Progress in phenanthroline-derived extractants for trivalent actinides and lanthanides separation: where to next?
Xiaofan
Yang
 a,
Lei
Xu
b,
Dong
Fang
ac,
Anyun
Zhang
a,
Lei
Xu
b,
Dong
Fang
ac,
Anyun
Zhang
 a and
Chengliang
Xiao
a and
Chengliang
Xiao
 *ac
*ac
aCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310058, China. E-mail: xiaoc@zju.edu.cn
bInstitute of Nuclear-Agricultural Science, Key Laboratory of Nuclear Agricultural Sciences of Ministry of Agriculture and Zhejiang Province, Zhejiang University, Hangzhou 310058, China
cInstitute of Zhejiang University-Quzhou, Quzhou 324000, China
First published on 29th August 2024
Abstract
Spent nuclear fuel (SNF) released from reactors possesses significant radioactivity, heat release properties, and high-value radioactive nuclides. Therefore, using chemical methods for reprocessing can enhance economic efficiency and reduce the potential environmental risks of nuclear energy. Due to the presence of relatively diffuse f-electrons, the chemical properties of trivalent lanthanides (Ln(III)) and actinides (An(III)) in SNF solutions are quite similar. Separation methods have several limitations, including poor separation efficiency and the need for multiple stripping agents. The use of novel multi-dental phenanthroline-derived extractants with nitrogen donor atoms to effectively separate An(III) over Ln(III) has been widely accepted. This review first introduces the development history of phenanthroline-derived extractants for extraction and complexation with An(III) over Ln(III). Then, based on structural differences, these extractants are classified into four categories: nitrogen-coordinated, N,O-hybrid coordinated, highly preorganized structure, and unsymmetric structure. Each category's design principles, extraction, and separation performance as well as their advantages and disadvantages are discussed. Finally, we have summarized and compared the unique characteristics of the existing extractants and provided an outlook. This work may offer a reliable reference for the precise identification and selective separation between An(III) and Ln(III), and point the way for future development and exploration.
1. Introduction
The world is accelerating its transition to clean energy with low-carbon emission sources like nuclear energy, which are poised to become key solutions for meeting future global electricity demands.1 Due to the significant advantages of nuclear power regarding cleanliness and stability, many countries are considering using nuclear energy to address their needs for climate change and energy security. The nuclear fuel cycle is one of the critical factors influencing the safe, stable, and sustainable development of nuclear power. Spent nuclear fuel (SNF) discharged after power generation contains a substantial amount of unused uranium (∼93%) and newly generated plutonium (∼1%), as well as small quantities of minor actinides (MAs, ∼0.1%) such as americium and curium, and fission products (FPs) including strontium, cesium, and lanthanides (∼5%).2,3 These minority elements (MAs and FPs) are present in the high-level liquid waste (HLLW) after SNF is processed through the Plutonium Uranium Recovery by Extraction (PUREX) process.4 Although these elements are present in relatively small masses, their high radioactivity and long half-lives necessitate the use of Partitioning and Transmutation (P&T) strategies or other methods to achieve safe management of high-level radioactive materials.5,6The actinides and lanthanides contained in HLLW are collectively referred to as f-block elements due to their possession of f-electrons.7 In acidic solutions, the most stable state of americium, curium, and later actinides and lanthanides is the trivalent ion with quite similar ionic radii (RLa(III)–Lu(III) = 1.03–0.86 Å, RAm(III)–Cf(III) = 0.98 – 0.95 Å, CN = 6), which results in analogical chemical properties.8,9 However, neptunium exists as Np(IV), Np(V) and Np(VI) in acidic solution, and its chemical properties are quite different from those of Am(III) and later actinides.10 According to the hard–soft acid–base (HSAB) theory, because the 5f orbitals of trivalent actinide ions (An(III)) are more diffuse than the 4f orbitals of trivalent lanthanide ions (Ln(III)), the An(III) are considered as softer acids than Ln(III).11,12 Softer An(III) ions are more likely to coordinate with softer donor atoms such as nitrogen and sulfur atoms, whereas harder donor atoms like oxygen atoms could coordinate with An(III) and Ln(III) together.13,14 Spectroscopic experiments and density functional theory (DFT) calculations also indicate that the mixing between the 5f and 6d orbitals of An(III) with the molecular orbitals of ligands is greater than that between the 4f and 5d orbitals of Ln(III).15–17 This results in stronger covalent bonding in An(III)–ligand complexes, which may be a key factor influencing their coordination ability.18 Precisely identifying the subtle differences in chemical properties between An(III) and Ln(III), and subsequently developing efficient separation methods based on these differences, has been a critical scientific challenge in the field of nuclear chemistry and radiochemistry.19
Researchers have also explored some separation methods based on altering the oxidation states of actinide elements.20–22 For example, using cluster complex precipitation for An(VI) ions and employing graphene oxide membranes for selective screening between actinyl ions and Ln(III).23–25 These new methods offer advantages such as high selectivity and efficiency, artificial precision control, and fast separation rates. However, they also have some drawbacks, including the need for additional oxidation steps, complicated operational procedures, and poor stability of high-valent ions.26 Overall, the mainstream approach for separating An(III) and Ln(III) remains solvent extraction based on a binary organic-aqueous phase system.27 The purpose of solvent extraction is to extract most of the target substances such as An(III) into the organic phase as much as possible, which means that their distribution ratios should be at least greater than 1. In contrast, the extraction capacity of non-target substances such as Ln(III) should be as weak as possible, and their distribution ratios should be well below 1. The ultimate aim of the study is to wholly separate An(III) such as Am(III) and Cm(III) from Ln(III) (La(III), Eu(III), and Gd(III), etc.). Due to the similar outer electronic structure and chemical properties, Am(III)(5f7 7S2) and Eu(III)(4f7 6S2) are usually selected as representatives of An(III) and Ln(III) in experimental and theoretical studies, respectively. Then the separation ability of An(III) and Ln(III) was evaluated by measuring the SFAm/Eu value. This method is favored due to its advantages of continuous operation, large processing capacity, operation simplicity, and mature equipment, which make it highly valuable for research and promising practical applications.28,29
One of the key points in the development of solvent extraction methods is the design of organic extractants that exhibit strong affinity and high selectivity for the target elements, along with a thorough investigation of their extraction mechanisms and coordination modes with metal ions.30,31 The TALSPEAK (trivalent actinide lanthanide separation with phosphorus-reagent extraction from aqueous komplexes) process developed during cold war employs the lipophilic di-(2-ethylhexyl) phosphoric acid (HDEHP, Fig. 1(a)) extractant, which contains P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordinating groups, and selects the hydrophilic diethylenetriamine-N,N,N′,N′′,N′′-pentaacetic acid (DTPA, Fig. 1(a)) that contains soft N donor atoms as the actinide masking agent.32,33 This process has demonstrated a good separation ability between Am(III) and Ln(III) (SFAm(III)/Ln(III) ≥ 100).34 However, due to its narrow operational pH range and slow phase separation and mass transfer kinetics, it is challenging to apply this method on an industrial scale. Diglycolamide (DGA, Fig. 1(b)) extractants only containing CHON atoms that can be completely incinerated without producing harmful waste have been selected as potential extractants for actinide ions and showed a promising extraction performance for Am(III). However, since the donor atoms of DGA extractants are relatively hard O atoms, their separation ability for An(III) and Ln(III) is inadequate and requires combination with other extractants or masking agents to achieve effective separation.35 Due to containing softer S donor atoms, the bis(2,4,4-trimethylpentyl)dithiophosphinic acid (purified Cyanex 301, Fig. 1(c)) exhibits a strong affinity and separation capability for Am(III) (SFAm(III)/Eu(III) ≥ 5900) and does not require the addition of a hydrophilic masking or stripping agent during extraction, which leads to a simpler extraction system.36,37 Nonetheless, it also has drawbacks such as low extraction acidity, a narrow pH range, and poor stability of the extractant, necessitating further improvements.38
O coordinating groups, and selects the hydrophilic diethylenetriamine-N,N,N′,N′′,N′′-pentaacetic acid (DTPA, Fig. 1(a)) that contains soft N donor atoms as the actinide masking agent.32,33 This process has demonstrated a good separation ability between Am(III) and Ln(III) (SFAm(III)/Ln(III) ≥ 100).34 However, due to its narrow operational pH range and slow phase separation and mass transfer kinetics, it is challenging to apply this method on an industrial scale. Diglycolamide (DGA, Fig. 1(b)) extractants only containing CHON atoms that can be completely incinerated without producing harmful waste have been selected as potential extractants for actinide ions and showed a promising extraction performance for Am(III). However, since the donor atoms of DGA extractants are relatively hard O atoms, their separation ability for An(III) and Ln(III) is inadequate and requires combination with other extractants or masking agents to achieve effective separation.35 Due to containing softer S donor atoms, the bis(2,4,4-trimethylpentyl)dithiophosphinic acid (purified Cyanex 301, Fig. 1(c)) exhibits a strong affinity and separation capability for Am(III) (SFAm(III)/Eu(III) ≥ 5900) and does not require the addition of a hydrophilic masking or stripping agent during extraction, which leads to a simpler extraction system.36,37 Nonetheless, it also has drawbacks such as low extraction acidity, a narrow pH range, and poor stability of the extractant, necessitating further improvements.38
 | ||
| Fig. 1 Representative extractants for An(III)/Ln(III) separation. (a) HDEHP and DTPA, (b) DGA, (c) purified Cyanex 301, (d) BTP, (e) BTBP, and (f) BTPhen. | ||
Based on previous research results, researchers have proposed several design principles for extractants used in the separation between An(III) and Ln(III):13,39,40 (1) the extractant should remain stable under highly acidic conditions (3–4 M HNO3), resisting decomposition or protonation; (2) the extractant should exhibit strong extraction capability for the target An(III) ion; (3) the extractant should have weak extraction capability for non-target ions, such as Ln(III) and other fission products, or should allow easy stripping of these ions to achieve optimal separation; (4) the extractant should have a fast extraction speed and reach extraction equilibrium in a short period; (5) the extraction system should be as simple as possible, minimizing the introduction of salting-out agents, synergic extractants, masking agents, or pH buffers; (6) the organic solvent should have high solubility for the lipophilic extractants and low miscibility with the aqueous phase to facilitate phase separation.
Over the past few decades, researchers have discovered that certain multidentate chelating extractants with nitrogen-containing heterocyclic skeletons (such as pyridine, bipyridine, and phenanthroline) can meet these requirements to some extent.41,42 The 2,6-bis(1,2,4-triazin-3-yl)pyridine (BTP, Fig. 1(d)) extractants, composed of a pyridine skeleton and triazine side chains, are tridentate chelating ligands that can form [M(BTP)3]3+ complexes with An(III) or Ln(III).43 Because BTP molecules completely replace water molecules bound to the An(III) or Ln(III), the complexes formed by BTP are highly stable, which leads to strong extraction and separation capabilities but poor stripping efficiency.44,45 When the pyridine skeleton is replaced with bipyridine, the 6,6′-bis(1,2,4-triazin-3-yl)-2,2′-bipyridine (BTBP, Fig. 1(e)) extractants could be designed, which are tetradentate chelating ligands to f-elements.46,47 The BTBP extractants can form M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 extracted complexes with An(III) and Ln(III), which makes the concentration reduction of extractants caused by radiolysis have less effect on their extraction capacity.45 The increased number of nitrogen donor atoms significantly enhances the stripping ability of An(III). However, the bipyridine skeleton requires twisting during the extraction process to achieve a suitable coordination configuration with An(III) and Ln(III). Therefore, the BTBP extractants exhibit slow extraction kinetics and require the addition of N,N′-dimethyl,N,N′-dioctylhexylethoxy malonamide (DMDOHEMA) or N,N,N′,N′-tetraoctyl diglycolamide (TODGA) as phase transfer agents to shorten the extraction equilibrium time.48
2 extracted complexes with An(III) and Ln(III), which makes the concentration reduction of extractants caused by radiolysis have less effect on their extraction capacity.45 The increased number of nitrogen donor atoms significantly enhances the stripping ability of An(III). However, the bipyridine skeleton requires twisting during the extraction process to achieve a suitable coordination configuration with An(III) and Ln(III). Therefore, the BTBP extractants exhibit slow extraction kinetics and require the addition of N,N′-dimethyl,N,N′-dioctylhexylethoxy malonamide (DMDOHEMA) or N,N,N′,N′-tetraoctyl diglycolamide (TODGA) as phase transfer agents to shorten the extraction equilibrium time.48
Researchers have developed a series of phenanthroline-derived extractants to effectively address these issues, such as 2,9-bis(1,2,4-triazin-3-yl)-1,10-phenanthroline (BTPhen, Fig. 1(f)), which have a preorganized phenanthroline skeleton and conjunct side chains with nitrogen or oxygen donor atoms.49–51 These extractants have the following advantages:51–53 (1) the nitrogen-containing phenanthroline skeleton provides nitrogen donor atoms and improves separation efficiency; (2) the multidentate chelating structures of phenanthroline-derived extractants enhance the resistance to the radiolysis influence on the extraction by changing the structures of the extracted complexes; (3) various substituents can be attached to the side chains to modify the electron cloud density at the coordination sites and improve solubility in the organic phase; (4) by adjusting the structure of the skeleton and substituents, the configuration of the extractant can be controlled, thereby influencing the kinetics and steric hindrance during the coordination process.
Based on various design concepts, dozens of phenanthroline-derived extractants with different structures have been designed and synthesized successfully.54 Our research group has also worked in this area for many years and achieved numerous contributions.55 This feature article will systematically introduce existing phenanthroline-derived extractants with good extraction and separation performance for An(III) and Ln(III) by categorizing them according to design principles. This paper is not a complete summary of the existing research, but a selection of the classic and important literature reports. The focus of this paper is to study the relationship between the structure of phenanthroline-derived extractants and their extraction and separation effect towards An(III) and Ln(III) in molecular organic solvents and refine the design concept and research ideas of extractants. The content of this paper does not involve the research of new solvents such as ionic liquids (IL) and deep eutectic solvents (DES), the decomposition effect caused by acids and radiation, extractant-modified solid-phase materials, and the development of separation processes. We aim to summarize the key issues and propose promising solutions in the construction of novel phenanthroline-derived extractants, thereby guiding the design of efficient future separation extractants.
2. Nitrogen-coordinated extractants
2.1. Phenanthroline-derived pyridine (DPP) ligand
The earliest developed purely nitrogen-coordinated phenanthroline-derived ligand was 2,9-di(pyrid-2-yl)-1,10-phenanthroline (DPP, Fig. 2(a)), which was used to study the photophysical and electrochemical properties of Ru(II) complexes.56 The DPP exhibits strong binding affinity towards divalent metal ions with large radii, thus was utilized for chelation-enhanced fluorescence (CHEF) detection of Cd(II) in solution systems.57 The DPP consists of a phenanthroline skeleton and two pyridine side chains that can rotate to some extent, which creates a large coordination cavity. Researchers note that the stable phenanthroline skeleton and pyridine side chains of DPP could synergistically participate in coordination.58 The nitrogen donor atoms of DPP result in lower affinity for harder metal ions compared to softer ions and enhance the separation capability between them. Unlike flexible amide ligands (such as DGA and NTAmide) that can easily adjust their configuration according to the coordination space of the target ion, or macrocyclic crown-ether ligands that only coordinate with metal ions of specific radii due to their fixed-sized cavities, DPP can be considered as a semi-rigid ligand with a certain degree of preorganization.59 The orientation of the pyridine side chains may undergo slight adjustments during coordination with the irreversible phenanthroline skeleton, which makes DPP inclined to complex metal ions that roughly match the coordination cavity size. Based on the selectivity of cavity size, DPP is expected to preferentially complex metal ions with larger radii.60 | ||
Fig. 2 (a) DPP, (b) variation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1(DPP) for some Ln(III) ions as a function of the number 1/r+ (r+ = ionic radius for eight-coordination) in the Ln(III) ion, and (c) structural parameters of interest in [Ln(DPP)(H2O)5]3+ complexes for Ln = La, Sm, Gd, Eu, and Lu.61 (Copyright © 2013, American Chemical Society.) K1(DPP) for some Ln(III) ions as a function of the number 1/r+ (r+ = ionic radius for eight-coordination) in the Ln(III) ion, and (c) structural parameters of interest in [Ln(DPP)(H2O)5]3+ complexes for Ln = La, Sm, Gd, Eu, and Lu.61 (Copyright © 2013, American Chemical Society.) | ||
Based on the aforementioned design concept, Carolan et al. fitted the stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k) of complexes formed by DPP and Ln(III), and correlated them with ionic radii of Ln(III).61 The peak of the log
k) of complexes formed by DPP and Ln(III), and correlated them with ionic radii of Ln(III).61 The peak of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values appears at Sm(III), indicating that DPP has the strongest coordination ability with Sm(III) (Fig. 2(b)). Theoretical calculations reveal that from La(III) to Lu(III), the ideal coordination bond lengths show a trend of initial shortening followed by lengthening influenced by spatial strain effects. The calculations also suggest that the Sm(III) matches the chelation cavity of DPP better than other Ln(III) ions, whose coordination bond angles deviate from the ideal 120°, thereby reducing the stability of the [Ln(DPP)(H2O)5]3+ complexes (Fig. 2(c)). Because the radius of Am(III) is similar to that of Sm(III) (RAm(III) = 1.09 Å, RSm(III) = 1.08 Å, CN = 8), the authors suppose that the coordination bond strength between DPP and Am(III) should also be stronger than with other Ln(III) ions. Therefore, DPP may have potential for the selective separation of Am(III) from Ln(III).
k values appears at Sm(III), indicating that DPP has the strongest coordination ability with Sm(III) (Fig. 2(b)). Theoretical calculations reveal that from La(III) to Lu(III), the ideal coordination bond lengths show a trend of initial shortening followed by lengthening influenced by spatial strain effects. The calculations also suggest that the Sm(III) matches the chelation cavity of DPP better than other Ln(III) ions, whose coordination bond angles deviate from the ideal 120°, thereby reducing the stability of the [Ln(DPP)(H2O)5]3+ complexes (Fig. 2(c)). Because the radius of Am(III) is similar to that of Sm(III) (RAm(III) = 1.09 Å, RSm(III) = 1.08 Å, CN = 8), the authors suppose that the coordination bond strength between DPP and Am(III) should also be stronger than with other Ln(III) ions. Therefore, DPP may have potential for the selective separation of Am(III) from Ln(III).
2.2. Phenanthroline-derived triazine (BTPhen) extractants
The second key class of purely nitrogen-coordinated phenanthroline-derived extractants features a phenanthroline skeleton with 1,2,4-triazine side groups, specifically 2,9-bis(1,2,4-triazin-3-yl)-1,10-phenanthroline (BTPhen) extractants.62 The design concept of BTPhen is inspired by the DPP and BTBP ligands. Replacing the freely rotating bipyridine skeleton in BTBP with the conformationally locked phenanthroline skeleton could prevent the trans- and cis-transformation. This modification not only reduces the energy barrier required for the extractant's conformational rotation but also significantly enhances the extraction rate of extractants for An(III) and Ln(III).63In 2011, Lewis et al. developed an efficient synthetic method to successfully synthesize the 2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (CyMe4-BTPhen, Fig. 3(a)) extractant and demonstrated its strong extraction ability for Am(III) while maintaining high Am(III)/Eu(III) separation efficiency (DAm(III) ≈ 1000, DEu(III) ≈ 5, SFAm(III)/Eu(III) > 200, 1 M HNO3).49 Due to the preorganized phenanthroline skeleton, the CyMe4-BTPhen exhibits significantly enhanced extraction rates, which reaches the extraction equilibrium in approximately 15 minutes. Within just 5 minutes of contact time between the organic and aqueous phases, the DAm of CyMe4-BTPhen exceeds 10. This indicates that there is no need for a phase transfer agent to improve the extraction kinetics, which is helpful to simplify the extraction system and avoid consequent issues such as decomposition caused by high acidity and radiation. Researchers further confirmed the clear relationship between the preorganization degree of the extractant and the complexation rate with metal ions through a combination of experimental and computational methods.64–66 To achieve the cis-conformation required for complex formation, the BTBP must overcome the rotational energy barrier of bipyridine (∼12 kcal mol−1), whereas BTPhen does not.
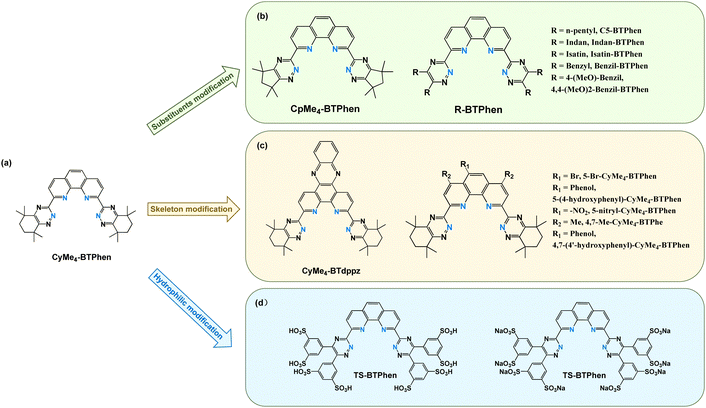 | ||
| Fig. 3 (a) CyMe4-BTPhen and its modified derivates: (b) substituent modified BTPhen extractants, (c) skeleton modified BTPhen extractants, and (d) hydrophilic modified BTPhen extractants. | ||
Using the similar synthetic method of CyMe4-BTPhen, a series of alkyl- or aryl-substituted BTPhen derivatives (Fig. 3(b)) were designed and synthesized.67 The n-pentyl substituted C5-BTPhen exhibited a lower extraction capacity for Am(III) compared to CyMe4-BTPhen, and its separation ability also decreased (DAm(III) < 100, DEu(III) ≈ 1, SFAm(III)/Eu(III) < 100, 1 M HNO3). Other substituted BTPhen extractants also showed varying degrees of decreased separation efficiency for Am(III) and Eu(III).68 Under 3 M HNO3 conditions, the SFAm(III)/Eu(III) values of heterocyclic substituted Indan-BTPhen and Isatin-BTPhen are only 2 and 4, respectively, which indicate negligible separation capability. The benzyl-substituted Benzil-BTPhen shows a less degree of decline (SFAm(III)/Eu(III) = 16), while only 4,4-(MeO)2-Benzil-BTPhen exhibited a satisfactory separation performance (SFAm(III)/Eu(III) = 284). Alkyl-substituted Me4-BTPhen and Et4-BTPhen also experienced slight reductions in separation ability with SFAm(III)/Eu(III) values of 122 and 105, respectively.
It is worth mentioning that when the functional groups on the CyMe4-BTPhen extractant's triazine side chains are replaced with five-membered aliphatic rings (2,9-bis(5,5,7,7-tetramethyl-6,7-dihydro-5H-cyclopenta[e]-1,2,4-triazin-3-yl)-1,10-phenanthroline, CpMe4-BTPhen, Fig. 3(b)), the extraction efficiency for Am(III) and Eu(III) will decrease by an order of magnitude but the separation ability remains unchanged (DAm(III) ≈ 100, DEu(III) ≈ 0.5, SFAm(III)/Eu(III) > 200, 1 M HNO3).69 This is because the distribution ratio for Eu(III) significantly decreases, thereby reducing the co-extraction effect between CyMe4-BTPhen and Eu(III). Additionally, Am(III) reaches extraction equilibrium faster than Eu(III) (tAm(III) ≈ 10 min, tEu(III) ≈ 20 min) with the separation factor being highest after a shaking time of 5 minutes (SFAm(III)/Eu(III) ≈ 300, 1 M HNO3). These characteristics could be considered in the subsequent design of separation processes to further enhance the separation efficiency of Am(III) from Eu(III).
In addition to side chain modifications, researchers have also attempted to modify the phenanthroline skeleton of CyMe4-BTPhen with various electron-donating or electron-withdrawing functional groups to investigate whether these substituents could enhance the extraction and separation capabilities of BTPhen extractants for Am(III) and Eu(III). When dipyridophenazine (dppz) is used to replace phenanthroline, the synthesized bis(1,2,4-triazin-3-yl)-dipyridophenazine (CyMe4-BTdppz, Fig. 3(c)) extractant exhibits a dramatic decrease in extraction for both Am(III) and Eu(III) (DAm(III) ≈ 2, DEu(III) ≈ 0.02, SFAm(III)/Eu(III) > 100, 1 M HNO3).70 Although the extractant concentration was lower than in previous experiments (5 mM), merely reducing the extractant concentration would not cause such a significant drop. This indicates that such drastic alterations to the skeleton considerably weaken the extraction and separation performance of BTPhen extractants. Therefore, subsequent efforts have focused on functional group substitutions that maintain the integrity of the phenanthroline skeleton.
Modifying the phenanthroline skeleton of C5-BTPhen with bromine at the No. 5 and No. 6 positions yielded BrC5-BTPhen and Br2C5-BTPhen.71 These modifications did not change the extraction capability for Am(III) but significantly reduced the extraction of Eu(III), thereby enhancing their separation factor (SFC5-BTPhen < 100, SFBrC5-BTPhen ≈ 150, SFBr2C5-BTPhen ≈ 450, 1 M HNO3). This can be attributed to the electron-withdrawing effect of bromine, which reduces the electron cloud density of phenanthroline and decreases the complexation ability with Ln(III). Introducing electron-withdrawing bromine atoms or electron-donating phenol groups at the No. 5 position of the phenanthroline skeleton of CyMe4-BTPhen resulted in 5-bromine-2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (5-Br-CyMe4-BTPhen, Fig. 3(c)) and 5-(4-hydroxyphenyl)-2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (5-(4-hydroxyphenyl)-CyMe4-BTPhen, Fig. 3(c)).72 5-Br-CyMe4-BTPhen showed an unchanged extraction ability for Am(III) but reduced extraction ability for Eu(III), which results in a higher separation factor than that of CyMe4-BTPhen (SFAm(III)/Eu(III) ≈ 680, 3 M HNO3). Conversely, 5-(4-hydroxyphenyl)-CyMe4-BTPhen not only significantly increased the distribution ratio for Am(III) but also slightly enhanced Eu(III) extraction, leading to a lower-than-expected separation factor (DAm(III) > 1000, DEuv > 3, SFAm(III)/Eu(III) ≈ 320, 3 M HNO3). The authors also demonstrate that the substituents on the phenanthroline skeleton could improve the separation between Am(III) and Cm(III), although co-extraction still occurs. Li et al. introduced a nitro group (–NO2) at the same position (No. 5) and synthesized 5-nitryl-2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (5-nitryl-CyMe4-BTPhen, Fig. 3(c)).73 The nitro group significantly reduced the solubility of the extractant thus restricts experiments to a 5 mM concentration in n-octanol. As a strong electron-withdrawing group, the nitro group greatly reduced the electron cloud density of the phenanthroline skeleton, leading to the lowest extraction capacity for Am(III) among the four CyMe4-BTPhen derivatives. However, due to the substantial decrease in Eu(III) extraction, the separation factor increased significantly (DAm(III) ≈ 20, DEu(III) ≈ 0.02, SFAm(III)/Eu(III) ≈ 870, 1 M HNO3).
Besides the grafting of the No. 5 and No. 6 position on phenanthroline, Prof. Harwood's group developed a convenient and efficient method for modifying the No. 4 and No. 7 positions of the phenanthroline in BTPhen extractants.74,75 They synthesized 4,7-methyl-2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (4,7-Me-CyMe4-BTPhen, Fig. 3(c)) and 4,7-(4′-hydroxyphenyl)-2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-1,2,4-benzotriazin-3-yl)-1,10-phenanthroline (4,7-(4′-hydroxyphenyl)-CyMe4-BTPhen, Fig. 3(c)). The experimental results indicate that the methyl substituents have a little impact on the extraction and separation. Similar to the No. 5 and No. 6 position results, the phenol substituent at the No. 4 and No. 7 positions increased the extraction capacity for Am(III) but also reduced the separation ability.
All the results mentioned above aimed to develop lipophilic BTPhen extractants with good solubility in organic solvents (primarily n-octanol). Because BTPhen extractants exhibit a strong affinity for Am(III) in both homogeneous and heterogeneous systems, researchers have leveraged this characteristic to modify them to enhance water solubility by introducing strongly polar groups. This led to synthesizing a series of highly effective hydrophilic masking and stripping agents for An(III). Lewis et al. first introduced benzene sulfonic acid groups onto the triazine sided chains and synthesized tetra sulfonated BTPhen ligands (TS-BTPhen, Fig. 3(d)) as hydrophilic Am(III) masking agents.76 When cooperating with the lipophilic TODGA extractant, the TS-BTPhen could effectively complex with Am(III) and successfully prevent its co-extraction with Ln(III) into the organic phase (SFEu(III)/Am(III) > 900, 0.28 M HNO3). As the acidity of the solution increased, the masking effect of TS-BTPhen gradually diminished due to the enhanced extraction capability of TODGA (SFEu(III)/Am(III) ≈ 65, 1.04 M HNO3). Overall, the masking effect of TS-BTPhen was superior to that of traditional hydrophilic An(III) complexing agents like DTPA and EDTA with an effectively functioned acidity over a wider range.77,78 Subsequent research also confirmed that TS-BTPhen exhibits slight differences in complexation abilities between Am(III) and Cm(III) and enhances the separation efficiency of Cm(III) from Am(III) (SFCm(III)/Am(III) ≈ 4.6, 0.5 M HNO3).79
2.3. Phenanthroline-derived nitrogen heterocycle extractants
Another type of pure nitrogen-coordinated phenanthroline-derived extractant substitutes the triazine side chains of BTPhen with various nitrogen heterocycles. A representative example is 2,9-di(quinazolin-2-yl)-1,10-phenanthroline (BQPhen, Fig. 4(a)).80 The design concept of this extractant is that only one nitrogen atom from the 2N side of triazine in BTPhen participated in coordination, potentially maintaining a strong binding affinity with An(III) and Ln(III) with reduced nitrogen atoms. Additionally, the introduction of quinazoline side chains renders BQPhen as a fully aromatic ligand, which potentially enhances its chemical and radiolytic stability compared to non-aromatic counterparts. Moreover, due to variations in its solubility across different solvents, integrating BQPhen into the family of phenanthroline-derived extractants broadens the choice of diluents in solvent extraction. Experimental results demonstrate that the stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) of complexes formed by BQPhen with Am(III) are approximately 10 and 100 times higher than those with Eu(III) and Nd(III), respectively. Another approach involves grafting pyrazoles onto the phenanthroline skeleton to synthesize 2,9-bis(1H-pyrazol-3-yl)-1,10-phenanthroline extractant (BPPhen, Fig. 4(b)), which is another tetradentate N-coordinating extractant.81 By extending alkyl chains on the side groups, the solubility of BPPhen in organic solvents can be significantly enhanced. Under conditions where 2-bromohexanoic acid serves as a synergistic agent, BPPhen exhibits a certain degree of separation efficiency between Am(III) and Eu(III) at lower acidity (SFAm(III)/Eu(III) ≈ 100, 0.5 M HNO3) with its extraction and separation capabilities decreasing as acidity increases.
β) of complexes formed by BQPhen with Am(III) are approximately 10 and 100 times higher than those with Eu(III) and Nd(III), respectively. Another approach involves grafting pyrazoles onto the phenanthroline skeleton to synthesize 2,9-bis(1H-pyrazol-3-yl)-1,10-phenanthroline extractant (BPPhen, Fig. 4(b)), which is another tetradentate N-coordinating extractant.81 By extending alkyl chains on the side groups, the solubility of BPPhen in organic solvents can be significantly enhanced. Under conditions where 2-bromohexanoic acid serves as a synergistic agent, BPPhen exhibits a certain degree of separation efficiency between Am(III) and Eu(III) at lower acidity (SFAm(III)/Eu(III) ≈ 100, 0.5 M HNO3) with its extraction and separation capabilities decreasing as acidity increases.
Similar to the design strategy of TS-BTPhen, modification of bistriazolyl-phenanthroline (BTrzPhen) ligands with highly polar groups such as hydroxyl, sulfonic acid, and carboxyl groups yield hydrophilic masking agents with a strong affinity for An(III). Hydrophilic BTrzPhen derivatives with dihydroxy and tetrahydroxy modifications exhibit moderate masking capabilities for Am(III) at low acidity (Fig. 4(c)).82 The increased hydroxylation levels affect their separation efficiency (SF2OH-BtrzPhen = 36, SF4OH-BtrzPhen = 47, 0.33 M HNO3). The sulfonic acid-modified 2,9-bis[1-(2-(4-sulfonatophenyl)ethyl)-1H-1,2,3-triazol-4-yl]-1,10-phenanthroline (DS-BTrzPhen, Fig. 4(c)) achieves a peak separation factor under 0.05 M HNO3 (SFEu(III)/Am(III) ≈ 300) with negligible extraction of Am(III) and Eu(III) at lower acidities (DAm(III), Eu(III) < 0.1, 0.001–0.01 HNO3). Amino acid-modified 2,9-bis[1-(3-amino-3-carboxyl-propyl)-1H-1,2,3-triazol-4-yl]-1,10-phenanthroline (DAA-BTrzPhen, Fig. 4(c)) shows increasing extraction capacities for Am(III) and Eu(III) with increasing acidity, however, the separation efficiency decreases accordingly, peaking at 0.05 M HNO3 (SFEu(III)/Am(III) = 84.4).83 Overall, these phenanthroline-derived heterocyclic ligands (both lipophilic and hydrophilic) demonstrate selective binding affinities for Am(III), but their extraction and separation capabilities are slightly weaker than those of BTPhen extractants. Moreover, their optimal operational acidity is relatively low, typically at or below 0.5 M HNO, which will potentially limit their suitability for real HLLW processing requirements (typically 3–4 M HNO3).31
3. N,O-Hybrid coordinated extractants
3.1. Phenanthroline-derived dicarboxylic acid (PDA) ligands
N donor atoms are softer bases and readily coordinate with softer acids like Am(III). With high electron cloud density, O donor atoms exhibit a strong coordination ability with metal ions. By providing two N donor atoms within a phenanthroline skeleton and allowing the side chains to contribute two O donor atoms, a series of N,O-hybrid tetradentate chelating ligands with potential coordination and separation abilities for An(III) over Ln(III) can be developed.51,55The 1,10-phenanthroline-2,9-dicarboxylic acid (PDA, Fig. 5(a)) is a classic N,O-mixed phenanthroline-derived ligand used for chelating large-radius ions, which has carboxylic acid side chains.84 Molecular mechanics (MM) calculations indicate that after deprotonation, PDA's carboxylate side chains are highly constrained, remaining coplanar with the phenanthroline skeleton. Theoretical calculations show that the distance between the two O donor atoms on PDA's side chains is above 4.8 Å. Therefore, only metal ions with radii around 1.0 Å can fit this coordination cavity. Compared to small-radius metal ions (R < 0.8 Å), PDA should exhibit strong selectivity for large-radius metal ions (R ≈ 1.0 Å). Due to steric effects, PDA forms five-membered chelate rings with minimal strain when coordinating large-radius metal ions, and the rigidity of the phenanthroline skeleton enhances this effect.85
 | ||
| Fig. 5 (a) PDA; (b) unique structure and characteristics of PDAM55 (Copyright © 2023 Elsevier); (c) alkyl substituted DAPhen; (d) DIPhen; (e) aromatic substituted DAPhen; (f) hydrophilic DAPhen and DIPhen masking agents: DS-Ph-DAPhen, nOH-DAPhen, But-acid-DIPhen, and amino-But-DIPhen. | ||
Hancock et al. measured the stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k) of PDA complexes with metal ions of varying radii, including Ca(II) (R = 1.0 Å, CN = 6), Gd(III) (R = 1.05 Å, CN = 8), Th(IV) (R = 1.13 Å, CN = 10), and Bi(III) (R = 1.17 Å, CN = 8), and found a general positive correlation between log
k) of PDA complexes with metal ions of varying radii, including Ca(II) (R = 1.0 Å, CN = 6), Gd(III) (R = 1.05 Å, CN = 8), Th(IV) (R = 1.13 Å, CN = 10), and Bi(III) (R = 1.17 Å, CN = 8), and found a general positive correlation between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and the ionic radius.86 However, for excessively large ions, like Pb(II) (R = 1.19 Å, CN = 6) and Ba(II) (R = 1.35 Å, CN = 6), the log
k and the ionic radius.86 However, for excessively large ions, like Pb(II) (R = 1.19 Å, CN = 6) and Ba(II) (R = 1.35 Å, CN = 6), the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values dramatically decrease, which may be due to the reduced matching degree of the excessive ionic size and the ligand cavity. Some researchers also suggest that PDA's coordination ability with f-block elements is positively correlated with the ionic potential (Z/r) of the metal ions.87 Subsequent studies confirmed that PDA also has a strong affinity for larger Am(III) ions (R = 1.09 Å, CN = 8), but it shows negligible separation capability for Nd(III) (R = 1.11 Å, CN = 8).88
k values dramatically decrease, which may be due to the reduced matching degree of the excessive ionic size and the ligand cavity. Some researchers also suggest that PDA's coordination ability with f-block elements is positively correlated with the ionic potential (Z/r) of the metal ions.87 Subsequent studies confirmed that PDA also has a strong affinity for larger Am(III) ions (R = 1.09 Å, CN = 8), but it shows negligible separation capability for Nd(III) (R = 1.11 Å, CN = 8).88
3.2. Phenanthroline-derived diamide (DAPhen) extractants
By introducing electron-withdrawing amide groups into the side chains of PDA, the synthesized 1,10-phenanthroline-2,9-dicarboxamide (PDAM, Fig. 5(b)) becomes a non-protonatable tetradentate chelating ligand.89 This characteristic allows it to maintain strong coordination ability under highly acidic conditions. Compared to phenanthroline, the O donor atoms in PDAM's amide groups exhibit stronger coordination capabilities, which results in highly stable metal ion complexes. Similar to PDA, the chelating cavity formed by PDAM's phenanthroline skeleton and amide side chains is relatively large (≈4.82 Å), which imparts selectivity for large-radius metal ions. More importantly, the strong electron-withdrawing nature of the amide groups reduces the electron cloud density on the O donor atoms, thereby decreasing their basicity. This makes them more suitable for binding with the weaker acids, An(III), rather than the stronger acids, Ln(III).90 Consequently, PDAM demonstrates some An(III)/Ln(III) separation capability (SFAm(III)/Eu(III) = 7.4, 0.01 M HNO3).91 Additionally, PDAM is neither easily protonated nor prone to ionizing its protons, which results in significantly lower solubility in the aqueous phase compared to acidic ligands like PDA. While this trait is disadvantageous for the development of hydrophilic ligands, it aids in the development of lipophilic extractants based on PDAM.Alkylation modifications on the amide side chains of PDAM yield a series of lipophilic 2,9-diamide-1,10-phenanthroline (DAPhen) extractants.92,93 The first developed extractant was N,N′-dioctyl-2,9-diamide-1,10-phenanthroline (Oct-Oct-DAPhen, Fig. 5(c)) with octyl substituents.94 Using o-nitrophenyl hexyl ether (NPHE) as the solvent and cesium commo-3,3-cobalta-bis(8,9,12-tribromo-1,2dicarba-closo-dodecarborane)ate(1-) (Br-Cosan) as a synergist, this extractant exhibited good extraction and separation capabilities for Am(III) and Eu(III) under low acidity conditions (DAm(III) = 8.16, DEu(III) = 0.23, SFAm(III)/Eu(III) = 34.8, 0.1 M HNO3). Subsequent synthesis of N,N′-diisobutyl-2,9-diamide-1,10-phenanthroline (iBu-iBu-DAPhen, Fig. 5(c)) did not achieve such an effective separation (DAm(III) = 4.62, DEu(III) = 1.05, SFAm(III)/Eu(III) = 4.4, 0.01 M HNO3), possibly due to the use of n-dodecane as a less polar solvent.91 Other researchers later systematically studied the effects of different solvents (1-(trifluoromethyl)-3-nitrobenzene (F-3), nitrobenzene, and n-dodecane) on the extraction and separation efficiency of N,N′-didodecyl-2,9-diamide-1,10-phenanthroline (Dodec–Dodec-DAPhen, Fig. 5(c)).95 They found that solvents with strong polarity and high dielectric constants (F-3 and nitrobenzene) could enhance the extraction and separation capabilities of Dodec–Dodec-DAPhen.
Without any salting-out agents or synergic extractants, the (1,10-phenanthroline-2,9-diyl)bis(pyrrolidin-1-ylmethanone (Pyr-DAPhen, Fig. 5(c)) extractant with five-membered cycloalkyl substituents, was shown to have weak extraction abilities for Am(III) and Eu(III), but decent separation capability (DAm(III) ≈ 0.8, DEu(III) ≈ 0.02, SFAm(III)/Eu(III) ≈ 55, 0.1 M HNO3).96 The distribution ratio of Am(III) and Eu(III) can be improved by introducing a methyl group with electron-donating capacity in the pyrrolidine side chains (DAm(III) = 3.2, DEu(III) = 0.12, SFAm(III)/Eu(III) = 27, 3.0 M HNO3).97 Several other dialkyl-substituted DAPhen extractants have been synthesized, but their An(III)/Ln(III) separation and extraction performance have not been reported.98,99
Researchers have also explored phenanthroline-derived diimide extractants with only one alkyl substituent on the amide. N2,N9-didecyl-2,9-diimide-1,10-phenanthroline (Dec-DIPhen, Fig. 5(d)) and N2,N9-dibutyl-2,9-diimide-1,10-phenanthroline (But-DIPhen, Fig. 5(d)) have been synthesized.91,100 In the 1 M HClO4 system, the Dec-DIPhen shows good extraction and separation performance (DAm(III) = 10.3, DEu(III) = 0.2, SFAm(III)/Eu(III) = 51) with the aid of a salting-out agent (2 M LiClO4). The But-DIPhen achieved some extraction and separation effects under high nitric acid conditions (DAm(III) ≈ 1.6, DEu(III) ≈ 0.1, SFAm(III)/Eu(III) = 16, 4.0 M HNO3). Overall, since alkyl groups are weak electron-donating groups, modifying different alkyl groups has a minimal effect on the electron cloud density of the amide N atoms and carbonyl O donor atoms. Alkyl-substituted DAPhen extractants still suffer from weak extraction abilities and poor separation performance, requiring synergic extractants or salting-out agents for assistance. Thus, further improvements and enhancements are necessary.
This phenomenon saw an improvement in 2014. Based on the HSAB theory, Xiao et al. introduced tolyl groups with strong electron-donating properties as side chain substituents and creatively synthesized N,N′-diethyl-N,N′-ditolyl-2,9-diamide-1,10-phenanthroline (Et-Tol-DAPhen, Fig. 5(e)) extractant.101 This extractant combines N donor atoms on the preorganized phenanthroline skeleton with O donor atoms from the amide side chains, exhibiting advantages such as resistance to protonation, strong extraction ability, and fast extraction rate. When using cyclohexanone as the solvent, the Et-Tol-DAPhen demonstrated strong extraction and separation abilities for Am(III) even under high acidity conditions (DAm(III) ≈ 6, DEu(III) < 0.1, SFAm(III)/Eu(III) = 67, 1.0 M HNO3). When the solvent is switched to F-3, its extraction capability will be further improved (DAm(III) ≈ 20, 2.0 M HNO3). More importantly, Et-Tol-DAPhen also exhibited strong extraction abilities for U(VI) and Pu(IV) under high acid conditions (DU(VI) ≈ 100, DPu(IV) > 100, 2.0 M HNO3).102,103 This indicated that Et-Tol-DAPhen has the potential for lanthanide/actinide group separation and might be useful in Group Actinides Extraction (GANEX) processes.104,105 In counter-current cascade extraction experiments, Et-Tol-DAPhen achieved a high extraction rate for Am(III) with complete extraction in three stages (>99%, 3 M HNO3). However, Et-Tol-DAPhen showed an undesirable co-extraction for light lanthanides (ELa(III) = 25.89%, ECe(III) = 8.69%), which necessitated further adjustments and improvements.106
Extending the alkyl side chains can enhance its solubility in n-octanol, which potentially avoids the toxicity and environmental risks associated with using F-3 as a solvent. Based on this concept, the N,N′-dioctyl-N,N′-diphenyl-2,9-diamide-1,10-phenanthroline (Oct-Ph-DAPhen, Fig. 5(e)) indeed exhibited high solubility in n-octanol (> 50 mM), but its extraction ability was significantly weakened, which required the addition of 30% TBP as a synergist to achieve the desired extraction and separation effect (DAm(III) ≈ 6, DEu(III) < 0.3, SFAm(III)/Eu(III) ≈ 20, 2.0 M HNO3).107 When the side chain substituents were the aromatic tetrahydroquinoline groups, the 2,9-diacyl-bis((3,4-dihydroquinoline-1(2H)-yl)-1,10-phenanthroline (QL-DAPhen, Fig. 5(e))) also exhibited notable separation abilities at high acidity (DAm(III) = 17, DEu(III) = 0.37, SFAm(III)/Eu(III) ≈ 40, 3.0 M HNO3).108 Furthermore, the QL-DAPhen's extraction rate was very fast (t ≈ 5 min) due to the limitation of the extractant configuration by the tetrahydroquinoline group with high steric resistance.
Similar to BTPhen, a series of hydrophilic An(III) selective masking agents or stripping agents can be obtained when DAPhen extractants are modified with strong polar groups such as benzene sulfonic acid and hydroxyl. One representative of this group is disulfonated N,N′-diphenyl-2,9-diamide-1,10-phenanthroline (DS-Ph-DAPhen, Fig. 5(f)).109 When used in conjunction with TODGA, DS-Ph-DAPhen can significantly inhibit the extraction of Am(III) into the organic phase. So a good separation effect of Am(III) and Eu(III) was obtained under low acidity conditions (SFEu(III)/Am(III) > 200, 0.05–0.3 M HNO3). Increasing the concentration of the masking agent will shorten the TODGA's extraction efficiency for Eu(III), thus reducing the separation capability (SFEu(III)/Am(III) = 800→300, [DS-Ph-DAPhen] = 1→5 mM).
Hydroxyl groups are also widely used to enhance the hydrophilicity of masking agents. Different research groups have synthesized N,N′-bis(n-hydroxyethyl)-2,9-dicarboxamide-1,10-phenanthroline (nOH-DAPhen, Fig. 5(f)) ligands with two, four, and six hydroxyl groups for selectively complexing Am(III) over Eu(III) in acidic solutions. The 2OH-DAPhen masking agent shows good masking effects for Am(III) under moderate acidity, and those with longer carbon chains exhibit better separation performance (SFEu(III)/Am(III) = 162–264, 1.25 M HNO3).110 Researchers believe this is due to the stronger electron-donating ability of long-chain alkyl groups, but the fundamental reason remains to be verified. Contrary to expectations, masking agents with more hydroxyl groups showed decreased solubility in acidic solutions, likely due to the spatial structure of the ligand. Both 4OH-DAPhen and 6OH-DAPhen maintained good masking effects under similar conditions with separation factors exceeding 100.111 It is noteworthy that all these experiments involving hydrophilic masking agents included 1 M NaNO3 as a salting-out agent, which significantly enhances the extraction ability of the extractants with Eu(III). Without the salting-out agent, the separation efficiency of the TODGA + 6OH-DAPhen extraction system would be greatly reduced (DEu(III) = 34.3, DAm(III) = 0.82, SFEu(III)/Am(III) > 40, 1.0 M HNO3).112
Using phenanthroline-derived diimide extractants as a foundation, the introduction of carboxyl and amino groups, which can ionize and gain protons, leads to the creation of two types of hydrophilic An(III) masking agents: N2,N9-dibutyric acid-2,9-diimide-1,10-phenanthroline (But-acid-DIPhen, Fig. 5(f)) and N2,N9-diaminobutyl-2,9-diimide-1,10-phenanthroline (Amino-But-DIPhen, Fig. 5(f)). But-acid-DIPhen exhibits a significant selective masking ability for Am(III) under medium to high acidity conditions (SFEu(III)/Am(III) = 120, 1.5 M HNO3).113 This effect is likely due to the ionization of the carboxyl group on the side chain, which promotes the formation of dimers in the aqueous phase, thereby enhancing the stability of the complex under highly acidic conditions. Researchers also believe that But-acid-DIPhen differentially masks Cm(III) and Am(III), although their overall distribution ratios are very low (DCm(III) = 0.055, SFCm(III)/Am(III) = 4.4, 1.5 M HNO3). The design concept of Amino-But-DIPhen is opposite to that of But-acid-DIPhen. Here, the inventors utilized the characteristic that amino groups readily protonate under acidic conditions. By grafting the amino group at the end of the alkyl side chains, the Amino-But-DIPhen ligand will carry positive charges, which significantly increase the solubility of the masking agent in dilute nitric acid. By increasing the concentration of the masking agent, the separation efficiency of the extraction system can be substantially enhanced ([Amino-But-DIPhen] = 90 mM, SFEu(III)/Am(III) > 290, 1.25 M HNO3).114
In summary, both DAPhen-based lipophilic extractants and hydrophilic masking agents exhibit a strong affinity for An(III) and achieve favorable An(III)/Ln(III) separation. However, lipophilic DAPhen extractants also show significant coextraction capabilities for light lanthanides such as La(III). Additionally, the hydrophilic DAPhen-based masking agents require pairing with salting-out agents like NaNO3 to achieve effective separation. Consequently, there is still room for further improvement and enhancement in these systems.
3.3. Phenanthroline-derived organophosphorus extractants
Organophosphorus extractants containing P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordinating groups have been widely used over the past few decades to extract and separate U(VI) and Pu(IV) in the nuclear fuel cycle.115 They have also been tested for separating An(III) from Ln(III). Due to the strongly electronegative O donor atoms, the organophosphorus extractants exhibit a strong extraction capability for actinides but lack selectivity for lanthanides. In contrast, N,O-hybrid coordination phenanthroline-derived extractants offer better An(III)/Ln(III) separation capabilities, protonation resistance, and fast extraction rates, but their extraction ability for target ions is slightly insufficient.
O coordinating groups have been widely used over the past few decades to extract and separate U(VI) and Pu(IV) in the nuclear fuel cycle.115 They have also been tested for separating An(III) from Ln(III). Due to the strongly electronegative O donor atoms, the organophosphorus extractants exhibit a strong extraction capability for actinides but lack selectivity for lanthanides. In contrast, N,O-hybrid coordination phenanthroline-derived extractants offer better An(III)/Ln(III) separation capabilities, protonation resistance, and fast extraction rates, but their extraction ability for target ions is slightly insufficient.
To combine the advantages of phenanthroline-derived extractants and organophosphorus extractants, our research group designed and synthesized a series of phenanthroline-derived organophosphorus extractants, which feature a phenanthroline skeleton with organophosphorus side chains. We systematically studied their extraction and separation capabilities for An(III)/An(IV)/An(VI) and Ln(III), as well as their coordination mechanisms. Employing a relatively simple synthesis method with a high yield, involving a one-step Pd-catalyzed C–P coupling reaction (Fig. 6(a)),116 Xu and colleagues selected ethyl and n-butyl as substituents of side chains and firstly synthesized tetraethyl (1,10-phenanthrolin-2,9-diyl)phosphonate (C2-POPhen, Fig. 6(b)) and tetrabutyl-(1,10-phenanthrolin-2,9-diyl)phosphonate (C4-POPhen, Fig. 6(b)). The alkyl side chains enhance the solubility of the extractants in organic solvents, while the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordinating groups provide high extraction capabilities. Without the need for synergic extractants or salting-out agents, C2-POPhen demonstrated good extraction and separation capabilities for Am(III) and Eu(III) under high acidity conditions (DAm(III) ≈ 40, DEu(III) ≈ 5, SFAm(III)/Eu(III) ≈ 7, 2.0 M HNO3).117 By extending the alkyl side chain, the extraction capacity of C4-POPhen significantly improved, while the separation ability remained unchanged (DAm(III) > 200, DEu(III) ≈ 30, SFAm(III)/Eu(III) ≈ 7, 2.0 M HNO3).118 DFT calculations also confirmed that n-butyl is the optimal substituent for POPhen extractants, and further increasing the length of the alkyl substituents is not beneficial for improving the extraction capacity.119 Coordination chemistry experiments and calculations also confirmed that C2-POPhen and C4-POPhen can form two types of complexes with Am(III) and Eu(III), with M
O coordinating groups provide high extraction capabilities. Without the need for synergic extractants or salting-out agents, C2-POPhen demonstrated good extraction and separation capabilities for Am(III) and Eu(III) under high acidity conditions (DAm(III) ≈ 40, DEu(III) ≈ 5, SFAm(III)/Eu(III) ≈ 7, 2.0 M HNO3).117 By extending the alkyl side chain, the extraction capacity of C4-POPhen significantly improved, while the separation ability remained unchanged (DAm(III) > 200, DEu(III) ≈ 30, SFAm(III)/Eu(III) ≈ 7, 2.0 M HNO3).118 DFT calculations also confirmed that n-butyl is the optimal substituent for POPhen extractants, and further increasing the length of the alkyl substituents is not beneficial for improving the extraction capacity.119 Coordination chemistry experiments and calculations also confirmed that C2-POPhen and C4-POPhen can form two types of complexes with Am(III) and Eu(III), with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios of 1
L ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The counter anions in the solution system significantly influence the coordination mode and strength between Ln(III) and ligands.120
2, respectively. The counter anions in the solution system significantly influence the coordination mode and strength between Ln(III) and ligands.120
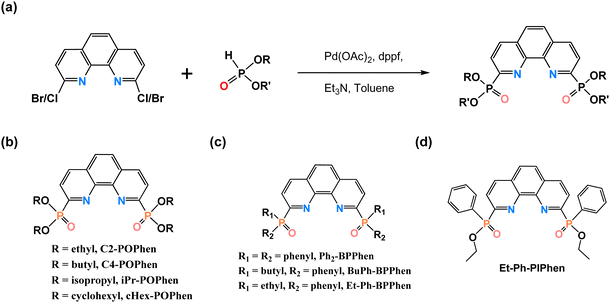 | ||
| Fig. 6 (a) Synthesis method of phenanthroline-derived organophosphorus extractants; (b) POPhen; (c) BPPhen; (d) Et-Ph-PIPhen. | ||
To study the steric hindrance effects of substituents on POPhen extractants, researchers synthesized tetraisopropyl-1,10-phenanthroline-2,9-diylbis(phosphonate) (iPr-POPhen, Fig. 6(b)) and tetracyclohexyl-1,10-phenanthroline-2,9-diylbis(phosphonate) (cHex-POPhen, Fig. 6(b)) using similar methods.121 Even at lower concentrations (2 mM), iPr-POPhen maintained a strong extraction capacity (DAm(III) ≈ 500, DEu(III) ≈ 50, SFAm(III)/Eu(III) ≈ 10, 2.0 M HNO3). The introduction of cyclohexyl groups with large steric hindrance on the side chain in cHex-POPhen did not significantly change the extraction capacity for Am(III) but slightly improved the separation ability (SFAm(III)/Eu(III) ≈ 14, 2.0 M HNO3), which achieved the highest separation factor among POPhen extractants.
Another class of phenanthroline-derived organophosphorus extractants includes bisphosphine oxide phenanthroline (BPPhen) extractants, where alkyl or aryl substituents are directly bonded to the phosphorus atoms. For traditional neutral organophosphorus extractants, as the RO-groups are increasingly replaced by R-groups (R = alkyl- or aryl-), the electron cloud density on the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups increases and enhances the extraction capability but reduces the selectivity of extractants.122 Inspired by the triphenylphosphine oxide (TPPO) with strong coordination and extraction abilities, our research group synthesized the 2,9-bis(diphenylphosphine oxide)-1,10-phenanthroline (Ph2-BPPhen, Fig. 6(c)) extractant. The Ph2-BPPhen exhibits strong co-extraction capabilities for Am(III) and Eu(III) from acidic solutions with distribution ratios peaking around 1 M HNO3 (DAm(III) > 800, DEu(III) ≈ 400, SFAm(III)/Eu(III) > 2).123 Further experiments showed that Ph2-BPPhen also has strong extraction abilities for higher-valent actinides (U(IV) and Th(IV)) under high acidity conditions (DU(VI) > 400, DTh(IV) > 800, 4.0 M HNO3).124 Based on the design concept of the TRPO process, Ph2-BPPhen could potentially be used for the co-separation of lanthanides and actinides and achieving non-α waste in HLLW.125,126
O functional groups increases and enhances the extraction capability but reduces the selectivity of extractants.122 Inspired by the triphenylphosphine oxide (TPPO) with strong coordination and extraction abilities, our research group synthesized the 2,9-bis(diphenylphosphine oxide)-1,10-phenanthroline (Ph2-BPPhen, Fig. 6(c)) extractant. The Ph2-BPPhen exhibits strong co-extraction capabilities for Am(III) and Eu(III) from acidic solutions with distribution ratios peaking around 1 M HNO3 (DAm(III) > 800, DEu(III) ≈ 400, SFAm(III)/Eu(III) > 2).123 Further experiments showed that Ph2-BPPhen also has strong extraction abilities for higher-valent actinides (U(IV) and Th(IV)) under high acidity conditions (DU(VI) > 400, DTh(IV) > 800, 4.0 M HNO3).124 Based on the design concept of the TRPO process, Ph2-BPPhen could potentially be used for the co-separation of lanthanides and actinides and achieving non-α waste in HLLW.125,126
When one phenyl group was replaced with a butyl group, the resulting 2,9-bis(butylphenylphosphine oxide)-1,10-phenanthroline (BuPh-BPPhen, Fig. 6(c)) extractant showed a slight decrease in extraction without significant improvement in separation (DAm(III), Eu(III) > 200, 2.0 M HNO3).127 Similar to the difference between C2-POPhen and C4-POPhen, the extraction capacity of the 1,10-phenanthroline-2,9-diyl)bis(ethyl(phenyl)phosphine oxide) (Et-Ph-BPPhen, Fig. 6(c)) extractant was further reduced (DAm(III), Eu(III) > 100, 2.0 M HNO3). This indicates that the length of alkyl chains affects the extraction capacity of phenanthroline-derived organophosphorus extractants but has little impact on their separation ability. Compared to Et-Ph-BPPhen, the diethyl (1,10-phenanthroline-2,9-diyl)bis(phenylphosphinate) (Et-Ph-PIPhen, Fig. 6(d)) extractant showed some improvement in separation ability (DAm(III) > 600, DEu(III) ≈ 200, SFAm(III)/Eu(III) ≈ 4, 2.0 M HNO3).128 The number of oxygen atoms attached to phosphorus atoms on the side chain will affect the separation ability of the extractant, likely related to the soft and hard nature of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Additionally, some researchers used DFT as the research method to theoretically analyze the impact of substituents on the extraction and separation performance of BPPhen extractants.129,130 Their results need further experimental validation.
O groups. Additionally, some researchers used DFT as the research method to theoretically analyze the impact of substituents on the extraction and separation performance of BPPhen extractants.129,130 Their results need further experimental validation.
Overall, phenanthroline-derived organophosphorus extractants partially combine the advantages of N,O-hybrid coordination phenanthroline-derived extractants and traditional organophosphorus extractants. Their synthesis methods are relatively simple and efficient, and they exhibit strong extraction capabilities and rapid extraction rates for f-block elements under high acidity conditions. However, their separation ability for Am(III)/Eu(III) is suboptimal and requires further improvement. Additionally, research is ongoing into the extraction and separation performance of these phenanthroline-derived organophosphorus extractants with other actinide elements (such as U(VI) and Pu(IV)), in order to lay a solid foundation for the subsequent development of separation processes based on such extractants.131,132
4. Highly preorganized extractants
4.1. Highly preorganized nitrogen-coordinated ligands
According to the chelating ligand design strategy, to enhance the coordination ability of the ligand with target ions, besides increasing the number of donor atoms and changing the types of donor atoms, another effective method is to improve the preorganization of the ligand. Flexible ligands can naturally twist in a solution environment.133 The donor atoms (such as O/N/S) on the ligand tend to repel each other due to charge repulsion and actuate ligand to form a stable conformation with minimal repulsion. However, when multidentate chelating ligands coordinate with metal ions, the various parts of the ligand need to twist to form a coordination cavity to accommodate the target ion. Pre-fixing the ligand's conformation and thereby improving its preorganization will contribute to the ligand coordination with the metal ion without requiring additional conformational changes during coordination.134 This design concept has been widely used in past ligand development. For example, replacing the bipyridine skeleton in CyMe4-BTBP with phenanthroline significantly increases the extraction efficiency and rate. Grafting catenoid extractants such as CMPO onto preorganized pillararenes also enhances their extraction and separation capabilities.135The previous text explained that the DPP ligand has a large coordination cavity, which results in a strong complexation ability with metal ions of larger radii.61 Additionally, DPP has four nitrogen donor atoms, which suggests potential An(III)/Ln(III) separation capability. Therefore, our research group aimed to further enhance preorganization of DPP (L1) by bridging the phenanthroline skeleton and pyridine side chains using 1,2-ethanediyl. This led to the design and synthesis of 12-(pyridin-2-yl)-5,6-dihydroquinolino[8,7b][1,10]phenanthroline (L2, Fig. 7(a)) and 5,6,11,12-tetrahydrobenzo[2,1-b:3,4-b′]bis([1,10]phenanthroline (L3, Fig. 7(a)) with different preorganization degrees.136 The structures of the complexes formed by these three ligands with Eu(III) are similar, which indicates the preorganized modification does not affect the coordination mode between the ligands and Ln(III). Due to different levels of side chain restriction, the degree of preorganization for the three ligands is: L3 > L2 > L1, corresponding to their coordination abilities with Eu(III). DFT calculations revealed the reasons for the differences in coordination abilities. When coordinated with metal ions, the free ligand will experience a conformational change process. The nitrogen donor atoms on the side chains needs to shift from an external to an internal position. Ligands with lower preorganization (L1) encounter higher energy barriers during this process, whereas a fully preorganized ligand (L3) does not, thus exhibiting stronger coordination abilities.
 | ||
| Fig. 7 (a) Side chain modified highly preorganized DPP-derived ligands; (b) skeleton-modified highly preorganized DPP-derived ligands; (c) BLPhen. | ||
Another strategy to enhance the preorganization of DPP is to replace its two pyridine side chains with a phenanthroline section, which reduces the number of rotatable single bonds and increases the preorganization degree. Based on this concept, 8,9-dihydro-diquino[8,7-b:7′,8′-j][1,10]phenanthroline (BIPhen, Fig. 7(b)) was developed. Compared to DPP, the presence of two phenanthroline groups in the BIPhen significantly increases its preorganization and enhances the stability of complexes with large metal ions (r > 0.9 Å), and concurrently improves its selectivity for Sm(III) and Gd(III).137 Since the ionic radius of Am(III) is very close to that of Sm(III), the size-based selectivity of BIPhen to Sm(III)/Gd(III) should be translated into enhanced Am(III)/Gd(III) selectivity. By restricting the only rotatable single bonds in the two phenanthroline groups of BIPhen, a fully preorganized 8,9-dihydro-diquino[8,7-b:7′,8′-j][1,10]phenanthroline (EBIP, Fig. 7(b)) ligand can be obtained.138 In EBIP, there are no freely rotatable single bonds and the four nitrogen donor atoms are completely coplanar, which makes it the most highly preorganized phenanthroline-derived ligand. EBIP exhibits a higher coordination ability with Ln(III) than BIPhen with no significant change in selectivity among the elements.139 Furthermore, since no conformational changes are required, the coordination rate of EBIP is much higher than that of BIPhen.
Kong et al. also investigated the complexation and separation performance of bis(pyrazole) phenanthroline-derived extractant (BPPhen) and derivatives with different preorganization degrees for Am(III) and Eu(III) by using theoretical calculations.140 They concluded that modifying the skeleton with bridging structures rather than modifying the side chains is more effective in enhancing the extraction and separation capabilities of extractants. Additionally, they found that increasing the preorganization of the pyrazole rings in the side chains of BPPhen derivatives decreases their extraction efficiency. This result may be due to the smaller coordination cavity formed by the phenanthroline skeleton and pyrazole side chains of BPPhen, which does not favor matching with Am(III). Therefore, increasing the preorganization of BPPhen is detrimental to its coordination and separation with Am(III). These theoretical findings and inferences require further experimental validation.
4.2. Highly preorganized N,O-hybrid coordinated extractants
The phenanthroline-derived N,O-hybrid extractants like DAPhen have been proven to effectively extract actinides such as Am(III) and achieve a good separation effect between actinides and lanthanides. However, their extraction capability still needs improvement. To address this issue, Jansone-Popova et al. used a Rh(III)-catalyzed C–H bond cleavage reaction to link the phenanthroline skeleton with diamide side chains and synthesized a series of highly preorganized bislactam-1,10-phenanthroline (BLPhen) extractants.141 Solvent extraction experiments demonstrated a significant enhancement in the extraction capabilities of BLPhen-type extractants for both Am(III) and Eu(III). The compound (4aS,8bS,12aS,18aS)-13,18-dihexyl-1,2,3,4,4a,8b,9,10,11,12,12a,13,18,18a-tetradecahydro-1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,12-dimethanodiquinolino[3,4-b:4′,3′-j][1,10]phenanthroline-14,17-dione (3a, Fig. 7(c)) can co-extract Am(III) and Eu(III) with no separation ability (DAm(III), Eu(III) > 1000, 3.0 M HNO3). The extraction ability of 2,11-dihexyl-3,4,9,10-tetraphenyl-2,11-dihydrodipyrido[3,4-b:4′,3′-j][1,10]phenanthroline-1,12-dione (4a, Fig. 7(c)) is also markedly improved but retains some separation capability (DAm(III) > 3000, DEu(III) ≈ 17, SFAm(III)/Eu(III) > 200, 3.0 M HNO3). Single-crystal structures of Ln(III) with BLPhen complexes show that the N and O donor atoms in the ligand are coplanar, which indicates that the high preorganization of the ligand prevents twisting and shifting of the amide side chains during coordination, thereby enhancing the ligand's binding ability with target ions. The results of the DFT calculation also support this conclusion.142
9,12-dimethanodiquinolino[3,4-b:4′,3′-j][1,10]phenanthroline-14,17-dione (3a, Fig. 7(c)) can co-extract Am(III) and Eu(III) with no separation ability (DAm(III), Eu(III) > 1000, 3.0 M HNO3). The extraction ability of 2,11-dihexyl-3,4,9,10-tetraphenyl-2,11-dihydrodipyrido[3,4-b:4′,3′-j][1,10]phenanthroline-1,12-dione (4a, Fig. 7(c)) is also markedly improved but retains some separation capability (DAm(III) > 3000, DEu(III) ≈ 17, SFAm(III)/Eu(III) > 200, 3.0 M HNO3). Single-crystal structures of Ln(III) with BLPhen complexes show that the N and O donor atoms in the ligand are coplanar, which indicates that the high preorganization of the ligand prevents twisting and shifting of the amide side chains during coordination, thereby enhancing the ligand's binding ability with target ions. The results of the DFT calculation also support this conclusion.142
Subsequent studies revealed that 3a and 4a exhibit a strong affinity for light lanthanides with larger ionic radii. Notably, the 4a extractant shows significantly higher extraction ability for light lanthanides from La(III) to Nd(III) compared to heavier lanthanides beyond Sm(III) (DLa(III)-Nd(III) > 100, SFLa(III)/Lu(III) > 1.7 × 104, 0.9 M HNO3).143 This phenomenon supports the hypothesis that highly preorganized BLPhen extractants have a stronger affinity for trivalent f-block elements with larger radii. Coordination chemistry experiments and theoretical calculations indicate that BLPhen forms complexes with Ln(III) as [Ln(BLPhen)(NO3)3] and [Ln(BLPhen)2(NO3)2]+, where the coordination mode of nitrate (monodentate or bidentate) depends on the ionic radius of the Ln(III) ion.144,145
To address the low solubility of BLPhen in organic solvents, researchers synthesized a branched alkyl-substituted tetradecahydro-1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,12-dimethanodiquinolino[3,4-b:4′,3′-j][1,10]phenanthroline-14,17-dione, which demonstrated better solubility in an Isopar L-Exxal 13 mixture. However, it did not show significant selectivity for separating Am(III) and Eu(III) (DAm(III), Eu(III) > 200, 3.0 M HNO3).146 Another approach involved grafting hydroxyl groups onto BLPhen to enhance its water miscibility, which results in a series of hydrophilic masking agents. Utilizing the “tug-of-war” effect of different ligands in aqueous and organic phases, the hydrophilic 3,4,9,10-tetrakis((2-hydroxyethoxy)methyl)-2,11-dihydrodipyrido[3,4-b:4′,3′-j][1,10]phenanthroline-1,12-dione (aqBLPhen) masking agent can synergistically interact with lipophilic extractants, thereby enhancing the intra-group separation of rare earth elements.147 It would be valuable to further investigate the masking effects of these hydrophilic BLPhen series masking agents on Am(III) and Eu(III), as they might yield unexpectedly positive results.
9,12-dimethanodiquinolino[3,4-b:4′,3′-j][1,10]phenanthroline-14,17-dione, which demonstrated better solubility in an Isopar L-Exxal 13 mixture. However, it did not show significant selectivity for separating Am(III) and Eu(III) (DAm(III), Eu(III) > 200, 3.0 M HNO3).146 Another approach involved grafting hydroxyl groups onto BLPhen to enhance its water miscibility, which results in a series of hydrophilic masking agents. Utilizing the “tug-of-war” effect of different ligands in aqueous and organic phases, the hydrophilic 3,4,9,10-tetrakis((2-hydroxyethoxy)methyl)-2,11-dihydrodipyrido[3,4-b:4′,3′-j][1,10]phenanthroline-1,12-dione (aqBLPhen) masking agent can synergistically interact with lipophilic extractants, thereby enhancing the intra-group separation of rare earth elements.147 It would be valuable to further investigate the masking effects of these hydrophilic BLPhen series masking agents on Am(III) and Eu(III), as they might yield unexpectedly positive results.
Overall, further modifications to the known phenanthroline-derived extractants can lead to synthesizing highly preorganized extractants. Experimental results generally demonstrate that increasing the preorganization of the extractants can significantly enhance their coordination ability with metal ions, thereby improving extraction efficiency. However, the current research is relatively limited with the sparse available data. Future work should build on this design concept by developing various highly preorganized phenanthroline-derived extractants based on known extractants like BTPhen and POPhen, and apply them to the separation of various metal ions, including actinides and rare earth elements. It is important to acknowledge that highly preorganized extractants often suffer from low solubility in organic solvents and other limitations that hinder further improvements in separation performance, which should also be addressed in future research.
5. Unsymmetric extractants
The previously mentioned extractants are all composed of a phenanthroline skeleton combined with two identical side chains. Except for some special cases (such as 5-Br-CyMe4-BTPhen), most phenanthroline-derived extractants exhibit C2 symmetry.55 The synthesis of these extractants typically involves first establishing the phenanthroline skeleton and then grafting the side chains with the same donor atoms and substituents on both sides. Modifying or adjusting the substituents or structure of the side chains can produce a series of unsymmetric phenanthroline-derived extractants (Fig. 8(a)). These unsymmetric extractants break the symmetry of the original extractants, which facilitates the binding of solvents around the extractants and complexes, thereby enhancing their solubility in organic solvents.Currently, the design strategies for unsymmetric phenanthroline-derived extractants primarily fall into two categories: The first involves modifying the substituents on existing extractants. This modification method does not significantly affect the main performance of the extractants. Instead, it aims to regulate the binding and separation capabilities of the extractant with the target metal ions through the electron-withdrawing or electron-donating effects and steric effects of different substituents. The second strategy directly alters the main structure of the extractant. This approach significantly impacts the fundamental properties of the extractant, which leads to the design of a series of novel unsymmetric phenanthroline-derived extractants by combining the phenanthroline skeleton with different side chains (such as amides, triazines, etc.).
5.1. Substituent modified extractants
Although the first design strategy appears to involve minimal structural modification of the extractant, a synthetic method for selectively modifying only one side of the extractant's identical side chains has yet to be developed. For example, the BTP series of extractants was invented in the 1970s with a simpler structure than that of the phenanthroline-derived extractants. Researchers have continuously focused on synthesizing symmetrical BTP series extractants with different structures.45 In 2024, Carrick and colleagues applied a new synthesis method using 2-cyano-6-methylpyridine as the starting material to efficiently obtain dozens of unsymmetric BTP extractants with different substituents on the side chains.148 They confirmed that the unsymmetric substituents indeed improve the extractant's solubility.It must be acknowledged that synthesizing unsymmetric extractants with different substituents by grafting side chains is indeed a significant challenge. The first phenanthroline-derived extractants with different substituents were developed by our group in this year: N2,N2,N9-triethyl-N9-tolyl-1,10-phenanthroline-2,9-dicarboxamide (DE-ET-DAPhen, Fig. 8(b)) and N2-ethyl-N9,N9-dioctyl-N2-tolyl-1,10-phenanthroline-2,9-dicarboxamide (DO-ET-DAPhen, Fig. 8(b)).149 These DAPhen extractants were designed based on Alk–Alk-DAPhen and Et-Tol-DAPhen by combining their high solubility and strong extraction capabilities. At the same concentration, DE-ET-DAPhen has a stronger extraction capacity than DO-ET-DAPhen (DAm(III)(Et) > 2, DAm(III)(Oct) < 1, [L] = 10 mM, 3.0 M HNO3), which is consistent with previously reported phenomena for DAPhen extractants. This may be due to the greater steric hindrance from the longer carbon chains, which reduces the extraction capability of extractants. The unsymmetric DE-ET-DAPhen extractant shows high solubility in the environmentally friendly solvent n-octanol. By increasing the extractant concentration, the desired extraction and separation performance can be achieved (DAm(III) ≈ 10, DEu(III) ≈ 0.4, SFAm(III)/Eu(III) > 20, [L] = 50 mM, 3.0 M HNO3).
Some researchers also used the DFT calculation method to study the effect of unsymmetric substituents on the coordination and separation ability of 2,9-bis(1H-pyrazol-3-yl)-1,10-phenanthroline (BPPhen) with Am(III) and Eu(III).150 They found that side chain substituents can affect the coordination ability of BPPhen derivatives without drawing regular conclusions from various computational results. Additionally, the designed ligands contained -OOH groups that are difficult to stabilize in the aqueous phase, rendering the study of little practical value as experimental guidance.
5.2. Side chain combination extractants
The attempt to design and synthesize unsymmetric extractants by introducing different structural side chains onto nitrogen heterocyclic skeletons began in 2003. Hudson et al. combined BTP with terpyridine to design a class of extractants known as 6-(5,6-dialkyl-1,2,4-triazin-3-yl)-2,2′-bipyridines (R,hemi-BTPs).151 These extractants have three nitrogen donor atoms and exhibit extraction and separation capabilities for Am(III) and Eu(III) under low acidity conditions with 2-bromodecanoic acid as a synergist. Researchers also found that changes in substituents affect the structure of the complexes, indicating that unsymmetric extractants might have significantly different coordination modes compared to symmetric analogs. Subsequently, our group combined phosphorus oxide and triazine side chains to design two pyridine-derived tridentate chelating extractants: diphenyl(6-(5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanobenzo-[1,2,4]triazin-3-yl)pyridin-2-yl)phosphine oxide (Ph2-MTP) and butylphenyl(6-(5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanobenzo-[1,2,4]triazin-3-yl)pyridin-2-yl)phosphine oxide (BuPh-MTP).152 Both MTP extractants showed strong extraction capabilities for Am(III) over Eu(III), demonstrating a degree of separation selectivity. The structure of the [M(MTP)3]3+ complex formed by MTP with metal ions is more similar to that of BTP than to aromatic phosphine oxides of pyridine (PyPO).The first unsymmetric phenanthroline-derived extractants with different side chain structures have recently been reported by our group. We combined amide and carboxylic acid side chains to synthesize 9-(N,N-dialkylcarbamoyl)-1,10-phenanthroline-2-carboxylic acid (DEAPA and DOAPA, Fig. 8(c)).153 DOAPA exhibits good solubility in n-octanol and can selectively separate U(VI) from Th(IV). Initially, extraction is performed at high acidity (4 M HNO3), followed by stripping with 1 M HNO3 to achieve the separation of a small amount of U(VI) from a large amount of Th(IV). Due to the presence of both neutral coordinating amide side chains and ionizable carboxylic acid side chains, these extractants possess characteristics of both acidic and neutral extractants. Under low acidity conditions, the extractant tends to lose protons and bind with metal ions through an ion exchange mechanism. When the acidity is higher than 1 M HNO3, DEAPA and DOAPA behave as neutral extractants and form complexes with metal ions and nitrates.
The strong extraction capability of DOAPA is attributed to its structural features, such as electron donor ability and spatial effects. On one hand, the electron-donating ability of the octyl group is stronger than that of the ethyl group, resulting in higher electron density on the amide oxygen atom, which facilitates complexation with actinide ions. On the other hand, actinide ions in solution are surrounded by solvent molecules. The hydrophobicity of the longer alkyl chain can displace these solvent molecules (desolvation), which further aids in coordination with metal ions. Additionally, the longer alkyl chain of DOAPA allows the ligand to better dissolve in the organic phase exhibits higher interfacial activity, and enhances the extraction process.
When DOAPA is saponified, the resulting 9-(N,N-dioctylcarbamoyl)-1,10-phenanthroline-2-sodium carboxylate (NaDOAPA) shows affinity for heavy lanthanide ions.154 In acidic solutions (2 ≤ pH ≤ 5), NaDOAPA demonstrates the ability to separate heavy lanthanides from light and middle lanthanides, with an average separation factor of 2.5 for adjacent heavy lanthanides. However, it should be noted that due to the strong coordinating ability of the O atoms in the side chains of DEAPA and DOAPA extractants, they lack the ability to separate An(III) from Ln(III). Introducing side chains containing softer N donor atoms might be a good improvement strategy.
By introducing different side chains containing either soft N-atom functional groups or hard O-atom functional groups at the No. 2 and No. 9 positions on the phenanthroline skeleton, novel unsymmetric phenanthroline-derived extractants can be synthesized. On the one hand, the unsymmetric extractants synthesized by this strategy are expected to reduce the extraction capacity of pure nitrogen-coordinated extractants (such as CyMe4-BTPhen) and improve the stripping performance. On the other hand, it is possible to obtain a higher Am(III) distribution ratio and separation factor than the symmetric N,O-hybrid extractants (such as DAPhen and POPhen), so as to achieve a perfect combination of the two types of extractants.
Based on this design strategy, Wang et al. conducted experiments. They selected an amide side chain containing an O donor atom and a pyrazole side chain containing a N donor atom and synthesized the N,N-diethyl-9-(5-ethyl-1H-pyrazol-3-yl)-1,10-phenanthroline-2-carboxamide (Et–Et-APPhen, Fig. 8(d)) and N,N-dihexyl-9-(5-hexyl-1H-pyrazol-3-yl)-1,10-phenanthroline-2-carboxamide (Hex–Hex-APPhen, Fig. 8(d)) extractants.155 Unfortunately, without using 2-bromohexanoic acid as a lipophilic anion source, these two N,O-hybrid unsymmetric extractants exhibited weak extraction capabilities for Am(III) and Eu(III), although they maintained a moderate separation ability (DAm(III) ≈ 0.5, DEu(III) ≈ 0.02, SFAm(III)/Eu(III) > 30, 1.0 M HNO3). Other researchers used DFT calculations to theoretically predict the extraction and separation characteristics of N-ethyl-9-(1H-pyrazol-3-yl)-N-(p-tolyl)-1,10-phenanthroline-2-carboxamide.156 They found that it has strong coordinating abilities with Am(III) and Eu(III), but a weaker separation ability compared to its bipyridine-based analogs. These predictions have yet to be experimentally confirmed.
Does this mean that using side chains with different donor atoms to design unsymmetric phenanthroline-derived extractants is an unreasonable approach? Our recent research provides a definitive answer to this question. Designing an extractant that combines extraction efficiency, stripping performance, and selectivity for An(III)/Ln(III) separation is a significant challenge. We believe that unsymmetric extractants composed of N-heterocyclic skeletons (pyridine, bipyridine, phenanthroline) with amide and triazine functional groups can achieve this goal. Based on the above design concept and objectives, we first designed a series of unsymmetric extractants: 2-N-ethyl-N-(tolyl)carboxamide-6-(5,5,8,8-tetramethylcyclohexyl-[1,2,4]triazin-3-yl)-pyridine (Et-Tol-CyMe4-ATP, Fig. 8(e)), 6-N-ethyl-N-(tolyl)carboxamide-6′-(5,5,8,8-tetramethylcyclohexyl-[1,2,4]triazin-3-yl)-2,2′-bipyridine (Et-Tol-CyMe4-ATBP, Fig. 8(e)), and 2-N-ethyl-N-(tolyl)carboxamide-9-(5,5,8,8-tetramethylcyclohexyl-[1,2,4]triazin-3-yl)-1,10-phenanthroline (Et-Tol-CyMe4-ATPhen, Fig. 8(e)).157 Then, we used theoretical calculation-assisted prediction to screen these extractants for their binding capacity and separation potential with Am(III) and Eu(III). It was found that Et-Tol-CyMe4-ATPhen undoubtedly exhibited the best extraction and separation performance. Subsequently, we developed a synthesis method for these extractants based on the de novo construction of the phenanthroline skeleton, which allows for the simple and efficient synthesis of unsymmetric extractants with different side chains. Extraction experiments showed that Et-Tol-CyMe4-ATPhen indeed demonstrated excellent Am(III) and Eu(III) extraction and separation performance over a wide acidity range, maintaining good performance even at high solution acidity. Additionally, Et-Tol-CyMe4-ATPhen exhibited the ability to separate the entire lanthanide series (DAm(III) > 40, DLn(III) < 0.2, SFAm(III)/Eu(III) > 280, 2.0 M HNO3) and effectively avoided the co-extraction with partial lanthanides seen in DAPhen and BTPhen extractants. More importantly, without the need for additional chemical reagents, the majority of Am(III) could be stripped using dilute nitric acid (SAm(III) > 92%, 0.001 M HNO3), which laid a solid foundation for the subsequent development of separation processes.
Coordination chemistry studies also showed that the unsymmetric Et-Tol-CyMe4-ATPhen can form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with Ln(III) as a tetradentate ligand, similar to DAPhen and BTPhen. Another recently published article focused on using DFT calculations to study the coordination performance differences between Et-Tol-CyMe4-ATPhen, CyMe4-BTPhen, and Et-Tol-DAPhen.158 Given that Et-Tol-CyMe4-ATPhen is the extractant capable of meeting the requirements of extraction, separation, and stripping simultaneously, we believe it has significant application value and represents a key choice for the future of An(III)/Ln(III) separation processes.
2 complexes with Ln(III) as a tetradentate ligand, similar to DAPhen and BTPhen. Another recently published article focused on using DFT calculations to study the coordination performance differences between Et-Tol-CyMe4-ATPhen, CyMe4-BTPhen, and Et-Tol-DAPhen.158 Given that Et-Tol-CyMe4-ATPhen is the extractant capable of meeting the requirements of extraction, separation, and stripping simultaneously, we believe it has significant application value and represents a key choice for the future of An(III)/Ln(III) separation processes.
6. Conclusion and outlook
In summary, phenanthroline-derived extractants exhibit strong extraction capabilities, rapid extraction rates, and good separation abilities for actinides with large radii, which proves they are highly valuable for An(III)/Ln(III) extraction and separation. Based on different development strategies, this paper categorizes the tetradentate chelating phenanthroline-derived extractants developed over the past decade into four types: nitrogen-coordinated extractants, N,O-hybrid coordinated extractants, highly preorganized extractants, and unsymmetric extractants. The design concepts and development histories of each series of extractants are thoroughly explained with representative phenanthroline-derived extractants illustrated as examples. The structure-activity relationships of these extractants are systematically summarized and analyzed in detail. These experimental and computational results not only aid in screening efficient An(III)/Ln(III) separation extractants but also provide a data foundation and reference for subsequent process development.Based on the design principles summarized in this paper, I believe the promising development directions for novel phenanthroline-derived extractants are as follows:
(1) Introducing substituents with electron-donating or electron-withdrawing capabilities and varying steric hindrance at different positions on the phenanthroline skeleton can precisely modulate the extraction and separation performance of extractants. This strategy can be extended from nitrogen-coordinated BTPhen extractants to N,O-hybrid extractants like DAPhen and POPhen, which potentially enhance the extraction and separation performance of various derivative extractants.
(2) The current variety of extractant side chains containing N or O donor atoms is still limited. Developing diverse side chains with potential coordination capabilities (e.g., various nitrogen heterocycles and sulfur-containing groups) can expand the database of extractant structure and performance relationships and lay the foundation for discovering more efficient extractants.
(3) Regulating the degree of preorganization of extractants significantly impacts their extraction and separation performance. By varying the number of restrictive structures to obtain new extractants with different degrees of preorganization, it might be possible to achieve a balance in extraction and separation performance.
(4) Extractants based on unsymmetric design principles can combine the advantages of two side chains while avoiding their drawbacks, which presents high scientific and practical value. Developing various novel unsymmetric extractants is both urgent and important, which represents a promising future direction.
(5) With the rapid development of big data and artificial intelligence (AI), AI has already been applied successfully in fields like drug design. Introducing advanced computational techniques into the design of novel extractant structures could significantly accelerate and enhance the efficiency of extractant development. I eagerly hope radiochemists will engage in more interdisciplinary collaborations and cultivate versatile talents to explore and implement these advancements.
Author contributions
Xiaofan Yang: conceptualization and writing – original draft; Lei Xu: writing – review & editing; Dong Fang: resources; Anyun Zhang: project administration; Chengliang Xiao: funding acquisition, supervision, and writing – review & editing.Data availability
No original research data were used in this feature article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number: U2067213, 22206168).References
- F. Gralla, D. J. Abson, A. P. Møller, D. J. Lang and H. von Wehrden, Renewable Sustainable Energy Rev., 2017, 70, 1251–1265 CrossRef
.
- R. C. Ewing, Nat. Mater., 2015, 14, 252–257 CrossRef CAS PubMed
.
- J. Bruno and R. C. Ewing, Elements, 2006, 2, 343–349 CrossRef CAS
.
- P. Baron, S. M. Cornet, E. D. Collins, G. DeAngelis, G. Del Cul, Y. Fedorov, J. P. Glatz, V. Ignatiev, T. Inoue, A. Khaperskaya, I. T. Kim, M. Kormilitsyn, T. Koyama, J. D. Law, H. S. Lee, K. Minato, Y. Morita, J. Uhlíř, D. Warin and R. J. Taylor, Prog. Nucl. Energy, 2019, 117, 103091 CrossRef CAS
.
- C. D. Bowman, Annu. Rev. Nucl. Part. Sci., 1998, 48, 505–556 CrossRef CAS
.
- NEA, Potential Benefits and Impacts of Advanced Nuclear Fuel Cycles with Actinide Partitioning and Transmutation, OECD Publishing, Paris, 2011 Search PubMed.
- N. Agmon, J. Am. Chem. Soc., 2017, 139, 15068–15073 CrossRef CAS PubMed
.
- M. Rahm, R. Hoffmann and N. W. Ashcroft, Chem. – Eur. J., 2016, 22, 14625–14632 CrossRef CAS PubMed
.
- D. Lundberg and I. Persson, Coord. Chem. Rev., 2016, 318, 131–134 CrossRef CAS
.
-
Z. Yoshida, S. G. Johnson, T. Kimura and J. R. Krsul, in The Chemistry of the Actinide and Transactinide Elements, ed. L. R. Morss, N. M. Edelstein and J. Fuger, Springer, Netherlands, Dordrecht, 2006, pp. 699–812 Search PubMed
.
- D. Lundberg, J. Radioanal. Nucl. Chem., 2018, 316, 849–854 CrossRef CAS PubMed
.
- J. A. Peters, K. Djanashvili, C. F. G. C. Geraldes and C. Platas-Iglesias, Coord. Chem. Rev., 2020, 406, 213146 CrossRef CAS
.
- A. Leoncini, J. Huskens and W. Verboom, Chem. Soc. Rev., 2017, 46, 7229–7273 RSC
.
- M. Ephritikhine, Coord. Chem. Rev., 2016, 319, 35–62 CrossRef CAS
.
- J. Su, E. R. Batista, K. S. Boland, S. E. Bone, J. A. Bradley, S. K. Cary, D. L. Clark, S. D. Conradson, A. S. Ditter, N. Kaltsoyannis, J. M. Keith, A. Kerridge, S. A. Kozimor, M. W. Löble, R. L. Martin, S. G. Minasian, V. Mocko, H. S. La Pierre, G. T. Seidler, D. K. Shuh, M. P. Wilkerson, L. E. Wolfsberg and P. Yang, J. Am. Chem. Soc., 2018, 140, 17977–17984 CrossRef CAS PubMed
.
- Y. Zhang, W. Duan, Y. Yang, T. Jian, Y. Qiao, G. Ren, N. Zhang, L. Zheng, W. Yan, J. Wang, J. Chen, S. G. Minasian and T. Sun, Inorg. Chem., 2022, 61, 92–104 CrossRef CAS PubMed
.
- Y. Zhang, W. Duan, Y. Yang, Z. Zhao, G. Ren, N. Zhang, L. Zheng, J. Chen, J. Wang and T. Sun, Inorg. Chem., 2024, 63, 2597–2605 CrossRef CAS PubMed
.
- J. Dognon, Coord. Chem. Rev., 2017, 344, 150–162 CrossRef CAS
.
- A. Peterson and J. N. Wacker, Nat. Rev. Chem., 2024, 8, 408–409 CrossRef PubMed
.
- W. H. Runde and B. J. Mincher, Chem.
Rev., 2011, 111, 5723–5741 CrossRef CAS PubMed
.
- C. J. Dares, A. M. Lapides, B. J. Mincher and T. J. Meyer, Science, 2015, 350, 652–655 CrossRef CAS PubMed
.
- K. McCann, B. J. Mincher, N. C. Schmitt and J. C. Braley, Ind. Eng. Chem. Res., 2017, 56, 6515–6519 CrossRef CAS
.
- J. D. Burns and B. A. Moyer, Inorg. Chem., 2016, 55, 8913–8919 CrossRef CAS PubMed
.
- H. Zhang, A. Li, K. Li, Z. Wang, X. Xu, Y. Wang, M. V. Sheridan, H. S. Hu, C. Xu, E. V. Alekseev, Z. Zhang, P. Yan, K. Cao, Z. Chai, T. E. Albrecht-Schonzart and S. Wang, Nature, 2023, 616, 482–487 CrossRef CAS PubMed
.
- Z. Wang, L. Huang, X. Dong, T. Wu, Q. Qing, J. Chen, Y. Lu and C. Xu, Nat. Commun., 2023, 14, 261 CrossRef CAS PubMed
.
- Z. Wang, J. Lu, X. Dong, Q. Yan, X. Feng, H. Hu, S. Wang, J. Chen, J. Li and C. Xu, J. Am. Chem. Soc., 2022, 144, 6383–6389 CrossRef CAS PubMed
.
- A. Geist, J. Adnet, S. Bourg, C. Ekberg, H. Galán, P. Guilbaud, M. Miguirditchian, G. Modolo, C. Rhodes and R. Taylor, Sep. Sci. Technol., 2021, 56, 1866–1881 CrossRef CAS
.
- G. Modolo, A. Wilden, A. Geist, D. Magnusson and R. Malmbeck, Radiochim. Acta, 2012, 100, 715–725 CrossRef CAS
.
- M. Miguirditchian, V. Vanel, C. Marie, V. Pacary, M. Charbonnel, L. Berthon, X. Hérès, M. Montuir, C. Sorel, M. Bollesteros, S. Costenoble, C. Rostaing, M. Masson and C. Poinssot, Solvent Extr. Ion Exch., 2020, 38, 365–387 CrossRef CAS
.
- C. Ebenezer and R. V. Solomon, Comments Inorg. Chem., 2024, 1–75 Search PubMed
.
- Y. Wang, K. M. Shield and R. J. Abergel, Sep. Purif. Rev., 2024, 53, 119–137 CrossRef CAS
.
- M. Nilsson and K. L. Nash, Solvent Extr. Ion Exch., 2007, 25, 665–701 CrossRef CAS
.
- B. Weaver and F. A. Kappelmann, United States, 1964.
- K. L. Nash, Solvent Extr. Ion Exch., 2014, 33, 1–55 CrossRef
.
- S. A. Ansari, P. Pathak, P. K. Mohapatra and V. K. Manchanda, Chem. Rev., 2012, 112, 1751–1772 CrossRef CAS PubMed
.
- Y. Zhu, J. Chen and R. Jiao, Solvent Extr. Ion Exch., 1996, 14, 61–68 CrossRef CAS
.
- N. P. Bessen, J. A. Jackson, M. P. Jensen and J. C. Shafer, Coord. Chem. Rev., 2020, 421, 213446 CrossRef CAS
.
- J. Chen, R. Jiao and Y. Zhu, Solvent Extr. Ion Exch., 1996, 14, 555–565 CrossRef CAS
.
- A. Geist and P. J. Panak, Solvent Extr. Ion Exch., 2021, 39, 128–151 CrossRef CAS
.
- M. V. Evsiunina, P. I. Matveev, S. N. Kalmykov and V. G. Petrov, Moscow Univ. Chem. Bull., 2021, 76, 287–315 CrossRef
.
- P. G. Sammes and G. Yahioglu, Chem. Soc. Rev., 1994, 23, 327 RSC
.
- Z. Kolarik, Chem. Rev., 2008, 108, 4208–4252 CrossRef CAS PubMed
.
- Z. Kolarik, U. Müllich and F. Gassner, Solvent Extr. Ion Exch., 1999, 17, 23–32 CrossRef CAS
.
- Z. Kolarik, U. Mullich and F. Gassner, Solvent Extr. Ion Exch., 2010, 17, 1155–1170 CrossRef
.
- P. J. Panak and A. Geist, Chem. Rev., 2013, 113, 1199–1236 CrossRef CAS PubMed
.
- T. Retegan, C. Ekberg, I. Dubois, A. Fermvik, G. Skarnemark and T. J. Wass, Solvent Extr. Ion Exch., 2007, 25, 417–431 CrossRef CAS
.
- M. G. B. Drew, M. R. S. J. Foreman, C. Hill, M. J. Hudson and C. Madic, Inorg. Chem. Commun., 2005, 8, 239–241 CrossRef CAS
.
- A. Geist, C. Hill, G. Modolo, M. R. S. J. Foreman, M. Weigl, K. Gompper and M. J. Hudson, Solvent Extr. Ion Exch., 2006, 24, 463–483 CrossRef CAS
.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T. Vu and J. Simonin, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed
.
- M. J. Hudson, L. M. Harwood, D. M. Laventine and F. W. Lewis, Inorg. Chem., 2013, 52, 3414–3428 CrossRef CAS PubMed
.
- E. M. Archer, S. S. Galley, J. A. Jackson and J. C. Shafer, Solvent Extr. Ion Exch., 2023, 41, 697–740 CrossRef CAS
.
- G. Accorsi, A. Listorti, K. Yoosaf and N. Armaroli, Chem. Soc. Rev., 2009, 38, 1690 RSC
.
- C. Queffélec, P. B. Pati and Y. Pellegrin, Chem. Rev., 2024, 124, 6700–6902 CrossRef PubMed
.
- M. Alyapyshev, V. Babain and D. Kirsanov, Energies, 2022, 15, 7380 CrossRef CAS
.
- L. Xu, X. Yang, A. Zhang, C. Xu and C. Xiao, Coord. Chem. Rev., 2023, 496, 215404 CrossRef CAS
.
- R. Zong and R. P. Thummel, J. Am. Chem. Soc., 2004, 126, 10800–10801 CrossRef CAS PubMed
.
- G. M. Cockrell, G. Zhang, D. G. VanDerveer, R. P. Thummel and R. D. Hancock, J. Am. Chem. Soc., 2008, 130, 1420–1430 CrossRef CAS PubMed
.
- R. Zong, G. Zhang, S. V. Eliseeva, J. G. Bünzli and R. P. Thummel, Inorg. Chem., 2010, 49, 4657–4664 CrossRef CAS PubMed
.
- R. D. Hancock and A. E. Martell, Chem. Rev., 1989, 89, 1875–1914 CrossRef CAS
.
- R. D. Hancock, Chem. Soc. Rev., 2013, 42, 1500–1524 RSC
.
- A. N. Carolan, G. M. Cockrell, N. J. Williams, G. Zhang, D. G. VanDerveer, H. Lee, R. P. Thummel and R. D. Hancock, Inorg. Chem., 2013, 52, 15–27 CrossRef CAS
.
- F. W. Lewis, M. J. Hudson and L. M. Harwood, Synlett, 2011, 2609–2632 CAS
.
- D. M. Whittaker, T. L. Griffiths, M. Helliwell, A. N. Swinburne, L. S. Natrajan, F. W. Lewis, L. M. Harwood, S. A. Parry and C. A. Sharrad, Inorg. Chem., 2013, 52, 3429–3444 CrossRef CAS PubMed
.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, A. Wilden, M. Sypula, G. Modolo, T. Vu, J. Simonin, G. Vidick, N. Bouslimani and J. F. Desreux, Procedia Chem., 2012, 7, 231–238 CrossRef CAS
.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, V. Hubscher-Bruder, V. Videva, F. Arnaud-Neu, K. Stamberg and S. Vyas, Inorg. Chem., 2013, 52, 4993–5005 CrossRef CAS PubMed
.
- C. Xiao, C. Wang, J. Lan, L. Yuan, Y. Zhao, Z. Chai and W. Shi, Radiochim. Acta, 2014, 102, 875–886 CrossRef CAS
.
- L. M. Harwood, D. M. Laventine, A. Afsar and M. J. Hudson, Heterocycles, 2012, 86, 1419 CrossRef PubMed
.
- M. A. Higginson, N. D. Kyle, O. J. Marsden, P. Thompson, F. R. Livens and S. L. Heath, Dalton Trans., 2015, 44, 16547–16552 RSC
.
- A. V. Zaytsev, R. Bulmer, V. N. Kozhevnikov, M. Sims, G. Modolo, A. Wilden, P. G. Waddell, A. Geist, P. J. Panak, P. Wessling and F. W. Lewis, Chem. – Eur. J., 2020, 26, 428–437 CrossRef CAS PubMed
.
- J. Westwood, L. M. Harwood, A. Afsar, J. Cowell, P. Distler and J. John, Lett. Org. Chem., 2018, 15, 340–344 CrossRef CAS
.
- A. Afsar, D. M. Laventine, L. M. Harwood, M. J. Hudson and A. Geist, Chem. Commun., 2013, 49, 8534 RSC
.
- A. Afsar, L. M. Harwood, M. J. Hudson, J. Westwood and A. Geist, Chem. Commun., 2015, 51, 5860–5863 RSC
.
- M. Li, X. Liu, Q. Yang, Y. Liu, J. Nie, S. Yang, Y. Yang, F. Lou, S. Xiao, Y. Ouyang and G. Ye, Molecules, 2022, 27, 1786 CrossRef CAS PubMed
.
- A. C. Edwards, C. Wagner, A. Geist, N. A. Burton, C. A. Sharrad, R. W. Adams, R. G. Pritchard, P. J. Panak, R. C. Whitehead and L. M. Harwood, Dalton Trans., 2016, 45, 18102–18112 RSC
.
- A. C. Edwards, A. Geist, U. Mullich, C. A. Sharrad, R. G. Pritchard, R. C. Whitehead and L. M. Harwood, Chem. Commun., 2017, 53, 8160–8163 RSC
.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, A. Geist, V. N. Kozhevnikov, P. Distler and J. John, Chem. Sci., 2015, 6, 4812–4821 RSC
.
- P. Kaufholz, G. Modolo, A. Wilden, F. Sadowski, D. Bosbach, C. Wagner, A. Geist, P. J. Panak, F. W. Lewis and L. M. Harwood, Solvent Extr. Ion Exch., 2016, 34, 126–140 CrossRef CAS
.
- J. Jose, T. Prathibha, N. S. Karthikeyan, K. A. Venkatesan, S. Sriram, H. Seshadri, B. Venkatachalapathy and C. Ravichandran, J. Radioanal. Nucl. Chem., 2020, 326, 1819–1829 CrossRef CAS
.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, A. Afsar, D. M. Laventine, K. Šťastná, J. John and P. Distler, Solvent Extr. Ion Exch., 2018, 36, 115–135 CrossRef CAS
.
- Y. Yang, J. Liu, L. Yang, K. Li, H. Zhang, S. Luo and L. Rao, Dalton Trans., 2015, 44, 8959–8970 RSC
.
- Y. Liu, X. Yang, S. Ding, Z. Wang, L. Zhang, L. Song, Z. Chen and X. Wang, Inorg. Chem., 2018, 57, 5782–5790 CrossRef CAS PubMed
.
- A. C. Edwards, P. Mocilac, A. Geist, L. M. Harwood, C. A. Sharrad, N. A. Burton, R. C. Whitehead and M. A. Denecke, Chem. Commun., 2017, 53, 5001–5004 RSC
.
- Y. Q. Wan, H. Hao, L. Yu, Z. P. Wang and P. Mocilac, RSC Adv., 2023, 13, 21982–21990 RSC
.
- D. L. Melton, D. G. VanDerveer and R. D. Hancock, Inorg. Chem., 2006, 45, 9306–9314 CrossRef CAS PubMed
.
- N. E. Dean, R. D. Hancock, C. L. Cahill and M. Frisch, Inorg. Chem., 2008, 47, 2000–2010 CrossRef CAS PubMed
.
- N. J. Williams, N. E. Dean, D. G. VanDerveer, R. C. Luckay and R. D. Hancock, Inorg. Chem., 2009, 48, 7853–7863 CrossRef CAS PubMed
.
- Y. Yang, Z. Zhang, L. Yang, J. Liu, C. Xu, S. Luo and L. Rao, Inorg. Chem., 2019, 58, 6064–6074 CrossRef CAS PubMed
.
- M. D. Ogden, S. I. Sinkov, M. Nilson, G. J. Lumetta, R. D. Hancock and K. L. Nash, J. Solution Chem., 2013, 42, 211–225 CrossRef CAS
.
- D. Merrill, J. M. Harrington, H. Lee and R. D. Hancock, Inorg. Chem., 2011, 50, 8348–8355 CrossRef CAS PubMed
.
- D. Merrill and R. D. Hancock, Radiochim. Acta, 2011, 99, 161–166 CrossRef CAS
.
- D. Manna, S. Mula, A. Bhattacharyya, S. Chattopadhyay and T. K. Ghanty, Dalton Trans., 2015, 44, 1332–1340 RSC
.
- B. Chen, J. Liu, L. Lv, L. Yang, S. Luo, Y. Yang and S. Peng, Inorg. Chem., 2019, 58, 7416–7425 CrossRef CAS PubMed
.
- X. Zhang, X. Kong, L. Yuan, Z. Chai and W. Shi, Inorg. Chem., 2019, 58, 10239–10247 CrossRef CAS PubMed
.
- M. Galletta, S. Scaravaggi, E. Macerata, A. Famulari, A. Mele, W. Panzeri, F. Sansone, A. Casnati and M. Mariani, Dalton Trans., 2013, 42, 16930–16938 RSC
.
- N. Tsutsui, Y. Ban, H. Suzuki, M. Nakase, S. Ito, Y. Inaba, T. Matsumura and K. Takeshita, Anal. Sci., 2020, 36, 241–245 CrossRef CAS PubMed
.
- R. Meng, L. Xu, X. Yang, M. Sun, C. Xu, N. E. Borisova, X. Zhang, L. Lei and C. Xiao, Inorg. Chem., 2021, 60, 8754–8764 CrossRef CAS PubMed
.
- P. S. Lemport, M. V. Evsiunina, P. I. Matveev, V. S. Petrov, A. S. Pozdeev, E. K. Khult, Y. V. Nelyubina, K. L. Isakovskaya, V. A. Roznyatovsky, I. P. Gloriozov, B. N. Tarasevich, A. S. Aldoshin, V. G. Petrov, S. N. Kalmykov, Y. A. Ustynyuk and V. G. Nenajdenko, Inorg. Chem. Front., 2022, 9, 4402–4412 RSC
.
- X. Zhang, L. Yuan, Z. Chai and W. Shi, Sci. China: Chem., 2018, 61, 1285–1292 CrossRef CAS
.
- H. Wang, T. Cui, J. Sui, P. Mocilac, Y. Wang and Z. Guo, Sep. Purif. Technol., 2022, 282, 120046 CrossRef CAS
.
- Y. Liu, M. Bao, L. Wang, Y. Kang, Y. Dou, J. Qin, F. Guo, H. Hao, Z. Wang, X. Tang, J. Chen, L. Wang and C. Xu, Chem. Eng. J., 2024, 485, 149730 CrossRef CAS
.
- C. Xiao, C. Wang, L. Yuan, B. Li, H. He, S. Wang, Y. Zhao, Z. Chai and W. Shi, Inorg. Chem., 2014, 53, 1712–1720 CrossRef CAS PubMed
.
- X. Zhang, L. Yuan, Z. Chai and W. Shi, Sep. Purif. Technol., 2016, 168, 232–237 CrossRef CAS
.
- X. Zhang, Q. Wu, J. Lan, L. Yuan, C. Xu, Z. Chai and W. Shi, Sep. Purif. Technol., 2019, 223, 274–281 CrossRef CAS
.
- R. Taylor, M. Carrott, H. Galan, A. Geist, X. Hères, C. Maher, C. Mason, R. Malmbeck, M. Miguirditchian, G. Modolo, C. Rhodes, M. Sarsfield and A. Wilden, Procedia Chem., 2016, 21, 524–529 CrossRef
.
- T. Lyseid Authen, A. Wilden, D. Schneider, F. Kreft, G. Modolo, M. R. StJ Foreman and C. Ekberg, Solvent Extr. Ion Exch., 2022, 40, 189–202 CrossRef CAS
.
- X. Yang, P. Ren, Q. Yang, J. Geng, J. Zhang, L. Yuan, H. Tang, Z. Chai and W. Shi, Inorg. Chem., 2021, 60, 9745–9756 CrossRef CAS PubMed
.
- X. Yang, Y. Liu, W. Tao, S. Wang, P. Ren, S. Yang, L. Yuan, H. Tang, Z. Chai and W. Shi, J. Environ. Chem. Eng., 2022, 10, 108401 CrossRef CAS
.
- S. Wang, C. Wang, X. Yang, J. Yu, W. Tao, S. Yang, P. Ren, L. Yuan, Z. Chai and W. Shi, Inorg. Chem., 2021, 60, 19110–19119 CrossRef CAS PubMed
.
- P. Ren, P. Huang, X. Yang, Y. Zou, W. Tao, S. Yang, Y. Liu, Q. Wu, L. Yuan, Z. Chai and W. Shi, Inorg. Chem., 2021, 60, 357–365 CrossRef CAS PubMed
.
- Y. Liu, Y. Kang, M. Bao, H. Cao, C. Weng, X. Dong, H. Hao, X. Tang, J. Chen, L. Wang and C. Xu, J. Hazard. Mater., 2024, 462, 132756 CrossRef CAS PubMed
.
- Q. Wu, H. Hao, Y. Liu, L. Sha, W. Wang, W. Shi, Z. Wang and Z. Yan, Inorg. Chem., 2024, 63, 462–473 CrossRef CAS PubMed
.
- S. Scaravaggi, E. Macerata, M. Galletta, E. Mossini, A. Casnati, M. Anselmi, F. Sansone and M. Mariani, J. Radioanal. Nucl. Chem., 2015, 303, 1811–1820 CAS
.
- D. Tian, Y. Liu, Y. Kang, Y. Zhao, P. Li, C. Xu and L. Wang, ACS Cent. Sci., 2023, 9, 1642–1649 CrossRef CAS PubMed
.
- H. Cao, Y. Kang, B. Li, Y. Liu, M. Bao, H. Li, Y. Zheng, L. Wang, C. Weng, X. Tang, L. Wang and C. Xu, Inorg. Chem., 2024, 63, 10511–10518 CrossRef CAS PubMed
.
- X. Yang, L. Xu, A. Zhang and C. Xiao, Chem. – Eur. J., 2023, 29, e202300456 CrossRef CAS PubMed
.
- G. G. Zakirova, D. Y. Mladentsev and N. E. Borisova, Tetrahedron Lett., 2017, 58, 3415–3417 CrossRef CAS
.
- L. Xu, N. Pu, Y. Li, P. Wei, T. Sun, C. Xiao, J. Chen and C. Xu, Inorg. Chem., 2019, 58, 4420–4430 CrossRef CAS PubMed
.
- L. Xu, N. Pu, G. Ye, C. Xu, J. Chen, X. Zhang, L. Lei and C. Xiao, Inorg. Chem. Front., 2020, 7, 1726–1740 RSC
.
- C. Ebenezer and R. V. Solomon, Polyhedron, 2021, 210, 115533 CrossRef CAS
.
- X. Yang, L. Xu, Y. Hao, R. Meng, X. Zhang, L. Lei and C. Xiao, Inorg. Chem., 2020, 59, 17453–17463 CrossRef CAS PubMed
.
- P. I. Matveev, P. Huang, A. A. Kirsanova, I. V. Ananyev, T. B. Sumyanova, A. V. Kharcheva, E. Y. Khvorostinin, V. G. Petrov, W. Shi, S. N. Kalmykov and N. E. Borisova, Inorg. Chem., 2021, 60, 14563–14581 CrossRef CAS PubMed
.
- P. A. Yudaev, N. A. Kolpinskaya and E. M. Chistyakov, Hydrometallurgy, 2021, 201, 105558 CrossRef CAS
.
- L. Xu, X. Yang, Z. Wang, S. Wang, M. Sun, C. Xu, X. Zhang, L. Lei and C. Xiao, Inorg. Chem., 2021, 60, 2805–2815 CrossRef CAS PubMed
.
- X. Yang, L. Xu, S. Wang, D. Fang, A. Zhang and C. Xiao, Sep. Purif. Technol., 2025, 354, 128802 CrossRef CAS
.
- W. Jianchen and S. Chongli, Solvent Extr. Ion Exch., 2001, 19, 231–242 CrossRef CAS
.
- J. Chen, X. He and J. Wang, Radiochim. Acta, 2014, 102, 41–51 CrossRef CAS
.
- L. Xu, Y. Hao, X. Yang, Z. Wang, C. Xu, N. E. Borisova, M. Sun, X. Zhang, L. Lei and C. Xiao, Chem. – Eur. J., 2021, 27, 10717–10730 CrossRef CAS PubMed
.
- X. Yang, S. Wang, L. Xu, Q. Yan, C. Xu, P. Matveev, L. Lei and C. Xiao, Inorg. Chem. Front., 2022, 9, 4671–4684 RSC
.
- C. Ebenezer and R. V. Solomon, ChemistrySelect, 2022, 7, e202200446 CrossRef CAS
.
- C. Ebenezer and R. V. Solomon, New J. Chem., 2022, 46, 5761–5770 RSC
.
- X. Yang, S. Wang, L. Xu, C. Liu, C. Jin, A. Zhang and C. Xiao, Sep. Purif. Technol., 2023, 320, 124124 CrossRef CAS
.
- X. Yang, D. Fang, S. Wang, Z. Tian, L. Xu, J. Liu, A. Zhang and C. Xiao, Chem. Commun., 2024, 60, 5042–5045 RSC
.
- R. D. Hancock, D. L. Melton, J. M. Harrington, F. C. McDonald, R. T. Gephart, L. L. Boone, S. B. Jones, N. E. Dean, J. R. Whitehead and G. M. Cockrell, Coord. Chem. Rev., 2007, 251, 1678–1689 CrossRef CAS
.
- R. D. Hancock, J. Chem. Educ., 1992, 69, 615 CrossRef CAS
.
- L. Chen, Y. Cai, W. Feng and L. Yuan, Chem. Commun., 2019, 55, 7883–7898 RSC
.
- D. Fang, X. Yang, J. Li, Z. Zhang, Y. Gao and C. Xiao, Inorg. Chem., 2024, 63, 8171–8179 CrossRef CAS PubMed
.
- L. E. Tucker, G. C. Littman, S. Uritis, J. W. Nugent, R. P. Thummel, J. H. Reibenspies, S. B. Jones, H. Lee and R. D. Hancock, Inorg. Chem., 2020, 59, 13117–13127 CrossRef CAS PubMed
.
- S. Uritis, R. P. Thummel, H. Lee and R. D. Hancock, Inorg. Chim. Acta, 2022, 530, 120670 CrossRef CAS
.
- S. Uritis, R. P. Thummel, S. B. Jones, H. S. Lee and R. D. Hancock, Eur. J. Inorg. Chem., 2024, 27, e202300633 CrossRef CAS
.
- X. Kong, Q. Wu, J. Lan, C. Wang, Z. Chai, C. Nie and W. Shi, Inorg. Chem., 2018, 57, 14810–14820 CrossRef CAS PubMed
.
- S. Jansone-Popova, A. S. Ivanov, V. S. Bryantsev, F. V. Sloop, R. Custelcean, I. Popovs, M. M. Dekarske and B. A. Moyer, Inorg. Chem., 2017, 56, 5911–5917 CrossRef CAS PubMed
.
- X. P. Lei, Q. Y. Wu, C. Z. Wang, J. H. Lan, Z. F. Chai, C. M. Nie and W. Q. Shi, Dalton Trans., 2022, 51, 16659–16667 RSC
.
- M. R. Healy, A. S. Ivanov, Y. Karslyan, V. S. Bryantsev, B. A. Moyer and S. Jansone-Popova, Chem. – Eur. J., 2019, 25, 6326–6331 CrossRef CAS PubMed
.
- T. D. N. Reddy, A. S. Ivanov, D. M. Driscoll, S. Jansone-Popova and D. Jiang, ACS Omega, 2022, 7, 21317–21324 CrossRef CAS PubMed
.
- T. D. N. Reddy, A. S. Ivanov, D. M. Driscoll, S. Jansone-Popova and D. Jiang, J. Mol. Liq., 2023, 387, 122573 CrossRef CAS
.
- Y. Karslyan, F. V. Sloop, L. H. Delmau, B. A. Moyer, I. Popovs, A. Paulenova and S. Jansone-Popova, RSC Adv., 2019, 9, 26537–26541 RSC
.
- K. R. Johnson, D. M. Driscoll, J. T. Damron, A. S. Ivanov and S. Jansone-Popova, JACS Au, 2023, 3, 584–591 CrossRef CAS PubMed
.
- E. A. Agyei and J. D. Carrick, Tetrahedron, 2024, 155, 133872 CrossRef CAS
.
- S. Wang, X. Yang, Y. Liu, L. Xu, C. Xu and C. Xiao, Inorg. Chem., 2024, 63, 3063–3074 CrossRef CAS PubMed
.
- Q. Huang, S. Wu, Y. T. Liang and A. Y. Li, Comput. Theor. Chem., 2024, 1238, 114694 CrossRef CAS
.
- M. J. Hudson, M. G. B. Drew, M. R. S. Foreman, C. Hill, N. Huet, C. Madic and T. G. A. Youngs, Dalton Trans., 2003, 1675–1685 RSC
.
- Y. Miao, L. Xu, X. Yang, S. Wang, J. Zhang, C. Xu and C. Xiao, Inorg. Chem., 2022, 61, 17911–17923 CrossRef CAS PubMed
.
- S. Wang, X. Yang, L. Xu, Y. Miao, X. Yang and C. Xiao, Ind. Eng. Chem. Res., 2023, 62, 15613–15624 CrossRef CAS
.
- S. Wang, X. Yang, L. Xu and C. Xiao, Ind. Eng. Chem. Res., 2024, 63, 10773–10781 CrossRef CAS
.
- H. Wang, P. Gao, T. Cui, D. Wang, J. Liu, H. He, Z. Chen, Q. Jin and Z. Guo, Dalton Trans., 2024, 53, 601–611 RSC
.
- Y. Chen, C. Wang, L. Zhang, Q. Wu, J. Lan, Z. Chai and W. Shi, Dalton Trans., 2024, 53, 746–7413 RSC
.
- X. Yang, D. Fang, L. Chen, Y. Liu, S. Wang, L. Xu, A. Zhang, J. Su, C. Xu and C. Xiao, Research Square, 2024 DOI:10.21203/rs.3.rs-4355272/v1
.
- L. Su, Q. Wu, C. Wang, J. Lan and W. Shi, Inorg. Chem., 2024, 63, 9478–9486 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |







