Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-performance asymmetric supercapacitor device with nickel–cobalt bimetallic sites encapsulated in multilayered nanotubes†
Rahul
Patil‡
 a,
Lingaraj
Pradhan‡
bc,
Babasaheb M.
Matsagar
a,
Lingaraj
Pradhan‡
bc,
Babasaheb M.
Matsagar
 d,
Omnarayan
Agrawal
a,
Kevin C.-W.
Wu
d,
Omnarayan
Agrawal
a,
Kevin C.-W.
Wu
 de,
Bikash Kumar
Jena
*bc and
Saikat
Dutta
de,
Bikash Kumar
Jena
*bc and
Saikat
Dutta
 *a
*a
aElectrochemical Energy & Sensor Research Laboratory, Amity Institute of Click Chemistry Research & Studies, Amity University Noida, India. E-mail: sdutta2@amity.edu
bMaterials Chemistry Department, CSIR-Institute of Minerals and Materials Technology, Bhubaneswar-751013, India
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India. E-mail: bikash@immt.res.in
dDepartment of Chemical Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan
eDepartment of Chemical Engineering and Materials Science, Yuan Ze University, Chung-Li, Taoyuan, Taiwan
First published on 10th August 2023
Abstract
Enhanced faradaic capacitance can be achieved by using a metallic heterostructure as a result of the intrinsic activity of the electrodes. Optimizing the multimetallic heterostructure is key to improving the intrinsic activity of the electrodes. Herein, a consolidated strategy of a multimetallic design with predominantly nickel, cobalt, and zinc centers on multiwalled nanotubes was developed via pyrolysis of a trimetallic metal–organic framework at 800 °C. The resulting C-ZIF-800 material offers a multiwalled nanotube-like structure that facilitates the encapsulation of multimetallic sites with predominantly nickel and cobalt centers on a nanotube-like N–C interface. XRD, HRTEM, XPS, and EXAFS spectroscopic analysis support the metallic valence state of the Ni and Co centers encapsulated in the N–C nanotubes. C-ZIF-800 offers an outstanding specific capacitance of 849.47 F g−1 at a current density of 1 A g−1. Based on three-electrode electrochemical measurements, an asymmetric supercapacitor (ASC) device (C-ZIF-800//AC) was fabricated with a wide potential window of 1.7 V. The device offers an excellent specific capacity of 599.7 C g−1 at 0.25 A g−1 with an exceptional energy density of 141.59 W h kg−1 at a power density of 212.5 W kg−1, and remaining 4250 W kg−1 after retaining 81.93 W h kg−1 of energy density. The as-fabricated device demonstrated its excellent potential for future energy storage applications by illuminating a red LED light for 60 min by combining two devices in series.
Introduction
Wide-voltage aqueous asymmetric supercapacitors (ASCs) break the energy storage restrictions of symmetric cells while offering promising future electronic systems with high energy-power density and cycle life due to their facile fabrication, operational safety, and high ionic conductivity.1,2 Unidirectional charging in ASCs, however, depends on the coupling of a carbon molecular sieve with ordered mesoporous carbon, which can act as a capacitive analog of semiconductor-based diodes.3 Using two different electrodes, ASCs can expand the window of operating voltage beyond the thermodynamic decomposition voltage of electrolytes by enhancing the energy storage capacity of symmetric systems.4 To the hierarchical architecture and high surface area are attributed the greater cycling safety and effective electrochemical performance with high specific capacitance and highest capacity retention by porous bilayers of NiZn(CO3)(OH)2–Ni2(CO3)(OH)2 on Ni foam.5,6 NiO/Ni-MOF ASCs exhibit an energy density of 31.3 W h kg−1 for cyclic stability with capacitance retention of 88.7% over 2000 cycles.7 Nitrogen-doped graphene has been used as a host to encapsulate metal nanoparticles, such as nickel–cobalt nitride, as a core–shell structure for flexible solid-state ASC devices with exceptional power density.8 The structure–property correlations of metal–organic-framework-derived superstructure materials help in an exploration of their potential as supercapacitors and capacitive-deionization electrodes.9,10 Capacitance-dependent supercapacitors with a transformative improvement in performance require high energy density and a graphitic-type structure with metal loading.11 Differentiation between double-layer and pseudocapacitive charge storage forms the basis of non-faradaic surface-controlled kinetics. In contrast, pseudocapacitive to battery-type charge storage is called the faradaic process, which runs on diffusion-controlled kinetics.12 Combining faradaic reactions and capacitive electrode charging not only for a monovalent cation or anion, but for use in an ASC is an evolutionary step for enhanced capacitance.12 The capacitance of the electrochemical interface is separated into faradaic pseudocapacitance and non-faradaic double-layer capacitance; however, faradaic intercalation depends on confinement by the microenvironment.13 Ni-based pseudocapacitive electrode materials remain challenging owing to their weak electrical conductivity, resulting in large volume expansion during repeated intercalation/deintercalation processes.14 Higher specific capacitance and nearly ideal capacitive behavior in aqueous electrolytes render electrode materials for high-power charge storage with the electrochemical capacitance of both faradaic and non-faradaic types for clarifying the capacitive mechanism.15 Full exposure of metal sites in the faradaic reaction with excellent pseudocapacitance performance, such as Ni-NOG-1.5 with a gravimetry capacitance of 532 F g−1 at a current density of 1 A g−1 with an ASC assembled with Ni-NOG-1.5 and activated carbon considered as the negative electrode.16 A low capacitive anode material significantly affects the performance of an ASC, wherein low-electrical conductivity limits capacitive energy storage.2,17–19 The accelerated ion diffusion and charge mobility support an enhanced specific capacitance and cycle stability to provide an ultrahigh energy density by introducing abundant oxygen vacancies.20 This extends to an adaptive pore structure for capacitive electrode materials to achieve ultrahigh energy