Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synergistic or antagonistic effect of lanthanides on Rose Bengal photophysics in upconversion nanohybrids?†
Juan
Ferrera-González
 ,
María
González-Béjar
,
María
González-Béjar
 * and
Julia
Pérez-Prieto
* and
Julia
Pérez-Prieto
 *
*
Instituto de Ciencia Molecular (ICMol), Departamento de Química Orgánica, Universitat de València, C/ Catedrático José Beltrán, 2, Paterna, Valencia 46980, Spain. E-mail: maria.gonzalez@uv.es; julia.perez@uv.es
First published on 20th November 2023
Abstract
A nanohybrid made of a xanthenic dye, rose bengal, grafted to an ytterbium and erbium codoped upconversion nanoparticle (UCNP) served as a proof-of-concept to evaluate the fundamental mechanisms which govern the dye photophysics upon interaction with the UCNP. Both photoactive lanthanides strongly influence the singlet and triplet excited states of rose bengal.
Introduction
Upconversion nanoparticles (UCNPs) are nanoparticles able to convert low energy photons into higher energy photons. The process, termed upconversion (UC), occurs due to the unique properties of photoactive lanthanide ions (Ln3+), which are the dopants of a transparent low-phonon energy matrix. UC is a nonlinear phenomenon and, usually, gives rise to multiple long-lifetime narrow emission bands in the ultraviolet–visible–near infrared (UV–vis–NIR) region upon NIR excitation.1,2Furthermore, upconversion nanohybrids (UCNHs), which combine UCNPs and photoactive species (e.g., dyes, organometallic complexes, or other photoactive nanoparticles) have been used for applications as sensors and bioimaging or therapeutic agents (photothermal, photodynamic, chemotherapy, radiotherapy and so on).3–9 Often, the photophysical interaction of both constituents in the UCNHs gives rise to an additive or synergistic effect to improve the optical features or generate new ones.
Most of the UCNHs reported so far include chromophores (usually organic dyes) and use energy transfer (trivial or resonant) processes which occur from the Ln3+ upconversion emission in the UCNP to the chromophore (NIR sensitization of the chromophore) or vice versa (dye-sensitized UCNP) by selective excitation of the desired counterpart.10–14 Some UCNHs, upon selective excitation of the UCNP and subsequent energy transfer to the chromophore, can generate triplet states after intersystem crossing. This chromophore can then react with oxygen to produce reactive oxygen species (ROS).4,15 Among the chromophores used to fabricate UCNHs are pyropheophorbide a,16 cationic porphyrin TMPyP4,17 diiodo-BODIPY,18 Rose Bengal (RB),19 methylene blue,20 hypericin,21 chlorine e6,22 and merocyanine 540.23
Great efforts have been made to study, model and improve the energy transfer process from the UCNP Ln3+ doping to the chromophore.11,24–30 More than 5000 research articles about UCNHs composed of UCNPs and dyes have been already published,‡ but only five of them analyzed experimentally the effect of Ln3+ on the dye excited state photophysics. It is desirable to gain a deeper understanding of the effect that the photoactive lanthanide doping of the UCNP has on the photophysical properties of chromophores. In this context, it was reported that the presence of heavy lanthanides, in particular Gd3+, in the UCNP favored the intersystem crossing of a dye anchored to the UCNP surface (IR-806) through the heavy atom effect, eventually leading to dye triplet formation.31 Later, the enhancement of intersystem crossing was confirmed for other lanthanide cations (Tb3+, Eu3+, Gd3+ and Yb3+) on films of Ln3+-doped nanoparticles with aromatic molecules; remarkably, this enhancement was not only linked to the heavy atom effect, but also to cations with unpaired 4f electrons (dye photophysics remained identical for Y3+ and Lu3+ doped nanoparticles). Moreover, heavy lanthanides with unpaired electrons also enabled the observation of the triplet exciton absorption transition (S0 → Tn) and the triplet state absorption deactivation lifetime decreased with respect to that of the pristine dye due to energy transfer to the Ln3+ ions.32 Afterwards, by knowing the effect that some lanthanides have on the intersystem crossing of dyes, a smart UCNH was developed to enable sensitization of a lanthanide with no intermediate energy levels (Tb3+ and Eu3+) in a core–shell–shell UCNH through dye triplet excited states.33 More recent research attributes, once again, the heavy atom effect as responsible for the intersystem crossing enhancement.34,35 Although the exact mechanism behind these observations is still controversial, all these publications clearly demonstrate that Ln3+ in the UCNP influences the dye photophysics.
Moreover, the restriction of movement of the dye anchored to the surface, the interaction with the surface36 and the potential dye aggregation37,38 raise serious doubts as to whether its behavior is the same as when it is free in solution. Consequently, dye photophysics can change considerably and should be reported for each UCNH.
Herein, we have selected a colloidal UCNH, commonly used in the field, as a model to evaluate the photophysical processes and phenomena that take place between its counterparts. Specifically, the UCEr@RB UCNH is composed of β-NaYF4:Yb3+(20%),Er3+(2%), a UCNP of ca. 20 nm and RB adsorbed on the surface. UCEr@RB has been selected based on the relatively high resonant energy transfer from Er3+ to RB and its reproducible and easy synthesis.39 In this system, RB absorption overlaps the main UC emission of Er3+ (520–540 nm). Moreover, RB photophysics is well known and presents a high intersystem crossing quantum yield (>90%)40 and 1O2 generation (68–80%).41
UCNHs made of UCNPs and RB have been investigated in the past.19,42–45 Most of them detected 1O2 by using optical probes (e.g., 1,3-diphenylisobenzofuran, DPBF) or by detecting 1O2 phosphorescence.§,19 Core–shell structures with increased concentrations of donor lanthanides in the shell are beneficial for resonant energy transfer to dyes.24 Also, the thickness of a silica shell coating the UCNP (NaYF4:Yb3+(20%),Er3+(2%)) influences the energy transfer to RB on the surface.45 The distance exerted an opposite influence between UC luminescence (reducing surface effects and solvent deactivation) and the energy transfer efficiency. The best energy transfer occurred with a 6 nm coating, leading to an emission of RB sensitized by UCNP with a lifetime on the microsecond scale.
