Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bifacial dye-sensitized solar cells for indoor and outdoor renewable energy-based application
Jessica
Barichello
 *ab,
Paolo
Mariani
b,
Luigi
Vesce
*ab,
Paolo
Mariani
b,
Luigi
Vesce
 b,
Donatella
Spadaro
a,
Ilaria
Citro
a,
Fabio
Matteocci
b,
Donatella
Spadaro
a,
Ilaria
Citro
a,
Fabio
Matteocci
 b,
Antonino
Bartolotta
a,
Aldo
Di Carlo
b,
Antonino
Bartolotta
a,
Aldo
Di Carlo
 bc and
Giuseppe
Calogero
bc and
Giuseppe
Calogero
 *a
*a
aCNR-IPCF, Viale F. Stagno D’Alcontres 37, 98158 Messina, Italy. E-mail: jessica.barichello@uniroma2.it; giuseppe.calogero@cnr.it
bCHOSE—Centre for Hybrid and Organic Solar Energy, Department of Electronic Engineering, University of Rome “Tor Vergata”, 00133 Roma, Italy
cISM-CNR, Istituto di Struttura della Materia, Consiglio Nazionale delle Ricerche, Via del Fosso del Cavaliere 100, 00133, 8 Roma, Italy
First published on 21st December 2023
Abstract
Bifacial solar cells (BFSCs) offer a way to boost electrical power generation for each unit area compared to traditional monofacial cells without a significant increase in production costs. These cells are designed to absorb sunlight from both front and rear sides, making them more efficient in converting light into electricity while exploiting the reflected solar radiation. The third-generation PV solar cells are widely known for the flexibility and the colour tunability allowing them to be designed as BFSCs. This article focuses on semi-transparent bifacial dye sensitized solar cells (BFDSCs), which stand out for their low production cost, for the possibility of being environmentally friendly and for the excellent conversion efficiency even in diffused light conditions, balancing both efficiency and transparency for a variety of applications. Their semi-transparent nature and the adaptability to different sunlight angles make BFDSCs ideal for both indoor and outdoor environments. This review highlights the newest breakthroughs in DSSC and provides an overview of the latest research on innovative electrodes, electrolytes, and dyes. Moreover, the review delves into current and potential uses of BFDSCs in areas such as agriculture, building integration, and smart devices.
1. Introduction
Over the past few decades, the rising demand for energy, coupled with the diminishing fossil resources and the imperative to reduce annual CO2 emissions, has driven a shift towards renewable and eco-friendly energy sources. Solar energy stands out as a universally accessible, clean, and cost-effective resource for all of humanity. Using non-fossil fuel sources, such as solar energy, provides an economically viable solution to combat climate change. The sun delivers approximately 13.6 TW of carbon-free energy to the Earth every hour, which exceeds the annual energy consumption of the entire human population, estimated at around 13 TW h.1 Harvesting just a tiny fraction of this enormous energy amount could be sufficient to satisfy the entire global energy demand.2 In this context, renewable solar photovoltaics (PV), as one model to convert solar energy directly into electricity, will play a key role in decarbonizing our energy production in the coming decades.To address this challenge, PV technologies have experienced rapid growth: from 2005 to 2019, global PV production surged from approximately 4 TW h to ∼681 TW h.3 In 2019, around 11% of global primary energy (encompassing electricity, transport, and heating) came from renewable technologies.4 Furthermore, the International Energy Agency (IEA) predicts that renewables account for almost 40% of global electricity output in 2027, making up for declining shares of coal, natural gas and nuclear.5
To date, silicon-based solar cells are still the dominant technology in the PV market with power conversion efficiencies (PCE) up to 26.6%.6 Comparable results have been achieved by a second generation of single- and multi-junction thin films solar cells (e.g., cadmium telluride (CdTe)), gallium arsenide (GaAs), cadmium telluride/cadmium selenide (CdTe/CdS), copper indium gallium selenide (CIGS, etc.),7 although more expensive due to their manufacturing and material costs. In all instances, the PCE values of single junction solar cell technologies remain still below the 33%, according to the Shockley–Queisser limit,8 and significant efforts have been made to approach this limit. In the quest to find cheap and eco-friendly solar energy conversion solutions, some interesting and unconventional approaches have been explored.
Novel materials synthesis, as well as efficient device architectures with minimal environmental impact, have been developed by numerous research groups. Among the various alternative approaches, dye-sensitized solar cells (DSSCs), due to their inexpensive/non-toxic starting materials, easy fabrication process, and significant aesthetic value, have been identified as one of the most competitive PV technologies for indoor applications and the Internet of Things (IoT).9
Mimicking the fundamental pathways of natural photosynthesis (i.e., light harvesting, charge separation and catalysis effects), by synergistically combining a molecular system with nanoparticles, DSSCs, belonging to the 3rd PV generation, offer a fresh perspective compared to inorganic p–n junction solar cells, stand out in terms of cheapness, light manipulation and long-term stability. Furthermore, in contrast to conventional solar cells (SCs), DSSCs maintain good PCE under different light conditions and, more interestingly, they can be designed to be flexible and colorless or with a colorful appearance. These attributes make them appealing for architectural integration in both residential and commercial buildings10 (known as building-integrated photovoltaics: BIPV) and portable applications, showing great potential in the realm of IoT, encompassing smart homes, healthcare, transportation, and much more (see Fig. 1).
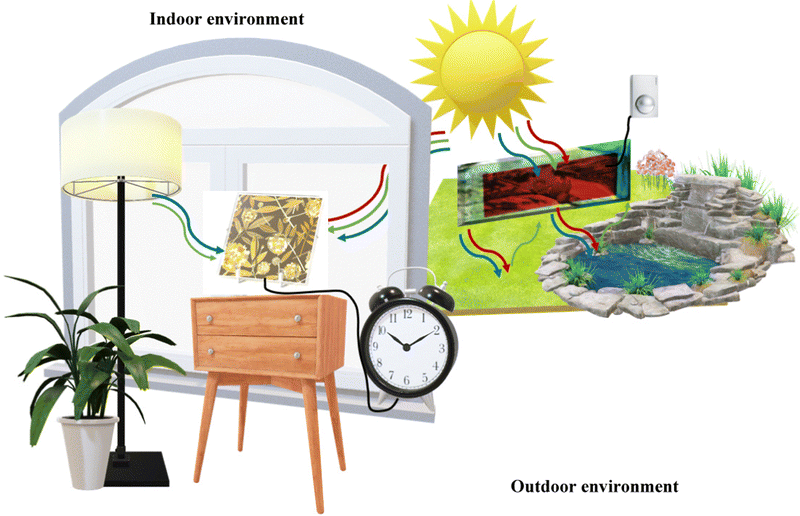 | ||
| Fig. 1 A Hana Ankara lamp (modified image from https://www.sony.com/ja/pressroom/pict_data/images/sonota/200812_Hana-Akari_1.jpg) that powers a small electronic device absorbing light from the front and rear side in an indoor environment while a BFDSC (modified image from ref. 35) is powering a movment sensor in an outdoor environment. | ||
Additionally, beyond their capability to meet energy demands, a particular choice of dye enables the proper filtering of incident solar radiation, making DSSCs ideal for agrivoltaic applications. Agrivoltaics refers to the dual use of land for solar photovoltaic power generation and agriculture. This approach presents a promising solution to minimize the competition between PV power generation and agricultural activities. Agrivoltaics can assist farmers in lowering their energy expenses and carbon footprint while enhancing their energy independence.11
Since the first paper of Grätzel and co-workers,12 the progress in the field of DSSCs has been accompanied by impressive advancement in the PCE, passing from ∼7.12% up to ∼14%,13,14 surpassing amorphous silicon.15,16 With common and commercial red and orange dyes, DSSCs have reached an average visible transmittance (AVT) of 20–30%,17 while an outstanding AVT higher than 60%,18 has been reported using a select dye system which absorbs in the UV and near-IR region. However, though significant breakthroughs have been obtained and years of thriving growth can be expected, the need to improve the cell's PCE beyond 20% is of critical importance for the scale-up industrial applications of this technology.
In view of this, with the goal of increasing the quantity of light harvesting, and hence the PCE value, several approaches like complementary dyes combination, either as a dye cocktail or arranged in multiple dye layers, the use of functionalized organic dye molecules in a rigid panchromatic structure,19 as well as strategies to enhance the optical path of incident light, via porous back-scattering films or submicron particles of high refractive index, have been considered.20,21 Strong interest has also been paid to the development of tandem-DSSCs (T-DSSCs) configuration,22 an alternative option to increase the device efficiency beyond the thermodynamic limit of single junction. However, although great steps have been made, actual results are still not so satisfactory.23 In the expectation to optimize solar cell architecture by using ecological criteria, the recent past has seen the rediscovered of bifacial solar cells (BFSCs) concept. A technology whose emergence can be traced back to a patent by Hiroshi in 196624 and following scientific reports by Luque, Cueva et al.25,26 which, demonstrated significant higher electrical power production in BFSCs with respect to their monofacial counterpart. However, notwithstanding these premises, this technology was long ignored, except for some niche applications.27 Bifacial technology gained new attention by academic and PV communities from 2009, especially when companies (like Yingli Solar, PVG Solutions Inc., Sanyo) began to commercialize bifacial modules based on crystalline silicon (c-Si) bifacial structures28–30 (e.g., PERC: passivated emitter rear contact; PERL: passivated emitter rear locally diffused; PERT: passivated emitter rear totally diffused; etc.31). Bifacial technology is perceived to be the most suitable choice for next-generation solar farms, thanks to its increased energy yield and low levelized cost of energy (LCOE32). The International Technology Roadmap for Photovoltaic (ITRPV) forecasts that bifacial solar cells are projected to hold over 80% of the global market share by 2032.33
In contrast to monofacial counterpart, which only collect photons reaching the device's front side, BFSCs capture photons from both the front and rear sides of the cell, allowing a better utilize of diffuse sky radiation and reflected light from surroundings (albedo), also offering the opportunity to lower the area-related costs. The additional rear side irradiance, as well as the device deployment (e.g., orientation, tilt angle, etc.) and their synergic effects, generating a greater number of charge-carriers promotes a short-circuit current density enhancement and thus of overall PCE. The semi-transparent DSSC technology indeed is an ideal candidate to be applied as BFSC however, the nature and opaqueness of electrolytes and/or counter electrode (CE) can create dramatic losses in the cell performance during backside illumination owing to screening and reflecting effects. As a result, the number of photons which reach the dye molecules, for the front illumination is greater with respect to that of the back side which, implies a quite low response of rear efficiency in relation to the front efficiency (bifaciality factor, BFF34), both being subject to the same irradiance. The engineering of transparent and cost-effective materials and components to fulfil high transmittance requirements, has been a constant mission to open transformative opportunities in BFDSCs field.
In 2011, BFDSCS with transparent polyaniline CE and PCE values of 6.54% and 4.26% for front- and rear-side illumination, respectively, and BFF ≈ 65% were reported by Tai et al.35 Duan et al.36 reported optically transparent CEs based on binary-alloy metal selenides M–Se (M = Co, Ni, Cu, Fe, Ru). Irrespective to the M–Se alloy, their fabricated BFDSCs showed front and rear conversion efficiencies significantly higher than those obtained using CE based on the most widely utilized platinum. Miranda-Munoz and co-workers,37 enhanced the PCE of BFDSCs by 25% and 33% in front- and rear-illumination, respectively, compared to an equivalent standard cell, by random dispersion of scattering particles in the nanostructured TiO2 photoanode. By judicious choice of electrolyte, CE, and photoanode, Venkatesan et al.,38 reported BFDSCs exhibiting PCE values as high as 20.65% and 17.31% under front-and rear-illumination, respectively, in indoor condition.
Currently, amorphous and crystalline silicon dominates the photovoltaic market. However, even in bifacial form, Si-based PV retains its intrinsic opacity properties, which make it unsuitable for BIPV or indoor applications.39 In addition, unlike DSSC technology, silicon is very sensitive to the angle of incidence of light radiation.40,41 Third-generation PV technologies, such as organic photovoltaic (OPV), DSSC, and perovskite solar cells (PSCs), in a bifacial configuration offer several advantages for flexible, lightweight, and semi-transparent applications.27 Recent PSCs have reached record efficiencies of over 26%.16 This technology can also achieve high light transmittance and coloured-semitransparency, making PSCs suitable for indoor and BIPV applications.42 PSCs can also be fabricated in a bifacial configuration without the need for additional material, and they are not as sensitive to the angle of incidence of light as silicon solar cells. However, the main challenge to the commercialization of PSCs is their stability.43 Perovskites are sensitive to moisture and light, which can degrade their performance over time. In OPV field, Burgués-Ceballos et al.44 explore the optimal trade-off between average AVT and PCE for various semi-transparent PV applications. While some OPV cells can achieve PCE and AVT values exceeding 5% and 40%, respectively, suitable for applications like skylights, PV windows, and smart windows, OPV solar modules still lack a satisfactory balance between these parameters.45 Despite the plethora of papers detailing with the advances made in DSSCs technological applications and designs, to our best knowledge, there are no published reviews focused on specific materials capable to emphasize/improve the bifacial feature in terms of PCE and transparency.27,46,47 Here we present an update overview of current efforts in the advancement of this transitioning technology, highlighting the optical properties of each device component to reach the best compromise between BFF and overall PCE, also discussing the optimum indoor/outdoor conditions. Paragraph number 2 focus on the working system of the DSSC technology, the main figures of merit (FoM) in BFDSCs and the albedo's effect in order to facilitate the discussion and understanding of subsequent sections. The associated properties and issues correlated to the device's main components are given and critically discussed in Sections 3.1–3.5. The use of this captivating technology as potential contender in the BIPV, as well as in the IoT and in Agrivoltaic field is presented in Section 4. Section 5 is devoted to the new trend in combining multiple types of devices into one unit, in order to enhance and/or diversified function of the integrated device. In this framework we are going to deal with the promises offered by the DSSC/DSSC and DSSC/Perovskite tandem devices. Finally, in Section 6 the challenges and outlook in BFDSCs field are featured on the basis of current development.
2. Operating principles of a DSSC and a BFDSC
In this paragraph, we delve into the operating principle of a DSSC cell by exploring its primary components and the suppression effects that arise. We introduce the most commonly used figures of merit in the literature that emphasize transparency. Subsequently, we analyse the albedo effect, which is crucial for the operation of bifacial PV devices.2.1 Components and working system of a DSSC
A DSSC consists of three main components sandwiched between two conductive sheet glasses (Fig. 2):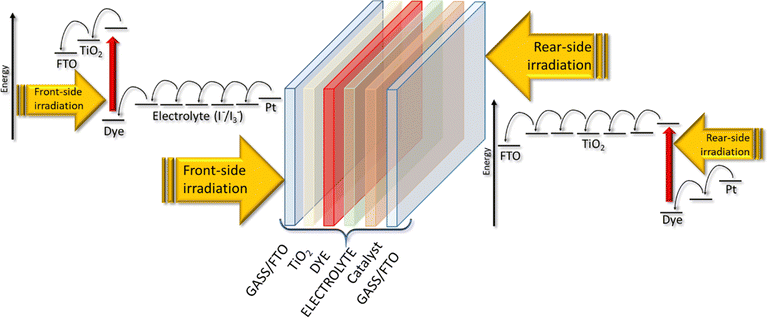 | ||
| Fig. 2 Electron's motion in a BFDSC when irradiated from front and rear side (modified image from ref. 53). | ||
• A mesoporous semiconductor layer, typically composed of TiO2, serves as the working electrode (WE) upon which the dye is anchored.
• A liquid, gel, or solid electrolyte with a redox couple, commonly composed of iodide/triiodide ions.
• A catalyst layer, usually made of platinum. This layer is the counter electrode (CE).
Upon encountering the anchored dye on the semiconductor, photons initiate the generation of electron–hole pairs within the dye molecule. These photo-excited electrons (eqn (1)), occupying energy levels higher than the conduction band (CB) edge of TiO2, swiftly transition into the TiO2's CB. This process, characterized by a typical time constant k of approximately 10−12 seconds under one sun illumination (irradiation power of 100 mW cm−2, temperature of 25 °C, and spectral intensity distribution of the light source matching the AM 1.5 global standard solar spectrum),48 occurs rapidly (eqn (2)).49
Due to the photo-induced charge carrier transfer from the dye to the semiconductor oxide, the electronic density in TiO2 increases giving rise to an electrochemical potential difference (DV) between TiO2 and the electrolyte. The regeneration of oxidized dye molecules is achieved through electron transfer from the electrolyte mediator. The cathode catalyzes the reduction of I3− (eqn (4)). Thus, in this circumstance, the entire cycle is regenerative.49
| Dye + hv → dye* light absorption (excitation) | (1) |
| Dye* + TiO2 → dye+ + eCB− (TiO2) electron injection (k ≈ 10−12 s) | (2) |
| 2Dye+ + 3I− → 2dye + I3− dye regeneration (k ≈ 10−6 s) | (3) |
| I3− + 2e−catalyst → 3I− electrolyte regeneration (reduction) | (4) |
| Dye* → dye decay of the dye excited state (k ≈ 10−10 s) | (5) |
| Dye+ + eCB− (TiO2) → dye + TiO2 recombination (back reaction, k ≈ 10−4 s) | (6) |
| I3− + 2e−CB (TiO2) → 3I− + TiO2 electron capture from mediator (dark current, k ≈ 10−2 s) | (7) |
The PV figure of merit are explained below:
– ISC (A) (short circuit current): represents the peak current produced when the cell is in a short-circuited state. JSC (A m−2) is the short circuit current density.
– VOC (V) (open circuit voltage): denotes the highest voltage when the circuit is open.
– I–V curve (or current–voltage characteristic) is the relationship, between the current (I) through a circuit (or device) and the corresponding voltage (V) across it. Typically, is represented as a graph where V is the abscissa and I the ordinate. In this graph, when I = 0 then V is equal to VOC and when V = 0 then I is equal to ISC. Within the graph, two coordinates can be identified: IMPP (or IMAX) and VMPP (or VMAX), the current and the voltage at maximum power point, respectively.49
– FF (fill factor): PMPP/ISC·VOC, where PMPP (or PMAX) is the available power in correspondence of the maximum power point (in detail, PMPP is equal to the product IMPP·VMPP). Closer is the value of PMPP with respect the product ISC·VOC, more efficient – in terms of electric power generated – is the device.
– PCE (power conversion efficiency): is defined as the ratio between POUT and PIN, where POUT is equal to the product JSC·VOC·FF and PIN is the incident light, which corresponds to 1000 W m−2, also known as 1 Sun (AM 1.5G condition). PCE is the most widely used figure of merit in the PV scientific community.
In BFDSC, the front PCE is the obtained photoconversion efficiency when the photoelectrode side is illuminated. The rear PCE is when the cell is illuminated from the side of the counter electrode. As matter of fact, being the front side the one that collects sunlight by direct incidence, results PCEfront ≥ PCErear.
In Fig. 2, all the components of a DSSC are shown schematically, and the movement of electrons when light reaches both sides of the device is presented.53 The thickness of the TiO2 layer determines the amount of incident light absorbed, regardless of the illumination side. When light passes through a thin-thick dye-sensitized porous TiO2 layer, 50% of the incident light is absorbed in accordance with the Beer–Lambert law.54 This means that photo-excitation in the porous TiO2 electrode primarily occurs near the side exposed to light. During front-side irradiation, the electron path within the TiO2 conduction band is shorter, while the hole paths in the electrolyte, which extend from the reduced dye to the platinum counter electrode, are longer compared to rear-side irradiation. This effect can minimize the disparity between conversion efficiencies for front- and rear-side irradiation, leading to improved performance and cost-effectiveness for large scale solar power generation systems. Optimizing the thickness of the porous TiO2 layer is crucial for enhancing DSSC performance. The photovoltaic characteristics demonstrate substantial variation depending on the thickness of the porous TiO2. A pioneering study53 revealed a linear decrease in open-circuit photovoltage (VOC) with increasing TiO2 film thickness. Under front-side irradiation, a 2.4 μm-thick dye-sensitized porous TiO2 layer can absorb 50% of the incident light, creating a dye-excited region on the FTO side and a non-excited region on the electrolyte side. In open-circuit conditions, electrons injected from the dye into the TiO2 layer spread uniformly from the FTO side to the electrolyte side within the porous TiO2 layer.55 Consequently, increasing the thickness results in a larger non-excited area, further lowering the VOC upon averaging the electron density in the non-excited region.
2.2 Enhancing BFDSC: a figure of merit approach
Figures of merit (FoMs) provide meaningful metrics to assess the performance of different solar devices for a specific application. In the case of BFDSCs, appropriate FoMs are needed to assess the influence of bifacial illumination and quantify the additional irradiance on the cell's rear side. Unlike their monofacial counterparts, there is not yet a universally accepted standard in the PV community for characterizing the performance of BFDSCs.Table 1 provides a summary of key FoMs that underscore the bifaciality feature in DSSC technology. These metrics provide meaningful tools to assess the capabilities of various BFDSCs designs and materials choices.
| FoMs | Description |
|---|---|
| BFF | Bifaciality factor. |
| Ratio of rear PCE to front PCE at equal irradiance. | |
| Range 0–100%. | |
| AVT | Average visible transmittance on the whole visible spectra from 380 to 780 nm. |
| Range 0–100%. | |
| LUE | Light utilization efficiency. |
| Trade off relationship between AVT and PCE. | |
| CRI | Colour rendering index. |
| 0–100 scale. | |
| Quantitively description of how accurately the colour of a given object is rendered. | |
| CCT | Correlated colour temperature. |
| Degrees Kelvin (K). | |
| It allows to understand of whether the colour is neutral, bluish white or reddish white. | |
| TED | Transparency, efficiency, diffused light efficiency. |
| Geometric mean of transparency, efficiency and diffused light. |
In this section, we will provide a description of the most widely used metrics in the DSSC literature such as BFF, AVT, LUE, and CRI. The other metrics mentioned, CCT56 and TED57 will not be discussed here, as they are rarely present in the DSSC field at the moment and therefore outside the scope of this review. We provide the relevant references in case the reader wishes to delve deeper.
The “bifaciality factor”, BFF (%)58 defined as the ratio between the rear and front efficiencies at the same irradiance (eqn (8)), have been used by several academic researchers:
| BFF = [(PCErear/PCEfront)] × 100 | (8) |
The opportunity to integrate DSSCs in BIPV, IoT, and greenhouses necessitates not only maximizing visible light harvesting for effective power generation but also ensuring visual comfort through suitable average visible transmittance (AVT) and a high color rendering index (CRI). Therefore, the aesthetic quality of BFDSCs is often quantified using these two main FoMs. Usually, the SCs transparency is estimated by AVT features in the 380–780 nm visible wavelength range, where the human eye is sensitive. In this framework, AVT becomes a critical FoM to assess the transparency level of BFDSCs. AVT value (%) is based on the integration of the device's transmission spectrum, the light source's spectrum and the spectral response of human eyes (photopic response) according to the following:59,60
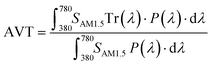 | (9) |
Traverse et al.62 proposed a revolutionary FoM, named light utilization efficiency and defined as the product of AVT and PCE. The LUE (= AVT·PCE) provides a metric for comparing, on the same scale, high-performance transparent photovoltaics without neglecting efficiency in favour of transparency and vice versa.
Spectral quality FoMs assess the color rendering fidelity of transmitted or reflected light compared to natural daylight. This aesthetic performance is especially important for architectural integration.
On the other hand, the color rendering index (CRI) is a further essential FoM, particularly in BIPV, where it is the most aesthetic criteria used to quantifies the accuracy of a light sources to reproduce faithfully the color of a given object. However, since the color is a perception, impossible to measure, CRI is evaluated on the comparison with a natural or standard light source.63 In other words, the CRI indicates quantitatively the color rendering ability by comparing the color of an object seen under a generic lighting with the color seen under a reference illumination source. Sunlight, which accurately renders the colors of objects, serves as a benchmark for all light sources. The CRI value ranges from 0 to 100. For glazing applications, a CRI of 80 or higher is typically considered acceptable, while a CRI above 90 is regarded as optimal.64 CRI value is calculated within an evolution of functions as an average score on eight different test colour samples.56
2.3 Albedo's effect
In real-world scenarios, the bifacial property of the device is utilized to harness rear irradiance, also known as the albedo effect. This effect stems from the reflection of sunlight off nearby surfaces, such as the ground or buildings, which can significantly enhance the power output of bifacial solar panels. The albedo effect is particularly pronounced in environments with high reflectivity, such as deserts or areas with snow cover58 (Fig. 3a). Fig. 3a shows the spectral reflectivity data for the chosen surface materials (grass, tree, snow, white marble, white dune and asphalt) obtained from the Ecostress Spectral Library provided by the NASA Jet Propulsion Laboratory.65 Measuring albedo involves the use of an albedometer, a specialized instrument that captures the reflected and incident radiation. The calculation of albedo incorporates various parameters, including the azimuth angle, the zenith angle, in addition, the radiance in watts per square meter and steradian, which includes direct and diffuse components. For a more in-depth understanding of the albedo calculation formula, refer to this ref. 27.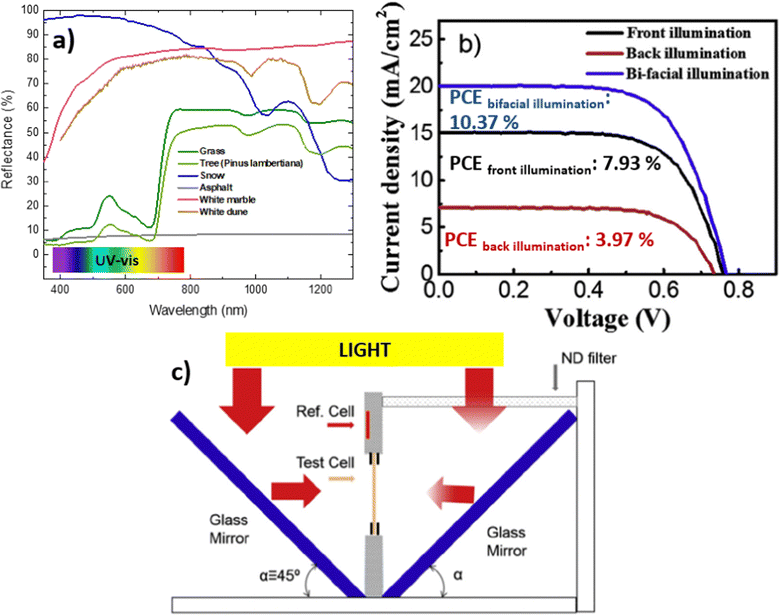 | ||
| Fig. 3 (a) Reflectance data for different surface materials downloaded from Ecostress Spectral Library provided by the NASA Jet Propulsion Laboratory.65 (b) J–V curves of a solid state BFDSC device by a back illumination, front illumination and bi-facial illumination (modified from ref. 68). (c) Schematic illustration of the light-splitting design in the bifacial cell tester, with simulated sunlight irradiating on both sides of the cell (modified image from ref. 68). | ||
The effectiveness of utilizing the bifacial feature for different PV technologies is primarily determined by their respective absorption spectra. DSSC can absorb across the entire visible spectrum and into the near-infrared region, depending on the chosen dye. Perovskite solar cells (PSCs) operate similarly, with their band gap adjusted to enhance absorption in specific regions of the spectrum.66
Amorphous silicon absorbs primarily in the shorter wavelengths of the visible spectrum, while crystalline silicon absorbs in the longer wavelengths and into the near-infrared region.
Analysis of albedo reflectance values has demonstrated that snow tends to exhibit a bias toward reflecting shorter wavelengths, particularly ultraviolet (UV) light. This preferential reflection of UV light favors hydrogenated amorphous silicon (a-Si:H) PV cells over other PV material technologies due to the energy gap values of the semiconductor. Conversely, paints tend to reflect preferentially within specific spectral ranges depending on their color, highlighting the strong situational dependence of PV technologies. These findings suggest the potential for categorizing bifacial PV module technologies based on their compatibility with diverse geographic, climatic, and ground characteristics, enabling the optimization of PV systems for specific micro-environments.27,67 In this research work focused on solid-state DSSC, Hwang et al. simulated various rear reflectance (albedo) conditions and realized a cell tester for J–V characterization of bifacial cells.68 The synergistic effects of irradiation from both sides result in improved sunlight utilization and enhanced PCE (Fig. 3b). When the device is measured simultaneously from both sides, thereby harnessing the Albedo effect, a remarkable increase in PCE is observed, reaching a maximum PCE of 10.4% compared to 7.9% and 3.9% achieved from the front and rear side, respectively. The bifacial cell tester is composed of a vertical sample holder that stands perpendicular to the base plate (Fig. 3c). Two mirror glasses are positioned symmetrically at a 45° angle to the sample holder. The sample holder includes means for contact with the photovoltaic cell on both sides, a reference cell for determining the irradiance after reflection on the mirror, and clamps to support the cell itself. To reduce the light irradiance on the rear side, various neutral density (ND) filters can be placed in front of the mirrors.
A recent paper,69 focusing on bifacial PSCs, provides compelling evidence of the correlation between PCE and Albedo. The study meticulously demonstrates how the PCE trend consistently rises in response to increasing Albedo values.69
3. Bifacial components
In DSSCs, a liquid, gel, or solid electrolyte containing the redox couple fills the space between the two electrodes or active layer (WE and CE). Each part significantly influences the cell transparency. There is considerable interest in optimizing the transparency of the entire device, as well as the way in which the various parts of the DSSC correlate with each other. This section aims to provide a comprehensive overview of the optical properties of each individual component of DSSCs (CE, electrolyte, and WE) in order to exploit the bifaciality feature. We have reviewed recent advancements in engineering electrolytes and redox couples that exhibit exceptional transparency across visible wavelengths. This is aimed at minimizing light filtering and absorption. Ensuring high transparency for each component is crucial for enhancing rear-side illumination and the overall performance of bifacial devices.3.1 Transparent conductive oxide (TCO)
The working electrode (WE) or counter electrode (CE) of a DSSC consist of a transparent conductive oxide (TCO) glass with a deposited semiconductor layer on the first and a deposited catalyser on the second. TCO are widely utilized as transparent electrodes in optoelectronic devices to fabricate flat panel displays such as smartphones, light emitting diodes and thin film solar cells.70 By doping intrinsically or extrinsically simple oxides, the electronic properties of a metal are obtained maintaining the transparency.71 Fluorine-doped tin oxide (FTO) and indium tin oxide (ITO) are the most utilized semiconductor-based ternary compounds49 in thin-film solar cell of the third generation. ITO is widely exploited in DSSC but also in PSC42 as TCO or top electrode due to the capacity to produce high visible light transmittance (85%), low electrical resistivity (10 Ω), and good adhesion strength to various substrates. However, several drawbacks are discouraging its application since indium is a critical raw material. In terms of average abundance, indium is a scarce element in the Earth's crust and from a geopolitical point of view, China possess most of the indium reserves and its supply risk has become a major concern in the global market.72 Furthermore, indium suffers of high prices, a demanding process, toxicity potential and sensitivity to both acid and basic environments.80 On the other hand, FTO that is still the reference and the most utilized TCO, possess a higher work function than ITO (4.9 eV vs. 4.8 eV for ITO), an improved thermal stability, mechanical and chemical durability, less toxicity, and low price if compared to previously mentioned ITO.73 Several TC materials have been proposed as electrode including carbon-based nanomaterials (carbon nanotubes and graphene).74–77 However, not only the PCE values are mediocre, but also the sheet resistance does not meet the requirements for PV applications (RS = 10 Ω and T = 90%).3.2 Counter-electrodes for bifacial application
The counter-electrode (CE) plays a crucial role in determining the bifaciality and photovoltaic performance of DSSCs. An ideal CE should exhibit high electrical conductivity, electrocatalytic activity, long-term stability, and optical transparency. However, simultaneously optimizing all these properties is challenging. Pt (platinum) is a commonly used catalyst for DSSCs.78 However, it is a costly and rare metal with limited resistance to corrosion from electrolyte redox solutions. This has motivated exploration of alternative cost-effective CE materials with bifacial capabilities, including conducting polymers,79 carbon-based materials,80–82 inorganic compounds, and their composites (Fig. 4).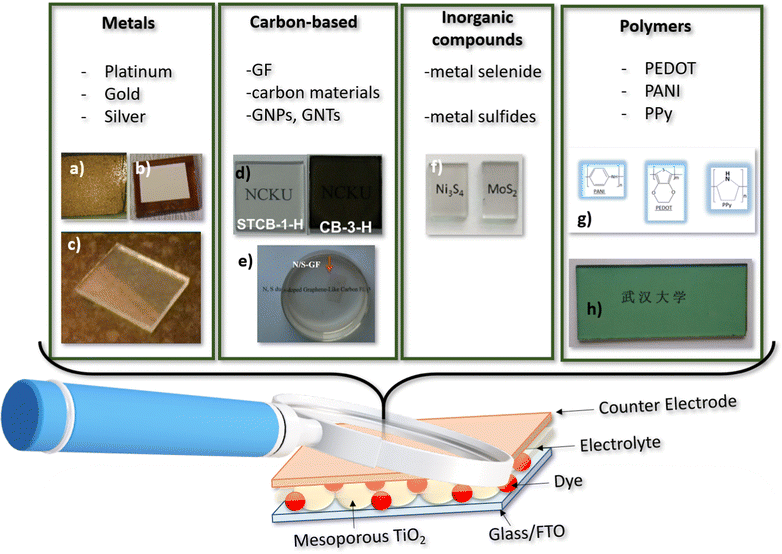 | ||
| Fig. 4 Counter electrode categories from left to right: metals; carbon based compound; inorganic metal compounds and polymers. (a) Gold counter electrode reproduced and adapted from ref. 85, under Creative Commons BY-4.0 license; (b) silver counter electrode reproduced and adapted from ref. 87, under Creative Commons BY-4.0 license; (c) platinum counter electrode reproduced and adapted from ref. 93, under Creative Commons BY-4.0 license; (d) semitransparent carbon based electrodes reproduced from ref. 97 with permission from the Royal Society of Chemistry; (e) graphene films counter electrode reproduced and adapted with permission from ref. 106 Copyright © 2015 Elsevier B.V. All rights reserved; (f) metal sulfides counter electrode reproduced and adapted from ref. 112 copyright © 2015 Published by Elsevier Ltd; (g) PANI, PEDOT and Ppy molecules; (h) PANI counter electrode Reprinted (adapted) with permission from ref. 35 Copyright {2011} American Chemical Society. | ||
In DSSC ambient, the CE collects the photogenerated electrons from the external circuit and reduces the redox species that function as mediators in the sensitizer's regeneration after electron injection, which appreciably impacts the PCE. In addition to the properties already mentioned, an ideal bifacial CE must have Tr ≈ 80% at λ ≈ 550 nm, sheet resistance (RS) < 20 Ω and charge transfer resistance (RCT) in the range 2–3 Ω cm2.83 It is well recognized that in DSSCs, the charge carriers transport from the WE to CE is hindered by multiple resistances; RS and RCT being those strongly enslaved to the CE. RS significantly governs the FF losses: at smaller RS corresponds higher FF, resulting in higher PCE. In other words, a lower RS indicates a higher conductivity of the catalytic CEs. On the other hand, RCT reflects the charge transfer dynamic on the CE surface during a photoactivated process and, in this regard, smaller RCT indicates better charge-transfer ability, faster diffusion process and lower photogenerated carrier recombination, meaning enhanced catalytic activity. RCT can be explained in terms of exchange current density (J0), as assessed by the following: RCT = (R·T/n·F·J0), where R, T, n, and F are the gas constant, temperature, number of electrons transferred in the elementary electrode reaction (n = 2) and the Faradays constant, respectively.84
Unfortunately, the simultaneous fulfilment of all the above requirements is almost unreachable. In the following paragraphs, a full description of the counter electrode advancement of each category, metals, carbon-based, inorganic compounds and polymers, are presented.
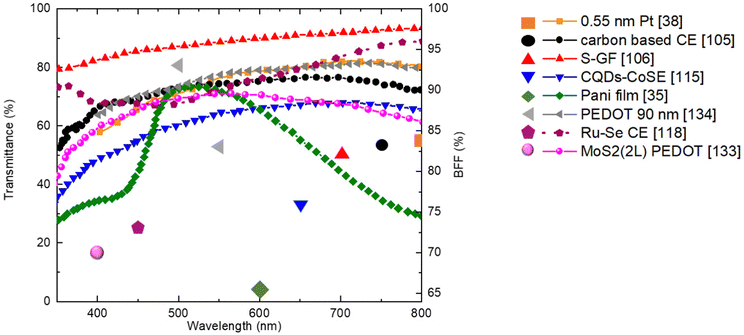
Among metals, platinum demonstrates the highest catalytic activity,93 towards I3− reduction, stability in I−/I3− electrolytes,94 and printability on rigid/flexible substrates. Pt CEs have enabled PCEs over 11% with I−/I3− redox shuttles.95 However, Pt may lack transparency and it is expensive. One effective strategy to balance conductivity and transmittance is the use of ultra-thin sputtered Pt layers. Venkatesan et al.38 fabricated BFDSCs with Pt thicknesses varying from 0.55 nm to 12.1 nm on FTO glass substrates using magnetron sputtering deposition. The extremely thin 0.5 nm Pt CE achieved an impressive AVT of 76% in the visible range along with front and rear PCEs of 20.65% and 17.31%, respectively, under 200 lux indoor lighting (Table 2). This corresponded to a very high 84% BFF, among the best for state-of-the-art BFDSC systems.
| CE | WE | Electrolyte | Dye | PCE MAX (%) | BFF (%) | Ref. | Year | |
|---|---|---|---|---|---|---|---|---|
| Metals CE | 0.55 nm Pt (200 lux) | TiO2 (ML 8 μm) | Co(II)/Co(III), TBP, LiClO4 | Y123 | 20.65 | 84 | 38 | 2019 |
| Pt | TiO2/SiO2 (16 μm/3 μm) | I2, PMII, GuSCN, MB | Z907Na | 6.54 | 91 | 53 | 2008 | |
| Pt | TiO2 (ML) | LiI, I2 | N719 | 7.29 | 80 | 96 | 2018 | |
| Carbon-base CE | S-GF | TiO2 (10 μm ML/2 μm SL) | I2, LiI, TBP, GuSCN, BMII | N719 | 3.86 | 83 | 105 | 2015 |
| N-GFs | TiO2 (ML 12 μm) | I2, Lii, GuSCN, BMII, TBP in ACN | N719 | 3.12 | 83 | 106 | 2015 | |
| Cu2−xSe@N-doped Carbon | — | — | N719 | 7.61 | 76 | 109 | 2020 | |
| Inorganic compound | CQDs-CoSe | TiO2 (ML) | NaI, KI, LiI, I2, TBP | N719 | 8.54 | 76 | 115 | 2018 |
| Ru–Se alloy | TiO2 (ML 10 μm) | — | N719 | 8.72 | 68 | 118 | 2018 | |
| PR-MoS2 | TiO2 (14 μm ML/3 μm SL) | GuSCN, PMII, I2,TBP in ACN:VN (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) 15) |
N719 | 8.77 | 55 | 124 | 2020 | |
| Polymers | MoS2/PEDOT | TiO2 (ML) | LiI, I2, TBP, GuSCN, BMII | N719 | 7 | 68 | 132 | 2020 |
| Pedot 90 nm (2000 lux) | TiO2 (ML 4 μm/SL 3.5 μm/ML 4 μm) | — | Y123 | 26 | 88 | 134 | 2022 | |
| Pedot 90 nm (200 lux) | 24.16 | 93 | ||||||
| PANI | TiO2 (ML) | PMII, LiI, I2, GuSCN, TBP | N3 | 6.54 | 65 | 35 | 2011 | |
| PEDOT | TiO2 (ML 6 μm) | Co(bpy)3(PF6)2, Co(bpy)3(PF6)3, LiClO4, TBP | Y123 | 8.65 | 85 | 133 | 2018 |
The enhanced transparency enabled more rear-side light harvesting while the continuous ultra-thin Pt layer provided sufficient conductivity and electrocatalytic activity. Another synthesis route is photofabrication of Pt CEs by UV irradiation of hexachloroplatinic acid (H2PtCl6·6H2O) solutions deposited on rigid or flexible substrates. Popoola et al.96 adopted this approach to produce flexible Pt CEs with better 400–700 nm transmittance than reference ITO/PET and FTO/glass substrates. BFDSCs with the photo-fabricated CEs achieved front and rear PCEs of 7.29% and 5.85% (Table 2), along with 78–86% BFF under simulated solar illumination. In contrast, thermally processed Pt CEs showed only 2.71% rear PCE, illustrating the strong impact of synthesis method on bifacial performance.
The initial exploration of graphene-based materials (e.g., graphene nanotubes (GNTs), graphene nanoplatelets (GNPs), functionalized graphene sheets (FGSs), etc.) as transparent CEs in DSSC environments can be traced back to the work of Hong et al.98 Subsequent research has shown that combining two distinct carbon materials, such as carbon nanotubes (CNTs) and graphene, can enhance the electrochemical activity of the CE.99,100 CEs based on micron-thick graphene films have exhibited charge transfer resistances (RCT) comparable to those of Pt-based CEs, with values potentially less than 1 Ω cm2 for the reduction of I3−.101 Kaven et al.,102 while studying the electrochemical performance of graphene-based CEs in DSSCs using Cu(II/I) based electrolytes, reported remarkable catalytic activity, comparable to electrodeposited PEDOT catalysts and superior to Pt catalysts. When graphene-based CEs were incorporated into advanced DSSC configurations, PCE values as high as 14.7% were achieved,13 surpassing the previous PCE record of 13%.103
Bu and his team104 demonstrated the potential of transparent carbon-based CEs in the BFDSCs domain. Employing a straightforward and cost-effective “in situ carbonization” method and carefully adjusting the precursor's concentration/composition, they fabricated carbon-based CEs with excellent optical transmittance across the 400–800 nm wavelength range. These CEs also exhibited acceptable conductivity (RS = 4.14 Ω) and catalytic activity towards I3− reduction (RCT = 12.5 Ω cm2). Their BFDSCs achieved PCE values of 5.04% and 6.07% under rear- and front-side illumination, respectively, with a BFF of 83%.
In 2015, Xu et al.105 introduced chemical vapour deposition (CVD)-derived doped Graphene Films (GFs) as promising transparent CEs for BFDSCs (Fig. 4e). Specifically, they fabricated nitrogen-doped (N-GF), sulfur-doped (S-GF), and dual-doped nitrogen/sulfur (N/S-GF) films on FTO substrates using the CVD method. These doped GFs were used as CEs in DSSCs with an I−/I3− redox couple and N719 sensitizer. The prepared doped GFs exhibited excellent AVT in the 350–1000 nm range, with optical transmittance values at 550 nm of 87.96%, 84.93%, and 87.05% for S-GF, N-GF, and N/S-GF, respectively. DSSCs with these CEs displayed front and rear PCE values of 3.86%, 3.74%, 4.22% and 3.19%, 3.07%, 3.45%, respectively, leading to BFF values of 83%, 82%, and 82% for S-GF, N-GF, and N/S-GF. Although the doped GFs exhibited higher RCT values compared to the Pt-CE (RCT = 2.70 Ω cm2), they all demonstrated significant electrocatalytic activity at the electrode/electrolyte interface. The RCT values were 2.85 Ω cm2, 5.30 Ω cm2, and 25.83 Ω cm2 for N/S-GFs, N-GFs, and S-GFs, respectively.
Yang et al.,106 utilized N-GFs, grown by CVD method, as transparent CEs in BFDSCs (Table 2). Their experimental results proved superior charge transfer at the graphene/electrolyte interface compared to untreated graphene, reflecting better electro-catalytic activity. It is well recognized that, electrical conductivity as well as catalytic activity are affected and often enhanced by nitrogen atom introduction into the graphene hexagonal lattice, giving rise to additional electron/ion pair as well as structural deformation via local strains.107,108 The BFDSCs based on N-GFs as CEs exhibited better thermal stability together with higher transparency and BFF (83%) compared to Pt-CE (58%) and PCE value ∼3 times higher than the devise using pristine graphene CE.
In 2020 Ou109et al., with the aim to realize transparent high-performance and low-cost Pt-free CEs, realized a Co2+/Co3+ mediated BFDSCs with carbon nanosheets as CE. Co2+/Co3+ redox mediator is lighter in colour compared to I−/I3− redox shuttles and exhibits weaker absorption in the 390–480 nm λ range. Co-mediated BFDSCs, using synthesized Cu2−xSe@N-doped carbon nanosheets CEs, reached PCE values of 7.61% and 5.82% under front and rear irradiation (Table 2), respectively. The obtained PCE values were comparable to those of standard Pt based device. The resulting excellent PCErear value has been attributed to the weak absorption of the Co2+/Co3+ electrolyte, as well as to the high optical transparency of the Cu2−xSe@N-doped carbon nanosheets and its outstanding catalytic ability (RCT = 4.4 Ω cm2) to reduce Co3+ ions. The large SSA110 and the uniform dispersion of Cu2−xSe nanoparticles provides numerous catalytic sites for Co3+ reduction, offering the desired superior electrocatalytic ability.
With a focus on electron density enrichment at CE surface, in order to enhance its catalytic performances without sacrificing the transparency, Duan and co-workers114 developed an arrangement of tandem carbon quantum dots (CQDs) structure with CoSe to produce transparent CEs for BFDSCs. The excellent ability of CQDs to photo-generate electrons upon excitation from 350 nm to visible light enables their applications in photocatalysis. Additionally, their capability to act as electron mediators enhances the photocatalytic activity of other photocatalyst.115 Due to these unique properties the manufactured CQDs-CEs showed in visible-light region optical transparency as high as ∼70%. The assembled BFDSCs exhibited superior catalytic activity of the CQDs-CE and achieved PCE values of 8.54% and 6.55% for front and rear illumination, respectively, outperforming BFDSCs utilizing CoSe-based CEs (Table 2).
A quite similar strategy was also proposed by Zhang et al.116 who reported BFDSCs with transparent sulphur-doped carbon quantum dots (S-CQDs)/metal selenide CEs. By a mild solution route and electrodeposition onto transparent metal selenide, CEs were prepared for high-performance BFDSCs applications. A well distribution of S-CQDs favoured light harvesting as well as fast electron transport from S-CQDs to catalyst, elevated the catalytic activity upon rear illumination testifying high electron concentration at CE surface. Using standard Pt-based CE, the DSSC yields a BFF of 56% while, by integrating S-CQDs with Pt CE, the final light-inducing S-CQDs/Pt tailored bifacial device increments the BFF to 62%. The maximized front and rear efficiencies are 9.15% and 6.26% for b-SC with Pt-free SCQDs/RuSe CE, respectively. The rear efficiency of 6.26% reported here116 is in a very high level among state-of-the-art bifacial DSSC platforms. High PCEs and catalytic behavior of a Pt-free CE has been reported in DSSCs employing Ru–Se based CEs (RuSe, Ru2Se, RuSe2, RuSe3), optimized by tuning via electrodeposition both stoichiometric Ru/Se ratio and bilayer number.117RCT values of 7.53, 1.68, 8.61 and 41.02 Ω cm2 for Ru2Se, RuSe, RuSe2 and RuSe3 electrodes, respectively, revealed impressive catalytic activity of Ru–Se based CE. Lower RCT implies lower resistance during charge diffusion processes and faster catalytic kinetics.118 The superior catalytic activity of Ru–Se CE toward I3− reduction, and its high transmittance (> 0%) produced, in the corresponding BFDSC, PCEs of 8.72% and 5.9% with a BFF ∼ 68% (Table 2). Liu et al.119 synthesized via hydrothermal method, CEs based on ternary (CoM)0.85Se (M = Ni, Ru, Fe) alloys. The constructed CEs showed good AVT, reaching 80% for (CoNi)0.85Se based CE, which was benefit for the light harvesting from device rear side. All DSSCs based on the manufactured CEs, showed impressive PCEs for both front and rear sides, higher compared to the reference DSSC based on Pt-CE (PCEfront 6.72%, PCErear 3.16%). In details, DSSCs based on (CoNi)0.85Se, (CoRu)0.85Se, (CoFe)0.85Se alloy CEs displayed PCEfront of 9.16%, 8.09%, 7.58% and PCErear of 3.86%, 3.31%, 3.51%, respectively. Quasi-solid-state BFDSCs utilizing CoSe2 nanorods (approximate lengths and widths of 70–500 nm and 20–60 nm, respectively) as CEs and polyvinylidene fluoride (PVDF) as quasi-solid-state electrolyte, have been proposed by Xia et coworkers120 to improve PCE and long-term stability. PVDF and CoSe2 were chosen owing to the good chemical stability and excellent optical transparency and flexibility of the former,121 and high electrocatalytic ability for I3− reduction of the latter.122 CoSe2 based CE showed a remarkably lower RS value (2.84 Ω) compared to Pt based CE (4.35 Ω), testifying its smaller bulk resistance, higher conductivity and easier electron transfer capability. Also, the RCT value (2.54 Ω cm2), representing the CE/electrolyte interface charge transfer velocity, was smaller than that of the Pt based CE (3.07 Ω cm2), demonstrating superior electrocatalytic reduction activity.
The resulting BFDSCs reached the PCE values for the front and rear irradiation of 8.02% and 4.22%, respectively (BFF = 50%). In addition, the CoSe2 based CE exhibited rapid photocurrent response and long-term photovoltaic stability.
DSSC-based on highly transparent MoS2 based CEs, directly electrodeposited on FTO glass substrates via potential-reversal (PR) deposition mode, have been proposed by Chang et al.123 The assembled CEs showed good transparence (∼80%) and power/weight ratio together with high electrocatalytic activity, mainly attributed to a removal of Mo excess by implementing anodic bias as well as to the high surface area of the uniform thin layer. The assembled with PR-MoS2 based CE reached satisfactory PCEs of 8.77% and 4.82% (BFF = 55%), rather equivalent with those of Pt reference device (9.01% and 5.67%) (Table 2).
Conducting polymers like PANI, PEDOT and PPy (Fig. 4g) have attracted great interest as Pt-free CEs owing to their facile synthesis, good conductivity, tunable morphology, and potential transparency.124,125 However, their catalytic activity is generally lower than Pt, so they are often combined with other nanomaterials. Polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT) and polypyrrole (PPy) are among the most intensively studied conducting polymers for DSSC applications.
Aniline type conducting polymers included several analogous. Discovered over 150 years ago, PANI have attract more and more interest in the PV community in the past 50 years, owing their outstanding properties which could benefit the advancement of BFDSCs, offering appreciable PCEs from both rear- and front-side of the device. In addition, PANI can be synthesized in various porose nanostructure with different morphologies and high SSA, improving the catalytic activity.126
In 2011,35 transparent PANI based CEs was synthesized by “in situ” polymerization of aniline monomers on FTO glass substrate (Fig. 4h). The grains of PANI films appeared homogeneous and smooth, with grains size of ∼250 nm, composed of smaller granules with diameters of ∼20 nm and cauliflowerlike-shaped. This kind of morphology, allowing high SSA, supported the favourable PANI's catalytic activity. Moreover, the PANI's thin film showed excellent transparency in the 450–750 nm λ range with a maximum transmittance of ∼74% at ∼510 nm and PCE of ∼4.26% illuminated from rear (BFF ∼65%). Later Wu and colleagues,127 using modified PANI with 4-aminothiophenol (4-ATP/PANI), as transparent electrocatalytic CE for BFDSCs, reporting PCE values of 6.70% and 4.15% (BFF of 62%), and 8.35% from both-sides. 4-ATP was used to diminish the unfavourable PANI/FTO interfacial resistance and accelerate the charge transport between them. The device high PCE, was attributed to fine microporous structure, high transmittance, good electrocatalytic activity to I3− reduction, low charge transfer resistance on electrolyte/electrode interface of the 4-ATP/PANI based CE. In the design of CEs, various chemical dopants including SO42−, ClO4−, BF4−, Cl− p-toluenesulfonate (TsO), etc.,128 have been incorporate or embed with PANI polymer chain in order to improve the PCE of PANI BFDSCs. As an example, SO42− doped PANI film exhibited porous morphology, higher reduction current for the I3− reduction and lower RCT (1.3 Ω cm2) than the Pt based CE (2.3 Ω cm2).129
PEDOT is a further frequently used conducting polymer suitable for large scale and cost-effective productions with excellent transparency and conductivity (300–500 S cm−1),130 much higher than that of various counterparts (e.g., PANI, polypirrole (PPy), polythiophene, etc.). However, its catalytic activity remains lower compared to that of Pt based CE or other metals-based CEs. Therefore, other catalytic materials have been combined with PEDOT to better utilize its superior properties in BFDSCs development. Xu et al.131 reported highly transparent PEDOT:PSS films (78% ≤ Tr ≤ 83.5% at ∼550 nm), prepared by spin coating and treatment with small amount of additives (iodine, IOD, DMSO and PEG200-) for CE's catalysts use in BFDSCs configurations.
The constructed BFDSCs, reached PCEs ranging from 4.36% to 5.29% and from 2.36% to 2.62% for front and rear illumination, respectively, and BFF varying from 45 to 55%, depending on the transmittances of the modified PEDOT:PSS films. The PCE values of the modified PEDOT:PSS films has been attribute to a high surface roughness, responsible of an enhanced catalytic activity. Moreover, the BFDSCs with the modified PEDOT:PSS films showed comparable PCEfront to that of conventional Pt based DSSCs, but higher PCErear than the latter (1.66%). Xu et al.132 for the first time, synthesized transparent organic/inorganic hybrid composite films MoS2/PEDOT with the aim to merge the conductivity and electrocatalytic ability of the two components. Nanosized MoS2 synthesized by hydrothermal method were deposited onto an FTO glass substrate by spin-coating. Then, on the top of the obtained MoS2 layer, PEDOT film was electrochemically polymerized to form the composite MoS2/PEDOT CEs. MoS2 as transition-metal compound, exhibits excellent conductivity and catalytic properties, moreover, it improves the continuity of the PEDOT film and reduces the pores at PEDOT film surface, improving the conductivity of the PEDOT/MoS2 composite film. BFDSCs using MoS2/PEDOT composite CE exhibited PCEfront of ∼7% and PCErear∼ of 4.82% (BFF ∼ 70%) (Table 2) together a good transmittance (∼70%) at ∼510 nm. Finally, the measured RS (5.26 Ω), proved the excellent conductivity and catalytic properties of the composite CEs.
In 2018, Kang et al.133 preparing DSSCs consisting of PEDOT based CE, Co(bpy)33+/2+electrolyte and Y123 dye, reached PCErear higher of ∼82% compared to that of reference Pt based CE under front-illumination. The constructed device achieved the remarkable PCE values of ∼8.65% and ∼7.48% with a BFF of ∼85%.
PEDOT CEs for indoor applications, synthesized via electrochemical deposition onto FTO glass, have been recently proposed by Venkatesan et al.134 By optimizing amount, polymerization time and thickness (90 nm) of the PEDOT layers, the prepare CEs showed higher Tr (∼70%), ion diffusivity (4.34 × 10−9 cm−2 s−1) and ionic conductivity (5.41 × 10−5 S cm−1), as well as low RCT (2.38 Ω cm2) at the CE/electrolyte interface than those of the reference cell (Pt-based CEs). Front and rear PCEs of 24.16% and 22.45%, respectively, (BFF = 93%) were obtained for DSSC using the CE with 90 nm thickness PEDOT layer, under light illumination of 200 lux (Table 2).
Polypyrrole PPy, is another class of conducting polymers that has attracted interests in DSSC technology. Produced by the pyrolysis of tetrahydropyrrole gives rise to highly conductive materials. Differently by their doped film which are blue or black, the pristine PPy films appear yellow but darken in air due to oxidation. In DSSCs ambient, the main issues of PPy CEs are correlated to their high RCT, and no good enough conductivity,124 which in turn are correlated to the morphology as well as to the synthesis procedure. In this framework, chemical dopants, incorporate or embed with PPy polymer chain, to enhance the PPy conjugation are often used.135,136 Wu et al.,137 first reported the use of PPy as CE materials in DSSCs in 2008. Bu et al.138 prepared CEs for BFDSCs, by in situ polymerization of PPy monomer on FTO glass substrate. The BFDSCs based on these CEs showed PCEs of 5.74% and 3.06% front- and rear-side illumination, respectively, improving those of a reference Pt-based DSSCs.
The opto-electrochemical features of PPy films displayed a transmittance that gradually increases with decreasing the monomer concentration vs. a JSC which follows the reverse behaviour, and RCT (2.46 Ω cm2) comparable to that of Pt (2.53 Ω cm2), proving an adequate catalytic activity, thanks to PPy films SSA and porous structure.
Wang et al.139 study showed that FeS2 nanocrystal (NC) ink is a promising, cost-effective alternative. It exhibits excellent electrochemical catalytic activity and stability in reducing triiodide to iodide. Recently, Yao and co-workers,140 combined pyrrole with MoS2 by a reflux method and next “in situ” polymerization, to make highly transparent PPy-MoS2 based CEs.
By comparing the as prepared CEs with those without MoS2 addition, a larger maximum bifacial PCE of ∼9.48% under both-side irradiation was observed.
MoS2 is characterized by high transparency and large SSA which provides a large number of I3− reduction active sites and favours electron transfer as well as excellent catalytic properties.141
In addition, the covalent bonds between N and Mo atoms, allows to control the grow of PPy onto the MoS2 surfaces as well as the CE transparency. Moreover, PPy and MoS2 as electron donors and electron acceptors, respectively can form a conductive matrix by antibonding interactions Modπ/Npπ which increases the electron delocalization and the PPy conductivity. The higher optical transmittance (∼70% in visible region) and the best PCEfront and PCErear, were obtained employing PPy-4 wt%MoS2 complex CE. Fig. 5 aims to gather the best proposed counter-electrodes in the literature in terms of transmittance and BFF obtained values. In Table 2, we report a summary of promising semi-transparent CE and their PV performance with all detailed information regarding WE, dye and electrolyte.
3.3 Transparent electrolyte
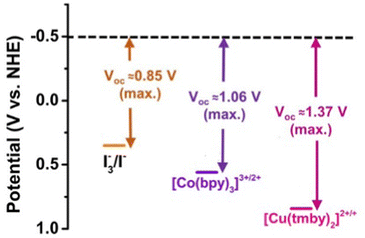
In the quest for sustainable energy solutions, electrochemical systems, and photovoltaics, particularly DSSCs, have been focal points of scientific exploration. A critical component in DSSCs is the electrolyte, which facilitates redox reactions, regenerates the oxidized dye, and maintains charge neutrality by transferring electrons to the counter electrode. The performance of DSSCs is closely related to the viscosity, dielectric properties and conductivity of the electrolyte, which determine the rate and quantity of charge transfer. Adjusting the proportion of electrolytes, particularly the concentrations of redox couple, can significantly impact the performance of DSSCs. For instance, increasing the concentration of the reducing specie from 0.1 to 0.8 mol L−1 can effectively improve the JSC and consequently the PCE of the cell. However, the VOC remains almost unchanged with variations in concentration of redox couple.142 The iodide/triiodide (I−/I3−) redox couples, or iodine-based electrolytes (IBEs), are frequently used due to their favourable kinetics and high carrier collection efficiencies, despite drawbacks such as low redox potential, corrosive tendencies, and competitive blue light absorption.143 Indeed, the transmittance spectrum of IBEs typically shows UV-Visible absorption ranging from 300–500 nm, contingent upon solvent composition and solution concentration. Yet, this attribute might sometimes compromise the PCE of BFDSCs during rear illumination. This is due to potential obstruction of optical penetration and light absorption by the IBE. Functioning like a filter, the IBE may obstruct the blue-green light region while enabling the transmission of red light. Despite this, these impediments can be effectively addressed through astute optimization strategies.144 These strategies are expected to include enhanced optical transparency at ideal conductive concentrations, superior physical and chemical attributes such as solubility, non-volatility, and thermal stability, and advancements in cumulative PCE and BFF under rear illumination. To address these challenges, there has long been a growing interest among researchers to investigate transition metal complexes as promising alternatives for redox mediators.145The liquid electrolyte (LE) such as IBE, presents some problems for long term stability and device sealing in addition to those mentioned above. The use of solid state146(SSE) or quasi solid68 electrolytes (QSSE) is encouraged to overcome these limits also in BFDSCs. The development of DSSC using polymer-based electrolytes, for example, such as a gel gives rise to a quasi-solid-state DSSC, and the use of a pure polymer electrolyte (without solvent), gives a solid-state DSSC.147 Electrolytes based on both organic solvents and ionic liquids can be gelated, polymerized, or dispersed with polymeric materials. Both types of liquids have been used as starting materials, and the inclusion of gelating or polymeric agents transforms the electrolyte into a quasi-solid electrolyte.148 Typically, these quasi-solid electrolytes show conversion efficiencies that are lower than that of the liquid redox electrolyte. This effect can be attributed to limitations in the mobility of the redox couple components in QSSE. Another strategy for the development of solid-state devices involves the use of transition metal complexes as an alternative redox mediator to the iodine–iodide couple. These complexes offer elevated redox potentials, corrosion resistance, and could be modified by change the ligands to find a solution to the blue light absorption issue of I−/I3− couples, which could lead to notable efficiency improvements in DSSCs.149 In particular, some transition-metal complexes such as Co3+/Co2+ (complex) and Cu2+/Cu+ (complex) have been employed and investigated as redox shuttles in DSSC electrolytes.150,151 The optical redox properties of these complexes are pivotal for enhancing the PCE in BFDSCs. Given the bifacial configuration of BFDSCs, the incorporation of materials with superior transparency is crucial to ensure effective light propagation to the photoactive layer from both directions. Some of these transition metal complexes, because of their lower light absorbance, can play an essential role in BFDSCs as highly transparent electrolytes. Furthermore, they offer significant advantages in aesthetic and integrated applications such as BIPV, urban-street PV applications, automotive industry, and more, where light management and the transmission/absorption properties under rear-light illumination are crucial.152,153 Cobalt polypyridine salts are noteworthy for their high electrocatalytic activity towards the reduction of triiodide, making them attractive candidates for DSSCs. Equally, copper polypyridine salts present similar electrocatalytic capacities, thereby underlining their increasing importance in DSSC research and application.145 The design and optimization of cobalt and copper polypyridine complexes are guided by the principles of ligand field theory. This theory elucidates the manner in which interactions between the metal ion and surrounding ligands affect the energy levels of the metal ion's d orbitals, in turn influencing the chemical and physical properties of the complex. These cobalt and copper polypyridine complexes have been the focus of extensive research over recent years, considering their promising applications as electrolytes in DSSCs. These complexes have different absorption spectra, which can be attributed to the different electronic configurations and relative energy levels of the two ions.
In the case of cobalt polypyridine complexes, the cobalt ion Co+2 has a d7 electronic configuration, when the ligand field is weak, it has five electrons in the t2g orbitals and two electrons in the eg orbitals resulting in a high-spin configuration. Beside when the ligand field is strong, only one electron occupies the eg orbitals, resulting in a low-spin configuration as reported in Fig. 6. A notable drawback of Co complexes is the slow mass transport and substantial reorganization between d7 (high-spin) and d6 (low-spin) states, which confines the VOC to a maximum of 1 V. In the case of copper polypyridine complexes, the copper ion has a d9 electronic configuration, which means that it has one electron in the t2g orbitals and two electrons in the eg orbitals. The remaining six electrons can occupy either the t2g or the eg orbitals, again depending on the ligand field strength. When the ligand field is weak, the six electrons occupy the eg orbitals, resulting in a high-spin configuration. However, when the ligand field is strong, the six electrons occupy the t2g orbitals, resulting in a low-spin configuration. The difference in the electronic configuration and relative energy levels of the d orbitals of cobalt and copper ions can be explained by the ligand field strength of the surrounding polypyridine ligands. The stronger ligand field of the polypyridine ligands in cobalt polypyridine complexes results in a larger energy separation between the t2g and eg orbitals of the cobalt ion, which leads to a stronger MLCT band in the absorption spectrum. On the other hand, the weaker ligand field of the polypyridine ligands in copper polypyridine complexes results in a smaller energy separation between the t2g and eg orbitals of the copper ion, which leads to a weaker MLCT band in the absorption spectrum. In conclusion, the application of ligand field theory to cobalt and copper polypyridine complexes provides insights into the electronic structure and properties of these complexes. Here we report some of the important history of these two families of ions salts culminated in the production of remarkably efficient DSSCs, as evidenced by the selection of some DSSCs with PCE ≥ 12%144 featured in Table 3.