density.21Integrated carbon nanotubes (CNTs) and nickel–cobalt–phosphate (NiCo–P) offer a maximum capacitance retention rate of 86% with excellent energy density in an ASC with 92.4% retention of capacitance upon 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles.22 The transition of the NF/MnP/NiCoP composite occurs in different charge/discharge cycles wherein the specific capacitance was enhanced with extended charge/discharge cycles.23 The Co/Ni ratio significantly affects the gravimetry capacitance of the Ni4−xCoxWO4/HPC electrode. The Ni3Co1WO4/HPC electrode offers an excellent gravimetry capacitance of 1084 F g−1 (364.5 C g−1) at a current density of 0.5 A g−1 in 6 M KOH due to a unique nanostructure with synergistic effects between nickel and cobalt ions.24 The nickel–cobalt phosphate clustered nanoparticle offers pseudocapacitive behavior with a maximum specific capacitance of 2228 F g−1 (891 C g−1) at 1.5 A g−1. Moreover, an aqueous hybrid asymmetric supercapacitor (AHAS) device exhibits a higher specific capacitance of 185 F g−1 with an energy density of 65.7 W h kg−1 at 2.2 kW kg−1 power density with capacitance retention of 97%.25 Towards enhanced capacitance, three-dimensional structured diatoms MnFeOx composed of MnO with FeOOH nanorods exhibited high capacitance.26 Similarly, FeOOH/PPy on a diatomic ternary complex was assembled to form oxygen vacancies and mesopores for enhanced supercapacitor features.27 Consequently, phosphorus vacancies regulate the interfacial coupling of a biotemplated CoP@FeP2 heterostructure with boosted pseudocapacitive kinetics for ASCs with a high energy density of 45.5 W h kg−1 at a high power density via defect regulation.28 Similar co-regulation of 3D cobalt phosphide in a vanadium-doped V-CoPx hierarchical heteroatomic electrode exhibited excellent energy density and cyclic stability.29 NiO also plays a major role in enhancing pseudocapacitance.30 Ternary nanocomposites also play a significant role in supercapacitor electrodes.31,32
000 charge–discharge cycles.22 The transition of the NF/MnP/NiCoP composite occurs in different charge/discharge cycles wherein the specific capacitance was enhanced with extended charge/discharge cycles.23 The Co/Ni ratio significantly affects the gravimetry capacitance of the Ni4−xCoxWO4/HPC electrode. The Ni3Co1WO4/HPC electrode offers an excellent gravimetry capacitance of 1084 F g−1 (364.5 C g−1) at a current density of 0.5 A g−1 in 6 M KOH due to a unique nanostructure with synergistic effects between nickel and cobalt ions.24 The nickel–cobalt phosphate clustered nanoparticle offers pseudocapacitive behavior with a maximum specific capacitance of 2228 F g−1 (891 C g−1) at 1.5 A g−1. Moreover, an aqueous hybrid asymmetric supercapacitor (AHAS) device exhibits a higher specific capacitance of 185 F g−1 with an energy density of 65.7 W h kg−1 at 2.2 kW kg−1 power density with capacitance retention of 97%.25 Towards enhanced capacitance, three-dimensional structured diatoms MnFeOx composed of MnO with FeOOH nanorods exhibited high capacitance.26 Similarly, FeOOH/PPy on a diatomic ternary complex was assembled to form oxygen vacancies and mesopores for enhanced supercapacitor features.27 Consequently, phosphorus vacancies regulate the interfacial coupling of a biotemplated CoP@FeP2 heterostructure with boosted pseudocapacitive kinetics for ASCs with a high energy density of 45.5 W h kg−1 at a high power density via defect regulation.28 Similar co-regulation of 3D cobalt phosphide in a vanadium-doped V-CoPx hierarchical heteroatomic electrode exhibited excellent energy density and cyclic stability.29 NiO also plays a major role in enhancing pseudocapacitance.30 Ternary nanocomposites also play a significant role in supercapacitor electrodes.31,32
Herein, we describe a strategy for deriving an Ni and Co metal encapsulated N–C porous matrix of C-ZIF-800 from M-ZIF via thermal pyrolysis at 800 °C. C-ZIF-800 contains a multiwalled carbon nanotube shell of several hundred nanometers in length. It offers predominant Ni and Co sites due to the thermal pyrolysis-driven reduction of M2+ to M (Ni, Co) (Scheme 1). C-ZIF-800 was extensively characterized by surface spectroscopy (XPS, XAS, Raman) and transmission electron microscopy (HR-TEM) with high-resolution techniques confirming the presence of an MWCNT-like morphology with encapsulated Ni and Co sites. A pseudocapacitor-type electrode, which further demonstrates the charge–discharge mechanism, follows faradaic behavior by using C-ZIF-800. The ASC performance was tested in an asymmetric coin cell (CR2032) wherein activated carbon (AC) acts as an anode and C-ZIF-800 as a cathode, to light a 1.5 V LED with extremely high brightness for about 60 minutes.
 | ||
| Scheme 1 Illustration of the solvothermal treatment of M-ZIF followed by pyrolysis at 800 °C under argon. | ||
The PXRD patterns of C-ZIF-700 to 800 samples exhibit three significant peaks at 2θ 45, 52, 76 corresponding to metallic nickel intensity for the (111), (200), and (220) planes (Fig. 1(a)). The XRD of M-ZIF resembles a few similar planes of ZIF-833 and ZIF-67,34 conforming to a stable trimetallic framework (Fig. S1, ESI†). The BET surface area measured by N2 sorption (Fig. 1(b)) for C-ZIF-800 exhibits a surface area of 208.28 m2 g−1. C-ZIF-800 shows a type-IV isotherm, including a hysteresis loop with adsorption–desorption of mesopores at higher P/P0, suggesting the presence of mesopores. The pore distribution of C-ZIF-800 (Fig. 1(c)) was confirmed on the basis of a Barrett–Joyner–Halenda (BJH) model showing an identical pore width (1 nm) in the micropore region, including mesoporosity with a peak for pore dimensions of 3–10 nm. C-ZIF-800 was further confirmed from the Raman scattering spectrum (Fig. 1(d)) in which the D, G, and 2D bands appeared. The ID/IG ratio was found to be 0.90, confirming high graphitic degree in the material. Additionally, peaks at 520 cm−1 correspond to crystalline Ni–O lattice35 vibrations in the C-ZIF-800 sample. Combining XRD and Raman analysis, the predominant Ni-sites consist of crystalline Ni nanoparticles and Ni–oxide in C-ZIF-800.