All this considered, there is still an important lack of knowledge about how the photophysics of chromophores can be affected when anchored to the UCNP surface in colloidal dispersions.
This work presents a comparative analysis between free RB and RB in a UCNH and focusses on obtaining the most complete overview of the photophysical processes that could take place between the photoactive counterparts in UCEr@RB. It is of fundamental importance to understand the overall photophysics of the nanohybrid and develop nanomaterials with appropriate photoactive properties.
Does the combination of RB with UCNPs generate a synergistic or an antagonistic effect on RB photophysics in these “well-known” UCNHs?
Results and discussion
Nanohybrid synthesis and characterization
One batch of β-NaYF4:Yb3+(28%),Er3+(3%) UCNPs were synthesized by thermal decomposition (see Table S1†).46 The hexagonal prisms had a size of 21.0 ± 0.8 × 18.9 ± 0.7 nm (Fig. S1†). Subsequently, the UCNPs were treated with NOBF4 to eliminate oleate ligands from the surface,47 giving rise to oleate-free UCNPs (UCEr). Then, UCEr was exposed to an excess of RB (42 mM) in DMF under shaking for 24 hours, followed by washing with DMF until the supernatant showed negligible RB absorption. The resulting UCNHs, UCEr@RB, were redispersed in DMF.Dynamic light scattering (DLS) measurements showed that the average hydrodynamic diameter (Dh) of UCEr@RB increases slightly (either by intensity or number) (Fig. S2:† 27.9 ± 0.3 and 86.3 ± 0.8 nm for UCEr and UCEr@RB, respectively). This reflected the UCNP coverage with the dye. Moreover, the average polydispersity index (PDI) for UCEr and UCEr@RB was 0.238 ± 0.015 and 0.344 ± 0.006, respectively. The acceptable monodispersity of the samples supports that the degree of RB functionalization among UCNPs is fairly homogeneous.
Photophysics of UCEr@RB
The photophysical interaction between UCEr and RB in UCEr@RB has been studied and is presented in the next two sections. N,N-Dimethylformamide (DMF) has been the solvent of choice because it allows the preparation of concentrated, low-scattering dispersions of UCEr@RB. Fig. 1 shows the energy diagrams for the photophysical processes that can be observed for UCEr@RB exciting either the dye (λexc = 560 nm) or the UCNP (λexc = 980 nm).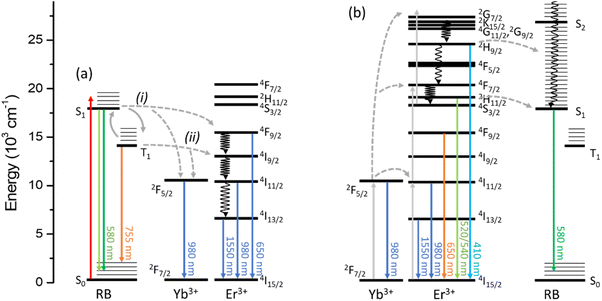 | ||
| Fig. 1 Energy diagram of photophysical processes observed for UCEr@RB under N2 exciting the (a) dye (λexc = 560 nm) or the (b) UCNP (λexc = 980 nm). | ||
Photophysics of RB in the UCNH
The photophysical properties of RB in the UCEr@RB dispersion were studied and compared with those of RB in solution to evaluate the effect that aggregation and photoactive lanthanide doping of UCEr have on the photophysical properties of RB. The contribution of each excited state is summarized in Table 1. Energy transfer from RB to UCEr in the UCNH is also discussed.| Sample | Φ F ± SDa | τ F ± SDa (ns) | Φ isc ± SD | τ T ± SDa (μs) | τ Bl ± SDa (μs) | k q(O2)c ± SDa (108 M−1s−1) | I P (a.u.) | τ P ± DSa (μs) | I DF (a.u.) |
|---|---|---|---|---|---|---|---|---|---|
| a SD: standard deviation of the measurement. b Average lifetime of a biexponential fitting (τ1 = 46 ± 2(8%); τ2 = 265 ± 3(92%)). c Extrapolated from two values. | |||||||||
| RB | 0.420 ± 0.003 | 2.2 ± 0.1 | 0.45 ± 0.03 | 241 ± 7 | 244 ± 2 | 9.98 ± 0.04 | 5.15 | 250 ± 2 | 15.5 |
| UCEr@RB | 0.174 ± 0.002 | 1.5 ± 0.1 | 0.35 ± 0.03 | 258 ± 3 | 247 ± 4b | 3.58 ± 0.04 | 3.73 | 165 ± 2 | 6.1 |
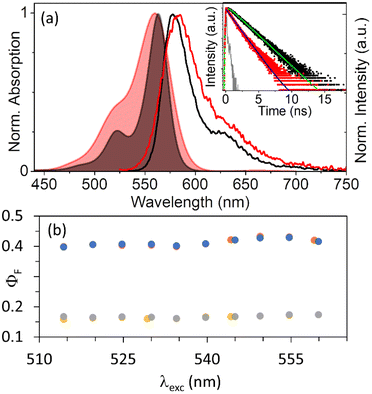 | ||
| Fig. 2 (a) Normalized absorption (colored areas) and emission (lines, λexc = 522 nm) of RB (black) and UCEr@RB (red). Raw attenuance data are shown in Fig. S3.† Inset: fluorescence lifetime kinetics (dots) and fitting (lines) of RB (black, 2.7 × 10−7 M) and UCEr@RB (red, <1 mg mL−1) under N2; λexc = 560 nm, NKT laser 5.5 MHz; λem = 580 nm. (b) Fluorescence quantum yield of RB under air (blue dots) and N2 (orange dots) and UCEr@RB under air (grey dots) and N2 (yellow dots) versus excitation wavelength (A = 0.1 at 560 nm). | ||
Xanthenic dyes form H-type aggregates.48–53 For example, xanthenes are aggregated when encapsulated in cucurbit[8]uril.54,55 The aggregation is evidenced by an increase in the relative absorption of the shoulder (absorption of the aggregates) as compared to the maximum absorption of the monomer. Although RB is a dianionic xanthenic dye that is highly soluble in polar solvents, it can also form H-type aggregates51,53 in protic polar solvents,51 in the presence of alkali metal ions56,57 or by interaction with positively charged surfaces.50,52,53,58–60 The formation of RB aggregates drastically reduced the emission quantum yield and also the quantum yield of singlet oxygen generation.52 Control experiments have shown that RB does easily aggregate in DMF (see the ESI and Fig. S4†). In this case, for UCEr@RB and RB, the ratio between λmax and the shoulder changes drastically (A560/A522 = 2.5 and A563/A522 = 3.8, respectively). In addition, the emission spectrum of RB in UCEr@RB is slightly red shifted (λmax = 583 vs. 578 nm for RB) while maintaining an identical shape. The fluorescence quantum yield (ΦF) of RB (0.42) is reduced to 0.17 when RB is anchored to the UCNP surface (different λexc values were tested from 510 to 560 nm) (Fig. 2b). Garcia et al.54 reported a drastic reduction, similar to the one observed here, which was attributed to the self-deactivation effect due to π–π interactions in the aggregates. The increase in the A563/A522 absorption ratio, the bathochromic shift of RB emission in UCEr@RB and the ΦF reduction indicate the presence of aggregates. This is consistent with the presence of aggregates in ground state due to the high RB concentration when functionalizing the UCEr (see the ESI†).
A concentration of 3 × 10−6 M mg−1 UCNH has been calculated for RB (ca. 25 molecules/UCNP), considering an identical molar absorption coefficient for RB when anchored to the surface to that free in solution. Thus, only ca. 3% of the surface would be covered by RB (calculations in the ESI†). Even assuming that either the molar absorption coefficient of RB reduced by half due to the aggregation effect49,50 or that the functionalization occurred exclusively on the lateral faces of the hexagonal prism,61–63 the coverage was low (4.7% and 3.4%, respectively).
Note that a low functionalization does not exclude the presence of dye aggregates on the surface. In fact, dimer formation on positively charged surfaces has been observed previously, even when only 1% of the active sites were occupied.64
RB fluorescence lifetime (τF) was shorter in UCEr@RB (1.5 ns) than in solution (2.2 ns) (Table 1 and Fig. 2a). The singlet excited state can be deactivated by different pathways: fluorescence emission, intersystem crossing, non-radiative deactivation and/or possible energy transfer. The decrease in the ΦF of RB in the UCNH together with a ca. 30% decrease in its lifetime indicated the presence of phenomena that effectively quench the singlet excited state 1RB. This can be attributed to different factors. One of them is RB aggregation, as mentioned above. Additionally, an improvement in the intersystem crossing efficiency when RB is anchored to the UCNP surface could be due to the heavy atom effect of the lanthanides,31,65 or/and an energy transfer from 1RB to the lanthanides (despite their low absorption coefficient). Both have been studied and are discussed in the following sections (see RB triplet state, internal conversion and energy transfer).
| ΦF + ΦISC + ΦIC = 1 | (1) |
In contrast, the Φic value of RB in UCEr@RB was 0.48. A lower Φic than the one obtained for free RB was expected for RB in UCEr@RB, because RB was anchored to the UCNP surface. The hypotheses of 1RB singlet deactivation due to the heavy atom effect of the UCNP and dye aggregation would result in an increase in the Φisc. Clearly this was not the case, thus leaving a possible energy transfer from 1RB to Ln3+ as the preferred explanation. In this context, a new term (ηetΦet), which considers the efficiency of the energy transfer (ηet) and the energy transfer quantum yield to the Ln3+ (Φet), should be added to eqn (1). If the Φic values of RB in solution and in UCEr@RB were identical, a contribution of 0.35 may be attributed to ηetΦet. This photophysical pathway is discussed in the energy transfer section.
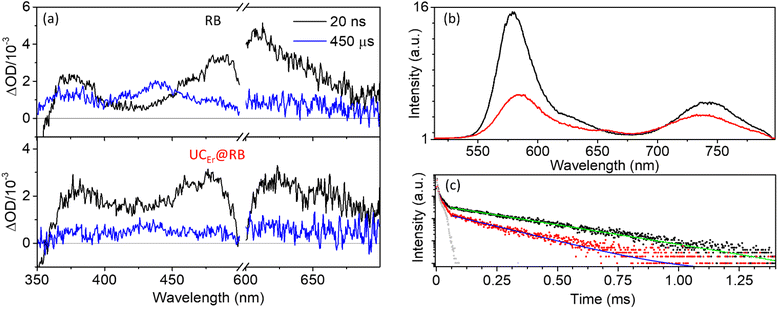
The kinetics of the singlet bleaching at 560 nm (τBl) matched well with the absorption of the triplet at 610 nm (τT = 240–260 μs) and was very similar for free RB and RB in the UCNH (Fig. S6† and Table 1). However, the bleaching of RB at 560 nm was much weaker when RB is in the UCNH due to scattering and fitted a biexponential equation.
The intersystem crossing (or triplet formation) quantum yield (Φisc) of RB in UCEr@RB was slightly lower (0.35), i.e., less 3RB was formed as compared to RB (0.45, Table 1). As expected, RB Φisc values in aprotic polar solvents, such as DMF here reported or ACN (Φisc = 0.4),68 were lower than in protic polar solvents (Φisc > 90%).40
Moreover, the rate constant for O2 quenching of 3RB, kq(O2) (Table 1 and Fig. S7†), revealed a fast process, close to a diffusion-controlled mechanism (kdiff(DMF) ≈ 8 × 109 M−1 s−1).¶ Remarkably, this process was ca. three times slower for 3RB in UCEr@RB, probably due to the restricted mobility of RB when grafted to the UCNP and the formation of aggregates, which limited the diffusion rate.
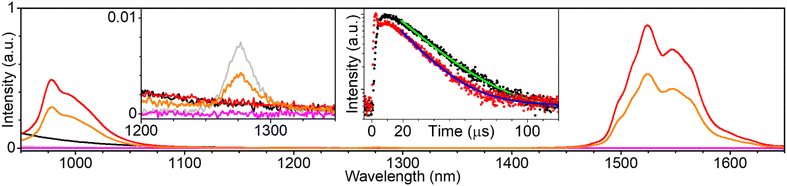
Laser induced emission measurements showed that RB exhibits room temperature phosphorescence together with thermally activated delayed fluorescence, as previously reported for other xanthenic dyes, such as eosin.69 The phosphorescence of 3RB under N2 began at ca. 670 nm and extended to ca. 1300 nm (vide infra) with a maximum at ca. 750 nm (755 and 753 nm for RB and UCEr@RB, respectively; Fig. 3b). The phosphorescence intensity of RB in the UCNH was weaker and its lifetime (τP) was shorter than that of RB (Fig. 3c and Table 1). These results point out that there are other mechanisms that deactivate the 3RB in the UCNH as compared to those of RB in solution. In addition, the weaker intensity of the delayed fluorescence of RB in UCEr@RB emphasized, once again, the existence of a 1RB deactivation process.
The NIR emission spectrum of RB (Fig. 4) showed that the tail of the phosphorescence lengthened up to ca. 1300 nm, while under air it was almost completely deactivated and the emission band characteristic of 1O2 phosphorescence centered at 1275 nm was observed (scheme in Fig. S8†).
Upon energy transfer from RB, 4F9/2 (655 nm) would be most likely the populated energy level of Er3+. Control experiments showed that the emission bands at 975 and 1550 nm were weaker when exciting the UCEr at 655 nm than the UCEr@RB nanohybrid at 560 nm (Table S3†). Therefore, these bands have been generated due to an efficient antenna effect from RB to the photoactive lanthanide ions in the UCNH (Fig. 1a).
Moreover, the emission intensities of these NIR bands at 975 nm (Yb3+ emission and 3RB phosphorescence) and 1550 nm (Er3+ emission) were greatly influenced by the atmosphere (N2/air).
The emission bands registered under air originated exclusively from an energy transfer process from 1RB (pathway i in Fig. 1a), since 3RB was efficiently deactivated under air (calculated O2 quenching efficiency >0.99). Accordingly, the emission bands under an inert atmosphere are due to the energy transfer from 1RB (46%) and 3RB (54%) to the photoactive lanthanides (pathways i and ii in Fig. 1a). This result is consistent with ca. 50% reduction of the RB fluorescence and phosphorescence lifetimes.
The emission kinetic profiles at 975 nm and 1550 nm under N2 were fitted to a biexponential decay function. At 975 nm, the biexponential behavior was due to the co-detection of two species: Yb3+ and 3RB, a short one of 52 μs (65%) and a long one of 171 μs (35%) attributed to 3RB phosphorescence. In fact, under air the kinetic profile fitted to a monoexponential decay (48 μs; Fig. S9 and Table S4†). Similarly, at 1550 nm, Er3+ emission presented two components; the shortest component was quenched under air (209 μs versus 305 μs; Fig. S9 and Table S4†), again highlighting the participation of the 3RB in the process.
In the presence of air, 1O2 phosphorescence was observed at 1275 nm with the characteristic lifetime in DMF (19–23 μs; inset of Fig. 4). The relative intensity of this emission under air was lower for UCEr@RB (7.5 × 10−2 a.u. in RB vs. 4.3 × 10−2 a.u. in UCEr@RB; Fig. 4). This result is attributed to a lower generation of singlet oxygen by triplet–triplet energy transfer from 3RB on the surface of the UCNP as compared to RB in solution. Consequently, if the final purpose of the UCNH were to efficiently generate 1O2 (e.g., for photodynamic therapy), the decrease of Φisc and kq(O2) will dramatically influence the performance of the UCNH.
Photophysics of UCEr in the UCNH
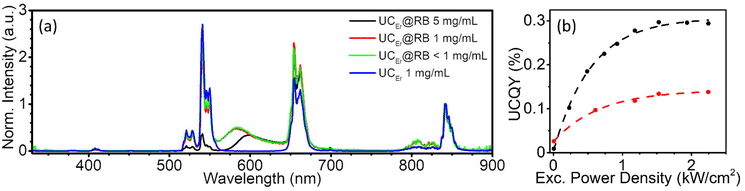
Upon NIR excitation at 980 nm, UCEr transfers energy to RB in UCEr@RB. Note that high chromophore absorption (despite low concentrations of UCNP) can produce secondary inner-filter effects or/and emission self-absorption that modify the shape of the emission spectrum. It is convenient to prepare diluted dispersions. Fig. 5a shows the emission spectrum of UCEr and UCEr@RB at 1 mg mL−1 and at 5 mg mL−1 in which the inner-filter effect for UCEr@RB can be appreciated.UC intensity and lifetimes were not affected by the gas atmosphere (N2 or air). As previously reported, in the UCNH, the relative intensity of the UCNP emission affected by RB absorption (520–540 nm) decreased slightly as compared to the UCEr emission (Fig. 5a), and a new emission centered at 584 nm appeared. This new band can be attributed to the sensitized emission of RB from the Er3+ of the UCNP, since it presents a lifetime in the order of the UC emissions of the Er3+ (51 μs; Table 2) in comparison to the conventional fluorescence of RB by direct excitation (1.5 ns). Note that the broad-emission tail of RB emission overlaps with the Er3+ emission at 655 nm (therefore, the Er3+ emission intensity increased in Fig. 5a).
Likewise, the presence of RB in the UCNH produced a slight reduction in the lifetime of the bands affected by RB absorption with respect to the precursor UCEr (Table 2 and Fig. S10†). Although the presence of the dye on the surface of the UCNP did not seem to greatly affect either the relative intensities of the Er3+ UC emissions or its deactivation kinetics, it did affect the overall performance of the UC process. The presence of the dye reduced ca. 50% of the upconversion quantum yield (UCQY) of the UCNH with respect to the UCEr (Table 2 and Fig. 5b). This is an expected result because more non-radiative deactivation phenomena will be possible by adding more electronic states to the system (with RB).
The 3RB generation sensitized by UC emissions was not observed by transient absorption spectroscopy at 980 nm (5 mJ per laser pulse). Likewise, an attempt was made to record the emission of 1O2 in a dispersion of UCNH bubbled with O2 for 20 minutes, but no signal could be detected. Although it has been widely reported that PS-functionalized UCNPs are capable of generating ROS,44,70–74 the sensitized formation of 3RB and 1O2 could not be spectroscopically observed under our experimental conditions, probably because the instrumentation is not sensible enough to detect these species for our system.
Conclusions
In summary, the results here described shed light on unresolved questions when functionalizing an UCNP with a xanthenic dye, such as RB. The photophysics of RB can be drastically affected by the aggregation and interaction with the UCNP surface and the presence of photoactive lanthanide cations (Yb3+, Er3+) doping the UCNP matrix.The low functionalization degree and RB aggregation in UCEr@RB are attributed to the high concentration of RB in DMF used for functionalization. Equally important, the dynamics of RB excited states were roundly changed: ΦF and Φisc decreased (specially the ΦF) and both 1RB and 3RB excited states were deactivated by means of energy transfer processes from the dye to the photoactive lanthanides in the UCNPs (lower emission intensity and shorter fluorescence and phosphorescence lifetimes). As far as we know, this is the first experimental demonstration of the equal contribution of 1RB and 3RB to the antenna effect from RB to Yb3+ and Er3+, which results in NIR-II emissions. Indeed, as previously reported for similar UCNHs, the UC emission spectra showed little influence on the intensity ratio of the Ln3+ emission bands for the nanohybrid (when avoiding the secondary inner filter effect), and a new sensitized long-lived emission from RB was detected due to a resonant energy transfer from Er3+ to 1RB. Moreover, the absolute external UCQY of the system decreased to half as compared to that of the pristine UCNP. Therefore, rather than a synergistic or additive effect, in this UCNH, we observed an antagonistic effect which limits its potential application as a bioimaging or 1O2 generation agent.
All in all, it was demonstrated that the dye photophysics in solution cannot be taken for granted when functionalizing UCNPs, and every case should be studied in advance to ensure a synergistic rather than an antagonistic effect. This knowledge is of utmost importance to design efficient functional UCNHs. In the case of designing bioimaging probes, a reduction in the dye ΦF will be an additional limitation to the low UCQY of the UCNP. Similarly, as shown here for the UCEr@RB nanohybrid, if the aim is to generate 1O2, the reduction in the dye Φisc together with the slower diffusion of the colloid will negatively affect the 1O2 quantum yield. In this example, the doping of the UCNP with Gd3+ may be a plausible and rational solution. In any case, the effects of Gd3+ doping, UCNP size, dye loading, and concentration used for the functionalization process, the distance between Ln3+ and dyes, and chromophores with different ratios of singlet/triplet need to be studied in the future to design UCNHs.
Author contributions
Juan Ferrera-González: investigation, methodology, data curation, visualization, and writing – original draft; María González-Béjar: conceptualization, supervision, and writing – review & editing; Julia Pérez-Prieto: conceptualization, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by MINECO (grant number PID2020-115710GB-I00); Agencia Estatal de Investigación-AEI (grant number MMCIU Unit of Excellence “Maria de Maeztu” CEX2019-000919-M); Ministerio de Educación, Cultura y Deporte (FPU grant J.F.-G.); and Generalitat Valenciana (grant numbers CIPROM/2022/57 and IDIFEDER/2018/064), all of them partially co-financed with FEDER funds. This study forms part of the Advanced Materials programme (MFA/2022/051) and was supported by MICIN with funding from the European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat Valenciana. TEM and ICP-MS were performed in Servicio Central de Soporte a la Investigación Experimental (SCSIE, University of Valencia).References
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS PubMed.
- G. Tessitore, G. A. Mandl, M. G. Brik, W. Park and J. A. Capobianco, Nanoscale, 2019, 11, 12015–12029 RSC.
- X. Li, F. Zhang and D. Zhao, Chem. Soc. Rev., 2015, 44, 1346–1378 RSC.
- M. R. Hamblin, Dalton Trans., 2018, 47, 8571–8580 RSC.
- M. González-Béjar, L. Francés-Soriano and J. Pérez-Prieto, Front. Bioeng. Biotechnol., 2016, 4, 47 Search PubMed.
- X. Zhu, J. Zhang, J. Liu and Y. Zhang, Adv. Sci., 2019, 6, 1901358 CrossRef CAS PubMed.
- B. Chen and F. Wang, Trends Chem., 2020, 2, 427–439 CrossRef CAS.
- W. Xu, X. Chen and H. Song, Nano Today, 2017, 17, 54–78 CrossRef CAS.
- W. Xu, H. Liu, D. Zhou, X. Chen, N. Ding, H. Song and H. Ågren, Nano Today, 2020, 33, 100892 CrossRef CAS.
- E. Andresen, U. Resch-Genger and M. Schäferling, Langmuir, 2019, 35, 5093–5113 CrossRef CAS PubMed.
- F. Pini, L. Francés-Soriano, V. Andrigo, M. M. Natile and N. Hildebrandt, ACS Nano, 2023, 17, 4971–4984 CrossRef CAS PubMed.
- L. Francés-Soriano, N. Estebanez, J. Pérez-Prieto and N. Hildebrandt, Adv. Funct. Mater., 2022, 32, 2201541 CrossRef.
- J. Ferrera-González, M. González-Béjar and J. Pérez-Prieto, in Dyes and Photoactive Molecules in Microporous Systems. Structure and Bonding, ed. F. López Arbeloa, Springer, Cham, 2020, pp. 371–396 Search PubMed.
- X. Wang, R. R. Valiev, T. Y. Ohulchanskyy, H. Ågren, C. Yang and G. Chen, Chem. Soc. Rev., 2017, 46, 4150–4167 RSC.
- Y. Liu, X. Meng and W. Bu, Coord. Chem. Rev., 2019, 379, 82–98 CrossRef CAS.
- A. Zhou, Y. Wei, B. Wu, Q. Chen and D. Xing, Mol. Pharm., 2012, 9, 1580–1589 CrossRef CAS PubMed.
- Q. Yuan, Y. Wu, J. Wang, D. Lu, Z. Zhao, T. Liu, X. Zhang and W. Tan, Angew. Chem., Int. Ed., 2013, 52, 13965–13969 CrossRef CAS PubMed.
- M. González-Béjar, M. Liras, L. Francés-Soriano, V. Voliani, V. Herranz-Pérez, M. Duran-Moreno, J. M. Garcia-Verdugo, E. I. Alarcon, J. C. Scaiano and J. Pérez-Prieto, J. Mater. Chem. B, 2014, 2, 4554–4563 RSC.
- K. Liu, X. Liu, Q. Zeng, Y. Zhang, L. Tu, T. Liu, X. Kong, Y. Wang, F. Cao, S. A. G. G. Lambrechts, M. C. G. G. Aalders and H. Zhang, ACS Nano, 2012, 6, 4054–4062 CrossRef CAS PubMed.
- F. Chen, S. Zhang, W. Bu, Y. Chen, Q. Xiao, J. Liu, H. Xing, L. Zhou, W. Peng and J. Shi, Chem. – Eur. J., 2012, 18, 7082–7090 CrossRef CAS PubMed.
- X. Yang, Q. Xiao, C. Niu, N. Jin, J. Ouyang, X. Xiao and D. He, J. Mater. Chem. B, 2013, 1, 2757–2763 RSC.
- Y. Il Park, H. M. Kim, J. H. Kim, K. C. Moon, B. Yoo, K. T. Lee, N. Lee, Y. Choi, W. Park, D. Ling, K. Na, W. K. Moon, S. H. Choi, H. S. Park, S.-Y. Yoon, Y. D. Suh, S. H. Lee and T. Hyeon, Adv. Mater., 2012, 24, 5755–5761 CrossRef PubMed.
- P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526–4527 CrossRef CAS PubMed.
- A. M. Kotulska, A. Pilch-Wróbel, S. Lahtinen, T. Soukka and A. Bednarkiewicz, Light: Sci. Appl., 2022, 11, 1–14 CrossRef PubMed.
- A. Pilch-Wróbel, A. M. Kotulska, S. Lahtinen, T. Soukka and A. Bednarkiewicz, Small, 2022, 18, 2200464 CrossRef PubMed.
- S. Melle, O. G. Calderón, M. Laurenti, D. Mendez-Gonzalez, A. Egatz-Gómez, E. López-Cabarcos, E. Cabrera-Granado, E. Díaz and J. Rubio-Retama, J. Phys. Chem. C, 2018, 122, 18751–18758 CrossRef CAS.
- R. Marin, L. Labrador-Paéz, A. Skripka, P. Haro-González, A. Benayas, P. Canton, D. Jaque and F. Vetrone, ACS Photonics, 2018, 5, 2261–2270 CrossRef CAS.
- O. Dukhno, F. Przybilla, M. Collot, A. Klymchenko, V. Pivovarenko, M. Buchner, V. Muhr, T. Hirsch and Y. Mély, Nanoscale, 2017, 9, 11994–12004 RSC.
- F. Pini, L. Francés-Soriano, N. Peruffo, A. Barbon, N. Hildebrandt and M. M. Natile, ACS Appl. Mater. Interfaces, 2022, 14, 11883–11894 CrossRef CAS PubMed.
- S. Bhuckory, S. Lahtinen, N. Höysniemi, J. Guo, X. Qiu, T. Soukka and N. Hildebrandt, Nano Lett., 2023, 23, 2253–2261 CrossRef CAS PubMed.
- D. J. Garfield, N. J. Borys, S. M. Hamed, N. A. Torquato, C. A. Tajon, B. Tian, B. Shevitski, E. S. Barnard, Y. D. Suh, S. Aloni, J. B. Neaton, E. M. Chan, B. E. Cohen and P. J. Schuck, Nat. Photonics, 2018, 12, 402–407 CrossRef CAS.
- S. Han, R. Deng, Q. Gu, L. Ni, U. Huynh, J. Zhang, Z. Yi, B. Zhao, H. Tamura, A. Pershin, H. Xu, Z. Huang, S. Ahmad, M. Abdi-Jalebi, A. Sadhanala, M. L. Tang, A. Bakulin, D. Beljonne, X. Liu and A. Rao, Nature, 2020, 587, 594–599 CrossRef CAS PubMed.
- S. Han, Z. Yi, J. Zhang, Q. Gu, L. Liang, X. Qin, J. Xu, Y. Wu, H. Xu, A. Rao and X. Liu, Nat. Commun., 2021, 12, 1–9 CrossRef PubMed.
- P. Zhang, J. Ke, D. Tu, J. Li, Y. Pei, L. Wang, X. Shang, T. Guan, S. Lu, Z. Chen and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202112125 CrossRef CAS PubMed.
- X. Wang, X. Wang, G. V. Baryshnikov, R. R. Valiev, R. Fan, S. Lu, H. Ågren and G. Chen, Chem. Eng. J., 2021, 426, 131282 CrossRef CAS.
- K. R. Gopidas and P. V. Kamat, J. Phys. Chem., 1989, 93, 6428–6433 CrossRef CAS.
- C. Nasr, D. Liu, S. Hotchandani and P. V. Kamat, J. Phys. Chem., 1996, 100, 11054–11061 CrossRef CAS.
- I.-I. S. Lim, F. Goroleski, D. Mott, N. Kariuki, W. Ip, J. Luo and C.-J. Zhong, J. Phys. Chem. B, 2006, 110, 6673–6682 CrossRef CAS PubMed.
- V. Muhr, C. Würth, M. Kraft, M. Buchner, A. J. Baeumner, U. Resch-Genger and T. Hirsch, Anal. Chem., 2017, 89, 4868–4874 CrossRef CAS PubMed.
- L. Ludvíková, P. Friš, D. Heger, P. Šebej, J. Wirz and P. Klán, Phys. Chem. Chem. Phys., 2016, 18, 16266–16273 RSC.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS.
- W. Liu, Y. Zhang, W. You, J. Su, S. Yu, T. Dai, Y. Huang, X. Chen, X. Song and Z. Chen, Nanoscale, 2020, 12, 13948–13957 RSC.
- F. Jin, J. Qi, D. Liu, Y. You, G. Shu, Y. Du, J. Wang, X. Xu, X. Ying, J. Ji and Y. Du, J. Controlled Release, 2021, 337, 90–104 CrossRef CAS PubMed.
- X. Chen, Y. Zhang, X. Zhang, Z. Zhang and Y. Zhang, Microchim. Acta, 2021, 188, 1–10 CrossRef PubMed.
- Y. Wang, K. Liu, X. Liu, K. Dohnalová, T. Gregorkiewicz, X. Kong, M. C. G. Aalders, W. J. Buma and H. Zhang, J. Phys. Chem. Lett., 2011, 2, 2083–2088 CrossRef CAS.
- Z. Li and Y. Zhang, Nanotechnology, 2008, 19, 16–21 Search PubMed.
- A. Dong, X. Ye, J. Chen, Y. Kang, T. Gordon, J. M. Kikkawa and C. B. Murray, J. Am. Chem. Soc., 2011, 133, 998–1006 CrossRef CAS PubMed.
- N. J. Hestand and F. C. Spano, Chem. Rev., 2018, 118, 7069–7163 CrossRef CAS PubMed.
- B. Mendes, S. Kassumeh, A. Aguirre-Soto, Q. Pei, B. Heyne and I. E. Kochevar, Photochem. Photobiol., 2021, 97, 718–726 CrossRef CAS PubMed.
- M. E. Daraio and E. San Román, Helv. Chim. Acta, 2001, 84, 2601–2614 CrossRef CAS.
- D. Xu and D. C. Neckers, J. Photochem. Photobiol., A, 1987, 40, 361–370 CrossRef CAS.
- E. Alarcón, A. M. Edwards, A. Aspée, C. D. Borsarelli and E. A. Lissi, Photochem. Photobiol. Sci., 2009, 8, 933–943 CrossRef PubMed.
- H. B. Rodríguez, M. G. Lagorio and E. S. Román, Photochem. Photobiol. Sci., 2004, 3, 674–680 CrossRef PubMed.
- P. Montes-Navajas, A. Corma and H. Garcia, ChemPhysChem, 2008, 9, 713–720 CrossRef CAS PubMed.
- P. Montes-Navajas, M. González-Béjar, J. C. Scaiano and H. García, Photochem. Photobiol. Sci., 2009, 8, 1743–1747 CrossRef CAS PubMed.
- O. Valdes-Aguilera and D. C. Neckers, J. Photochem. Photobiol., A, 1989, 47, 213–222 CrossRef CAS.
- O. Valdes-Aguilera and D. C. Neckers, J. Phys. Chem., 2002, 92, 4286–4289 CrossRef.
- M. B. E. Turbay, V. Rey, N. M. Argañaraz, F. E. Morán Vieyra, A. Aspée, E. A. Lissi and C. D. Borsarelli, J. Photochem. Photobiol., B, 2014, 141, 275–282 CrossRef CAS PubMed.
- Y. Litman, M. G. Voss, H. B. Rodríguez and E. S. Román, J. Phys. Chem. A, 2014, 118, 10531–10537 CrossRef CAS PubMed.
- P. V. Kamat and W. E. Ford, Chem. Phys. Lett., 1987, 135, 421–426 CrossRef CAS.
- W. Ren, S. Wen, S. A. Tawfik, Q. P. Su, G. Lin, L. A. Ju, M. J. Ford, H. Ghodke, A. M. Van Oijen and D. Jin, Chem. Sci., 2018, 9, 4352–4358 RSC.
- H. Na, K. Woo, K. Lim and H. S. Jang, Nanoscale, 2013, 5, 4242–4251 RSC.
- S. Lahtinen, A. Lyytikäinen, H. Päkkilä, E. Hömppi, N. Perälä, M. Lastusaari and T. Soukka, J. Phys. Chem. C, 2017, 121, 656–665 CrossRef CAS.
- W. E. Ford and P. V. Kamat, J. Phys. Chem., 1989, 93, 6423–6428 CrossRef CAS.
- G. A. Hebbink, L. Grave, L. A. Woldering, D. N. Reinhoudt and F. C. J. M. Van Veggel, J. Phys. Chem. A, 2003, 107, 2483–2491 CrossRef CAS.
- S. L. Murov, I. Carmichael and G. L. Hug, in Handbook of Photochemistry, Marcel Dekker, INC., New York, 2nd edn, 1993, pp. 1–53 Search PubMed.
- H. C. Junqueira, D. Severino, L. G. Dias, M. S. Gugliotti and M. S. Baptista, Phys. Chem. Chem. Phys., 2002, 4, 2320–2328 RSC.
- F. Stracke, M. Heupel and E. Thiel, J. Photochem. Photobiol., A, 1999, 126, 51–58 CrossRef CAS.
- C. A. Parker and C. G. Hatchard, Trans. Faraday Soc., 1961, 57, 1894–1904 RSC.
- C. Wang, L. Cheng and Z. Liu, Theranostics, 2013, 3, 317–330 CrossRef PubMed.
- N. M. Idris, M. K. Gnanasammandhan, J. Zhang, P. C. Ho, R. Mahendran and Y. Zhang, Nat. Med., 2012, 18, 1580–1585 CrossRef CAS PubMed.
- C. Wang, H. Tao, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 6145–6154 CrossRef CAS PubMed.
- D. Zhang, L. Wen, R. Huang, H. Wang, X. Hu and D. Xing, Biomaterials, 2018, 153, 14–26 CrossRef CAS PubMed.
- Y. Liu, X. Meng and W. Bu, Coord. Chem. Rev., 2019, 379, 82–98 CrossRef CAS.
- S. L. Murov, I. Carmichael and G. L. Hug, Handbook of Photochemistry, Marcel Dekker, INC., New York, 2nd edn, 1993 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, UCEr TEM images, UCEr ICP-MS analysis, absorption of RB, attenuance of UCEr@RB, RB concentration study in DMF, calculation of RB molecules per UCNP, TAS spectra and kinetics of RB and UCEr@RB, scheme of RB photophysical processes under N2 and air, kq(O2) Stern–Volmer plot, NIR-II emission kinetics, upconversion and downshifting emission kinetics of UCEr and UCEr@RB and their fitting parameters. See DOI: https://doi.org/10.1039/d3nr03774f |
| ‡ Search performed in Web of Science (Clarivate Analytics) reported 5242 results with the following input data: database: all; type of document: article; topic: upconversion nanoparticles; keywords should include: upconverting nanoparticles, nanohybrid, nanosystem, nanoplatform, nanostructure, heterostructure; keywords must include: dye. Date of search: 2023-10-11. |
| § Set up: O2-saturated D2O colloidal dispersion with no reported concentration; excitation: a CW 980 nm laser diode with no reported power density; detection: a liquid nitrogen cooled InGaAs detector. |
| ¶ Calculated from equation kdiff = 8RT·103/(3η), R being the gas constant, T being the temperature and η being the viscosity of the solvent.75 |
| This journal is © The Royal Society of Chemistry 2023 |