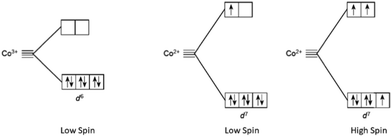 | ||
| Fig. 6 High spin and low spin cobalt ions configuration for Co2+ and Co3+ in presence of field ligand. | ||
| EL | DYE | PCE (%) | Ref. | Year |
|---|---|---|---|---|
| Cu(tmby)2+/2+, LiTSFI, CEMI | SL9/SL10 | 15.2 | 169 | 2022 |
| I2, LiI, TBP | Y123 | 12.7 | 145 | 2022 |
| Cu(tmby)2+/2+, LiTSFI, MBI | MS5/XY1b | 13.5 | 14 | 2021 |
| Co(bpy)32+/3+, LiTSFI, TBP | SGT-149/SGT-021 | 14.2 | 168 | 2020 |
| Cu(tmby)22+/3+, LiTSFI, NMB | R7/Y123 | 12.7 | 167 | 2020 |
| Cu(tmby)22+/3+, LiTSFI, MBI | XY1b/Y123 | 13.1 | 166 | 2018 |
| Co(bpy)32+/3+, LiClO4, TBP | SGT-021-HCA4 | 12.0 | 150 | 2017 |
| Co(phen)32+/3+, LiClO4 | ADEKA-1/LEG4 | 14.3 | 13 | 2015 |
| Co(phen)32+/3+, LiClO4 | ADEKA-1/SFD-5 | 12.8 | 160 | 2015 |
| Co(bpy)32+/3+ | GY50 | 12.7 | 159 | 2014 |
| Co(bpy)32+/3+, LiTSFI, TBP | SM371 | 12.0 | 103 | 2014 |
By adeptly coordinating the properties of the dye and cobalt redox mediator, they successfully circumvented constraints introduced by recombination and mass-transport. The researchers adopted organic dyes D29 and D35, which possess greater extinction coefficients than conventional ruthenium sensitizers. This allowed for the incorporation of thinner TiO2 films.157
They introduced insulating butoxyl chains onto the dye, a strategy that curtailed recombination and permitted the utilization of redox pairs with superior diffusion coefficients and optimally suited redox potential. This strategic approach resulted in enhanced photocurrent and photovoltage. Crucially, the team minimized the recombination and mass transport issues typically associated with the electrolyte by expertly adjusting the steric bulk of both the dye and the redox mediator.
They examined a series of cobalt complexes featuring distinct redox potentials as one-electron outer-sphere redox mediators in DSSCs. Cobalt complexes, including [Co(bpy)3]3+/2+, [Co(dmb)3]3+/2+, [Co(dtb)3]3+/2+, and [Co(phen)3]3+/2+ demonstrated standard redox potentials of 0.56, 0.43, 0.43, and 0.62 V, respectively, all utilizing hexafluorophosphate (PF6−) as the counterion. Theoretically, the VOC value of the devices should increase along with the cobalt complexes' redox potential. However, an optimal VOC and JSC were achieved from the [Co(bpy)3]3+/2+ redox couple due to the optimal driving force for dye regeneration, diminished mass transport restriction attributable to the less bulky [Cu(bpy)3] complexes, and recombination reduction due to the inclusion of steric bulk in the D35 dye.156 Overall, among investigated dyes the D–π–A structure-based Zn–porphyrin sensitizer emerges as a promising dye for cobalt complex redox electrolytes, illustrating the potential to achieve remarkable efficiencies. The cobalt complex [Co(bpy)3]3+/2+(B(CN)4)−3/2 exhibits a standard redox potential of 0.57 V (vs. NHE) and is composed by a bidentate ligand, 2,2′-bipyridine (bpy), and tetracyanoborate (B(CN)4)− as the counterion. Employing this redox couple and the donor–π-bridge–acceptor (D–π–A) structured Zn porphyrin dye YD2-o-C8 with the addition of an organic dye has been achieved the efficiency to 12.5% under simulated 1-sun (AM 1.5G) illumination.158 The dye YD2-o-C8, complete with octyloxy groups, allows slower recombination of the injected electron in TiO2 with Co3+(bpy)3 and absorbs light across the entire visible range. Employing the D–π–A structured Zn–porphyrin SGT-021 dye and cobalt complex redox shuttle [Co(bpy)3]3+/2+(B(CN)4)−3/2, Kang et al. achieved a 12.1% efficiency under 1-sun illumination.150 Yella et al. with Co(bpy)32+/3+ and GY50 achieved a PCE of 12.7%.159
Mathew and co-workers achieve a PCE of 12% and 13% with Co(bpy)32+/3+ and SM371 and SM315 respectively103 Kakiage et al with Co(Cl-phen)32+/3+ and ADEKA-1 achieved a PCE of 12.5%13 while co-sensiting with SFD5 and employing Co(phen)32+/3+ measured a PCE of 12.8%.160 Finally combining ADEKA-1/LEG4 and Co(phen)32+/3+ found a PCE of 14.3%13 (Table 3).
Cong et al.164 discovered that the device using the [Cu(bpye)2]2+/+ redox shuttle resulted in higher power conversion efficiency (PCE) and open-circuit voltage (VOC) than Cobalt redox electrolytes. This outcome was primarily due to the [Cu(bpye)2]2+/+ complex's more positive redox potential (approximately 0.59 V). The [Co(bpy)3]3+/2+ redox pair, while useful, posed a challenge in terms of mass transport that could possibly be addressed by altering the ligand of the Co complex to bpye. Pradhan et al.,165 through their research using various redox electrolytes, found that the [Cu(dmb)]2+/+ redox shuttle presented less mass transport issues, better diffusion, and required a relatively low driving force (around 0.1 V) for efficient dye regeneration when compared to the [Co(bpy)3]3+/2+ redox shuttles. Recently, studies have shown promising results with the use of copper complexes in combination with additives to improve the stability and performance of copper-based DSSCs.145
Cao and his team166 achieved remarkable performance by enhancing the light-harvesting efficiency through co-sensitization of two D–π–A structured organic dyes, namely Y123 and XY1b, combined with the co-adsorbent CDCA. Notably, they also achieved a high fill factor (0.79) for copper electrolytes, attributable to improved mass transport brought about by a high concentration of the redox pair and the absence of TiCl4 post-treatment. Their unique direct contact device design allowed the redox shuttle to diffuse through TiO2 nanopores, significantly reducing the Warburg resistance by shortening the diffusion path.
In 2020, Ren's team documented a DSSC boasting an efficiency of 12.7% by leveraging the [Cu(tmby)2]2+/+ redox electrolyte in conjunction with co-sensitized organic blue dye R7 and Y123 dye.167 Subsequently, in 2021, Zhang and his colleagues utilized the [Cu(tmby)2]2+/+ redox mediator in conjunction with the D–π–A structure-based organic dye MS5.14
Owing to the higher positive redox potential of the [Cu(tmby)]2+/[Cu(tmby)]+ complexes (approximately 0.87 V versus NHE), several high-efficiency porphyrin dyes such as SGT-021 (EHOMO ≈ 0.82 V),150 SM315103 (EHOMO ≈ 0.89 V) and some organic dyes including SGT-149 (EHOMO ≈ 0.84 V)168 cannot be investigated with this Cu redox shuttle because of the insufficient driving force for dye regeneration, in particular with SGT-149, a hexyloxy-phenyl substituted fluorenyl derivative, was demonstrated high PCEs of 11.7% with a [Co(bpy)3]2+/3+ redox electrolyte. Moreover, co-sensitization of SGT-149 with SGT-021 porphyrin dye, achieved through a simple “cocktail” method, yielded an outstanding PCE of 14.2% with the same cobalt-based electrolyte.
Recently, the use of a Cu complex, the [Cu(tmby)2]2+/[Cu(tmby)2]+ redox shuttle, has been reported to achieve a power conversion efficiency (PCE) of 15.2% under 1-sun illumination with VOC over 1 V in DSSCs.169 While this redox shuttle significantly enhanced VOC, further improvement in the JSC is challenging. This difficulty arises due to the competition between Cu complex absorption and the need for a dye with a higher positive HOMO level in the visible light spectrum, which restricts complete utilization of the entire visible light range. For the future there is a need for research to identify an appropriate redox shuttle with a redox potential ranging between 0.45 and 0.65 V, or alternatively, to modify a ligand to adjust the redox potential of Cu complexes, enabling the use of most porphyrin and organic dyes.
Concluding, the prevailing role of iodine as the redox mediator in DSSCs is being increasingly challenged. The drive for enhanced stability and cost-effectiveness has spurred considerable research efforts towards iodine-free redox couples. Transition metal complexes, notably copper and cobalt-based couples, have emerged as compelling alternatives, demonstrating favourable electrochemical attributes and resilience.
The efficiency of DSSCs hinges significantly on redox kinetics, or the speed at which electron transfers occur within the redox pair. Thus, a thorough understanding and optimization of these dynamics remains integral to enhancing DSSC performance. In Fig. 7 are reported the potential diagram for I3−/I−, [Co(bpy)]3+/2+ and [Cu(tmby)2]2+/+.
Concurrent with the rise of bifacial DSSCs is the promising exploration into iodine-free redox pairs, a research direction showing great potential to boost stability and reduce the overall expense of DSSCs. Copper and cobalt-based redox couples, showcasing both advantageous electrochemical characteristics and robustness, are promising contenders to replace iodine. In Table 3, we summarize the main outstanding PV performances of DSSC reporting the utilized dye and electrolyte.
3.4 Working electrode (WE)
After 30 years of study, the most efficient utilized wide band gap semiconductor in a DSSC WE is TiO2,170 in anatase phase, with nanocrystalline nanoparticles at high porosity. Titanium oxide is white, insoluble in water and, as a dye, finds a wide range of application in paintings, sun-creme, food colouring. Early investigations focused on the optimization of the TiO2 layer in terms of particle sizes, film porosity, TiCl4 treatment,53 thickness, doping171,172 and introduction of a reflecting layer with the aim of scattering light (SL).53 However, some properties of TiO2 are not ideal.173 With a band gap of 3.2 eV, TiO2 anatase absorbs to wavelengths below 390 nm, generating highly reactive holes that can induce destructive oxidation reactions with organic components of DSSCs. To counteract these detrimental effects and ensure long-term stability under full sunlight exposure, UV filters are commonly employed in DSSC device studies. Additionally, incorporating an ultrathin layer of Al2O3 or MgO can effectively minimize the photocatalytic action of TiO2.174Employing a mesoporous semiconductor with a wider bandgap offers distinct benefits. SnO2, with a bandgap of 3.6 eV, absorbs less UV light from the solar spectrum compared to TiO2. While SnO2 has been successfully implemented in DSSCs, its conduction band lies approximately 0.5 V more positive than that of TiO2, hindering its performance due to reduced voltage output. Coating SnO2 with an ultrathin metal oxide layer, such as ZnO, Al2O3, or MgO, significantly enhances the voltage output.174,175 ZnO has been extensively investigated as a nanostructured electrode in DSSC devices, with a wide range of morphologies explored.176 Despite purported advantages in electron transport properties, ZnO has not demonstrated superior performance compared to traditional mesoporous TiO2 electrodes. This is attributed to electron transport not being a limiting factor for DSSCs under operational conditions (at the maximum power point).173 While other metal oxides can serve as mesoporous electrodes in DSSC devices, their performance generally lags behind that of TiO2 counterparts.
In BFDSCs, that absorb light from both front and rear sides, additional factors must be considered in TiO2 photoanode design to maintain transparency while ensuring adequate electron transport.
The TiO2 film thickness is a critical component that impacts DSSC parameters. An increase in TiO2 thickness results in a loss of internal resistances at the TiO2/dye/electrolyte interface; consequently, the electron lifetime enhances, leading to improved electron diffusion and transport, and therefore overall PCE growth. On the contrary, exceeding TiO2 thickness causes electronic recombination that negatively interferes with DSSC performance. The TiO2 paste contains mostly 15–20 nm nanoparticles, and a precise formulation is needed when high PCE and transmittance are required. With a few microns of semiconductor layer, 75–80% light transmittance is attained in the visible spectrum.177 In an early work of Tagliaferri et al.,177 it is evident how the TiO2 thickness and the immersion time impacts in the colour tonality therefore on the transmittance of the photoanode (Fig. 8a). However, when the TiO2 thickness and the dye loading increase, light transmission decreases, according to Lambert–Beer law, whilst the efficiency of the corresponding cells rises. On the other hand, a strong dependence on the dye type was observed.
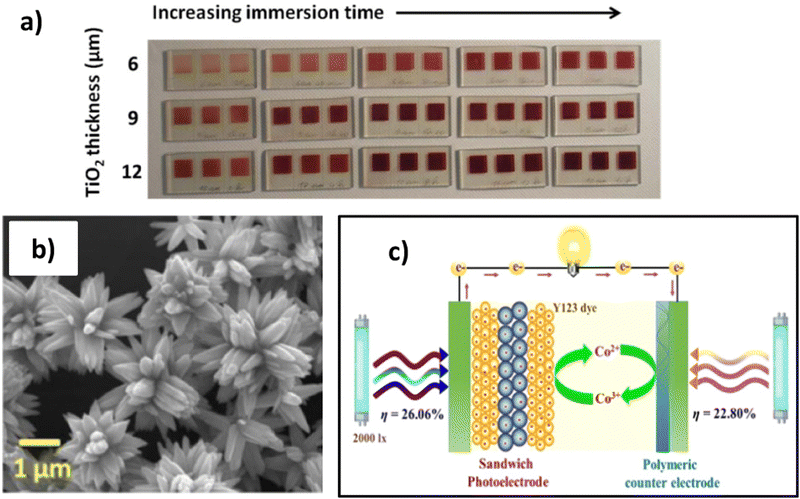 | ||
| Fig. 8 (A) Comparison of fabricated WE with N719 dye considering the TiO2 thickness and the immersion time. Reproduced and adapted from ref. 177. (B) SEM image of ZnO micro-flowers applied as SL from ref. 179 under Creative Commons BY-4.0 license (c) sandwich photoelectrode structure realized by ML–SL–ML from ref. 134 Copyright © 2022 American Chemical Society. | ||
Dyes with absorption peaks shifted with respect to the eye sensitivity factor of the photopic view's observer (eye responsivity) can perform better in term of transparency vs. efficiency. Larger TiO2 nanoparticles (>100 nm) are typically applied in a diffusive scattering layer, which is an effective collector of solar intensity within the device. When a SL is present in a TiO2 stack, the photocurrent significantly improves, but a reduction in transparency is evident.178 In 2008, a pioneering work by Ito et al.,53 authors manage the TiO2 thickness to meet an optimum between max PCE and BFF reporting a considerable BFF of 91%. The new architecture is based on a mesoporous TiO2 layer sensitized with a high stable Ru dye (Z907Na) in combination with an ionic-liquid (IL) electrolyte and SiO2 layer between the electrodes to prevent unwanted back currents. Their device showed a maximum of PCE ∼ 6.54% for incident light hitting front or rear surface. Further structure detailed are in Table 2.
Recently, with the use of an organic yellowish dye, Y123, Professor Lee's group38 aimed to optimize device's parameters such as TiO2 and platinum thickness and electrolyte composition to develop a BFDSC device.
Concerning the scaffold study, they found 6 μm of main TiO2 layer (ML) and 4 μm of SL as optimal in terms of efficiency for Y123 dye. The exploitation of a scattering layer is not always suitable for a bifacial device; therefore, they opted for 8 μm of main layer to attain satisfactory results (from the front and rear side) reaching a BFF of 83%.38
However, TiO2 as semiconductor can be always considered an appropriate material for a BFDSC and the only issue concerns the SL application. As previously reported,38 a thick SL is not suitable for a BFDSC.
Recently, through a surface engineering of the semiconductor layer, ZnO micro-flowers were added with the aim to scatter the light without harming the AVT179 (Fig. 8b). The negative shift of the TiO2-ZF conduction band leads to minor transport time and to mitigate recombination effect. The total BFF reached the 70% value proving the transparency of the ZnO layer. This may be a promising frontier for further application. Moreover, a newly developed sandwich photoelectrode (ML 4 μm-SL 3.5 μm-ML 4 μm) has been utilized for reaching a BFF of 93% under 200 lux with a max PCE of 24.16. This new structure is constructed by introducing an additional ML behind the SL of the traditional DSSC structure (ML/SL), creating a sandwich (ML/SL/ML) architecture of the TiO2 film (Fig. 8c). Results demonstrate rear illuminated light can be harvested more efficiently by this photoelectrode because the light is first absorbed by the final ML before the SL effect.134,180
However, the choice of dye significantly impacts light transmittance, while bifaciality remains unaffected.
3.5 The dye
In this recent work, a Ru complex dark dye, C106, obtained a BFF of 93% when measured in a white background.144 Dye's choice, as previously discussed, doesn’t affect the bifaciality feature while the application in BIPV as façades or indoor smart device may become crucial. The aim is to briefly discuss the three categories of dyes widely used in DSSC while a detailed description can be found here.49,56A sustainable dye for DSSC should possess the following features:
• absorbance properties in the visible spectra and near the IR region;
• a high molar extinction coefficient to harvest the light;
• the LUMO and HOMO level of the dye must match respectively with the conduction band of the semiconductor and with the energy level of the redox mediator;
• a robust bond between the dye and the semiconductor surface facilitates electron transfer.
Indeed, in order to efficiently inject the electron, the LUMO level of the excited dye should be 0.2–0.3 eV above the conduction band of TiO2 while the HOMO level must lay below the energy level of the redox electrolyte in order to have an efficient dye regeneration.
Anchoring the adsorbing dye molecules onto the surface of semiconducting metal oxide nanoparticles, which collectively form the working electrode of a DSSC, is paramount for initiating an electrical current by injecting electrons from the dye into the conduction band of the metal oxide. Conventionally, carboxylic acid and cyanoacrylic acid groups have been the mainstay anchors in DSSC research. However, the recent surge in DSSC exploration has ushered in a plethora of novel anchor groups. The molecular structures and optoelectronic properties of a broad variety of DSSC anchors including pyridine, phosphonic acid, tetracyanate, perylene dicarboxylic acid anhydride, 2-hydroxybenzonitrile, 8-hydroxyquinoline, pyridine-N-oxide, 3-hydroxy-N-methylpyridinuim, catechol, hydroxamate, sulfonic acid, acetylacetanate, boronic acid, nitro, tetrazole, rhodanine, and salicylic acid substituents have been widely discussed in this review.181 A strategic selection of anchors can bestow upon the solar cell customized performance attributes, including enhanced stability and optimized conversion efficiency.
The dye structure has an important role in their overall performance. A good blocking behavior is crucial for successful use in combination with the novel redox mediators or hole conductors. Steric groups can slow down the electron transfer between TiO2 and oxidized redox mediator or hole conductor.173 Co-sensitization is a proven strategy for enhancing the performance of DSSCs. Selecting appropriate dyes with complementary absorption spectra enables the capture of a broader range of sunlight, resulting in strong and panchromatic light absorption. By employing a combination of dyes, co-sensitization can effectively expand the light harvesting window of DSSCs, leading to improved PCE and overall device performance. Moreover, co-sensitization has the beneficial effect of decreasing dye aggregation.182,183 Dye aggregation is a molecular phenomenon that plays an essential rule in the DSSC photovoltaic performances. The molecular interactions between dye molecules are governed by the chemical structure of the dye, including its polarity and the types of substituents it possesses.184 The assumption of a uniformly spaced dye monolayer at the dye⋯TiO2 interface may not hold if dye clustering occurs laterally, disrupting the monolayer structure. Additionally, dye aggregation in the longitudinal direction would invalidate the assumption of homogeneous dye distribution. A dye molecule that binds to another dye rather than the TiO2 surface would render itself non-photovoltaic since it cannot interact with the TiO2 surface for electron injection and also obstructs electrolyte ions from reaching and regenerating the cognate dye on the TiO2 surface. Furthermore, a dye molecule that binds too close to another dye molecule on the TiO2 surface may experience dye⋯dye interactions that could positively or negatively impact their charge characteristics. The molecular factors that cause dye aggregation, and ways by which dye aggregation can be suppressed are deeply discussed in this review.184 An interesting approach is to use dyes with different binding groups that do not compete for the same binding sites on TiO2. Shibayama et al. successfully combined the black dye (with carboxylic acid binding groups) with an organic dye possessing a pyridine binding group.185 They demonstrated that the dyes adsorbed to different binding sites.
There are three classes of possible NIR sensitizers: polymethines, phthalocyanines and porphyrins. The NIR dyes and their performances are described in detail in this review56 while, in this section, we will mention the main characteristic of each class and the best obtained performances.
Polymethines are a class of dyes initially investigated for silver halide photography and as probes for biological systems190,191 when integrated with other high band gap semiconductor materials. They possess a high molar extinction coefficient and are extremely versatile: the central heart, the length of the numerous conjugated chains and the nature of the side groups are easily modifiable. The latter concludes that shifting the transition level from s0 to s1, therefore from the visible to the NIR region, is possible. Self-aggregation is a negative aspect of this class of dye that is limiting both the efficiency and the transparency; this can be solved with the addition of a disaggregating agent, chenodeoxycholic acid (CDCA) or with the introduction of a lateral bulky unit or alkaline chains.184 VG10 is a molecular engineered near-IR squaraine sensitizers, containing the novel benzoindolenine moiety functionalized with COOH as an efficient attaching group. This blue dye shows panchromatic light harvesting in the range between 400 to 800 nm and it detects the record PCE of 6.2% that is an outstanding value for this class of dye considering the easy and efficient low-cost synthesis process.192 Recently, a polymethine cyanine structure (VG20-Cx) has been proposed18 to render DSSCs fully transparent and colorless. The selective NIR-DSSC can display 3.1% PCE and a value approaching the 78% AVT reaching a CRI of 92.1.
Phthalocyanines are 2D tetrapyrrole macroheterocycles with 18 delocalized p-electrons. Their optical and electronic properties can be tailored through structural engineering, enabling absorption extending into the NIR region. They possess a high molar extinction coefficient, chemical and thermal stability.193,194 These also show a tendency to aggregate and poor solubility. Currently, the highest PCE is 6.4% given by PcS20.195
Porphyrins (Pors) are tetrapyrrolic macroheterocycle containing 18 delocalized p-electrons, synthetic analogues of Pcs. Pors have a high molar extinction coefficient and absorb in the visible spectrum but with appropriate structural engineering modifications, many porphyrins can extend the absorption spectrum up to the NIR region. Chemical and thermal stability are shown, and aggregation is lower if compared to PCs. The highest reported PCE for this class of dyes is 13%,85 one of the highest detected for DSSCs. The latest result was possible thanks to the introduction of the proquinoidal benzo thia-diazole (BTD) unit directly connected to one meso position, and a donor amine N-linked to the macrocycle on the other meso side. This led to the creation of the SM315 dye, with the push and pull porphyrin structure. By extending the ability to absorb light from the limit of 600 to the limit of 800 nm SM315 reaches high IPCE values across the whole visible wavelength range.
These DSSCs exhibit a reversible colour change and self-adjustable light transmittance upon irradiation, achieving a PCE of up to 4.17%.
By employing the well-established donor-π-conjugated-bridge-acceptor architecture for the dyes, where the π-conjugated bridge is substituted with a photochromic moiety, researchers have successfully developed DSSCs that can alter their colour and dynamically adjust their light transmittance upon exposure to sunlight (Fig. 9a). Exploring this concept, Fauvel et al.202 proposed that the replacement of the diphenyl-amine group with modified carbazole units allows for the tuning of the dye's optical properties to better match the photopic response of the human eye. The incorporation of carbazole units enables the lowering of the HOMO energy level of the dyes, thereby expanding the range of compatible redox systems beyond tri-iodide/iodide-based electrolytes. Some of the dyes show a maximum PCE of about 3% in opaque cells and 2.3% in transparent devices while an outstanding AVT above 50% and a CRI of 95 have been obtained (Fig. 9b).
 | ||
Fig. 9 Colour change of a semi-transparent NPI-based mini-module under natural light. Evolution of the colour of an NPI-based solar semi-transparent mini-module when exposed to natural light at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Interval times between pictures (from left to right) of 30, 30 and 240 °C. Interval times between pictures (from left to right) of 30, 30 and 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s cumulatively (modified image from ref. 196). (b) Picture of a colored butterfly taken through the mini-module (total area: 23 cm2) in the non-activated state (top) and activated state (bottom). It is reported the obtained AVT and CRI values with the use of a photocromic dye (modified image from ref. 202). s cumulatively (modified image from ref. 196). (b) Picture of a colored butterfly taken through the mini-module (total area: 23 cm2) in the non-activated state (top) and activated state (bottom). It is reported the obtained AVT and CRI values with the use of a photocromic dye (modified image from ref. 202). | ||
4. Application
In the following sections, we discuss the applications of BFDSC, with a focus on BIPV, agrivoltaic and IoT.4.1 BIPV
In Europe, electricity usage in buildings will surge at a rate five times faster than the growth of natural gas consumption by 2050 due to the implementation of short-term policies aimed at curtailing the import of natural gas from Russia.203 From the last BIPV market analysis, the global building-integrated photovoltaics market was valued at USD 19 billion in 2022 and is projected to reach a staggering USD 143.99 billion by 2032.204 While PV modules integrated into building envelopes can serve as multifunctional components, fulfilling roles like heat insulation, noise reduction, privacy enhancement, and weather protection, BIPV systems are primarily installed to generate electricity on-site, with minimal impact on the building's standard functionalities.205 BIPV windows are required to provide natural light while controlling solar heat in one system. Accordingly, PV devices must have a minimum transparency of 25–38%61 to be installed as tiles, modules, and solar glazing products.BIPV windows can be developed in two types: spacing non-transparent solar cell to achieve transparency or the use of transparent devices as window.206 Among PV technologies, c-Si has poor building integration in respect to the transparency requirement. The mutual distance between the cells can be settled to mimic semi-transparency but the pattern produces shades not comfortable for the internal living;61,207,208 c-Si can guarantee daylighting by distributing the cells but affecting the natural viewing.209 Besides, tilt angle and low irradiation levels complicate their integration in architectural glazing. In the last years, the new PV technologies like hydrogenated a-Si,210 CIGS, organic211 and perovskite solar cells have been considered as good candidates for BIPVs applications. Currently, BIPV systems are considered to have a lifespan of 25–30 years.
BFDSCs, as discussed before, have peculiar and promising features for BIPV applications. The transition between small laboratory scale and industry (such as BIPV application) implies the development of modules and panels. Most of the manufacturing steps for modules, such as the deposition of semiconductors and catalyzer, can be adopted from already developed techniques and methods in other industrial sectors.212 However, only the electrolyte injection and sealing have to be optimized for this purpose. From laboratories cell (<1 cm2) to modules the layout optimization is crucial. Dye sensitized solar modules (DSSMs) are made with a number of interconnected cells stripes on the same substrate which can be either connected in parallel (currents of the cells are summed) or series (voltages of the cells are summed). In this book chapter,213 a detailed explanation of parallel connection, series monolithic, series W-type connection and series Z-type connection DSSMs is present. Despite the type of connection used, a good sealing is decisive for guarantee a life span of more than 5 years.
From the beginning of the century, some works evaluated DSSC glazing performance in real outdoor condition. Lee et al.206 tested DSSM, by a Korean company, applied to a full-scale mock-up facility to verify the power performance of transparent DSSC BIPV glazing over a 2 year period (Fig. 10). Four and two DSSC BIPV windows were installed on the vertical south wall and on the 30° sloped roof, respectively. Over the two-year study period, the average daily power yield for the vertical DSSC BIPV window varied between 1.75 and 3.93 kWh per kWp per day, with an average of 2.53 kWh per kWp per day. In contrast, the average daily power yield for the 30-degree sloped DSSC BIPV window ranged from 2.16 to 5.34 kWh per kWp per day, averaging 3.60 kWh per kWp per day. However, DSSM demonstrates lower power efficiency compared to a-Si, c-Si, or CIGS, necessitating enhanced installation for equivalent power generation.
 | ||
| Fig. 10 DSSC BIPV mock-up. Reproduced from ref. 206. Copyright Clearance Center's RightsLink®. | ||
Kim et al.214 realized a large-area Z-type transparent DSSM of 900 cm2 for producing DSSC-based BIPV windows. Authors utilized a thin layer of TiO2 (4–5 μm), Z907 dye, a standard iodine-based electrolyte and, as counter electrode, a Pt layer. The module reached 3.19% of PCE but no AVT value is reported.
Godfroy et al.,215 with the aim to realize semi-transparent DSSC devices, focus on the synthesis and structure–properties relationships of benzothiadiazole-based organic dyes. Authors selected the YKP-88 dye for its superior performance and stability in laboratory testing and compared it against the reference N719 dye in a W-type series-connected module of 14.08 cm2 active area. In order to achieve an acceptable degree of semi-transparency in the visible spectrum, the thickness of the titania electrode was maintained at 7–8 μm without the use of a scattering layer. While YKP-88 dye exhibited a higher PCE of 8.7% compared to N719 dye's 6.3%, it was N719 dye that demonstrated a higher AVT of 34% compared to YKP-88's 26%.
In the last decade, first installation prototypes on buildings are reported. In 2014, Solaronix installed 300 m2 of W-connected modules (35 × 50 cm2 size for each) on Swiss Tech Convention Center façade at EPFL campus (Fig. 11a).170
Established in Italy, the dyepower consortium made significant strides in 2015, achieving a PCE of 5.6% on a Z-connected 600 cm2 device fabricated within their pilot line facility.216 These devices demonstrated remarkable resilience, successfully passing the stringent UV preconditioning test, humidity freeze test, and damp heat test outlined in the IEC 61646 Standard. Additionally, a comprehensive assessment of the environmental footprint of semi-transparent DSSCs, was conducted demonstrating their potential as a sustainable energy solution.217 H. Glass realized a huge DSSC installations on Science Tower in Austria (Fig. 11b) consisting of 896 W-connected red DSSC devices of 0.6 m2 area each (in total approx. 500 m2) while Dongjin Semichem realized a DSSC installation at the Solar Pavillon at Roskilde University in Denmark, consisting of 196 W-connected red DSSC panels of area 900 cm2 each (in total approx. 180 m2).170
Recently, Vesce et al.218 demonstrated a reliable, reproducible and industrially compatible method to fabricate under ambient air conditions large-area DSSM (400 cm2) and dye sensitized solar panel (DSSP) (0.2 m2) containing an organic inhouse made dye. The finest module efficiency was more than 5%, AVT is 35.7% (higher than any previous reported semi-transparent DSSM), LUE is above 1.8% and the modules are stable in temperature (ISOS-D-2 test) and under light soaking (ISOS-L-1 test)219 for more than 1000 h.
Interestingly, Roy et al.220 characterized and analyzed the color and semi-transparent properties of a N719-based DSSC using CRI and CCT values after two years of ambient exposure. They tested three different TiO2 thicknesses: 3.5, 6, and 10 μm. The study on the DSSC glazing after ambient exposure revealed a decrease in AVT over two years, while there was an improvement in color properties (CRI and CCT). This suggests that both CCT and CRI are spectrally dependent parameters. The authors concluded that the thinnest TiO2 layer, which experienced a 30% increase in CRI and only a 11.4% decrease in AVT, is the most suitable for DSSC integration in warmer seasons and hot climates.
4.2 Agrivoltaic
With the current energy and environmental crisis, soil preservation is of utmost importance. Indiscriminate deforestation to make room for photovoltaic installations is not the optimal choice in a world desperately in need of oxygen, which only plants can provide. The coined terminology Agrivoltaic expresses the struggle to combine the intended land for crops production with the supply of clean energy through panels. In just eight years, the global installed agrivoltaic capacity has rapidly reached 2900 MW in 2020 starting from 5 MW in 2012.221Commercial greenhouses interior conditions, as lighting, heating, cooling and ventilation for maximum yield require a significant energy consumption leading to unsuitable cultivated products. Several facilities such as fans, window-opening motors, artificial lighting, irrigation and automation equipment need electricity power. PV panels can support an electricity demand in greenhouses.
Generally, a greenhouse is made of transparent plastic or glass, therefore the PV integration on the roof or façades may compete with plants for lights, resulting in negative effects on crop growth.
The so-called photosynthetically active region (PAR) of plants is between 400 and 750 nm but not all the radiation is efficiently converted for the entire PAR wavelengths. The most common photosynthetic pigments are chlorophyll-a and chlorophyll-b, which absorb light mainly in two regions of the visible spectrum with two high peaks around 425–475 nm and 625–750 nm.
Commercially available silicon-based solar modules were tested concluding that the covered area of the greenhouse roofs should not exceed the 20–30% of the total roof area.222 Si-based modules are opaque and inhibit plant growth and productivity through excessive shading. Recently, Alinejad et al.223 investigated on PV application in a rose greenhouse assessing different parameters such as temperature, relative humidity, natural gas consumption, electricity consumption, and carbon dioxide (CO2) emission reduction. They identified an optimum PV coverage of 19.2% on the roof, resulting in no significant change in the illumination level on the plant canopy. Moreover, they demonstrated an annual reduction of natural gas consumption, electricity demand, and CO2 emission by 3.57%, 45.5%, and 30.56 kg m−2 respectively. In earlier research, Ureña-Sánchez224 tested two sides of a greenhouse with 10% roof coverage and compared different parameters especially the crop growth, to a conventional side of the greenhouse. Due to 9.8% radiation blocking caused by the solar panel, a small reduction in the transmitted light through the polythene cover was detected but this did not affect marketable production. While the largest tomatoes were obtained under conventional greenhouse conditions, notable distinctions in mean fruit mass and diameter were observed for fruits grown under the PV panel-covered greenhouse. However, these smaller fruits did not influence the final price. Similarly, Kavga et al.225 tested a roof coverage of 12.4% to compare the growth of lettuce (Lactuca sativa L.) and rocket (Eruca sativa Mill.) under polycrystalline silicon (pc-Si) panel shading versus an unshaded control. PV installations caused a favorable temperature reduction of 2.2 and 2.9 °C when temperatures reached peak levels during the spring and summer seasons, consequently resulting in energy and money savings for greenhouse temperature control. For plant growth, the PV presence reduced the PAR by 7.2% and 10.5% for lattice and rocket respectively. The most crucial commercial crop attribute (plant and stem fresh weight and the number of mature leaves) was unaffected under the PV greenhouse for lettuce, whose ability to adapt to varying solar radiation environments is well documented. However, all rocket salad growth characteristics appeared diminished under PV conditions compared to the unshaded control.
OPV shows interesting properties such as transparency and flexibility, enabling use in transparent façades or roofs. Zisisa et al.226 conducted an experimental study integrating semi-transparent OPVs based on a blend of regular poly(3-hexylthiophene) (P3HT) and phenyl-C61-butyric acid methyl ester (PCBM) on the rooftop of a Mediterranean greenhouse, covering 22%. The optical transmittance of the manufactured OPVs is around 18–20% in the PAR region. They cleverly tested pepper plants, which thrive in partial shade rather than direct sunlight. By absorbing ultraviolet radiation, the OPV materials favoured the shaded plants, enabling them to build longer central stems and produce more fruit, yielding increased mass of horticultural products. Ultimately, the cultivated plants under OPV shading showed better performance.
Several research groups are focusing on DSSC application in greenhouse environments, but there are few real-life tests. A prominent research direction involves manipulating absorbed light through spectral engineering to mitigate transmittance. In a pioneering work, Ru-based JJ-7 and JJ-9 sensitizers were proposed as efficient DSSC sensitizers for greenhouses. JJ-7 and JJ-9 dyes show a transmittance of 62.0% and 61.0% at 660 nm, and 18.0% and 15.0% at 440 nm, respectively, when deposited on 5 μm-thick TiO2 films.227
Recently, researchers have aimed to develop metal-free organic dyes for economic and environmental reasons. Dessi et al. proposed D–A–π–A dyes for greenhouse application. First, the organic thiazolo dye TTZ8–12, with a push–pull D–π–A structure, improved light absorption in the green visible range while maintaining ∼25–40% transmittance in the blue (425–475 nm) and 83% transmittance in the red (625 nm), achieving ∼6% efficiency in small-area devices with 5 μm228 TiO2. Efficiency then reached 8% by adding an extra acceptor between the donor and π spacer.229
Another spectral engineering approach was contemplated Chalkias et al.230 proposing new low-cost triphenylamine dyes and a highly transparent I2-free redox mediator as an alternative to the yellowish I2 electrolyte. TPA dyes mainly absorb in the blue, providing high red transparency. A key contribution was using an I2− free electrolyte that maintained device efficiency while improving current density by avoiding competition with the dye for blue light absorption. They reported a maximum 6.1% efficiency with the semi-transparent combination (TPA-1 and I2-free electrolyte) and crop growth factor G of almost 35%, claiming the best combined agrivoltaic performance known at the time.
The crop growth factor G (eqn (10)) was conceived by Emmot's group231 to assess the impact of transparent solar cells on plant growth:
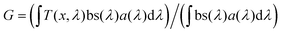 | (10) |
 | ||
| Fig. 12 (a) and (b) DSSM application in an Aquaponic greenhouse.17 (c) The detailed construction drawings of PDMG prototype. Reproduced with permission ref. 233 Elsevier. | ||
Roslan's group constructed a portable dye-sensitized solar cell mini green house (PDMG) with commercial DSSC panels by Solaronix (Fig. 12c), comparing growth of Misai Kucing, an anti-inflammatory/anti-gout tea plant, to a standard greenhouse. DSSC shading beneficially lowered heat and increased mid-day humidity. Misai Kucing grew equally well in the PDMG and control.233
4.3 Internet of things
The field of photovoltaics provides opportunity to make our buildings “smart” and our portable devices “independent”. Moreover, effective energy sources can be developed for ambient indoor use.77 To address this, ambient light photovoltaic cells have been developed to power autonomous IoT devices9 capable of machine learning, enabling on-device artificial intelligence234 implementation. IoT is a promising DSSC application area, though bifaciality is not mandatory.The Internet of Things consists of connected physical objects and sites with digital identities that communicate to provide automation services. It is estimated 75 billion IoT devices will be part of daily life in 2025, assisting in.9,235 This extends beyond current internet-connected devices like smartphones, desktops, tablets and laptops to unsupported appliances like air conditioners, coffee makers, and washing machines, plus environmental sensors. The cloud infrastructure is the IoT platform where devices communicate; wireless networking technologies include Wi-Fi, Bluetooth, LPWAN, cellular, and Ethernet. Most IoT devices require low, continuous power. Indoor PV technologies, especially DSSCs with high indoor performance, are a perfect match to supply this market. Lux refers to indoor light intensity based on the human eye response spectrum. DSSCs efficiently operate over a wide range from dim 50 lux to bright 1000 lux. Freitag et al. showed under 1000 lux DSSCs exceed conventional Si and GaAs PV thin films with remarkable 28.9% efficiency.234 Cao et al. improved the PCE to 32% under 1000 lux. Using a dye cocktail composed of Y123 and XY1b, along with a Cu(II)/Cu(I) redox-based electrolyte, the device benefits from the use of PEDOT as the counter electrode. This choice reduces the diffusion path of the redox mediator to only the mesoporous TiO2 film, thereby attenuating the Warburg resistance and boosting the photovoltaic performance. The authors claim promising applications in the IoT domain.166 Sensing fading of natural dyes via IoT and refilling to restore efficiency was suggested. Butein and isobutrin from Butea monosperma were extracted as sensitizers. When the natural dye faded, it was refilled, restoring (even improving) initial performance. The authors proposed IoT sensing of dye fading beyond a critical value, triggering refill from a reservoir.236
Researchers have developed a prototype of a fully self-powered intelligent IoT node that can infer information based on a pre-trained artificial neural network (Fig. 13). The node, which is based on an ATmega328P microcontroller, is powered by five 3.2 cm2 XY1:L1-sensitized solar cells (total 16 cm2) illuminated with 1000 lux fluorescent light. DSSCs utilizing XY1 and L1 dyes maintain an impressive photovoltage of over 910 mV under 1000 lux of fluorescent illumination, translating into remarkable conversion efficiencies of 34.0%, 32.7%, and 31.4% at 1000, 500, and 200 lux, respectively. The cells generate enough energy to power the node's microcontroller, sensors, and wireless communication circuitry.9
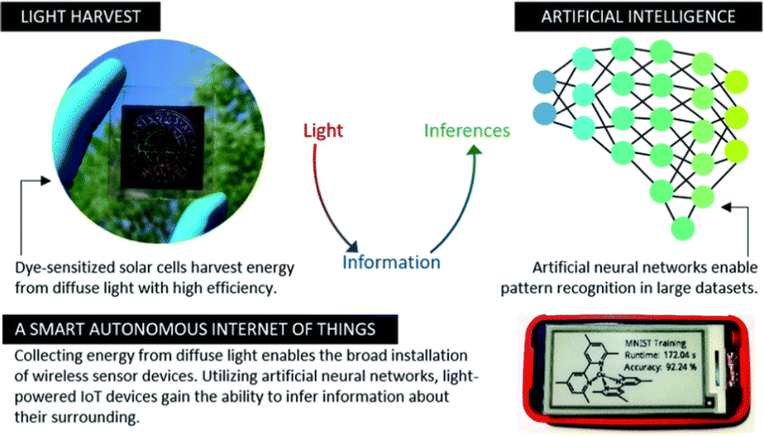 | ||
| Fig. 13 Fully autonomous IoT devices powered by harvested ambient light directly convert photons into computational information. Reproduced with permission of ref. 9 (Licensed Under a Creative Commons Attribution 3.0 Unposter Licence). | ||
Established companies such as RICOH and EXEGER have introduced DSSCs to the market, using them in a variety of IoT applications, including wearable electronics and smart sensors.
Ricoh237 launched solid-state DSSC-powered devices supporting the IoT era, eliminating battery replacement and waste while generating clean, sustainable energy.
