 Open Access Article
Open Access ArticleBioaccumulation and human risk assessment of inorganic nanoparticles in aquaculture species†
Cristian
Suárez-Oubiña
 a,
Paloma
Herbello-Hermelo
a,
Paloma
Herbello-Hermelo
 a,
Natalia
Mallo
a,
Natalia
Mallo
 b,
María
Vázquez
b,
María
Vázquez
 b,
Santiago
Cabaleiro
b,
Santiago
Cabaleiro
 b,
Raquel
Domínguez-González
b,
Raquel
Domínguez-González
 a,
Antonio
Moreda-Piñeiro
a,
Antonio
Moreda-Piñeiro
 *a and
Pilar
Bermejo-Barrera
*a and
Pilar
Bermejo-Barrera
 *a
*a
aTrace Element, Spectroscopy and Speciation Group (GETEE), Institute of Materials (iMATUS), Department of Analytical Chemistry, Nutrition and Bromatology, Faculty of Chemistry, Universidade de Santiago de Compostela, Avenida das Ciencias, s/n, 15782, Santiago de Compostela, Spain. E-mail: antonio.moreda@usc.es; pilar.bermejo@usc.es
bCentro Tecnológico del Cluster de la Acuicultura (CETGA), Punta Couso S-N, Ribeira 15965, Spain
First published on 14th June 2024
Abstract
The escalating use of inorganic nanoparticles (NPs) in various applications raises concerns regarding their potential environmental release and subsequent bioaccumulation in the food chain, posing a risk to human health. This study aimed to assess the bioaccumulation potential of titanium dioxide (TiO2) and silver (Ag) NPs in three commercially relevant aquatic species: sea bream, sea bass, and Japanese carpet shell, and evaluate the associated human health risks through dietary exposure. Bioaccumulation patterns were evaluated in target organs (liver, kidney, and muscle) of sea bream and sea bass following dietary exposure to varying concentrations of NPs (0.25–1.5 mg kg−1) for extended durations (up to 90 days). While moderate bioaccumulation was observed in non-edible organs like kidneys and livers, no significant accumulation was detected in the muscle tissue, even at high exposure levels. Conversely, bioaccumulation of both TiO2 and Ag NPs was evident in the soft tissues of Japanese carpet shell (maximum concentrations: 2.5 × 1010 g−1 for Ag NPs and 8.0 × 106 g−1 for TiO2 NPs). In vitro studies utilizing the Caco-2 human intestinal model revealed limited transcellular transport of NPs from both fish and shellfish muscle tissue (less than 34% for TiO2 NPs in sea bream and less than 61% and 4% for TiO2 NPs and Ag NPs, respectively, in Japanese carpet shell). These findings suggest that, while bioaccumulation may occur in certain species and organs, the human health risk associated with dietary exposure to NPs from commonly consumed fish appears to be low due to limited intestinal uptake. However, further research is necessary to elucidate the long-term consequences of chronic exposure and potential health effects.
Environmental significanceThe increase in the use of nanomaterials in several manufactured goods and the potential release of nanomaterials into the environment concerns the scientific community due to the possible risk to humans and the marine ecosystem. We have studied the bioaccumulation of titanium dioxide and silver nanoparticles (TiO2 NPs and Ag NPs) in aquaculture species such as sea bass (Dicentrarchus labrax), sea bream (Sparus aurata), and Japanese carpet shell (Ruditapes philippinarum). Results have revealed that TiO2 NPs and Ag NPs are not bioaccumulated in fish's flesh, whereas moderate bioaccumulation was observed in the kidneys and liver (non-edible parts). However, Ag NPs and TiO2 NPs can be bioaccumulated in Japanese carpet shell, although human in vitro bioavailability studies have shown low bioavailability. |
Introduction
Nanotechnology is now an emerging area of study with many applications in science and technology. According to the European Commission (EC) the term “nanomaterial” embraces all materials specified by EC Recommendation 2022/C229/01 of 10 June 2022 as “a natural, incidental or manufactured material consisting of solid particles that are present, either on their own or as identifiable constituent particles in aggregates or agglomerates, and where 50% or more of these particles in the number”.1 Based on the StatNano database,2 silver nanoparticles (Ag NPs) and titanium dioxide nanoparticles (TiO2 NPs) are widely used in several industrial sectors such as cosmetics, textile, and medicine. In the case of Ag NPs, about 50% of all nanomaterials-based products used in medicine contain Ag NPs in their formulations.2Due to the large-scale production and use of NPs their release and presence in the environment is expected. However, the assessment of the true impacts of the presence of NPs in the aquatic environment is difficult because the released NPs vary their characteristics when interacting with natural components of aquatic ecosystems. As examples, dissolved compounds in both lake and seawater promote TiO2 NPs aggregation and sedimentation,3 whereas interactions between Ag NPs and some dissolved organic compounds with oxidative properties promote dissolution (generation of hazardous dissolved ions).4 In this regard, some studies have suggested that Ag NPs in environmentally relevant concentrations are not stable in seawater for more than three day.5 Since NPs are emerging pollutants, NPs background levels in seawater are nowadays low. Besides, although fish feeding with fish feed containing NPs is not usual, Khosravi-Katuli et al.6 have reported that animals in aquaculture facilities could be exposed to NPs if nanomaterials are used for seawater treatment and fishpond sterilization, and also by accidental discharges near the catchment area. Therefore, there is concern about the potential effect of NPs in aquaculture species and several research have explored this scenario by performing exposure experiments by keeping the specimens in water containing NPs suspensions.7–10 Moreover, cultured animals could be also exposed to NPs by ingestion since NPs could be accidentally or intentionally (use of feed additives) present in some feed's formulations.11–13
Chemical composition, size, shape, surface coating or modification, solubility properties, and other physical and chemical properties are important factors that condition the danger of NPs.14,15 To increase the available data about the possible toxicity of NPs, the European Food Safety Agency (EFSA) has published a guidance on the risk assessment of nanomaterials16 which encompass several assays for nanotoxicity assessment (bio-accessibility and bioavailability, among others). Bioaccumulation can be defined as the build-up of chemicals, usually harmful, in the body of an organism from different exposure sources (mainly water, air, and diet) that are not metabolized or excreted, and consequently accumulate in the organisms over time.17 Regarding human consumption, the term bio-accessibility refers to the fraction of a compound that is released from the food matrix in the gastrointestinal tract and consequently, is available for absorption. In addition, the term bioavailability names the fraction of a compound that can be taken up by the body, enter circulation and being able to have an active effect.16,18 The knowledge of these three parameters enables to assess the risk related to pollutants in the environment and humans. Caco-2 cells are typically used as a model to perform bioavailability studies since they are derived from human colorectal adenocarcinoma and exhibit remarkable morphological and physiological similarities to the human intestine.19 Previous studies with Caco-2 cultures and Caco-2/HT29 cultures for transcellular transport and uptake of NPs can be found elsewhere.20–23
Regarding marine organisms, the small size and rapid development of zebrafish embryos make these species the preferred candidates for bioaccumulation studies.24 In addition, some investigations have been conducted in fish organs, such as rainbow trout,25 goldfish,26,27 and zebrafish,28 which have shown potential toxicity and effective bioaccumulation after NPs exposure. Other studies have stated that the gills, intestine, liver, and brain are the most affected organs in fish species. Oxidative stress is a potential mechanism of NPs toxicity in fish, as well as the induction of new enzymes involved in antioxidant defences.29 However, the concentration of metals and NPs in the natural environment (especially in the aquatic environment) is quite low, far from the high dose conditions required for acute toxicity testing.
