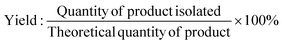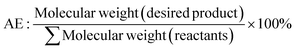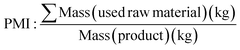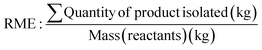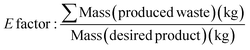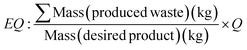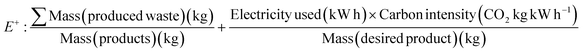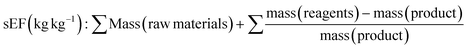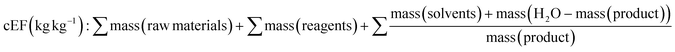Open Access Article
Open Access ArticleA tutorial review for research laboratories to support the vital path toward inherently sustainable and green synthetic chemistry
Sarah M.
Kernaghan
 ,
Tracey
Coady
,
Tracey
Coady
 ,
Michael
Kinsella
,
Michael
Kinsella
 and
Claire M.
Lennon
and
Claire M.
Lennon
 *
*
Pharmaceutical and Molecular Biotechnology Research Centre, South East Technological University, Cork Road, Waterford City, Co. Waterford X91 K0EK, Ireland. E-mail: Claire.Lennon@setu.ie
First published on 15th January 2024
Abstract
For synthetic chemistry to become inherently sustainable and green in its design and implementation, each research group must reflect, (re)assess practices, (re)define goals, embed new concepts, commit to a new mindset, and implement necessary change. This is a complex challenge, requiring measurable actions as starting points and during the implementation phase. This tutorial review aims to provide insight and background context into this area, highlighting several starting points to be considered by synthetic chemistry research laboratories. It is designed for new researchers to review at the start of their journey and to inform existing researchers of key methodologies for application in their research. Metrics, sustainable research design and solvent considerations are discussed, along with background understanding on commitment to change. The review also presents practical aspects to be applied within a laboratory and its daily practices and operations, a key element of any synthetic chemistry research.
Sustainability spotlightTo continue to contribute solutions to societal issues and support sustainable development, synthetic chemistry which generates key functional products that by their nature and in their production are inherently green and sustainable is required. Development of valuable technologies is needed, but we must assess and adapt the way our synthetic chemistry achieves this. Research groups in their design, education, mindset, and laboratory practices have a clear role, bringing innovation and skills, helping to achieve the UN Sustainable Development Goals (SDGs) of 12: ensure responsible consumption and production patterns, and 3: ensure healthy lives and promote well-being for all at all ages. This tutorial review provides concepts for consideration by synthetic research groups as starting elements on their journey, including aspects employed at an industrial level. We acknowledge these are not standalone solutions, however, are starting points, for reflection, discussion within teams, and implementation from which work can grow and expand. |
1. Introduction
Increasing awareness of the depletion of our global finite resources and our climate and biodiversity crises requires evaluation of our practices on an individual and industrial level. Chemistry has contributed significantly to societal development enhancing human lives, however in 1998 Anastas and Warner outlined that the chemical industry and chemists themselves had reached an ultimatum where the hazards associated with their industry; from an environmental, legislative, economic or social standpoint, must be considered with clear need to adapt and change.1 The concept of Green Chemistry, and “benign by design” syntheses pioneered in the 1990's, involves the reduction or elimination of the use of hazardous chemicals when manufacturing chemical products, along with most importantly, clear consideration for improvement in safety and environmental impact in the design phase. In 1998, Anastas and Warner, in the publication of Green Chemistry, Theory and Practice defined 12 guiding principles, outlined within Fig. 1, for safer, cleaner, more efficient processing and product development, aiming to reduce waste and energy usage.1 The Green Chemistry Institute (GCI), a non-profit corporation, to promote and advance green chemistry was established in 1997 and joined the American Chemical Society (ACS) to implement its goals on a global level in 2001.2 As an example of commitment to change within sectors, to enhance green chemistry within the field of pharmaceutics, a Pharmaceutical Roundtable (ACS GCIPR) was developed in 2005 and outlined their outreach towards the following areas; to advance research, define and deliver tools for innovation, promote training and education, and enable collaboration on a global level.3,4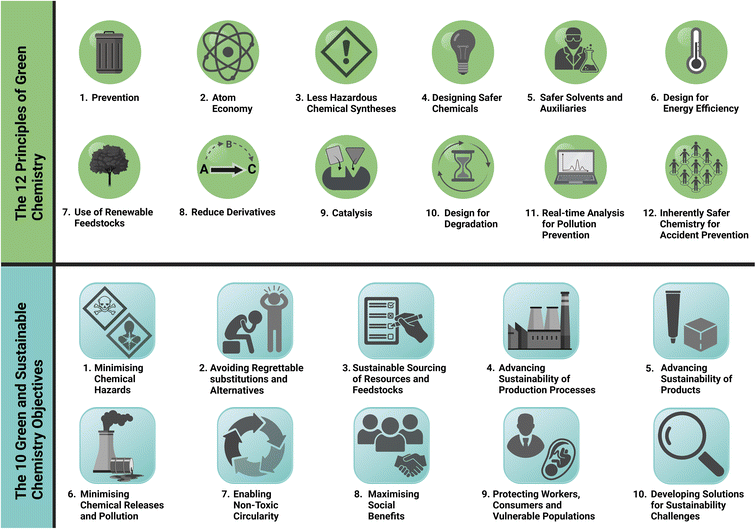 | ||
| Fig. 1 The 12 principles of green chemistry presented by Anastas and Warner in 1998 and the UN 10 green and sustainable chemistry objectives presented in the UN Environment Programme, Green and Sustainable Chemistry: Framework Manual. The figure has been created using https://www.biorender.com/.1,16 | ||
The concept of sustainable chemistry has also developed with definitions proposed for this area. As examples, the Organisation for Economic Co-operation and Development (OECD) provide a definition of sustainable chemistry as “a scientific concept that seeks to improve the efficiency with which natural resources are used to meet human needs for chemical products and services. …encompasses the design, manufacture and use of efficient, effective, safe and more environmentally benign chemical products and processes”.5 Sustainable chemistry works to support development within society but in a manner that remains within ecological limits.6 Within the special edition of Chemical Reviews in 2018, a definition was proposed for sustainable chemistry with respect to a chemical, reaction or process as “sustainable chemistry should use resources, including energy, at a rate at which they can be replaced naturally, and the generation of waste cannot be faster than the rate of their remediation”.7 The author comments that the target for chemicals, reactions and processes should be to aim that they are both green and sustainable in design and use. Reflection and assessment on the clear need for a chemical solution is also a consideration within sustainable chemistry, and if deemed required then detailed evaluation of product function and service must be undertaken.8 A key remit is the management of chemicals, and if new products are deemed necessary, innovation is necessary in their manufacturing processes, with the goal to protect the environment and society. A discussion of the respective development and definition of green chemistry and sustainable chemistry, their context, similarities, intersection and differences are provided by Zuin et al. in their 2021 review paper.9
Green chemistry with its clear framework, guiding principles for design, life cycle consideration from source of chemical across the process to product, and drive for continuous improvement in products and process is a central pillar of sustainable chemistry.10 Along with green chemistry there are other interdisciplinary supporting and empowering elements needed such as education, policy, awareness and equity required to ensure chemistry supports sustainability in a global sense.11 Anastas and Zimmerman further built on the principles and concept of green chemistry and green engineering, as pillars of sustainable chemistry to define their metaphoric “The Periodic Table of elements of Green and Sustainable Chemistry”.12 This represents how chemistry and engineering, humanitarian aims, enabling systems and noble goals can aid chemists to work to a sustainable future. It also demonstrates the need for thinking beyond the discipline of chemistry with connection between science and technology, the environment, social and policy development paramount. Such complete thinking applied to chemistry is needed for it to play its role to achieve the 17 United Nations (UN) Sustainable Development Goals and their indicators.13
A 2023 publication, by Lane et al. discusses how green chemistry can contribute to environmental and social justice.14 The authors discuss how traditional chemistry, manufacturing and the waste generated has in part contributed to environmental injustices and provide insight into how green chemistry applied with systems thinking approaches could work to counteract these issues. They highlight that evolution towards a sustainable and circular economy, realistically is not a choice but a necessity.14 Laskar et al. provide insight into how integration of environmental and social justice concepts within the chemistry educational curricula with training on green chemistry will provide key skills to future scientists to guide their work.15
The International Union of Pure and Applied Chemistry (IUPAC) established a Committee on Green Chemistry for Sustainable Development in 2017 to highlight, promote and develop their objectives within Green and Sustainable Chemistry.17 The UN Environment Programme in their The Green and Sustainable Chemistry: Framework detail that innovation in chemistry can contribute to sustainable development and achievement of SDG's and targets, with the education sector a key enabling contributor. It has outlined 10 Green and Sustainable Objectives and guiding considerations which chemists at all levels can consider in their research design and where products need to be synthesised the 12 Principles of Green Chemistry provide a clear guiding ethos (Fig. 1).16,18
For chemistry to support sustainable development, it needs to aim to be self-reflective, understanding the needs outlined above and hence inherently green and sustainable in its design and daily working practices. This will require commitment, developing researcher education, building awareness of issues and respective solutions and taking input from industries. It must be acknowledged however, that such change could be difficult from the perspective of knowledge, time, cost, perceived need for results/output, publishing successes and precedence. There can be a sense of lack of experience, resources, and education in sustainability for research leads and students and hence a lack of confidence in implementing change. It can also appear daunting to meet all required areas within the definition of sustainability i.e., society, environment and economic and this must be acknowledged. Movement into areas such as catalysis and biocatalysis will clearly support the development of highly efficient methods and technologies and the effects of this cannot not be underestimated, but this can take time, resources, and collaboration for research groups to establish.
However, we believe it is possible to target immediate starting points for reflection, utilising well established available tools and literature to support learning. In this manner, this tutorial review is aimed as a support, to provide starting insights to undergraduate, postgraduate, academic and industry researchers for reflection and to prompt manageable change in this area. It consists of five key areas, each reviewed, to allow reflection individually or preferably, as collective themes. The areas include background context to legislation, commitments and common goals, process metrics and toolkits, sustainable product design concepts and solvent. The final aspect is practical change which can be applied within a laboratory and its daily practices to become more sustainable in its operations. Our background lies in synthetic organic chemistry research aligned to fine chemicals and pharmaceuticals, therefore examples related to this underpin sections and are provided as potential options for consideration within related synthetic applications. Excellent detailed perspectives and reviews also include green chemistry metrics, solvent and the future of chemical production in their considerations for industry.19–21 Our approach also includes these thematic areas from the perspective of pointing to suitability for direct application and adoption within the research laboratory and ethos of research groups. There are also clear learning support tools and guides available on websites such as the ACS GCI or Beyond Benign22,23 and within each section where possible, examples of further specific reviews which provide a higher level of detail are provided.
2. Legislation, directives, commitments and common goals
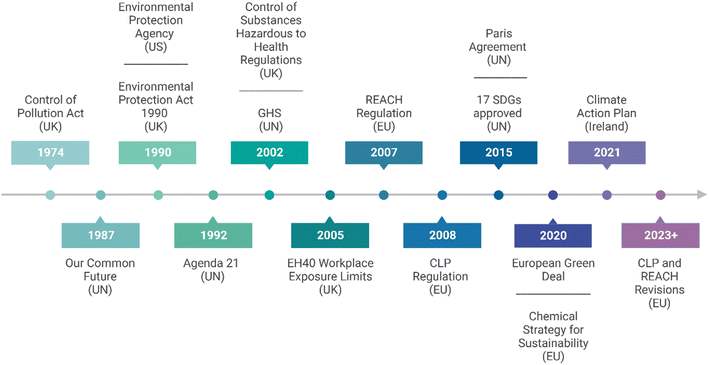
The ability to protect the environment while developing chemicals/materials, efficient processing methodologies and economies has been an ongoing challenge for countries and manufacturing industries. Legislation and regulation have been implemented to address this with respect to waste, pollution prevention, safety and handling of chemicals and insight to this is provided in this section. The Pollution Prevention Act of 1990 in the US noted the correlation between the production of pollution and the subsequent expense of attempting to control this waste.24 In response the US Environmental Protection Agency (EPA) was delegated the role of enforcing source reduction rather than a waste management approach. In the same year, the UK government updated their 1974 Control of Pollution Act under the legislation ‘The Environmental Protection Act 1990’. As with the US legislative counterpart, this regulation acknowledged the duty of care on producers and importers to risk assess, prevent pollution and dispose of controlled waste in an authorised manner.25There has also been a transition towards a global standardisation relating to the communication of the physical, health and environmental hazards associated with chemicals. The ‘Global Harmonized System’ (GHS) of classification and labelling of chemicals was developed by the UN as an international outline of criteria for chemical hazard testing and classification.26 The European Union (EU) used this GHS agreement when implementing their Classification, Labelling and Packaging (CLP) regulation in 2008, helping to identify hazards and inform of the intrinsic dangerous properties of chemicals to manufacturers, distributors and downstream users.27 This built on the previous year's EU legislation the REACH Regulation (Registration, Evaluation, Authorisation and Restriction of Chemicals), to hold companies responsible for identifying and managing the risks and hazards of any chemicals they manufacture or import.28 This directive encourages the substitution of hazardous substances for less dangerous alternatives, although also acknowledges the need for these replacements to be economically and technically feasible. This is an important consideration as the sustainability of a chemical process cannot only be defined by the human health and environmental consequences but must consider the efficiency and economic viability of a synthetic methodology.29,30 Complementary to REACH are the two UK regulations, Control of Substances Hazardous to Health (COSHH) and EH40/2005 Workplace Exposure Limits (WELs).31,32 COSHH also calls for a risk assessment, however, this is to be carried out by the employer rather than the manufacturer. This is significant in the context of safety as human health should be addressed for both those directly involved through occupational exposure and those indirectly affected through pollution or accidental release of toxic chemicals.