Applications include sensors for temperature, humidity, illumination, pressure, and optical sensing or remote controllers moreover a DSSC-integrated desk that can recharge a detachable mobile battery under the desktop. Exeger,238 a Swedish company, upgraded through a superior design capability, the best of existing DSSC advancing in the flexibility and the PCE. Powerfoyle™ is thin (1.3 mm), flexible, durable and can look like steel, leather or carbon fiber. They spread this PV DSSC based material to a wider range of consumers electronics and IoT devices.
5. Bifacial enabling technologies
Next-generation solar cells adhere to the trend of integrating multiple energy device types into single, consolidated systems. Single-junction (SJ) PV cells, however, exhibit significant energy losses due to the thermalization of high-energy photons and the inability to capture transmitted low-energy photons. This phenomenon leads to the Shockley–Queisser (S–Q) limit, which represents the theoretical maximum efficiency. For single-junction silicon solar cells, which are commonly fabricated using silicon with a bandgap of 1.1 eV, the Shockley–Queisser (S–Q) limit is approximately 32%. Last record PCE of single junction crystalline silicon solar cell reached the 26%16 due to internal parasitic effect and light reflection. Tandem technology appears a promising strategy to overcome the S–Q limit. The purpose of a tandem device is extending, by integrating more PV cells, the light absorption range, limiting the loss of photons. Tandem solar cells can be divided in two categories: 4-terminal (4-T) tandems mechanically stack two different devices, while 2-terminal (2-T) monolithic structures directly fabricate the top cell on the bottom (Fig. 14). Conventional series-connected 2-T tandems offer advantages such as fewer fabrication steps, resulting in reduced manufacturing costs and energy consumption, and fewer parasitic light effects due to fewer layers. However, paying attention to the choice of the material and the chosen processes is mandatory. For example, the manufacturing processes of the top cell must not deteriorate the bottom one with high temperature or pressure. In contrast, 4-T structures avoid these limitations but suffer optical losses from interlayer materials.In 2T tandem solar cells, two sub-cells (top and bottom) are connected in series. Each sub-cell selectively absorbs a specific portion of the solar spectrum, maximizing energy conversion efficiency. The top sub-cell, featuring a wider bandgap, effectively captures and converts high-energy photons associated with shorter wavelengths, such as ultraviolet and visible light. This allows lower-energy photons, characterized by longer wavelengths like infrared radiation, to pass through and reach the bottom sub cell. The bottom sub-cell, equipped with a narrower bandgap, efficiently absorbs and converts these lower-energy photons. However, the series connection means current through both cells must be equal, which can limit efficiency if the currents are mismatched.
In 4T tandem solar cell, top and bottom cells operate independently, each with separate terminals, eliminating the current-matching requirement and allowing maximum power point operation. As in 2T cells, the top cell has a larger bandgap, converting high-energy photons, while allowing lower energy photons to reach the bottom cell with a lower bandgap. Both 2T and 4T designs employ a wider range of the solar spectrum, resulting in enhanced efficiency compared to single-junction cells. The selection of materials is influenced by factors such as material compatibility, fabrication complexity, and the balance between efficiency and cost. Solar cells are typically connected in series to achieve higher voltage output. Photocurrent matching is crucial to minimize performance losses and ensure optimal efficiency.
At present, the world record power conversion efficiency (PCE) for a multi-junction solar cell stands above 39%.16,239 Since the advent of DSSCs, integration with materials like Si, GaAs, and perovskite has targeted energy harvesting, conversion and storage.240,241 As semi-transparent cells with tunable light filtering via dye selection, DSSCs are strong tandem candidates. Here we discuss results based on materials used.
5.1 Tandem DSSC (T-DSSC)
In early work,242 a T-DSSC achieved 1.45 V and over 10% efficiency. N719 was utilized as the top sensitizer over NKX2677 due to its high molar extinction coefficient, ensuring sufficient light reaches the bottom cell. Black dye absorbed 600–800 nm in the bottom cell. Thickness optimization involved employing a thinner 6.5 μm TiO2 layer for the top sub-cell and a 34 μm layer for the bottom sub-cell. Subsequently, the Sewaga group utilized a Ru(II) DX1 sensitizer with strong absorption in the 320 nm UV and 500–900 nm ranges as the bottom dye in combination with N719 as the top dye, achieving an impressive 11.4% efficiency. Barawal et al.243 developed a TCO-less T-DSSC, with photons crossing just one TCO substrate before the bottom electrode, using a flexible metal mesh to replace one TCO. To achieve current matching, TiO2 layers with thicknesses of 1 and 16 μm were employed in conjunction with D131 and N719 dyes. Thinner TiO2 improves top cell transparency. Eom et al.244 synthesized SGT-136 and SGT-137 D–π–A sensitizers to improve SGT-130's π-bridge. A parallel-connected tandem device employing an SGT-137 top cell and an SGT-021 bottom cell achieved a remarkable current density of 22.06 mA cm−2 and an efficiency of 14.64%, representing one of the highest PCE values reported for T-DSSCs. Recently, a T-DSSC exceeded 33%245 dim-light efficiency using the Y123 dye in both cells with a Co2+/3+ electrolyte, which avoids iodine light absorption limiting photon transmission.In the last year, Mounika et al.246 demonstrated the integration of an LG6 dye sensitizer for near-infrared light utilization and an N719 dye sensitizer for effective visible light capture in the bifacial tandem cell design. The bifacial tandem DSSC–DSSC was fabricated by sandwiching two photoelectrodes (PE) (N719 dye-anchored PE and LG6 dye-anchored PE) between one common counter electrode (Fig. 15). The T-DSSC exhibits a high PCE of 12.76% with a current density of 27.61 mA cm−2.
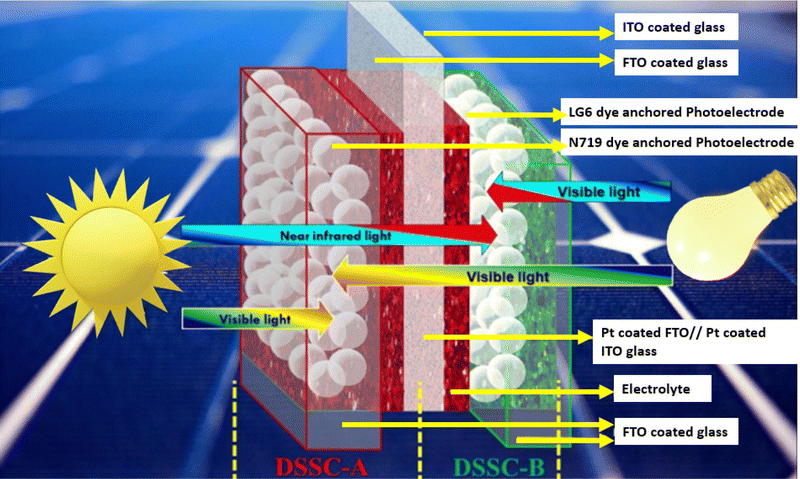 | ||
| Fig. 15 Bifacial tandem DSSC–DSSC device structure modified from ref. 246. | ||
In a similar vein, Gianola et al.247 introduced the Hole Tandem Bifacial configuration (HTB-DSSC), a two-compartment cell design that incorporates a bifacial semi-transparent shared counter electrode (Pt-based) between two identical semi-transparent photoanodes. Using common N719 dye and I-based electrolyte, the HTB-DSSC achieved a PCE of 13.19% under front illumination. This value further enhanced to 14.96% by integrating a reflecting mirror on the cell's backside to harness the albedo effect.
5.2 Tandem DSSC and thin film PV
In 2002, a model-based study248 suggested optimal tandem cell bandgaps of 1.1 and 1.7 eV for bottom and top cells. Early work interpreted this by applying 1.04–1.67 eV bandgap CIGS thin film cells as bottom cells and N719-based DSSCs as top cells, with N719's HOMO–LUMO gap matching the model. Liska et al.249 reached 15% efficiency and 1.45 V of VOC, while Park et al.250 later obtained similar VOC but slightly lower efficiency. Both devices utilized ∼12 μm TiO2 in the top DSSC, resulting in a lack of transparency in the materials. A DSSC employing 4 μm TiO2 film sensitized with light red Y123 dye was rendered semi-transparent by employing a cobalt electrolyte, offering enhanced transparency and reduced corrosivity compared to iodine-based electrolytes. PEDOT:PSS replaced platinum as a more transparent CE. This Y123-based DSSC combined with a CIGS bottom cell showed high stability over 1000 hours under ambient conditions.251 Based on electrochemical impedance spectroscopy and photoelectrochemical cell measurement, the enhanced stability of the tandem cell is attributed to minimal corrosion by the cobalt-based polypyridine complex redox couple. In an early study,252 stacked multijunction tandem solar cells were fabricated by mechanically assembling DSSCs as the top cell and a GaAs/AlXGa(1−X)As graded solar cell (GGC) as the bottom cell. These cells were proposed for water-splitting applications, leveraging the remarkable open circuit voltage of 1.85. Three organic dyes, namely D131, D102, and D205, each with distinct absorption spectra, were employed in the DSSCs to ensure a balanced photocurrent density between the DSSC and the GGC. The open-circuit photovoltages for the GGC and the DSC with D131 were measured at 1.11 V and 0.76 V, respectively. This resulted in a combined VOC of 1.85 V and a photovoltaic conversion efficiency of 7.63% for the tandem cell. Finally, successful solar-to-hydrogen conversion is achieved through the utilization of a T-DSSC/Si device.253 An advanced DSSC/Si monolithic tandem cell is introduced, employing PEDOT:FTS as an interfacial catalytic layer. This layer, characterized by enhanced transparency and reduced charge-transfer resistance compared to traditional Pt as discussed before, plays a crucial role. Additionally, the precise adjustment of dye adsorption on TiO2 nanoparticle surfaces ensures accurate current matching between the two sub-cells.Through these strategic approaches, the DSSC/Si tandem cell demonstrates a significantly superior PCE of 18.1%, outperforming stand-alone SJ devices of DSSCs (≈11.4%) or Si (≈12.3%) cells. Moreover, the 2-T DSSC/Si solar cells exhibit a notable VOC value of 1.36 V. These tandem solar cells, externally connected to a Pt electro-catalyst, serve as water splitting cells. Solar-to-hydrogen conversion is successfully achieved at 0.65 V vs. Pt bias.
This study240 presents the successful fabrication of high-performance four-terminal solution-processed tandem solar cells, employing DSSCs as top-cells and lead sulfide (PbS) colloidal quantum dot solar cells (CQDSCs) as bottom-cells. To optimize light transmission, the TiO2 scattering layer in the DSSCs was eliminated. Additionally, the impact of quantum dot size on PbS bottom-cell performance was investigated. The combination of the XL dye mixture and 890 nm PbS QDs yielded a remarkable PCE of over 12%, demonstrating a significant advancement compared to individual DSSC or CQDSC sub-cells.
5.3 Tandem DSSC and perovskite solar cells
In regard to the latest PSC, several record efficiencies for perovskite/silicon tandem cells reflect the race to surpass the most commercially available silicon-based devices. The most recent PCE record for a T-PSC/Silicon tandem cell reached 33.9% in 2023.16 Despite their exceptional light absorption capabilities in the visible spectrum (400–800 nm), perovskites like MaPbI3 and FaPbI3 do not effectively capture NIR radiation, which contributes to about 45% of the sun's energy up to 1700 nm. By mixing lead with tin, researchers were able to move the perovskite photo-response until region spectrum where human cone are not sensitive, 1060 nm.254,255 Unlucky, the mixed Pb/Sn compounds do not follow a linear trend in term of performance that resulted lower in comparison with remarkable results of reference perovskite. Recently, a tandem solar cell perovskite/DSSC has been proposed. A double cation perovskite ((FAPbI3)0.85(MAPbBr3)0.15), exhibiting a broad absorption spectrum in the visible range, was employed as the top sub-cell. Two dyes, thioindigo and N719, both absorbing in the visible range similar to perovskite, were tested in the bottom sub-cell of a DSSC. With both dyes, the photocurrent was constrained by the low current of the DSSC, resulting in a low PCE of 10.54% for the PSC/DSSC tandem device. However, a more effective approach would involve optimizing critical parameters such as the bandgaps of the absorbing materials. Considering that standard and efficient PSCs absorb within the visible range, a suitable compromise would be to employ a NIR-absorbing dye in the DSSC. Recently, a NIR-selective DSSC has been presented for the first time. The NIR-selective DSSC is colourless and transparent and impressive high AVT and CRI values of 76% and 92 have been demonstrated respectively.18 Despite the modest PCE of 3.1%, which is not competitive with those present in the literature, the considerable high AVT and CRI values worth of investigation in terms of aesthetic and integration opportunities such as in tandem device and BIPV. The NIR selective sensitizer, VG20-Cx, is based on a cyanine structure and by molecular engineering, has been developed with its strong and sharp S0–S1 transition in the near-infrared region (λ = 832 nm) and a minimized S0–Sn contribution in the far blue where the human retina is poorly sensitive. Opening rooms for the progress of efficient colourless NIR-DSSC request the design of specific transparent materials with tailored properties. Although a wide variety of NIR dyes have been synthesized for DSSCs to achieve selective NIR-DSSCs, the dye needs to exhibit almost zero absorption in the entire spectral region covered by human cones. Polymethine, phthalocyanines, and porphyrins56 appear a promising class of dye due to the energetic levels that can ensure an efficient injection while having a bandgap sufficiently narrow to selectively absorb the NIR region.6 Future prospects and concluding thoughts
Bifacial dye-sensitized solar cells (BFDSCs) have emerged as frontrunners in third-generation photovoltaic technologies. Their unparalleled adaptability, compared to traditional solar cells, pave the way for diverse applications, as highlighted in Sections 4.1–4.3. The purpose of this review was to consolidate all the information from the literature concerning the characteristics of engineered materials, synthesized dyes, and different structures to enhance bifaciality and transparency. Several times, it has been emphasized how the catalyst plays a crucial role in ensuring the bifaciality of the BFDSC. Although expensive platinum remains one of the most efficient catalysts and, with optimized thickness, maintains good transparency, the PEDOT polymer emerges as an ideal competitor. The thickness of titania affects the cell's transparency, as does the choice of dyes. The application of NIR dyes will find future applications in tandem solar cells, thanks to the DSSC's ability to tune light absorption. However, the most notable feature of DSSC is the aesthetic appeal provided by its colors and semi-transparency. The second part of the review is dedicated to exploring adaptability in residential contexts, such as building façades. The challenge of applying DSSC in BIPV arises from the technology's current low efficiency. A viable application can be niche items like lamps or power sources for small devices or IoT. Furthermore, the integration of DSSCs into wearable electronics holds immense promise for the future of personal electronics. By harnessing the power of ambient light, DSSCs can provide a sustainable and renewable energy source for a wide range of wearable devices, from smartwatches and fitness trackers to smart clothing and healthcare monitoring systems.In the realm of agrivoltaic, even though the same concern of low efficiency persists, making it challenging for massive energy production, DSSCs can serve as shading inside greenhouses for crops that aren't significantly affected by filtered light. This could help lower internal temperatures. Additionally, in these contexts, DSSCs could power IoT devices such as humidity and temperature sensors.
List of abbreviations
| 2T | Two terminals |
| 4T | Four terminals |
| a-Si | Amorphous silicon |
| AVT | Average visible transmittance |
| BFDSCs | Bifacial DSSCs |
| BFF | Bifaciality factor |
| BFSCs | Bifacial solar cells |
| BIPV | Building-integrated photovoltaics |
| CB | Conduction band |
| CCT | Correlated color temperature |
| CdS | Cadmium selenide |
| CdTe | Cadmium telluride |
| CE | Counter electrode |
| CIGS | Copper indium gallium selenide |
| CQDs | Carbon quantum dots |
| CRI | Color rendering index |
| c-Si | Cristalline silicon |
| CV | Cyclic voltammetry |
| DSSCs | Dye-sensitized solar cells |
| DSSMs | Dye sensitized solar modules |
| EIS | Electrochemical impedance spectroscopy |
| FF | Fill factor |
| FoM | Figure of merit |
| FTO | Fluorine-doped tin oxide |
| GaAs | Gallium arsenide |
| GGC | GaAs/AlXGa (1×)As graded solar cell |
| GNTs | Graphene nanotubes |
| GuSCN | Guanidinium thiocyanate |
| HOMO | Highest occupied molecular orbital |
| IBEs | Iodine-based electrolytes |
| IoT | Internet of things |
| IPCE | Incident photon to current efficiency |
| ITO | Indium-doped tin oxide |
| J SC | Short circuit current density |
| LUE | Light utilization efficiency |
| LUMO | Lowest unoccupied molecular orbital |
| MB | Methylbenzimidazole |
| MISCN | 1-Ethyl-3-methylimidazolium thiocyanate |
| ML | Main layer |
| m-SC | Monofacial solar cell |
| M–Se | Metal selenide |
| N/S-GF | Nitrogen/sulphur dual-doped graphene film |
| N-GF | Nitrogen doped graphene films |
| OPV | Organic photovoltaics |
| PANI | Polyaniline |
| PCE | Power conversion efficiency |
| Pc-Si | Polycrystalline silicon |
| PE | Photoelectrode |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PET | Polyethylene terephthalate |
| PMII | 1-Propyl-3-methyl-imidazolium iodide |
| PPy | Polypyrrole |
| PSC | Perovskite solar cells |
| Pt | Platinum |
| PV | Photovoltaic |
| PVDF | Polyvinylidene fluoride |
| QSSE | Quasi solid state electrolyte |
| R CT | Charge transfer resistance |
| R S | Series resistance |
| SC | Solar cell |
| S-CQDs | Sulphur-doped carbon quantum dots |
| SEM | Scanning electron microscope |
| S-GF | Sulphur doped graphene films |
| SL | Scattering layer |
| SSA | Specific surface area |
| TBP | 4-tert-Butyl-pyridine |
| TCE | Transparent conductive electrode |
| TCO | Transparent conductive oxide |
| t-DSSC | Tandem DSSC |
| TED | Transparency, efficiency, diffused light efficiency |
| TEM | Transmission electron microscopy |
| VN | Valeronitrile |
| V OC | Open circuit voltage |
| WE | Working electrode |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the project Best4U “Tecnologia per celle solari bifacciali ad alta Efficienza a 4 terminali per utility scale”. European project financed by Italy's Ministry of Education (Ministero dell’Istruzione, dell’Università e della Ricerca, or MIUR) and the European Community through the European Regional Development Fund.Notes and references
- A. Bartolotta and G. Calogero, in Solar Cells and Light Management: Materials, Strategies and Sustainability, ed. F. Enrichi and G. C. Righini, Elsevier, Amsterdam, Netherlands, 2020, ch. 4, pp. 107–161, ISBN: 9780081027622 Search PubMed.
- N. Armaroli and V. Balzani, Energia oggi e domani. Prospettive, sfide, speranze, Bononia University Press, 2004 Search PubMed.
- Key world energy statistics 2021, International Energy Agency, https://www.iea.org/reports/key-world-energy-statistics-2021, (Last access 29/11/2023).
- H. Ritchie, M. Roser and P. Rosado, Energy, Our World in Data, 2020 – https://ourworldindata.org/energy, (Last access 3/12/2023).
- IEA, Renewables 2022, Analysis and forecast to 2027, OECD Publishing, Paris, International Energy Agency, https://www.iea.org/reports/renewables-2022, (Last access 29/11/2023).
- K. Yoshikawa, H. Kawasaki, W. Yoshida, T. Irie, K. Konishi, K. Nakano, T. Uto, D. Adachi, M. Kanematsu, H. Uzu and K. Yamamoto, Nat. Energy, 2017, 2, 17032 CrossRef CAS.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta, E. D. Dunlop, D. H. Levi and A. W. Y. H. Baillie, Prog. Photovoltaics, 2017, 25, 3–13 Search PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef CAS.
- H. Michaels, M. Rinderle, R. Freitag, I. Benesperi, T. Edvinsson, R. Socher, A. Gagliardi and M. Freitag, Chem. Sci., 2020, 11, 2895–2906 RSC.
- P. Selvaraj, H. Baig, T. K. Mallick, J. Siviter, A. Montecucco, W. Li, M. Paul, T. Sweet, M. Gao, A. R. Knox and S. Sundaram, Sol. Energy Mater. Sol. Cells, 2018, 175, 29–34 CrossRef CAS.
- W. Liu, L. Liu, C. Guan, F. Zhang, M. Li, H. Lv, P. Yao and J. Inghenoff, Sol. Energy, 2018, 162, 84–94 CrossRef.
- B. O’Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J.-I. Fujisawa and M. Hanaya, Chem. Commun., 2015, 51, 15894–15897 RSC.
- D. Zhang, M. Stojanovic, Y. Ren, Y. Cao, F. T. Eickemeyer, E. Socie, N. Vlachopoulos, J.-E. Moser, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nat. Commun., 2021, 12, 1777 CrossRef CAS PubMed.
- W. Qarony, M. I. Hossain, M. K. Hossain, M. J. Uddin, A. Haque, A. R. Saad and Y. H. Tsang, Results Phys., 2017, 7, 4287–4293 CrossRef.
- National Renewable Energy Laboratory (NREL) website: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf, (Last access 29/11/2023).
- J. Barichello, L. Vesce, P. Mariani, E. Leonardi, R. Braglia, A. Di Carlo, A. Canini and A. Reale, Energies, 2021, 14, 6393 CrossRef CAS.
