Open Access Article
Open Access ArticleMethionine and its hydroxy analogues: the paths toward their sustainable chemical synthesis†
Sergio
Calderon-Ardila
 a,
Didier
Morvan
b,
Olivier
Péruch
b,
Virginie
Bellière-Baca
b,
Michiel
Dusselier
a,
Didier
Morvan
b,
Olivier
Péruch
b,
Virginie
Bellière-Baca
b,
Michiel
Dusselier
 *a and
Bert F.
Sels
*a and
Bert F.
Sels
 *a
*a
aCenter for Sustainable Catalysis and Engineering, Katholieke Universiteit Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: michiel.dusselier@kuleuven.be; bert.sels@kuleuven.be
bAdisseo France SAS, Antony Parc 2, 10 Place du Général de Gaulle, 92160 Antony, France
First published on 21st February 2024
Abstract
Methionine (Met) and its hydroxy analogue (MHA) are important components of a multimillion tonne scale commodity market of supplements used in human and livestock nutrition. Currently the industrial chemical synthesis of Met and MHA depends on petroleum-derived feedstock, such as propene. Additionally, the conventional synthetic methods involve dealing with highly toxic compounds, such as acrolein or cyanide. Substituting the conventional processes with new ones dependent on bio-based feedstocks, and involving safer chemistries, will be of great importance to reach a sustainable future. This review discusses the current chemical processes to synthesize Met and MHA and critically assesses different approaches aiming to improve the sustainability of its key feedstock, namely acrolein, or the overall synthesis. The strengths, weaknesses, future opportunities and threats for each synthetic approach are highlighted.
1. Introduction
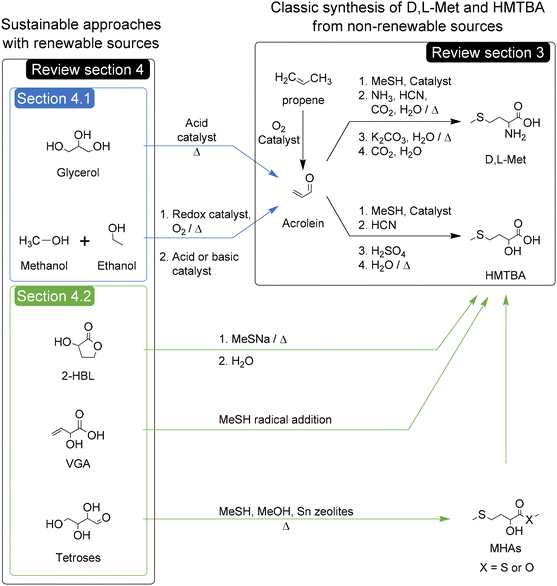
Creating a sustainable world is currently one of the most pressing challenges for humanity. Substituting processes dependent on petroleum for new ones dependent on renewable bio-based resources is a key step to achieving a more sustainable future, provided that the new processes prioritize atom economy and minimize overall greenhouse gas emissions. Great attention has been paid to the achievements leading said substitution for a variety of chemical species used to produce, or used directly as, polymers,1–4 fuels,5–8 and surfactants,9,10 among others.11–15Perhaps less visible are the efforts to create sustainable chemical processes for the synthesis of amino acids,16 and particularly methionine and its hydroxy analogues. These compounds constitute a commodity market with a scale of MT (megatonnes) per year that are chemically produced using non-renewable feedstock such as propene.17,18
In this review, we will focus on the renewable chemical synthesis of D,L-methionine (D,L-Met) and methionine hydroxy analogues (MHAs). First, we will provide a broad context that will allow the identification of the importance of these compounds en route to a sustainable future for humanity. For this purpose we will assess the importance of these compounds in the production of animal protein for human consumption, along with a description of the physical and chemical properties of D,L-Met and MHAs and their biological roles. Then we will briefly review the industrial chemical production of Met and MHAs (as depicted in Scheme 1) in order to identify the sustainability challenges of these processes. In a following section we will critically review the state of the art of approaches with potential to improve, directly or indirectly, the sustainability of the chemical synthesis of D,L-Met and MHAs. It is worth noting that for the purpose of this manuscript, the potential improvements in sustainability are mainly attributed, but not limited, to improvements in the renewability of the synthesis of the C4 backbone of Met and MHAs. The sustainability aspects of the synthesis of methanethiol (MeSH) fall outside the scope of this review. Specifically, this section will focus on discussing the advances in the chemical conversion of glycerol to acrolein, methanol and ethanol to acrolein, and the conversion of bio-based molecules and carbohydrates to MHAs. The potential and drawbacks of these approaches for replacing the current non-renewable routes will be discussed. In the following section we will present several sustainability metrics of the most relevant industrial and renewable processes for the synthesis of D,L-Met and D,L-HMTBA. This sustainability section will also present a qualitative assessment of different reaction parameters, reactants’ safety and risk of depletion of elements present in the catalysts used in each process. Apart from helping us to rank the processes according to their sustainability, the assessment will allow us to identify reaction parameters or process conditions that could be changed to further improve the greenness of the processes. Furthermore, this review will expose some outlooks and topics that could be ripe for further investigation to improve the feasibility of the sustainable approaches in the years to come.
2. The importance of methionine and its hydroxy analogues in the context of a sustainable future
2.1. Feeding the world by 2050 and beyond
The world's population has been increasing for almost a century and, although its rate of growth is decelerating, it is projected to keep growing from 7.7 billion in 2019 to close to 10 billion by 2075.19 This increase in population poses mounting challenges to governments and societies to guarantee food security for the whole human race. Comparing the consumption per capita of major food commodities, it turns out that developing and developed nations currently consume comparable amounts of carbohydrates and vegetable fats, while the consumption of animal-based protein such as meat and milk is remarkably lower in developing countries, see Fig. 1. It has been identified that developing nations that increase their wealth per capita also tend change their diets towards higher consumption of animal-based protein, reducing food insecurity and resembling the developed countries. This trend is expected to persist among developing countries at least up to 2080, driven primarily by the projected reduction of poverty in said nations during the years to come.20 In this regard, Vranken et al. have identified that meat consumption per capita stagnates or even decreases when nations have gross domestic products (GDP) per capita around and above 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–53
000–53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dollars (based on purchasing power parity (PPP) at constant 2005 international dollars).21 Although these results suggest that global consumption of meat, and possibly other animal-based proteins such as milk and dairy, could eventually decrease as poverty is reduced worldwide in the next decades, the high GDP per capita required for observing this effect, at around 44
000 dollars (based on purchasing power parity (PPP) at constant 2005 international dollars).21 Although these results suggest that global consumption of meat, and possibly other animal-based proteins such as milk and dairy, could eventually decrease as poverty is reduced worldwide in the next decades, the high GDP per capita required for observing this effect, at around 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dollars (PPP at constant 2005 international dollars), suggests that a sizable reduction in meat consumption of global scale could be far away from happening. This conclusion becomes clearer when observing that the GDP of most nations is well below this figure and the global GDP per capita sits currently at 16
000 dollars (PPP at constant 2005 international dollars), suggests that a sizable reduction in meat consumption of global scale could be far away from happening. This conclusion becomes clearer when observing that the GDP of most nations is well below this figure and the global GDP per capita sits currently at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 dollars (PPP at constant 2017 international dollars).22
997 dollars (PPP at constant 2017 international dollars).22
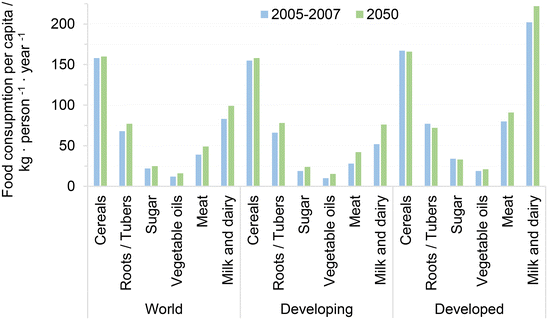 | ||
| Fig. 1 Consumption per capita of major food commodities. Adapted from ref. 20 with permission from the Food and Agriculture Organization of the United Nations, copyright 2012. | ||
The complete substitution of animal-based protein for plant-based protein does not seem the most sustainable solution. Models attempting to increase land productivity and reduce waste indicate that the most efficient use of land requires 12% of dietary protein to be obtained from animal sources, especially for populations of 40 million or more. Under these conditions, livestock located on land unsuitable for staple food crops can optimally consume by-products from crops located elsewhere and provide protein without competing for land with crops.23,24 Additionally, the future of the use of land to produce plant-based protein seems to be complicated by the decline in yield of staple food crops as a consequence of climate change.25,26 In a scenario where agriculture will not expand to current forest areas and therefore will not damage the environment by harming water quality, biodiversity, wildlife habitats and carbon capture capacity,27 climate change will produce an increase in land unsuitable for crops, and a sustainable and efficient use of land would require that a higher proportion of dietary protein is obtained from animal sources.
On the other hand, Willett et al. have recently proposed a universal healthy reference diet that aims to meet the United Nations sustainable development goals (SDGs) while also reducing health problems associated with inadequate nutrition and eating behaviors.28 The 2500 kcal per day diet consists mainly of vegetables, whole grains, dairy, fruits and legumes, along with small amounts of meat (particularly very low amounts of meat from cattle, sheep and pigs), starch-containing vegetables and added sugars. Under certain scenarios, the healthy reference diet and some variants thereof allow the reduction of the environmental effects of food production to reach the SDGs set for 2050. The authors stress that achieving the positive environmental effects of such healthy diet depends on substantial improvements in food production practices such as closing crop yield gaps to at least 75% and improving water, nitrogen, phosphorus and feed efficiency, among others. Interestingly, the results of these authors also showed that under scenarios with best production practices, the reference diets involving animal-based protein have better biodiversity preservation outcomes than the vegetarian and vegan variants.
Therefore, providing sufficient and constant access to safe and affordable animal-based protein is and will continue to be one of the most pressing challenges to achieve a sustainable future with adequate human nutrition worldwide. It turns out that scientific and technological advances will be fundamental to achieving this goal.
2.2. Optimizing livestock nutrition to improve the sustainability of animal-based protein production
The law of the minimum introduced by Carl Sprengel in 1828 for plant nutrition, and later popularized by Justus von Liebig for other agricultural systems, states that the growth of the system is not determined by the total amount of nutrients available but rather by the amount of the most deficient nutrient.29 This concept was applied by several researchers, mainly between 1900 and 1960, to establish the nutritional importance of alpha-amino acids (AAs) in mammals and distinguish the essential AAs from the non-essential, as presented in Table 1.30,31| Amino acid | Human (adult) | Chicken | Hen | Salmon | Swine | Cattle | Shrimp |
|---|---|---|---|---|---|---|---|
| Nutritionally essential alpha-amino acids are those that cannot be synthesized through the metabolic pathways of the organism at a sufficient rate to supply the demand of other metabolic functions. Conversely, non-essential amino acids can be synthesized by the organism. | |||||||
| Histidine | ± | + | ± | + | + | + | + |
| Isoleucine | + | + | + | + | + | + | + |
| Leucine | + | + | + | + | + | + | + |
| Lysine | + | + | + | + | + | + | + |
| Methionine | + | + | + | + | + | + | + |
| Phenylalanine | + | + | + | + | + | + | + |
| Threonine | + | + | + | + | + | + | + |
| Tryptophan | + | + | + | + | + | + | + |
| Valine | + | + | + | + | + | + | + |
| Arginine | ± | + | ± | + | + | + | + |
| Alanine | · | · | · | · | · | · | · |
| Asparagine | · | · | · | · | · | · | · |
| Aspartic acid | · | · | · | · | · | · | · |
| Cysteine | · | · | · | · | · | · | · |
| Glutamic acid | · | · | · | · | · | · | · |
| Glutamine | · | · | · | · | · | · | · |
| Glycine | · | + | · | · | · | · | · |
| Proline | · | · | · | · | · | · | · |
| Serine | · | · | · | · | · | · | · |
| Tyrosine | · | · | · | · | · | · | · |
| Selenocysteine | · | · | · | · | · | · | · |
It is well established that lysine and methionine are typically the first, second or third limiting AAs in the natural feedstuffs used in livestock nutrition, i.e. these are the essential AAs found in the smallest amount in the feedstuff, which can limit the growth and performance of farm animals.17,35,36 Supplementing natural feedstuffs with the corresponding limiting AA creates balanced feeds that allow achievement of optimal development and protein yields in livestock for human consumption37,38 while optimizing feed and water consumption and minimizing livestock waste.17,39–41 Consequently, AA supplementation in livestock feedstuffs, particularly with methionine and lysine, is key to achieving both the “zero hunger” and the “responsible consumption and production” SDGs of the United Nations.42
2.3. Methionine: properties and biological roles
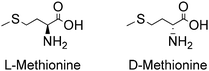
2-Amino-4-(methylthio)-butyric acid, commonly known as methionine (Met), is one of the two sulphur-containing proteinogenic alpha AAs. It has a four-carbon backbone and a sulphide functional group, besides the carboxylic acid and amino functional groups present in all AAs. It is classified as a nonpolar aliphatic AA. The carbon bearing its amino functional group is chiral, so Met can exist as L- and D-isomers, as shown in Fig. 2.Table 2 presents some of their physicochemical properties, along with those of the racemic mixture D,L-Met. Although enantiomers are expected to have identical physical properties in liquid and gas phase, and therefore L-Met, D-Met and D,L-Met are expected to have identical properties in said phases, differences in properties such as melting point, solubility in water and change of enthalpy of sublimation observed for the solid compounds are considered to arise from differences in the crystalline structure of the homochiral crystals L-Met and D-Met, as well as of the heterochiral crystal D,L-Met.43
As for all other alpha AAs, only the L-enantiomer of Met is found in proteins. The D-enantiomers are found only in other components and metabolic products of lower organisms such as bacteria, fungi, insects and certain plants.47–49 However, D-Met is the only D-enantiomer of an alpha AA that can be used by animals and humans as a nutritional AA, given the metabolic ability to convert D-Met into L-Met via transamination.50,51 The D-enantiomers of all other alpha AAs are typically catabolized by D-amino acid oxidase and D-aspartate oxidase.48
Methionine is an essential AA for animals and humans, i.e. their metabolic pathways cannot synthesize it and it needs to be ingested via the diet to maintain the biological functions of the organism. Plants and microorganisms such as bacteria can synthesize methionine from aspartate and a sulphur source, typically cysteine, which is synthesized using inorganic sulfate (SO42−) in plants as well as hydrogen sulfide (H2S) or methanethiol (CH3SH) in bacteria.52–54
For the biosynthesis of proteins in the ribosome, L-Met is encoded in mRNA by the AUG codon, which is also the most common initiator codon. Apart from its presence in proteins, L-methionine plays some indirect roles in animal and plants.55,56L-Met makes part of S-adenosyl-methionine (SAM), a cofactor with different biological functions. In bacteria, SAM is involved in terminating mRNA transcription via the SAM riboswitch.57 In eukaryotic cells, SAM works as a methyl group donor to different compounds such as nucleic acids, proteins, lipids and metabolites.56 In plants, SAM is involved in the biosynthesis of ethylene.56 As L-Met is involved in the biosynthesis of cysteine,58L-Met indirectly participates in the synthesis of glutathione which plays a key role in the control of oxidative stress.55 Likewise, L-Met is also indirectly involved in the synthesis of taurine which plays many different roles in biological functions such as osmoregulation, cardiovascular function and the formation and maintenance of muscle, among others.59
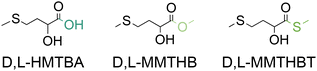
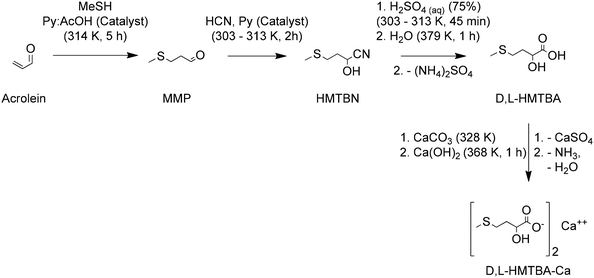
On the other hand, 2-hydroxy-4-(methylthio)butanoic acid (HMTBA), one of the methionine hydroxy analogues (MHAs) presented in Fig. 3, is of nutritional importance since it can be converted to L-Met by animals.