Sustainable development was defined by the Our Common Future/Brundtland report in 1987 as “meeting the needs of the present without compromising the ability of future generations to meet their own needs”.33 Agenda 21, the global UN action plan for human impact on the environment and development adopted in 1992 in Rio de Janeiro and Johannesburg in 2002, detailed specific aims and actions for the protection of water quality, and the management of toxic chemicals and hazardous wastes with requirements for safe substitutes for chemicals with long life cycles.34 In 2015 the UN 2030 Agenda for Sustainable Development, outlined 17 Sustainable Development Goals (SDGs), with 179 targets, which countries committed to collectively implement by 2030. The previous UN Agenda 21 was used as a foundation to inform and develop a blueprint for challenging current global practices, creating sustainable opportunities, ensuring equality for all global citizens, and offering pathways for reaching environmental, social and economic sustainable targets.13,35 Several of the SDGs are clearly relevant to all sectors of the chemical industry including scaled production and academic research such as; climate action (SDG 13), life below water (SDG 14), and life on land (SDG 15). Additionally, by targeting an improvement in human health through novel treatments, sustainable energy utilisation and toxic waste reduction, progress could be made towards: good health and well-being (SDG 3), clean water and sanitisation (SDG 6), affordable and clean energy (SDG 7) and sustainable cities and communities (SDG 11). Improvements in sustainability within the chemical sector could provide economic benefits and fulfil; industry innovation and infrastructure (SDG 9) and most importantly responsible consumption and production (SDG 12).13,36,37
The Paris Agreement in 2015 was a strategic and pioneering binding agreement which for the first time brought all nations together to tackle and take adaptive measures towards climate change.38 To align with its objectives the international community has outlined and published national guidelines on how they will reduce greenhouse gas emissions and achieve a circular economy; such as in our country, Ireland, with its Climate Action and Low Carbon Development (Amendment) Act 2021 and associated Climate Action Plans of 2021 and 2023 requiring all industries and sectors to play their role.39,40
A timeline of discussed legislation and directives established over the last 50 years is presented in Fig. 2. As time has progressed so has the need to revisit regulations and introduce amendments and modifications to ensure that the current legislation remains sufficient. Building upon the EU's rich history of chemical protective regulatory frameworks, the EU have highlighted the need for innovation and evolution of existing policies.41 Many regions share the common goal of becoming a sustainable and resource efficient competitive economic system, with the EU hoping to make this vision a reality through their European Green Deal and Chemical Strategy for Sustainability, introduced in 2020. Some of the key actions focus on investing in sustainable-by-design chemicals, promoting the EU's network and supply of critical chemicals and continuing to encourage a leading role in chemical safety and sustainability.41 As a consequence of this work, CLP and REACH regulations are currently undergoing revisions to ensure that hazard communication is clear, legal obligations clarified and new hazard classes are included such as persistent bioaccumulative, endocrine disrupting and persistent and mobile.42,43
To move forward, education is critical. The United Nations Global Action Programme (UN GAP) on Education for Sustainable Development (ESD), is a comprehensive initiative aimed at advancing sustainable development through education. It encourages the integration of sustainability principles within education, fostering a global commitment to transform learning for a more sustainable future.44 Its key objectives include promoting policy dialogue, capacity building, and knowledge sharing to empower individuals with the skills and values needed to address global challenges. The European Sustainability Competence Framework (GreenComp) is closely connected to ESD within the European Union. GreenComp defines essential skills, knowledge and attitudes needed for individuals to promote sustainability across sectors, guiding educators, employers and policymakers in developing programmes aligned with ESD objectives.45
Guided by these, national policies in ESD have been developed, as an example, our country, Ireland launched “ESD to 2030” which encompasses education, including third-level, research, and community engagement46 and builds on the achievements of the previous strategy (2014-2020). Sustainability and inclusion must underpin education, including chemistry, from teaching and research to governance and community partnerships. Chemistry education that incorporates ESD will cultivate a new generation of chemists who are not only skilled in traditional chemical disciplines but are also aware of and committed to sustainable practices and ethical considerations. By fostering ESD leadership within educational institutions and promoting collaborative involvement in decision-making, ESD encourages critical thinking, raises awareness of the environmental and ethical dimensions of chemical research and applications, empowers citizens to explore innovative visions and solutions: ensuring society is well-prepared for sustainable future challenges.47
3. Green chemistry metrics, toolkits and life cycle analysis
3.1 Development and standardisation of metrics
As a starting point within synthetic methodology development programmes, to implement green and sustainable chemistry concepts, it is necessary to assess reactions and developed processes routinely using suitable metrics, and most importantly reflect on the results and their significance. In an academic or discovery research setting, synthesis reactions and processes often employ smaller scale reactions, less efficient work-ups, resource intensive purifications, with less application of such measurements. Processes at industrial level for example, to produce pharmaceuticals, move through discovery, development and scale-up phases where reactions are refined, improved, chemicals altered, and a focus brought to greener, more efficient methods. The level of application of metrics significantly increases within the development and pre-commercial stage to yield arguably greener processes. Within a green and sustainable chemistry mindset the use of metrics however, should not be limited to industrial development settings but expanded in discovery and academic synthetic chemistry research to drive reflection and change. Examples of structure-, reaction-, and process-based metrics are illustrated in Table 1 and discussed in this section. It is important to understand the development of such metrics, hence, to design appropriate combinations and toolboxes for research applications and there are some excellent examples of publications discussing metrics in this regard.19,20,48,49Traditionally, product yield has been used by chemists to determine how efficient their chemical synthesis reaction is with its formula shown as eqn (1) in Table 1.50 A caveat of this key metric is that yield ignores the use of auxiliaries and doesn't consider the hazardous nature of the chemicals employed in the reaction.51 Barry Trost introduced the atom economy metric in 1991, where the molecular weight of the desired product is divided by the total sum of all the reactants' molecular weights in the stoichiometric reaction (Table 1, eqn (2)).19,52 Maximum efficiency can be achieved through minimum production of innocuous by-products or discovering an in-house or marketable function.52,53 Importantly, this metric can be calculated prior to experiments; allowing chemists to identify atom inefficient reactions and strategically design or optimise the reaction before they begin.54 Assumptions are made such as 100% chemical yield and that exact stoichiometric amounts of reactants are used. Solvents and auxiliary reagents such as acids or bases used in the work-up are not included, since they are not present in the stoichiometric equation.36,55 Reaction Mass Efficiency (RME) (Table 1, eqn (4)) was later introduced to build upon the work of Trost and his atom economy calculations. The RME was proposed by Constable et al. as an industrial method to consider yield and excess reagents that would remain in the product, that could be utilised by chemists, process chemists and chemical engineers.56
The development of waste-based metrics has been important to quantify and measure greener process and synthetic route development. The environmental factor (E factor) metric (ideal = 0) was introduced by Sheldon to account for the actual amount of waste produced in a chemical process per kilo of product (Table 1, eqn (6)).58 The E factor did not include water and included the concept of solvent recycling (an assumption could be made that 90% of solvent could be recycled with 10% loss inputted in the waste factor calculation). In an academic or early development research setting, however such recycling is much less likely. Sheldon published a comparison of E factors for various specialities of the chemical industry in 2007, highlighting that though oil and the bulk commodity chemical sectors have the largest production in tonnage, their manufacturing processes are extremely efficient when compared to the fine chemical and pharmaceutical sectors.51,61 Within bulk and oil refining the implementation of catalysis and continuous flow have had a strong impact increasing efficiency.62–65 Contrastingly, multi-step synthesis and purification to produce more complex fine chemicals and pharmaceuticals result in much higher E factors and environmental and economic cost; in part due to the large quantities of solvent required.
It must be clearly stated that correctly specifying the boundary or starting point of the analysis is critical to ensure accurate reflection of the E factor.19 The E factor of production of the starting material should not be ignored, particularly in industrial pharmaceutical synthesis where outsourcing of such materials for production by a contract supplier may be completed. An addendum of E factor was introduced, an environmental quotient (EQ), where Q represents an arbitrary unfriendliness multiplier to acknowledge that not all waste is equal, with various factors such as toxicity and ease of recycling coming into play (Table 1, eqn (7)).58
Originally energy was taken into consideration when comparing the E factor values between various industry sectors since this input produces carbon dioxide waste. This was not fully feasible however as the energy may not be devoted to individual product production but used to drive multi-purpose manufacturing sites.51 Often energy consumption is much less considered in academic research laboratory settings, with product and impurity profile given priority, and the use of holding or accepted times such as “overnight” common. While energy consumption may seem much less when compared to industrial applications, this is an important area for all laboratories to consider in a complete approach to sustainability and small changes, reaction understanding, and process development that can lead to benefits. As an example, McElroy et al. note that running reaction at reflux can result in a 6-fold increase in energy consumption when compared to running the reaction at 5 °C below this point.66 This will be discussed further in a later section.
The lack of inclusion of energy demands, electrical power and emissions within the E factor was noted in 2019.59 An adapted E factor, the E+ factor presented by the authors includes the emissions caused by electricity generation and evaluated the complete production of a recombinant enzyme in laboratory setting and its reaction (Table 1, eqn (8)). It was observed that the electricity emissions contributed to >80% of the total waste generated. The authors also highlighted the impact of not including waste generated in the enzyme preparation in the E factor metric for their chosen reaction which clearly significantly reduced the value.59 The authors also note that processes including biocatalysis and catalysis should be clearly and critically evaluated for their environmental consequences before labelling them “green”.
An extension to the E-factor concept relating to organocatalysis was introduced by Antenucci et al.67 Here, the authors went beyond the classification of various organocatalysts as “green” purely arising from their metal free and natural scaffold origins. The authors recognised that organocatalysts are becoming more complex, designed, and engineered to be more reactive and enantioselective with the drive for more efficient catalysts, with less consideration for the sustainability of organocatalyst synthesis. The authors global E-factor (EG-factor) expanded to include the synthetic route of the catalyst in a more complete manner when compared to the classical E-factor.
The authors calculated the EG-factor for a series of reactions and organocatalysts. One example compared the use of a tert-butyldiphenylsilyl(TBDPS)-protected proline catalyst to a prolinothioamide alternative in the reaction of 4-nitrobenzaldehyde with cyclohexanone and found a 9-fold increase in EG relative to the proline analogue, resulting from the 3-step synthesis of the thioamide catalyst (Scheme 1). The prolinothioamide catalysed process would have had a much higher EG-factor than 95 but in this case, solvent intensive column chromatography was avoided in its synthesis as the authors could purify the catalyst by crystallisation, recover and reuse the catalyst using an acid-base extraction process. The authors applied the EG to further selected examples and highlighted the need to consider the sustainability of the complete process, including the preparation of catalysts, particularly where a system may be scaled for industrial use.68
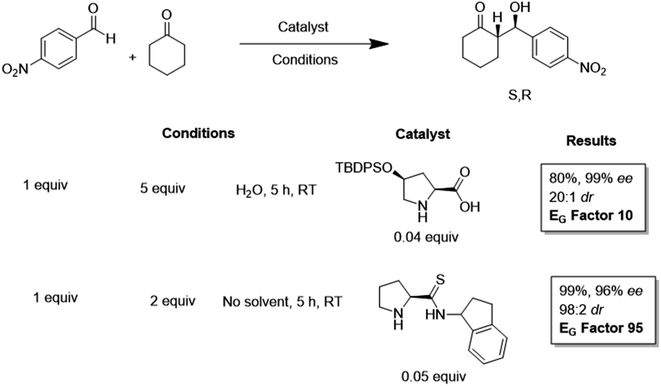 | ||
| Scheme 1 Comparison of global E-factors for TBDPS proline and prolinothioamido organocatalysts when promoting the Aldol reaction of p-nitrobenzaldehyde with cyclohexanone. Note: TBDPS = tert-butyldiphenylsilyl.67 | ||
In 2017 Roschanger et al. presented two adapted E factor metrics. These consisted of the simple E factor sEF and complete E factor cEF (Table 1, eqn (9) and eqn (10)).60 The cEF includes all process materials involved in the chemical reaction, raw materials, solvents, reagents, water, and final product. The simple E factor sEF does not include water and solvents and links to the early development phase where changes in solvent occur. From a commercial production perspective, the E factor can lie between the sEF and cEF and a recycling E factor can be generated once solvent recovery process is developed and loss quantified. Both the sEF and cEF are highly applicable for use in research laboratory settings across the complete process including the chosen method of purification, commonly flash chromatography.
Further metrics developed by the pharmaceutical industry are the Green Aspirational Levels of GAL, innovative GAL and iGAL 2.0 which allow standardisation, benchmarking within the pharmaceutical industry and assessment of synthetic routes for relative “greenness”.69–71 To develop the GAL the authors considered the compound structure and its complexity and its significant impact on the chemistry required for synthesis. The iGAL 2.0 presented in 2022 aims to significantly reduce process waste and fulfill UN SDG 12—in ensuring sustainable consumption and production.71 Yield and the term convergence are key indicators with Relative Process Greenness used as a measure of improvement or deficit (to 100%). Usefully both the iGAL and iGAL 2.0 generate a benchmarking scorecard, a visual representation of the impact of change. These metrics can provide insight in academic synthetic research and could offer potential to assess developments in synthetic routes, promoting process innovation and improvement, utilising industry standards to ensure relevance.
An alternative metric that does not target waste is Process Mass Intensity (PMI) (Table 1, eqn (3)). Here the total mass in a process is divided by the mass of the product, where the ideal value is 1. This includes reagents, reactants, catalysts, solvents and includes reaction work-up and purification processes. Solvent and work-up can largely impact PMI,57,72 these are particularly significant contributors in synthetic research laboratories where operations such as flash chromatography are still applied as purification tools. The PMI metric can be broken into components of the overall value, to view and separately compare different parts of the process i.e., PMI = PMI reaction reagents and catalyst + PMI solvent + PMI work-up etc. which can highlight areas for improvement.73 Monteith et al. noted benchmarking to literature and comparison of different synthetic procedures using PMI in the discovery laboratory requires further consideration in the context of yield, molecular weight of reagent and products and reaction concentration variations. The authors note that PMI as a single metric is valuable, however as with all metrics this should not be evaluated in isolation but assessed with other factors applied in a complete way.