- W. Naim, V. Novelli, I. Nikolinakos, N. Barbero, I. Dzeba, F. Grifoni, Y. Ren, T. Alnasser, A. Velardo, R. Borrelli, S. Haacke, S. M. Zakeeruddin, M. Graetzel, C. Barolo and F. Sauvage, JACS Au, 2021, 1, 409–426 CrossRef CAS PubMed.
- M. D. Akhtaruzzamana, M. D. Shahiduzzamand, V. Selvanathana, K. Sopiana, M. I. Hossaine, N. Aminf and A. K. Mahmud Hasana, Appl. Mater. Today, 2021, 25, 101204 CrossRef.
- Y. H. Sim, M. J. Yun, S. I. Cha, S. H. Seo and D. Y. Lee, ACS Omega, 2018, 3, 698–705 CrossRef CAS PubMed.
- J. M. Miranda-Munoz, S. Carretero-Palacios, A. Jiménez-Solano, Y. Li, G. Lozano and H. Mìguez, J. Mater. Chem. A, 2016, 4, 1953–1961 RSC.
- J. Chantana, Y. Kawano, T. Nishimura, A. Mavlonov and T. Minemoto, Opt. Mater., 2021, 113, 110819 CrossRef CAS.
- A. Kolay, P. Ghosal and M. Deepa, ACS Sustainable Chem. Eng., 2020, 8, 8593–8603 CrossRef CAS.
- M. Hiroshi, US Pat., 3,278, 811, 1966 Search PubMed.
- A. Luque, A. Cuevas and J. M. Ruiz, Sol. Cells, 1980, 2, 155–166 CrossRef.
- A. Cuevas, A. Luque, J. Eguren and J. Del Alamo, Sol. Energy, 1982, 19, 419–420 CrossRef.
- R. Guerrero-Lemus, R. Vega, T. Kim, A. Kimm and L. E. Shephard, Renewable Sustainable Energy Rev., 2016, 60, 1533–1549 CrossRef CAS.
- R. Kopecek, 2nd Bifi PV Workshop, Chambery, France, 2014.
- G. Romijn, 3rd Bifi PV Workshop, Miyazaki, Japan, 2016.
- H. Fang, 4th Bifi PV Workshop, Denver, USA, 2018.
- B. L. Smith, M. Woodhouse, K. A. W. Horowitz, T. J. Silverman, J. Zuboy and R. M. Margolis, National Renewable Energy Laboratory (NREL), NREL/TP-7A40-78173 2021, https://www.nrel.gov/docs/fy22osti/78173.pdf.
- M. T. Patel, M. R. Khan, X. Sun and M. A. Alam, Appl. Energy, 2019, 247, 467–479 CrossRef.
- International Technology Roadmap for Photovoltaic- Results 2021 including maturity report 2022, Thirteenth edition, November 2022.
- J. M. Gaby, G. J. Janssen, K. E. J. Tool, E. J. Kossen, B. B. Van Aken, A. J. Carr and I. G. Romijn, Energy Procedia, 2017, 124, 76–83 CrossRef.
- Q. Tai, B. Chen, F. Guo, X. Xu, H. Hu, B. Shebo and X.-Z. Zhao, ACS Nano, 2011, 5, 3795 CrossRef CAS PubMed.
- Y. Duan, Q. Tang, J. Liu, B. He and L. Yu, Angew. Chem., 2014, 53, 14569–14574 CrossRef CAS PubMed.
- J. M. Miranda-Munoz, S. Carretero-Palacios, A. Jiménez-Solano, Y. Li, G. Lozano and H. Mıguez, J. Mater. Chem. A, 2016, 4, 1953–1961 RSC.
- S. Venkatesan, W.-H. Lin, H. Teng and Y.-L. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 42780–42789 CrossRef CAS PubMed.
- N. Roslan, M. E. Yàacob, M. A. M. Radzi, Y. Hashimoto, D. Jamaludin and G. Chen, Renewable Sustainable Energy Rev., 2018, 92, 171–186 CrossRef.
- S. Pal, A. Reinders and R. Saive, IEEE J. Photovolt., 2020, 10(6), 1803–1815 Search PubMed.
- J. Balenzategui and F. Chenlo, Sol. Energy Mater. Sol. Cells, 2005, 86(1), 53–83 CrossRef CAS.
- J. Barichello, D. Di Girolamo, E. Nonni, B. Paci, A. Generosi, M. Kim, A. Levtchenko, S. Cacovich, A. Di Carlo and F. Matteocci, Sol. RRL, 2023, 7, 2200739 CrossRef CAS.
- L. Duan, D. Walter, N. Chang, J. Bullock, D. Kang, S. Pheng Phang, K. Weber, T. White, D. Macdonald, K. Catchpole and H. Shen, Nat. Rev. Mater., 2023, 8, 261–281 CrossRef CAS.
- I. Burgués-Ceballos, L. Lucera, P. Tiwana, K. Ocytko, L. W. Tan, S. Kowalski, J. Snow, A. Pron, H. Bürckstümmer, N. Blouin and G. Morse, Joule, 2021, 5, 2261–2272 CrossRef.
- J. J. Bezerra Galdino, O. de Castro Vilela, N. Fraidenraich and L. A. Gómez-Malagón, Sol. Energy Mater. Sol. Cells, 2023, 257, 112359 CrossRef.
- N. Gao, L. Huang, T. Li, J. Song, H. Hu, Y. Liu and S. Ramakrishna, J. Appl. Polym. Sci., 2020, 137, 48443 CrossRef CAS.
- D. Devadiga, M. Selvakumar, P. Shetty and M. S. Santhosh, J. Electron. Mater., 2021, 50, 6 CrossRef.
- J. L. Gray, The Physics of the solar cell, in Handbook of photovoltaic Science of Engineering, ed. A. Luque and S. Hegedus, John Wiley & Sons Ltd, 2003 Search PubMed.
- G. Calogero, A. Bartolotta, G. Di Marco, A. Di Carlo and F. Bonaccorso, Chem. Soc. Rev., 2015, 44, 3244–3294 RSC.
- K. Kalyasundaran and M. Grätzel, Coord. Chem. Rev., 1998, 117, 347–414 CrossRef.
- M. Grätzel, Prog. Photovoltaics, 2006, 14, 429–442 Search PubMed.
- M. K. Nazeeruddin, P. Pechy, P. Liska, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef CAS PubMed.
- S. Ito, S. M. Zakeeruddin, P. Comte, P. Liska, D. Kuang and M. Gratzel, Nat. Photonics, 2008, 2, 693–698 CrossRef CAS.
- W. Kubo, S. Kambe, S. Nakade, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, J. Phys. Chem. B, 2003, 107, 4374–4381 CrossRef CAS.
- U. Würfel, J. Wagner and A. Hinsch, J. Phys. Chem. B, 2005, 109, 20444–20448 CrossRef PubMed.
- F. Grifoni, M. Bonomo, W. Naim, N. Barbero, T. Alnasser, I. Dzeba, M. Giordano, A. Tsaturyan, M. Urbani, T. Torres, C. Barolo and F. Sauvage, Adv. Energy Mater., 2021, 11, 2101598 CrossRef CAS.
- S. O. Abdellatif, S. Josten, A. S. G. Khalil, D. Erni and F. Marlow, IEEE J. Photovoltaics, 2020, 10, 522–530 Search PubMed.
- W. Gu, T. Ma, S. Ahmed, Y. Zhang and J. Peng, Energy Convers. Manage., 2020, 223, 113283 CrossRef.
- R. R. Lunt, Appl. Phys. Lett., 2012, 101, 043902 CrossRef.
- Z. Hu, J. Wang, X. Ma, J. Gao, C. Xu, K. Yang, Z. Wang, J. Zhang and F. Zhang, Nano Energy, 2020, 78, 105376 CrossRef CAS.
- P. Boyce, N. Eklund, S. Magnum, C. Saalfield and L. Tang, Light. Res. Technol., 1995, 8(2783), 145–152 CrossRef.
- C. J. Traverse, R. Pandey, M. C. Barr and R. R. Lunt, Nat. Energy, 2017, 2, 849–860 CrossRef.
- M. R. Hunt and R. W. Pointer, The Reproduction of Colour, John Wiley & Sons Inc., Chichester, UK, 2011 Search PubMed.
- B. Commault, T. Duigou, V. Maneval, J. Gaume, F. Chabuel and E. Voroshazi, Appl. Sci., 2021, 11, 11598 CrossRef CAS.
- Jet Propulsion Laboratory, Ecostress Spectral Librabry, version 1.0, https://speclib.jpl.nasa.gov/library, (Last accessed 16th November 2023).
- V. Carpenella, F. Ripanti, E. Stellino, C. Fasolato, A. Nucara, C. Petrillo, L. Malavasi and P. Postorin, J. Phys. Chem. C, 2023, 5(127), 2440–2447 CrossRef.
- M. P. Brennan, A. L. Abramase, R. W. Andrews and J. M. Pearce, Sol. Energy Mater. Sol. Cells, 2014, 124, 111–116 CrossRef CAS.
- D.-K. Hwang, J. Eun Nam, H. Jeong Jo and S.-J. Sung, J. Power Sources, 2017, 361, 87–95 CrossRef CAS.
- Q. Jiang, Z. Song, R. C. Bramante, P. F. Ndione, R. Tirawat, J. J. Berry, Y. Yan and K. Zhu., Joule, 2023, 7(7), 1543–1555 CrossRef CAS.
- D. Ebner, M. Bauch and T. Dimopoulos, Opt. Express, 2017, 25(8), A240–A252 CrossRef CAS PubMed.
- K. Kawajiri, K. Tahara and S. Uemiya, Resour. Environ. Sustain., 2022, 7, 100047 Search PubMed.
- Asia Nikkei, 2019, https://asia.nikkei.com., (Last access 3/12/2023).
- R. G. Gordon, MRS Bull., 2000, 25, 52–57 CrossRef CAS.
- F. Bonaccorso, Int. J. Photoenergy, 2010, 727134 Search PubMed.
- Z. Wu, Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzle, Science, 2004, 305, 1273–1276 CrossRef CAS PubMed.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. I. Song, Y.-J. Kim,K., S. Kim, B. Özyilmaz, J.-H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 4, 574–578 CrossRef PubMed.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- B. Ó. Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- M. Dhonde, K. Sahu, M. Das, A. Yadav, P. Ghosh and V. V. S. Murty, J. Electrochem. Soc., 2022, 169, 066507 CrossRef CAS.
- S. Bellani, A. Bartolotta, A. Agresti, G. Calogero, G. Grancini, A. Di Carlo, E. Kymakis and F. Bonaccorso, Chem. Soc. Rev., 2021, 50, 11870–11965 RSC.
- J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan, G. Luo, Y. Lin, Y. Xie and Y. Wei, Chem. Soc. Rev., 2017, 46, 5975 RSC.
- Q. Tai and X.-Z. Zhao, J. Mater. Chem., 2014, 2, 13207–13218 RSC.
- J. E. Trancik, S. C. Barton and J. Home, Nano Lett., 2008, 8, 982–987 CrossRef CAS PubMed.
- T. N. Murakami and M. Grätzel, Inorg. Chim. Acta, 2008, 361, 572–580 CrossRef CAS.
- M. Vafaiee, M. Vossoughi, R. Mohammadpour and P. Sasanpour, Sci. Rep., 2019, 9, 2985 CrossRef PubMed.
- S. Gullace, F. Nastasi, F. Puntoriero, S. Trusso and G. Calogero, Appl. Surf. Sci., 2020, 506, 144690 CrossRef CAS.
- Ç. Cetinkaya, E. Çokduygulular, B. Kınacı, F. Güzelçimen, Y. Özen, H. I. Efkere, I. Candan, S. Emik and S. Özçelik, Sci. Rep., 2021, 11, 13079 CrossRef PubMed.
- H. Dong, Z. Wu, Y. Gao, A. El-Shafei, B. Jiao, Y. Dai and X. Hou, Org. Electron., 2014, 15, 1641–1649 CrossRef CAS.
- N. Mustaffa, M. Y. A. Rahman and A. A. Umar, Ionics, 2018, 24, 3665–3671 CrossRef CAS.
- F. Navarro Pardo, D. Benetti, H. G. Zhao, V. M. Castaño, A. Vomiero and F. Rosei, J. Power Sources, 2016, 335, 138–145 CrossRef CAS.
- U. A. Kamarulzaman, M. Y. A. Rahman, M. S. Su’ait and A. A. Umar, Optik, 2023, 276, 170658 CrossRef CAS.
- R. G. Charles, P. Douglas, J. A. Baker, M. J. Carnie, J. O. Douglas, D. J. Penney and T. M. Watson, J. Cleaner Prod., 2018, 202, 1167–1178 CrossRef CAS.
- C. Zhao, Y. Shi, Z. Zhong and T. Ma, Chin. J. Catal., 2014, 35, 219–226 CrossRef CAS.
- S. Thomas, T. G. Deepak, G. S. Anjusree, T. A. Arun, S. V. Naira and A. S. Nair, J. Mater. Chem., 2014, 2, 4474–4490 RSC.
- L. Y. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. F. Zhang, X. D. Yang and M. Yanagida, Energy Environ. Sci., 2012, 5, 6057 RSC.
- I. K. Popoola, M. A. Gondal, J. M. AlGhamdi and T. F. Qahtan, Sci. Rep., 2018, 8, 12864 CrossRef PubMed.
- I.-P. Liu, Y.-C. Hou, C.-W. Li and Y.-L. Lee, J. Mater. Chem. A, 2017, 5, 240 RSC.
- W. Hong, Y. Xu, G. Lu, C. Li and G. Shi, Electrochem. Commun., 2008, 10, 1555–1558 CrossRef CAS.
- Z. Yang, M. Liu, C. Zhang, W. W. Tjiu, T. Liu and H. Peng, Angew. Chem., Int. Ed., 2013, 52(14), 3996–3999 CrossRef CAS PubMed.
- Y. Jo, J. Y. Cheon, J. Yu, H. Y. Jeong, C.-H. Han, Y. Jun and S. H. Joo, Chem. Commun., 2012, 48, 8057–8059 RSC.
- B. E. Hardin, H. J. Snaith and M. D. McGehee, Nat. Photonics, 2012, 6, 162–169 CrossRef CAS.
- L. Kavan, Y. Saygili, F. Freitag, S. M. Zakeeruddin, A. Hagfeld and M. Grätzel, Electrochim. Acta, 2017, 227, 194–202 CrossRef CAS.
- S. Mathew, A. Yella, P. Gao, R. H. Baker, B. F. E. Curchod, N. A. Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- C. Bu, Y. Liu, Z. Yu, S. You, N. Huang, L. Liang and X.-Z. Zhao, ACS Appl. Mater. Interfaces, 2013, 5, 7432–7438 CrossRef CAS PubMed.
- X. Xu, W. Yang, Y. Li, Z. Tu, S. Wu, X. Ma, F. Yang, L. Zhang, S. Chen, A. Wang, H. Liu and P. Richard, Chem. Eng. J., 2015, 267, 289–296 CrossRef CAS.
- W. Yang, X. Xu, Z. Tu, Z. Li, B. You, Y. Li, S. I. Raj, F. Yang, L. Zhang, S. Chen and A. Wang, Electrochim. Acta, 2015, 173, 715–720 CrossRef CAS.
- D. Yu, E. Nagelli, F. Du and L. Dai, J. Phys. Chem. Lett., 2010, 1, 2165–2173 CrossRef CAS.
- D. S. Su, J. Zhang, B. Frank, A. Thomas, X. Wang, J. Paraknowitsch and R. Schlögl, ChemSusChem, 2010, 3, 169–180 CrossRef CAS PubMed.
- J. Ou, B. Hu, S. He, W. Wang and Y. Han, Sol. Energy, 2020, 201, 693–700 CrossRef CAS.
- J. Ou, J. Xiang, J. Liu and L. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 14862–14870 CrossRef CAS PubMed.
- Z. Hu, K. Xia, J. Zhang, Z. Hu and Y. Zhu, Electrochim. Acta, 2015, 170, 39–47 CrossRef CAS.
- Y. Duan, Q. Tang, J. Liu, B. He and L. Yu, Angew. Chem. Int. Ed., 2014, 53, 14569–14574 CrossRef CAS PubMed.
- M. Du, X. Li, H. Pang and Q. Xu, EnergyChem, 2023, 5(2), 100083 CrossRef CAS.
- J. Duan, Y. Zhao, B. He and Q. Tang, Electrochim. Acta, 2018, 278, 204–209 CrossRef CAS.
- H. Jung, V. S. Sapner, A. Adhikari, B. R. Sathe and R. Patel, Front. Chem., 2022, 10, 881495 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhao, J. Duan and Q. Tang, Electrochim. Acta, 2018, 261, 588–595 CrossRef CAS.
- T. Zhang, J. Bai, P. Yang, F. Li, X. Jin, Q. Zhang and Q. Li, Sol. Energy, 2018, 161, 159–162 CrossRef CAS.
- K. Xiong, G. Li, C. Jin and S. Jin, Mater. Lett., 2016, 164, 609–612 CrossRef CAS.
- T. Liu, Y. Zhao, J. Duan, B. He, J. Zheng and Q. Tang, Sol. Energy, 2018, 170, 762–768 CrossRef CAS.
- J. Xia, Q. Wang, Q. Xu, R. Yu, L. Chen, J. Jiao, S. Fan and H. Wu, Appl. Surf. Sci., 2020, 530, 147238 CrossRef CAS.
- C. Feng, G. Zhao, Y. Li, H. Cheng and Z. S. Wang, J. Power Sources, 2019, 426, 16–22 CrossRef CAS.
- W. L. Lu, R. Jiang, X. Yin and L. Y. Wang, Nano Res., 2019, 12, 159–163 CrossRef CAS.
- C. Y. Chang, K. S. Anuratha, Y.-H. Lin, Y. Xiao, P. Hasin and J.-Y. Linet, Sol. Energy, 2020, 206, 163–170 CrossRef CAS.
- K. Saranya, M. Rameez and A. Subramania, Eur. Polym. J., 2015, 66, 207–227 CrossRef CAS.
- S. S. B. Gunasekera, I. R. Perera and S. S. Gunathilaka, Solar Energy: Systems, Challenges, and Opportunities, 2020, ed. H. Tyagi, P. R. Chakraborty, S. Powar and A. Kumar Agarwal, pp. 345–371 Search PubMed.
- Q. Li, J. Wu, Q. Tang, Z. Lan, P. Li, J. Lin and L. Fan, Electrochem. Commun., 2008, 10, 1299–1302 CrossRef CAS.
- J. Wu, Y. Li, Q. Tang, G. Yue, J. Lin, M. Huang and L. Meng, Sci. Rep., 2014, 4, 4028 CrossRef PubMed.
- Z. Li, B. Ye, X. Hu, X. Ma, X. Zhang and Y. Deng, Electrochem. Commun., 2009, 11, 1768–1771 CrossRef CAS.
- R. S. Moraes, E. Saito, D. M. G. Leite, M. Massi and A. S. da Silva Sobrinho, Appl. Surf. Sci., 2016, 364, 229–234 CrossRef CAS.
- L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef CAS.
- S. Xu, Y. Luo, G. Liu, G. Qiao, W. Zhong, Z. Xiao, Y. Luo and H. Ou, Electrochim. Acta, 2015, 156, 20–28 CrossRef CAS.
- T. Xu, D. Kong, H. Tang, X. Qin, X. Li, A. Gurung, K. Kou, L. Chen, Q. Qiao and W. Huang, ACS Omega, 2020, 5, 8687–8696 CrossRef CAS PubMed.
- S. Kang, J. Kim, J.-Y. Kim, M. J. Lee, J. Kang, Y. J. Son, J. Jeong, S. H. Park, M. J. Ko and Y.-E. Sung, ACS Appl. Mater. Interfaces, 2018, 10, 8611–8620 CrossRef PubMed.
- S. Venkatesan, W.-H. Lin, T.-H. Hsu, H. Teng and Y.-L. Lee, ACS Sustainable Chem. Eng., 2022, 10, 2473–2483 CrossRef CAS.
- S. S. Jeon, C. Kim, J. Ko and S. S. Im, J. Phys. Chem. C, 2011, 115(44), 22035–22039 CrossRef CAS.
- S. S. Jeon, C. Kim, J. Kob and S. S. Im, J. Mater. Chem., 2011, 21, 8146 RSC.
- J. Wu, Q. Lin, L. Fan, Z. Lan, P. Li, J. Lin and S. Hao, J. Power Source, 2008, 181, 172–176 CrossRef CAS.
- C. Bu, Q. Tai, Y. Liu, S. Guo and X. Zhao, J. Power Sources, 2013, 221, 78–83 CrossRef CAS.
- D.-Y. Wang, Y. T. Jiang, C.-C. Chen., K.-C. Ho and H.-L. Chou, Angew. Chem., Int. Ed., 2013, 52, 6694–6698 CrossRef PubMed.
- X. Yao, B. He, L. Cui, J. Ti, H. Chen, Y. Duan and Q. Tang, Catal. Commun., 2022, 163, 106403 CrossRef CAS.
- J. Li, X. Li, T. Wang, P. He, F. Li, W. Chen and E. Wang, ACS Appl. Energy Mater., 2019, 2, 5824–5834 CrossRef CAS.
- P. Gu, D. Yang, X. Zhu, H. Sun, P. Wangyang, J. Li and H. Tian, AIP Adv., 2017, 7, 105219 CrossRef.