The aim of this research has been the evaluation of Ag NPs and TiO2 NPs bioaccumulation in cultured fish sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata), and in Japanese carpet shell (Ruditapes philippinarum) through controlled-NPs exposure experiments. In addition, human risk assessment of potential consumption of marine products with high Ag NPs and TiO2 NPs contents has also been studied. To this end, in vitro bio-accessibility and bioavailability (Caco-2 cells model for transcellular transport assessment) assays were carried out.
Material and methods
Sea bream, sea bass, and Japanese carpet shell exposure trials and sample preparation
Exposure trials for sea bass (Dicentrarchus labrax), sea bream (Sparus aurata), and Japanese carpet shell (Ruditapes philippinarum) with NPs were carried out by personnel qualified in animal experimentation, in authorized facilities of Centro Tecnológico de Acuicultura, CETGA (Ribeira, A Coruña, Spain). All experimental procedures were carried out in accordance with European Union and Spanish Regulations (Council Directive 2010/63/EU (European Union, 2010) and R.D. 53/2013 (BOE, 2013), respectively), for the protection of animals used for experimental purposes.Regarding assays with sea bass and sea bream, TiO2 NPs and Ag NPs were incorporated to commercial fish feed pellets (details are given in ESI†) following the method described in previous investigation,30 whereas Japanese carpet shells were fed with microalgae mixture (Isochrysis galbana (T-ISO) and Phaeodactylum tricornutum (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v)).
50, v/v)).
Experiments were performed with fifty individuals in 400 L open circuit tanks (sea bass, average initial weight of 121.6 g), and one hundred and twenty individuals in open circuit 300 L tanks (sea bream, average initial weight of 7.7 g) for 90 days. Sampling was performed each 15 days obtaining exposure times of 0 (experiment beginning), 15, 30, 45, 60, 75, and 90 days. Different exposure NPs concentrations were tested for both cultured species: 0 (control tanks), and 0.25, 0.75 and 1.5 mg kg−1 (concentration referred as the mg of Ag NPs or TiO2 NPs per kg of fish). Regarding Japanese carpet shell specimens, assays were carried out with forty individuals in 50 L closed circuit tanks. Detailed information regarding exposure trials and sample preparation can be found in ESI† section.
Microwave assisted acid digestion and enzymatic hydrolysis procedures
Microwave assisted acid digestion was performed for further assessing total silver and titanium contents. The procedure consisted of treating in triplicate approximately 1.000 g of homogenised tissue (muscle + skin from fish and soft tissues from Japanese carpet shell), with a nitric acid/hydrogen peroxide mixture under microwave energy (detailed information can be found in ESI† section). A similar procedure was performed for liver and kidney tissues from fish, although only one replicate (0.1500 g) per specimen was used for digestion (small sample size).The isolation of Ag NPs and TiO2 NPs from seafood tissues was performed by enzymatic hydrolysis (pancreatin plus lipase) in accordance with previous developments31,32 (detailed information can be found in ESI† section).
Culinary treatments: grilling and boiling
Studies with liver and kidney tissues from fish were discarded since fish's kidney/liver do not offer nutritional interest. Because of the low Ag bioaccumulation in sea bass's muscle + skin and Ti bioaccumulation in sea bream's muscle + skin, the effect of the culinary treatment was only carried out for muscle + skin from sea bream specimens exposed to 1.5 mg TiO2 NPs kg−1 for 75 days. Sea bream muscle + skin from unexposed specimens (control at 90 days) was also used in the study for comparative purposes. Regarding Japanese carpet shell, the effect of grilling was only tested in samples obtained after 28-day exposure to Ag NPs (0.1 and 1.0 mg kg−1), and in samples from experiments with the maximum TiO2 NPs dose (1.0 mg kg−1) after 21-day and 28-day exposure.For all cases, two sample pools were prepared, and total Ti and Ag contents (and TiO2 NPs and Ag NPs concentrations) were assessed in raw and cooked samples in duplicate (the remaining pooled samples were kept for further bio-accessible and bioavailable experiments). Grilling and boiling were carried out without using oil and spices. In accordance with the literature,33 grilling treatment was done for 5.0 min, allowing then grilled samples to cool at room temperature. Boiling procedure for sea bream's flesh was carried out by applying heat to 300 mL of ultrapure water inside a cooking pot. Temperature (boiling temperature within the 90–100 °C range) was controlled with a thermometer, and once the boiling temperature was achieved, the fish tissues were immersed a cooked for 10 min. Then, samples were placed onto Petri dishes and left to cool at room temperature and then at 30–40 °C in an oven to dry the cooked sample.
In vitro digestion procedure: bio-accessibility assays
An in vitro digestion approach that mimics the environment of the stomach (use of pepsin at acid pH) and intestines (use of pancreatin and bile salts at neutral pH) was used as a model of human gastrointestinal process.34 Detailed information can be found in ESI† section.The bio-accessibility ratio was calculated according to eqn (1):18
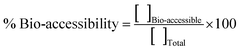 | (1) |
Caco-2 cellular transport assays
After Caco-2 monolayer development (see details in ESI† section), cellular transport was performed by loading 1.5 mL of bio-accessible fraction (section 2.4), previously denatured and adjusting the osmolarity at 280–300 mΩ cm2, at the basolateral chamber of six-well Transwell® (see details in ESI† section). The transwell plates were then placed in a temperature-controlled environment (37 °C, 95% relative humidity, and 5% CO2 flow) for 2.0 h (see detail in ESI† section).The basolateral and apical [2.0 mL of Hanks' Balanced Salt Solution (HBSS)] solutions were carefully removed and kept for analysis. Each bio-accessible fraction was subjected to the cellular transport procedure in triplicate which allows six independent measurements (two bio-accessible fractions per sample). At least two blanks were subjected to the same process in each set of samples.
The bioavailability (transcellular transport across the intestinal epithelium) of total Ti and Ag, and TiO2 NPs and Ag NPs was obtained by applying eqn (2):18
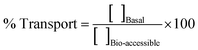 | (2) |
ICP-MS measurements
The determination of the total Ag and Ti contents in the acid digests, bio-accessible fractions and apical and basolateral were performed under the ICP-MS operating conditions detailed in Table 1. Details on instrument components as well as daily performance are given in ESI† section. Rhodium was used as an internal standard for Ag determination under KED work mode using helium at 4.5 mL min−1 as a collision gas, whereas scandium was the selected internal standard for Ti determination under Dynamic Reaction Cell technology by using ammonia at 1.0 mL min−1 as a reaction gas (the ammonia adduct Ti(NH)(NH3)4, mass-charge ratio of 131, was recorded). The standard addition method was used for determinations covering range concentrations from 0.1 to 10 μg L−1 for Ag and Ti. The limit of detection and quantification of the method are listed in Table S1 (ESI†).| Spray chamber type | QuartzCyclonic |
|---|---|
| PC3x Peltier Cooler System | 4 °C |
| Nebulizer type | Concentric Meinhard™ |
| RF power (W) | 1600 |
| Ar plasma gas flow rate (L min−1) | 15 |
| Ar auxiliary gas flow rate (L min−1) | 1.2 |
| Ar nebulizer gas flow (L min−1) | 1.14 |
| Sample loop (μL) | 100 |
| Dwell time (ms) | 50 |
| Analyte (m/z) | Ag (107) |
| Internal standard (m/z) | Rh (103) |
| Mode | KED or collision mode |
| Helium flow rate (mL min−1) | 4.5 |
| Analyte (m/z) | Ti (131) |
| Internal standard (m/z) | Sc (45) |
| Mode | Dynamic reaction cell technology |
| Ammonia flow rate (mL min−1) | 1.0 |
| Ion-product registered | 48Ti(NH)(NH3)4 |
| Rejection parameter q | 0.20 |
| Quadrupole ion deflector (V) | Set for maximum ion transmission |
Single particle-ICP-MS measurements
The determinations for Ag NPs and TiO2 NPs aiming particle number concentrations and size distributions were performed by ICP-MS operating in the single particle mode (spICP-MS) under operating conditions summarized in Table 2. Details on operating conditions as well as daily performance are given in ESI† section. Transport efficiency (TE%) was assessed by the particle frequency method (see details in ESI† section), resulting in values close to 8.0%.| Analyte (m/z) | Ti (131) |
|---|---|
| Density (g cm−3) | 4.23 |
| Mass fraction | 59.90% |
| Sample flow rate (mL min−1) | ≈0.18 |
| Transport efficiency (%) | ≈8% |
| Dwell time (μs) | 100 |
| Mode | Dynamic reaction cell technology |
| Ammonia flow rate (mL min−1) | 0.75 |
| Ion-product registered | 48Ti(NH)(NH3)4 |
| Rejection parameter q | 0.20 |
| Quadrupole ion deflector (V) | Set for maximum ion transmission |
| Analyte (m/z) | Ag (107) |
|---|---|
| Density (g cm−3) | 10.49 |
| Mass fraction | 100% |
| Sample flow rate (mL min−1) | ≈0.20 |
| Transport efficiency (%) | ≈8% |
| Dwell time (μs) | 50 |
| Mode | Standard |
| Quadrupole ion deflector (V) | Set for maximum ion transmission |
Calibrations were performed using ultrapure water and 1.0% (v/v) glycerol covering ionic Ti and Ag concentrations within the 0.1–10 μg L−1 range. Several reagent blanks were also analysed throughout the work. Extracts containing TiO2 NPs and/or Ag NPs were dispersed before analysis by using ultrasound. The limit of detection (number concentration and size) and quantification (number concentration) of the method are listed in Table S1 (ESI†).
Statistical analysis
Fish and clam growth parameters (weight gain (WG), feed conversion ratio (FCR), and specific growth rate (SGR)) were analysed using one-way analysis of variance (ANOVA) following three replicates per treatment (n = 3). Statistically significant differences (p < 0.05) between groups were identified using Fisher's Least Significant Difference (LSD) post-hoc test (STATGRAPHICS Centurion XVI). Mean values with standard deviations are presented in Tables S2 and S3.†Total Ag and Ti concentrations, as well as Ag NPs and TiO2 NPs levels, were also analysed by one-way ANOVA after different cooking procedures. Similar to growth parameters, statistically significant differences (p < 0.05) were identified.
Results and discussion
Several studies regarding NPs bioaccumulation in biota under controlled laboratory conditions have been reported. The conclusions obtained from these studies depend greatly on the fish/mollusc species under investigation (carp fish,7,35,36 rainbow trout,9,37 silver barb,10 Persian sturgeon,38 zebrafish,39 goldfish,26,27 turbot,30,40 mussel,41–43 red swamp crayfish,44 clam,45–48 and oyster42), as well as the NPs exposure doses (from μg L−1 to mg L−1) and the exposure time (from hours to several days). On the other hand, water salinity9,38 and the presence of organic matter35,39 have also been found as important parameters affecting the bioaccumulation trends. However, there are two key features to highlight when interpreting the results of these studies. First, the way in which NPs are administered to animals. In most cases, NPs are added to the water in the tanks so, depending on the salinity of the water, the added NPs will be less available for the animals (the higher the salinity of the water, the higher of NPs agglomeration and settlement at the bottom of the tank9). A more realistic situation would be NPs integration into the feed to ensure their intake. Second, another important point is the analytical methodology used for NPs assessment. Although the animals were exposed to NPs, most of studies assess the total metal content in the animal's tissues (NPs number concentration is not obtained). Therefore, the determination of total metal contents for experiments with NPs such as Ag NPs, which are easily ionised during animal metabolism (and when are suspended in water), leads to un-realistic values of NPs bioaccumulation since a great proportion of the measured bioaccumulated fraction encompasses ionic metal.Bioaccumulation: total Ag and Ti
 | ||
| Fig. 1 Titanium concentrations in sea bream kidney (A), liver (B), and muscle + skin (C) after several exposure conditions. | ||
Regarding liver tissues, Fig. S1 (ESI†) shows that Ag is bioaccumulated from the beginning of the experiment (sampling at 15 days) with maximum bioaccumulation in the middle of the exposure time (between 15 and 60 days) and decreasing at the end of the experiment (sampling at 75 and 90 days) when using the highest exposure doses (0.75 and 1.5 mg kg−1). The maximum Ag bioaccumulation was observed after 30 days of exposure at 0.75 mg kg−1 (4.60 μg Ag g−1, Fig. S1, ESI†). This trend is also observed for Ti bioaccumulation in sea bream's liver (Fig. 1) although the maximum Ti bioaccumulation was observed after 60 days of exposure at 1.5 mg kg−1 (1.14 ± 0.09 μg Ti g−1). Although the exposure time and the fish species were different, maximum bioaccumulation ratios in the middle of the assays have been reported by Xiao et al.39 for zebrafish exposed to Ag NPs, and by Ribeiro et al.26 for goldfish also exposed to Ag NPs. In general, higher Ag bioaccumulation in sea bass's liver (within the 2.0–4.5 μg Ag g−1 range, Fig. S1 – ESI†) than Ti in sea bream's liver (within the 0.5–1.5 μg Ti g−1 range, Fig. 1) were found, and high bioaccumulation was observed when using the highest exposure doses.
However, clear trends for Ag and Ti bioaccumulation in kidney tissues were not found (Fig. S1 – ESI† and Fig. 1) and similar bioaccumulation concentrations can be observed from the middle to the end of the experiment (within the 15–90 days range in the case of Ag, and between 45 and 90 days for Ti mainly when using the highest dose).
The lower Ag and Ti bioaccumulation at large exposure times is not because fish eat less feed, since the weight of the fish increases according to the time of exposure and feeding (Fig. S2 and S3, ESI†). On the other hand, the evaluation of growth parameters (culture conditions) in fish growth (WG, FCR, and SGR) are similar in all cases (Table S2 and S3, ESI†). No significant differences were observed for any parameter, in both studies. Furthermore, no mortality and anomalous behaviour were registered.
These findings agree with the literature, which shows intestine, liver, kidney, and gills as the target organs for Ag NPs and TiO2 NPs bioaccumulation. Therefore, moderate-high Ag bioaccumulation has been reported in carp fish's liver7,36 and gill,36 rainbow trout's liver and kidney,9,37 turbot's liver,30 and in liver, kidney and gill from Persian sturgeon,38 zebrafish39 and goldfish.26 Regarding TiO2 NPs, exposure assays with goldfish have shown Ti bioaccumulation the intestine and gill,27 whereas liver was the target organ in the case of turbot.30,40
 | ||
| Fig. 2 Silver (A), titanium (B), Ag NPs (expressed as Ag mass) (C), and TiO2 NPs (expressed as Ti mass) (D) concentrations in Japanese carpet shells after several exposure conditions. | ||
A decrease in weight (Fig. S6, ESI†) and no variation in shell length (Fig. S7, ESI†) was observed for specimens exposed to Ag NPs, where no significant differences were observed (Table S6 and S7, ESI†). The shells from the specimens exposed to the highest Ag NPs dose were found to be brittle. This finding has also been reported by Liu et al.48 for the freshwater clam Corbicula fluminea, and the authors claim that the easily broken shells after exposure at high Ag NPs doses is consequence of induced calcospherite disintegration phenomena (loss of calcium) by Ag NPs. Nevertheless, no mortalities were observed in both clam assays.
Total Ti contents were found to gradually increase along the exposure trial, and higher bioaccumulation (Fig. 2B) was also observed when exposed at the highest TiO2 NPs dose (1.0 mg kg−1). These findings are quite similar to those reported by Kuehr et al.46 for the freshwater clam Corbicula fluminea using TiO2 NPs of two different sizes. Comparisons with other studies were not possible since most of them assess Ti bioaccumulation41,42,44,45 only at the end of the exposure trials and bioaccumulation trends were not reported.
The weight of the clams exposed to TiO2 NPs were found be constant throughout the exposure trial, but shell length increased slightly (Fig. S4, ESI†). No significant differences were observed between the weight of clams fed with 0, 0.1 and 1 mg TiO2 NPs L−1 at any time point (Table S4, ESI†). However, regarding shell length (Fig. S5, ESI†), significant differences were observed between treated and control groups on days 7 and 14, although such differences disappear on days 21 and 28 (Table S6, ESI†).
In conclusion, results suggest that NPs bioaccumulation in Japanese carpet shells depends on the NPs type, and a greater concern is expected for TiO2 NPs since, in addition to their higher bioaccumulation, TiO2 NPs tend to bioaccumulate in this mollusc over time.
Bioaccumulation: Ag NPs and TiO2 NPs
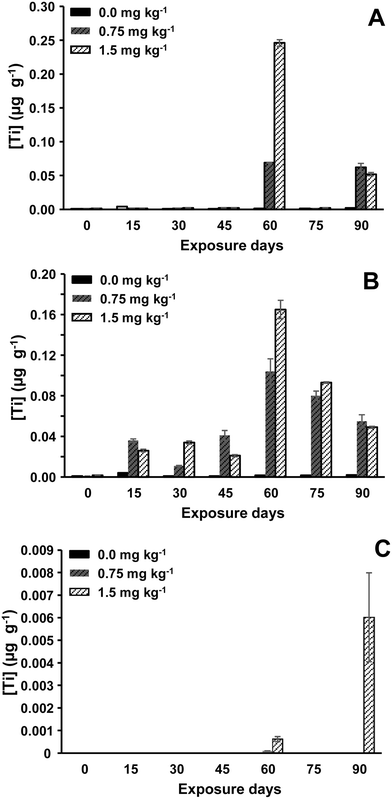 | ||
| Fig. 3 TiO2 NPs concentrations (expressed as Ti mass) in sea bream kidney (A), liver (B), and muscle + skin (C). | ||
Findings showed that Ag NPs bioaccumulation in liver was found to be higher than in the kidney, outcomes that agree with results obtained for total Ag in sea bass (Fig. S1, ESI†). Regarding sea bass's liver tissues, a rapid increase in Ag NPs levels (sampling within 15–45 days) was again observed, followed by a clear Ag NPs concentration decrease. Hence, the highest level of Ag NPs concentration was measured for sea bass's liver tissues sampled after 45 days of exposure at 0.75 mg kg−1 (0.57 ± 0.00043 μg g−1, expressed as Ag mass). High Ag NPs concentrations were also observed after 15 days of exposure at 0.25 mg kg−1 (0.20 ± 0.00032 μg g−1, expressed as Ag mass) and at 0.75 mg kg−1 (0.41 ± 0.00013 μg g−1, expressed as Ag mass). Therefore, Ag NPs bioaccumulation in sea bass's liver appears to be higher at low Ag NPs concentrations and at small exposure times. However, results for sea bass's kidney are quite different, and the highest Ag NPs bioaccumulation was observed for long exposure time and also at the highest Ag NPs concentrations (Fig. S8, ESI†). These findings confirm a more reliable interpretation of NPs bioaccumulation when assessing NPs instead of total metal contents.
Regarding Ag NPs size, Table S8 (ESI†) lists the mean Ag NPs sizes in kidney and liver tissues from sea bass after several Ag NPs doses and exposure times. Mean sizes quite lower than the size used for the exposure assays (100 nm) were measured: mean sizes within the 19–24 nm range for kidney tissue and from 24 to 41 nm in liver tissues. These findings imply that Ag NPs are highly ionized when they are added to the exposure tanks (during the exposure period) and/or during Ag NPs absorption (bioaccumulation) by sea bass specimens.
Lower TiO2 NPs bioaccumulation in sea bream's liver than that found for Ag NPs bioaccumulation in sea bass was observed, showing values of 106–107 particles per gram for TiO2 NPs vs. 109 particles per gram for Ag NPs (mass concentration between 0 and 0.3 μg g−1 for TiO2 NPs vs. 0.02 and 0.57 μg g−1 for Ag NPs). High TiO2 NPs concentrations were measured in sea bream's liver and kidney tissues than in muscle + skin (Fig. 3), and the highest TiO2 NPs levels in the liver were noticed after 60- and 75-days exposure at the highest TiO2 NPs concentrations (0.75 and 1.5 mg kg−1). Similarly, the highest TiO2 NPs levels in sea bream's kidney tissues were also observed at the end of the experiment (sampling at 60 days) and for exposure at 1.5 mg TiO2 kg−1. On the other hand, TiO2 NPs assessment in muscle + skin from sea bream was also possible in sea bream specimens exposed to the largest TiO2 NPs doses and results in Fig. 3 did not show a bioaccumulation trend since the quantified TiO2 NPs number concentrations were close to the limit of detection of the method (6.97 × 105 TiO2 NPs g−1 as listed in Table S1, ESI†).
Finally, mean TiO2 NPs sizes throughout the exposure trial are given in Table S8 (ESI†). The measured mean TiO2 NPs sizes were higher (from 111 to 141 in kidney tissues and between 106 and 161 nm in liver tissues) than the nominal TiO2 NPs size used for feeding the seabream specimens. As explained before, the prepared citrate-45 nm TiO2 NPs shows a high agglomeration degree, which can remain and probably increase when the material is suspended in water and/or is absorbed (bioaccumulated) by the fish. Our results contrast greatly with those reported by Gallocchio et al.,41 who have reported unchanged NP sizes for clams exposed to TiO2 NPs.
Effect of the cooking procedure
Culinary processes, such as grilling and boiling, lead to water loss and therefore, the percentage of moisture of the raw samples must be considered to compare the results (total metal and nanoparticles content in the raw and cooked samples). The calculated moisture percentages are listed in Table S10.† Results for total Ti contents in sea bream samples are given in Table 3 and Fig. S9A, ESI,† whereas results for TiO2 NPs concentrations (expressed as Ag/Ti mass considering the mean size and assuming a solid spherical shape of Ag NPs and TiO2 NPs) are given in Table 3 (Fig. S9B, ESI,† plots the values as number concentrations). Total Ti contents were found to be statistically significant similar (95% confidence interval) when sea bream samples were subjected to grilling (p-value of 0.1873 > 0.05). However, boiling led to a decrease on total Ti contents (the difference was statistically significant at a 95% confidence interval). Regarding TiO2 NPs, both culinary processes led to a decrease on the TiO2 NPs contents (Table 3) and TiO2 NPs were not detected in the boiled sample.| ID sample | Total metal contenta (μg g−1) | Nanoparticles concentrationa,b (μg g−1) | ||||
|---|---|---|---|---|---|---|
| Sample | Bio-accessible fraction | Bio-accessibility ratio (%) | Sample | Bio-accessible fraction | Bio-accessibility ratio (%) | |
| a Expressed as the mean ± SD of three replicates. b Ag NPs/TiO2 NPs concentrations expressed as Ag/Ti mass taking into account the mean size and assuming a solid spherical shape of the Ag NPs/TiO2 NPs. c CA0.1_28 (Japanese carpet shell, 0.1 mg kg−1, 28 days exposure) and CA1.0_28 (Japanese carpet shell, 1.0 mg kg−1, 28 days exposure). d CT1.0_21 (Japanese carpet shell, 1.0 mg kg−1, 21 days exposure) and CT1.0_28 (Japanese carpet shell, 1.0 mg kg−1, 28 days exposure). e SBr1.5_90 (Sea bream, 1.5 mg kg−1, 90 days exposure). f <LOQ. g Not calculated. | ||||||
| Japanese carpet shell (Ag)c | ||||||
| CA0.1_28 raw | 12.8 ± 3.00 | 9.70 ± 0.971 | 76 | 5.95 ± 1.37 | 1.00 ± 0.33 | 17 |
| CA0.1_28 grilled | 11.9 ± 1.90 | 9.27 ± 1.39 | 78 | 0.876 ± 0.107 | 0.570 ± 0.0945 | 65 |
| CA1.0_28 raw | 5.60 ± 2.41 | 4.33 ± 1.93 | 77 | 1.52 ± 0.301 | 0.270 ± 0.00205 | 18 |
| CA1.0_28 grilled | 10.5 ± 4.84 | 9.56 ± 2.43 | 91 | 0.882 ± 0.0667 | 0.501 ± 0.0656 | 57 |
| Japanese carpet shell (Ti)d | ||||||
| CT1.0_21 raw | 3.38 ± 0.53 | 0.696 ± 0.132 | 21 | 0.229 ± 0.0526 | 0.191 ± 0.0226 | 83 |
| CT1.0_21 grilled | 2.95 ± 0.23 | 0.80 ± 0.16 | 27 | 0.0647 ± 0.00390 | 0.0494 ± 0.00448 | 76 |
| CT1.0_28 raw | 6.07 ± 0.887 | 0.922 ± 0.0709 | 15 | 0.466 ± 0.302 | 0.289 ± 0.0897 | 62 |
| CT1.0_28 grilled | 4.18 ± 0.962 | 0.590 ± 0.108 | 14 | 0.168 ± 0.0105 | 0.127 ± 0.00237 | 76 |
| Sea bream (Ti)e | ||||||
| SBr1.5_90 raw | 0.489 ± 0.0212 | 0.376 ± 0.0752 | 77 | 0.124 ± 0.00104 | 0.125 ± 0.0133 | 101 |
| SBr1.5_90 grilled | 0.861 ± 0.405 | 0.227 ± 0.0383 | 27 | 0.00723 ± 0.00517 | 0.00766 ± 0.00562 | 106 |
| SBr1.5_90 boiled | 0.222 ± 0.0354 | 0.0881 ± 0.00762 | 41 | 0.0288 ± 0.00353 | —f | —g |
Regarding Japanese carpet shells (Table 3 and Fig. S10, ESI†), grilling was not found to change the total Ti and Ag contents [the ANOVA test at a 95% significant level showed p-values higher than 0.05 (0.6833 and 0.1916 for total Ag, and 0.2669 and 0.0666 for total Ti)]. However, TiO2 NPs and Ag NPs (expressed as Ag/Ti mass) were lower in grilled samples than those calculated in the raw ones (Table 3).
Human oral bio-accessibility from exposed sea bream and Japanese carpet shell
The total Ti contents in the bio-accessible fractions (Fig. S9A, ESI†) are lower than those found in sea bream's muscle + skin sample (Table 3), which implies a bio-accessibility ratio of 77% in raw sea bream flesh, and lower bio-accessibility ratios (41% and 27%) for cooked sea bream flesh. In general, we can conclude that the fraction of Ti that can be released from the matrix sample under gastro-intestinal conditions is moderate, which is a positive issue regarding human risk assessment. However, results are quite different for TiO2 NPs, and therefore bio-accessible TiO2 NPs, expressed as Ti mass considering the mean size and a solid spherical shape of the TiO2 NPs, in raw and grilled sea bream's flesh led to bio-accessible ratios of 100% (101 and 106% for raw and grilled samples, Table 3).Regarding Japanese carpet shell (Table 3 and Fig. S10, ESI†), moderate bio-accessibility ratios (within the 15–21% and 14–27% ranges for raw and grilled shellfish, respectively) were observed for total Ti, whereas higher bio-accessibilities ratios were obtained for total Ag (76–77% for raw shellfish and 78–91% for grilled pooled samples). Results for Ag NPs bio-accessibility (concentrations referred to Ag mass, Table 3 and Fig. S10, ESI†) were found to be lower in raw shellfish than in grilled samples, and bio-accessibility ratios of 17 and 18% (raw samples) against 35 and 57% (grilled samples) were obtained. However, TiO2 NPs bio-accessibility (expressed as Ti mass) gave similar bio-accessibility ratios for raw and frilled samples (62 and 83% for raw shellfish, and 76% for grilled seafood, as listed in Table 3).
Human oral bioavailability (transcellular transport) from exposed sea bream and Japanese carpet shell
Transcellular transport ratios (bioavailability ratios) for TiO2 NPs, expressed as Ti mass considering the mean size and a solid spherical shape of the TiO2 NP, were 8 and 34% for raw and grilled sea bream, respectively, whereas total Ti transcellular transport ratios were quite higher (87 ± 11 and 67 ± 9% for raw and grilled sea bream samples, respectively). Regarding Japanese carpet shell tissues exposed to TiO2 NPs (1.0 mg kg−1 and 21-day exposure, and 1.0 mg kg−1 and 28-day exposure), Ti transcellular transport ratios were moderate (within the 37–66% and 20–33% ranges for raw and grilled samples, respectively) when assessing total Ti, and also when considering TiO2 NPs (within the 44–61% for raw shellfish and from 34 to 55% for grilled samples). Lower transcellular transport ratios were achieved for Ag in raw and grilled samples: from 7 to 9% for total Ag, and within the 3–4% for Ag NPs. The culinary treatment (grilling) does not appear to alter the transcellular transport ratio for Ag: between 15 to 20% for total Ag, and within the 0.2–1% for Ag NPs.As listed in Table S11, ESI,† Ag NPs sizes are quite similar in the bio-accessible and basolateral fractions (from 30 to 37 nm in the bio-accessible fraction and from 21 to 33 nm in the basolateral solutions). However, the size of TiO2 NPs is dependent on the environment and TiO2 NPs sizes are quite higher in the basolateral fractions (from 110 to 147 nm) than in the bio-accessible fractions (within the 73–121 nm range). The agglomeration phenomena must be favoured by the substances present in the basolateral liquid.
Limitations and future directions
The main limitations of the topic addressed in this work lie in the lack of a standardized protocol to carry out NPs exposure trials. Differences on NPs bioaccumulation ratios can be attributed to the NPs administration procedure. Nanoparticles are directly added to the water in the experimental tanks in most of reviewed papers, being less available to the individuals than when NPs are present in the feed (feed pellets for feeding fish, or microalgae previously exposed to NPs for feeding mollusc). In addition, standardized conditions (doses and exposure times) are needed for more realistic comparisons.Another issue to highlight is the potential differences raised from assessing total metal contents or NPs (particle number concentrations). Most research use ICP-MS that give total metals content (ionic plus nanoparticulate species) and therefore they do not give a realistic measure of NPs bioaccumulation itself (NPs can be ionized in the feed tanks or once they are incorporated by the biota).
Finally, there are not studies of human oral bioavailability of NPs, and studies, mainly using cellular models that offer a more realistic approximation of bioavailability, are needed for an accurate NPs risk assessment. Other important issue is the potential interaction of NPs with other food components which can change the bioavailability ratios. A recent study by Li et al.49 has shown that polyphenols human oral bioavailability is reduced in the presence of TiO2 NPs.
Conclusions
This study investigated the bioaccumulation potential of nanoparticles in commonly consumed fish and shellfish. Controlled dietary exposure revealed moderate bioaccumulation of NPs in non-edible organs (kidneys and livers) of sea bass and sea bream, but minimal presence in their muscle (edible flesh) even at high doses and extended durations (up to 90 days). Conversely, Japanese carpet shells accumulated NPs in their soft tissues. However, this is unlikely to pose a significant health risk under normal environmental conditions, as in vitro studies showed limited human uptake (bioavailability) of NPs from both fish and shellfish, even after cooking. Bioavailability from fish muscle was below 67% for total titanium and 34% for titanium dioxide nanoparticles (TiO2 NPs). Similarly, bioavailability from shellfish was generally low, except for TiO2 NPs in cooked Japanese carpet shells, which showed comparable levels to total titanium (around 33%). These findings suggest minimal risk for human consumers of fish exposed to environmental NPs. However, shellfish exposed to very high NP concentrations (exceeding realistic environmental levels) may warrant further investigation.Research involving human participants and/or animals
The activities performed in the current research (fasting and sacrifice), are included in the article 1.5f of the Council Directive 2010/63/EU regarding the protection of animals used for experimental purposes, as they are considered practices not likely to cause pain, suffering, distress, or lasting harm equivalent to, or higher than, that caused by the introduction of a needle. Therefore, there was no need to have a specific approval by the competent Spanish authority to complete these experiments. Nevertheless, fish were sacrificed by personnel qualified in animal experimentation, in accordance Spanish Ministerial Order ECC/566/2015.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
Authors thank funding from Interreg (10.1339/100013276) ACUINANO, reference 07-12-ACUINANO_1_E; Agencia Estatal de Investigación (10.13039/501100011033) FOODNANORISK, reference PID2021-125276NB-I00); and Conselleria de Cultura, Educación e Ordenación Universitaria Xunta de Galicia (10.13039/501100008425) Grupo de Referencia Competitiva, reference ED431C 2022/029.References
- E. C. (2022/C229/01), Commission Recommendation of 14 June 2021 on the definition of nanomaterial (2022/C229/01), Official Journal of the European Union, C229 (14 June 2021) 1–5 Search PubMed.
- Nanotechnology Products Database | STATNANO, https://product.statnano.com/ (accessed September 6, 2023) Search PubMed.
- P. Prasher, M. Sharma, H. Mudila, A. Verma and P. Bhatt, Silver nanoparticles in natural ecosystems: Fate, transport, and toxicity, in Green Synthesis of Silver Nanomaterials, ed. K. A. Abd-Elsalam, Elsevier Amsterdam, Netherlands, 2022 Search PubMed.
- J. R. Bathi, F. Moazeni, V. K. K. Upadhyayula, I. Chowdhury, S. Palchoudhury, G. E. Potts and V. Gadhamshetty, Sci. Total Environ., 2021, 793, 148560 CrossRef CAS PubMed.
- A. Wimmer, A. Urstoeger, N. C. Funck, F. P. Adler, L. Lenz, M. Doeblinger and M. Schuster, Water Res., 2020, 171, 115399 CrossRef CAS PubMed.
- K. Khosravi-Katuli, E. Prato, G. Lofrano, M. Guida, G. Vale and G. Libralato, Environ. Sci. Pollut. Res., 2017, 24, 17326–17346 CrossRef PubMed.
- M. A. Kakakhel, F. Wu, W. Sajjad, Q. Zhang, I. Khan, K. Ullah and W. Wang, Environ. Sci. Eur., 2021, 33, 14 CrossRef CAS.
- A. M. Yasuji and M. F. Vajargah, J. Environ. Treat. Tech., 2017, 5, 1–4 Search PubMed.
- H. S. Joo, M. R. Kalbassi, I.-L. Yu, J.-H. Lee and S. A. Johari, Aquat. Toxicol., 2013, 140, 398–406 Search PubMed.
- M. Yoo-iam, R. Chaichana and T. Satapanajaru, Chem. Speciation Bioavailability, 2014, 26, 257–265 CrossRef.
- E. Can, V. Kizak, M. Kayim, S. S. Can, B. Kutlu, M. Ates and N. Demirtas, J. Mater. Sci. Eng., 2011, 5, 605–609 Search PubMed.
- E. G. Canli, H. B. Ila and M. Canli, Environ. Sci. Pollut. Res., 2019, 26, 938–945 CrossRef CAS PubMed.
- M. Connolly, D. Hernández-Moreno, E. Conde, A. Garnica, J. M. Navas, F. Torrent, I. Rucandio and M. L. Fernandez-Cruz, Environ. Sci. Eur., 2022, 34, 1 CrossRef CAS.
- L. Truong, T. Zaikova, B. L. Baldock, M. Balik-Meisner, K. To, D. M. Reif, Z. C. Kennedy, J. E. Hutchison and R. L. Tanguay, Nanotoxicology, 2019, 13, 879–893 CrossRef CAS PubMed.
- X. Liu, E. Dumitrescu, A. Kumar, D. Austin, D. Goia, K. N. Wallace and S. Andreescu, Environ. Pollut., 2019, 248, 627–634 CrossRef CAS PubMed.
- EFSA Scientific Committee, Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health, EFSA J., 2021, 19(8), 6768 Search PubMed.
- K. Chojnacka and M. Mikulewicz, Bioaccumulation, in Encyclopedia of Toxicology, ed. P. Wexler, Academic Press, 3rd edn, 2014, pp. 456–460 Search PubMed.
- S. Perales, R. Barbera, J. M. Lagarda and R. Farré, J. Agric. Food Chem., 2005, 53, 3721–3726 CrossRef CAS PubMed.
- E. Fröhlich and E. Roblegg, Arch. Toxicol., 2016, 90, 2297–2314 CrossRef PubMed.
- M. Saez-Tenorio, J. Domenech, A. García-Rodríguez, A. Velázquez, A. Hernández, R. Marcos and C. Cortés, Food Chem. Toxicol., 2019, 123, 258–267 CrossRef CAS PubMed.