The ACS GCIPR has chosen PMI as their primary green metric that is mass-based as they believe this will encourage a broader approach encompassing increased process innovation, resource optimisation and efficiency rather than focusing solely on end-of-pipe waste reduction.57 A PMI calculator and PMI predictor (requiring a proposed route from retrosynthesis), aimed primarily for use at route design phase, are available on the ACS GCIPR website.74 Such predictive tools can drive and support innovative route development. At an industrial level, Merck & Co won the EPA Presidential Award Green Chemistry Challenge: Greener Synthetic Pathways Award in 2021 for their Green and Sustainable production process for Gefapixant Citrate.75 The reduction of PMI from 366 to 88 was achieved and commodity chemicals applied in the synthetic route. A key element was the application of a SMART PMI tool that set aspirational PMI targets for the molecule from its chemical structure. This was correlated with data from a series of established processes. Compound complexity and molecular weight were considered, and its application for Gefapixant Citrate prompted the redesign of the manufacturing route.37,49,75
3.2 Combination of metrics and toolkits
Rather than a single metric being used in isolation to determine “greenness”, a series should be compared and analysed in a complete approach. The University of York and CHEM21 consortium have developed a ‘Metrics Toolkit’.66 for use at various stages in the development timeline of pharmaceuticals (screening, development, kilo/pilot plant and manufacturing) to allow comparison between processes to assess improvements. It combines quantitative (efficiency and mass-based) metrics and qualitative insights and was designed to be actively used at the bench. One objective in its development was to train researchers to analyse synthetic routes in the context of sustainability and environmental footprint. Raw material renewability, reagent preparation, purification, isolation, recycling, recovery of catalyst, energy, life cycle and solvent choice are considered. Health and safety via the GSH, and REACH considerations are included with safety study at the scale up and production stage. The toolkit applies metrics appropriate to the stage of the route across the passes or phases, with flag colour codes of green, red and amber for clear visual clarity within some parameters. The zero and first pass toolkits are practical for application as decision tools in synthetic research laboratory settings with metrics for first pass outlined in Fig. 3.66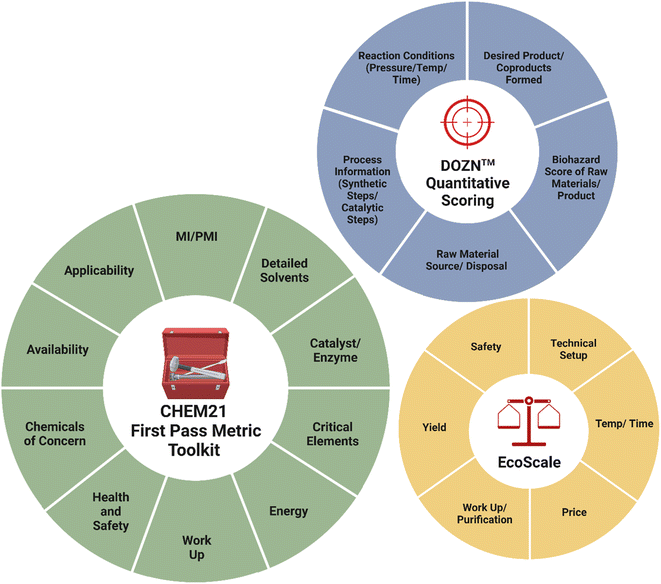 | ||
| Fig. 3 Key considerations from the CHEM 21 first pass toolkit, the EcoScale analysis tool and the DOZN™ quantitative scoring system relevant for synthetic chemistry researchers. Parameters adapted from 66,76,77 Image created using https://www.biorender.com/. | ||
Two further user-friendly tools for use in academic research setting both at undergraduate, postgraduate and researcher level are EcoScale and DOZN™ (Fig. 3).76,78 EcoScale is a post-synthesis evaluation tool that uses yield, safety, reaction conditions, cost, technical set-up and ease of work-up, to provide a score (from 100).54,76 Reactions are penalised according to their unfavourable safety, ecological and economic properties, with scores greater than 75 classed as excellent, those above 50 acceptable and <50 inadequate.76,79 As a further tool DOZN™ is a quantitative metric platform that defines how well a reaction aligns with the 12 principles of green chemistry (Fig. 3).77,79,80 The principles are categorised into 3 subgroups; those targeting improved resource use, those focused on reducing human and environmental hazards and increased energy efficiency.81 This calculator requires the user to add reagent information, reaction conditions, the products and monitoring systems. Unlike EcoScale, DOZN™ also evaluates if the raw materials are from renewable feedstocks encouraging the use of bio-renewable raw materials. The raw material source and their recyclability in the reaction, demonstrates how aligned a method is with the values of sustainability and a circular economy.81 Individual values are reported for each of the 12 principles, with an aggregate score of overall sustainability produced.
3.3 Life cycle analysis
While the metrics and toolkits above focus primarily on process development and assessment of reactions in the context of green chemistry, the assessment of sustainable research requires further and broader considerations.12 Life Cycle Assessments (LCA) are used in both industry and governmental organisations as a method of quantifying the impact of a service, production process or product.82,83 A cradle-to-grave LCA approach takes into consideration the environmental impacts, including the source of raw materials, how a product is used and its eventual disposal or recycling. A schematic presenting the LCA of a solvent as an example and the system boundaries of cradle-to-gate, gate-to-, cradle-to-grave is shown in Fig. 4 below.84,85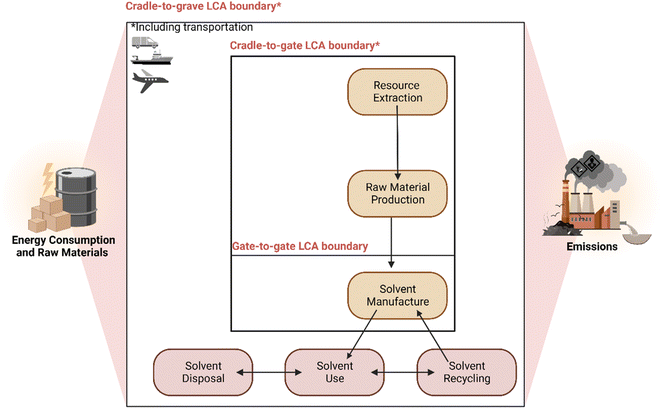 | ||
| Fig. 4 Boundary considerations when calculating the life cycle assessment of a solvent. Image created using https://www.biorender.com/. Adapted from ref. 84 and 85. | ||
The LCA considers energy, mass and larger quantifiable environmental factors for example, smog formation, global warming potential, human and eco-toxicity, acidification in addition to resource depletion and this is completed in four phases.84 The International Organisation for Standardisation (ISO) have published an LCA framework (ISO 14040).86 The first phase of LCA defines goals, scope, and boundaries. The second phase includes a detailed Life Cycle Inventory (LCI) and process of data collection, for example energy and material extraction, inputs, and releases. Phases 3 and 4 are impact assessments to analyse the relevance and implications of phase 2, with weightings and interpretation applying data to the LCA goal.
LCA has some constraints, it is time-intensive, complex, and different assessments of the same product can produce varying results due to its sensitivity and the amount and quality of the information inputted. There may also be limited data available for starting materials. In the context of pharmaceuticals, many rapid changes requiring evaluation occur within the early development phase timelines. As a result, evaluation limited to a cradle-to-gate or even gate-to-gate basis considering only the manufacturing may be completed; or developed metric systems and tool kits may be used which may then move to LCA at later stages.29,87
In practice, LCA is rarely employed as a routine practice in academic synthetic research, mainly due to the inherent complexities, the evolving nature of processes, their variability, and the scarcity of data and training in this domain. Comprehension of LCA will become increasingly necessary however to guarantee that developments effectively contribute to quantifiable improvements in sustainability.49 To support LCA, databases such as ecoQuery and ecoinvent are available and contain “datasets”, that simulate human activities and processes offering insights into environmental impacts and services.88 These datasets encompass details about industrial processes, including resource consumption, emissions to air/water/soil, electricity usage and the creation of products, co-products, and waste. Life Cycle Impact Assessment (LCIA) scores are available for each dataset for several impact assessment methods such as those outlined by the Intergovernmental Panel on Climate Change “IPCC 2021” and “ReCiPe”, which include categories such as climate change, human toxicity, water use and land use.89,90 The IPCC periodically publishes Assessment Reports (ARs) that present emission metrics for Global Warming Potential (GWP) and Global Temperature Change (WTP). However, there are limitations when using ecoQuery involving non-transparent gate-to-gate data blocks, diverse original data sources, and the inclusion of unrevealed proprietary data.91,92
Within literature Sheldon has summarised examples of LCA approaches applied and developed within the chemical and pharmaceutical industries.19,51 In addition, Khoo et al. provide an informative discussion of the evolution of LCA within the chemical industry, highlighting many examples of its application in solvent, synthesis, waste, chemical and pharmaceutical manufacture, and assessment of biomass derived chemicals. The authors note the varying level of complexities within types of LCA and the challenge associated with data selection, proposing criteria for life cycle inventory for a multi-disciplinary research team comprised of chemists, engineers, industry/technologist and environmental scientist.93 While LCA is clearly challenging at the academic or development phase, level 1 life cycle approach or thinking, or with education, level 2 or streamlined LCA as outlined by Khoo et al., offer opportunities. Examples which demonstrate use of such a streamlined approach in a pharmaceutical development setting discussed below, are Q-SA√ESS “Quick Sustainability Assessment via Experimental Solvent Selection” and the LCA-PMI tool developed by MSD.49,94
The LCA-based Q-SA√ESS cradle-to-gate methodology for solvent choice assessment for active pharmaceutical ingredient (API) production, by Isoni et al. examined information on human health, environment and cost for a series of solvents.94 A mini LCA database for solvent production (1 kg) was generated (including petroleum and biomass derived) as part of the assessment. Considerations were given to treatments such as incineration, recycling, and cleaning at the end of use. An excel spreadsheet tool allowed the user to provide inputs (mass and energy balances) from lab-scale tests, and simulations of how spent solvent was handled. The current list includes 10 solvents. Ultimately, the selection of solvent requires decisions, sustainability deliberations, and a consideration of trade-offs.
A streamlined PMI-LCA Tool, developed by MSD in collaboration with the ACS GCI Pharmaceutical Roundtable, allows the user to assess commodity raw materials and their life cycle in developed routes.49,95 LCA data was accumulated for materials across a series of indicators and used in combination with other metrics including PMI and energy to quickly determine points for attention. This approach is hence from cradle (including extraction and mining)-to-gate, as assessment includes the routes themselves via a user-friendly tool. The authors note adding a streamlined LCA process across the pharmaceutical supply chain could lead to increased availability of data for further raw materials and intermediates, shared in a similar manner to providing a certificate of analysis. Data could be collected as the materials are produced. At a commercial level however, it is likely this would require agreements between commodity manufacturers and suppliers and pharmaceutical companies regarding sharing such data.
4. Sustainability by design
In their discussion of the future of chemical products and processes, Zimmerman et al. present current issues with the sector in design, linear production route and focus on performance.12 The authors highlight the need for change and that the future design of products and processes requires increased performance, defined function and evaluation of toxicity, biodegradability and persistence. It is imperative that designers understand and evaluate these characteristics requiring that chemists collaborate and gain knowledge in toxicology, molecular and environmental mechanisms. This therefore requires an expansion in education. Additional areas for development include movement from fossil to renewable sources, utilisation of clean energy, development of new (bio)catalysts, design of energy efficient processes, new building blocks, and circular approaches.12 Synthetic chemists at all levels can take example from this discussion, working to evaluate their products comprehensively and critically, completing retrosynthesis to renewable materials, aiming to utilise their reaction by-products as a resource in a circular manner, reflecting on the concepts of linear and circular economy (Fig. 5 below), and sustainability within their research design.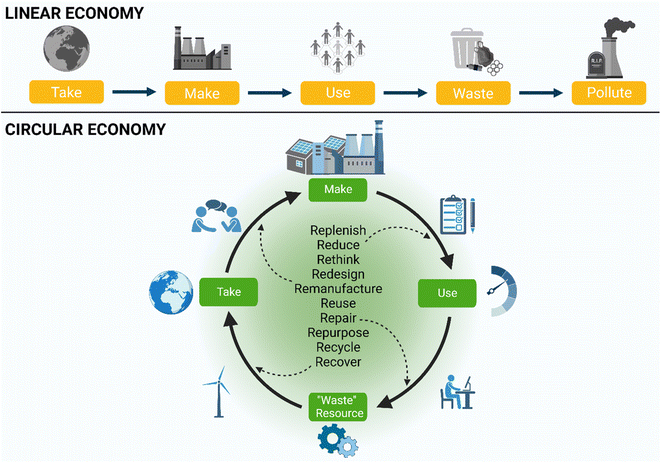 | ||
| Fig. 5 Schematic representing the linear vs. circular economy. Image created using https://www.biorender.com/. | ||
In the context of new medicines, a group of researchers within European Institutions have proposed 10 elements (including amongst these: ecological-environmental impact, medical need, green chemistry, cost, lean discovery process and responsible research and innovation) to ensure sustainability within pharmaceutical drug discovery to design and develop sustainable medicinal products.96 These are considered as connected and provide opportunities for reflection, discussion and consideration of sustainability leading from the discovery rather than the development and clinical phases, with awareness that as the need for new medicine increases there is significant impact on the ecosystem and health.96 Rose et al. outlined the need for process development chemists to introduce a sustainability mindset within their workflows, utilising green chemistry and new process innovations. A green-by-design approach to process development would assess and evaluate routes in development with metrics for environmental impact and sustainability. Importantly the culture must support and empower this when assessing metrics and alternatives.49 In their 2022 perspective, Moermond et al. propose a GREENER concept for API discovery and development. This includes a “benign-by-design” approach, with patient and environment considered, aiming for less impact on the environment of the API post-use.97 The ACS GCI Pharmaceutical Roundtable also provides information and discussion on the life cycle and fate of API post-use.98
In 2022, Bechu and co-workers presented a qualitative checklist which early career or senior researchers can employ to design and guide their research projects with the goal of developing safer and more sustainable products.99 Within the checklist there are five sections with 20 items for self-scored assessment. The authors suggest this is completed at the beginning, during and at the end of the research project. Elements include thinking beyond the discipline in the context of social, economic and environmental considerations, (reviewing SDG's, 12 principles of green chemistry and/or green engineering, understanding planetary boundaries, circularity, functionality, reviewing current at scale alternatives). The checklist questions and guides to fully define the research question with materials, chemicals, processes, metrics, accepted quality control procedures and standards outlined. This is supported by a detailed literature review. A key element is that the designer reflects and takes a holistic view of their project and potential impact if it were to be scaled, which can be less considered in academic research, verifying safety data, properties and considering chemical life cycle. The final stage of evaluation concerns communication to both scientific and non-scientific audiences. This tool could act as a valuable guide for undergraduates in the completion of research components at the advanced stage of their programme or within research internships.