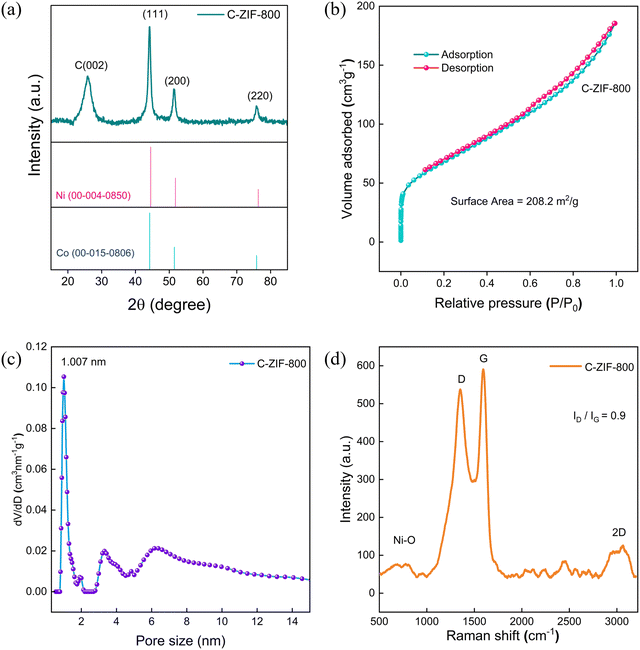 | ||
| Fig. 1 (a) PXRD patterns of C-ZIF-800. (b) N2 sorption isotherm of C-ZIF-800. (c) BHJ pore size distributions of C-ZIF-800. (d) Raman scattering spectrum of C-ZIF-800. | ||
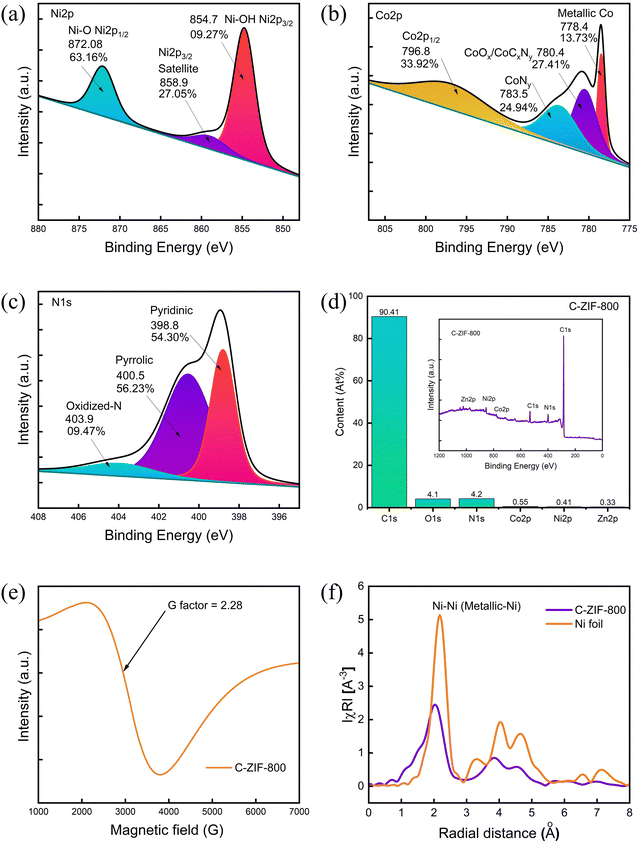
The deconvoluted spectral plots of the high-resolution X-ray photoelectron spectra (HR-XPS) of Ni 2p show a major peak at 854.7 eV (Fig. 2(a)), representing the formation of Ni–OH/Ni-O. Similarly, the Co 2p3/2 peak at 778.4 eV corresponds to metallic Co, along with peaks at 780.4 eV and 783.5 eV corresponding to CoOx/CoCxNy and CoNy (Fig. 2(b)). The Zn 2p spectra suggest the formation of Zn–O (∼1022 eV) (Fig. S2(a), ESI†). The deconvolution plot of N 1s (Fig. 2(c)) confirms the presence of pyridinic, pyrrolic, and oxidized N. Deconvolution of C 1s indicates three peaks associated with C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 284.7 eV, C–N/C–OH at 285.6 eV; and C(O)–O at 289.08 eV (Fig. S2(b), ESI†). Moreover, O 1s peaks are associated with –C
C at 284.7 eV, C–N/C–OH at 285.6 eV; and C(O)–O at 289.08 eV (Fig. S2(b), ESI†). Moreover, O 1s peaks are associated with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 531.7 eV and C–O at 533.1 eV (Fig. S2(c), ESI†). The survey spectra of C-ZIF-800 are presented in Fig. 2(d)) along with the elemental composition of each element in C-ZIF-800. The oxidation state of Ni species in C-ZIF-800 was further investigated with electron paramagnetic resonance (EPR) spectroscopy (Fig. 2(e)) at room temperature (298 K) with g value 2.28 assigned to an unpaired electron in the 3dx2−y2 orbital of Ni(II). The Fourier transformation of the phase-corrected extended X-ray absorption fine structure (EXAFS) spectra shows a peak at 2.10 Å (Fig. 2(f)) corresponding to an Ni–Ni interaction similar to Ni foil. Moreover, the broad region in the R-space curve at ∼1.5 Å suggests the formation of Ni–O. The XAS spectra of C-ZIF-800 contain relative intensities and edge positions (Fig. S5, ESI†) at 8334 eV, corresponding to a 3d and 4p orbital hybridization of Ni followed by a peak at 8340 eV before multiple scattering peaks above 8350 eV. Moreover, the energy absorption curve suggests a higher fraction of Ni–O bonds (Fig. S5, ESI†), which indicates the partially oxidized state of the Ni species in C-ZIF-800.
O at 531.7 eV and C–O at 533.1 eV (Fig. S2(c), ESI†). The survey spectra of C-ZIF-800 are presented in Fig. 2(d)) along with the elemental composition of each element in C-ZIF-800. The oxidation state of Ni species in C-ZIF-800 was further investigated with electron paramagnetic resonance (EPR) spectroscopy (Fig. 2(e)) at room temperature (298 K) with g value 2.28 assigned to an unpaired electron in the 3dx2−y2 orbital of Ni(II). The Fourier transformation of the phase-corrected extended X-ray absorption fine structure (EXAFS) spectra shows a peak at 2.10 Å (Fig. 2(f)) corresponding to an Ni–Ni interaction similar to Ni foil. Moreover, the broad region in the R-space curve at ∼1.5 Å suggests the formation of Ni–O. The XAS spectra of C-ZIF-800 contain relative intensities and edge positions (Fig. S5, ESI†) at 8334 eV, corresponding to a 3d and 4p orbital hybridization of Ni followed by a peak at 8340 eV before multiple scattering peaks above 8350 eV. Moreover, the energy absorption curve suggests a higher fraction of Ni–O bonds (Fig. S5, ESI†), which indicates the partially oxidized state of the Ni species in C-ZIF-800.