Dibner and Knight used chick liver homogenates to establish how the D- and L-enantiomers of HMTBA are converted to L-Met.60 They found that both enantiomers are first converted to 2-keto-4-(methylthio)butanoic acid (α-keto methionine) by enzymes that are enantiospecific. The conversion of L-HMTBA was catalyzed by L-α-hydroxy acid oxidase (L-HAOX, EC 1.1.3.15), an enzyme found in the peroxisomes, a organelle that in vertebrates is more abundant in liver and kidney cells. The conversion of D-HMTBA was catalyzed by D-2-hydroxy acid dehydrogenase (D-HADH, EC 1.1.99.6), an enzyme found in almost all cell tissues. The α-keto methionine intermediate is later converted to L-Met by transaminases in the presence of a sacrificial AA. The process is depicted in Scheme 2. Although the data of conversion of HMTBA into L-Met in ruminants vary greatly, it is well established that the conversion occurs in a large variety of tissues.61,62
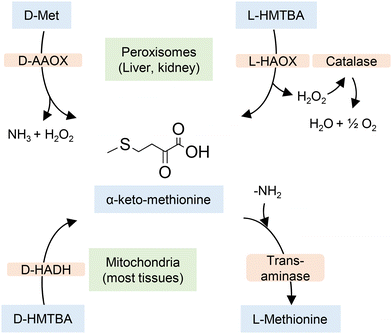 | ||
| Scheme 2 Conversion of D- and L-HMTBA and D-Met to L-Met in vertebrates. D-AAOX is D-amino acid oxidase. Based on ref. 50 and 60. | ||
Given its role as essential AA for different animal species, its low availability in natural livestock feedstuffs and its involvement in the sustainable production of animal protein for human nutrition, methionine (and HMTBA) is a compound of global relevance for a sustainable future. As will be discussed later, the conventional chemical synthesis of these molecules, on the other hand, is an industry of a scale of millions of tonnes per year that relies on non-renewable feedstocks and hazardous reagents. Consequently, achieving a sustainable future will require finding more renewable ways of synthesizing methionine and HMTBA or other MHAs such as MMTHB and MMTHBT (Fig. 3) that can be easily transformed to HMTBA through hydrolysis.
3. Industrial production of methionine and HMTBA
The production of methionine was recently reviewed by Willke in an excellent work that focuses on biological and biotechnological approaches for production of L-Met.18 The critical examination of the results published so far for the production of L-Met by fermentation of carbohydrates using wild and genetically modified organisms led the author to conclude that many of the best results reported are rather questionable. Several of the methods used for methionine quantification in the studies are known to give overestimations of methionine, which in many cases produced yields of methionine that are not possible according to mass balances in the reaction medium. Considering only the studies with reliable quantification methods, the author concluded that the highest yields of methionine of 26 mol% (concentration of 16 g L−1) and 19 mol% (concentration of 30 g L−1) have been obtained with genetically modified organisms, C. glutamicum by Moeckel et al.63 and E. coli by Discher et al.,64 respectively. The highest concentration of methionine of 35 g L−1 (yield of 16 mol%), obtained in a 60-hour process, has been achieved with a genetically modified C. glutamicum by Figge et al.65On the other hand, Willke also discussed in detail the advances of CJ CheilJedang in the production of L-Met through combined systems incorporating biotechnological and chemical steps. Its most important system comprises the fermentation of sugars to acetyl-homoserine through genetically modified organisms, followed by the enzymatic conversion of the homoserine to L-Met in the presence of MeSH.66–69 This technological platform has been used to build a plant for production of bio-methionine with a 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonne capacity in Malaysia. Willke asserted that while this approach may not provide economic advantages over the chemical synthesis of methionine, it could enhance the sustainability of its production.
000 tonne capacity in Malaysia. Willke asserted that while this approach may not provide economic advantages over the chemical synthesis of methionine, it could enhance the sustainability of its production.
However, the production of methionine through approaches using biological and biotechnological methods account for a very small fraction of the methionine produced every year. In 2018, close to 1.7 million tonnes of all forms of methionine, namely the enantiomerically pure L-Met, the racemic mixture of D- and L-Met (D,L-Met) and HMTBA, were produced worldwide,70 of which around 1.6 million tonnes correspond to DL-Met and HMTBA produced by chemical synthesis.17,18,71 Therefore, it is important to analyze and evaluate the strengths and weakness of the chemical synthesis of methionine and look for ways to overcome the latter.
Barger and Coyne reported one of the first chemical syntheses of methionine applying the Strecker reaction to 3-(methylmercapto)propionaldehyde (MMP, methional or 3-(methylthio)propanal). This and other methods provided yields below 6%, discouraging their use.72 It was only after the industrial production of acrolein, that was achieved by Degussa in 1942 by aldol reaction of formaldehyde and acetaldehyde, that further developments in the synthesis of methionine were made. Pierson et al. summarized the improvements made in this topic between 1938 and 1947 and proposed two improved methodologies to convert methanethiol and acrolein to D,L-Met through the hydantoin and the cyanohydrin pathways with yields of 73.5 and 75%, respectively.73
The chemical synthesis of methionine at industrial scale started in Germany in 1948 at Degussa with a process for D,L-methionine. A conventional process for production of D,L-Met starts by reacting acrolein with methanethiol (MeSH). Different variations for this reaction with yields around 95% are described by Hsu and Ruest.74 MMP is then subject to a Bucherer–Bergs reaction to form 5-(2-(methylthio)ethyl)hydantoin. Different variations of this reaction with yields around 97% are described by Geiger et al.75 Later the hydantoin is hydrolyzed in basic conditions to produce a salt of D,L-Met, which is finally converted to D,L-Met in acidic conditions. An important improvement to these last steps of the synthesis consist of minimizing the formation of salt by-products, e.g. Na2SO4. An efficient approach consists of performing the hydantoin hydrolysis with K2CO3 to later convert the salt of D,L-Met into D,L-Met using the CO2 by-product generated during hydrolysis. The KHCO3 is later converted to K2CO3, CO2 and water and recirculated to the system. This hydrolysis approach produces D,L-Met in yields around 99%.76 The high efficiency of the hydantoin hydrolysis is achieved by removal of the NH3 and CO2 by-products via distillation with concomitant sparging of the solution with an inert gas. The whole process, as shown in Scheme 3, has a yield of D,L-Met of 90%, calculated on acrolein.
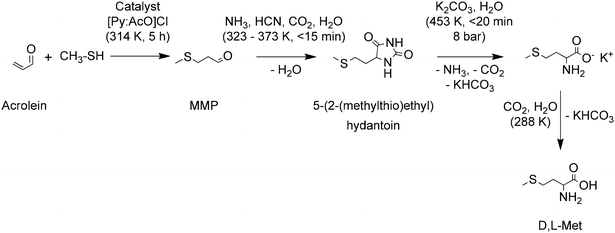 | ||
| Scheme 3 A chemical route for the industrial synthesis of D,L-Met. Based on ref. 74–76. | ||
Approaches for improving the safety of the process and reducing the formation of salt by-products have been developed. For instance, Adisseo developed a process that first converts acrolein to its cyanohydrin followed by reaction with MeSH to produce 2-hydroxy-4-(methylthio)butanenitrile (HMTBN), see Scheme 4. This variation avoids the formation of MMP which is a volatile, highly toxic and unstable compound. Instead, it forms the more stable cyanohydrin of acrolein. HMTBN is finally converted to D,L-Met through hydrolysis in the presence of an aqueous ammoniacal solution (NH3 and NH4HCO3). This last step replaces the formation of salt by-products for gaseous NH3 and CO2, that can later be used to regenerate the ammoniacal solution. The conversion of HMTBN into D,L-Met is believed to proceed through the formation of the 5-(2-(methylthio)ethyl)hydantoin; however, no evidence of its formation is provided. The process yields 44% of D,L-Met and 30% of by-products than can be converted to D,L-Met with further processing.77
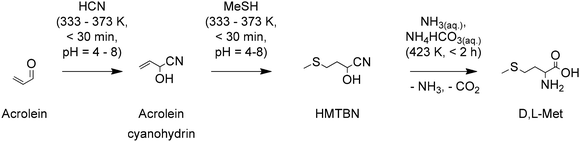 | ||
| Scheme 4 A cyanohydrins pathway for the synthesis of D,L-Met. Based on ref. 77. | ||
The chemical synthesis of D,L-HMTBA at industrial scale was developed by Monsanto in the United States in the 1950s. In 1956 the company patented a process that has become the industrial standard for the production of this MHA. This process also starts by producing MMP from acrolein and MeSH. MMP is then converted to its cyanohydrin (HMTBN) at 303–313 K in the presence of HCN and a catalytic amount of pyridine. Afterwards the nitrile group in the cyanohydrin is hydrolyzed to an amide group at the same low temperatures in the presence of a sub-stoichiometric amount of H2SO4. Then the amide is hydrolyzed to the corresponding carboxylic acid to form D,L-HMTBA under reflux of a larger aqueous dilution of the H2SO4 initially added. The previous step produces (NH4)2SO4 as by-product. Addition of CaCO3 removes the sulphate from the solution as CaSO4 and the remaining ammonium salt of D,L-HMTBA is converted to its calcium salt with concomitant elimination of gaseous NH3 in the presence of an aqueous solution of Ca(OH)2 at 368 K. The process yields 95% of the calcium salt of D,L-HMTBA, calculated on MMP, corresponding to a yield of 89% calculated on acrolein.78
Several variations to the synthesis of HMTBA are summarized by Hernandez and Moreno.79 These authors also developed, at the Sociedad de Desarrollo Técnico Industrial (now part of Adisseo), a synthesis to convert MMP into a solution with 80 wt% of monomeric HMTBA and 12 wt% of HMTBA oligomers. In their process, HMTBN is synthesized by a procedure similar to that shown in Scheme 5. Then, after the hydrolysis of HMTBN it uses NH4(OH) at a temperature below 343 K to neutralize the H2SO4. This allows the separation of an organic phase enriched in said monomeric D,L-HMTBA and an aqueous phase enriched in (NH4)2SO4. The patent also provides the preparation of a liquid solution with 88 wt% of monomeric D,L-HMTBA. Since HMTBA is the most abundant MHA produced at industrial scale, rather than its calcium salt, the sustainability assessment that will presented later for the synthesis of HMTBA corresponds to this process developed by Adisseo.
Although the efforts made so far to improve the sustainability and safety of the chemical synthesis of D,L-Met and HMTBA are significant, the processes still rely on feedstocks typically obtained from non-renewable sources such as acrolein (obtained from propene) and HCN (obtained from methane).80,81 Furthermore, acrolein and HCN are also very toxic compounds that, even though they can be generated on-site, avoiding their transportation, represent a considerable safety and hazard exposure risk for this industry.
Consequently, in the next section we will review the state of the art of three different approaches with potential to improve the sustainability and/or the safety of the chemical synthesis of D,L-Met and MHAs, namely the synthesis of acrolein and MHAs from bio-based molecules and the direct conversion of carbohydrates to MHAs.
4. Approaches for improving the renewability of the chemical synthesis of D,L-Met and MHAs
4.1. Acrolein from bio-based molecules
The synthesis of acrolein from renewable sources represents an indirect approach for improving the sustainability of the synthesis of D,L-Met and HMTBA. The conventional production of acrolein by oxidation of propene in the presence of catalytic metal oxides was summarized by Arntz et al. in 2007.80 In this review, we will focus in the chemical synthesis of acrolein from bio-based glycerol and renewables such as methanol and ethanol.Briefly, these authors examined the catalysts used in the gas-phase dehydration of glycerol to acrolein, which can be classified in three groups: (un)supported keggin-type heteropolyacids (HPAs), zeolites, and mixed metal oxides including phosphates and pyrophosphates. The best catalysts of the first group, namely CsSiW12O40 and Cs2.5H0.5PW12O40, have achieved yields and selectivities toward acrolein between 96 and 98% at temperatures between 523 and 548 K and short times on stream of 1 up to 3 hours.85,86 For the second group, different zeolites with Brønsted acidity such as H-beta, H-ZSM-5 and H-ZSM-11 produce yields and selectivities toward acrolein between 50% and 70%. The best results have been obtained with nanosized H-ZSM-11 producing a yield and selectivity towards acrolein of 78% at 593 K after 2 hours on stream.87 Using Pd-LaY zeolite and hydrogen as carrier gas, Pala-Rosas et al. obtained a constant yield of acrolein of 67% (selectivity of 87%) at 548 K after 3 hours in stream.88 The best catalysts of the third group are Fex(PO4)y, which reached yields and selectivities toward acrolein of up to 92% at 553 K after 5 hours on stream.89 The main advantage of the mixed metal oxide catalysts is their lower tendency to form cokes, which allows them to remain catalytically active and selective towards acrolein for longer times.
Yoda and Ootawa proposed two main reaction pathways for the gas-phase acid-catalyzed conversion of glycerol that are consistent with previous experimental results.90 Using in situ FT-IR spectrometry for the reaction catalyzed by H-Al-MFI (SiO2/Al2O3 = 27), the authors found that when glycerol interacts through its terminal alcohols with the acid sites in the zeolite, the reaction proceeds to form acetol, as shown in pathway 1 in Scheme 6. When glycerol interacts with the zeolite through its secondary alcohol the product formed is acrolein (pathway 2). Other authors have established that the reaction is more selective towards acetol in the presence of Lewis acidity and bases, while it is more selective towards acrolein in the presence of Brønsted acidity.86,91 Additional studies by Corma et al. and Suprun et al. have complemented the reaction pathways with zeolites and mixed oxides by establishing the most important reaction by-products including acetone, olefins, heterocycles and aromatics.92,93
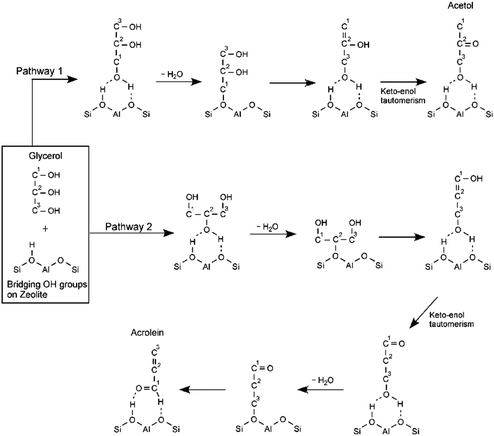 | ||
| Scheme 6 Proposed reaction pathways for the conversion of glycerol catalyzed by acid zeolites. Adapted from ref. 90 with permission from Elsevier, copyright 2009. | ||
In spite of the excellent catalytic results, the demonstrated techno-economic viability for the gas-phase synthesis of acrolein from glycerol (even at crude glycerol prices of 350 dollars per tonne),94 and the high prices of crude oil in the last decade (always above 39 dollars per barrel, annual average price for WTI), the production of acrolein from oil-based propene remains the industrial method of choice. Everything suggests that the future adoption of the glycerol to acrolein approach will require additional policy efforts intended to incentivize improvements in the sustainability of chemical processes of this kind and those depending on it, such as the synthesis of D,L-Met and MHAs.
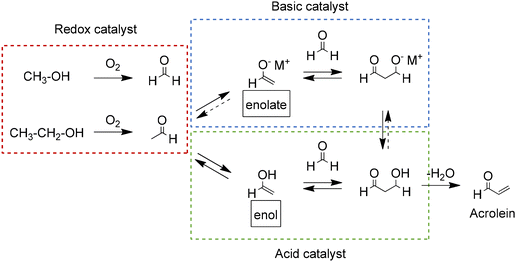 | ||
| Scheme 7 Proposed reaction pathways for the catalytic conversion of methanol and ethanol to acrolein. Adapted from ref. 95 with permission from The Royal Society of Chemistry, copyright 2017. | ||
Different catalysts have been tested in both the direct conversion of alcohols to acrolein and the two-step process using one catalyst for the oxidation reaction and another catalyst for the aldol reaction (see Table 3). For the direct conversion, FeMo composites of iron molybdate (Fe2(MoO4)3) and molybdenum oxide (MoO3) have achieved yields of acrolein between 36 and 39% with catalyst containing Mo to Fe ratio of 2.0 and 2.5 (FeMo2.0 and FeMo2.5). The high selectivity of these catalysts towards acrolein was attributed to the ability of molybdenum to catalyze the selective oxidation of the alcohols while being partially reduced from MoVI to MoV and MoIV. The participation of iron in the redox cycle is also expected, since it was reduced to FeII during reaction. The authors suggest that the aldol reaction to form acrolein is catalyzed by acid sites formed in the catalysts after partial reduction.95 Improvements in the yield of acrolein up to 40 and 42% were obtained after doping the FeMo catalysts with 1 mol% of lanthanum and cerium. The addition of these elements improved the aldol reaction of the process by increasing the amount of acid and basic sites in the catalysts.96 For the two-step process, yields of acrolein of up to 35% have been obtained using bare FeMo catalysts for the oxidation step and different amphoteric catalysts containing magnesium such as MgO supported on SiO2 and spinel (MgAl2O4). The high selectivity of the magnesium catalysts for the aldol reaction is attributed to their appropriate ratio of acid and basic sites.99,101
| Catalyst | T rxn (K) | TOS (h) | Conversion (%) | Y acrolein (%) | Ref. | |
|---|---|---|---|---|---|---|
| MeOH | EtOH | |||||
| T rxn = reaction temperature; TOS = time on stream; Yacrolein = yield of acrolein; FeMoX(Y) = Fe2(MoO4)3 + MoO3 catalyst with Mo/Fe ratio of X, synthesized at temperature Y (in kelvin); FeMoOx = iron molybdate; Mg/Si = MgO (3.7 wt%) supported on SiO2. | ||||||
| FeMo2.0 (723 K) | 593 | 2.5 | 100 | 100 | 36.5 | 95 |
| FeMo2.5 (673 K) | 593 | 2.5 | 100 | 100 | 39 | 95 |
| FeMoCe2.0 (673 K) | 593 | n.a. | 100 | 100 | 42 | 96 |
| FeMoLa2.5 (673 K) | 593 | n.a. | 100 | 100 | 40.5 | 96 |
| FeMoOx; Mg/Si | 533; 593 | ≥5 | 70 | 95 | 35 | 97 |
| FeMoOx; MgAl2O4 | 539; 558 | n.a. | 66 | 96 | 27 | 99 |
| FeMoOx; (0.8 Mg; 0.2 Mn)Al2O4 | 539; 558 | n.a. | 69 | 90 | 31 | 101 |
An important advantage of this approach is that the alcohols required for the process can be obtained from renewable sources. Ethanol is readily available from the fermentation of carbohydrates and significant advances have been made in the conversion of CO2 and methane to methanol.102–106 On the other hand, there is a long way to go to overcome some of the challenges that limit the yield of acrolein below 50%, namely the overoxidation of the alcohols to CO and CO2 and the low catalytic activity and selectivity of the catalysts for aldol reaction leading to acrolein (rather than by-products).
It is important to highlight that although the approaches converting glycerol and methanol and ethanol to acrolein can significantly improve the sustainability of the synthesis of D,L-Met and MHAs, these approaches do not eliminate the safety risk associated with working with acrolein and HCN. Therefore, alternative approaches that also address this issue, such as those discussed below, seem more benign from an environmental point of view.
4.2. Bio-based molecules to Methionine hydroxy analogues (MHAs)
Just as the industrial production of acrolein enabled the current developments in the synthesis of D,L-Met and HMTBA, recent advances in the chemical transformation of biomass-derived cellulose and carbohydrates to molecules that can be converted to MHAs can enable pre-existing and new routes for a more sustainable synthesis of HMTBA and other MHAs. 3-Hydroxydihydrofuran-2-(3H)-one (alpha-hydroxy-gamma-butyrolactone or 2-HBL) and 2-hydroxy-3-butenoic acid (vinyl glycolic acid or VGA) and analogues thereof such as methyl vinyl glycolate (MVG) are carbohydrate-derived molecules of particular interest given their potential for direct conversion to MHAs such as HMTBA and MMTHB.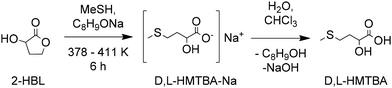 | ||
| Scheme 8 Conversion of 2-HBL to D,L-HMTBA. Based on ref. 107. | ||
Although Ruest did not specify the reaction mechanism, the formation of the sulphide could only occur if alkyl-O cleavage is preferred over acyl-O cleavage during lactone thiolysis under basic conditions. Plieninger had previously established that gamma-butyrolactone and alpha-amino gamma-butyrolactone (the lactone of homoserine) were transformed in the presence of MeSNa to intermediates resulting from the nucleophilic acyl addition of MeS−. As sulphides were formed after heating said intermediates (showing the preference for alkyl-O cleavage over acyl-O cleavage), the author indicated that a rearrangement occurred.108
To the best of our knowledge, further advances in the conversion of 2-HBL to MHAs were only reported in 2006, when Deck et al. described the conversion of 2-HBL to D,L-HMTBA in yields of up to 100% in the presence of MeSNa and polar aprotic solvents such as DMSO and DMF at temperatures below 464 K.109
Even though producing HMTBA from 2-HBL can considerably improve the safety of the synthesis of MHAs, additional studies of this transformation have been discouraged by several factors. Perhaps the most important factor is that the historically low prices of HMTBA and D,L-Met do not make the synthesis from 2-HBL economically attractive. In the last two decades, the European spot prices of HMTBA and D,L-Met have fluctuated between 1.6 and 4 euros per kg, with a small exception during a period between 2015 and 2016 when these commodities reached prices between 4 and 7 euros per kg.70 These economic factors motivated the study of the inverse process of converting HMTBA into 2-HBL, which has been achieved in the last decade with yields as high as 78% based on HMTBA.110
Another factor that prevented further exploration of the conversion of 2-HBL to MHAs is that until very recently 2-HBL used to be synthesized at industrial scale from feedstocks derived from oil such as maleic anhydride or gamma-butyrolactone.109–113 Therefore this way of producing 2-HBL would not radically improve the sustainability of the synthesis of MHAs when compared with the conventional production of D,L-Met and MHAs from acrolein obtained from non-renewables. This outlook has recently changed after the publication of works reporting the synthesis in good yields of 2-HBL from bio-based dihydroxyacetone (DHA) and formaldehyde (HCHO). Yamaguchi et al. were the first to report the conversion of DHA and formaldehyde to 2-HBL in yields up to 70% when catalyzed by SnCl4 in the presence of small amounts of water.114 Later Van de Vyver et al. reported a yield of 68% for the same reaction catalyzed by Sn-beta zeolite.115 They also proposed a reaction pathway involving a Sn-catalyzed soft enolization of DHA followed by aldol addition of formaldehyde to form a tetrose intermediate. This intermediate undergoes beta dehydration via retro-Michael reaction followed by cyclization, isomerization and a final 1,2-hydride shift to form 2-HBL (see Scheme 9 for the chemistry).
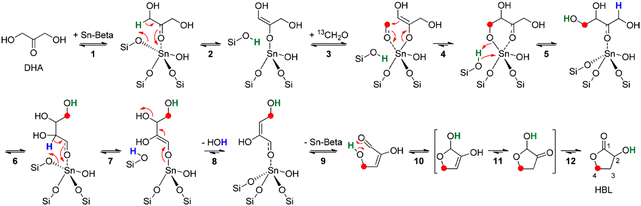 | ||
| Scheme 9 Reaction for the conversion of DHA and formaldehyde to 2-HBL catalyzed by Sn-beta zeolite. Reproduced from ref. 115 with permission from The American Chemical Society, copyright 2015. | ||
Using DFT calculations the authors discussed that this reaction pathway would be preferred over the one proposed by Yamaguchi et al. requiring an energetically unfavourable aldol addition of formaldehyde to pyruvic aldehyde. In follow-up work Yamaguchi et al. proposed another reaction pathway for their SnCl4 catalyzed system, involving the aldol addition of formaldehyde to 2-hydroxyacrylaldehyde.116 Likewise, they established the effects of addition of water, carbonyl compounds and Brønsted acids on the reaction rate and selectivity.116–118
These advances in the catalytic conversion of bio-based DHA and formaldehyde to 2-HBL have the potential to make a more sustainable synthesis of MHAs from 2-HBL a reality. On the one hand, the existence of this alternative route to produce 2-HBL can lower the spot price of this chemical. On the other hand, its inherent improvements to the sustainability of 2-HBL synthesis can be directly translated as an improvement to the sustainability of MHAs synthesis when compared with the conventional synthesis of D,L-Met and MHAs from acrolein obtained from non-renewables. Nonetheless, the synthesis of MHAs from renewable 2-HBL would indirectly, or directly, involve working with formaldehyde, a compound recognized as toxic and carcinogenic, representing an important safety drawback that could impede the implementation of this approach.119
An efficient process for the conversion of VGA and MVG to HMTBA and MMTHB, respectively, was developed at Novus International Inc in 1997 by Koenig.120 The reaction proceeds through a classical free-radical thiol–ene addition reaction between MeSH and VGA (or MVG) to yield the anti-Markovnikov product via addition of a thyil radical to the terminal vinyl carbon in the substrate. The product of this radical addition contains a terminal sulphide group and a secondary alkyl radical capable of generating more thyil radicals by abstraction of a hydrogen from MeSH, as shown in Scheme 10.121,122 Particularly, the authors used a radical initiator, such as azobisisobutyronitrile, that after activation by exposure to UV light or temperatures between 323–333 K converted MeSH and VGA (or MVG) to HMTBA (or MMTHB) in yields up to 85% after 6 hours of reaction.
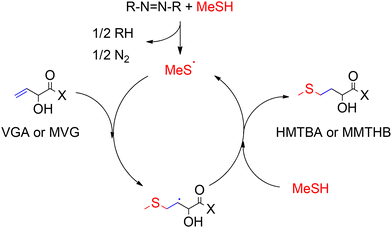 | ||
| Scheme 10 Reaction for the conversion of VGA and MVG to methionine hydroxy analogues HMTBA and MMTHB through free radical thiol–ene addition. X represents –OH or –OMe and R represents [(CH3)2C(CN)−]. | ||
Further interest in the conversion of VGA and MVG to methionine hydroxy analogues only appeared in recent years, after reports of the successful synthesis of these vinylic compounds from carbohydrates, as will be presented later. Researchers at Adisseo France S.A.S. have recently reported the conversion of MVG to MMTHB through free-radical thiol–ene addition where the radical initiator is composed of a persulfate (S2O82−) and a transition metal in elemental or oxidized form, particularly copper or iron species.123 The process yields up to 81% of MMTHB after 1 hour at 273 K. The advantage of this method in comparison with that developed by Novus is the replacement of the organic radical initiator for an inorganic one that is more stable and less expensive. However, this method requires working at temperatures below ambient conditions and, as the previous method, it also requires the removal of by-products generated by the initiator.
Adisseo has also reported the conversion of VGA and butanethiol (BuSH) to the corresponding MHA through free-radical thiol–ene addition in the presence of UV light with wavelength of 365 nm and HMTBA as a catalyst.124 After 1 hour of reaction at 305 K, a reaction without HMTBA reached a VGA conversion of only 63% and a yield of MHA of 51% (selectivity of 81%). In the presence of HMTBA the conversion of VGA was almost complete, yielding 96% of the MHA (selectivity of 98%), reflecting the catalytic ability of HMTBA to improve reaction rate and selectivity. Assuming that the results would be similar when replacing BuSH by MeSH, this process would offer several advantages in comparison with those mentioned above. This new process is not only faster and more selective, but it is also safer and cheaper as it does not require radical initiators or the removal of their by-products.
As mentioned before, the revived interest in the conversion of VGA and MVG to methionine hydroxy analogues has arisen from recent developments in the conversion of carbohydrates to said vinyl compounds. Conventionally, VGA is synthesized from acrolein and HCN in yields around 85%.120 The synthesis is similar to that presented in Scheme 4, by reacting acrolein and HCN to form acrolein cyanohydrin which is then hydrolyzed to VGA in the presence of a Brønsted acid. MVG is obtained from VGA by several methods such as Fischer esterification in the presence of a Brønsted acid and methanol under reflux.
The synthesis of MHAs from VGA and MVG obtained by these conventional methods would not significantly improve either the sustainability or the safety of the MHA synthesis. However, these parameters can substantially improve thanks to recent advances in the catalytic conversion of carbohydrates to VGA and MVG. Holm et al. were the first to report the formation of small amounts of MVG during the conversion of sucrose, glucose and fructose to methyl lactate in the presence of methanol and Lewis acid zeolites, particularly Sn-beta. When the reaction was performed with erythrose at 433 K for 20 hours, a yield of MVG of 56% was obtained.125 Holm et al. also found that Sn-beta catalyzed the aldol reaction of glycolaldehyde to form tetroses that are later transformed to MVG in yields up to 30%, in reactions in methanol at 413 K for 16 hours,126 see Scheme 11.
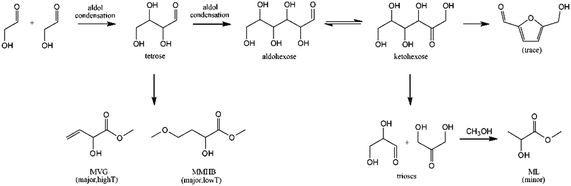 | ||
| Scheme 11 Proposed reaction pathway for the conversion of glycolaldehyde to using Sn-beta zeolite. Reproduced from ref. 126 with permission from The Royal Society of Chemistry, copyright 2012. | ||
Dusselier et al. later established the reaction pathways of the Sn-catalyzed conversion of tetroses and glycolaldehyde to alpha-hydroxy acids (AHAs) and derivatives with a four-carbon backbone such as MVG, 2-HBL and methyl-4-methoxy-2-hydroxybutanoate (MMHB).127,128 For the conversion of glycolaldehyde the authors also established that balancing Brønsted and Lewis acidity generated from homogeneous tin chlorides to a ratio of 3 (by adding or quenching HCl) enhanced the reaction rate for the formation of AHAs. The authors also found that the selectivity towards MVG analogues (and 2-HBL) improved in sterically hindered alcohols and nonprotic solvents like DMSO, see Scheme 12.
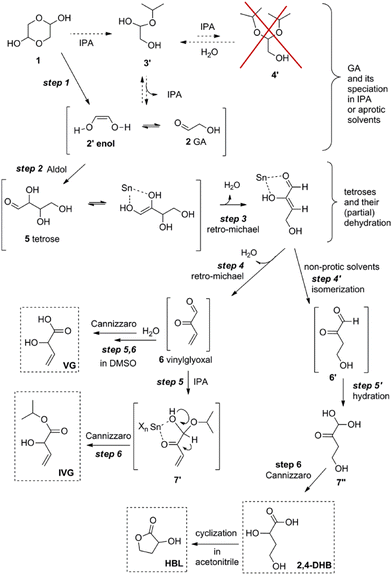 | ||
| Scheme 12 Reaction pathway for the conversion of glycolaldehyde in isopropyl alcohol or nonprotic solvents in the presence of homogeneous tin chlorides. Reproduced from ref. 128 with permission from The American Chemical Society, copyright 2013. | ||
Additional details of the transformation of tetroses to VGA and other AHAs in the presence of homogeneous tin chlorides have been recently published by Jensen et al.129 The authors performed NMR studies that confirmed the formation of the key intermediates vinyl glyoxal and 3-deoxythreosone (6 and 6′ in Scheme 12, respectively) that gives rise to VGA (or its derivatives) and 2-HBL, respectively. Thermodynamic and kinetic details of the transformation of tetroses to the most abundant AHA product, MMHB, are also presented.
So far, the best results for the catalytic conversion of carbohydrates to VGA and its analogues have been obtained with Sn zeolites. In addition to the 56% yield of MVG obtained by Holm et al. in the conversion of tetroses with Sn-beta at 433 K,125 De Clercq et al. demonstrated that confinement effects produced in different Sn zeolite frameworks affect the selectivity of the transformation of tetroses toward different AHAs.130 Mesoporous materials like Sn-MCM-41 and Sn-SBA-15, with mesopore diameters ranging between 2 to 6.5 nm and 2 to 30 nm, respectively, were more selective towards the bulkier AHA, namely MMHB. Conversely, microporous materials such as Sn-MFI and Sn-beta, the latter with 12-membered-ring channels with diameter of 6.6 to 7 Å (straight channels along [100] axis) and 5.6 Å (zigzag channels along [001] axis), had a higher selectivity towards a smaller AHA, namely MVG. The reaction was particularly selective towards MVG at high temperature, where a selectivity and yield of MVG of 50% was obtained at 433 K in one hour reactions.
Furthermore, Tolborg et al. reported the conversion of glycolaldehyde to VGA in yields of up to 44% in water at 373 K after 4.5 hours, using a hydrothermal Sn-MFI obtained by the base synthetic route.131 The authors found that the medium size channels in Sn-MFI, with 10-membered rings and average diameter of 5.5 to 6 Å, were more selective to convert glycolaldehyde into VGA given that these size restrictions in the zeolite framework prevented the formation of large carbohydrates (pentoses and hexoses) through aldol reaction. The slightly larger channels in a hydrothermal Sn-beta produced a lower selectivity towards VGA along with reaction products coming from the formation and transformation of hexoses.
It is reasonable to consider that the synthetic and mechanistic advances made so far in the catalytic transformation of carbohydrates to VGA and its analogues have the potential to foster both the already existing and new developments for more sustainable and safer synthesis of MHAs from bio-based VGA and MVG.
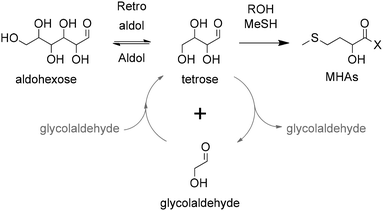 | ||
| Scheme 13 Direct chemical conversion of C2, C4 and C6 carbohydrates to MHAs catalyzed by Sn-zeolites. X represents MeS− or RO−. | ||
This approach has been scarcely explored and the first advances in this matter have recently been reported in patents.132,133 One of these documents discloses the use of different metallo-zeolites in the conversion of carbohydrates to MHAs in aqueous and alcoholic solutions containing small amounts of K2CO3.132 It was found that postsynthetic Sn-MFI and Sn-beta, prepared by wet incipient impregnation, were the most selective towards the formation of MHAs in methanolic solutions. For batch reactions, the highest yield of MHAs of 29% was obtained in the conversion of erythrulose with Sn-MFI at 373 K for 4 hours (selectivity of 36%). Sn-Beta yielded 24% of MHAs (selectivity of 31%) under the same conditions. Glycolaldehyde, glucose and sucrose produced significantly lower yields of MHAs under all reaction conditions tested with Sn-beta. It is worth noting that some results presented in this document are rather peculiar, such as the lower conversion of glycolaldehyde at 393 K when the reaction time is increased from 4 to 16 hours and when both temperature and reaction time are increased to 413 K and 16 hours. The document also revealed that under a continuous flow reactor the transformation of glycolaldehyde to MHAs considerably improved, yielding around 30 to 35% of MHAs in the presence of Sn-beta and methanol with 11 wt% of water in a process at 433 K. However, the low concentration of MeSH used in this continuous process limits the yield of MHAs that can be obtained to less than 25%, so that the results of this continuous process will need further validation.
The other patent shows that the presence of small amounts of water improved the conversion of carbohydrates to AHAs in both continuous flow and batch reactions catalyzed by Sn-beta in alcoholic solutions.133 The document states that the addition of small amount of water to the system also decreased carbon deposits and Sn leaching from the catalyst. The patent also asserts that the addition of water also benefits the conversion of glycolaldehyde to MHAs when catalyzed by Sn-beta in alcoholic solutions. The document reports yields of MHAs of 39% (selectivity of 40%) and 47% (selectivity of 47%) for batch reactions performed in methanol and ethanol, respectively, at 433 K for 16 hours. However, the results for these reactions performed with molar ratios of glycolaldehyde to MeSH of around 0.25–0.29 do not agree with additional results for reactions in methanol, where a molar ratio glycolaldehyde to MeSH of 0.2 led to a yield of MHAs as low as 8%. Conversely, an optimum yield of MHAs of 37% is reported for a reaction with a molar ratio glycolaldehyde to MeSH of 0.8. Due to the technical nature of the documents, both patents lack fundamental experiments and analysis that explain the transformation of carbohydrates to MHAs and the factors limiting the reaction selectivity.
More recently, our group established the major reaction pathways for the acid-catalyzed conversion of tetroses to MHAs derived from butanethiol (BuSH), as shown in Scheme 14.134
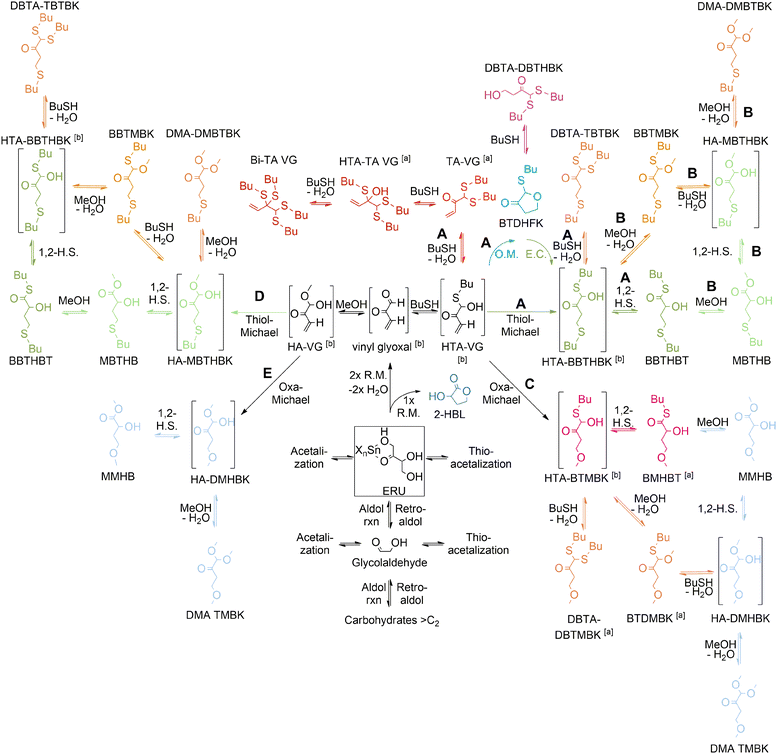 | ||
| Scheme 14 Reaction pathways for the acid-catalyzed conversion of tetroses in the presence of butanethiol and butanethiol/methanol mixtures. Reaction pathways: A (in BuSH as solvent), B and C (in BuSH rich medium), D (in BuSH poor medium) and E (in MeOH) Reproduced from ref. 134 with permission from John Wiley and Sons, copyright 2022. | ||
We found that when butanethiol is the only nucleophile in the solvent mixture (pathways A in Scheme 14), the conversion of tetroses to MHAs is kinetically and thermodynamically unfavourable in comparison with the formation of thioacetal by-products. It was found that Brønsted acids were highly selective to catalyze the thioacetalization side-reactions while Lewis acids were more selective towards the final 1,2-hydride shift reaction that produces MHAs from tetroses, especially tin, tungsten and molybdenum chlorides. However, said 1,2-hydride shift was found to be the rate-determining step of the reaction cascade, so that improving the reaction's selectivity towards MHAs required establishing strategies to either prevent the thioacetalization reactions or to minimize the formation of thioacetals by carefully choosing the reaction conditions so that their formation was thermodynamically less favorable.
In this sense, a higher selectivity towards MHAs was achieved by the addition of base, water or methanol to the reaction mixture. The addition of a catalytic amount of base minimized the occurrence of thioacetalization reactions by neutralization of Brønsted acidity that is generated by solvolysis of the Lewis acid catalyst. On the other hand, the addition of water and methanol affected the thermodynamic equilibrium of the thioacetalization reactions, disfavouring the formation of thioacetals and allowing the reaction intermediates HTA-BBTHBK and HA-MBTHBK to be converted to MHAs.
We also found that using methanol as reaction solvent had other benefits for the reaction. On the one hand, methanol established a nucleophile competition with BuSH for addition to reactive carbonyl intermediates, contributing to decreasing the formation of thioacetals and enhancing the selectivity towards MHAs (pathways B and D in Scheme 14). On the other hand, the incorporation of this green solvent allowed optimization of the use of the sulphur feedstock in the reaction, an important factor considering that the presence of thiols in waste streams can cause air pollution by formation of SOx and subsequent generation of acid rain.135,136 In the presence of polar protic solvents such as methanol, the catalyst choice narrowed to tinIV chlorides since the more oxophilic tungsten and molybdenum chlorides were more selective toward the formation of by-products containing an ether bond (i.e. preferring the reaction pathways C and E of Scheme 14) instead of the sulphide bond present in MHAs.
When the sulphur source was changed from BuSH to MeSH, aiming to synthesize MHAs of industrial relevance such as HMTBA, MMTHB and MMTHBT, we observed an improved selectivity towards the synthesis of MHAs.137 We correlated said enhanced MHA selectivity to an important change in the reaction pathways when MeSH is present, namely, MeSH does not lead to the formation of thioacetals of vinyl glyoxal (such as compound 11 in Scheme 15). We propose that both the lower nucleophilicity of MeSH and its harder nucleophile character, in comparison with BuSH, favours the thio-Michael addition reaction that convert 1 into 2a–2c (the last key intermediates before the final formation of MHAs). On the one hand, the less nucleophilic MeSH adds slower to the aldehyde carbon of vinyl glyoxal to form the hemithioacetal 1a, and the formation of the thioacetal 11 would be even slower (as it is the case for most thioacetalization reactions).138 As a result, one can expect that in the presence of MeSH there will be more hemithioacetal 1a available to perform the thiol-Michael reaction that leads to the key intermediates 2a–2c. Furthermore, the addition of a harder nucleophile (like MeSH or the even harder MeOH) to the aldehyde carbon in vinyl glyoxal helps to retain the electrophilicity of the terminal carbon in the enone on compound 1, preserving it as a notable electrophile for the desired thiol-Michael addition. Conversely, the more nucleophilic BuSH adds faster to the aldehyde carbon of vinyl glyoxal to yield both the hemithioacetal 1 and the thioacetal 11 (despite its softer nucleophile character suggesting that BuSH addition to the harder aldehyde electrophile in vinyl glyoxal is disfavoured). The softer nucleophile character of BuSH makes the terminal carbon of the enone in 1 less electrophilic (and even less electrophilic for the case of 11), converting the enone into a poorer Michael acceptor for the desired thiol-Michael reaction that yields compounds 2a–2c. Although the discussion about nucleophile and electrophile hardness was not included in our original manuscript, this additional concept helps to explain the results obtained, without changing the conclusions derived in the research.
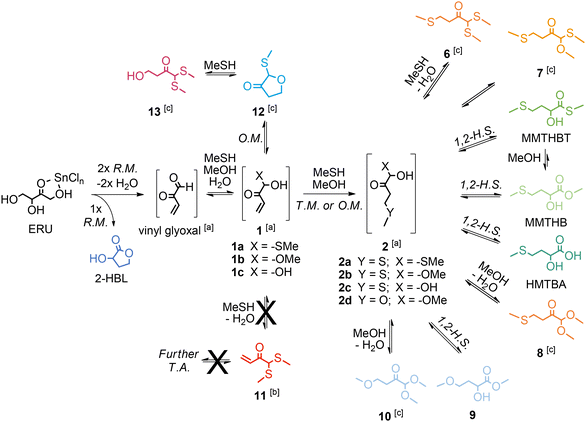 | ||
| Scheme 15 Reaction pathways for the conversion of tetroses to methionine hydroxy analogues in the presence of SnIV Lewis acid catalyst in MeSH/MeOH mixtures. Adapted from ref. 137 with permission from Elsevier, copyright 2023. | ||
We achieved the highest reported yield and selectivity towards MHAs of 38% in the conversion of tetroses catalyzed by SnIV Lewis acid in one hour batch reactions at 413 K, by combining parameters affecting the reaction selectivity, such as the ratio between MesH and MeOH, the pressure of inert gas and water content in the reaction mixture.
It is important to highlight that, despite our analytical efforts, the reaction pathways followed by roughly 30% of the carbon coming from the substrate remain unknown. It is well known that the high reactivity of tetroses during catalytic conversion leads to the formation of a significant number of minor by-products with very low yields, making their identification challenging.129,139 Consequently, additional characterization and quantification efforts will be required to complete the reaction networks of the conversion of tetroses in the presence of acid catalysts and thiols.
Although the conversion of carbohydrates to MHAs is a very recent approach, the results obtained so far are very promising and suggest different topics that will require further research in order to improve the reaction selectivity towards MHAs; in particular (i) establishing the reaction pathways of the minority products, (ii) studying other catalysts with different structural frameworks or different acid properties, (iii) performing in-depth spectroscopic studies of the interaction between thiols and Lewis acid catalysts in order to determine the effects of thiols in the catalyst's reactivity and stability, and (iv) assessing the effects of other solvents and other chemical species (that can be added to or removed from the system) in the reaction selectivity and catalyst stability, are of utmost importance. Whether Sn-beta remains as the most active and selective catalyst for this transformation, the scientific literature already provides different strategies aiming to preserve or improve its activity, selectivity or stability.140–143 Many of these strategies still need to be tested in a medium containing sulphur compounds.
5. The sustainability metrics of the old and new routes for chemical synthesis of D,L-Met and MHAs
We assessed the green chemistry metrics of the most relevant industrial processes for the synthesis of D,L-Met and HMTBA, as well as of the most novel renewable routes towards MHAs, as depicted in Scheme 16. Specifically, for the industrial processes we chose the synthesis of D,L-Met through the hydantoin and cyanohydrin processes, and the synthesis of HMTBA through the cyanohydrin process. For the renewable routes we selected the direct conversion of 2-HBL to HMTBA using MeSNa, the conversion of MVG to MMTHB using copper and persulfate as catalyst and the direct conversion of carbohydrates to MMTHB.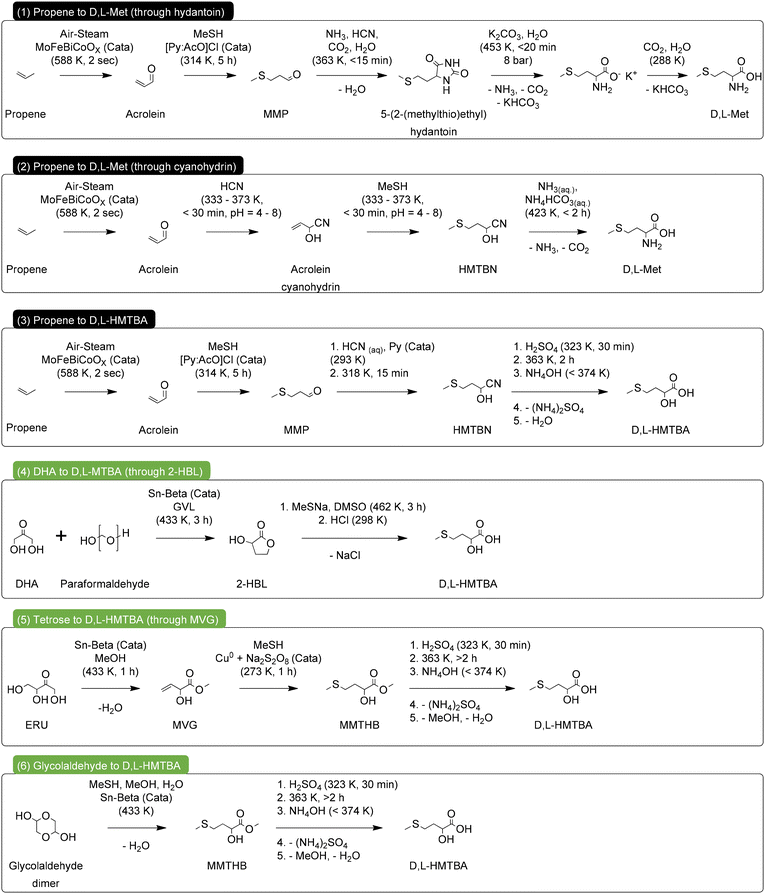 | ||
| Scheme 16 Industrial (1–3) and renewable (4–6) synthetic routes for the production of D,L-Met and HMTBA, used in the assessment of green chemistry metrics. | ||
We propose implementing minor additions to the different processes so that their comparison can be as equitable as possible. First, we decided that all processes should start from easily accessible carbon feedstock. Therefore, the industrial processes will start from propene as carbon source, while the renewable routes will start from carbohydrates. For the case of the industrial processes 1–3, we chose the conversion of propene to acrolein as described by Teshigahara and Kanuka.144 For the renewable processes 4–6, we chose conversion of dihydroxyacetone and paraformaldehyde to 2-HBL as described by Van De Vyver et al.,115 the conversion of erythrulose to MVG as described by Holm et al.,125 and the direct conversion of glycolaldehyde dimer to MMTHB as described by Sadaba Zubiri et al.133 Additionally, we decided that the target product of every route should be a free acid (D,L-Met or HMTBA) as is the case for the industrial processes 1–3 and the renewable process 4. Therefore, the renewable processes 5 and 6 include the necessary steps for hydrolysis of MMTHB into HMTBA. For the sake of ease of comparison, this hydrolysis step can be a replica of the hydrolysis step in process 3 converting HMTBN into HMTBA. The yield of the hydrolysis in processes 5 and 6 can be expected to be similar as in process 3, provided that sufficient reaction time is allowed.
We evaluated the sustainability metrics of these different processes using the zero and first passes of the CHEM21 metrics toolkit developed by McElroy et al.145 Readers are referred to said publication to delve into the specifics of this metrics toolkit. Briefly, the CHEM21 metrics toolkit provides both quantitative and qualitative results that facilitate establishing the sustainability of the different chemical processes as well as their safety risks. For the quantitative metrics of the different processes, we calculated yield, atom economy (AE), reaction mass intensity (RME), optimum efficiency (OE), process mass intensity (PMI) and renewables percentage (RP). AE, RME and OE are used to evaluate the efficiency of the synthetic pathways in terms of utilization of reactants (Jiménez-González et al. provide a clear definition for reactant146). AE provides the theoretical maximum efficiency of the reaction while RME provides the experimental efficiency (as it accounts for AE, yield and stoichiometry). OE indicates how efficient a synthetic pathway actually is, as percentage of its theoretical maximum efficiency. Therefore, OE is a useful metric to compare the efficiency of utilization of reactants among the different synthetic pathways. AE, RME and OE are important to evaluate the sustainability of reactions that are under early research stages in the lab, as these metrics only account for the mass of reactants and neglect the mass of other components such as solvents and work-up chemicals (which are typically used in excess when performing research at lab scale).
On the other hand, PMI metrics account (combined or separately) for other species present in the synthetic pathway such as reagents and catalysts (that when combined with the mass of reactants become PMI RRC), solvents (PMI Solv) and chemicals used in work up (PMI WU). Therefore, PMI metrics are more relevant to evaluate the sustainability of chemical processes that are beyond the early stages of research, such as the industrial processes 1–3 for production of D,L-Met and HMTBA.
The RP of each process was calculated considering species such as NH3, CO2, NH4OH, MeOH, HCHO, K2CO3 and NH4HCO3 as renewables. This consideration stems from the ongoing efforts to develop technologies for the production of green hydrogen as well as for CO2 capture and utilization,147–153 which hold the potential to render the production of the former compounds renewable. As a result, the RP values reported here will remain valid for the years to come.
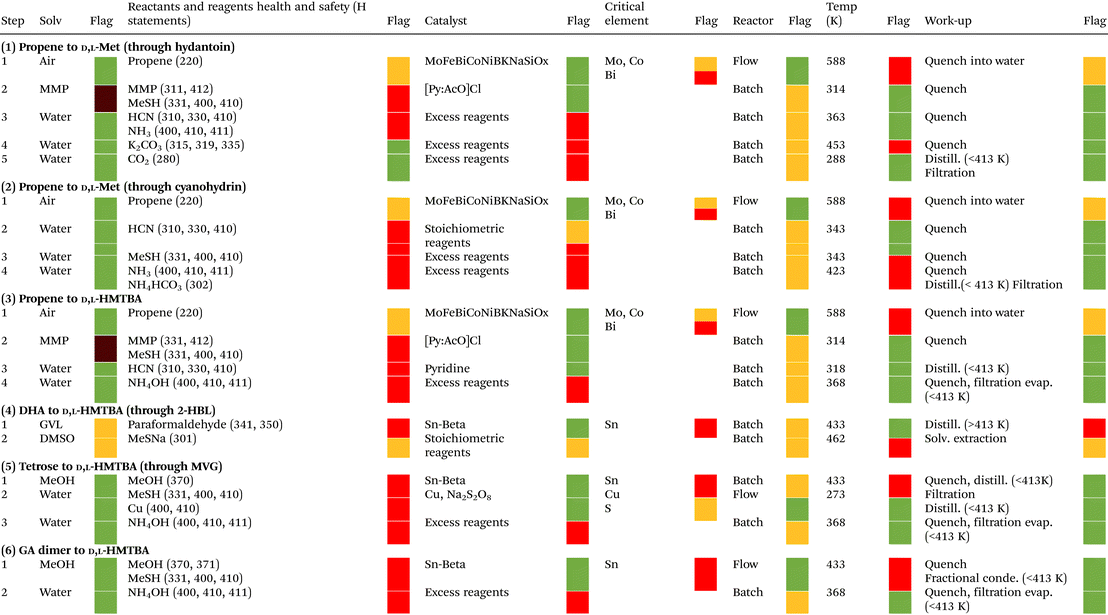
For the different processes we also evaluated the solvents, reactants and reagents, catalysts, reactor type, reaction temperature and workup, using the zero and first passes of the qualitative sustainability assessment of the CHEM21 metrics toolkit. The outcome of this evaluation is a coloured flag. In general terms, a green flag indicates that a given parameter or condition of the process follows the principles of green chemistry, and therefore is recommended. A red flag indicates the contrary, while an amber flag is given for conditions in-between. A brief explanation of the criteria used to assign the flags is presented in the footnote of Table 5, while a more detailed explanation can be found in the original manuscript of McElroy et al.145
Tables 4 and 5 present result summaries of the quantitative and qualitative sustainability assessment for the different processes for production of D,L-Met and HMTBA presented in Scheme 16. A extended version of the quantitative sustainability metrics is presented in Tables S1.†
| Metric | Yield (%) | AE (%) | RME (%) | OE (%) | PMI (g g−1) | PMI RRC (g g−1) | PMI Solv (g g−1) | PMI WU (g g−1) | RP (%) |
|---|---|---|---|---|---|---|---|---|---|
| AE: atom economy. RME: reaction mass efficiency. OE: optimum efficiency. PMI: process mass intensity. PMI RRC: PMI for reactants, reagents and catalysts. PMI solv: PMI of solvents. PMI WU: PMI of species used in reaction work-ups. RP: renewables percentage. | |||||||||
| Synthetic route | |||||||||
| (1) Propene to D,L-Met (through hydantoin) | 81.0 | 40.7 | 17.9 | 43.9 | 31.0 | 6.7 | 20.7 | 3.6 | 91.1 |
| (2) Propene to D,L-Met (through cyanohydrin) | 67.7 | 60.8 | 16.6 | 27.3 | 31.4 | 7.2 | 19.9 | 4.3 | 91.8 |
| (3) Propene to D,L-HMTBA | 71.8 | 50.0 | 37.2 | 74.4 | 11.1 | 3.9 | 3.3 | 3.9 | 66.0 |
| (4) DHA to D,L-HMTBA (through 2-HBL) | 68.0 | 66.3 | 38.3 | 57.7 | 144.8 | 3.5 | 122.1 | 19.1 | 92.5 |
| (5) Tetrose to D,L-HMTBA (through MVG) | 38.1 | 42.7 | 19.0 | 44.4 | 68.0 | 6.9 | 59.6 | 1.5 | 95.9 |
| (6) GA dimer to D,L-HMTBA | 35.2 | 42.7 | 12.7 | 29.6 | 650.5 | 14.5 | 634.5 | 1.5 | 98.5 |
| Description of criteria for flag assignment: for solvents, the flags are assigned according to the solvent selection guides created by Prat et al.154,155 and Alder et al.156 Reagents and reactants are evaluated according to their health and safety statements (H statements). Reagents and reactants with very serious health, environmental or safety concerns receive a red flag. Reagents and reactants with less serious concerns receive an amber flag, while those that do not receive a red or amber flag receive a green flag. Reactions involving catalysts that can be recovered receive a green flag, while those using catalysts that cannot be recovered are given an amber flag. In the absence of catalyst, an amber flag is given to reactions using stoichiometric amounts of reagents, and a red flag to reactions using excess reagents. Elements present in the catalysts are also classified according to their risk of depletion. A red flag is assigned if the remaining supply of an element (if recycling and discovery of new reserves are not taken into account) is between 5 and 50 years, an amber flag when it is between 51 and 500 years, and a green flag when it exceeds 500 years. The CHEM21 metrics toolkit assigns a green flag to reactions carried out in flow (continuous) reactors, and an amber flag to those carried out in batch reactors. The criteria for this assignment are based on the advantages known for continuous over batch reactors, such as higher efficiencies in production rates, energy management, solvent use and cleaning processes, among others. Reactions performed between 273K and 343 K receive a green flag, between 253 K and 272 K or between 344 K and 413 K receive an amber flag, and below 253 K and above 413 K receive a red flag. However, if the reaction is performed under reflux conditions it receives a red flag, while it receives a green flag if the reaction is performed 5 K or more below the boiling point of the solvent. For work-up processes, a green flag is given to operations such as quenching, filtration, centrifugation, crystallization, and evaporation or sublimation below 413 K. An amber flag is given to work-up operations such as solvent exchange and quenching into aqueous solution. A red flag is assigned to work-up operations such as chromatography, ion exchange, multiple recrystallization, and evaporations or sublimations above 413 K. |
|---|
|
For the industrial processes 1–3, the results of Table 4 clearly shows that the conversion of propene to HMTBA has the highest OE, giving its high RME and relatively high AE. Process 3 is also a clear winner when it comes to PMI metrics. Particularly, process 3 makes very efficient use of reactants, reagents and catalysts (PMI RRC), and solvents (PMI Solv). Yet one of the biggest penalties for process 3 is its relatively low RP, which arises from both its low global PMI (which makes significant the contribution of non-renewables such as propene, HCN and MeSH), and from the use of H2SO4 during the conversion of HMTBN into HMTBA. Replacing this hydrolysis step for the one in process 2 would increase the RP of process 3 to around 84%, but this would also negatively impact the global PMI of the process by increasing it to almost 30.
As mentioned before and as reflected in Table 5, processes 1–3 involve working with highly toxic chemicals such as HCN, acrolein and MMP. This constitutes a considerable safety penalty for these processes, which can be mitigated by synthetic routes that are safer by design (such as the renewable processes 5 and 6). Additionally, the production of acrolein in processes 1–3 has a sustainability penalty because it requires a multimetallic catalyst containing bismuth (Bi), molybdenum (Mo) and cobalt (Co). Bismuth is considered a critical element by the European Union, with high risk of depletion within the next 5–50 years (if recycling and discovery of new reserves are not taken into account). Mo and Co have a moderate risk of depletion within the next 51–500 years.
The results of Table 4 show that process 4 has the highest OE among the renewable processes 4–6. This is largely attributed to the relatively higher yield of process 4. Nonetheless, it is the conversion of DHA and paraformaldehyde into 2-HBL that limits the overall yield and OE of process 4 from being higher, despite the high AE of this reaction (see Table S1†). Even though process 4 already has a higher OE than the industrial processes 1 and 2, further improvements in the selective conversion of DHA into 2-HBL will be essential to make the OE of process 4 even higher, and possibly better than that of the industrial process 3.
Process 4 also has a high RP. This is a consequence of considering DMSO as a bio-based solvent, since its precursor dimethyl sulfide can nowadays be obtained in industrial amounts from bio-based sources. The possibility of improving the PMI Solv and PMI WU of the process by replacing GVL for DMSO in the synthesis of 2-HBL still remains to be tested. Conversely, it is not feasible to replace DMSO by GVL in the conversion of 2HBL to HMTBA because GVL is expected to react under the reaction conditions applied (as it is a lactone alike 2-HBL).
However, Table 5 shows that the main solvents used in process 4, GVL and DMSO, are not as green as many of the solvents used in the other processes assessed. Furthermore, as mentioned before, the use of paraformaldehyde represents a significant safety hazard that should not be overlooked. This poses a substantial safety penalty for this process, which must be addressed if this synthetic strategy is to be considered as part of the future of the sustainable chemical synthesis of Met and MHAs. Conversely, replacing MeSH for MeSNa increases the safety of this process, in spite of slightly lowering its AE. This reactant swap could improve the safety of the other processes for synthesis of D,L-Met and HMTBA, yet the consequences of this strategy in the reaction's selectivity need to be assessed. Finally, the use of a tin-containing zeolite creates a sustainability penalty for process 4, as tin presents high risk of depletion. This last drawback applies equally to processes 5 and 6. Despite this, the use of heterogeneous catalysts (such as tin-containing zeolites) offers advantages for the sustainability of the processes, as these catalysts can be recovered, regenerated, and recycled in the production process. Said advantages, however, only become evident in more complex sustainability assessments that were not implemented here, such as life cycle assessments and the second and third passes of the CHEM21 metrics toolkit.
On the other hand, the results of Table 4 demonstrate that even though processes 5 and 6 use the highest amount of renewables, with RPs above 95%, their low OEs decrease their sustainability when compared with processes 3 and 4. The most important factor affecting the OEs of these processes is that their RMEs are severely limited by the low yield of specific reactions, namely the conversion of tetroses to MVG in process 5 and the conversion of glycolaldehyde dimer to MMTHB in process 6 (see Table S1†). Said reactions have good AEs, of 76% and 82%, respectively, reflecting the efficiency of their synthetic approach. Actually, the main limitation in AE of processes 5 and 6 arises from the last hydrolysis step converting MMTHB into HMTBA. In light of the above, it is clear that improving the selectivity of the conversion of tetroses to MVG and the conversion of glycolaldehyde dimer to MMTHB is the key to substantially improving the sustainability of processes 5 and 6, respectively.
On the other hand, processes 5 and 6 not only have the highest RPs (see Table 4), but these synthetic routes are also advantageous in terms of safety as they avoid using many of the most hazardous and toxic chemical species involved in processes 1 to 4 (see Table 5). Additionally, the safety of process 5 can be further improved by replacing MeOH for other alcohol such as ethanol (EtOH) or isopropyl alcohol (IPA). IPA has also the potential to increase the reaction's selectivity toward the production of the isopropyl analogue of MVG, as it has been proved that the steric hindrance created by the isopropyl group impedes acetalyzation reactions that compete with reactions required for the conversion of carbohydrates to MHAs such as 1,2-hydride shift.128 The effects of alcohol replacement in process 6 remain to be verified since, as was mentioned before, the patent reporting reactions of this kind presents results that, according to other examples of the same document, could not be attained.133
Finally, Table 5 also shows that all steps in processes 5 and 6 are carried out at relatively low temperatures, below 434 K, which could potentially result in an energy advantage for these processes. However, the zero and first passes of the CHEM21 metrics toolkit do not allow for an adequate evaluation of the energy requirements of the processes, as they do not include, for example, the thermodynamics (exothermic vs. endothermic) of the reaction, or other related evaluations such as the CO2 footprint of the process. These types of assessments are only possible in the more complicated and time-consuming life cycle assessments and the second and third passes of the metrics toolkit. It remains an important task for the future to perform these intricate evaluations, in order to provide a clear view of the overall energy requirements of each process and their impact on process sustainability.
Even though our sustainability assessment does not allow us to establish with absolute certainty which process is the most sustainable and the safest for production of D,L-Met or HMTBA, we can conclude that process 3 is the most efficient industrial process in terms of reactants’ usage and process mass intensity, but it also uses a low percentage of renewable chemicals. On the other hand, process 4 is currently the most efficient process involving renewable feedstocks in terms of reactants’ usage, while it also has a high percentage of renewable chemicals. However, its use of paraformaldehyde creates a safety concern, and GVL and DMSO are not as green as other solvents used by other processes evaluated. Finally, future improvements in selectivity for the conversion of tetroses to MVG and the conversion of glycolaldehyde dimer to MMTHB have the potential to substantially improve the efficiency of reactants’ usage of processes 5 and 6, respectively. This, along with their use of less hazardous chemicals and green solvents, could give them the opportunity to replace methods to produce HMTBA such as process 3 and 4 in the years to come.
6. Conclusions and outlook
The chemical synthesis of methionine and MHAs is a process of key importance for reaching a sustainable future in line with the UN sustainability goals. After reviewing the current industrial methods for the chemical synthesis of D,L-Met and MHAs and critically assessing the most promising novel pathways to improve the sustainability and safety of their synthesis, we have identified the most significant strengths, weaknesses, opportunities and threats (SWOT) associated with each chemical route, as presented in Table 6. This SWOT analysis offers valuable insights to elucidate some key aspects of the future roadmap for the chemical synthesis of D,L-Met and MHAs.| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
| OE: optimum efficiency. PMI: process mass intensity. RP: renewables percentage. | |||
| Propene to D,L-Met (through hydantoin) | |||
| • High RP | • Non-renewable main feedstock | • Can benefit from incorporating acrolein synthesized from renewables (e.g. from glycerol). | • Production process can lose competitivity with price increases on oil and mining of metals used in catalyst |
| • Well-established industrial process | • Moderate OE and PMIs | • It might be phased out if the more sustainable and safer processes continue to improve or are incentivized through policy | |
| • Toxic chemicals (HCN, acrolein, MMP) | |||
| • Metals with high and moderate risk of depletion (Bi, Mo, Co) | |||
| Propene to D,L-Met (through cyanohydrin) | |||
| • High RP | • Non-renewable main feedstock | • Can benefit from incorporating acrolein synthesized from renewables (e.g. from glycerol). | • Production process can lose competitivity with price increases on oil and mining of metals used in catalyst |
| • Well-established industrial process | • Low OE | • It might be phased out if the more sustainable and safer processes continue to improve or are incentivized through policy | |
| • Avoids a toxic intermediate (MMP) | • Moderate PMIs | ||
| HMTBN hydrolysis method: | • Toxic chemicals (HCN, acrolein) | ||
| • Based on renewables | • Metals with high and moderate risk of depletion (Bi, Mo, Co) | ||
| • Prevents the formation of a solid salt byproduct | |||
| Propene to D,L-HMTBA | |||
| • Highest OE | • Non-renewable main feedstock | • Can benefit from incorporating acrolein synthesized from renewables | Production process can lose competitivity with price increases on oil and mining of metals used in catalyst |
| • Lowest PMIs | • Low RP | • Increase RP by replacing H2SO4 in the hydrolysis step with the one used in the cyanohydrin process | • It might be phased out if the more sustainable and safer processes continue to improve or are incentivized through policy |
| • Well-established industrial process | • Toxic chemicals (HCN, Acrolein, MMP) | ||
| • Metals with high and moderate risk of depletion (Bi, Mo, Co) | |||
| Glycerol to acrolein | |||
| • Renewable feedstock | • Fast deactivation of catalyst | • Catalyst deactivation can be mitigated by coupling the more stable and selective catalysts with both process modifications that minimize coke formation, and catalyst regeneration processes | • Supply of glycerol is controlled by other markets (biodiesel and detergents) and can be used in competitive markets such as 1,2-propanediol. |
| • High yield and selectivity | • Implementation might be uncertain without additional policies | ||
| • Technically and economically feasible | |||
| Alcohols to acrolein | |||
| • Partly renewable feedstock | • Modest yield and selectivity | • Use of renewables can be increased if MeOH is obtained from CO2, and EtOH from carbohydrate fermentation or CO2 | • Difficulty in finding catalysts that are both more active and selective for the conversion of alcohols to acrolein |
| • Catalyst with low activity and selectivity | • Projections suggest that the synthesis of MeOH and EtOH from CO2 reduction could be economically viable | ||
| DHA to D,L-HMTBA (through 2-HBL) | |||
| • High OE, RP | • Toxic chemicals (paraformaldehyde) | • Can benefit from incorporating formaldehyde synthesized from captured CO2 | • Implementation might be uncertain if the conversion of DHA and paraformaldehyde to 2-HBL cannot be improved through further research in catalysis and reaction conditions |
| • The replacement of MeSH for MeSNa increases process safety | • Solvents used are not very green (GVL and DMSO) | • Lowering PMI by replacing GVL with DMSO in 2-HBL synthesis | • It might be phased out if other renewable but safer processes continue to improve or are incentivized through policy |
| • Metal with high risk of depletion (Sn) | |||
| • High temperature required to convert 2-HBL into HMTBA | |||
| Tetrose to D,L-HMTBA (through MVG) | |||
| • High RP | • Conversion of carbohydrates to VGA/VGM has yield of 50% (moderate OE) | • Replacing MeOH with either EtOH or IPA can improve process safety and selectivity for the conversion of tetroses to MVG (increasing the process OE) | • Implementation may be uncertain if the conversion of tetroses to VGA/MVG cannot be improved through further research in catalysis and reaction conditions |
| • Renewable and safe reactants | • Metal with high risk of depletion (Sn) | ||
| Tetrose to D,L-HMTBA (through VGA in photocatalytic reaction) | |||
| • Renewable and safe reactants | • The product formed using BuSH is not approved for use in animals or humans | • Implementing photochemical flow reactors can enhance production volumes | • Implementation might not be feasible if replacing BuSH with MeSH does not produce the same results. |
| • Conversion of VGA to MHA: | • UV light of 365 nm poses some eye safety concerns | ||
| • 98% selectivity | • Batch photocatalytic reactions are limited to small production volumes | ||
| • Room temperature | |||
| • Fast reaction rate (1 h) | |||
| • Reaction is catalyzed by HMTBA | |||
| • Easier purification of product | |||
| GA dimer to D,L-HMTBA | |||
| • Highest RP | • Lowest OE | • It is very likely that additional studies on the reaction cascade and catalysts can improve the selectivity of the MMTHB synthesis | • Global economic instability may discourage industrial players from continuing the lengthy path of R&D needed to improve the direct conversion of carbohydrates to MMTHB through further catalysis research and process development |
| • Renewable and safe reactants | • Metal with high risk of depletion (Sn) | • Verifying the possibility to replace MeOH by EtOH or IPA to improve the safety and the selectivity of the conversion of glycolaldehyde to MMTHB. | |
In general, the oil-based approaches for the conversion of acrolein (or propene) to D,L-Met and HMTBA are well established in the industry. A sustainability assessment revealed that the processes involving the formation of hydantoin and cyanohydrin utilize a high percentage of renewable chemicals (RP) in the overall synthesis (despite propene, the initial feedstock, being non-renewable). Furthermore, the latter process not only avoids the formation of the toxic intermediate MMP but also implements a method for hydrolysis of HMTBN based on renewables, which does not lead to the formation of a solid salt byproduct. On the other hand, the process converting acrolein to HMTBA is the most efficient industrial process in terms of reactants’ usage and process mass intensity. We consider that there is significant potential to improve the low RP of this process by replacing the hydrolysis step in H2SO4 with the one used in the cyanohydrin process. Additionally, a major opportunity to enhance the sustainability of all approaches dependent on acrolein lies in the ongoing efforts to synthesize this species from renewables such as glycerol or alcohols.
However, all current oil-based approaches for the production of D,L-Met and HMTBA not only use non-renewable feedstock but also involve compounds with high safety risks such as hydrogen cyanide and acrolein. Furthermore, the conventional synthesis of acrolein from propene requires catalyst involving several metals with high and moderate risks of depletion. These weaknesses, coupled with the dependence of these synthetic approaches on the prices of both oil and the mining of critical metals, pose an important threat to the feasibility of the oil-based approaches for synthesizing D,L-Met and HMTBA. The risk of these approaches being phased out will be compounded if the more sustainable and safer processes continue to improve or are incentivized through legislation.
The production of acrolein from bio-based compounds presents great potential to indirectly improve the sustainability of Met and MHAs synthesis. The conversion of bio-based glycerol to acrolein is already a well-established approach that can produce yields and selectivities toward acrolein between 65 and 96%, depending on the catalyst used. Selected mixed metal oxides, such as Fex(PO4)y, can produce excellent results while remaining active for relatively long times given their low coke deposition. The deactivation of the catalysts, too fast for industrial standards, remains as the main technical weakness hindering the industrialization of the bio-based glycerol to acrolein approach. Additionally, an important external threat to the implementation of this approach is the reliance of glycerol supply from other industrial activities, such as the production of biodiesel and detergents, while glycerol can also be a feedstock for the production of other chemicals such as 1,2-propanediol. Therefore, in spite of the outstanding results and the demonstrated technical and economic feasibility for the synthesis of acrolein from bio-based glycerol, the petroleum-based approach is still the industrial process of choice. We consider that policies directed to provide additional incentives for improvements in the sustainability of the acrolein synthesis could be the deal breaker needed to unlock the final developments and industrial application of acrolein production from bio-based glycerol.
The catalytic conversion of ethanol and methanol to acrolein is a more recent approach that produces relatively modest yields and selectivities for acrolein between 30 and 42%. A significant opportunity to improve the sustainability of this approach for the synthesis of acrolein arises when ethanol is produced from either bio-based carbohydrates (e.g. through classic fermentation) or from CO2 coming from capture systems, and when methanol is also produced from captured CO2. Unfortunately, the different approaches for reducing CO2 to methanol are still too expensive to replace conventional methanol derived from methane (through synthesis gas).157,158 Theoretical efficiencies and future projections of technology and market improvements suggest that different synthetic platforms could make the reduction of CO2 to methanol and ethanol economically viable. Meanwhile, other fundamental problems of the conversion of these alcohols to acrolein appear as major threats that need to be addressed for this method for acrolein synthesis to become appealing for industrial application, especially the improvement of catalyst activity and selectivity towards the desired product.
The chemical conversion of selected bio-based molecules to MHAs has potential to directly improve the sustainability of MHAs as well as avoiding potential exposure to hazardous species such as acrolein and cyanide. Advances in the conversion of biomass-derived carbohydrates to 2-HBL and VGA (or MVG) are reviving the interest for developing synthetic routes converting these platform molecules to MHAs.
The sustainability assessment of processes for HMTBA synthesis involving renewable feedstock showed that a process involving the conversion of DHA and paraformaldehyde to 2-HBL with final conversion to HMTBA is the most efficient process of its class in terms of reactants’ usage, while it also involves a high percentage of renewable chemicals. Some strengths of this synthetic approach stem from the high yield and selectivity of the conversion of 2-HBL to HMTBA, reaching up to 100%. Furthermore, this reaction uses MeSNa as a sulphur source, which is safer than MeSH that is used in all the other processes assessed.
On the other hand, this synthetic approach also presents several weaknesses. The use of formaldehyde in the renewable synthesis of 2-HBL poses a considerable safety risk, as exposure to formaldehyde can be as hazardous as, and in some instances worse than, exposure to acrolein or cyanide. Therefore, the synthesis of HMTBA from 2-HBL could offer little or no safety improvement when compared with the conventional acrolein approaches. Additionally, all solvents used in this renewable approach raise some sustainability and safety concerns. For instance, the use of DMF as solvent in the conversion of 2-HBL to HMTBA allows the reaction to be performed at slightly lower temperatures. Nevertheless, we recommend preferring the greener solvent DMSO due to its higher safety and lower impact on health,155 even though it is acknowledged that DMSO is not the greenest polar aprotic solvent. Other drawbacks of this renewable approach concern the need for Sn, a metal with high risk of depletion, in the catalytic conversion of DHA and paraformaldehyde to 2-HBL, and the relatively high temperature required for the conversion of 2-HBL to HMTBA.
There are several opportunities that can improve the outlook of the synthesis of HMTBA through the renewable 2-HBL approach. Investigating the replacement of GVL by DMSO in the synthesis of 2-HBL is recommended. This solvent substitution could substantially improve the solvent PMI of the overall process, as it is the same solvent used in the subsequent step converting 2-HBL into HMTBA. Furthermore, this approach can benefit from the possibility of synthesizing formaldehyde from captured CO2 (either through the initial conversion of CO2 to MeOH, followed by oxidation to formaldehyde, or through the direct reduction of CO2 to formaldehyde). However, as stated before, the reduction of CO2 to MeOH remains too expensive, while the oxidation of MeOH to formaldehyde has problems with overoxidation to CO and CO2. Additionally, in spite of decades of research in the direct reduction of CO2 to formaldehyde, the fundamental problem of quenching the CO2 reduction at the aldehyde stage remains a major challenge.159
Consequently, additional advancements in both the renewable synthesis of formaldehyde and the conversion of DHA and paraformaldehyde to 2-HBL will be required before the conversion of bio-based 2-HBL to HMTBA can fully realize its sustainability potential and becomes a method of choice for the synthesis of MHAs.
The conversion of bio-based VGA (or MVG) to MHAs is another candidate with potential to replace the acrolein approach for synthesis of MHAs. Adisseo has developed two different strategies to convert VGA or MVG into MHAs using milder conditions than those required when starting from 2-HBL. The first one yields around 81% of MHA in 1 hour reactions at 273 K in the presence of catalytic amounts of persulfate and copper. The second yields around 96% of MHA in 1 hour reactions at 305 K in the presence of BuSH, UV light and HMTBA.
The sustainability assessment performed for a process involving the first strategy with prior synthesis of MVG from tetroses showed that the main strengths of the process are its high percentage of renewable chemicals and their safety. Conversely, the main weaknesses of the process lie in its first step of converting the carbohydrate to MVG. This reaction depends on Sn-containing catalysts, which involve a high risk of depletion. More importantly the conversion of carbohydrates to VGA and MVG has moderate yields of around 50%, so that further advancements in this field are required to improve the prospects for the application of this approach for the synthesis of MHAs. In this regard, we have identified that replacing MeOH with either EtOH or IPA in the conversion of tetroses to MVG has the opportunity to improve both the safety of the process, and the reaction selectivity towards MVG (thereby improving the process OE).
On the other hand, it remains to be proved whether the second strategy to convert VGA into MHA would produce similar results when replacing BuSH for MeSH. If this were the case, the second strategy would be the preferred choice due to its higher selectivity, fast reaction rate and safety (as it does not require a radical initiator). It is reasonable to assume that the second strategy would be cheaper as it does not require extensive removal of by-products. Additionally, the second strategy is greener as it is catalysed by the target reaction product (HMTBA) and it also avoids working below room temperature.160 It is necessary to highlight that this approach also has some weaknesses, the most important being the eye safety risk associated with work involving UV light (wavelength of 365 nm), and the small production volumes typical of photochemical reactions performed in batch. Nonetheless, this approach has the opportunity to increase the production volume by implementing photochemical flow reactors at high throughput.
Finally, the direct conversion of carbohydrates to MHAs is still in its infancy, yet the results reported so far demonstrate great potential for the future of this approach. The sustainability assessment for the conversion of glycolaldehyde to HMTBA demonstrates that this approach utilizes the highest percentage of renewables of all processes evaluated. Another strength of this approach concerns its safety, as it avoids many of the most hazardous and toxic chemical species involved in the other processes. We found that the main weaknesses of this approach concern its low OE (arising from the low yield of the conversion of glycolaldehyde to MMTHB) and the use of Sn-containing catalysts in the synthesis of MMTHB. We are confident that the catalytic and mechanistic advances achieved so far will guide future research to fully understand the reaction cascade and propose improvements to both the reaction conditions and catalytic system in order to optimize reaction selectivity towards MHAs and catalyst stability. In this regard, further investigation is needed to verify whether replacing MeOH with EtOH or IPA could enhance the selectivity of the reaction towards MMTHB, while also improving the safety of the synthesis. Perhaps the most significant threat to this synthetic approach involves the potential impact of global economic instability, which may discourage industrial players from persisting in the lengthy path of R&D that is still required to improve the direct conversion of carbohydrates to MHAs through further studies into its reaction conditions and suitable catalytic systems.
Although it falls outside the scope of this review, another remaining challenge for the sustainable synthesis of MHAs is related to the sustainability of the synthesis of MeSH.161 The industrial processes for the synthesis of MeSH use methanol or formaldehyde as carbon source, which can be obtained from renewables.162,163 On the other hand, the sulphur source of industrial choice is H2S, which is typically recovered from sour gas or synthesized from elemental sulphur and hydrogen at high temperature in the presence of a catalyst.164 The fight against climate change suggests that lower amounts of sour gas will be produced in the years to come, while the use of hydrogen as an energy vector will make H2 scarcer for the synthesis of H2S (actually the reverse process is under study for production of H2).165 Consequently, a more sustainable production of the sulphur source will be required, with bioprocesses using sulfate-reducing prokaryotes likely being a good candidate for this synthesis.
Author contributions
S. Calderon-Ardila: conceptualization, formal analysis, visualization, writing – original draft. D. Morvan: conceptualization, supervision, writing – review & editing. O. Péruch: conceptualization, writing – review & editing. V. Bellière-Baca: conceptualization, writing – review & editing. M. Dusselier: conceptualization, supervision, writing – review & editing. B. F. Sels: conceptualization, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. C. A. thanks KU Leuven for the Research Associate contract at the Center for Sustainable Catalysis and Engineering. We acknowledge Adisseo France SAS for the financial support for this work.References
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst, M. Maesen, H. Matsushima, J. M. Thevelein, K. Van Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385–1390 CrossRef CAS PubMed.
- H. Yang, B. Yu, X. Xu, S. Bourbigot, H. Wang and P. Song, Green Chem., 2020, 22, 2129–2161 RSC.
- Biodegradable Polymers in the Circular Plastics Economy, ed. M. Dusselier and J. Lange, Wiley, 2022 Search PubMed.
- T. V. Choudhary and C. B. Phillips, Appl. Catal., A, 2011, 397, 1–12 CrossRef CAS.
- S. Nanda, J. Mohammad, S. N. Reddy, J. A. Kozinski and A. K. Dalai, Biomass Convers. Biorefin., 2014, 4, 157–191 CrossRef CAS.
- G. Kumar, S. Shobana, W.-H. Chen, Q.-V. Bach, S. H. Kim, A. E. Atabani and J.-S. Chang, Green Chem., 2017, 19, 44–67 RSC.
- S. Kim, E. E. Kwon, Y. T. Kim, S. Jung, H. J. Kim, G. W. Huber and J. Lee, Green Chem., 2019, 21, 3715–3743 RSC.
- P. Foley, A. Kermanshahi-pour, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518 RSC.
- A. Bhadani, A. Kafle, T. Ogura, M. Akamatsu, K. Sakai, H. Sakai and M. Abe, Curr. Opin. Colloid Interface Sci., 2020, 45, 124–135 CrossRef CAS.
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, United States, 2004 Search PubMed.
- W. Schutyser, T. Renders, S. Van den Bosch, S.-F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- W. H. Faveere, S. Van Praet, B. Vermeeren, K. N. R. Dumoleijn, K. Moonen, E. Taarning and B. F. Sels, Angew. Chem., Int. Ed., 2021, 60, 12204–12223 CrossRef CAS PubMed.
- S. Gillet, M. Aguedo, L. Petitjean, A. R. C. Morais, A. M. da Costa Lopes, R. M. Łukasik and P. T. Anastas, Green Chem., 2017, 19, 4200–4233 RSC.
- M. H. Tran, D.-P. Phan and E. Y. Lee, Green Chem., 2021, 23, 4633–4646 RSC.
- N. Yan and Y. Wang, Chem, 2019, 5, 739–741 CAS.
- K. Drauz, I. Grayson, A. Kleemann, H.-P. Krimmer, W. Leuchtenberger and C. Weckbecker, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007, vol. 3, pp. 1–58 Search PubMed.
- T. Willke, Appl. Microbiol. Biotechnol., 2014, 98, 9893–9914 CrossRef CAS PubMed.
- P. Gerland, K. Andreev, L. Bassarsky, G. Bay, H. Cruz Castanheira, V. Gaigbe-Togbe, D. Gu, S. Hertog, N. Li, I. Ribeiro, T. Spoorenberg, P. Ueffing, M. Wheldon and L. Zeifman, World Population Prospects 2019 - Volume II: Demographic Profiles, Department of Economic and Social Affairs, Population Division, United Nations, 2019, vol. II Search PubMed.
- N. Alexandratos and J. Bruinsma, World agriculture towards 2030/2050. The 2012 Revision. ESA Working paper no. 12-03. FAO, Rome, 2012.
- L. Vranken, T. Avermaete, D. Petalios and E. Mathijs, Environ. Sci. Policy, 2014, 39, 95–106 CrossRef.
- International Comparison Program – World Bank; World Development Indicators Database – World Bank; Eurostat-OECD PPP, GDP per capita, https://data.worldbank.org/indicator/NY.GDP.PCAP.PP.KD, (accessed 13 January 2023).
- H. R. J. Van Kernebeek, S. J. Oosting, M. K. Van Ittersum, P. Bikker and I. J. M. De Boer, Int. J. Life Cycle Assess., 2016, 21, 677–687 CrossRef.
- H. H. E. van Zanten, H. Mollenhorst, C. W. Klootwijk, C. E. van Middelaar and I. J. M. de Boer, Int. J. Life Cycle Assess., 2016, 21, 747–758 CrossRef CAS.
- D. K. Ray, N. Ramankutty, N. D. Mueller, P. C. West and J. A. Foley, Nat. Commun., 2012, 3, 1293 CrossRef PubMed.
- X. Wang, C. Zhao, C. Müller, C. Wang, P. Ciais, I. Janssens, J. Peñuelas, S. Asseng, T. Li, J. Elliott, Y. Huang, L. Li and S. Piao, Nat. Sustainability, 2020, 3, 908–916 CrossRef.
- L. L. Sloat, S. J. Davis, J. S. Gerber, F. C. Moore, D. K. Ray, P. C. West and N. D. Mueller, Nat. Commun., 2020, 11, 1243 CrossRef CAS PubMed.
- W. Willett, J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, D. Tilman, F. DeClerck, A. Wood, M. Jonell, M. Clark, L. J. Gordon, J. Fanzo, C. Hawkes, R. Zurayk, J. A. Rivera, W. De Vries, L. M. Sibanda, A. Afshin, A. Chaudhary, M. Herrero, R. Agustina, F. Branca, A. Lartey, S. Fan, B. Crona, E. Fox, V. Bignet, M. Troell, T. Lindahl, S. Singh, S. E. Cornell, K. S. Reddy, S. Narain, S. Nishtar and C. J. L. Murray, Lancet, 2019, 393, 447–492 CrossRef PubMed.
- A. Jungk, J. Plant Nutr. Soil Sci., 2009, 172, 633–636 CrossRef CAS.
- R. H. McCoy, C. E. Meyer and W. C. Rose, J. Biol. Chem., 1935, 112, 283–302 CrossRef CAS.
- W. C. Rose, Physiol. Rev., 1938, 18, 109–136 CrossRef.
- Committee on Nutrient Requirements of Swine. Board on Agriculture and Natural Resources. Division on Earth and Life Studies. National Research Council, Ed., Nutrient Requirements of Swine, The National Academies Press, Washington, D.C., 11 (revise., 2012).
- Subcommittee on Dairy Cattle Nutrition. National Committee on Animal Nutrition. Board on Agriculture and Natural Resources. National Research Council, Ed., Requirements of Dairy Cattle, National Academy Press, Washington, D.C., 7 (revised., 2001).
- Committee on Nutrient Requirements of Fish and Shrimp. Board on Agriculture and Natural Resources. Division on Earth and Life Studies, Ed., Nutrient Requirements of Fish and Shrimp, The National Academies Press, Washington, D.C., 2011.
- C. M. Lyman, K. A. Kuiken and F. Hale, J. Agric. Food Chem., 1956, 4, 1008–1013 CrossRef CAS.
- X. Li, R. Rezaei, P. Li and G. Wu, Amino Acids, 2011, 40, 1159–1168 CrossRef CAS PubMed.
- W. G. Pond, D. B. Church, K. R. Pond and P. A. Schoknecht, Basic Animal Nutrition and Feeding, John Wiley & Sons, Hoboken, N.J., 5th edn, 2004 Search PubMed.
- C. G. Schwab and G. A. Broderick, J. Dairy Sci., 2017, 100, 10094–10112 CrossRef CAS PubMed.
- C. Lee, A. N. Hristov, K. S. Heyler, T. W. Cassidy, H. Lapierre, G. A. Varga and C. Parys, J. Dairy Sci., 2012, 95, 5253–5268 CrossRef CAS PubMed.
- W. K. Kim, C. A. Froelich, P. H. Patterson and S. C. Ricke, Worlds Poult. Sci. J., 2006, 62, 338–353 CrossRef.
- M. Alagawany, M. E. Abd El-Hack, M. Arif and E. A. Ashour, Environ. Sci. Pollut. Res., 2016, 23, 22906–22913 CrossRef CAS PubMed.
- United Nations, Transforming our world : the 2030 Agenda for Sustainable Development, UN Publishing, 2015, vol. A/RES/70/1 Search PubMed.
- J. Bernstein, Polymorphism in Molecular Crystals, Oxford University Press, 2007 Search PubMed.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, FL, USA, 2005 Search PubMed.
- V. B. Motalov, M. A. Korobov, A. M. Dunaev, V. V. Dunaeva, E. Y. Tyunina and L. S. Kudin, J. Chem. Eng. Data, 2022, 67, 1326–1334 CrossRef CAS.
- R. Sabbah and C. Minadakis, Thermochim. Acta, 1981, 43, 269–277 CrossRef CAS.
- S. K. Bhattacharyya and A. B. Banerjee, Folia Microbiol., 1974, 19, 43–50 CrossRef CAS PubMed.
- R. F. Barker and D. A. Hopkinson, Ann. Hum. Genet., 1977, 41, 27–42 CrossRef CAS PubMed.
- T. Robinson, Life Sci., 1976, 19, 1097–1102 CrossRef CAS PubMed.
- J. J. Dibner and F. J. Ivey, Poult. Sci., 1992, 71, 700–708 CrossRef CAS PubMed.
- L. D. Stegink, J. Moss, K. J. Printen and E. S. Cho, J. Nutr., 1980, 110, 1240–1246 CrossRef CAS PubMed.
- S. Ravanel, B. Gakière, D. Job and R. Douce, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 7805–7812 CrossRef CAS PubMed.
- H. Hesse, Trends Plant Sci., 2003, 8, 259–262 CrossRef CAS PubMed.
- M. P. Ferla and W. M. Patrick, Microbiology, 2014, 160, 1571–1584 CrossRef CAS PubMed.
- D. J. Reed and S. Orrenius, Biochem. Biophys. Res. Commun., 1977, 77, 1257–1264 CrossRef CAS PubMed.
- S. Roje, Phytochemistry, 2006, 67, 1686–1698 CrossRef CAS PubMed.
- W. C. Winkler, A. Nahvi, N. Sudarsan, J. E. Barrick and R. R. Breaker, Nat. Struct. Mol. Biol., 2003, 10, 701–707 CrossRef CAS PubMed.
- S. M. Sanderson, X. Gao, Z. Dai and J. W. Locasale, Nat. Rev. Cancer, 2019, 19, 625–637 CrossRef CAS PubMed.
- R. J. Huxtable, Physiol. Rev., 1992, 72, 101–163 CrossRef CAS PubMed.
- J. J. Dibner and C. D. Knight, J. Nutr., 1984, 114, 1716–1723 CrossRef CAS PubMed.
- G. E. Lobley, T. J. Wester, A. G. Calder, D. S. Parker, J. J. Dibner and M. Vázquez-Añón, J. Dairy Sci., 2006, 89, 1072–1080 CrossRef CAS PubMed.
- H. Lapierre, M. Vázquez-Añón, D. Parker, P. Dubreuil, G. Holtrop and G. E. Lobley, J. Dairy Sci., 2011, 94, 1526–1535 CrossRef CAS PubMed.
- B. Moeckel, W. Pfefferle, K. Huthmacher, C. Rueckert, A. Puehler, M. Binder, D. Greissinger and G. Thierbach, US2002110878 A1, Degussa AG, 2002.
- W. Dischert and R. Figge, WO2013001055 A1, Metabolic Explorer, 2013.
- R. Figge, P. Soucaille, G. Barbier, G. Bestel-Corre, C. Boisart and M. Chateau, WO2009043372 A1, Metabolic Explorer, 2009.
- S. Kim, K. Cho, Y. Shin, H. Um, K. Choi, J. Chang, Y. Cho and Y. Park, EP2046968 B1, CJ CheilJedang Corporation, 2013.
- B. Brazeau, Y. Shin, H. Um, J. Chang, K. Cho, Y. Cho, M. Desouza, H. Jessen, S. Kim and W. Niu, EP2808381 B1, CJ CheilJedang Corporation, 2016.
- S. Hong, I. Hwang, S. Lee, Y. Lee and J. Jung and A. Eyal, EP2658986 B1, CJ CheilJedang Corporation, 2020.
- G. Fremy, P. Barre, S. Kim and S. Lee, EP2751276 B1, CJ CheilJedang Corporation and Arkema France, 2023.
- FeedInfo, Overview of Current DL-methionine and L-lysine market, https://www.feedinfo.com/login-page/?returnUrl=/home/global-news/insight-overview-of-current-dl-methionine-and-l-lysine-market/122633, (accessed 14 January 2020).
- K. Araki and T. Ozeki, in Kirk-Othmer Encyclopedia of Chemical Technology, ed. J. I. Kroschwitz and M. Howe-Grant, John Wiley & Sons, New York, 4th edn, 1991, vol. 3, pp. 504–571 Search PubMed.
- G. Barger and F. P. Coyne, Biochem. J., 1928, 22, 1417–1425 CrossRef CAS PubMed.
- E. Pierson, M. Giella and M. Tishler, J. Am. Chem. Soc., 1948, 70, 1450–1451 CrossRef CAS PubMed.
- Y. C. Hsu and D. A. Ruest, US5925794, Novus International Inc., 1999.
- F. Geiger, B. Halsberghe, H. J. Hasselbach, K. Hentschel, K. Huthmacher, M. Körfer, S. Mannsfeld, H. Tanner, F. Theissen, J. Vanrobaeys and K. Willigerodt, EP1256571 B1, Degussa AG, 1996.
- H. Wagner, H. Tanner, E. Liebetanz, S. T. Mannsfeld and A. Pfeiffer, GB1296347 A, Degussa GmbH, 1970.
- C. Casse, WO2008006977 A1, Adisseo France S.A.S., 2008.
- E. S. Blake and R. J. Wineman, US2745745, Monsanto Chemical Company, 1956.
- J. A. Hernandez and L. R. Moreno, US4912257, Sociedad de Desarrollo Tecnico Industrial, 1990.
- D. Arntz, A. Fischer, M. Höpp, S. Jacobi, J. Sauer, T. Ohara, T. Sato, N. Shimizu and H. Schwind, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007, vol. 8, pp. 255–271 Search PubMed.
- F. Endter, Chem. Ing. Tech., 1958, 30, 305–310 CrossRef CAS.
- B. Katryniok, S. Paul, V. Bellière-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079 RSC.
- B. Katryniok, S. Paul and F. Dumeignil, ACS Catal., 2013, 3, 1819–1834 CrossRef CAS.
- S. T. Wu, Q. M. She, R. Tesser, M. Di Serio and C. H. Zhou, Catal. Rev., 2020, 62, 481–523 CrossRef CAS.
- M. H. Haider, N. F. Dummer, D. Zhang, P. Miedziak, T. E. Davies, S. H. Taylor, D. J. Willock, D. W. Knight, D. Chadwick and G. J. Hutchings, J. Catal., 2012, 286, 206–213 CrossRef CAS.
- A. Alhanash, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2010, 378, 11–18 CrossRef CAS.
- Y. Gu, N. Cui, Q. Yu, C. Li and Q. Cui, Appl. Catal., A, 2012, 429–430, 9–16 CrossRef CAS.
- I. Pala Rosas, J. Contreras, J. Salmones, C. Tapia, B. Zeifert, J. Navarrete, T. Vázquez and D. García, Catalysts, 2017, 7, 73 CrossRef.
- J. Deleplanque, J.-L. Dubois, J.-F. Devaux and W. Ueda, Catal. Today, 2010, 157, 351–358 CrossRef CAS.
- E. Yoda and A. Ootawa, Appl. Catal., A, 2009, 360, 66–70 CrossRef CAS.
- A. K. Kinage, P. P. Upare, P. Kasinathan, Y. K. Hwang and J.-S. Chang, Catal. Commun., 2010, 11, 620–623 CrossRef CAS.
- A. Corma, G. W. Huber, L. Sauvanaud and P. O'Connor, J. Catal., 2008, 257, 163–171 CrossRef CAS.
- W. Suprun, M. Lutecki, T. Haber and H. Papp, J. Mol. Catal. A: Chem., 2009, 309, 71–78 CrossRef CAS.
- L. Liu, X. P. Ye and J. J. Bozell, ChemSusChem, 2012, 5, 1162–1180 CrossRef CAS PubMed.
- A. Borowiec, J. F. Devaux, J. L. Dubois, L. Jouenne, M. Bigan, P. Simon, M. Trentesaux, J. Faye, M. Capron and F. Dumeignil, Green Chem., 2017, 19, 2666–2674 RSC.
- A. Borowiec, A. Lilić, J.-C. Morin, J.-F. Devaux, J.-L. Dubois, S. Bennici, A. Auroux, M. Capron and F. Dumeignil, Appl. Catal., B, 2018, 237, 149–157 CrossRef CAS.
- A. Lilić, S. Bennici, J. Devaux, J. Dubois and A. Auroux, ChemSusChem, 2017, 10, 1916–1930 CrossRef PubMed.
- A. Lilić, T. Wei, S. Bennici, J.-F. Devaux, J.-L. Dubois and A. Auroux, ChemSusChem, 2017, 10, 3459–3472 CrossRef PubMed.
- V. Folliard, G. Postole, J.-F. Devaux, J.-L. Dubois, L. Marra and A. Auroux, Appl. Catal., B, 2020, 268, 118421 CrossRef CAS.
- V. Folliard, G. Postole, L. Marra, J.-L. Dubois and A. Auroux, Catal. Sci. Technol., 2020, 10, 1889–1901 RSC.
- V. Folliard, G. Postole, L. Marra, J.-L. Dubois and A. Auroux, Appl. Catal., A, 2020, 608, 117871 CrossRef CAS.
- S. G. Jadhav, P. D. Vaidya, B. M. Bhanage and J. B. Joshi, Chem. Eng. Res. Des., 2014, 92, 2557–2567 CrossRef CAS.
- X. Jiang, X. Nie, X. Guo, C. Song and J. G. Chen, Chem. Rev., 2020, 120, 7984–8034 CrossRef CAS PubMed.
- S.-T. Bai, G. De Smet, Y. Liao, R. Sun, C. Zhou, M. Beller, B. U. W. Maes and B. F. Sels, Chem. Soc. Rev., 2021, 50, 4259–4298 RSC.
- B. E. R. Snyder, P. Vanelderen, M. L. Bols, S. D. Hallaert, L. H. Böttger, L. Ungur, K. Pierloot, R. A. Schoonheydt, B. F. Sels and E. I. Solomon, Nature, 2016, 536, 317–321 CrossRef CAS PubMed.
- B. E. R. Snyder, M. L. Bols, H. M. Rhoda, D. Plessers, R. A. Schoonheydt, B. F. Sels and E. I. Solomon, Science, 2021, 373, 327–331 CrossRef CAS PubMed.
- D. A. Ruest, EP0245231 B1, Monsanto Company, 1986.
- H. Plieninger, Chem. Ber., 1950, 83, 265–268 CrossRef CAS.
- P. Deck, K. M. Exner and B. Buschhaus, WO2008022953 A1, BASF AG, 2008.
- V. Henryon and J. Monbrun, US8822706 B2, Adisseo France S.A.S., 2012.
- U. Klar, W. Schwede, W. Skuballa, B. Buchmann and M. Schirner, DE19735575 A1, Schering AG, 1997.
- K. Lohbeck, H. Haferkorn, W. Fuhrmann and N. Fedtke, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- W. Schwarz, J. Schossig, R. Rossbacher, R. Pinkos and H. Höke, in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley, 2019, vol. 74, pp. 1–7 Search PubMed.
- S. Yamaguchi, K. Motokura, Y. Sakamoto, A. Miyaji and T. Baba, Chem. Commun., 2014, 50, 4600–4602 RSC.
- S. Van De Vyver, C. Odermatt, K. Romero, T. Prasomsri and Y. Román-Leshkov, ACS Catal., 2015, 5, 972–977 CrossRef CAS.
- S. Yamaguchi, H. Deguchi, S. Kawauchi, K. Motokura, A. Miyaji and T. Baba, ChemistrySelect, 2017, 2, 591–597 CrossRef CAS.
- S. Yamaguchi, T. Matsuo, K. Motokura, Y. Sakamoto, A. Miyaji and T. Baba, ChemSusChem, 2015, 8, 3661–3667 CrossRef CAS PubMed.
- S. Yamaguchi, T. Matsuo, K. Motokura, Y. Sakamoto, A. Miyaji and T. Baba, ChemSusChem, 2015, 8, 853–860 CrossRef CAS PubMed.
- National Toxicology Program, U.S. Deparment of Health and Human Services and Public Health Services, Formaldehyde, https://ntp.niehs.nih.gov/go/roc, (accessed 17 January 2023).
- K. E. Koenig, EP0961769 B1, Novus International Inc., 1997.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- B. H. Northrop and R. N. Coffey, J. Am. Chem. Soc., 2012, 134, 13804–13817 CrossRef CAS PubMed.
- V. Belliere-Baca, O. Peruch, D. Morvan and A. Petrelli, WO2020148509 A1, Adisseo France S.A.S., 2019.
- V. Belliere-Baca, S. Perdriau, M. Roudier and J. Monbrun, WO2020201412 A1, Adisseo France S.A.S., 2019.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS PubMed.
- M. S. Holm, Y. J. Pagán-Torres, S. Saravanamurugan, A. Riisager, J. A. Dumesic and E. Taarning, Green Chem., 2012, 14, 702–706 RSC.
- M. Dusselier, P. VanWouwe, F. DeClippel, J. Dijkmans, D. W. Gammon and B. F. Sels, ChemCatChem, 2013, 5, 569–575 CrossRef CAS.
- M. Dusselier, P. Van Wouwe, S. De Smet, R. De Clercq, L. Verbelen, P. Van Puyvelde, F. E. Du Prez and B. F. Sels, ACS Catal., 2013, 3, 1786–1800 CrossRef CAS.
- P. R. Jensen, E. Taarning and S. Meier, ChemCatChem, 2019, 11, 5077–5084 CrossRef CAS.
- R. De Clercq, M. Dusselier, C. Christiaens, J. Dijkmans, R. I. Iacobescu, Y. Pontikes and B. F. Sels, ACS Catal., 2015, 5, 5803–5811 CrossRef CAS.
- S. Tolborg, S. Meier, S. Saravanamurugan, P. Fristrup, E. Taarning and I. Sádaba, ChemSusChem, 2016, 9, 3054–3061 CrossRef CAS PubMed.
- I. Sadaba Zubiri, E. Taarning and D. Tzoulaki, US10189778 B2, Haldor Topsoe A/S, 2019.
- I. Sadaba Zubiri and E. Taarning, US10100007 B2, Haldor Topso A/S, 2018.
- S. Calderon-Ardila, J. Matthijssen, B. Van Huffel, O. Péruch, D. Morvan, V. Bellière-Baca, M. Dusselier and B. F. Sels, ChemCatChem, 2022, 14, e202101730 CrossRef CAS.
- R. J. Charlson, T. L. Anderson and R. E. McDuff, in International Geophysics, Academic Press Inc., 1992, vol. 50, pp. 285–300 Search PubMed.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. R. McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4(7) CAS.
- S. Calderon-Ardila, F. Rammal, E. Peeters, J. Van Waeyenberg, O. Péruch, D. Morvan, V. Bellière-Baca, M. Dusselier and B. F. Sels, Catal. Today, 2023, 418, 114137 CrossRef CAS.
- G. E. Lienhard and W. P. Jencks, J. Am. Chem. Soc., 1966, 88, 3982–3995 CrossRef CAS PubMed.
- Y. Yan, L. Feng, G. Li, S. Lin, Z. Sun, Y. Zhang and Y. Tang, ACS Catal., 2017, 7, 4473–4478 CrossRef CAS.
- E. Peeters, G. Pomalaza, I. Khalil, A. Detaille, D. P. Debecker, A. P. Douvalis, M. Dusselier and B. F. Sels, ACS Catal., 2021, 11, 5984–5998 CrossRef CAS.
- E. Peeters, I. Khalil, P. Eloy, S. Calderon-Ardila, J. Dijkmans, P. Ferrini, D. P. Debecker, R. A. Taylor, A. P. Douvalis, M. Dusselier and B. F. Sels, Chem. Mater., 2021, 33, 9366–9381 CrossRef CAS.
- E. Peeters, S. Calderon-Ardila, I. Hermans, M. Dusselier and B. F. Sels, ACS Catal., 2022, 12, 9559–9569 CrossRef CAS.
- P. Sun, C. Liu, H. Wang, Y. Liao, X. Li, Q. Liu, B. F. Sels and C. Wang, Angew. Chem., Int. Ed., 2023, 62, e202215737 CrossRef CAS PubMed.
- I. Teshigahara and N. Kanuka, US20050159620 A1, Mitsubishi Chemical Corporation, 2005.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- C. Jiménez-González, D. J. C. Constable and C. S. Ponder, Chem. Soc. Rev., 2012, 41, 1485–1498 RSC.
- S. S. Kumar and V. Himabindu, Mater. Sci. Energy Technol., 2019, 2, 442–454 Search PubMed.
- W. Khetkorn, R. P. Rastogi, A. Incharoensakdi, P. Lindblad, D. Madamwar, A. Pandey and C. Larroche, Bioresour. Technol., 2017, 243, 1194–1206 CrossRef CAS PubMed.
- G. Heremans, C. Trompoukis, N. Daems, T. Bosserez, I. F. J. Vankelecom, J. A. Martens and J. Rongé, Sustainable Energy Fuels, 2017, 1, 2061–2065 RSC.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Müller, Energy Environ. Sci., 2012, 5, 7281–7305 RSC.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernández, M. C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7, 130–189 RSC.
- A. Al-Mamoori, A. Krishnamurthy, A. A. Rownaghi and F. Rezaei, Energy Technol., 2017, 5, 834–849 CrossRef.
- A. I. Osman, M. Hefny, M. I. A. A. Maksoud, A. M. Elgarahy and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 797–849 CrossRef CAS.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- R. G. Grim, Z. Huang, M. T. Guarnieri, J. R. Ferrell, L. Tao and J. A. Schaidle, Energy Environ. Sci., 2020, 13, 472–494 RSC.
- Z. Huang, R. G. Grim, J. A. Schaidle and L. Tao, Energy Environ. Sci., 2021, 14, 3664–3678 RSC.
- S. Zhao, H. Liang, X. Hu, S. Li and K. Daasbjerg, Angew. Chem., Int. Ed., 2022, 61, e202204008 CrossRef CAS PubMed.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 1–7 Search PubMed.
- K.-M. Roy, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, pp. 629–648 Search PubMed.
- P. Gautam, Neha, S. N. Upadhyay and S. K. Dubey, Fuel, 2020, 273, 117783 CrossRef CAS.
- N. S. Shamsul, S. K. Kamarudin, N. A. Rahman and N. T. Kofli, Renewable Sustainable Energy Rev., 2014, 33, 578–588 CrossRef CAS.
- F. Pouliquen, C. Blanc, E. Arretz, I. Labat, J. Tournier-Lasserve, A. Ladousse, J. Nougayrede, G. Savin, R. Ivaldi, M. Nicolas, J. Fialaire, R. Millischer, C. Azema, L. Espagno, H. Hemmer and J. Perrot, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, pp. 459–460 Search PubMed.
- A. P. Reverberi, J. J. Klemeš, P. S. Varbanov and B. Fabiano, J. Cleaner Prod., 2016, 136, 72–80 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc03826b |
| This journal is © The Royal Society of Chemistry 2024 |