At a policy level, The European Green Deal and Chemicals Strategy for Sustainability, are signposting towards the goal that any future design, development, production and use of chemicals and materials achieves function without compromising on safety. This approach ensures chemicals and materials are Safe and Sustainable by Design (SSbD), offering dual benefits in terms of sustainability and economic impacts.41 The European Commission (EC) are working to develop a framework of guidance and process impact assessment for human health, climate, and the environment through the complete life cycle. The Joint Research Centre (JRC), report in 2022, provided a suggested design and assessment approach considering safety followed by environmental, social and economic considerations which is currently under review.100 The proposed SSbD framework has outlined a set of 8 design principles embodying green chemistry, green engineering, sustainable chemistry and EC policies (Fig. 6) which could act as a guiding framework in an academic or research setting.100 The entire chemical/material's life cycle understanding is paramount. Evaluation of the chemical/material is proposed, assessing adherence to SSbD principles and performance against safety and sustainability criteria.100
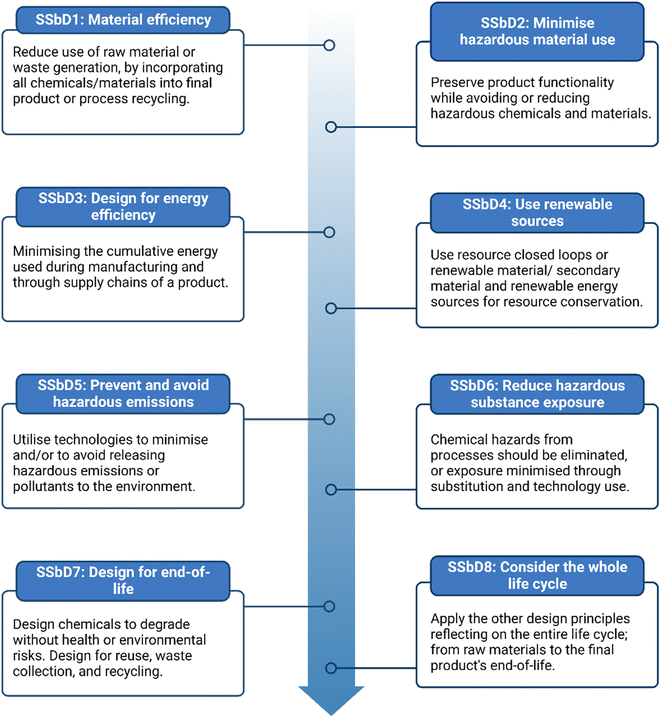 | ||
| Fig. 6 The eight SSbD principles as proposed by Caldeira et al. Image created using https://www.biorender.com/. Prepared with principles outlined in the JRC Report 2022.100 | ||
Discussion of the concept of sustainable by design is also provided in the UN The Green and Sustainable Chemistry: Framework.16
5. Solvent in synthetic chemistry
There are many domestic, academic and commercial uses of solvents as demonstrated by EU usage data for 2017 presented in Fig. 7.101 It has been estimated that the annual industrial-scale production of organic solvents is nearly 20 million metric tons.29,102 As outlined by Jiminez-Gonzalez “The most sustainable solvent is the solvent that is not used”.84 However, in many cases within synthetic chemistry for efficient reactivity and productivity solvent is a required. Therefore, the shift towards being safer and more sustainable in our solvent choice considering source and availability, toxicity, separability, usage volume in reaction and purification operations, along with investigation of alternative media are means to make improvements. Such alignment with green chemistry principle 5 applied at both large- and small-scale level will yield environmental and economic advantages. Solvents/auxiliaries are outlined in “The Periodic Table of Elements of Green and Sustainable Chemistry”, with aqueous biobased, ionic liquids/non-volatile, sub and supercritical and smart solvents included.103 It must be clear however that changing solvent to a “greener” solvent does not make a process or product green and sustainable, particularly if the product and overall process is toxic and hazardous to the environment. A detailed critical analysis and perspective of the challenges, questions and considerations in determining a ‘green’ solvent was provided by Winterton in 2021.104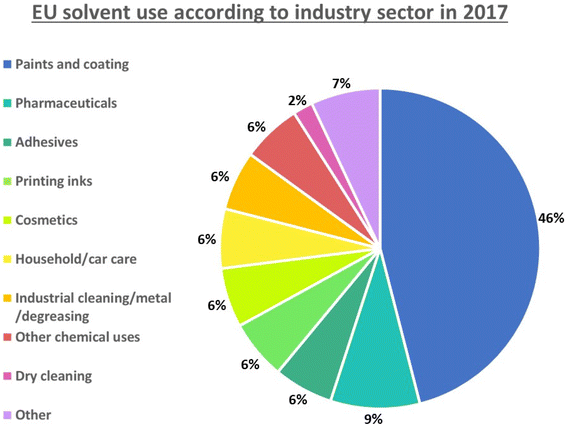 | ||
| Fig. 7 Solvent use according to industry sector within the EU in 2017. (Data taken and adapted from European Solvents Industry Group, 2018).101,104 | ||
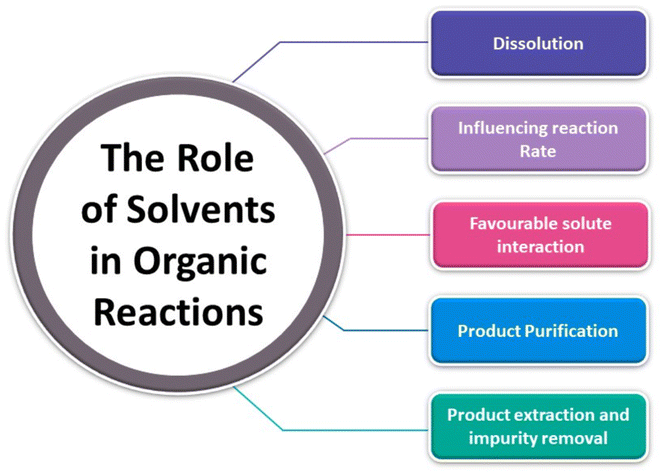
Within a synthetic context, solvents can be key for reaction progress and critical in steps such as extraction and purification (Fig. 8 below). Often excess solvents are required to afford a product with sufficient purity, and they offer beneficial traits during the reaction such as selectivity control, improved handling and mixture viscosity.105 Solvent removal, however, is an energy intensive process. Solvent also plays a clear role to avoid cross contamination during the cleaning of reactors and equipment in industrial settings.
Solvent use and choice within academic and discovery research laboratories can differ somewhat to commercial production scale, due often to literature precedent detailing a particular solvent. The concentration of reactions are less optimised, larger volumes used during work-up extractions and crystallisations, and significant volumes of different solvent mixtures utilised in flash chromatography, a regularly used method of purification. There is also acceptance of more toxic, less sustainable solvents in reactions and work-ups due to assumed relatively small scale. In their comprehensive review of ethereal and polar aprotic solvents for example, Jorden et al. note that in 2020 within the process chemistry journal, organic process research and development, the most common dipolar aprotic solvent employed was acetonitrile while in J. Med. Chem. and Angewandte Chemie DMF was most prominent.106,107 The authors provide a detailed comprehensive discussion of alternative solvent selections for potential replacement of dipolar aprotic and ethereal solvents in medicinal chemistry with a supporting selection guide.107 The solvent also needs to be considered across the complete and full process rather than the reaction media only.
There is not one property or parameter that defines every aspect of what makes a solvent applicable for a reaction or unit operation. Along with EHS (environment, health and safety) and life cycle there are various physio-chemical properties that are considered, such as boiling point, density, viscosity, Hansen parameters, Abraham parameters and solvatochromic parameters such as Kamlet–Taft and ETN.108–110 The term ETN is a normalised polarity parameter, while Kamlet Taft combines α, β and π which represent hydrogen bond donating ability (acidity or proticity), hydrogen bond accepting ability (basicity) and dipolarity/polarizability respectively.111–114 Each of these parameters uses two reference solvents to define the scale, with most solvents ranking between 0 and 1. Kamlet–Taft parameters are useful and have been employed to gain insight into reaction trends and profiles in the context of enantioselectivity and substrate solubility.115
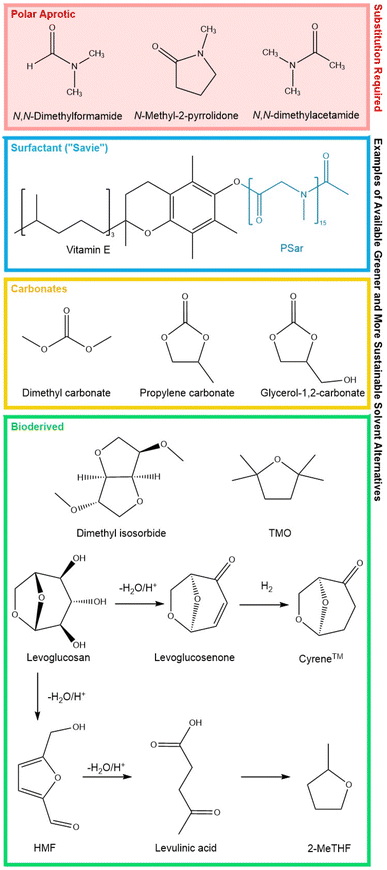
Sustainable yet chemically viable replacements are required for several solvent classes, with a clear example of the dipolar aprotic solvents, particularly N-methyl-2-pyrrolidone, N,N-dimethylacetamide and N,N-dimethylformamide; NMP, DMAc, and DMF, respectively (Fig. 9). These three solvents pose a concern for reproductive and developmental toxicity, have become more regulated in subsequent years and several have been included on the EU's ‘Candidate List of substances of very high concern for Authorisation’.116,117 Environmental concerns are also associated with the large quantities of water used during work-up and hence solvent separation from streams post this, and their disposal via incineration methods.72,118
While DMSO (Fig. 9) could be considered a renewable solvent, as it can be sourced as a by-product of the papermaking industry oxidising dimethyl sulfide, there are concerns regarding its difficult removal, decomposition at elevated temperatures (∼200 °C) and potential for SOx gas upon incineration.105,119 Acetonitrile is a dipolar aprotic solvent which has been deemed by some solvent selection guides as relatively “green” with some issues, while GSK and Q-SA√ESS categorise it as amber for problematic.94,120,121 This is an important solvent within the pharmaceutical industry but suffers from difficulty in its recovery processes particularly from water, in its life cycle and has suffered from supply issues.122 There is research ongoing for alternative routes to its production including from biomass sources and more efficient options for recovery. It is key that any solvent replacements maintain desired characteristics but are benign in nature, sustainable in their life-cycle, biodegradable and are subject to a wide range of various toxicological testing.29
Within solvent selection there is no single broad-spectrum sustainable solvent suitable for all reactions and purifications, therefore each candidate must be evaluated using metrics on a specific process/product basis.123 Switching to a more EHS favourable solvent in a process may come at a cost of decreased yield and purity in a reaction, requiring more energy or resulting in more waste than the original solvent. Hence, chemists must have an awareness of the sacrifices that may be required, and which route will be the solution for optimum sustainability.29,87 In their editorial note on solvents and expectations for publication in ACS Sustainable Chemistry and Engineering, the authors outline requirements for solvents with respect to sustainability i.e. solvent needs to efficiently support the reaction to deliver a product which is needed, the impact and life cycle of the solvent on the environment and its ecology should be negligible, and the solvent should be highly economically feasible.123 They also acknowledge that meeting these is not trivial and can seem unattainable but are a point which researchers can work to travel toward. Acknowledging challenges around solvent choice and use, the remainder of this section is outlined in the manner of questions and answers to aid reflection.
5.1 How can researchers be informed about their solvent choice?
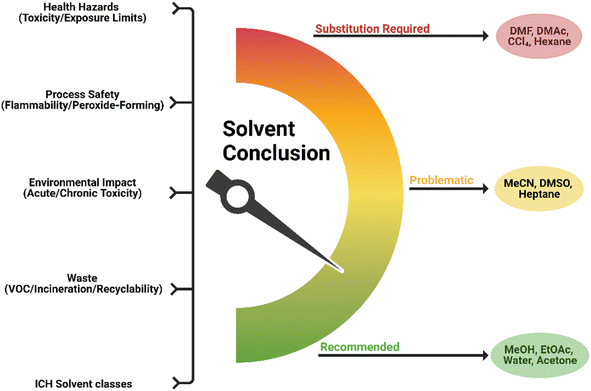
As a result of solvents being the significant mass component in a pharmaceutical process, for many years companies such as GSK, Pfizer, AstraZeneca and Sanofi have taken lead by producing solvent selection guides to aid more sustainable choices during process design and development.108,120,124,125 Academic institutes such as ETH Zurich and Rowan University have generated valuable solvent guides, with support tools to aid the screening of solvents and to analyse solvent waste treatment options.126–129 A comprehensive review of solvent selection tools with extension to neoteric and bioderived solvents was presented by Byrne et al. in 2016.105Many solvent selection guides classify solvents based on their various chemical families such as halogenated, alcohols and esters. Most solvent selection tools employ a colour-coded system (as outlined in Fig. 10 below) where green illustrates the preferred solvent, yellow is associated with solvents that are useable but have some associated issues and red represents undesirable for use due to serious implications, therefore substitutions are advised.124,125 A composite colour is evaluated based on health hazards such as toxicity and exposure limits; process safety including flammability, peroxide formation; and environmental concerns through acute and chronic toxicity impacts. In their medicinal chemistry selection guide, Pfizer chose to include heavily restricted chemicals to reinforce by their colour coding that these solvents were unsustainable options. Within two years of implementation, Pfizer saw a 50% reduction in chlorinated solvent and reduced the use of an undesirable ether by 97%.125 Due to the highly regulated nature of pharmaceutical manufacturing, some guides such as Sanofi's, opted to include ICH (The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) residual solvents classes in their tables.108,124
In addition to the previously mentioned areas, GSK included a waste category that assesses incineration, ease of recycling and VOC.120,130 This methodology calls for a heedful approach to the consequences of solvent properties within a reaction and post-use. When comparing the contribution from solvent manufacture, internal processes and non-solvent chemical manufacture before waste treatment, GSK found that solvents are responsible for 50% of the greenhouse gas emissions, 70% of the life cycle photochemical ozone creation potential, and 75% of the energy use, in cradle-to-gate LCA of a typical API.131 Considering the large influence of solvents on the LCA, these values were introduced into the solvent guide in 2004 which was subsequently updated in 2016 to a total of 154 solvents.120,130
Contradicting information may be presented through different solvent guides due to the lack of consensus on scoring and the assignment of acceptable alternatives. Acetone is an example that many guides classify as a recommended solvent, while AstraZeneca and GSK have labelled it as associated with issues due to impacted waste scores concerning VOC potential and biotreatment.120,130,132 Common agreements within guides suggest that preferred solvents for pharmaceutical processes consist largely of alcohols and esters for example n-butanol and ethyl acetate, and water.
There have been several collaborative solvent selection guides produced, including by CHEM21.133 The ACS GCIPR released an online solvent tool, originally built by AstraZeneca, which allows solvents to be compared according to their EHS and various engineering properties such as viscosity.108,134 In addition to these qualities, solvents are primarily mapped according to correlations within their physical properties through multivariate modelling of principal component analysis (PCA). Solvents cannot be ranked according to one single parameter that will encompass every aspect of what makes one a suitable candidate in a reaction. This database therefore provides a method of identifying nearest neighbours and those in opposing quadrants, increasing awareness of available alternatives. The utilisation of PCA solvent maps allows optimisation of a reaction to be carried out by a design of experiment (DoE) approach rather than a more laborious one variable at a time (OVAT) strategy.132,135 The availability and application of such selection tools and guides is highly beneficial (Fig. 11).