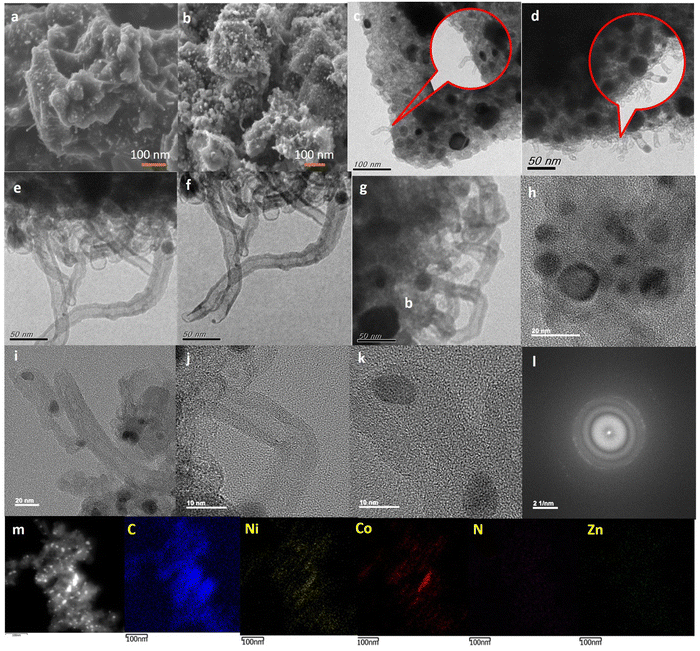
Field-emission scanning electron microscopy (FE-SEM) of C-ZIF-700 and C-ZIF-800 reveals rectangular-shaped particles like that of the parent M-ZIF, resulting in a fused layered topology with a porous surface (Fig. 3(a) and (b)). The porosity level was further increased with the temperature of pyrolysis (800 °C) resulting in C-ZIF-800 with no major change in overall morphology (Fig. 3(b)). The porous architecture of C-ZIF-700, as found in the SEM images, is consistent with the porosity profile from the BJH pore profile (Fig. 1(c)). Transmission electron microscopy (TEM) analysis of C-ZIF-700 showed a multiwall carbon layer containing a tube-like morphology (Fig. 3(c)) which holds Ni sites in encapsulated form (Fig. 3(d)). An increase in carbon nanotube volume is expected to increase at higher pyrolysis temperatures by a hard-templating effect of ZnO. The formation of the highly porous architecture of C-ZIF-800 offers a multiwalled nanotube morphology, which is enhanced at a higher pyrolysis temperature of 800 °C. Therefore, hard templating by ZnO supports the formation of an MWCNT architecture longer than 100 nm (Fig. 3(e) and (f)), in which continuous multiwalled tube-like growth is evident. A connection between MWCNT and double-layer capacitance can be assumed based on the experimental results for electrochemical impedance and capacitance. The encapsulation of Ni and Co centers is predominantly shown in Fig. 3(g) and (h), in which metal nanoparticles are located within the nanotube extensions. The encapsulation of metal centers is further shown in Fig. 3(i), followed by Fig. 3(j), in which the multiwalled architecture of MWCNT is evident. The walls of these tubes are clear as uniform distances are maintained by the several layers. Fig. 3(k) shows that the MWCNT-like tubes hold multiple metal sites encapsulated in a tube-like architecture. HR-TEM of C-ZIF-800 nanoparticles and corresponding fast Fourier transform (FFT) reveal d-spacings of 0.21 nm for Ni (111) and Co (200), and 0.32 nm for (N–C) (Fig. 3(l)). FFT images, generated from the sample analysis, provide diffraction patterns in reciprocal space of the selected region of the HR-TEM image. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) electron image of C-ZIF-800 Fig. 3(m) and corresponding C, N, Ni, Co, Zn elemental maps show the predominance of Ni-sites in the nanotube architecture. We determined the average crystallite size of Ni, Co nanoparticles as 11.2 nm (Fig. S6, ESI†), whereas the crystallite size (D) of Ni, Co nanoparticles obtained from the XRD peaks (Fig. 1(a) and Equation 8, ESI†) is 7.0 nm, which is slightly smaller than the particle size observed from the TEM image. Such a variation in the crystallite size (D) with TEM particle size was noticed in the case of CeO2.36
Electrochemical measurement
Three-electrode test
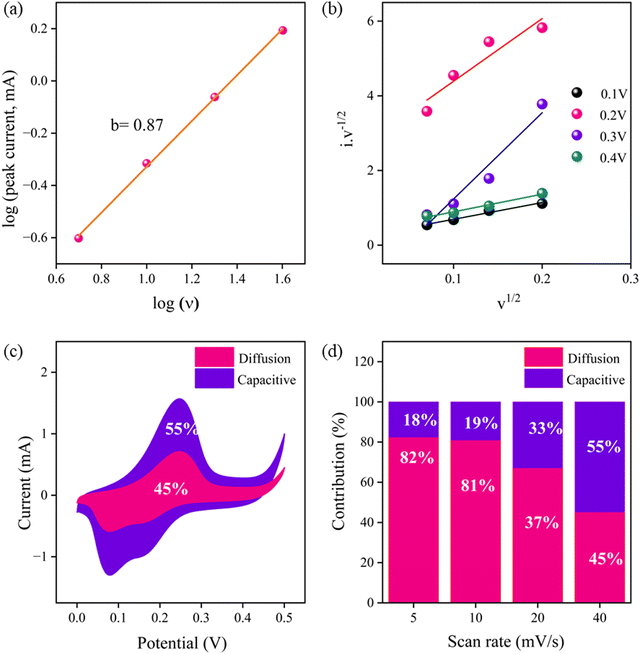
For preliminary analysis of the supercapacitance properties, three-electrode electrochemical measurement was carried out using catalyst-modified glassy carbon (GC), Ag/AgCl in 3 M saturated KCl solution, and platinum wire as the working, reference, and counter electrode, respectively. The energy storage performance of the as-prepared trimetallic (Ni, Co, Zn) M-ZIF, C-ZIF-700 and C-ZIF-800 was studied by taking 1 M KOH as the electrolyte. The activity of the M-ZIF electrode towards supercapacitance is optimized by varying the temperature, i.e., 700 °C and 800 °C and Co ratio. The supercapacitive performance of the C-ZIF-800 electrode is compared with M-ZIF and C-ZIF-700 at a sweep rate of 100 mV s−1 (Fig. S7(a), ESI†). With increasing temperature and Co ratio, the area of the cyclic voltammetry (CV) curve increases. Therefore, C-ZIF-800 is considered the standard electrode material for all electrochemical measurements. The area of the CV curve of the C-ZIF-800 hybrid electrode is much higher than those of M-ZIF and C-ZIF-700, which indicates higher capacitive behaviour. A well-defined pair of redox peaks characteristic of pseudocapacitor behaviour can be observed for all the electrode materials. It can be observed from the CV curve that the current values for oxidation and reduction peaks are augmented with an increase in sweep rates; this signifies that the charge-storage processes are of the diffusion control type. However, with an increase in the sweep rate, the oxidation and reduction peak potentials are slightly displaced towards higher and lower potentials, respectively. This may be due to the strengthened electric polarization and the possible kinetic irreversibility at the electrode and electrolyte