- C. Gitrowski, A. R. Al-Jubory and R. D. Handy, Toxicol. Lett., 2014, 226, 264–276 CrossRef CAS PubMed.
- A. Abdelkhaliq, M. van der Zande, A. K. Undas, R. J. B. Peters and H. Bouwmeester, Nanotoxicology, 2020, 14, 111–126 CrossRef CAS PubMed.
- Z. M. Song, N. Chen, J. H. Liu, H. Tang, X. Deng, W. S. Xi, K. Han, A. Cao, Y. Liu and H. Wang, J. Appl. Toxicol., 2015, 35, 1169–1178 CrossRef CAS PubMed.
- C. Bai and M. Tang, J. Appl. Toxicol., 2020, 40, 37–63 CrossRef CAS PubMed.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS PubMed.
- F. Ribeiro, C. Pinheiro, M. Monteiro, C. A. M. Van Gestel, A. M. V. M. Soares and S. Loureiro, Environ. Sci. Pollut. Res., 2022, 29, 56079–56089 CrossRef CAS PubMed.
- M. Ates, V. Demir, R. Adiguzel and Z. Arslan, J. Nanomater., 2013, 2013, 460518 Search PubMed.
- M. Asztemborska, M. Jakubiak, R. Stęborowski, E. Chajduk and G. Bystrzejewska-Piotrowska, Water, Air, Soil Pollut., 2018, 229, 208 CrossRef PubMed.
- B. J. Shaw and R. D. Handy, Environ. Int., 2011, 37, 1083–1097 CrossRef CAS PubMed.
- M. J. Araújo, M. L. Sousa, E. Fonseca, A. B. Felpeto, J. C. Martins, M. Vázquez, N. Mallo, L. Rodriguez-Lorenzo, M. Quarato, I. Pinheiro, M. V. Turkina, J. J. López-Mayán, E. Peña-Vázquez, M. C. Barciela-Alonso, M. Spuch-Calvar, M. Oliveira, P. Bermejo-Barrera, S. Cabaleiro, B. Espiña, V. Vasconcelos and A. Campos, Chemosphere, 2022, 308, 136110 CrossRef PubMed.
- M. V. Taboada-López, N. Alonso-Seijo, P. Herbello-Hermelo, P. Bermejo-Barrera and A. Moreda-Piñeiro, Microchem. J., 2019, 148, 652–660 CrossRef.
- M. V. Taboada-López, P. Herbello-Hermelo, R. Domínguez-González, P. Bermejo-Barrera and A. Moreda-Piñeiro, Talanta, 2019, 195, 23–32 CrossRef PubMed.
- G. D. T. M. Jayasinghe, P. Herbello-Hermelo, R. Domínguez-González, P. Bermejo-Barrera and A. Moreda-Piñeiro, J. Agric. Food Chem., 2021, 69, 11451–11460 CrossRef CAS PubMed.
- M. V. Taboada-López, B. H. Leal-Martínez, R. Domínguez-González, P. Bermejo-Barrera, P. Taboada-Antelo and A. Moreda-Piñeiro, Talanta, 2021, 233, 1–32 CrossRef PubMed.
- L. Azeez, H. K. Aremu and O. A. Olabode, Bull. Environ. Contam. Toxicol., 2022, 108, 694–701 CrossRef CAS PubMed.
- S. Vali, G. Mohammadi, K. R. Tavabe, F. Moghadas and S. S. Naserabad, Ecotoxicol. Environ. Saf., 2020, 194, 110353 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle, B. P. Eynon and R. D. Handy, Environ. Sci.: Nano, 2019, 6, 1393 RSC.
- A. Banan, A. Forouharmehr, M. R. Kalbassi, M. Esmaeilbeigi, M. Bahmani, M. Y. Sadati, A. S. Kolok and E. G. Rogan, Reg. Stud. Mar. Sci., 2022, 52, 102264 Search PubMed.
- B. Xiao, X. Wang, J. Yang, K. Wang, Y. Zhang, B. Sun, T. Zhang and L. Zhu, Ecotoxicol. Environ. Saf., 2020, 194, 110454 CrossRef CAS PubMed.
- E. Fonseca, M. Vázquez, L. Rodriguez-Lorenzo, N. Mallo, I. Pinheiro, M. L. Sousa, S. Cabaleiro, M. Quarato, M. Spuch-Calvar, M. A. Correa-Duarte, J. J. López-Mayán, M. Mackey, A. Moreda, V. Vasconcelos, B. Espiña, A. Campos and M. J. Araújo, J. Hazard. Mater., 2023, 458, 131915 CrossRef CAS PubMed.
- F. Gallocchio, G. Biancotto, A. Moressa, F. Pascoli, T. Pretto, A. Toffan, G. Arcangeli, F. Montesi, R. Peters and A. Ricci, Food Chem., 2020, 323, 126841 CrossRef CAS PubMed.
- J. J. Doyle, J. E. Ward and R. Mason, Mar. Environ. Res., 2015, 110, 45–52 CrossRef CAS PubMed.
- S. Zimmermann, N. Ruchter, K. Loza, M. Epple and B. Sures, Environ. Pollut., 2017, 222, 251–260 CrossRef CAS PubMed.
- M. A. El-Atti, M. M. A. Desouky, A. Mohamadien and R. M. Said, Egypt. J. Aquat. Res., 2019, 45, 11–18 CrossRef.
- I. Marisa, V. Matozzo, A. Martucci, E. Franceschinis, N. Brianese and M. G. Marin, Mar. Environ. Res., 2018, 136, 179–189 CrossRef CAS PubMed.
- S. Kuehr, B. Meisterjahn, N. Schröder, B. Knopf, D. Völker, K. Schwirn and C. Schlechtriem, Environ. Sci.: Nano, 2020, 7, 535 RSC.
- F. Aouini, C. Trombini, M. Sendra and J. Blasco, Mar. Environ. Res., 2019, 152, 104783 CrossRef CAS PubMed.
- W. Liu, Z. Zeng, A. Chen, G. Zeng, R. Xiao, Z. Guo, F. Yi, Z. Huang, K. He and L. Hu, J. Environ. Chem. Eng., 2018, 6, 4236–4244 CrossRef CAS.
- Q. Li, M. Duan, L. Liu, X. Chen, Y. Fu, J. Li, T. Zhao and D. J. McClements, J. Agric. Food Chem., 2021, 69, 9661–9670 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00167b |
| This journal is © The Royal Society of Chemistry 2024 |