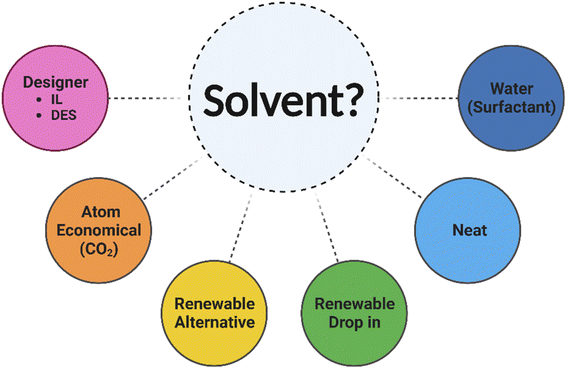 | ||
| Fig. 11 Considerations when exploring future solvent options in synthesis. Image created using https://www.biorender.com/. | ||
5.2 Can water be used as solvent?
Some well-known organic reactions have been shown to occur with water as solvent, in some cases improving the reaction e.g. Wittig and Diels Alder reactions.136–138 The advantages of applying water in place of organic solvent are low toxicity, lack of flammability, relatively low cost, work-up and abundance.139,140 There are some disadvantages however such as challenges in removal via distillation, waste-water treatment, reactivity of reagents with water and solubility. In their review on micellar catalysis, Lipschutz et al. point out that chemists often think of water only in the context of work-up rather than as reaction medium.141 Changing this perspective and applying reactions in water can yield highly efficient reactions in terms of selectivity, with significant reduction in waste from both a solvent use and total water volume requirement perspective. In the context of widespread application, outside of reactivity there can be the perception that the lack of solubility of starting materials will yield a lack of reaction.There are several ways of considering reactions completed in water; where water acts as a medium but without solvation of components at the interface to the bulk liquid water phase (“on water”); where water acts as the medium with solvation of components (this can be via support from added species) (“in water”) or where water is added to the main reaction medium (“with water”). The difference between “on water” and “in water” is not so clear, as there may be limited amounts of reactant dissolved.142 A detailed review in 2016 of “on water” reactions by Butler and Coyne discusses both “in-” and “on water” systems defining these based on physical requirements at the organic–water interface, solubility, and the transition state and its position in bulk water or at the organic side of the interface respectively.143
To aid water solubility and support reactivity the addition of designer surfactants can be beneficial. Several groups have developed impressive novel surfactants for example, TPGS-750-M which can self-assemble and form mini-reaction systems.144 In such micellar catalysts, substrates are solubilised and protected with a significant reduction in PMI over the reaction. While these aim to be greener solvent alternatives, the presence of components within the surfactant can impact biodegradability and waste treatment. A more polar preparation, applicable in a range of reactions was presented by Kincaid et al. called “Savie” in 2023 (Fig. 9), prepared from sarcosine and vitamin E, which offers significant potential, and a 4-fold reduced E-factor in its preparation compared to TPGS-750-M.145
5.3 Can reactions be done in the absence of solvent (neat)?
Solvents facilitate a reaction by enabling mass and heat transport and allow for reactant and product separation, but this can represent 80–90% of the total mass of a reaction, 70–85% of the waste,84 and require energy consumption for removal.29 Therefore, the option to perform a chemical process via a solvent free route should be considered. Viscosity related issues such as poor diffusion and heat transfer, however, can occur and can potentially be combatted with non-conventional heating such as sonochemical, mechanochemical or microwave assisted approaches,146–148 or by utilising one of the reactants as solvent.115Applying this combination Belluati et al. recently reported the sustainable synthesis of isosorbide, a platform chemical derived from lignocellulose biomass.149 The versatility and scope to derivatise isosorbide makes it an attractive synthetic target and is a precursor for the synthesis of other molecules such as the cardiovascular drug isosorbide-5-mononitrate.150 The authors combined several methods for its production including the use of microwave, applying a heterogeneous catalyst, without solvent, in a one-pot approach. Good yield and selectivity >85% were obtained with yields of 47% achieved from glucose. To counteract the challenge of mass transfer associated with performing neat reactions, a temperature above the melting point of glucose was chosen to avoid degradation (155 °C).
Other techniques to drive reactions in a solvent free approach at smaller scale include extruder chemistry and light (photochemistry/photocatalysis).151 Where one or more reactants are in liquid form, a solvent-free process appears more straightforward but, the work-up and product purification must be considered as, solvent may be needed at these later points. While this is attracting attention at laboratory scale, fewer solvent free processes are reported at large scale, however.
5.4 What raw materials and processes can be used to generate more sustainable solvents?
The production of solvent, existing, “repurposed” and completely new alternatives with a sustainable life cycle requires the use of alternative, renewable and abundant raw materials and production methods.104 Biomass as such a source including lignocellulose containing-agricultural and forestry waste, plants, algae, by-products from the food industry, and even municipal waste offers potential to act as feedstock for a biorefinery capable of sustainably transforming it to valuable chemicals, solvents, energy and fuels. With the correct infrastructure in place, the aim would for this manufacturing process to generate zero waste in a fully circular manner. In their presentation of their developed BioLogicTool to aid in developing routes from biomass to products, Lie et al. present an extensive list of 59 platform molecules (including solvents) which may be derived from biomass and its constituents utilising processing via appropriate methods and routes.152For synthetic laboratories there has been an increase in the commercial availability of biomass derived solvents, which can act as direct drop-in replacements for traditional solvents such as methanol, ethanol, n-butanol and acetone. In addition, alternatives transformed from platform molecules of biomass-derived origin such as 2-methyl tetrahydrofuran (2-MeTHF), 2,2,5,5-tetramethyloxolane (TMO), and dihydrolevoglucosenone (Cyrene™) (Fig. 9) are potential sustainable alternatives.29 From the perspective of dipolar aprotics, Cyrene™ is a bio-based, water soluble, high boiling solvent that can be prepared from waste cellulose in two steps as illustrated (Fig. 9) This solvent is marketed as an alternative to harmful dipolar aprotic solvents, which are toxic, derived from petrochemical feedstocks and can degrade to CO2.153,154 In tests, Cyrene™ produced a negative result for mutagenic activity when screened according to Ames Mutagenicity testing. This is however, one test of a panel that screens for genotoxicity and traditional polar aprotic solvents like NMP, DMAc and DMF also obtained negative results for mutagenicity in this assay.153,155–157
In a further application outside of synthesis, Cyrene™ may be a potential direct replacement for DMSO for antibacterial drug screening in some pathogens.102,105,119 Due to DMSO's radical scavenging ability, it can offer protection from intracellular reactive oxygen species (ROS) mediated programmed bacterial cell death and in contrast, Cyrene™ does not offer this interfering protection.119 A detailed review of the development and application of this solvent was presented by Stini et al. in 2022, with examples of its use in several types of organic reactions as solvent and as reactant. The authors notably also discuss reactions which are not effective due to the solvents nature as a ketone bearing two α-reactive positions and properties such as water solubility.158
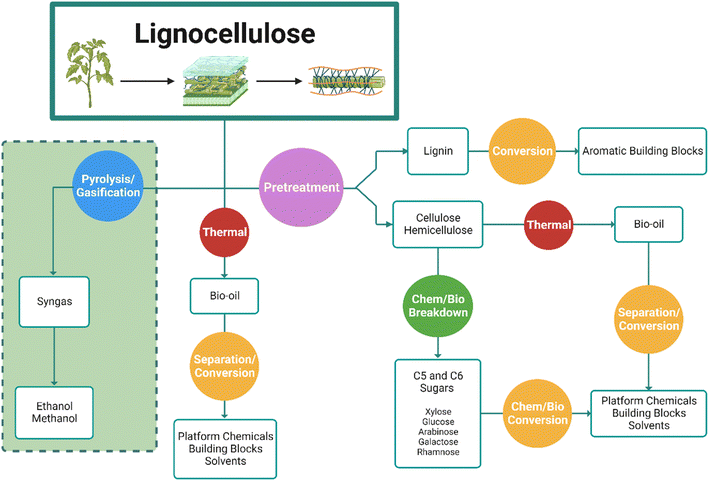
To grasp the production of solvents and chemicals derived from biomass, it is crucial to consider the raw materials, manufacturing, conversion processes, the toxicity, persistence and safety profile of products and the complete life cycle. Biomass feedstock offering potential is fiberous lignocellulose material, which contains three polymeric components (Fig. 12) i.e. cellulose, hemicellulose, and lignin which may each be transformed into valuable products. Further high value products in smaller quantities such as proteins can also be isolated from this material. Cellulose and hemicellulose can be converted into C5 and C6 sugars which via chemical and fermentation pathways (Fig. 12) can be transformed into to alcohols and diols, hydrocarbons and other platform molecules.159
Processing of lignocellulose material to obtain cellulose and hemicellulose as separate components requires pretreatment, followed by depolymerisation and biological or chemical conversion to fuels, solvents, and building block molecules, all which must be considered. Lignocellulose itself or cellulose may be subjected to pyrolysis to generate bio-oils containing valuable compounds such as levoglucosan, amongst many other products.160 The high level of functionalisation in such materials is beneficial. In their 2015 review Isikgor and Remzi Becer provide a comprehensive breakdown of lignocellulose material, and its potential to form over 200 compounds via a multitude of platforms. Lignin also offers potential to generate aromatic building blocks amongst other products.161
From a sustainability perspective there are many considerations required, particularly the source and nature of the biomass feedstock, its cultivation, the processes employed chemically and biocatalytically for conversion and ultimately the safety, environmental and toxicity profile of the products and intermediates generated. There should be a clear benefit with consideration for the use of the solvent itself and while biomass and an energy efficient transformation process could be developed to as an alternative to yield an established product, this product itself may have issues. A concern with first generation biomass sources of sugars, starches and vegetable oils lies in their cultivation, land use and potential for competition with food production. Second generation sources such as valorised agricultural and forestry waste streams for example, straw, rice or stover, could offer relatively abundant material, in a potentially circular manner, to supply lignocellulose biorefineries.162 The land use, agricultural practices, growth and harvesting in the generation of the biomass materials must also be considered in the complete LCA along with yield of product, economics and composition. Significant contributions and variation in global warming potential, acidification potential and eutrophication potential can occur depending on agricultural practices. Khoo et al. have completed LCA for bioderived methanol, formic acid, and acetone in comparison to petroleum derivatives.163 The authors have also analysed the production of bioderived 2-MeTHF from levulinic acid via C5/C6 sugars from lignocellulose sources.164 This work highlighted the importance of choosing the biomass source, its availability and the conversion technology. As a third-generation source of fuel and chemicals algal biomass has been proposed as a possible feedstock. This can yield high-carbohydrate content, lacks lignin, can consume CO2 while using non-arable land for production. This method however requires high investment costs, is challenging to scale and can yield low biomass concentration.165
Ewing et al. detail significant work in the development of fermentation processes,166 and combination of these with biocatalytic and chemocatalytic biorefinery technologies, could result in efficient conversion to valuable products in a circular approach. Direct conversion of biomass to syngas (CO, CO2 and H2) alternatively (Fig. 12, green shaded area), effectively removes functionalisation to yield inputs to generate biomethanol, hence functionalisation of this to products through the established petrochemical chain is also possible. Here non-fossil feedstock can utilise the traditional petrochemical network repurposing equipment. However, the complexity in renewable feedstocks could be considered as highly beneficial, opening doorways to new chemical building blocks.
Along with biomass as a renewable resource, CO2 may act as a feedstock for chemical and solvent production, and as a source of C1 chemicals such as methanol (with conversion to formaldehyde and formic acid). The production of organic carbonates, potentially useful solvents, utilising CO2 in their manufacture is also possible. These solvents can have low eco-toxicity, low viscosity and show good biodegradability. They can be acyclic or cyclic with examples of dimethyl carbonate, glycerol carbonate and propylene carbonate shown in Fig. 9.48,167,168 As an example, propylene carbonate is an appealing solvent due to both its solvation properties and its benign nature. This polar aprotic solvent offers low viscosity, high chemical stability and a high dielectric constant, while exhibiting biodegradable and noncorrosive characteristics.169 Application of this cyclic carbonate has been reported in hydrogenations, Suzuki–Miyaura cross couplings, biocatalysis, photodegradation and as a promising co-solvent for aqueous rechargeable zinc-ion batteries.170–173 This solvent is also identified as carbon-dioxide neutral as it can manufactured with 100% atom economy from CO2 and propylene epoxide.171 A caveat of this promising solvent is its high boiling point and therefore the associated high energy inputs that would be required for solvent removal and recycling.167 This reaffirms that the specific requirements of a process and the wider life cycle analysis must be considered and described when reporting the use of a “green” solvent. Further information on this solvent class and their CO2 mitigation production strategies can be found in a report by Pescarmona.167
5.5 Are there completely new types of solvent in development?
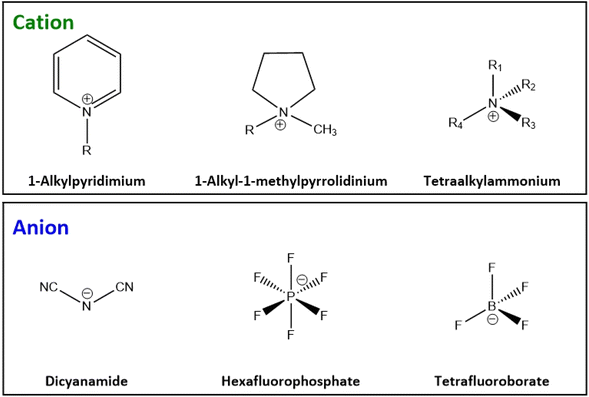
Ionic liquids (IL) began to gain momentum as new designer solvents over two decades ago.174,175 This group of chemicals are composed of a large asymmetric organic cation, combined with an inorganic or organic anion. This partnership results in a molten salt, liquid at room temperature.176 The behaviour of IL can be altered depending on the structure of the cation and anion, but many offer beneficial traits of low vapour pressure, non-flammability at ambient conditions, high chemical and thermal stability, and good solvation ability for a large selection of compounds.29 Many of these attributes are beneficial from a safety perspective compared to volatile organic solvents that are utilised in industry, which carry a risk of toxicity and flammability through vapours.177 Some of the commonly employed cations and anions in ionic liquids are shown in Fig. 13.178In the context of “greenness” however the manufacturing process to generate IL from their origins can be laboursome with large numbers of steps, involving non-renewable petrochemical feedstocks and the production of harmful and toxic intermediates.179 The resulting IL products can often have EHS implications through their environmental persistence and bioaccumulation, and transdermal toxicity.178,180 They can be corrosive, requiring specialised containers, piping and equipment.181 Kunz reported that molecular solvents are 5–20 times cheaper than ILs, without there being a clear sustainable justification for their use.176
Another form of nontraditional or neoteric solvents are deep eutectic solvents (DES) which consist of a homogeneous mixture of two or more compounds that has a combined melting point temperature lower than the two individual components and below that of the ideal liquid mixture (Fig. 14).111,182 This mixture can contain a diverse range of constituents, described as Brønsted or Lewis acids and bases, may contain inorganic or organic cation and anions and are obtained from both ionic and non-ionic molecules.183,184 Representative examples of components are shown in Fig. 15. These characteristics result in strong intermolecular interactions depending on the components185 and can result in a steep reduction in the combined melting point as shown in Fig. 14.186,187 Choline chloride (ChCl) and urea formed one of the first DES reported by Abbott et al. in 2003.188 When these two chemicals are mixed at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at 80 °C a DES is produced with a melting point of 12 °C, rather than the individual freezing temperatures of 302 °C (ChCl) and 133 °C (urea).
2 at 80 °C a DES is produced with a melting point of 12 °C, rather than the individual freezing temperatures of 302 °C (ChCl) and 133 °C (urea).
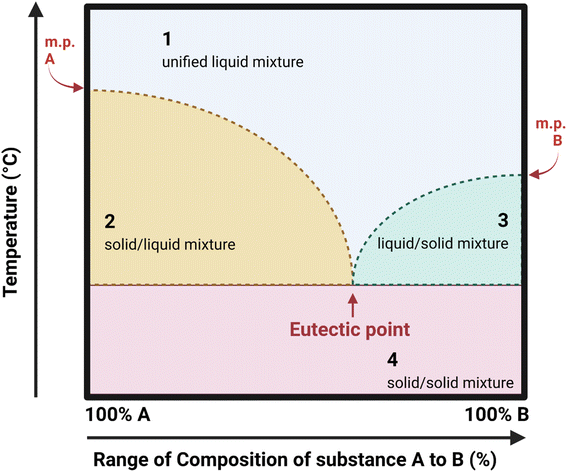 | ||
| Fig. 14 Phase diagram representing the variation in form of a mixture of substance A and B as composition and temperature vary. Image made using https://www.biorender.com/ and adapted from ref. 187 and 189. | ||
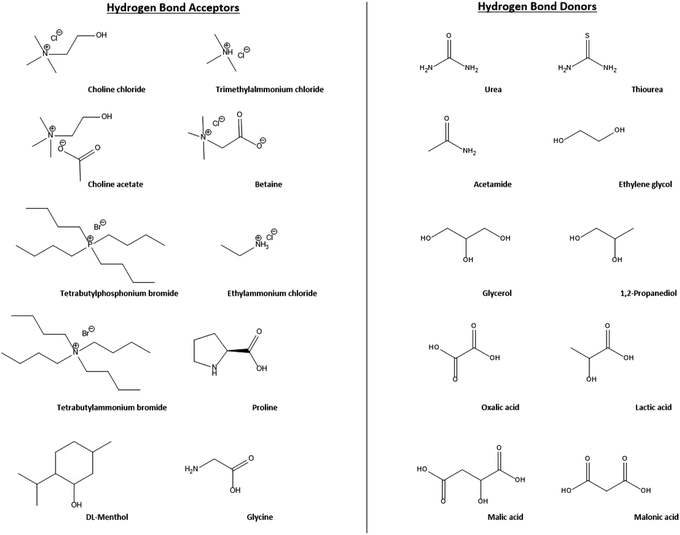 | ||
| Fig. 15 The structures of various common hydrogen bond acceptors and donors used for DES preparation.197,198 | ||
Tertiary DES are not as researched as in-depth as binary interactions but are gaining attention through favourable properties for example reduced viscosity.189–193 Often a third component introduced is water, to reduce viscosity, however disruption of the DES will occur at high water contents.194–196
As solvents, DES can be described based on their hydrophobic or hydrophilic nature. HBAs such as tetrabutylphosphonium bromide, tetrabutylammonium bromide, and DL-menthol, can be combined with various long alkyl chain fatty acid and carboxylic acid HBDs to produce hydrophobic solvents. Less bulky organic acids e.g. lactic acid have been employed as HBDs with hydrophobic HBAs e.g. DL-menthol to produce hydrophobic DES with lower viscosity.182,199 Hydrophilic DES are more common, having originally received most of the research due to the wide range of candidates that can be utilised.111,200 Proposed in 2011, by Choi et al. natural deep eutectic solvents (NADES) have constituents that are entirely composed of natural products like sugars, sugar organic acids, choline derivatives, amino acids, and alcohols, which often are present in living cells at high concentrations.174,193,201
When compared, DES have been described as superior analogues to ILs as they offer a tailorable, stable solvent where physiochemical properties of pH, polarity and viscosity can be adjusted through modifying the compounds, molar ratios, temperature, water content and the use of co-solvents.198,202 They have been assessed and are under assessment in chemocatalytic, biocatalytic and as extraction solvents.203
It can be noted that DES adhere to several of the principles of green chemistry in their preparation and can be generated from renewable sources. The atom economy of formation is 100% as the starting materials are incorporated into the final product,186,204 without waste or purification processes. Their preparation is usually carried out mixing two components with heat and stirring.197,202,205–209 Eutectic solvents, however, are infamous for their high viscosity values which may be a reaction limiting factor.210 Viscosity can be influenced through component choice, water content and molar ratios. They may also be hygroscopic, recovery via distillation is difficult and during preparation side reactions are possible.211
While many of the pure DES components are classified as Generally Recognised as Safe (GRAS) by the United States Food and Drug Administration (FDA), assumptions of DES characteristics cannot and should not be made based upon the properties of the components used to make the mixture. In terms of biodegradability and toxicity, DES combinations must be evaluated on a case-by-case basis, considering the partners, preparation method, the water content and concentration. This aspect is vital for large scale industrial application of ILs and DESs. In addition, Martínez et al. noted in their review that the physical properties of viscosity, pH and water content may impact the testing protocol (particularly diffusion in solid media) and must be considered during any toxicity or biodegradability assessment.212 The authors suggest incubation of a range of microbes for assessment in liquid, preferably, culture medium, with monitoring of growth in the presence of DES.
In their report Jung et al. compared how IC50 values varied between the pure components, the formed choline chloride-based DES and the hydrated DES system.213 In several cases evaluated, lower amounts of DES were needed to produce cytotoxic effects in human liver hepatocellular cell line (HepG2) and human embryonic kidney 293 cell line (HEK293T) than the individual constituents. Similarly, Juneidi et al. observed that some DES displayed higher toxicity towards the fungi strain Aspergillus niger than the individual components, although they found this trend reversed when comparing LC50 values from DES and component mixtures on Cyprinus carpio fish. A common theme was that DES with metal salts exhibited significantly higher toxicity across species so should not be included under the innocuous DES umbrella term often applied.214 The variation between some DES formulations and individual components does not necessarily mean that all variants will fall into this pattern. Therefore, DES combinations must be evaluated for their toxicity on a case-by-case basis, considering the binding partners, concentration, water content and various living organisms.196
Compared to common organic solvents, there is limited available data on the solvatochromic parameters for DES and the values available may differ due to the various compounds that can be used as probes during measurement readings.197,215 Values of Kamlet–Taft and ETN solvatochromic parameters are reported by Florindo et al. for various DES combinations.109 A current issue with DES is high cost of preparation, but this is offset by atom economy and the non-toxic nature of the starting materials, several of which could be sourced from non-fossil biomass resources. Increased applications, with complete and thorough evaluation, may yield increased demand for DES that will see reduced cost and uptake for the synthesis laboratory and could also see commercial processing within the bio/pharmaceutical industry. In their review Chen and Mu provide suggested strategies to overcome current issues with DES and IL to increase their applicability.211
6. Laboratory considerations for a complete approach to green and sustainable synthetic chemistry
As discussed above, it is imperative that researchers understand how their work in its design and operation contributes to environmental emissions. However, it is important that we clearly understand the footprint of our practical day-to-day research laboratory environment, which itself is energy and resource intensive.216 Implementing sustainable practices within the research laboratory is crucial to address global challenges, minimise the environmental footprint and promote long term sustainability. There are several articles and guides available which provide more detail on laboratory greening and sustainability initiatives.217,218 As an example, Durgan et al. recently published a clear guide to develop sustainable science in your organisation.219 Greever et al. discussed how efforts at an institutional level, focused on incorporating green chemistry promotes the reduction of hazardous waste, reducing energy and water use.220 Such changes implemented at scale within an organisation can result in a positive impact.221While lab sustainability initiatives usually begin as grassroots movements, there are many organisations offering pilot schemes, sharing resources and offering formal certification to support work.221 Joining a scheme such as the My Green Lab programs, Laboratory Efficiency Assessment Framework (LEAF), groups and national and international networks, as examples Sustainable European Laboratories network (SELs) and within our country Ireland, Irish Green Labs, can help guide, organize, share and reward work.222–226 These programmes and Networks work by engaging institutional stakeholders and working scientists that impact decisions at the laboratory level. The shift towards conducting sustainable science means that there is a growing number of organisations to share such information, ideas, and resources. Scientists can promote these changes by advocating for such programmes within their institutions.
To begin such an initiative, it is important to set clear and measurable goals, considering factors such as reducing energy consumption, minimising waste generation, and integrating sustainable development principles into research projects. Establishing a team or committee comprising individuals from each group, research centre and various departments within the organization is paramount. This should include members from outside of the scientific domain. This cooperative approach encourages the sharing of ideas and expertise, facilitating the development and implementation of a wider array of sustainable initiatives. A key aspect is education and awareness among researchers and staff about the importance of sustainable practices in daily laboratory operations. Necessary actions in the transition towards creating a sustainable laboratory relating to energy, waste, water, procurement, green chemistry, education, and outreach are outlined below in Fig. 16.
 | ||
| Fig. 16 Overview of aspects of sustainability for consideration within the day-to-day operation of synthetic research laboratories. | ||
Strategies can be employed across different areas including energy, water, waste, and procurement. In the context of energy conservation, for example, the efficiency of Ultra Low Temperature (ULT) freezers depends on factors like model, age, capacity, and environment. While lower temperatures like −80 °C were promoted, they consumed 30% more energy without clear benefits. Many labs have returned to the original temperatures and signed up to the Freezer challenge to save energy and costs due to sustainability concerns.227 Furthermore, labs should adopt effective maintenance practices for fridges and freezers, such as creating a cold storage inventory and regular defrosting, is crucial. Promoting awareness about switching off equipment, encouraging fume hood best practices, and adopting energy-saving behaviours through communication channels can contribute to broader energy-saving efforts.228 To ensure measurable progress, quantifiable data should be generated using tools such as energy monitoring plugs or meters, which can consider, Heating, Ventilation, and Air Conditioning (HVAC) systems over defined periods, facilitating both estimation of savings and behavioural change monitoring. Additionally, utilising energy auditing services such as “Green Light Laboratories Limited” may further help to reduce costs and meet climate change regulations.229
In terms of water usage, employing water-efficient equipment such as purification systems, cooling systems, and autoclaves, can significantly reduce consumption. Recycling water through recirculating pumps for experiments and utilising oil-free vacuum pumps for filtrations as opposed to water vacuum aspiration also yield benefits. Alternatively, “The Findenser™”, can eliminate the requirement for water-cooled condensers in more than 95% of reflux reactions. The glass column and aluminium jacket featuring “fins”, efficiently dissipates heat in laboratory settings, providing cooling capacity comparable with water-cooled systems.230 Furthermore, fitting low-flow aerators can save significant quantities of tap water.
Waste reduction can be achieved by strategically reusing materials, avoiding surplus chemical purchases, minimizing chemical orders, and shifting from disposable plastics to autoclavable glassware, all contributing to lowering hazardous waste generation.231–234 Most lab recycling is done through supplier take-back programmes. Single-use nitrile gloves from the laboratory may be recycled by participating in “The RightCycle” Kimberly-Clark programme.235 Researchers deposit uncontaminated gloves into specific bins. Once collected, the gloves are ground into new materials and products such as patio furniture and planters.236 Scientists are encountering rising prices and supply shortages for liquid helium which is essential for cooling superconducting magnets such as nuclear magnetic resonance spectrometers. It is crucial to prevent the needless evaporation of liquid helium which has a boiling point of −269 °C and tends to evaporate during typical lab operations. Helium can be recaptured, with up to 95% of it re-liquified and stored for future use. However, this recycling technology is costly and often primarily found in industry and large universities.237
Incorporating the selection of sustainable lab materials during the practical design phase of a laboratory itself is a pivotal step in achieving environmentally responsible and efficient lab operations. By considering sustainability from the outset, architects, researchers and laboratory planners can make informed choices that minimise environmental impact and promote the principles of green and sustainable chemistry. One such example would be Trespa® TopLab® Plus Align, which is an innovation that incorporates up to 85% bio-based carbon content including up to 50% lignin, in place of phenol in its core resins of laboratory worktops. Installing a Variable Air Volume (VAV) rather than a Constant Air Volume (CAV) can significantly reduce the strain on a HVAC.238,239
Finally in terms of procurement, scientists can influence policy decisions by requesting transparency in suppliers' sustainable product ranking systems, potentially using labels such as the ACT (Accountability, Consistency, and Transparency) label.240 The ACT label is intended to empower scientists to make informed purchasing decisions by providing them with reliable and standardised information about the environmental impact of laboratory products. Practices such as maintenance of an inventory, consolidated orders, take-back programs, and selecting high-energy-rated equipment during procurement can also reduce the carbon footprint associated with purchasing, contributing to long-term energy and water waste reduction.234
7. Conclusion
Our generation is the first to comprehend the gravity of climate change and the last with a chance to limit severe consequences. Resolving these issues requires collective action, as scientists we need to take practical steps as a means of driving transformative change, using our research to develop solutions to problems which do so in a way to preserve our environment and society. This tutorial review provides concepts for consideration by synthetic research groups for this transformative journey, starting points for education and reflection of researchers which have established and growing resources for consideration.Traditionally in terms of toxicology, the emphasis is on assessing the potential risks of chemicals to human health and the environment. Making change in the development of our chemicals, in their synthesis and in the operation of our daily practices, can reduce the need for complex risk assessments and mitigation measures associated with hazardous substances. This broader sustainable approach will ensure minimal negative impacts on both human health, society, and the environment. Incorporating green and sustainable practices, and importantly, reflection and education in our research, aiming for synthetic chemistry that is beneficial and productive, with reduced life cycle impacts of raw materials, processes and products will clearly support industries, employees, and society.
Conflicts of interest
The authors declare no competing financial interest. Dr Tracey Coady sits on the engagement and education working group of the Irish Green Labs Network. The PMBRC have completed the MyGreen Labs Certification process for their pharmaceutical/biomedical and synthesis laboratories and are also a member of the Irish Green Labs Network. This publication has been prepared solely by the authors listed, without any involvement in the researching, writing, reviewing, editing or publication process by the network and certification organisation respectively.Acknowledgements
Funding for this work was provided by the South East Technological University PhD Scholarship Programme (WIT_2021_013)References
- P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- American Chemical Society, ACS Green Chemistry Institute, https://www.acs.org/content/acs/en/greenchemistry/about.html#history, accessed 21 July 2023.
- ACS Green Chemistry Institute Pharmaceutical Roundtable, No Title, https://www.acsgcipr.org/, accessed 21 July 2023.
- S. G. Koenig, D. K. Leahy and A. S. Wells, Org. Process Res. Dev., 2018, 22, 1344–1359 CrossRef CAS.
- Organisation for Economic Co-operation and Development, Sustainable chemistry, https://www.oecd.org/chemicalsafety/risk-management/sustainable-chemistry/, accessed 21 July 2023.
- O. Hutzinger, Environ. Sci. Pollut. Res., 1999, 6, 123 CrossRef CAS PubMed.
- I. T. Horváth, Chem. Rev., 2018, 118, 369–371 CrossRef PubMed.
- K. Kümmerer, Angew. Chem., Int. Ed., 2017, 56, 16420–16421 CrossRef PubMed.
- V. G. Zuin, I. Eilks, M. Elschami and K. Kümmerer, Green Chem., 2021, 23, 1594–1608 RSC.
- C. Hogue, Differentiating between green chemistry and sustainable chemistry in Congress, https://cen.acs.org/environment/green-chemistry/Differentiating-between-green-chemistry-sustainable/97/web/2019/07, accessed 21 July 2023.
- P. T. Anastas and J. B. Zimmerman, Curr. Opin. Green Sustainable Chem., 2018, 13, 150–153 CrossRef.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed.
- United Nations General Assembly, Transforming Our World: the 2030 Agenda for Sustainable Development, 2015 Search PubMed.
- M. K. M. Lane, H. E. Rudel, J. A. Wilson, H. C. Erythropel, A. Backhaus, E. B. Gilcher, M. Ishii, C. F. Jean, F. Lin, T. D. Muellers, T. Wang, G. Torres, D. E. Taylor, P. T. Anastas and J. B. Zimmerman, Nat. Sustain., 2023, 6, 502–512 CrossRef.
- G. A. Lasker, K. E. Mellor and N. J. Simcox, Green Chem. Lett. Rev., 2019, 12, 178–186 CrossRef CAS PubMed.
- United Nations Environment Programme, Green and Sustainable Chemistry: Framework Manual, 2021 Search PubMed.
- International Union of Pure and Applied Chemistry, Interdivisional Committee on Green Chemistry for Sustainable Development (ICGCSD), https://iupac.org/body/041/, accessed 11 September 2023.
- P. T. Anastas, ChemSusChem, 2009, 2, 391–392 CrossRef CAS PubMed.
- R. A. Sheldon, ACS Sustain. Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- S. Kar, H. Sanderson, K. Roy, E. Benfenati and J. Leszczynski, Chem. Rev., 2022, 122, 3637–3710 CrossRef CAS PubMed.
- Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. W. Zhang and B. W. Cue, Wiley, 2018 Search PubMed.
- Beyond Benign, Beyond Benign Green Chemistry Education, https://www.beyondbenign.org/, accessed 11 September 2023.
- ACS Green Chemistry Institute Pharmaceutical Roundtable, Guides and Metrics, https://learning.acsgcipr.org/guides-and-metrics/, accessed 11 September 2023.
- United States Environmental Protection Agency, Pollution Prevention Act of 1990, https://www.epa.gov/p2/pollution-prevention-act-1990, accessed 19 July 2023.
- Environmental Protection Act 1990, London, 1990.
- United Nations, Global Harmonized System of Classification and Labelling of Chemicals (GHS), New York and Geneva, 2023 Search PubMed.
- European Parliament and of the Council of the European Union, Regulation (EC) No 1272/2008 of the European Parliament, 2008 Search PubMed.
- European Commission, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/4, 2006 Search PubMed.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- P. T. Anastas, in Benign by Design Chemistry, ACS Symposium Series, ed. P. T. Anastas and C. A. Farris, American Chemical Society, Washington, DC, 1994, pp. 2–22 Search PubMed.
- Health and Safety Executive, EH40/2005, London, 4th edn, 2020 Search PubMed.
- Health and Safety Executive, Control of Substances Hazardous to Health, London, 6th edn, 2013 Search PubMed.
- United Nations World Commission on Environment and Development, Report of the World Commission on Environment and Development: Our Common Future, 1987 Search PubMed.
- United Nations Division for Sustainable Development, in United Nations Conference on Environment & Development, Rio de Janerio, 1992 Search PubMed.
- I. Hristov and A. Chirico, Sustainability, 2019, 11, 5742 CrossRef.
- T.-L. Chen, H. Kim, S.-Y. Pan, P.-C. Tseng, Y.-P. Lin and P.-C. Chiang, Sci. Total Environ., 2020, 716, 136998 CrossRef CAS PubMed.
- J. Becker, C. Manske and S. Randl, Curr. Opin. Green Sustainable Chem., 2022, 33, 100562 CrossRef CAS.
- United Nations, Paris Agreement, 2015 Search PubMed.
- Government of Ireland, Climate Action Plan 2021, 2021 Search PubMed.
- Government of Ireland, Climate Action Plan 2023, 2023 Search PubMed.
- European Commission, Chemicals Strategy for Sustainability towards a Toxic-free Environment, Brussels, 2020 Search PubMed.
- European Chemicals Agency, New hazard classes 2023, https://echa.europa.eu/new-hazard-classes-2023, accessed 19 July 2023.
- European Commission, Proposal for a Regulation of the European Parliament and of the Council Amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures, 2022 Search PubMed.
- UNESCO, UNESCO Global Action Programme on Education for Sustainable Development: Information Folder, 2016 Search PubMed.
- G. Bianchi, U. Pisiotis and M. Cabrera Giraldez, GreenComp, the European Sustainability Competence Framework, Publications Office of the European Union, Luxemburg, 2022 Search PubMed.
- Government of Ireland, ESD to 2030: Second National Strategy on Education for Sustainable Development, 2022 Search PubMed.
- Government of Ireland, ESD to 2030: Implementation Plan 2022–2026, 2022 Search PubMed.
- S. M. Gade, V. B. Saptal and B. M. Bhanage, Catal. Commun., 2022, 172, 106542 CrossRef CAS.
- H. B. Rose, B. Kosjek, B. M. Armstrong and S. A. Robaire, Curr. Res. Green Sustain. Chem., 2022, 5, 100324 CrossRef CAS.
- A. Burrows, J. Holman, A. Parsons, G. Pilling and G. Price, Chemistry3: Introducing Inorganic, Organic and Physical Chemistry, Oxford University Press, 2017 Search PubMed.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- B. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS PubMed.
- A. P. Dicks and A. Hent, in Green Chemistry Metrics, Springer, Cham, 2015, pp. 17–44 Search PubMed.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- R. A. Sheldon, Proc. Acad. Sci., 2000, 3, 541–551 CAS.
- F. Tieves, F. Tonin, E. Fernández-Fueyo, J. M. Robbins, B. Bommarius, A. S. Bommarius, M. Alcalde and F. Hollmann, Tetrahedron, 2019, 75, 1311–1314 CrossRef CAS.
- F. Roschangar, J. Colberg, P. J. Dunn, F. Gallou, J. D. Hayler, S. G. Koenig, M. E. Kopach, D. K. Leahy, I. Mergelsberg, J. L. Tucker, R. A. Sheldon and C. H. Senanayake, Green Chem., 2017, 19, 281–285 RSC.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- D. Dallinger and C. O. Kappe, Curr. Opin. Green Sustainable Chem., 2017, 7, 6–12 CrossRef.
- J. A. Bennett, Z. S. Campbell and M. Abolhasani, Curr. Opin. Chem. Eng., 2019, 26, 9–19 CrossRef.
- M. Baumann, T. S. Moody, M. Smyth and S. Wharry, Synth., 2021, 53, 3963–3976 CrossRef CAS.
- R. A. Sheldon and D. Brady, ChemSusChem, 2022, 15, e202102628 CrossRef CAS PubMed.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- A. Antenucci, S. Dughera and P. Renzi, ChemSusChem, 2021, 14, 2785–2853 CrossRef CAS PubMed.
- A. Antenucci and S. Dughera, Catalysts, 2023, 13, 102 CrossRef CAS.
- F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC.
- F. Roschangar, Y. Zhou, D. J. C. Constable, J. Colberg, D. P. Dickson, P. J. Dunn, M. D. Eastgate, F. Gallou, J. D. Hayler, S. G. Koenig, M. E. Kopach, D. K. Leahy, I. Mergelsberg, U. Scholz, A. G. Smith, M. Henry, J. Mulder, J. Brandenburg, J. R. Dehli, D. R. Fandrick, K. R. Fandrick, F. Gnad-Badouin, G. Zerban, K. Groll, P. T. Anastas, R. A. Sheldon and C. H. Senanayake, Green Chem., 2018, 20, 2206–2211 RSC.
- F. Roschangar, J. Li, Y. Zhou, W. Aelterman, A. Borovika, J. Colberg, D. P. Dickson, F. Gallou, J. D. Hayler, S. G. Koenig, M. E. Kopach, B. Kosjek, D. K. Leahy, E. O'Brien, A. G. Smith, M. Henry, J. Cook and R. A. Sheldon, ACS Sustain. Chem. Eng., 2022, 10, 5148–5162 CrossRef CAS.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- E. R. Monteith, P. Mampuys, L. Summerton, J. H. Clark, B. U. W. Maes and C. R. McElroy, Green Chem., 2020, 22, 123–135 RSC.
- ACS Green Chemistry Institute Pharmaceutical Roundtable, PMI Calculator, https://members.acsgcipr.org/tools-development/pmi-calculator/, accessed 17 August 2023.
- United States Environmental Protection Agency, Green Chemistry Challenge: 2021 Greener Synthetic Pathways Award, https://www.epa.gov/greenchemistry/green-chemistry-challenge-2021-greener-synthetic-pathways-award, accessed 18 August 2023.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 1–7 Search PubMed.
- A. DeVierno Kreuder, T. House-Knight, J. Whitford, E. Ponnusamy, P. Miller, N. Jesse, R. Rodenborn, S. Sayag, M. Gebel, I. Aped, I. Sharfstein, E. Manaster, I. Ergaz, A. Harris and L. Nelowet Grice, ACS Sustain. Chem. Eng., 2017, 5, 2927–2935 CrossRef CAS.
- E. Ponnusamy and J. Whitford, J. med. clin. res. rev., 2017, 1, 1–6 CrossRef.
- Merck, DOZN™ Quantitative Green Chemistry Evaluator, https://www.sigmaaldrich.com/IE/en/services/software-and-digital-platforms/dozn-tool, accessed 15 August 2023.
- N. J. O'Neil, S. Scott, R. Relph and E. Ponnusamy, J. Chem. Educ., 2021, 98, 84–91 CrossRef.
- E. S. Ponnusamy and J. Whitford, Int. J. Inf. Res. Rev., 2018, 5, 5147–5153 Search PubMed.
- L. Jacquemin, P.-Y. Pontalier and C. Sablayrolles, Int. J. Life Cycle Assess., 2012, 17, 1028–1041 CrossRef CAS.
- M. A. Curran, Curr. Opin. Chem. Eng., 2013, 2, 273–277 CrossRef.
- C. Jimenez-Gonzalez, Curr. Opin. Green Sustainable Chem., 2019, 18, 66–71 CrossRef.
- D. R. G. de Faria, J. L. de Medeiros and O. Q. F. Araújo, J. Cleaner Prod., 2021, 278, 123966 CrossRef CAS.
- International Organization for Standardization, ISO 14040:2006 Environmental Management — Life Cycle Assessment — Principles and Framework, 2006 Search PubMed.
- T. Welton, Proc. R. Soc. A, 2015, 471, 20150502 CrossRef PubMed.
- Ecoinvent, Ecoinvent Database, https://ecoinvent.org/the-ecoinvent-database/, accessed 14 November 2023.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- Intergovernmental Panel on Climate Change (IPCC), in Climate Change 2021 – the Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 2023, pp. 923–1054 Search PubMed.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123–145 RSC.
- Y. Wu and D. Su, in Sustainable Product Development, ed. D. Su, Springer International Publishing, Cham, 2020, pp. 39–55 Search PubMed.
- H. H. Khoo, V. Isoni and P. N. Sharratt, Sustain. Prod. Consum., 2018, 16, 68–87 CrossRef.
- V. Isoni, L. L. Wong, H. H. Khoo, I. Halim and P. Sharratt, Green Chem., 2016, 18, 6564–6572 RSC.
- E. C. Sherer, A. Bagchi, B. Kosjek, K. M. Maloney, Z. Peng, S. A. Robaire, R. P. Sheridan, E. Metwally and L.-C. Campeau, Org. Process Res. Dev., 2022, 26, 1405–1410 CrossRef CAS.
- E. Wynendaele, C. Furman, B. Wielgomas, P. Larsson, E. Hak, T. Block, S. Van Calenbergh, N. Willand, M. Markuszewski, L. R. Odell, G. J. Poelarends and B. De Spiegeleer, Med. Drug Discovery, 2021, 12, 100107 CrossRef.
- C. T. A. Moermond, N. Puhlmann, A. R. Brown, S. F. Owen, J. Ryan, J. Snape, B. J. Venhuis and K. Kümmerer, Environ. Sci. Technol. Lett., 2022, 9, 699–705 CrossRef CAS PubMed.
- ACS Green Chemistry Institute Pharmaceutical Roundtable, The Fate of APIs, https://learning.acsgcipr.org/life-cycle-impacts-and-environmental-fate-of-pharmaceuticals/the-fate-of-apis/, accessed 20 November 2023.
- A. Bechu, K. Mittal, K. Xu, R. Yekani, G. P. Demopoulos, A. Moores and N. Basu, ACS Sustain. Chem. Eng., 2022, 10, 14658–14664 CrossRef CAS.
- C. Caldeira, I. Garmendia Aguirre, D. Tosches, L. Mancini, E. Abbate, L. Farcal, D. Lipsa, K. Rasmussen, H. Rauscher, J. Riego Sintes and S. Sala, Sustainable by Design Chemicals and Materials - Review of Safety and Sustainability Dimensions, Aspects, Methods, Indicators, and Tools., Publications Office of the European Union, Luxembourg, 2022 Search PubMed.
- European Solvents Industry Group, Discover Solvents, https://www.esig.org/discover-solvents/, accessed 18 August 2023.
- J. Clark, T. Farmer, A. Hunt and J. Sherwood, Int. J. Mol. Sci., 2015, 16, 17101–17159 CrossRef CAS PubMed.
- P. T. Anastas and J. B. Zimmerman, Green Chem., 2019, 21, 6545–6566 RSC.
- N. Winterton, Clean Technol. Environ. Policy, 2021, 23, 2499–2522 CrossRef PubMed.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- A. Jordan, P. Stoy and H. F. Sneddon, Chem. Rev., 2021, 121, 1582–1622 CrossRef CAS PubMed.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Chem. Rev., 2022, 122, 6749–6794 CrossRef CAS PubMed.
- L. J. Diorazio, D. R. J. Hose and N. K. Adlington, Org. Process Res. Dev., 2016, 20, 760–773 CrossRef CAS.
- C. Florindo, A. J. S. McIntosh, T. Welton, L. C. Branco and I. M. Marrucho, Phys. Chem. Chem. Phys., 2018, 20, 206–213 RSC.
- J. T. Gorke, F. Srienc and R. J. Kazlauskas, Chem. Commun., 2008, 1235 RSC.
- L. Percevault, E. Limanton, F. Gauffre, C. Lagrost and L. Paquin, Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances, Springer International Publishing, Cham, 2021, Vol. 56 Search PubMed.
- J. P. Wojeicchowski, D. O. Abranches, A. M. Ferreira, M. R. Mafra and J. A. P. Coutinho, ACS Sustain. Chem. Eng., 2021, 9, 10240–10249 CrossRef CAS.
- D. Kundu, P. S. Rao and T. Banerjee, Ind. Eng. Chem. Res., 2020, 59, 11329–11339 CrossRef CAS.
- M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
- K. Tseke, C. Lennon, J. O’Mahony and M. Kinsella, Eur. J. Org. Chem., 2021, 2021, 5746–5842 CrossRef.
- J. A. Tickner, R. V. Simon, M. Jacobs, L. D. Pollard and S. K. van Bergen, Green Chem. Lett. Rev., 2021, 14, 21–42 Search PubMed.
- European Chemicals Agency, Candidate List of substances of very high concern for Authorisation, https://echa.europa.eu/candidate-list-table, accessed 19 August 2023.
- R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- J. E. Camp, S. B. Nyamini and F. J. Scott, RSC Med. Chem., 2020, 11, 111–117 RSC.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- I. F. McConvey, D. Woods, M. Lewis, Q. Gan and P. Nancarrow, Org. Process Res. Dev., 2012, 16, 612–624 CrossRef CAS.
- D. T. Allen, N. Gathergood, P. Licence and B. Subramaniam, ACS Sustain. Chem. Eng., 2020, 8, 14627–14629 CrossRef CAS.
- D. Prat, O. Pardigon, H. W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- C. S. Slater and M. Savelski, J. Environ. Sci. Health, Part A, 2007, 42, 1595–1605 CrossRef CAS PubMed.
- G. Koller, U. Fischer and K. Hungerbühler, Ind. Eng. Chem. Res., 2000, 39, 960–972 CrossRef CAS.
- Eidgenössische Technische Hochschule Zürich, EHS Tool, https://emeritus.setg.ethz.ch/research/downloads/software---tools/ehs-tool.html, accessed 29 August 2023.
- Eidgenössische Technische Hochschule Zürich, Ecosolvent, https://emeritus.setg.ethz.ch/research/downloads/software---tools/ecosolvent.html, accessed 29 August 2023.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854 RSC.
- C. Jiménez-González, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2004, 9, 114–121 CrossRef.
- S. G. Sveegaard and R. Kristiansen, Org. Process Res. Dev., 2021, 25, 68–74 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- ACS Green Chemistry Institute Pharmaceutical Roundtable, Solvent Tool, https://www.acsgcipr.org/tools-for-innovation-in-chemistry/solvent-tool/, accessed 21 July 2023.
- P. M. Murray, F. Bellany, L. Benhamou, D.-K. Bučar, A. B. Tabor and T. D. Sheppard, Org. Biomol. Chem., 2016, 14, 2373–2384 RSC.
- D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816–7817 CrossRef CAS.
- J. Dambacher, W. Zhao, A. El-Batta, R. Anness, C. Jiang and M. Bergdahl, Tetrahedron Lett., 2005, 46, 4473–4477 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 1991, 24, 159–164 CrossRef CAS.
- H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114–120 CrossRef CAS.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai and M. Cortes-Clerget, Chem. – Eur. J., 2018, 24, 6672–6695 CrossRef CAS PubMed.
- M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC.
- R. N. Butler and A. G. Coyne, Org. Biomol. Chem., 2016, 14, 9945–9960 RSC.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed.
- J. R. A. Kincaid, M. J. Wong, N. Akporji, F. Gallou, D. M. Fialho and B. H. Lipshutz, J. Am. Chem. Soc., 2023, 145, 4266–4278 CrossRef CAS PubMed.
- I. C. B. Martins, M. C. Oliveira, H. P. Diogo, L. C. Branco and M. T. Duarte, ChemSusChem, 2017, 10, 1360–1363 CrossRef CAS PubMed.
- V. V Namboodiri and R. S. Varma, Org. Lett., 2002, 4, 3161–3163 CrossRef PubMed.
- R. S. Varma and V. V Namboodiri, Chem. Commun., 2001, 643–644 RSC.
- M. Belluati, S. Tabasso, F. Bucciol, T. Tabanelli, F. Cavani, G. Cravotto and M. Manzoli, Catal. Today, 2023, 418, 114086 CrossRef CAS.
- F. Aricò, Curr. Opin. Green Sustainable Chem., 2020, 21, 82–88 CrossRef.
- P. Cintas, S. Tabasso, V. V Veselov and G. Cravotto, Curr. Opin. Green Sustainable Chem., 2020, 21, 44–49 CrossRef CAS.
- Y. Lie, P. Ortiz, R. Vendamme, K. Vanbroekhoven and T. J. Farmer, Ind. Eng. Chem. Res., 2019, 58, 15945–15957 CrossRef CAS.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- N. Guajardo and P. Domínguez de María, Mol. Catal., 2020, 485, 110813 CrossRef CAS.
- European Chemicals Agency, 1-methyl-2-pyrrolidone, https://echa.europa.eu/registration-dossier/-/registered-dossier/15493/7/7/1, accessed 19 August 2023.
- European Chemicals Agency, N,N-dimethylacetamide, https://echa.europa.eu/registration-dossier/-/registered-dossier/15266/7/7/1, accessed 19 August 2023.
- European Chemicals Agency, N,N-dimethylformamide, https://echa.europa.eu/registration-dossier/-/registered-dossier/15093/7/7/1, accessed 19 August 2023.
- N. A. Stini, P. L. Gkizis and C. G. Kokotos, Green Chem., 2022, 24, 6435–6449 RSC.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- I. Itabaiana Junior, M. Avelar Do Nascimento, R. O. M. A. De Souza, A. Dufour and R. Wojcieszak, Green Chem., 2020, 22, 5859–5880 RSC.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- A. K. Chandel, V. K. Garlapati, A. K. Singh, F. A. F. Antunes and S. S. da Silva, Bioresour. Technol., 2018, 264, 370–381 CrossRef CAS PubMed.
- H. H. Khoo, W. L. Ee and V. Isoni, Green Chem., 2016, 18, 1912–1922 RSC.
- H. H. Khoo, L. L. Wong, J. Tan, V. Isoni and P. Sharratt, Resour., Conserv. Recycl., 2015, 95, 174–182 CrossRef.
- M. Benedetti, V. Vecchi, S. Barera and L. Dall'Osto, Microb. Cell Fact., 2018, 17, 173 CrossRef CAS PubMed.
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Green Chem., 2022, 24, 6373–6405 RSC.
- P. P. Pescarmona, Curr. Opin. Green Sustainable Chem., 2021, 29, 100457 CrossRef CAS.
- S.-H. Pyo, J. H. Park, T.-S. Chang and R. Hatti-Kaul, Curr. Opin. Green Sustainable Chem., 2017, 5, 61–66 CrossRef.
- M. C. Bryan, P. J. Dunn, D. Entwistle, F. Gallou, S. G. Koenig, J. D. Hayler, M. R. Hickey, S. Hughes, M. E. Kopach, G. Moine, P. Richardson, F. Roschangar, A. Steven and F. J. Weiberth, Green Chem., 2018, 20, 5082–5103 RSC.
- B. Kakoty, R. Vengarathody, S. Mukherji, V. Ahuja, A. Joseph, C. Narayana, S. Balasubramanian and P. Senguttuvan, J. Mater. Chem. A, 2022, 10, 12597–12607 RSC.
- A. Czompa, B. L. Pásztor, J. A. Sahar, Z. Mucsi, D. Bogdán, K. Ludányi, Z. Varga and I. M. Mándity, RSC Adv., 2019, 9, 37818–37824 RSC.
- H. Cumming and S. N. Marshall, J. Biotechnol., 2021, 325, 217–225 CrossRef CAS PubMed.
- P. Magalhães, J. Ângelo, O. C. Nunes and A. Mendes, Appl. Surf. Sci., 2018, 458, 597–602 CrossRef.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustain. Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- G. Kaur, H. Kumar and M. Singla, J. Mol. Liq., 2022, 351, 118556 CrossRef CAS.
- W. Kunz and K. Häckl, Chem. Phys. Lett., 2016, 661, 6–12 CrossRef CAS.
- M. Bystrzanowska, F. Pena-Pereira, Ł. Marcinkowski and M. Tobiszewski, Ecotoxicol. Environ. Saf., 2019, 174, 455–458 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- P. G. Jessop, Green Chem., 2011, 13, 1391 RSC.
- S. S. de Jesus and R. Maciel Filho, Renewable Sustainable Energy Rev., 2022, 157, 112039 CrossRef CAS.
- E. K. Ardakani, E. Kowsari, A. Ehsani and S. Ramakrishna, Microchem. J., 2021, 165, 106049 CrossRef CAS.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, J. Solution Chem., 2019, 48, 962–982 CrossRef CAS.
- F. G. Calvo-Flores and C. Mingorance-Sánchez, ChemistryOpen, 2021, 10, 815–829 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- X. Xiong Chang, N. Mujawar Mubarak, S. Ali Mazari, A. Sattar Jatoi, A. Ahmad, M. Khalid, R. Walvekar, E. C. Abdullah, R. R. Karri, M. T. Siddiqui and S. Nizamuddin, J. Ind. Eng. Chem., 2021, 104, 362–380 CrossRef CAS.
- C. Andrew, E. E. Etim, O. A. Ushie and J. N. Job, Int. J. Adv. Res. Chem. Sci., 2017, 4, 23–30 Search PubMed.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- A. Bjelić, B. Hočevar, M. Grilc, U. Novak and B. Likozar, Rev. Chem. Eng., 2020, 38, 243–272 CrossRef.
- F. S. Mjalli and O. U. Ahmed, Korean J. Chem. Eng., 2016, 33, 337–343 CrossRef CAS.
- H. Sereshti, G. Abdolhosseini, S. Soltani, F. Jamshidi and N. Nouri, J. Sep. Sci., 2021, 44, 3626–3635 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- B. Nian, C. Cao and Y. Liu, Int. J. Biol. Macromol., 2019, 136, 1086–1095 CrossRef CAS PubMed.
- R. Amoroso, F. Hollmann and C. Maccallini, Molecules, 2021, 26, 6286 CrossRef CAS PubMed.
- L. A. Rodrigues, M. Cardeira, I. C. Leonardo, F. B. Gaspar, I. Radojčić Redovniković, A. R. C. Duarte, A. Paiva and A. A. Matias, J. Mol. Liq., 2021, 335, 116201 CrossRef CAS.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef CAS.
- M. Zhang, X. Zhang, Y. Liu, K. Wu, Y. Zhu, H. Lu and B. Liang, Environ. Sci. Pollut. Res., 2021, 28, 35537–35563 CrossRef CAS PubMed.
- B. D. Ribeiro, C. Florindo, L. C. Iff, M. A. Z. Coelho and I. M. Marrucho, ACS Sustain. Chem. Eng., 2015, 3, 2469–2477 CrossRef CAS.
- C. Florindo, L. C. Branco and I. M. Marrucho, ChemSusChem, 2019, 12, 1549–1559 CrossRef CAS PubMed.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. C. E. Arends, G.-J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Food Chem., 2015, 187, 14–19 CrossRef CAS PubMed.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org. Chem., 2016, 2016, 612–632 CrossRef CAS.
- J. Cao and E. Su, J. Cleaner Prod., 2021, 314, 127965 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- V. Vatanpour, A. Dehqan and A. R. Harifi-Mood, J. Membr. Sci., 2020, 614, 118528 CrossRef CAS.
- K. Shahbaz, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, Fluid Phase Equilib., 2012, 319, 48–54 CrossRef CAS.
- C. Lu, J. Cao, N. Wang and E. Su, Medchemcomm, 2016, 7, 955–959 RSC.
- K. Shahbaz, F. S. Mjalli, G. Vakili-Nezhaad, I. M. AlNashef, A. Asadov and M. M. Farid, J. Mol. Liq., 2016, 222, 61–66 CrossRef CAS.
- J. A. Kist, H. Zhao, K. R. Mitchell-Koch and G. A. Baker, J. Mater. Chem. B, 2021, 9, 536–566 RSC.
- Y. Chen and T. Mu, Green Chem. Eng., 2021, 2, 174–186 CrossRef.
- G. M. Martínez, G. G. Townley and R. M. Martínez-Espinosa, Heliyon, 2022, 8, e12567 CrossRef PubMed.
- D. Jung, J. B. Jung, S. Kang, K. Li, I. Hwang, J. H. Jeong, H. S. Kim and J. Lee, Green Chem., 2021, 23, 1300–1311 RSC.
- I. Juneidi, M. Hayyan and M. A. Hashim, RSC Adv., 2015, 5, 83636–83647 RSC.
- W. E. Waghorne, Pure Appl. Chem., 2020, 92, 1539–1551 CrossRef CAS.
- N. Borgermann, A. Schmidt and J. Dobbelaere, One Earth, 2022, 5, 18–21 CrossRef.
- M. Palmieri, B. Lasserre, D. Marino, L. Quaranta, M. Raffi and G. Ranalli, Sustainability, 2023, 15 Search PubMed.
- PMBRC multidisciplinary team, The SETU Greener Lab Guide, http://pmbrc.org/wp-content/uploads/HERE.pdf.
- J. Durgan, M. Rodríguez-Martínez and B. Rouse, Immunol. Cell Biol., 2023, 101, 289–301 CrossRef PubMed.
- C. Greever, K. Ramirez-Aguilar and J. Connelly, FEBS Lett., 2020, 594, 3079–3085 CrossRef CAS PubMed.
- J. Dobbelaere, J. B. Heidelberger and N. Borgermann, J. Cell Sci., 2022, 135, jcs259645 CrossRef CAS PubMed.
- Irish Green Labs, https://irishgreenlabs.org/, accessed 19 July 2023.
- LEAF - Laboratory Efficiency Assessment Framework, https://www.ucl.ac.uk/sustainable/leaf-laboratory-efficiency-assessment-framework, accessed 19 July 2023.
- My Green Lab, https://www.mygreenlab.org/, accessed 19 July 2023.
- Sustainable Energy Authority of Ireland, Training, https://www.seai.ie/business-and-public-sector/small-and-medium-business/supports/training/, accessed 19 July 2023.
- Sustainable European Laboratories (SELs) Network, SELs Network-Working towards sustainable life science in Europe, https://sels-network.org/, accessed 30 August 2023.
- My Green and The International Institute for Sustainable Laboratories, Freezer Challenge, https://www.freezerchallenge.org/, accessed 16 November 2023.
- E. Mills and D. Sartor, Energy, 2005, 30, 1859–1864 CrossRef CAS.
- Green Light Laboratories Limited, Green Light Laboratories, http://greenlightlabs.co.uk/, accessed 20 November 2023.
- Heidolph, Findenser™, https://heidolph-instruments.com/en_US/products/Findensertm%7Ec27942, accessed 14 November 2023.
- M. A. Urbina, A. J. R. Watts and E. E. Reardon, Nature, 2015, 528, 479 CrossRef CAS PubMed.
- G. Bistulfi, Nature, 2013, 502, 170 CrossRef CAS PubMed.
- H. Ritchie, V. Samborska and M. Roser, Plastic Pollution, 2023, https://ourworldindata.org/plastic-pollution, accessed 5 December 2023.
- J. Bowler, Nature, 2022 DOI:10.1038/d41586-022-02092-1.
- Kimberly-Clark Professional, Rightcycle By Kimberly-Clark Professional™, https://www.kcprofessional.com/en-gb/solutions/rightcycle-by-kimberly-clark-professional, accessed 14 November 2023.
- L. Howes, ACS Cent. Sci., 2019, 5, 1904–1906 CrossRef CAS PubMed.
- Nature, 2017, 547, p. 6, DOI:10.1038/547006a.
- Trespa, Trespa® Toplab® Plus Align, https://www.trespa.com/products/trespa-toplab-plus-align, accessed 14 November 2023.
- TION, What Is Variable Air Volume And Why Is It Important?, https://www.tion.co.uk/education/what-is-variable-air-volume-and-why-is-it-important/, accessed 14 November 2023.
- My Green Lab, ACT The Environmental Impact Factor Label, https://act.mygreenlab.org/, accessed 11 September 2023.
| This journal is © The Royal Society of Chemistry 2024 |