- F. I. Chowdhury, M. Buraidah, A. Arof, B.-E. Mellander and I. Noor, Sol. Energy, 2020, 196, 379–388 CrossRef CAS.
- J. Barichello, S. Gullace, A. Cusimano, G. Di Marco, F. Matteocci and G. Calogero, Appl. Sci., 2022, 12, 3159 CrossRef CAS.
- H. K. Kim, ACS Omega, 2023, 8, 6139–6163 CrossRef PubMed.
- Y.-F. Chiang, C.-H. Tsai, P. Chen and T.-F. Guo, Sol. Energy, 2012, 86, 1967–1972 CrossRef CAS.
- A. F. Nogueira, C. Longo and M.-A. De Paoli, Coord. Chem. Rev., 2004, 248, 1455–1468 CrossRef CAS.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110(11), 6595–6663 CrossRef CAS PubMed.
- K. Yoo, N. C. D. Nath, H. C. Kang, S. Muthu and J.-J. Lee, ECS J. Solid State Sci. Technol., 2021, 10, 025007 CrossRef CAS.
- S. H. Kang, M. J. Jeong, Y. K. Eom, I. T. Choi, S. M. Kwon, Y. Yoo, J. Kim, J. Kwon, J. H. Park and H. K. Kim, Adv. Energy Mater., 2017, 7(7), 1602117 CrossRef.
- S. Hattori, Y. Wada, S. Yanagida and S. J. Fukuzumi, J. Am. Chem. Soc., 2005, 127(26), 9648–9654 CrossRef CAS PubMed.
- G. P. Kini, S. J. Jeon and D. K. Moon, Adv. Funct. Mater., 2021, 31, 2007931 CrossRef CAS.
- Q. Liu, L. G. Gerling, F. Bernal-Texca, J. Toudert, T. Li, X. Zhan and J. Martorell, Adv. Energy Mater., 2020, 10, 1904196 CrossRef CAS.
- H. Nusbaumer, J.-E. Moser, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Gratzel, J. Phys. Chem. B, 2001, 105, 10461 CrossRef CAS.
- S. A. Sapp, C. M. Elliot, C. Contado, S. Caramori and C. A. Bignozzi, J. Am. Chem. Soc., 2002, 124, 11215–11222 CrossRef CAS PubMed.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, J. Am. Chem. Soc., 2010, 132(46), 16714–16724 CrossRef CAS PubMed.
- D. P. Hagberg, X. Jiang, E. Gabrielsson, M. Linder, T. Marinado, T. Brinck, A. Hagfeldt and L. Sun, J. Mater. Chem., 2009, 19, 7232 RSC.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334(6056), 629–634 CrossRef CAS PubMed.
- A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 2973–2977 CrossRef CAS PubMed.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, T. Kyomen and M. Hanaya, Chem. Commun., 2015, 51, 6315–6317 RSC.
- V. P. S. Perera and K. Tennakone, Sol. Energy Mater. Sol. Cells, 2003, 79, 249–255 CrossRef CAS.
- K. Tennakone, G. R. R. A. Kumara, A. R. Kumarasinghe, K. G. U. Wijayantha and P. M. Sirimanne, Semicond. Sci. Technol., 1995, 10, 1689–1693 CrossRef.
- G. R. R. A. Kumara, A. Konno, G. K. R. Senadeera, P. V. V. Jayaweera, D. B. R. A. De Silva and K. Tennakone, Sol. Energy Mater. Sol. Cells, 2001, 69, 195–199 CrossRef CAS.
- J. Cong, D. Kinschel, Q. Daniel, M. Safdari, E. Gabrielsson, H. Chen, P. H. Svensson, L. Sun and L. Kloo, J. Mater. Chem. A, 2016, 4(38), 14550–14554 RSC.
- S. C. Pradhan, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2018, 6(44), 22204–22214 RSC.
- Y. Cao, Y. Liu, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Joule, 2018, 2(6), 1108–1117 CrossRef CAS.
- Y. Ren, N. Flores-Díaz, D. Zhang, Y. Cao, J. D. Decoppet, G. C. Fish, J. E. Moser, S. M. Zakeeruddin, P. Wang, A. Hagfeldt and M. Grätzel, Adv. Funct. Mater., 2020, 30(50), 2004804 CrossRef CAS.
- J. M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, Adv. Energy Mater., 2020, 10(15), 2000124 CrossRef CAS.
- Y. Ren, D. Zhang, J. Suo, Y. Cao, F. T. Eickemeyer, N. Vlachopoulos, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nature, 2023, 613, 60 CrossRef CAS PubMed.
- A. B. Muñoz-García, I. Benesperi, G. Boschloo, J. J. Concepcion, J. H. Delcamp, E. A. Gibson, G. J. Meyer, M. Pavone, H. Pettersson, A. Hagfeldt and M. Freitag, Chem. Soc. Rev., 2021, 50, 12450 RSC.
- J. Barichello, P. Mariani, F. Matteocci, L. Vesce, A. Reale, A. Di Carlo, M. Lanza, G. Di Marco, S. Polizzi and G. Calogero, Nanomaterials, 2022, 12, 267 CrossRef CAS PubMed.
- C. Clavero, Nat. Photonics, 2014, 8, 95–103 CrossRef CAS.
- G. Boschloo, Front. Chem., 2014, 7, 2296–2646 Search PubMed.
- A. Kay and M. Grätzel, Chem. Mater., 2022, 14, 2930–2935 CrossRef.
- G. Kumara, K. Tennakone, V. P. S. Perera, A. Konno, S. Kaneko and M. Okuya, J. Phys. D: Appl. Phys., 2001, 34, 868–873 CrossRef CAS.
- Q. F. Zhang, C. S. Dandeneau, X. Y. Zhou and G. Z. Cao, Adv. Mater., 2009, 21, 4087–4108 CrossRef CAS.
- R. Tagliaferro, D. Colonna, T. M. Brown, A. Reale and A. Di Carlo, Opt. Express, 2013, 21(3), 3235 CrossRef CAS PubMed.
- S. Hore, C. Vetter, R. Kern, H. Smitc and A. Hinsch, Sol. Energy Mater. Sol. Cells, 2006, 90, 1176–1188 CrossRef CAS.
- S. Sasidharan, S. C. Pradhan, A. Jagadeesh, B. N. Nair, A. A. P. Mohamed, K. N. Narayanan Unni, S. Soman and U. N. S. Hareesh, ACS Appl. Energy Mater., 2020, 3, 12584–12595 CrossRef CAS.
- S. Venkatesan, Y.-S. Cho, I. P. Liu, H. Teng and Y.-L. Lee, Adv. Opt. Mater., 2021, 9, 2100936 CrossRef CAS.
- L. Zhang and J. M. Cole, ACS Appl. Mater. Interfaces, 2015, 7, 3427–3455 CrossRef CAS PubMed.
- R. Y. Ogura, S. Nakane, M. Morooka, M. Orihashi, Y. Suzuki and K. Noda, Appl. Phys. Lett., 2009, 94, 073308 CrossRef.
- Y. Hao, Y. Saygili, J. Y. Cong, A. Eriksson, W. X. Yang, J. B. Zhang, E. Polanski, K. Nonomura, S. M. Zakeeruddin, M. Grätzel, A. Hagfeldt and G. Boschloo, ACS Appl. Mater. Interfaces, 2016, 8, 32797–32804 CrossRef CAS PubMed.
- L. Zhang and J. M. Cole, J. Mater. Chem. A, 2017, 5, 19541–19559 RSC.
- N. Shibayama, H. Ozawa, M. Abe, Y. Ooyama and H. Arakawa, Chem. Commun., 2014, 50, 6398–6401 RSC.
- S. Armeli Minicante, E. Ambrosi, M. Back, J. Barichello, E. Cattaruzza, F. Gonella, E. Scantamburlo and E. Trave, J. Phys. D: Appl. Phys., 2016, 49, 295601 CrossRef.
- G. Calogero, J. Barichello, I. Citro, P. Mariani, L. Vesce, A. Bartolotta, A. Di Carlo and G. Di Marco, Dyes Pigm., 2018, 155, 75–83 CrossRef CAS.
- X.-F. Wang, C. H. Zhan, T. Maoka, Y. Wada and Y. Koyama, Chem. Phys. Lett., 2007, 447, 79–85 CrossRef CAS.
- P. Sanjay, K. Deepa, J. Madhavan and S. Sentill, Mater. Lett., 2018, 219, 158–162 CrossRef CAS.
- C. Encinas, E. Otazo, L. Rivera, S. Miltsov and J. Alonso, Tetrahedron Lett., 2002, 43, 8391 CrossRef CAS.
- Z. Li, Z. Hui Jin, K. Kasatani and H. Okamoto, Phys. B, 2006, 382, 229 CrossRef CAS.
- J. Park, N. Barbero, J. Yoon, E. Dell’Orto, S. Galliano, R. Borrelli, J.-H. Yum, D. Di Censo, M. Grätzel, M. K. Nazeeruddin, C. Barolo and G. Viscardi, Phys. Chem. Chem. Phys., 2014, 16, 24173 RSC.
- M. Urbani, G. De La Torre, M. K. Nazeeruddin and T. Torres, Chem. Soc. Rev., 2019, 48, 2738 RSC.
- C. C. Leznoff and A. B. P. Lever, Phthalocyanines, Wiley VCH, New York, 1997, pp. 1–4 Search PubMed.
- T. Ikeuchi, H. Nomoto, N. Masaki, M. J. Griffith, S. Mori and M. Kimura, Chem. Commun., 2014, 50, 1941 RSC.
- Q. Huaulmé, V. M. Mwalukuku, D. Joly, J. Liotier, Y. Kervella, P. Maldivi, S. Narbey, F. Oswald, A. J. Riquelme, J. A. Anta and R. Demadrille, Nat. Energy, 2020, 5, 468–477 CrossRef PubMed.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741–1754 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- Y. Zhuge, D. Xu, C. Zheng and S. Pu, Anal. Chim. Acta, 2019, 1079, 153–163 CrossRef CAS PubMed.
- R. A. Evans, T. L. Hanley, M. A. Skidmore, T. P. Davis, G. K. Such, L. H. Yee, G. E. Ball and D. A. Lewis, Nat. Mater., 2005, 4, 249–253 CrossRef CAS PubMed.
- D. Kim, K. Jeong, J. E. Kwon, H. Park, S. Lee, S. Kim and S. Y. Park, Nat. Commun., 2019, 10, 3089 CrossRef PubMed.
- S. Fauvel, A. J. Riquelme, J. M. Andrés Castán, V. Mwatati Mwalukuku, Y. Kervella, V. Kumar Challuri, F. Sauvage, S. Narbey, P. Maldivi, C. Aumaître and R. Demadrille, Chem. Sci., 2023, 14, 8497 RSC.
- International Energy Outlook 2023, https://www.eia.gov/outlooks/ieo/ (Last access 3/12/2023).
- Building Integrated Photovoltaic Market Size Report, 2030, https://www.grandviewresearch.com/industry-analysis/building-integrated-photovoltaics-bipv-market, (Last access 3/12/2023).
- S. Barkaszi and J. Dunlop, Sol. Energy, 2001, 333–338 Search PubMed.
- H. M. Lee and J. H. Yoon, Appl. Energy, 2018, 225, 1013 CrossRef.
- T. Miyazaki, A. Akisawa and T. Kashiwagi, Renewable Energy, 2005, 30, 281 CrossRef.
- C. Hachem, A. Athienitis and P. Fazio, Energy Procedia, 2014, 57, 1815 CrossRef.
- A. Ghosh, S. Sundaram and T. K. Mallick, Renewable Energy, 2019, 131, 730 CrossRef.
- F. Martellotta, A. Cannavale and U. Ayr, Energy Procedia, 2017, 126, 219 CrossRef CAS.
- S. Feroze, A. Distler, K. Forberich, I. Ahmed Channa, B. Doll, C. J. Brabec and H. J. Egelhaaf, Sol. Energy, 2023, 263, 111894 CrossRef CAS.
- P. Mariani, L. Vesce and A. Di Carlo, Semicond. Sci. Technol., 2015, 30, 104003 CrossRef.
- L. Vesce, A. Guidobaldi, P. Mariani and A. Di Carlo, in Scaling-up of Dye Sensitized Solar Modules, ed. M. L. Parisi and R. Basosi, World Scientific Reference of Hybrid Materials, 2019, ch. 3, vol. 2 Search PubMed.
- H. Kim, J. Jo, G. Lee, M. Shin and J. C. Lee, Sol. Energy, 2017, 155, 585 CrossRef CAS.
- M. Godfroy, J. Liotier, V. M. Mwalukuku, D. Joly, Q. Huaulmé, L. Cabau, C. Aumaitre, Y. Kervella, S. Narbey, F. Oswald, E. Palomares, C. A. González Flores, G. Oskam and R. Demadrille, Sustainable Energy Fuels, 2021, 5, 144 RSC.
- D. Magistri, A. Lembo, V. Mirruzzo, G. Viscardi, P. Mariani, R. Manfredi, V. Liparoti, A. Lanuti, C. Bignozzi, T. M. Brown, L. Bonandini, E. Busatto, D. Colonna, A. Di Carlo, A. Giglielmotti, A. Guidobaldi, R. Boaretto, D. Prencipe, C. Barolo, R. Tagliaferro, S. Caramori, L. Vesce, G. Soscia, A. Smarra, R. Riccitelli and A. Reale, HOPV15, 2015 Search PubMed.
- M. L. Parisi, S. Maranghi, L. Vesce, A. Sinicropi, A. Di Carlo and R. Basosi, Renewable Sustainable Energy Rev., 2020, 121, 109703 CrossRef.
- L. Vesce, P. Mariani, M. Calamante, A. Dessì, A. Mordini, L. Zani and A. Di Carlo, Sol. RRL, 2022, 2200403 CrossRef CAS.
- M. V. Khenkin, E. A. Katz, A. Abate, G. Bardizza, J. J. Berry, C. Brabec, F. Brunetti, V. Bulović, Q. Burlingame and A. Di Carlo, et al. , Nat. Energy, 2020, 5, 35–49 CrossRef.
- A. Roy, A. Ghosh, S. Bhandari, P. Selvaraj, S. Sundaram and T. K. Mallick, J. Phys. Chem. C, 2019, 123, 23834–23837 CrossRef CAS.
- H. Dinesh and J. M. Pearce, Renewable Sustainable Energy Rev., 2016, 54, 299–308 CrossRef.
- M. Kumar, D. Haillot and S. Gibout, Sol. Energy, 2022, 232, 18–34 CrossRef.
- T. Alinejad, M. Yaghoubi and A. Vadiee, Renewable Energy, 2020, 156, 1–13 CrossRef CAS.
- R. U. Sánchez, Á. J. C. Ferre, J. P. Alonso and Á. C. Ortega, Sci. Agric., 2012, 69(4), 233–239 CrossRef.
- A. Kavga, G. Trypanagnostopoulos, G. Zervoudakis and Y. Tripanagnostopoulos, Not. Bot. Horti Agrobot. Cluj-Napoca, 2018, 46(1), 206–212 CrossRef CAS.
- C. Zisisa, E. M. Pechlivania, S. Tsimikli, E. Mekeridisb, A. Laskarakisa and S. Logothetidis, Mater. Today: Proc, 2019, 19, 65–72 Search PubMed.
- J.-J. Kim, M. Kang, O. K. Kwak, Y.-J. Yoon, K. S. Min and M.-J. Chu, Int. J. Photoenergy, 2014, 1–7 Search PubMed.
- A. Dessì, M. Calamante, A. Sinicropi, M. L. Parisi, L. Vesce, P. Mariani, B. Taheri, M. Ciocca, A. Di Carlo, L. Zani, A. Mordini and G. Reginato, Sustainable Energy Fuels, 2020, 4, 2309 RSC.
- A. Dessì, D. A. Chalkias, S. Bilancia, A. Sinicropi, M. Calamante, A. Mordini, A. Karavioti, E. Stathatos, L. Zani and G. Reginato, Sustainable Energy Fuels, 2021, 5, 1171 RSC.
- D. A. Chalkias, C. Charalampopoulos, A. K. Andreopoulou, A. Karavioti and E. Stathatos, J. Power Sources, 2021, 496, 229842 CrossRef CAS.
- C. J. M. Emmott, J. A. Röhr, M. Campoy-Quiles, T. Kirchartz, A. Urbina, N. Ekins-Daukes and J. Nelson, Energy Environ. Sci., 2015, 8, 1317 RSC.
- A. Mourtzikou, D. Sygkridou, T. Georgakopoulos, G. Katsagounos and E. Stathatos, World Academy of Science, Engineering and Technology, Int. J. Struct. Constr. Eng., 2020, 14, 3 Search PubMed.
- N. Roslan, M. E. Ya’acob, D. Jamaludin, Y. Hashimoto, M. H. Othman, A. N. Iskandar, M. R. Ariffin, M. H. Ibrahim, J. Mailan and A. H. Jamaluddin, Agronomy, 2021, 11, 631 CrossRef.
- M. Freitag, J. Teuscher, Y. Saygili, X. Zhang, F. Giordano, P. Liska, J. Hua, S. M. Zakeeruddin, J.-E. Moser, M. Grätzel and A. Hagfeldt, Nat. Photonics, 2017, 11, 372–378 CrossRef CAS.
- A. Aslam, U. Mehmood, M. H. Arshad, A. Ishfaq, J. Zaheer, A. Ul Haq Khan and M. Sufyan, Sol. Energy, 2020, 207, 874–892 CrossRef CAS.
- M. Bradha, N. Balakrishnan, A. Suvitha, T. Arumanayagam, M. Rekha, P. Vivek, P. Ajay, V. Sangeetha and A. Steephen., Environ. Dev. Sustain., 2022, 24, 8807–8822 CrossRef.
- RICOH, https://industry.ricoh.com/en/dye-sensitized-solar-cell, (Last access 3/12/2023).
- EXEGER, https://www.exeger.com/powerfoyle/applications, (Last access 3/12/2023).
- J. F. Geisz, R. M. France, K. L. Schulte, M. A. Steiner, A. G. Norman, H. L. Guthrey, M. R. Young, T. Song and T. Moriarty, Nat. Energy, 2020, 5(4), 326–335 CrossRef CAS.
- L. Yuan, H. Michaels, R. Roy, M. Johansson, V. Öberg, A. Andruszkiewicz, X. Zhang, M. Freitag and E. M. J. Johansson, ACS Appl. Energy Mater., 2020, 3(4), 3157–3161 CrossRef CAS.
- Y. Z. Zheng, X. Tao, J. W. Zhang, X. S. Lai and N. Li, J. Power Sources, 2018, 376, 26–32 CrossRef CAS.
- T. Yamaguchi, Y. Uchida, S. Agatsuma and H. Arakawa, Sol. Energy Mater. Sol. Cells, 2009, 93, 733–736 CrossRef CAS.
- A. K. Baranwal, T. Shiki, Y. Ogomi, S. S. Pandey, T. Ma and S. Hayase, RSC Adv., 2014, 4, 47735 RSC.
- Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim and H. K. Kim, J. Mater. Chem. A, 2017, 5, 2297 RSC.
- S. Venkatesan, T.-H. Hsu, X.-W. Wong, H. Teng and Y.-L. Lee, Chem. Eng., 2022, 446(4), 137349 CrossRef CAS.
- P. Muni Mounika, M. Ambapuram, G. Maddala, S. Kalvapalli, M. K. Ganesha, A. K. Singh, N. Thota, M. Naga Rajesh, L. Giribabu, K. Venkateswarlu and R. Mitty, ACS Appl. Electron. Mater., 2023, 5, 5661–5667 CrossRef.
- G. Gianola, R. Speranza, F. Bella and A. Lamberti, Sol. Energy, 2023, 265, 112116 CrossRef CAS.
- T. J. Coutts, K. A. Emery and J. S. Ward, Prog. Photovoltaics, 2002, 10, 195–203 CAS.
- P. Liska, K. R. Thampi, M. Grätzel, D. Brémaud, D. Rudmann, H. M. Upadhyaya and A. N. Tiwari, Appl. Phys. Lett., 2006, 88, 203103 CrossRef.
- W. S. Jeong, J. W. Lee, S. Jung, J. H. Yun and N.-G. Park, Sol. Energy Mater. Sol. Cells, 2011, 95, 3419–3423 CrossRef CAS.
- S. Y. Chae, S. J. Park, O. S. Joo, Y. Jun, B. K. Min and Y. J. Hwang, Sci. Rep., 2016, 6, 30868 CrossRef CAS PubMed.
- S. Ito, I. M. Dharmadasa, G. J. Tolan, J. S. Roberts, G. Hill, H. Miura, J.-H. Yum, P. Pechy, P. Liska, P. Comte and M. Grätzel, Sol. Energy, 2011, 85, 1220–1225 CrossRef CAS.
- J. Kwon, M. J. Im, C. U. Kim, S. H. Won, S. B. Kang, S. H. Kang, I. T. Choi, H. K. Kim, I. H. Kim, J. H. Park and K. J. Choi, Energy Environ. Sci., 2016, 9, 3794 RSC.
- H. Feng, C. C. Stoumpos, R. P. H. Chang and M. G. Kanatzidis, J. Am. Chem. Soc., 2014, 136(22), 8094–8099 CrossRef PubMed.
- R. K. Kothandaraman, Y. Jiang, T. Feurer, A. N. Tiwari and F. Fu, Small Methods, 2020, 4, 2000395 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |