Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cell culture models for assessing the effects of bioactive compounds in common buckwheat (Fagopyrum esculentum): a systematic review
Sara Margherita
Borgonovi
,
Stefania
Iametti
,
Anna Ramona
Speranza
and
Mattia
Di Nunzio
 *
*
Department of Food, Environmental and Nutritional Sciences (DeFENS), University of Milan, via Celoria 2, 20133 Milan, Italy. E-mail: mattia.dinunzio@unimi.it
First published on 20th February 2024
Abstract
Common buckwheat (CBW) is grown and consumed worldwide. In addition to its already established reputation as an excellent source of nutrients, CBW is gaining popularity as a possible component of functional foods. Whereas human studies remain the gold standard for evaluating the relationship between nutrition and health, the development of reliable in vitro or ex vivo models has made it possible to investigate the cellular and molecular mechanisms of CBW effects on human health. Herein is a systematic review of studies on the biological effect of CBW supplementation, as assessed on various types of cellular models. Although the studies reported here have been conducted in very different experimental conditions, the overall effects of CBW supplementation were found to involve a decrease in cytokine secretion and oxidation products, related mainly to CBW polyphenols and protein or peptide fractions. These chemical species also appeared to be involved in the modulation of cell signaling and hormone secretion. Although further studies are undoubtedly necessary, as is their extension to in vivo systems, these reports suggest that CBW-based foods could be relevant to maintaining and/or improving human health and the quality of life.
1. Introduction
Pseudocereals are among the main components of many functional foods associated with several benefits to human health.1Fagopyrum esculentum (common buckwheat, CBW) is a pseudocereal native to southwest Asia, now grown in temperate climates and valued for its high levels of fiber, vitamins, and proteins with a balanced amino acid composition and high biological value. CBW also contains significant amounts of bioactive components such as polyphenols, phytosterols, squalene, and fagopyritols.2 Since it does not contain gluten, CBW may be consumed harmlessly by people with celiac disease, who have a limited selection of suitable food that responds to their peculiar needs.3Several health-related benefits (hypotensive, hypoglycemic, hypocholesterolemic, neuroprotective) were associated with CBW and with its milling and processing by-products, thus bringing into the limelight the potential of CBW in the formulation of functional foods4 and therefore leading to an increase in their agricultural, industrial, and pharmacological use. However, there is still some misinformation and a general lack of knowledge about how CBW-based or CBW-enriched foods can be advantageously exploited and included in the human diet.5
Although human studies remain the gold standard for assessing the association between nutrients and health, the continuing progress of consistent in vitro/ex vivo models allowed us to investigate the cellular/molecular mechanisms of the reported effects of specific food compounds. The use of models represents a primary – and unquestionably necessary – step when exploring the health-promoting properties of bioactive species. As an additional advantage, the use of cellular models also facilitates the exploration of the possible synergies among individual compounds – or classes of compounds – that are present in foods.6
Cell cultures are most frequently used in clinical settings to develop model systems for studying fundamental traits of cell biology,7 for simulating disease mechanisms,8 or for assessing the toxicity or the safety of specific molecules.9 Additionally, the homogeneity of clonal cell populations or of cell types in well-defined culture systems eliminates confounding genetic or environmental factors, enabling the collection of data with levels of reproducibility and consistency that are not possible when studying whole organs or organisms.10
To date, several studies investigated the effect of single food bioactive compounds,11–13 food extracts,14,15 or food digests in cell cultures.16,17 Studies aimed at evaluating the nutritional and healthful properties of specific fractions/compounds in CBW are systematically reviewed here, to highlight any connection between their molecular properties and their effects on the investigated system. This may allow a somehow deeper insight into the molecular bases underpinning the potential advantages of using CBW as a key ingredient in functional foods.
2. Methodology
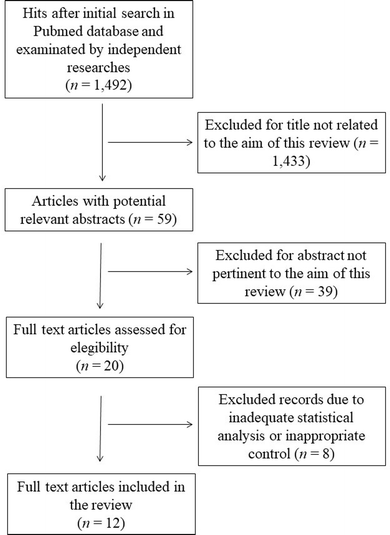
This systematic review was performed according to the preferred reporting items for systematic reviews and meta-analyses guidelines (PRISMA).18 The search was carried out by using the PubMed database in August 2023, and was carried out using the following keywords and Boolean operators: “common buckwheat” OR “Fagopyrum esculentum” NOT “review”. The initial search yielded 1492 hits. During the screening process (reviewing titles), 1433 records were excluded. After abstract analysis, another 39 articles were ousted. Altogether, 20 records were assessed for eligibility and 12 were included in the review. Chosen studies were published between 1995 and 2023 without restrictions on the timeframe or the publication status. Exclusion criteria were: (i) titles irrelevant to the research topic; (ii) abstract inappropriate or not related to the topic; (iii) use of Tartary buckwheat (Fagopyrum tataricum) or not-defined buckwheat species which may vary in the type and content of bioactive substances;19 (iv) studies or data comparing treated buckwheat or buckwheat in combination with other species; (v) studies involving single molecules or purified compounds; (vi) studies or data with inadequate statistical analysis or inappropriate controls. Reviews, letters, abstracts, and articles without a complete text in the English language were also excluded. Two of the authors (SMB and ARS) checked the titles and abstracts of studies, and disagreements between the two reviewers were resolved through senior authors acting as mediators (SI and MDN). The detailed selection process is presented in Fig. 1.3. Results
Using the criteria detailed under methodology, a total of 12 studies on the effects of CBW extract supplementation in cultured cells have been selected. The most relevant results are summarized in Table 1.| Ref. | Type of sample | Cell/tissue model | Concentration/time of incubation | Exogenous treatment | Results | Significance of findings |
|---|---|---|---|---|---|---|
| Effects are referred to respective unsupplemented control cells either in basal or stressed conditions. In the presence of more than one concentration/treatment time, only statistically significant changes are reported. When CBW-derived samples/concentrations/times are not reported, the effects are referred to all experimental conditions. Variation entity must be considered as approximately. ↑: increase; ↓: decrease; ↔: no effect; ALT: alanine transaminase; AP2: transcription factor AP2; AST: aspartate transaminase; CBW: buckwheat; C/EBPα: transcription factor CCAAT/enhancer binding protein α; CCK: cholecystokinin; COX-2: cyclooxygenase-2; Cu/Zn SOD: copper/zinc superoxide dismutase; CuNPs/TiO2: copper nanoparticles supported on titania; G6PDH: glucose-6-phosphate dehydrogenase; GPx: glutathione peroxidase; IL-12: interleukin-12; IL-1β: interleukin-1β; IL-6: interleukin-6; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharides; MDA: malondialdehyde; NF-κB: nuclear factor-κB; NO: nitric oxide; NOX4: nicotinamide adenine dinucleotide phosphate oxidase 4; p70S6K (Thr389): p70 S6 kinase at threonine 389; p70S6K (Thr421): p70 S6 kinase at threonine 421; p-Akt: phospho-protein kinase B; p-ERK: phospho-extracellular signal-regulated kinase; p-GSK-3: phospho-glycogen synthase kinase 3; p-IκB: phospho-inhibitor of κB; p-InsR: phospho-insulin receptor β; p-IRS-1: phospho-insulin receptor substrate 1; p-JNK: phospho-c-Jun N-terminal kinase; p-MKK4: phospho-mitogen-activated protein kinase kinase 4; p-p38 MAPK: phospho-p38 mitogen-activated protein kinase; p-p38: phospho-p38; p-p42/44 ERK: phospho-p42/44 extracellular signal-regulated kinase; PPARγ: peroxisome proliferator activated receptor γ; p-Stat3: phospho-signal transducer and activator of transcription 3; ROS: reactive oxygen species; t-BOOH: tert-butyl hydroperoxide; TNF-α: tumor necrosis factor-α. | ||||||
| Vogrinčič et al.22 | CBW flour hydroalcoholic extract | Human hepatic cancer cell line (HepG2) | 0.2%, 0.4%, and 1% (v/v) for 4 h or 24 h | Subsequent treatment with 400 μM t-BOOH for 20 min | Basal condition: ↔ DNA damage | Extract has high antigenotoxic activity |
| Oxidative condition: ↓ DNA damage by 20%, 33%, and 33% at 0.2%, 0.4%, and 1% for 24 h, respectively | ||||||
| Curran et al.31 | Dehulled CBW flour hydroalcoholic extract | Rat hepatic cancer cell line (H4IIE) | 0.1% and 0.4% (v/v) for 6 min | Co-supplementation with 250 nM insulin | Basal condition: ↔ p-InsR, p-IRS-1, p-Akt, p-GSK-3, p-Stat3, p-Src kinase, and p70S6K (Thr389) protein expression at 0.1% (v/v); ↑ p-p42/44 ERK, p-p38 MAPK, and p70S6K (Thr421) protein expression by 120–150, 5–14, and 10-fold at 0.1% (v/v), respectively; ↓ 3H-deoxyglucose uptake by 85% at 0.4% (v/v) | Extract inhibits basal and insulin-stimulated glucose uptake by acting on selected phosphorylation events |
| Insulin-supplemented condition: ↑ p-p42/44 ERK, p-p38 MAPK protein expression by 3 and 2-fold, respectively | ||||||
| Lee et al.30 | CBW sprout hydroalcoholic extract | Murine preadipocyte cell line (3T3-L1) | 50 μg mL−1 for 24 h | Not present | ↓ C/EBPα, PPARγ, AP2 gene expression by 40%, 40%, and 45%, respectively; ↔ lipid accumulation and adiponectin expression, GPx, and Cu/Zn SOD gene expression; ↓ ROS production by 20% and G6PDH and NOX4 gene expression by 25% and 85%, respectively | Extract has potential anti-adipogenesis activity with anti-oxidative properties |
| Wang et al.28 | CBW hull flavonoid aqueous extract | Human hepatic cancer cell line (HepG2) | 10 μg mL−1, 25 μg mL−1, and 50 μg mL−1 for 24 h | Co-supplementation with 200 mM glucose | Diabetic condition: ↑ cell viability by 20%, 30%, and 60% at 10 μg mL−1, 25 μg mL−1, and 50 μg mL−1, respectively; ↑ SOD activity by 55% and 90%, CAT activity by 15% and 16%, and GPx activity by 15% and 25% at 25 μg mL−1 and 50 μg mL−1, respectively; ↓ MDA level by 18%, 40%, and 65% at 10 μg mL−1, 25 μg mL−1, and 50 μg mL−1; ↓ AST leakage by 30% and 45%, and ALT leakage by 14% and 25% at 25 μg mL−1, and 50 μg mL−1, respectively | CBW hull extract has considerable antioxidant an hepatoprotective potential |
| Kim et al.27 | CBW hull hydroalcoholic extract | Human breast cancer cell line (MCF-7) | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 30%, 65%, and 70% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | CBW hull extract shows anticancer properties against a variety of cancer cell lines, depending on the solvent used for preparation and fractionation |
| Hexane fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 55%, 70%, 90%, and 90% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 60%, 60%, 60%, and 70% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 35%, 70%, 95%, and 95% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 25%, 45%, and 60% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 2%, 10%, 30%, and 60% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| CBW hull hydroalcoholic extract | Human hepatic cancer cell line (Hep3B) | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 20%, 50%, 60%, and 65% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | ||
| Hexane fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 30%, 65%, 85%, and 85% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 50%, 65%, 65%, and 70% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 35%, 35%, 50%, and 50% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 60%, 75%, 75%, and 80% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 30%, 50%, and 70% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| CBW hull hydroalcoholic extract | Human lung cancer cell line (A549) | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 25%, 40%, 55%, and 60% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | ||
| Hexane fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 2%, 50%, 70%, and 85% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 3%, 50%, 60%, and 75% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 30%, 50%, 55%, and 70% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 5%, 45%, 45%, and 60% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 2%, 35%, 50%, and 60% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| CBW hull hydroalcoholic extract | Human gastric cancer cell line (A549) | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 55%, 80%, and 90% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | ||
| Hexane fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 20%, 40%, 60%, and 90% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 5%, 25%, 45%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 40%, 50%, 80%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 60%, 60%, 60%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 1%, 10%, 50%, and 65% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| CBW hull hydroalcoholic extract | Human cervical cancer cell line (HeLa) | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 30%, 50%, 70%, and 70% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | ||
| Hexane fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 35%, 40%, 45%, and 55% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 25%, 70%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 45%, 75%, 75%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 50%, 50%, 80%, and 80% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 20%, 25%, 40%, and 50% at 0.125 mg mL−1, 0.25 mg mL−1, 0.375 mg mL−1, and 0.5 mg mL−1, respectively | |||
| CBW hull hydroalcoholic extract | Human transformed primary embryonal kidney cells (293) | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 10%, 15%, 30%, and 40% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | ||
| Hexane fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 10%, 15%, 20%, and 25% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Chloroform fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 10%, 20%, 20%, and 30% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Ethyl acetate fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 15%, 20%, 25%, and 35% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Butanol fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 10%, 15%, 30%, and 30% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Water fractionated CBW hull hydroalcoholic extract | 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1 for 48 h | Not present | ↑ Cell proliferation inhibition by 10%, 15%, 15%, and 20% at 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1, and 1 mg mL−1, respectively | |||
| Nam et al.29 | CBW sprouts hydroalcoholic extract | Mouse macrophages cell line (RAW 264.7) | 62.5 μg mL−1, 125 μg mL−1 and 250 μg mL−1 for 24 h | Co-supplementation with 1 μg mL−1 LPS | Inflammatory condition: ↓ NO production by 15% at 125 μg mL−1; ↓ iNOS protein expression by 70% and 40% at 125 μg mL−1 and 250 μg mL−1, COX-2 protein expression by 25%, 60%, and 40% at 62.5 μg mL−1, 125 μg mL−1, and 250 μg mL−1, p-IκB protein expression by 25% at 250 μg mL−1, p-p38 protein expression by 40% at 62.5 μg mL−1, and p-MKK4 protein expression by 35% and 20% at 62.5 μg mL−1 and 250 μg mL−1, respectively; ↔ p-JNK and p-ERK protein expression; ↓ IL-6 secretion by 17% and 100% at 125 μg mL−1 and 250 μg mL−1, IL-12 secretion by 45%, and TNF-α secretion by 14% at 62.5 μg mL−1 and 250 μg mL−1, respectively; ↓ TNF-α mRNA expression by 20% at 250 μg mL−1; ↔ IL-6 and IL-12 mRNA expression | Extract can be a potential source of anti-inflammatory agents addressing macrophage-mediated inflammatory disorders |
| CBW sprouts hydroalcoholic extract | Primary BALB/c mice peritoneal macrophages | 62.5 μg mL−1, 125 μg mL−1 and 250 μg mL−1 for 24 h | Co-supplementation with 100 ng mL−1 LPS | Inflammatory condition: ↓ IL-6 secretion by 20%, 40%, and 45% at 62.5 μg mL−1, 125 μg mL−1, and 250 μg mL−1, IL-12 secretion by 20%, 60%, and 85% at 62.5 μg mL−1, 125 μg mL−1, and 250 μg mL−1, and TNF-α secretion by 10%, 15%, and 18% at 62.5 μg mL−1, 125 μg mL−1, and 250 μg mL−1, respectively | ||
| Metzger et al.21 | Insoluble CBW flour protein extract | Human intestinal cancer cell line (Caco-2) | 0.2% (w/v) for 90 min | Not present | ↓ Cholesterol uptake by 55% | CBW protein extract shows anti-cholesterol uptake properties |
| Capraro et al.23 | Albumin fraction from CBW flour | Human intestinal cancer cell line (Caco-2) | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ NF-κB activation | Intact and in vitro digested and fractionated CBW proteins show anti-inflammatory activity |
| Inflammatory condition: ↓ NF-κB activation by 60% | ||||||
| Fractioned CBW flour with very low-charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ NF-κB activation | |||
| Inflammatory condition: ↓ NF-κB activation by 55% | ||||||
| Fractioned CBW flour with low-charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ NF-κB activation | |||
| Inflammatory condition: ↓ NF-κB activation by 20% | ||||||
| Fractioned CBW flour with high-charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ NF-κB activation | |||
| Inflammatory condition: ↓ NF-κB activation by 25% | ||||||
| In vitro digested fractioned CBW flour albumins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ IL-8 activation | |||
| Inflammatory condition: ↓ IL-8 activation by 60% | ||||||
| In vitro digested fractioned C BW flour with very low charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ IL-8 activation | |||
| Inflammatory condition: ↓ IL-8 activation by 65% | ||||||
| In vitro digested fractioned CBW flour with low-charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ IL-8 activation | |||
| Inflammatory condition: ↓ IL-8 activation by 70% | ||||||
| In vitro digested fractioned CBW flour with high-charge globulins | 1 mg mL−1 for 4 h | Co-supplementation with 10 ng mL−1 IL-1β | Basal condition: ↔ IL-8 activation | |||
| Inflammatory condition: ↓ IL-8 activation by 40% | ||||||
| Song et al.20 | In vitro digested CBW protein extract | Mouse intestinal neuroendocrine cancer cell line (STC-1) | 5 mg mL−1 for 2 h | Not present | ↑ CCK secretion by 220% | In vitro digested CBW proteins show a CKK-mediated anorexigenic effect |
| Very low hydrophobicity in vitro digested CBW flour protein extract | 5 mg mL−1 for 2 h | Not present | ↔ CCK secretion | |||
| Low hydrophobicity in vitro digested CBW flour protein extract | 5 mg mL−1 for 2 h | Not present | ↔ CCK secretion | |||
| High hydrophobicity in vitro digested CBW flour protein extract | 5 mg mL−1 for 2 h | Not present | ↑ CCK secretion by 840% | |||
| Very high hydrophobicity in vitro digested CBW flour protein extract | 5 mg mL−1 for 2 h | Not present | ↑ CCK secretion by 570% | |||
| Sirotkin et al.26 | Dissolved CBW powder | Primary porcine ovarian granulosa cells | 10 μg mL−1 for 48 h | Co-supplementation with 1 μg mL−1, 10 μg mL−1, and 100 μg mL−1 CuNPs/TiO2 | Basal condition: ↑ cell viability and apoptosis by 4% and 15%, respectively; ↓ testosterone release by 60%; ↔ cell proliferation, progesterone, and estradiol release | CBW mitigates the adverse effect of exposure to CuNPs/TiO2 and modulates sexual hormone secretion |
| Cytotoxic condition: ↑ cell viability by 15% at 1 μg mL−1 and apoptosis by 4% at 100 μg mL−1 and, respectively; ↓ cell viability by 8% and 7% at 10 μg mL−1 and 100 μg mL−1, cell proliferation by 40%, 25%, and by 10% at 1 μg mL−1, 10 μg mL−1, and 100 μg mL−1, testosterone release by 75%, 50%, and by 45% at 1 μg mL−1, 10 μg mL−1, and 100 μg mL−1, and estradiol release by 20% and 40% at 10 μg mL−1 and 100 μg mL−1, respectively | ||||||
| Sirotkin et al.25 | Dissolved CBW powder | Primary porcine ovarian granulosa cells | 10 μg mL−1 for 48 h | Co-supplementation with 10 ng mL−1, 100 ng mL−1, and 1000 ng mL−1 xylene | Basal condition: ↑ proliferation and ↓ estradiol release by 5% and 50%, respectively; ↔ cell viability, progesterone, and estradiol release | CBW alleviates the detrimental effect of xylene exposure and moderates sexual hormone secretion |
| Cytotoxic condition: ↑ cell viability by 5% and 3%, apoptosis by 4% and 7%, ↓ progesterone release by 70% and 75% at 100 ng mL−1 and 1000 ng mL−1, respectively; ↑ cell proliferation and ↓ estradiol release by 23% and 18% at 100 ng mL−1, respectively | ||||||
| Sirotkin et al.24 | Dissolved CBW powder | Primary porcine ovarian granulosa cells | 10 μg mL−1 for 48 h | Co-supplementation with 10 ng mL−1, 100 ng mL−1, and 1000 ng mL−1 benzene | Basal condition: ↓ cell viability and proliferation by 32% and 6%, respectively | CBW mitigates the adverse effects of benzene exposure and controls sexual hormone secretion |
| Cytotoxic condition: ↑ cell viability by 5% and 7% and estradiol release by 40% and 35% at 100 ng mL−1 and 1000 ng mL−1; ↓ apoptosis by 5% and 6% at 100 ng mL−1 and 1000 ng mL−1, and progesterone release by 40% and 65% at 10 ng mL−1 and 100 ng mL−1; ↔ cell proliferation | ||||||
A first general observation is that the CBW-derived materials used for defining bioactivity were very different. Four studies were conducted on CBW flour,20–23 three on CBW powder,24–26 two on CBW hull27,28 and CBW sprout,29,30 and one on dehulled CBW flour.31 Moreover, the CBW extracts spanned a broad range of concentrations (from 10 μg mL−1 (ref. 24–26 and 28) to 5 mg mL−1 (ref. 20)) and supplementation times (from 6 minutes31 to 48 hours24–27).
Also, two studies were conducted on CBW protein extracts21,23 or on the products of their in vitro digestion protein extract,20,23 thus highlighting effects that may be mainly attributed to the protein fraction/peptide fraction. Conversely, five studies used hydroalcoholic extracts,22,27,29–31 and one was limited to an aqueous extract,28 so the reported effects may be mostly accredited to the phenolics fraction. Finally, three studies used a solubilized CBW powder,24–26 in which both phenolics and proteins/peptides could be present.
Moreover, four of the studies were conducted in basal conditions,20,21,27,30 whereas two studies included simultaneous or subsequent exogenous stress.28,29 A total of six studies compared both basal and exogenous stress responses.22–26,31
A further challenge to assessing some unifying mechanism is represented by the heterogeneity of cell models used in these studies. Seven studies used cell line models,20–23,28,30,31 three relied on primary cells,24–26 and two on both types of model cells.27,29
Among the studies involving cell cultures, studies, three were conducted on intestinal cells,20,21,23 four on hepatic cells,22,27,28,31 and one each for pre-adipocytic cells,30 breast cells,27 lung cells,27 gastric cells,27 cervical cells,27 and macrophages.29 Among primary cell studies, three were conducted on ovarian granulosa cells,24–26 and one on embryonal kidney cells27 and peritoneal macrophages.29
The studies considered cover a wide range of the biological effects induced by CBW extracts, and this is useful in providing an overview of the many facets of “bioactivity”. Five studies focused on cytotoxicity,24–28 four on hormone secretion,20,24–26 three on oxidative stress and antioxidant defenses,22,28,30 two on response to inflammatory stimuli23,29 and to changes in cell signalling pathways,29,31 whereas only one focused on CBW effects on cholesterol uptake.21
By piecing together, the most relevant information gathered in this review, it appears that the overall effects of CBW supplementation were consistently associated with a decrease in cytokine secretion,23,29 with low levels of intracellular oxidation products22,28,30 and – in general – with improved response to inflammatory agents. Modulation of the levels of molecules involved in cell signaling29,31 and changes in hormone secretion20,24–26 were also consistent in all the studies that addressed these parameters.
However, cytotoxicity data remain ambiguous, as contrasting effects on cell viability and/or proliferation were detected in cancer and primary cells.24–28 Further studies are needed to clarify these issues and to assess whether the observed effects may be of general relevance.
4. Discussion
Numerous facets of the complex relationship between nutrition and health have been clarified by the expansion of studies and investigations aimed at examining the molecular basis of functional nutrients and food components. When researching this subject, it should always be considered that foods – rather than specific single compounds – form the basis of the human diet. Therefore, it is crucial to show whether food ingredients in their entirety have any good effects on health.32 Also of relevance in real food systems are the interactions among potential or established bioactives. These interactions occur only in food and often have been proven to impair or promote specific biological activities.The increasing interest in pseudocereals in general, and on CBW in particular, was first based on their content of active components being higher than in other grain crops, such as modern wheat varieties,33 to the point of pseudocereals being described as “the grains of the twenty-first century”.34 Consuming CBW and CBW-enriched products has been linked to a variety of biological and physiological responses, including hypoglycemic,35 and anti-inflammatory effects,36 and there is a consensus on the phenolics and the proteins in CBW being responsible for a good share of these advantages.37,38
Phenolic compounds are present in pseudocereal grains mainly in two forms: soluble species (either free or conjugated to simple sugars and oligosaccharides), and insoluble species that are mostly bound to biopolymers.39 Due to their chemical nature,40 free polyphenols aglycones, along with their glycosides, can be readily extracted by solvents such as methanol, ethanol, acetonitrile, and acetone, used alone or mixed with water.41 In a recent paper, Borgonovi et al. reported that most phenolic compounds in CBW were in the free form rather than in the bound one (1421 μg per g dw vs. 55 μg per g dw, respectively). According to the same study, flavan-3-ols such as epicatechin-3-(3′′-O-methyl) gallate, epicatechin-O-3,4-dimethyl gallate, and catechin-glucoside were the most abundant species in CBW.42
Here we reviewed six studies in which the materials used for supplementing cell cultures were resembling a standard phenolics-rich extract.22,27–31 Vogrinčič et al.,22 Wang et al.,28 and Lee et al.30 showed that supplementation with aqueous/hydroalcoholic CBW extracts (from flour and hull) was able to reduce oxidative damage in basal or oxidative stress and to improve diabetes conditions in human hepatic and murine preadipocyte cell lines. This effect was also accompanied by an increase in cellular antioxidant defenses,28 and by a decrease in the expression of enzymes involved in the generation of reactive oxygen species (ROS).30 It is believed that dietary flavonoids exert powerful antioxidant action for protection against ROS/cellular oxidative stress by directly scavenging ROS and chelating metal ions relevant to ROS formation and stability.43 Polyphenols reduce free radicals by donating one electron to the phenolic OH group, and the aromatic group is kept stable by the resonance of the resulting aroxyl radicals.44 A radical form of the antioxidant is created after interaction with the initial reactive species and is stabilized by charge delocalization brought on by the interaction of the phenolic hydroxyl groups with the benzene ring's electrons.45 The amount and arrangement of the hydroxyl group determine the phenolic compounds’ antioxidant capacity, and their antioxidant activity is correlated with the number of hydroxyl groups present.46 In addition, polyphenols also exert their antioxidant effects in an indirect way, that involves the up regulation of antioxidant enzymes expression in vivo. Ajiboye et al. evidenced that polyphenolic extract of Sorghum bicolor grains enhances ROS detoxification in N-nitrosodiethylamine-treated rats by improving serum superoxide dismutase (SOD), catalase, glutathione (GSH) peroxidase, and GSH reductase activities.47 Similarly, type 2-diabetic Wistar rats given CBW hull flavonoid extract showed an increase in SOD activity and GSH content in serum.28 Also, several polyphenolic compounds have been shown to inhibit pro-oxidant enzymes such as lipoxygenase,48 cyclooxygenase,49 myeloperoxidase,50 NADPH oxidase,51 and xanthine oxidase,52 thus preventing the endogenous generation of ROS.
The studies reviewed here also report that different CBW extracts may inhibit proliferation27 and inflammatory response29 by appropriate modulation of signalling pathways29,31 in several cancer cell lines. Noteworthily, these activities were different when using polar (i.e., aqueous) extracts28 or extracts prepared by using alcohols or non-polar solvents.22,27,29–31 Of course, the chemical properties of the extraction media used in these studies resulted in the solubilization of different classes of compounds. In this regard, Meneses et al. evaluated the efficacy of different solvents and their mixtures for extracting antioxidant phenolic compounds from brewer's spent grains. Although all the produced extracts showed antioxidant activity, the extract prepared with aqueous acetone (60%, v/v) had the most elevated content of total phenols.53 In addition to the type of solvent used, the extraction methods can also produce extracts with varying concentrations of polyphenols. Dobrinčić et al. reported that microwave, ultrasound, and high-pressure-assisted extraction resulted in higher total polyphenol content in extracts compared to conventional heat-reflux extraction.54
Substantial progress has been made in outlining the mechanisms through which polyphenols inhibit cell proliferation and act on the cellular response to inflammatory stimuli. Flavan-3-ols – which are the most abundant class of phenolics in CBW42 – have been shown to inhibit cell proliferation through the modulation of multiple signalling pathways. For instance, Deguchi et al. evidenced that catechin supplementation determined a dose-dependent growth inhibition effect associated with phosphorylation of c-Jun N-terminal kinases/stress-activated protein kinase and of the p38 protein in human breast cancer cells.55 Catechins can exert significant anti-inflammatory properties by regulating the activation or deactivation of inflammation-related cell signalling pathways, such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), signal transducer activator of transcription 1 (STAT1) activation and the activator of transcription 1/3 pathways.56,57
In addition to polyphenols, recent research identified the potential health benefits of food proteins and bioactive peptides.58 The multifunctional properties, including antioxidant, antimicrobial, anti-hypertensive, and anti-diabetic activities demonstrated for some of these proteins and peptides, have led to CBW gaining importance as an ingredient for foods aiming at the prevention and/or management of various chronic diseases.38 In the reports reviewed here, evidence was provided for anti-inflammatory activity23 and inhibition of cholesterol uptake21 in intestinal cells both by fractionated and total CBW protein extracts. To date, very few plant proteins have been reported to possess anti-inflammatory properties in their intact form.23,59,60 The most studied peptide presently is lunasin, a biologically active peptide originally discovered as a 2S albumin protein first found in soybean and subsequently detected in cereals and pseudocereals.61 Various studies evidenced that lunasin supplementation to lipopolysaccharide (LPS)-stimulated macrophages resulted in the decrease of pro-inflammatory biomarkers associated with an inhibition of nuclear translocation of the p65 and p50 NF-κB subunits and the protein kinase B-mediated NF-κB pathway.62–65 This effect is mediated by the interaction between the Arg-Gly-Asp motif present in lunasin with αVβ3 integrin, which is reportedly associated with the activation of inflammatory pathways.66
Different mechanisms have been proposed for the reported hypocholesterolemic capacity of CBW proteins in vivo,67,68 which almost invariably appears to require intact proteins. One hypothesis assumes that insoluble and hydrophobic CBW proteins – as well as other specific plant proteins – may interfere with the organization of cholesterol-rich micelles, affecting their solubility and impairing their uptake by intestinal cells.21
In addition to intact proteins, bioactive peptides can be found in enzymatic protein hydrolysates and fermented products. Of course, peptides are also released during the gastrointestinal enzymatic digestion of proteins.69 Several studies evidenced that bioactive peptides from CBW protein hydrolysis possess in vitro radical scavenging properties,23,70,71 display a remarkable reducing power and metal ion chelating activity,58 and may be capable of inhibiting platelet aggregation72 and the activity of dipeptidyl peptidase IV.73
Capraro et al.23 reported that the anti-inflammatory activity of in vitro digested CBW proteins was higher than that measured – in an intestinal cell model – for the corresponding native intact proteins. The biological functions of peptides are governed by either the presence of a definite amino acid sequence or by the relative ratio among specific amino acids or amino acid classes.74 Song et al. evidenced that most of the peptides obtained after in vitro digestion of CBP were highly hydrophobic, due to the frequency of amino acid residues such as Pro, Phe, Gly, and Val.20 Hydrophobicity of the peptides has been reported as one of the major factors responsible for the anti-inflammatory responses, as most of the peptides with anti-inflammatory activity (independently of their size) were rich in hydrophobic amino acids. In oligopeptides, hydrophobic side chains were mainly clustered toward the N-terminal, while the C-terminal contained mainly polar side chains.75 The molecular mechanisms of the anti-inflammatory peptides at the cell level may include a modulation of NF-κB and mitogen-activated protein kinase pathway, a reduction of TNF-α induced inflammatory pathway, and an inhibition of both NO production and histamine release.75
One of the studies considered here also evidenced a hunger-suppressing effect of in vitro-digested CBW proteins on an intestinal cell model, that was attributed to the release of cholecystokinin (CCK).20 Phe and Try,76 as well as the soybean β51–63 peptide,77 can stimulate the release of CCK from intestinal cells through the mobilization of intracellular calcium and that this effect was abolished by a specific calcium-sensitive receptor antagonist.78 Making sense of all these observations may undoubtedly benefit from yet unexplored approaches based on the facile synthesis of definite amino acid sequences. Such an approach might elucidate the structural requirements for either the anti-inflammatory activity or the stimulation of intestinal hormone secretion by small peptides of both plant and animal origin.
Finally, this review includes studies in which cultured cells were supplemented with solutions/suspension of various types of CBW milling products in the absence of any prior extraction step.24–26 This experimental approach makes it next to impossible any attribute the reported biological effects to specific classes of bioactive compounds. However, this approach could provide some information on whether the possible simultaneous presence of different types of bioactive compounds may lead to a cellular response different from the one observed with individual classes of potential bioactives.
In conclusion, whereas progress in future studies should always consider purity/identity issues, the involved researchers should consider that the in vivo effects may be the result of a synergistic effect between the various bioactive compounds. A recent authoritative review has summarised as synergistic treatment approaches of polyphenols may be effective in the treatment of many diseases providing information about the benefits of these compounds in combination.79 The effects of these combinations may be greater than the sum of the separate effects of individual chemical species, but the possibility that the simultaneous presence of species addressing different molecular events in a conflicting way – and thus being useless from a health-promoting standpoint – should be considered as well.80 Although cell cultures are often used to evaluate the effectiveness and mechanism of action of bioactives in vitro, to avoid misleading results it is crucial to employ concentrations comparable to those found in vivo, which can vary from nM to μM.81,82 One major concern when using cell cultures to study biomarkers triggered by bioactive compounds is the cancer-related origin of many commercially available cell cultures respect primary non-cancerous cell. This is because several bioactive peptides and polyphenols selectively induce apoptosis in cancer cells by deregulating the cell cycle, making them potential anticancer agents.83–86 In particular, Sak et al.87 conducted a study reviewing the cytotoxicity of flavonoids on over 150 cell lines. The Authors concluded that the toxicity effect varied greatly depending on the type of flavonoid, dose, and cell line origin. In contrast, previous studies have reported that polyphenols increase cell viability in primary cells,24–26,29 highlighting the significance of cell type (primary vs. cell lines) in interpreting the biological effects of bioactive compounds. Also worth considering are issues related to the modulation of bioavailability of any bioactive (and the timing of their release in an active form from foodborne precursors) by the many other components, be they natural or man-made, that are almost unavoidable in most of the foods consumed by humans in all corners of an increasingly globalized world.
5. Conclusion
CBW shows promise as a natural source of physiologically active substances that have positive effects on human health, including anti-inflammatory, anti-tumor, and antioxidant properties. This suggests that CBW-based foods could be useful in promoting and maintaining consumers’ health and quality of life.This said, much work remains to be done to clarify several yet unaddressed issues. For instance, most of the reports reviewed here22,24–26,28–31 have assessed the effects of various extracts without considering the bioaccessibility of polyphenols.88 Future research examining the modifications that take place during digestion will be beneficial in determining the effectiveness of the bioactive substances included in food.
Also, various research groups have shown how technological processes, such as sprouting, fermentation, exogenous enzyme treatment, and thermal processing, can lead to an increase in the content of free (and thus more easily extractable) polyphenols in CBW, as well as facilitate the release of bioactive peptides from CBW proteins.42,89–91 These results emphasize the importance of addressing the role of technological processes in determining the overall bioactivity of foods. Investigation on this topic is currently undergoing novel popularity, also because of the increasing interest in: (i) advanced and sustainable methods for implementing optimal nutritional characteristics using bioprocesses and bio-processed ingredients; (ii) ongoing change in consumer needs, preferences, and expectations; (iii) of the impact of climate changes on the availability (and processing characteristics) of both established and novel plant-based raw materials.
In any case, further investigations are required, as in vivo studies in animal models, clinical trials and cohort studies are yet not available, in contrast with the promising – but far from exhaustive – data from cellular models and food extracts. In vivo approaches should allow also to address properly most of the bioavailability issues and to define more accurately the nature and the mechanism of action of bioactive species in CBW and their synergies. The resulting holistic view should – hopefully – confirm the health benefits of CBW consumption and provide a sound molecular basis for the determinants of the “bioactive quality” of this pseudocereal.
Author contributions
Conceptualization, S. I. and M. D. N.; methodology, S. M. B., A. R. S., and M. D. N.; validation, S. M. B., A. R. S., and M. D. N.; formal analysis, S. M. B., A. R. S. and M. D. N.; investigation, S. M. B., A. R. S., S. I., and M. D. N.; writing – original draft preparation, M. D. N.; writing – review and editing, S. I. and M. D. N.; supervision, M. D. N.; funding acquisition, S. I. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This investigation was supported in part by the National Recovery and Resilience Plan (NRRP), (Mission 4, Component 2, Investment 1.3 – Call for tender no. 341 of 15/03/2022) of the Italian Ministry of University and Research, funded by the European Union–NextGenerationEU, in the frame of the project “Research and innovation network on food and nutrition: Sustainability, Safety and Security (ON Foods)”.References
- M. Shahbaz, N. Raza, M. Islam, M. Imran, I. Ahmad, A. Meyyazhagan, K. Pushparaj, B. Balasubramanian, S. Park, K. R. R. Rengasamy, T. A. Gondal, A. El-Ghorab, M. A. Abdelgawad, M. M. Ghoneim and C. Wan, The nutraceutical properties and health benefits of pseudocereals: a comprehensive treatise, Crit. Rev. Food Sci. Nutr., 2023, 63, 10217–10229 CrossRef PubMed.
- S. A. Sofi, N. Ahmed, A. Farooq, S. Rafiq, S. M. Zargar, F. Kamran, T. A. Dar, S. A. Mir, B. N. Dar and A. Mousavi Khaneghah, Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: An updated overview, Food Sci. Nutr., 2023, 11, 2256–2276 CrossRef CAS PubMed.
- S. Yeşil and H. Levent, The influence of fermented buckwheat, quinoa and amaranth flour on gluten-free bread quality, LWT, 2022, 160, 113301 CrossRef.
- H. Yılmaz, Y. Nurcan and Ç. Meriç, Buckwheat: A Useful Food and Its Effects on Human Health, Curr. Nutr. Food Sci., 2020, 16, 29–34 CrossRef.
- D. Morales, M. Miguel and M. Garcés-Rimón, Pseudocereals: a novel source of biologically active peptides, Crit. Rev. Food Sci. Nutr., 2021, 61, 1537–1544 CrossRef CAS PubMed.
- S. Iametti, F. Bonomi and M. Di Nunzio, Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review, Int. J. Mol. Sci., 2022, 23, 1–19 Search PubMed.
- A. J. Valente, L. A. Maddalena, E. L. Robb, F. Moradi and J. A. Stuart, A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture, Acta Histochem., 2017, 119, 315–326 CrossRef CAS PubMed.
- M. Hebisch, S. Klostermeier, K. Wolf, A. R. Boccaccini, S. E. Wolf, R. E. Tanzi and D. Y. Kim, The Impact of the Cellular Environment and Aging on Modeling Alzheimer's Disease in 3D Cell Culture Models, Adv. Sci., 2023, 10, e2205037 CrossRef PubMed.
- M. Di Nunzio, V. Valli, L. Tomás-Cobos, T. Tomás-Chisbert, L. Murgui-Bosch, F. Danesi and A. Bordoni, Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives?, BMC Complementary Altern. Med., 2017, 17, 453 CrossRef PubMed.
- C.-P. Segeritz and L. Vallier, in Basic Science Methods for Clinical Researchers, ed. M. Jalali, F. Y. L. Saldanha and M. Jalali, Academic Press, Boston, 2017, pp. 151–172, DOI:10.1016/B978-0-12-803077-6.00009-6.
- M. Di Nunzio, F. Danesi and A. Bordoni, n-3 PUFA as regulators of cardiac gene transcription: a new link between PPAR activation and fatty acid composition, Lipids, 2009, 44, 1073–1079 CrossRef PubMed.
- M. Di Nunzio, D. van Deursen, A. J. Verhoeven and A. Bordoni, n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells, Br. J. Nutr., 2010, 103, 161–167 CrossRef CAS PubMed.
- Tjahjodjati, S. Sugandi, R. Umbas and M. Satari, The Protective Effect of Lycopene on Prostate Growth Inhibitory Efficacy by Decreasing Insulin Growth Factor-1 in Indonesian Human Prostate Cancer Cells, Res. Rep. Urol., 2020, 12, 137–143 CAS.
- M. Di Nunzio, G. Picone, F. Pasini, M. F. Caboni, A. Gianotti, A. Bordoni and F. Capozzi, Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell, Food Res. Int., 2018, 113, 392–400 CrossRef CAS PubMed.
- A. Nowak-Terpiłowska, I. Nowak, A. Feliczak-Guzik and M. Wyganowska, Analysis of the Impact of Ethanol Extract of Calendula officinalis L. on Human Fibroblast Cell Cultures Using the PANsys 3000 Device for Breeding and Visualization of Cells, Life, 2023, 13, 1–11 Search PubMed.
- M. Di Nunzio, A. Bordoni, F. Aureli, F. Cubadda and A. Gianotti, Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread, Nutrients, 2018, 10, 1–14 CrossRef PubMed.
- A. Kondrashina, E. Arranz, A. Cilla, M. A. Faria, M. Santos-Hernández, B. Miralles, N. Hashemi, M. K. Rasmussen, J. F. Young, R. Barberá, G. Mamone, L. Tomás-Cobos, S. Bastiaan-Net, M. Corredig and L. Giblin, Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility, Crit. Rev. Food Sci. Nutr., 2023, 1–19, DOI:10.1080/10408398.2023.2214628.
- A. Liberati, D. G. Altman, J. Tetzlaff, C. Mulrow, P. C. Gøtzsche, J. P. Ioannidis, M. Clarke, P. J. Devereaux, J. Kleijnen and D. Moher, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration, Br. Med. J., 2009, 339, b2700 CrossRef PubMed.
- Y. Liu, C. Cai, Y. Yao and B. Xu, Alteration of phenolic profiles and antioxidant capacities of common buckwheat and tartary buckwheat produced in China upon thermal processing, J. Sci. Food Agric., 2019, 99, 5565–5576 CrossRef CAS PubMed.
- H. Song, Q. Wang, Z. Shao, X. Wang, H. Cao, K. Huang, Q. Sun, Z. Sun and X. Guan, In vitro gastrointestinal digestion of buckwheat (Fagopyrum esculentum Moench) protein: release and structural characteristics of novel bioactive peptides stimulating gut cholecystokinin secretion, Food Funct., 2023, 14, 7469–7477 RSC.
- B. T. Metzger, D. M. Barnes and J. D. Reed, Insoluble fraction of buckwheat (Fagopyrum esculentum Moench) protein possessing cholesterol-binding properties that reduce micelle cholesterol solubility and uptake by Caco-2 cells, J. Agric. Food Chem., 2007, 55, 6032–6038 CrossRef CAS PubMed.
- M. Vogrinčič, I. Kreft, M. Filipič and B. Zegura, Antigenotoxic effect of Tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour, J. Med. Food, 2013, 16, 944–952 CrossRef PubMed.
- J. Capraro, S. Benedetti, G. C. Heinzl, A. Scarafoni and C. Magni, Bioactivities of Pseudocereal Fractionated Seed Proteins and Derived Peptides Relevant for Maintaining Human Well-Being, Int. J. Mol. Sci., 2021, 22, 1–14 Search PubMed.
- A. V. Sirotkin, M. Macejková, A. Tarko, Z. Fabova, A. Alrezaki, S. Alwasel and A. H. Harrath, Effects of benzene on gilts ovarian cell functions alone and in combination with buckwheat, rooibos, and vitex, Environ. Sci. Pollut. Res. Int., 2021, 28, 3434–3444 CrossRef CAS PubMed.
- A. V. Sirotkin, M. Macejková, A. Tarko, Z. Fabova, S. Alwasel and A. H. Harrath, Buckwheat, rooibos, and vitex extracts can mitigate adverse effects of xylene on ovarian cells in vitro, Environ. Sci. Pollut. Res. Int., 2021, 28, 7431–7439 CrossRef CAS PubMed.
- A. V. Sirotkin, M. Radosová, A. Tarko, Z. Fabova, I. Martín-García and F. Alonso, Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals, Nanomaterials, 2020, 10, 1–14 CrossRef PubMed.
- S. H. Kim, C. B. Cui, I. J. Kang, S. Y. Kim and S. S. Ham, Cytotoxic effect of buckwheat (Fagopyrum esculentum Moench) hull against cancer cells, J. Med. Food, 2007, 10, 232–238 CrossRef PubMed.
- H. Wang, S. Liu, Y. Cui, Y. Wang, Y. Guo, X. Wang, J. Liu and C. Piao, Hepatoprotective effects of flavonoids from common buckwheat hulls in type 2 diabetic rats and HepG2 cells, Food Sci. Nutr., 2021, 9, 4793–4802 CrossRef CAS PubMed.
- T. G. Nam, T. G. Lim, B. H. Lee, S. Lim, H. Kang, S. H. Eom, M. Yoo, H. W. Jang and D. O. Kim, Comparison of Anti-Inflammatory Effects of Flavonoid-Rich Common and Tartary Buckwheat Sprout Extracts in Lipopolysaccharide-Stimulated RAW 264.7 and Peritoneal Macrophages, Oxid. Med. Cell. Longevity, 2017, 2017, 9658030 Search PubMed.
- Y. J. Lee, K. J. Kim, K. J. Park, B. R. Yoon, J. H. Lim and O. H. Lee, Buckwheat (Fagopyrum esculentum M.) sprout treated with methyl jasmonate (MeJA) improved anti-adipogenic activity associated with the oxidative stress system in 3T3-L1 adipocytes, Int. J. Mol. Sci., 2013, 14, 1428–1442 CrossRef CAS PubMed.
- J. M. Curran, D. M. Stringer, B. Wright, C. G. Taylor, R. Przybylski and P. Zahradka, Biological response of hepatomas to an extract of Fagopyrum esculentum M. (buckwheat) is not mediated by inositols or rutin, J. Agric. Food Chem., 2010, 58, 3197–3204 CrossRef CAS PubMed.
- A. Bordoni, F. Danesi, M. Di Nunzio, A. Taccari and V. Valli, Ancient wheat and health: a legend or the reality? A review on KAMUT khorasan wheat, Int. J. Food Sci. Nutr., 2017, 68, 278–286 CrossRef PubMed.
- P. Thakur, K. Kumar and H. S. Dhaliwal, Nutritional facts, bio-active components and processing aspects of pseudocereals: A comprehensive review, Food Biosci., 2021, 42, 101170 CrossRef CAS.
- C. Martínez-Villaluenga, E. Peñas and B. Hernández-Ledesma, Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods, Food Chem. Toxicol., 2020, 137, 111178 CrossRef PubMed.
- J. H. Chiang, X. Y. Hua, A. H. M. Yu, E. W. Y. Peh, E. E. See and C. Jeyakumar Henry, A Review on Buckwheat and Its Hypoglycemic Bioactive Components in Food Systems, Food Rev. Int., 2023, 39, 6362–6386 CrossRef CAS.
- J. A. Giménez-Bastida and H. Zieliński, Buckwheat as a Functional Food and Its Effects on Health, J. Agric. Food Chem., 2015, 63, 7896–7913 CrossRef PubMed.
- M. Kreft, Buckwheat phenolic metabolites in health and disease, Nutr. Res. Rev., 2016, 29, 30–39 CrossRef CAS PubMed.
- F. Zhu, Buckwheat proteins and peptides: Biological functions and food applications, Trends Food Sci. Technol., 2021, 110, 155–167 CrossRef CAS.
- V. Melini, F. Melini and R. Acquistucci, Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta, Antioxidants, 2020, 9, 1–30 Search PubMed.
- R. Tsao, Chemistry and biochemistry of dietary polyphenols, Nutrients, 2010, 2, 1231–1246 CrossRef CAS PubMed.
- E. Brglez Mojzer, M. Knez Hrnčič, M. Škerget, Ž. Knez and U. Bren, Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects, Molecules, 2016, 21, 1–38 CrossRef PubMed.
- S. M. Borgonovi, E. Chiarello, F. Pasini, G. Picone, S. Marzocchi, F. Capozzi, A. Bordoni, A. Barbiroli, A. Marti, S. Iametti and M. Di Nunzio, Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum), Foods, 2023, 12, 1–20 CrossRef PubMed.
- M. Rudrapal, S. J. Khairnar, J. Khan, A. B. Dukhyil, M. A. Ansari, M. N. Alomary, F. M. Alshabrmi, S. Palai, P. K. Deb and R. Devi, Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action, Front. Pharmacol., 2022, 13, 806470 CrossRef CAS PubMed.
- M. Platzer, S. Kiese, T. Herfellner, U. Schweiggert-Weisz, O. Miesbauer and P. Eisner, Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays, Molecules, 2021, 26, 1–17 CrossRef PubMed.
- M. Platzer, S. Kiese, T. Tybussek, T. Herfellner, F. Schneider, U. Schweiggert-Weisz and P. Eisner, Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study, Front. Nutr., 2022, 9, 882458 CrossRef PubMed.
- P. R. Johnsen, C. Pinna, L. Mattio, M. B. Strube, M. Di Nunzio, S. Iametti, S. Dallavalle, A. Pinto and H. Frøkiær, Investigation of the Effects of Monomeric and Dimeric Stilbenoids on Bacteria-Induced Cytokines and LPS-Induced ROS Formation in Bone Marrow-Derived Dendritic Cells, Int. J. Mol. Sci., 2023, 24, 1–16 Search PubMed.
- T. O. Ajiboye, Y. O. Komolafe, O. B. Oloyede, S. M. Ogunbode, M. D. Adeoye, I. O. Abdulsalami and Q. O. Nurudeen, Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats, Food Sci. Hum. Wellness, 2013, 2, 39–45 CrossRef.
- M. Lončarić, I. Strelec, T. Moslavac, D. Šubarić, V. Pavić and M. Molnar, Lipoxygenase Inhibition by Plant Extracts, Biomolecules, 2021, 11, 1–17 CrossRef PubMed.
- K. Owczarek and U. Lewandowska, The Impact of Dietary Polyphenols on COX-2 Expression in Colorectal Cancer, Nutr. Cancer, 2017, 69, 1105–1118 CrossRef CAS PubMed.
- J.-X. Li, R. Tian and N. Lu, Quercetin Attenuates Vascular Endothelial Dysfunction in Atherosclerotic Mice by Inhibiting Myeloperoxidase and NADPH Oxidase Function, Chem. Res. Toxicol., 2023, 36, 260–269 Search PubMed.
- M. Yousefian, N. Shakour, H. Hosseinzadeh, A. W. Hayes, F. Hadizadeh and G. Karimi, The natural phenolic compounds as modulators of NADPH oxidases in hypertension, Phytomedicine, 2019, 55, 200–213 CrossRef CAS PubMed.
- L. Liu, L. Zhang, L. Ren and Y. Xie, Advances in structures required of polyphenols for xanthine oxidase inhibition, Food Front., 2020, 1, 152–167 CrossRef.
- N. G. T. Meneses, S. Martins, J. A. Teixeira and S. I. Mussatto, Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer's spent grains, Sep. Purif. Technol., 2013, 108, 152–158 CrossRef CAS.
- A. Dobrinčić, M. Repajić, I. E. Garofulić, L. Tuđen, V. Dragović-Uzelac and B. Levaj, Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols, Processes, 2020, 8, 1008 CrossRef.
- H. Deguchi, T. Fujii, S. Nakagawa, T. Koga and K. Shirouzu, Analysis of cell growth inhibitory effects of catechin through MAPK in human breast cancer cell line T47D, Int. J. Oncol., 2002, 21, 1301–1305 CAS.
- M. Menegazzi, S. Mariotto, M. Dal Bosco, E. Darra, N. Vaiana, K. Shoji, A. A. Safwat, J. D. Marechal, D. Perahia, H. Suzuki and S. Romeo, Direct interaction of natural and synthetic catechins with signal transducer activator of transcription 1 affects both its phosphorylation and activity, FEBS J., 2014, 281, 724–738 CrossRef CAS PubMed.
- F. Y. Fan, L. X. Sang and M. Jiang, Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease, Molecules, 2017, 22, 1–29 Search PubMed.
- C.-H. Tang, J. Peng, D.-W. Zhen and Z. Chen, Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates, Food Chem., 2009, 115, 672–678 CrossRef CAS.
- A. C. Sullivan, P. Pangloli and V. P. Dia, Kafirin from Sorghum bicolor inhibition of inflammation in THP-1 human macrophages is associated with reduction of intracellular reactive oxygen species, Food Chem. Toxicol., 2018, 111, 503–510 CrossRef CAS PubMed.
- E. Lima-Cabello, S. Morales-Santana, R. C. Foley, S. Melser, V. Alché, K. H. M. Siddique, K. B. Singh, J. D. Alché and J. C. Jimenez-Lopez, Ex vivo and in vitro assessment of anti-inflammatory activity of seed β-conglutin proteins from Lupinus angustifolius, J. Funct. Foods, 2018, 40, 510–519 CrossRef CAS.
- M. Malaguti, G. Dinelli, E. Leoncini, V. Bregola, S. Bosi, A. F. Cicero and S. Hrelia, Bioactive peptides in cereals and legumes: agronomical, biochemical and clinical aspects, Int. J. Mol. Sci., 2014, 15, 21120–21135 CrossRef CAS PubMed.
- E. G. de Mejia and V. P. Dia, Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-kappaB pathway in the macrophage, Peptides, 2009, 30, 2388–2398 CrossRef PubMed.
- P.-Y. Huang and C.-C. Hsieh, Immunomodulatory effects of seed peptide lunasin in RAW264.7 macrophages in obese microenvironments, J. Funct. Foods, 2023, 108, 105719 CrossRef CAS.
- B. Hernández-Ledesma, C. C. Hsieh and B. O. de Lumen, Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages, Biochem. Biophys. Res. Commun., 2009, 390, 803–808 CrossRef PubMed.
- V. P. Dia, W. Wang, V. L. Oh, B. O. d. Lumen and E. G. de Mejia, Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity, Food Chem., 2009, 114, 108–115 CrossRef CAS.
- A. Cam and E. G. de Mejia, RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin, Mol. Nutr. Food Res., 2012, 56, 1569–1581 CrossRef CAS PubMed.
- I. Shimaoka, H. Tomotake, J. Kayashita, F. Yokoyama, M. Nakajoh and N. Kato, A Buckwheat Protein Product Suppresses Gallstone Formation and Plasma Cholesterol More Strongly than Soy Protein Isolate in Hamsters, J. Nutr., 2000, 130, 1670–1674 CrossRef PubMed.
- N. Yang, C. Li, K. Zhang, R. Jiao, K. Ma, R. Zhang, G. Ren and Z.-Y. U. Chen, Hypocholesterolemic activity of buckwheat flour is mediated by increasing sterol excretion and down-regulation of intestinal NPC1L1 and ACAT2, J. Funct. Foods, 2013, 6, 311–318 CrossRef.
- S. Fernández-Tomé and B. Hernández-Ledesma, Gastrointestinal Digestion of Food Proteins under the Effects of Released Bioactive Peptides on Digestive Health, Mol. Nutr. Food Res., 2020, 64, 2000401 CrossRef PubMed.
- Y. Ma and Y. L. Xiong, Antioxidant and Bile Acid Binding Activity of Buckwheat Protein in Vitro Digests, J. Agric. Food Chem., 2009, 57, 4372–4380 CrossRef CAS PubMed.
- Y. Ma, Y. L. Xiong, J. Zhai, H. Zhu and T. Dziubla, Fractionation and evaluation of radical-scavenging peptides from in vitro digests of buckwheat protein, Food Chem., 2010, 118, 582–588 CrossRef CAS PubMed.
- G. Yu, F. Wang, B. Zhang and J. Fan, In vitro inhibition of platelet aggregation by peptides derived from oat (Avena sativa L.), highland barley (Hordeum vulgare Linn. var. nudum Hook. f.), and buckwheat (Fagopyrum esculentum Moench) proteins, Food Chem., 2016, 194, 577–586 CrossRef CAS PubMed.
- F. Wang, G. Yu, Y. Zhang, B. Zhang and J. Fan, Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Oat (Avena sativa L.), Buckwheat (Fagopyrum esculentum), and Highland Barley (Hordeum vulgare trifurcatum (L.) Trofim) Proteins, J. Agric. Food Chem., 2015, 63, 9543–9549 CrossRef CAS PubMed.
- P. Ferranti, C. Nitride, M. A. Nicolai, G. Mamone, G. Picariello, A. Bordoni, V. Valli, M. Di Nunzio, E. Babini, E. Marcolini and F. Capozzi, In vitro digestion of Bresaola proteins and release of potential bioactive peptides, Food Res. Int., 2014, 63, 157–169 CrossRef CAS.
- S. Guha and K. Majumder, Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review, J. Food Biochem., 2019, 43, e12531 CrossRef PubMed.
- Y. Wang, R. Chandra, L. A. Samsa, B. Gooch, B. E. Fee, J. M. Cook, S. R. Vigna, A. O. Grant and R. A. Liddle, Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor, Am. J. Physiol.: Gastrointest. Liver Physiol., 2011, 300, G528–G537 CrossRef CAS PubMed.
- S. Nakajima, T. Hira, Y. Eto, K. Asano and H. Hara, Soybean beta 51–63 peptide stimulates cholecystokinin secretion via a calcium-sensing receptor in enteroendocrine STC-1 cells, Regul. Pept., 2010, 159, 148–155 CrossRef CAS PubMed.
- T. Hira, S. Nakajima, Y. Eto and H. Hara, Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells, FEBS J., 2008, 275, 4620–4626 CrossRef CAS PubMed.
- S. Mitra, A. M. Tareq, R. Das, T. B. Emran, F. Nainu, A. J. Chakraborty, I. Ahmad, T. E. Tallei, A. M. Idris and J. Simal-Gandara, Polyphenols: A first evidence in the synergism and bioactivities, Food Rev. Int., 2023, 39, 4419–4441 CrossRef CAS.
- R. Pezzani, B. Salehi, S. Vitalini, M. Iriti, F. A. Zuñiga, J. Sharifi-Rad, M. Martorell and N. Martins, Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective, Medicina, 2019, 55, 1–16 CrossRef PubMed.
- K. R. Patel, E. Scott, V. A. Brown, A. J. Gescher, W. P. Steward and K. Brown, Clinical trials of resveratrol, Ann. N. Y. Acad. Sci., 2011, 1215, 161–169 CrossRef CAS PubMed.
- G. Pereira-Caro, M. N. Clifford, T. Polyviou, I. A. Ludwig, H. Alfheeaid, J. M. Moreno-Rojas, A. L. Garcia, D. Malkova and A. Crozier, Plasma pharmacokinetics of (poly)phenol metabolites and catabolites after ingestion of orange juice by endurance trained men, Free Radical Biol. Med., 2020, 160, 784–795 CrossRef CAS PubMed.
- M. Di Nunzio, C. Loffi, S. Montalbano, E. Chiarello, L. Dellafiora, G. Picone, G. Antonelli, T. Tedeschi, A. Buschini, F. Capozzi, G. Galaverna and A. Bordoni, Cleaning the Label of Cured Meat; Effect of the Replacement of Nitrates/Nitrites on Nutrients Bioaccessibility, Peptides Formation, and Cellular Toxicity of In Vitro Digested Salami, Int. J. Mol. Sci., 2022, 23, 12555 CrossRef CAS PubMed.
- N. Gupta and S. S. Bhagyawant, Bioactive peptide of Cicer arietinum L. induces apoptosis in human endometrial cancer via DNA fragmentation and cell cycle arrest, 3 Biotech., 2021, 11, 63 CrossRef PubMed.
- M. Xie, D. Liu and Y. Yang, Anti-cancer peptides: classification, mechanism of action, reconstruction and modification, Open Biol., 2020, 10, 200004 CrossRef CAS PubMed.
- P. B. Bhosale, S. E. Ha, P. Vetrivel, H. H. Kim, S. M. Kim and G. S. Kim, Functions of polyphenols and its anticancer properties in biomedical research: a narrative review, Transl. Cancer Res., 2020, 9, 7619–7631 CrossRef CAS PubMed.
- K. Sak, Cytotoxicity of dietary flavonoids on different human cancer types, Pharmacogn. Rev., 2014, 8, 122–146 CrossRef CAS PubMed.
- F. Antognoni, R. Mandrioli, A. Bordoni, M. Di Nunzio, B. Viadel, E. Gallego, M. P. Villalba, L. Tomás-Cobos, D. L. Taneyo Saa and A. Gianotti, Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads, Nutrients, 2017, 9, 1–17 CrossRef PubMed.
- X. Gong, Q. An, L. Le, F. Geng, L. Jiang, J. Yan, D. Xiang, L. Peng, L. Zou, G. Zhao and Y. Wan, Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production, Crit. Rev. Food Sci. Nutr., 2022, 62, 2855–2871 CrossRef CAS PubMed.
- O. Kadiri, A review on the status of the phenolic compounds and antioxidant capacity of the flour: Effects of cereal processing, Int. J. Food Prop., 2017, 20, S798–S809 CrossRef CAS.
- K. Rafińska, O. Wrona, A. Krakowska-Sieprawska, J. Walczak-Skierska, A. Kiełbasa, Z. Rafiński, P. Pomastowski, M. Kolankowski and B. Buszewski, Enzyme-assisted extraction of plant material – New functional aspects of the process on an example of Medicago sativa L, Ind. Crops Prod., 2022, 187, 115424 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |