M. S. Duyar, Feasibility of switchable dual function materials as a flexible technology for CO2 capture and utilisation and evidence of passive direct air capture, Nanoscale, 2022, 14, 12620–12637 RSC.
- M. Zanatta, E. García-Verdugo and V. Sans, Direct Air Capture and Integrated Conversion of Carbon Dioxide into Cyclic Carbonates with Basic Organic Salts, ACS Sustainable Chem. Eng., 2023, 11, 9613–9619 CrossRef CAS PubMed.
- M. Yin, L. Wang and S. Tang, Stable Dicationic Covalent Organic Frameworks Manifesting Notable Structure-Enhanced CO2 Capture and Conversion, ACS Catal., 2023, 13, 13021–13033 CrossRef CAS.
- H.-H. Wang, L. Hou, Y.-Z. Li, C.-Y. Jiang, Y.-Y. Wang and Z. Zhu, Porous MOF with Highly Efficient Selectivity and Chemical Conversion for CO2, ACS Appl. Mater. Interfaces, 2017, 9, 17969–17976 CrossRef CAS PubMed.
- C. M. Nagaraja, R. Haldar, T. K. Maji and C. N. R. Rao, Chiral Porous Metal–Organic Frameworks of Co(II) and Ni(II): Synthesis, Structure, Magnetic Properties, and CO2 Uptake, Cryst. Growth Des., 2012, 12, 975–981 CrossRef CAS.
- B. Yu and L.-N. He, Upgrading carbon dioxide by incorporation into heterocycles, ChemSusChem, 2015, 8, 52–62 CrossRef CAS PubMed.
- J. L. Segura, S. Royuela and M. M. Ramos, Post-synthetic modification of covalent organic frameworks, Chem. Soc. Rev., 2019, 48, 3903–3945 RSC.
- M. Wilson, S. N. Barrientos-Palomo, P. C. Stevens, N. L. Mitchell, G. Oswald, C. M. Nagaraja and J. P. S. Badyal, Substrate-Independent Epitaxial Growth of the Metal–Organic Framework MOF-508a, ACS Appl. Mater. Interfaces, 2018, 10, 4057–4065 CrossRef CAS PubMed.
- Y.-L. Wu, G.-P. Yang, S. Cheng, J. Qian, D. Fan and Y.-Y. Wang, Facile Incorporation of Au Nanoparticles into an Unusual Twofold Entangled Zn(II)-MOF with Nanocages for Highly Efficient CO2 Fixation under Mild Conditions, ACS Appl. Mater. Interfaces, 2019, 11, 47437–47445 CrossRef CAS PubMed.
- H. Li, G. Deng, Z. Wang, K. Cheng, Z. Li, X. Luo, Y. Yang, Y. Sun, P.-Z. Li and Z. Wang, Effective CO2 Catalytic Conversion by Nitrogen-Rich Covalent Organic Framework-Immobilized Ag Nanoparticles, ACS Appl. Nano Mater., 2023, 6, 16424–16432 CrossRef CAS.
Footnote |
| † Current Address: Chemical & Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, 117585, Singapore. |
| This journal is © the Partner Organisations 2025 |