interface. The CV plots of C-ZIF-800, C-ZIF-700, and M-ZIF at different scan rates are presented in Fig. S7(b)–(d) (ESI†). The galvanostatic charge–discharge (GCD) curve of the C-ZIF-800 electrode is much slower and nonlinear, which indicates outstanding Coulombic efficiency and good reversibility. This nonlinear discharge curve with an obvious discharge plateau is the characteristic feature of a pseudocapacitor-type electrode, which further demonstrates that the charge–discharge mechanism follows the faradaic process. C-ZIF-800 achieves a capacitance value of 849.47 F g−1 at 1 A g−1, which is higher than those of C-ZIF-700 (198.27 F g−1) and M-ZIF (190.18 F g−1) (Fig. S8, ESI†). The GCD plots of C-ZIF-800, C-ZIF-700, and M-ZIF at different current densities are presented in Fig. S9(a)–(c) (ESI†). As depicted in the electrochemical impedance spectra (EIS) in Fig. S10 (ESI†), The C-ZIF-800 electrode exhibits a reduced iR-drop, indicating greater conductivity and a quicker I–V response. The EIS curves of M-ZIF, C-ZIF-700, and C-ZIF-800 were recorded in the frequency domains of 0.1 Hz and 100 kHz. The C-ZIF-800 catalyst possesses smaller Rs and Rct values than other electrode materials, suggesting higher electrochemical performance. The cyclability test of the C-ZIF-800 electrode exhibits long-term durability with capacitance retention of more than 100% after 5000 cycles, as shown in Fig. S11 (ESI†).To explore the detailed energy storage mechanism of as-prepared C-ZIF-800, the individual percentages of the diffusion-controlled contribution and surface-capacitive contribution were evaluated. The correlation between the scan rate and current from CV is represented using a power law in eqn (1):
| I = aνb | (1) |
To obtain the approximate percentage of the contribution, eqn (2) was taken into account:
| I = k1ν + k2ν1/2 | (2) |
Asymmetric supercapacitors
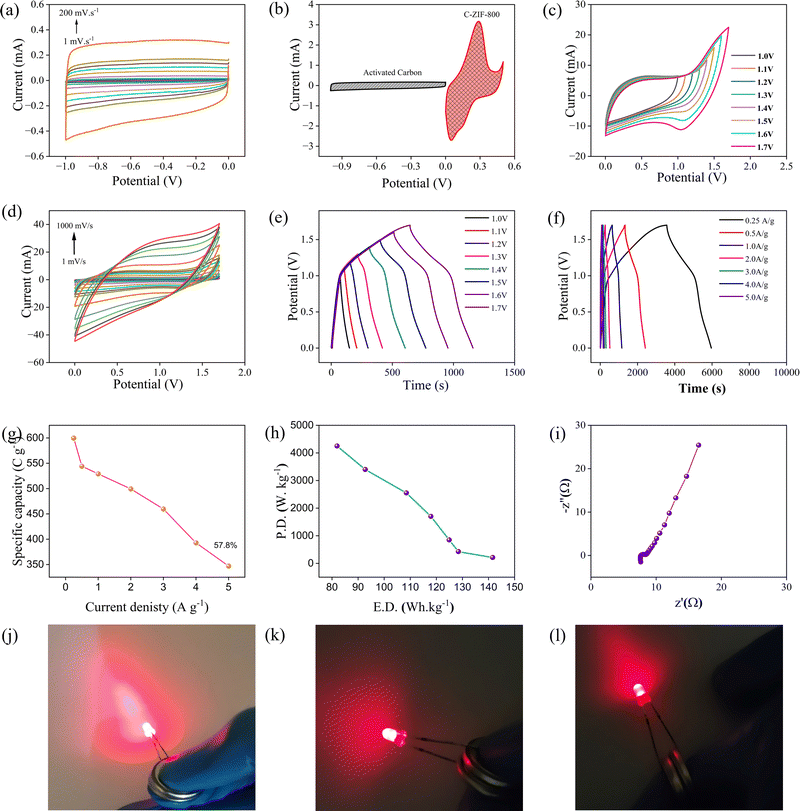
To investigate the performance of a two-electrode coin cell device, an asymmetric supercapacitor (ASC) device was built utilizing C-ZIF-800 as the positive electrode and activated carbon (AC) as the negative electrode in 1 M KOH aqueous electrolyte (CR2032).To construct the ASC device, a three-electrode electrochemical investigation analyzing the CV for AC was undertaken (Fig. 5(a)). Fig. 5(b) depicts the CV graphs of the C-ZIF-800 and AC electrodes in various potential windows at a sweep rate of 100 mV s−1 in three-electrode setups. Charge equilibrium was maintained for the two-electrode device by adjusting the mass loading of the active materials in accordance with eqn (S7) (ESI†). The optimization of the potential window for this ASC was performed in the potential range of 0–1.7 V (Fig. 5(c)). The CV curve has a nice quasi-rectangular shape between 0 and 1.7 V; hence, the 0–1.7 V potential window was maintained as the optimal potential window for further research into the device. Fig. 5(d) illustrates the CV curves of the ASC device at different scan speeds (1–1000 mV s−1). Fig. 5(e) depicts the GCD curves at several potentials ranging from 1.0 V to 1.7 V. To determine the capacitance value and specific capacity of the ASC device, a GCD analysis was undertaken at several current densities (Fig. 5(f)). The specific capacity was found 599.7, 544.1, 529.1, 499.4, 456.0, 392.8, and 347 C g−1 at the current density of 0.25, 0.5, 1, 2, 3, 4, and 5 A g−1, respectively (Fig. 5(g)). As shown in Fig. 5(g), the ASC device has a maximum specific capacity of 599.7 C g−1 at 0.25 A g−1, and the capacity value was maintained at 57.8% even after 5 A g−1 (370 C g−1). In addition, the ASC device provides an outstanding energy density of 141.59 W h kg−1 at a power density of 212.5 W kg−1, while retaining 81.93 W h kg−1 at a power density of 4250 W kg−1 (Fig. 5(h)). Table S1 (ESI†) provides a more extensive assessment of the published literature using a conventional two-electrode device. Due to the porosity network, the ASC device (C-ZIF-800/AC) has demonstrated excellent electrochemical performance. Fig. 5(i) depicts the electrochemical impedance spectroscopy (EIS) investigation conducted to determine the kinetic behavior of the as-prepared composites. The ASC exhibits a very low Rs of 7.62 and a minimum Rct of 0.72, which substantiates its exceptional electrical conductivity. Two ASCs were manufactured and the device was charged at a current density of 1 A g−1 using an electrochemical workstation in order to examine its practical capabilities further. The two ASCs were linked in series to illuminate a 1.5 V LED with great brightness at the time of illumination, indicating substantial storage capacity (Fig. 5(j)–(l)).
The combination of HRTEM, XPS, and EXAFS techniques supports the presence of Ni and Co centers in encapsulated form in N–C nanotubes in C-ZIF-800. The multimetallic design with the maximized number of favorable active sites offered may have optimized the electronic structure of the C-ZIF-800 electrode.
In the given case studies, Ni–Mn LDH/rGO/Ni(OH)2@Ni foam exhibits high-performance capacitance with a specific energy density of 0.23 mW h cm2 at a specific power density of 0.91 mW cm2.37 Nickel-nanoparticle-modified MnO nanosheet arrays wherein Ni NPs promote the accumulation and release of electrons at the interface of Ni/MnO, which contributes extra capacitance to the composite, are similar to the case of C-ZIF-800 with porous electrochemically exposed active sites.38 An asymmetric supercapacitor of Ni(OH)2/A/CNT in 6 M KOH within a 1.6 V window offers a maximum specific capacitance of 82.1 F g−1 with an energy density of 32.3 W h kg−1 at a power density of 504.8 W kg−1.39 The hierarchical structure in the combination of Ni and Ag nanoparticles on rGO offers a synergistic effect and retention of capacitance with supercapacitive performance.40,41 Our studies of several faradaic capacitance materials, such as Mn3O4 in a graphene matrix and MoS2-rGO, showed both experimental and theoretical insights into the origin of capacitance enhancement and storage.18,42,43 In Table S1 (ESI†), we have listed a series of Ni-centered MOF and CNT-based capacitance with specific capacitance, power density, and energy density, including capacitance retention cycle number to represent cyclic stability. The trend in specific capacitance suggests that C-ZIF-800 offers a unique morphology along with the key role of Ni metallic sites offering a synergistic effect of bimetallic (Ni and Co) interface on the multi-walled CNT of C-ZIF-800. The effect of Ni-O and Ni nanoparticles in the metallic valence state in a multimetallic design has not been extensively studied, so this report focuses on enhancing capacitance and the fabrication of an asymmetric supercapacitor. The enhanced capacitance of C-ZIF-800 was compared with other relevant Ni-centred electrodes, which suggests that Ni-metal sites with cobalt NPs and a conductive tube-like network in the N–C facilitate enhanced capacitance. In the case studies given in Table S1 (ESI†), in terms of capacitance electrode performance, the energy density and capacitance retention of C-ZIF-800 are far ahead, and it is perhaps successful as a result of the nanotube-like architecture with predominant Ni, Co sites along with a multimetallic design. An asymmetric supercapacitor based on bimetallic nanoparticles of Ni and Co on micro–meso carbon utilizes the synergistic effect between the bimetallic sites and support materials44 consisting of a hierarchical porous and unique nanoarchitecture, as in the case of C-ZIF-800 with a multi-walled nanotube network. The synergistic effect between Ni–Co bimetallic sites with a hierarchical porous support and unique tube-like morphology enables facile electron/ion transfer in the electrolyte, resulting in performance over an enlarged voltage window for powering an LED device for several minutes. Not only the bimetallic synergistic effect but also Ni-Co bimetallic sites offer rich sites for redox reactions with internal ultrahigh conductivity for faster electron transfer with an average thickness of electrode.45 In this work, Table S1 (ESI†) includes the electrochemical performance of C-ZIF-800 in three electrodes being retained for 5000 cycles. Therefore, the combined role of Ni–Co bimetallic sites with abundant pores for synergism between electron transfer events and protection of metallic centers in tubular encapsulation is considered to be responsible for the enhanced capacitance retention.
A connection between the MWCNT and double-layer capacitance can be assumed based on the experimental results for electrochemical impedance and capacitance. Such bimetallic C-ZIF-800 with CNT-type tubular architecture of N–C favors the electrical double-layer (EDL) mechanism of charge transfer, but the presence of predominant Ni metal nanoparticles and possible Ni–O bonding favors the faradaic mechanism of charge transfer.46 The smaller iR-drop of C-ZIF-800 suggests higher conductivity and rapid I–V response, as confirmed by the impedance spectra (Fig. 5(i)). Moreover, Ni 2p and Co 2p XPS spectral patterns confirm the formation of Ni(II)/Ni–O and Co metallic sites, which favors the faradaic pseudocapacitance mechanism for high charge storage. Based on the EIS and XPS observations, the charge storage mechanism depends on polarization, wherein counter-ion adsorption and co-ion desorption are key routes to ion-exchange. Pure counterion-adsorption in the positive electrode and excess ionic charge in the pores of C-ZIF-800 would balance the electronic charge in both electrodes.
Table S1 (ESI†) includes the energy density, power density of C-ZIF-800 as an ASC and cyclic stability in three-electrode measurements, the capacitance retention for 5000 cycles was witnessed. A valid comparison of our current findings with other relevant reports revealing the combined role of Ni–Co bimetallic sites along with single Ni or Co metal sites with abundant pores is included in Table S1 (ESI†). Abundant pores for synergism between electron transfer event and protection of metallic sites in a tubular structure that encapsulates the metals is considered to be responsible for the difference in enhanced capacitance retention. When comparing the capacitance retention percentage, a performance range of 80 of 90% was noted for most NiCo bimetallic capacitors.
Conclusions
In summary, a nanotube with multi-wall supports encapsulating Ni and Co in a metallic valence state was fabricated via the high-temperature pyrolysis of a trimetallic MOF in a porous graphene-like multi-walled N–C matrix. The porous N–C matrix offers adequate exposure to metallic sites, enabling effective modulation of the electronic structures of the metal centers. The rich electronics of Co and Ni-sites on the N–C matrix were confirmed using XPS, XAS, and HR-TEM techniques. Among the configurations, the graphitic N-decorated Ni and Co metallic sites enabled them to act as faradaic electrodes for longer-term charge–discharge stability than many reported materials, as shown in Table S1 (ESI†). Higher discharge power density, high capacitance, and better long-term stability compared to commercial Pt/C + Ru2O enable our strategy to provide highly active materials for capacitance-based charge storage. Finally, an ASC device (C-ZIF-800//AC) with a wide potential window of 1.7 V was successfully fabricated. The device offers an excellent specific capacity of 599.7 C g−1 at 0.25 A g−1 with an exceptional energy density of 141.59 W h kg−1 at a power density of 212.5 W kg−1, retaining 81.93 W h kg−1 at a power density of 4250 W kg−1. The as-fabricated device demonstrated its potential for future energy storage applications by illuminating a red LED light for 60 min by combining two devices in series. The novelty of the current work originates from the strategy of deriving a Ni–Co system with predominantly Ni centers on C-ZIF-800 encapsulated in a nanotube architecture, which was explored for ASC and LED studies.Conflicts of interest
Authors have no conflict of interest to declare.Acknowledgements
SD wishes to acknowledge research funding support by the Department of Biotechnology, Ministry of Science & Technology, Government of India for the grant number BT/RLF/Re-entry/41/2017 under DBT Ramalingaswami Re-entry Fellowship (2019-2024) and DBT-Energybioscience-Biofuels research grant (2022-2025 BT/PR38594/PBD/26/795/2020). B. K. J. acknowledges CSIR (HCP-42; OLP-48), NALCO, BRNS, MNRE, India for financial support. OA wishes to acknowledge the UGC-DAE-CSR Government of India and Smt. Babita Vinayak Salaskar from Beamline Development and application section, BARC for EXAFS measurements. KCW is thankful to the National Science and Technology Council (NSTC), Taiwan (111-2124-M-002-021 and 111-2628-E-002-008) for the funding support. Also, financial support by the Center of Atomic Initiative for New Materials, National Taiwan University, from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (111L900801), is acknowledged.References
- J. Huang, K. Yuan and Y. Chen, Adv. Funct. Mater., 2022, 32, 2108107 CrossRef CAS.
- S. K. Das, L. Pradhan, B. K. Jena and S. Basu, Carbon, 2023, 201, 49–59 CrossRef CAS.
- E. Zhang, N. Fulik, G.-P. Hao, H.-Y. Zhang, K. Kaneko, L. Borchardt, E. Brunner and S. Kaskel, Angew. Chem., Int. Ed., 2019, 58, 13060–13065 CrossRef CAS PubMed.
- Y. Shao, M. F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn and R. B. Kaner, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- D. Lee, S. Mathur and K. H. Kim, Nano Energy, 2021, 86, 106076 CrossRef CAS.
- S. Ding, J. An, D. Ding, Y. Zou and L. Zhao, Chem. Eng. J., 2022, 431, 134100 CrossRef CAS.
- G. Wang, Z. Yan, N. Wang, M. Xiang and Z. Xu, ACS Appl. Nano Mater., 2021, 4, 9034–9043 CrossRef CAS.
- J. Balamurugan, T. T. Nguyen, V. Aravindan, N. H. Kim and J. H. Lee, Adv. Funct. Mater., 2018, 28, 1804663 CrossRef.
- S. Zhang, M. Zheng, Y. Tang, R. Zang, X. Zhang, X. Huang, Y. Chen, Y. Yamauchi, S. Kaskel and H. Pang, Adv. Funct. Mater., 2022, 32, 2204714 CrossRef CAS.
- R. Patil, S. Liu, A. Yadav, N. Khaorapapong, Y. Yamauchi and S. Dutta, Small, 2022, 18, 2203147 CrossRef CAS PubMed.
- V. Šedajová, A. Bakandritsos, P. Błoński, M. Medveď, R. Langer, D. Zaoralová, J. Ugolotti, J. Dzíbelová, P. Jakubec, V. Kupka and M. Otyepka, Energy Environ. Sci., 2022, 15, 740–748 RSC.
- T. S. Mathis, N. Kurra, X. Wang, D. Pinto, P. Simon and Y. Gogotsi, Adv. Energy Mater., 2019, 9, 1902007 CrossRef CAS.
- S. Fleischmann, Y. Zhang, X. Wang, P. T. Cummings, J. Wu, P. Simon, Y. Gogotsi, V. Presser and V. Augustyn, Nat. Energy, 2022, 7, 222–228 CrossRef CAS.
- J. Zhao, B. Wu, X. Huang, Y. Sun, Z. Zhao, M. Ye and X. Wen, Adv. Sci., 2022, 9, 2201678 CrossRef CAS PubMed.
- S. Boyd, K. Ganeshan, W.-Y. Tsai, T. Wu, S. Saeed, D.-E. Jiang, N. Balke, A. C. T. van Duin and V. Augustyn, Nat. Mater., 2021, 20, 1689–1694 CrossRef CAS PubMed.
- L. Liu, Z. Xie, X. Du, D. Yu, B. Yang, B. Li and X. Liu, Chem. Eng. J., 2022, 430, 132815 CrossRef CAS.
- L. Pradhan, B. Nayak, A. Mukherjee, S. Basu, P. Bhanja and B. K. Jena, ACS Appl. Energy Mater., 2023, 6, 3347–3356 CrossRef CAS.
- S. K. Das, S. Kamila, B. Satpati, M. Kandasamy, B. Chakraborty, S. Basu and B. K. Jena, J. Power Sources, 2020, 471, 228465 CrossRef CAS.
- B. Mohanty, L. Giri and B. K. Jena, Energy Fuels, 2021, 35, 14304–14324 CrossRef CAS.
- L. Xu, W. Zhou, S. Chao, Y. Liang, X. Zhao, C. Liu and J. Xu, Adv. Energy Mater., 2022, 12, 2200101 CrossRef CAS.
- H. Ma, H. Chen, Y. Hu, B. Yang, J. Feng, Y. Xu, Y. Sun, H. Cheng, C. Li, X. Yan and L. Qu, Energy Environ. Sci., 2022, 15, 1131–1143 RSC.
- Y. Chen, H. Hou, B. Liu, M. Li, L. Chen, C. Chen, S. Wang, Y. Li and D. Min, Chem. Eng. J., 2023, 454, 140453 CrossRef CAS.
- X. Hong, C. Deng, G. Wang, X. Wang and W. Dong, Chem. Eng. J., 2023, 451, 139036 CrossRef CAS.
- F. Shi, S. Zhao, J. Yang, Y. Tong, J. Li, S. Zhai, X. Zhao, S. Wu, H. Li, Q. An and K. Wang, J. Mater. Chem. A, 2022, 10, 12679–12691 RSC.
- S. J. Marje, S. S. Pujari, S. A. Khalate, V. V. Patil, V. G. Parale, T. Kim, H.-H. Park, J. L. Gunjakar, C. D. Lokhande and U. M. Patil, J. Mater. Chem. A, 2022, 10, 11225–11237 RSC.
- K. Li, X. Liu, T. Zheng, D. Jiang, Z. Zhou, C. Liu, X. Zhang, Y. Zhang and D. Losic, Chem. Eng. J., 2019, 370, 136–147 CrossRef CAS.
- K. Li, S. Feng, C. Jing, Y. Chen, X. Liu, Y. Zhang and L. Zhou, Chem. Commun., 2019, 55, 13773–13776 RSC.
- K. Li, Z. Guo, Q. Sun, X. Dai, Y. Li, K. Yao, X. Liu, Z. Bao, J. Rao and Y. Zhang, Chem. Eng. J., 2023, 454, 140223 CrossRef CAS.
- K. Li, Y. Xiao, T. Zheng, Q. Sun, Y. Zhang, H. Teng, W. Wang, K. Yao, J. Rao and Y. Zhang, Appl. Surf. Sci., 2023, 622, 156950 CrossRef CAS.
- T. Munawar, M. Shahid Nadeem, F. Mukhtar, S. Manzoor, M. Naeem Ashiq and F. Iqbal, Mater. Sci. Eng., B, 2022, 284, 115900 CrossRef CAS.
- M. Naveed Ur Rehman, T. Munawar, M. S. Nadeem, F. Mukhtar, U. A. Akbar, S. Manzoor, A. S. Hakeem, M. N. Ashiq and F. Iqbal, Solid State Sci., 2022, 128, 106883 CrossRef CAS.
- T. Munawar, F. Mukhtar, M. S. Nadeem, S. Manzoor, M. N. Ashiq, M. Riaz, S. Batool, M. Hasan and F. Iqbal, Ceram. Int., 2022, 48, 19150–19165 CrossRef CAS.
- B. Ding, Z. Fan, Q. Lin, J. Wang, Z. Chang, T. Li, J. Henzie, J. Kim, H. Dou and X. Zhang, Small Methods, 2019, 3, 1900277 CrossRef CAS.
- R. Patil, N. Kumar, S. Bhattacharjee, H.-Y. Wu, P.-C. Han, B. M. Matsagar, K. C. W. Wu, R. R. Salunkhe, A. Bhaumik and S. Dutta, Chem. Eng. J., 2023, 453, 139874 CrossRef CAS.
- W. Liu, C. Lu, X. Wang, K. Liang and B. K. Tay, J. Mater. Chem. A, 2015, 3, 624–633 RSC.
- T. Munawar, A. Bashir, M. S. Nadeem, F. Mukhtar, S. Manzoor, M. N. Ashiq, S. A. Khan, M. Koc and F. Iqbal, Ceram. Int., 2023, 49, 8447–8462 CrossRef CAS.
- Y. Lin, S. Su, H. Dai, X. Xiang, P. Li and X. Zhu, J. Energy Storage, 2022, 56, 106138 CrossRef.
- M. Huang, Y. Lin, H. Huang, X. Fan, K. Shi, Z. Yang and W. Zhang, Electrochim. Acta, 2021, 383, 138353 CrossRef CAS.
- L. Sui, S. Tang, Y. Chen, Z. Dai, H. Huangfu, Z. Zhu, X. Qin, Y. Deng and G. M. Haarberg, Electrochim. Acta, 2015, 182, 1159–1165 CrossRef CAS.
- M. Chandel, P. Makkar and N. N. Ghosh, ACS Appl. Electron. Mater., 2019, 1, 1215–1224 CrossRef CAS.
- W. Li, L. Xin, X. Xu, Q. Liu, M. Zhang, S. Ding, M. Zhao and X. Lou, Sci. Rep., 2015, 5, 9277 CrossRef CAS PubMed.
- S. Kamila, B. Chakraborty, S. Basu and B. K. Jena, J. Phys. Chem. C, 2019, 123, 24280–24288 CrossRef CAS.
- S. Kamila, B. Mohanty, A. K. Samantara, P. Guha, A. Ghosh, B. Jena, P. V. Satyam, B. K. Mishra and B. K. Jena, Sci. Rep., 2017, 7, 8378 CrossRef PubMed.
- Y. Zhang, Y. Zhang, Y. Zhang, H. Si and L. Sun, Nano-Micro Lett., 2019, 11, 35 CrossRef CAS PubMed.
- Q. Yang, Y. Liu, M. Yan, Y. Lei and W. Shi, Chem. Eng. J., 2019, 370, 666–676 CrossRef CAS.
- A. C. Forse, C. Merlet, J. M. Griffin and C. P. Grey, J. Am. Chem. Soc., 2016, 138, 5731–5744 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ya00206c |
| ‡ Both the authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |