Active nanomotors surpass passive nanomedicines: current progress and challenges
Shuqin
Chen
ab,
Yuduo
Chen
ab,
Mingming
Fu
ab,
Qinghua
Cao
c,
Bo
Wang
c,
Wenjun
Chen
*ab and
Xing
Ma
 *abd
*abd
aSchool of Materials Science and Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen, 518055, China. E-mail: maxing@hit.edu.cn
bSauvage Laboratory for Smart Materials, School of Materials Science and Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen, 518055, China. E-mail: chenwenjun@hit.edu.cn
cSchool of Materials Engineering, Shanghai University of Engineering Science, Shanghai, 201620, China
dShenzhen Bay Laboratory, No. 9 Duxue Road, Shenzhen, 518055, China
First published on 13th April 2022
Abstract
Artificial nanomotors show advantages over traditional nanomedicines in biomedical applications due to their active locomotion by converting various energy sources into mechanical force in situ. Currently, nanomotors are attracting wide attention in diagnosis and therapy towards clinical applications. In this perspective, we summarize recent developments of nanomotors in biomedical applications, including targeted drug delivery, biological barrier penetration, and diagnostics and cancer treatment. The development of nanomotors in biomedicine still faces considerable challenges and practical difficulties towards clinical use, which are discussed in detail. Finally, we propose potential solutions that may help the development of nanomotors towards advanced diagnostics and therapy.
Introduction
A nanomotor, a miniaturized robot equipped with an engine, is capable of transferring diverse types of energy sources into mechanical force in situ, thus achieving self-propulsion.1–6 The development of nanomotors over the last decade has demonstrated their great potential for diverse applications in biomedical fields, such as targeted drug delivery,7–9 minimally invasive treatment,10,11 penetration of complex biological barriers,12–16 and deep tissue imaging.17–19 Attributed to their miniaturization and unique mobility, nanomotors are expected to actively target the lesion area, and accomplish specific tasks in a non-invasive way in future clinical applications.In contrast to traditional nanomedicines which are delivered passively by blood circulation, nanomotors are able to autonomously drive toward diseased regions, where only 0.7% administered dosages can be reached by traditional passive nano-carriers.20 For example, motile nanomotors as drug delivery carriers enable therapeutic medicines to enrich at the target site,21 thus enhancing the efficacy of the administered dosage and reducing side effects as well. In addition, the active propulsion of nanomotors can enhance the penetration of biological barriers such as mucus,14,22,23 cell membranes,16,24 and the blood–brain barrier.12,25 Nanomotors outperform nanomedicines not only in terms of therapeutic intervention, but also in bio-imaging for diagnostics.26–28 By guiding nanomotors to target sites, real-time imaging can be acquired, thus providing disease signals and precise positioning information inside the body, essentially guiding precise diagnosis and therapy.
In this perspective, we focus on the advanced applications of nanomotors in the biomedical field and discuss the superiorities of active nanomotors over traditional nanomedicines. Based on the state-of-the-art research on biomedical nanomotors, we discuss current challenges that hinder the development of nanomotors in biomedicine, and put forward feasible solutions in future.
Superiorities of nanomotors over nanomedicines
Active targeted drug delivery
Assuming that administered dosages of drug enter the body, they may not break through the circulatory system due to the hemodynamics of blood flow, or not penetrate target tumor tissues because of interstitial fluid pressure. Additionally, as the drug travels throughout the body in the bloodstream, unexpected side effects may occur to harm normal tissues and organs where drugs non-specifically gather. Although new strategies such as surface functionalization with targeting ligands may enhance the chances of traditional drug carriers reaching their target sites, there still exist hard-to-reach locations behind biological barriers and deep hypoxic tumor cores that can be hardly penetrated. These issues are especially challenging for the application of conventional medicine in practice. Therefore, it is vital to design an active delivery vehicle which is capable of enriching at target sites and then releasing the loaded drugs.Thus, the development of controllable drug delivery systems is one of the current major goals in biomedicine. Drug delivery takes many issues into account, such as non-specific aggregates in healthy organs, limited tissue penetration, and biocompatibility.29–31 Therefore, it is significant to solve these problems and deliver the drug to the target site for effective cure. Using cells or microorganisms as carriers is one of the promising approaches. The use of macrophages, red blood cell membranes, or leukocyte-derived membranes to camouflage the drug delivery system32–35 is also accessible because of their mutual recognition of target cells or tissues by membrane surface proteins. Thanks to advanced nanotechnology and nanomaterials, various types of nanomotors can be designed and manufactured according to the different demands to achieve specific functions.
Considering that sperm cells are more suitable for the female reproductive system environment than purely synthetic micromotors, Xu et al.36 proposed a sperm hybrid micromotor for targeted drug delivery (Fig. 1A). The drug delivery system consists of a magnetic tubular structure and bovine sperm cells loaded with chemotherapy drugs. The micromotors approach cancer cells under the control of magnetic force, and release sperm cells in situ, allowing the drug loaded sperm cells to kill the cancer cells. Experimental results show that this drug delivery system can be used to treat gynecological tumors and other diseases of the female reproductive tract. Compared with pure artificial micromotors, the sperm hybrid micromotor system not only has high drug loading and movement controllability, but also has the ability to suppress immune responses, making them more suitable for the host.
 | ||
| Fig. 1 (A) Schematic of the microfluidic chip for the transport and delivery of drug-loaded sperm nanomotors. Reproduced with permission.36 Copyright 2017, American Chemical Society. (B) Schematic diagram of urease-driven nanomotors penetrating bladder cancer spheroids. Reproduced with permission.38 Copyright 2018, American Chemical Society. (C) Schematic of the use of the micromotors for targeted delivery in the intestine. Reproduced with permission.41 Copyright 2019, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. (D) Schematic depicting multifunctional microrollers actuated magnetically for targeted cargo delivery against blood flow on the vessel wall. Reproduced with permission.43 Copyright 2020, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. | ||
In addition to using external thrust for drug delivery, the continuous driving force of self-propelled nanomotors can also aid the passive diffusive transport of drugs across biological barriers and facilitate tissue penetration.37 Hortelão et al.38 presented urease-driven nanomotors based on hollow silica nanoparticles with polyethylene glycol and a bladder cancer-specific antibody (anti-FGFR3) on the outer surface (Fig. 1B). The high urea concentration in the bladder provided a large amount of fuel for the actuation of the nanomotor, and the urease-driven nanomotor was active in the bladder. This active motion increased the penetration rate of the nanomotor, and the specific antibody enhanced its tumor-targeting ability as well, thereby enhancing the therapeutic efficacy.
Although many studies have shown the promotion of nanomotors as active carriers for targeted drug delivery, the imaging and motion control in the deep tissue of the living body are less studied,39,40 which leads to the incomplete transparency of drug accumulation and therapeutic effect in the deep tissue. To achieve effective control of biomedical nanomotors, Wu et al.41 proposed a photoacoustic computed tomography (PACT)-guided intestinal micromotor (Fig. 1C). The migration of the micromotor to the intestinal target region was visualized using PACT in real time. The platform established by the research team lays the foundation for the targeted delivery of drugs within deep tissues and opens up new horizons for precision medicine research.
The application of micro/nano-motors in the human body is mainly limited to stagnant or low-speed fluid environments. Their motion capability in the circulatory system is weakened as a result of blood flow, especially for robots with an overall size of less than 10 μm. However, the circulatory system is the natural fluid transport network of the human body, which can reach all organs and even the deepest tissues.42 So, it is of great significance for the nanomotors that can be preserved for a long time in a dynamic flow environment. Alapan et al.43 prepared micromotors composed of magnetically responsive half side on silica particles (Fig. 1D). The motion for targeted drug delivery could be activated by an external magnetic field and the targeting effect was achieved by surface functionalization of cell-specific antibodies. This multifunctional micromotor has the capability of countercurrent motion and controlled navigation in the blood flow plane, which sheds new light on navigation and cargo transportation of nanorobots within the circulatory system.
Micro/nano-motors can be designed in various forms to overcome many challenges in the field of drug delivery, such as nonspecific adsorption, systemic toxicity, and limited tumor penetration. The achievements of nanomotors in the field of targeted drug delivery display their prospects for wider development in biomedical areas.
Enhanced tissue penetration
The physiological environment of tissue is complex, which brings challenges to the biomedical application of nanomotors in practice. In recent years, many research studies have focused on exploring the application of nanomotors in real tissue environments, including skin tissue,44,45 mucosal tissue,14,46 ocular tissue,47 and the blood–brain barrier.12,13Fungi easily form biofilms under the skin barrier, making it difficult for antifungal drugs to penetrate skin tissue and reach the infection site.48 The emergence of self-propelling nanomotors provides new solutions to this problem. Recently, Dai et al.49 fabricated a parachute-like nanomotor (PNM) triggered by a near-infrared laser and loaded with miconazole nitrate (MN), denoted as PNM-MN (Fig. 2A). Under near-infrared laser irradiation, the nanomotors can achieve enhanced skin tissue penetration. In vivo experiments show that, compared with the low tissue penetration effect of free MN drugs, PNM-MN triggered by laser light can effectively penetrate the skin and deliver anti-fungal drugs to the infection site, achieving effective antibacterial and anti-inflammatory effects on skin surface tissue.
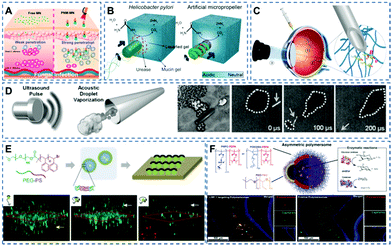 | ||
| Fig. 2 (A) Schematic diagram of nanomotors facilitating drug penetration into skin tissue. Reproduced with permission.49 Copyright 2021, American Chemical Society. (B) Mechanism for mucin penetration. Reproduced with permission.14 Copyright 2015, The Authors. (C) Schematic of the three-step targeted delivery procedure by helix nanomotors used for the slippery micropropellers. Reproduced with permission.47 Copyright 2018, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. (D) Schematic diagram of ultrasonic pulse-triggered propulsion of a self-fueled nanomotor. Reproduced with permission.7 Copyright 2012, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (E) Schematic diagram of the preparation process of a polymer Janus motor and the fluorescent Janus motor distribution for the different groups in the vasculature model. Reproduced with permission.50 Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (F) Schematic illustration of the components of asymmetric polymersome nanomotors. Immunofluorescence histologies show rat hippocampus sections of animals treated with asymmetric polymersome nanomotors loaded with Gox and Cat and pristine asymmetric polymersomes loaded with Gox and Cat. Reproduced with permission.12 Copyright 2017, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. | ||
Mucus as the human body's defence barrier, not only prevents the intrusion of pathogens, but also keeps drug carriers out. Walker's team14 utilized the strategy of Helicobacter pylori producing urea, increasing local pH to liquefy gastric mucus and overcoming the mucosal barrier. As shown in Fig. 2B, urease catalyses the hydrolysis of urea to release ammonia, followed by a local increase in pH that induces a gel–sol transition of urea. The gel–sol transition reduces the viscosity through liquefaction, enabling nanomotors to propel through gastric mucus under the control of an external thrust. The nanomotor can be used as a carrier to carry drugs and other particles. Under this propulsion mechanism, the transport of substances in gastric mucus can be realized.
The designability of nanomotors and the advantages of multiple propulsion methods make them shine in the field of tissue penetration. Wu et al.47 made a magnetic propulsion screw motor (Fig. 2C) that was driven through the vitreous humor by remote control, reaching the retina and performing non-invasive detection near the retina. The application of nanomotors offers endless possibilities for future ophthalmic treatments. In addition to the abovementioned magnetically controlled micro/nano-motors, Wang's team7 developed a micro-machine carrying an organic fuel source (perfluorocarbon emulsion), where organic fuel can vaporize under the action of ultrasonic waves and releases energy for high-speed propulsion (Fig. 2D).
In nanomedicine based cancer therapy, chemotherapeutics mainly reach target sites through the so-called enhanced permeation and retention (EPR) effect, but the efficiency of such passive drug delivery is very low. Therefore, Peng et al.50 proposed polymer-based Janus nanomotors for enhanced EPR (Fig. 2E). In the simulated tumor vascular model experiment, compared with the control group, more particles migrate to the bottom cavity in the experimental group, confirming the possibility of using polymer nanomotors to enhance the EPR effect.
The blood–brain barrier is the most important physiological barrier which regulates glucose from the blood to the brain. However, only a small percentage of the injected drug dose can reach the brain and the spinal cord. The current study shows that nanomotors can enhance the penetration and drug delivery across the blood–brain barrier. Joseph et al.12 encapsulated glucose and catalase into polymersomes (Fig. 2F), which have the ability to self-propel to high-concentration regions in the presence of a glucose gradient. Experiments showed that the chemotactic aggregates of nanomotors can penetrate the blood–brain barrier by self-propulsion, and the number of aggregates entering the rat brain parenchyma was increased four times as compared to that of non-chemotactic aggregates.
Enhanced cell membrane penetration
As a major biological barrier against drug delivery, the cell membrane ensures the relative stability of the intracellular environment and prevents the free entry of exogenous matter. However, the cell membrane limits the ability of cargos (including drugs, genes, and proteins) to exert their effects inside cells. Therefore, the capability to open the cell membrane by untethered nanomotors is of great significance in biomedical applications. In the field of nanomedicine, conventional strategies rely on endocytosis, which often results in low cytosolic release and long endocytosis duration. In contrast, the active nanomotors can convert other forms of energy into mechanical motion, hereby demonstrating the potential to address the issue of cellular penetration.Artificial nanomotors can be propelled by chemical fuels or by external physical fields, showing their advantages in enhanced cell membrane penetration. Catalytic reactions have been widely utilized in designing nanomotors in recent years, with hydrogen peroxide and urea being the most common fuels. Alzebeta et al.51 achieved nonviral transfection of a protein into a cell facilitated by active nanorobots. As shown in Fig. 3A, swarms of chemically powered nanorobots were internalized into cells for gene transfection. The rectangular Au@Ag nanorobots (68 nm × 33 nm) were synthesized by a three-step wet-chemical method. And they exhibited enhanced diffusion in the presence of hydrogen peroxide, which enabled significant cellular internalization within 10 min of incubation. Another self-propelled nanomotor was used for intracellular cargo release,52 as shown in the scheme in Fig. 3B. The nanomotors with a diameter of 418 ± 21 nm equipped with urease enzymes could reach a maximum diffusion coefficient of 1.47 ± 0.04 μm2 s−1 at 300 mM of urea.
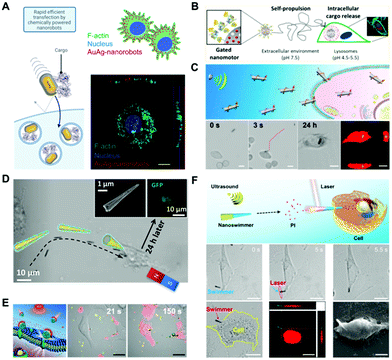 | ||
| Fig. 3 Enhanced penetration of the cell membrane by nanomotors. (A) Schematic depicting internalization of protein cargo carried by AuAg-nanorobots, and orthogonal projection of the Z-stack image showing that the AuAg-nanorobots reside within the cell. Reproduced with permission.51 Copyright 2021, American Chemical Society. (B) Enzyme-propelled nanomotor entering into the cytoplasm. Reproduced with permission.52 Copyright 2019, American Chemical Society. (C) Acoustic perforation of liquid metal nanomachines. Reproduced with permission.54 Copyright 2018, American Chemical Society. (D) Magnetic perforation through nanospears. Reproduced with permission.57 Copyright 2018, American Chemical Society. (E) Nanoparticles thermomechanically percolating through cell membranes. Reproduced with permission.60 Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (F) Photomechanical puncture of needle-like nanoswimmers, and images showing the movement of the nanoswimmers toward a HeLa cell under an acoustic field and images of the nanoswimmer after cell poration. Reproduced with permission.61 Copyright 2019, American Chemical Society. | ||
External physical fields such as acoustic, magnetic, and light fields were also often applied to open the cell membrane.53 As shown in Fig. 3C, the liquid metal nanorods propelled by ultrasound were applied for entering cells for intracellular photothermal therapy.54 The rod-like nanomotors with a length of 5.5 μm, and diameters of 389 ± 29 nm and 590 ± 38 nm for the two ends, respectively, were fabricated by a pressure-filter-template method. Under the actuation by an acoustic field, the nanorod drilled the cell membrane and entered the cytoplasm after 24 h. Acoustically propelled nanowires were also utilized to penetrate the cell membrane for intracellular gene55 and protein56 delivery.
Magnetic fields have shown advantages for powering micro/nano-robots for cell membrane poration because of the precision of motion control. Fig. 3D illustrates that the functionalized nanospheres can actively target and deliver genes into the cells under a gradient magnetic field.57 Other forms of magnetic robots capable of penetrating the cell membrane for drug delivery were reported.58,59 Additionally, the light field triggered photothermal effect was utilized for cell membrane opening as well. As shown in Fig. 3E, researchers developed a near-infrared (NIR) light powered membrane-coated nanomotor to thermomechanically percolate through cell membranes for cancer therapy.60 Afterwards, an efficient approach for cell membrane poration61 was exploited by the same research group (Fig. 3F). They fabricated a multilayer tubular nanoswimmer, which can be guided to the targeted cell by ultrasound. Then, they applied NIR light to irradiate a gold-containing nanoshell, which converted laser energy to mechanical force and penetrated the cell membrane within 0.1 s.
Taking advantage of the distinctive structure and propulsion mechanisms, active nanomotors present effective methods for cell membrane perforation, thus improving the performance in biomedical applications such as intracellular cargo delivery and subcellular surgery.
Improved imaging effect and therapeutic efficacy
Imaging enables visualization of the lesion site and provides useful information for treatment. So, imaging-guided therapy holds great potential in the field of precision medicine. At present, a variety of biological imaging technologies have been investigated for visualization of nanomotors, such as fluorescence imaging,62–65 magnetic resonance (MR) imaging,19etc. For instance, Zheng et al.19 prepared a near-infrared propulsion nanomotor based on Janus mesoporous silica nanoparticles (Fig. 4A). Because of Gd3+, the nanomotors can be used for providing high-resolution imaging. In vivo experiments showed that the nanomotors had stronger and broader MR signals in most areas of the tumor after near-infrared laser excitation. Through MR imaging technology, visualization of the nanomotors’ escaping from the tumor blood vessels and penetrating deeply into the tumor can be achieved.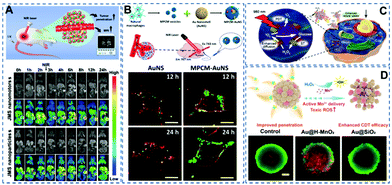 | ||
| Fig. 4 (A) Schematic illustration of the preparation process of JMS nanomotors and the NIR powered JMS nanomotors for deep tumor penetration and enhanced MR imaging. Reproduced with permission.19 Copyright 2021, Wiley-VCH GmbH. (B) Schematic diagram of the fabrication of a macrophage cell membrane camouflaged Au nanoshell nanomotor. Confocal laser scanning microscopy pictures showing the enhanced recognition of camouflaged nanomotors on 4T1 cancer cells at 12 and 24 h. Reproduced with permission.30 Copyright 2016, American Chemical Society. (C) Schematic illustration of the synergistic effect of self-accelerating cascade photodynamic starvation therapy by MOF based core–shell nanomotors. Reproduced with permission.66 Copyright 2019, Elsevier Ltd. (D) Schematic diagram of enhanced CDT based on MnO2 nanomotors, and live/dead staining of tumor spheroids after treating with nanomotors. Reproduced with permission.67 Copyright 2021, American Chemical Society. | ||
In recent decades, malignant tumors have been regarded as a major threat to human health. Numerous research teams have been looking for therapeutic strategies. Many new methods using nanomotors have been tested. Recently He et al.32 prepared a macrophage membrane-camouflaged Au nanoshell motor as a new photothermal conversion agent for in vivo photothermal therapy (Fig. 4B). This biomimetic strategy can significantly improve the efficacy of photothermal therapy against cancer tumors. In vivo PTT cancer treatment showed that near-infrared irradiation effectively inhibited tumor growth in mice. Ma et al.66 prepared a self-driven enzyme motor by simultaneously loading glucose oxidase and catalase on a metal–organic framework (Fig. 4C). Glucose oxidase catalyses the oxidative decomposition of cellular glucose and produces H2O2, achieving the effect of starvation therapy. Catalase catalyses H2O2 to provide the driving force and oxygen molecules, promoting the generation of singlet oxygen and the effect of photodynamic therapy. These two processes receive positive feedback that enhances the combined therapy effects of photodynamic and starvation treatments.
Moreover, the weak endocytosis ability of passive nanoparticles with low penetration ability would lead to an unsatisfactory anticancer effect. To solve this problem, Tu et al.67 fabricated nanomotors containing hollow nanomanganese dioxide particles (Fig. 4D). The enhanced diffusion of nanomotors was proved to be able to effectively enhance the penetration of nanomotors into tumor spheroids. During cellular uptake, intracellular overexpressed glutathione produces a large amount of toxic Fenton-like compounds, which have a strong scavenging effect on HO·, leading to an improved chemodynamic therapeutic effect.
Current challenges hindering the advancement of nanomotors in biomedicine
Taking advantage of nanomotors’ active propulsion, many applications in biomedicine have been inspired. Scientists made many attempts to drive nanomotors and applied them in different application scenarios. Nanomotors have been developed from having a single function to carrying out multiple tasks simultaneously, holding potential to solve current problems of nanomedicine, such as the low precision and long circulation durations. However, each kind of nanomotor aims at specific tasks, and the nanomotors have their own strengths and weaknesses. Therefore, some comprehensive issues need to be addressed about nanomotors’ journey in the human body.Lack of direct evidence showing nanomotors’ motion in a real physiological environment
Nanomotors outperform passive nanomedicine due to their autonomous motion. However, whether this locomotion in a real physiological environment is real or not is still a question. Besides, the safety of the active movement has not been completely systematically investigated. For example, unique gold nanostructures with surface nanospikes have been investigated in the biomedical field due to their potential mechanical killing effect.68 Nevertheless, due to the sharp tips on the surface, the nanospikes may induce damage to surrounding cells or normal tissue when they move under laser irradiation. The potential bio-safety problem caused by nanomotors’ mobility has not been proposed. Besides, the current research on nanomotors is often based on observations of two-dimensional planes or a few observations in three-dimensional situations. The in vivo environment is much more complex than simulated experimental conditions in vitro. For example, in blood, nanomotors might be attacked by immune cells, and the movement of the nanomotors is significantly inhibited by proteins attached to the surface. The problem of aggregation exists and the stability in biological media is unclear. In addition, certain disease sites such as tumor tissue have a totally different microenvironment from that of normal tissue. Therefore, the motion of nanomotors under real physiological conditions needs to be further explored with the development of nanotechnology and nanomaterials.The reason for enhanced cellular uptake is unclear
Although many investigations have proved that nanomotors can be internalized into cells, and the active actuation can further promote the uptake,66,69 the reason why the enhanced uptake process takes place and what is the influencing factor have not been identified explicitly. Although a myriad of nanomotors perform different tasks, they ultimately function by the interactions at the cellular or even subcellular level.70–72 Since nanomotors are considered as a more efficient and safer approach than nanomedicine for biomedical issues, their interactions with the cell membrane require a deeper understanding.Cell membranes are composed of phospholipid bilayers,73 forming an efficient barrier for selectively choosing entering molecules. Typically, the substance is modified with a protein to enter the inner side of the cell membrane, so as to facilitate the specific recognition with the cell membrane. Endocytosis is considered the main mechanism leading to engulfment of nanomotors.74 By endocytic vesicles, nanomotors are transported to specialized intracellular compartments. Besides, other types of mechanisms have also been reported, such as direct microinjection, and electroporation.75,76 For example, Geiser et al.77 reported that nano-sized substances can enter cell membranes by passive diffusion which emphasizes the role of thermal capillary waves and line tension. Furthermore, advances in chemical analysis and imaging reveal that several physiochemical properties of nanomotors, such as size, shape, surface charge, and functionalized molecules78–81 may also influence their cellular uptake.
At present, many research results show that both external field actuation and chemical reaction-induced self-propulsion of nanomotors contribute to enhanced cell membrane penetration and intracellular drug delivery. However, the underlying mechanism has not been studied in depth. Only by uncovering the cell membrane penetration mechanisms of nanomotors will the development of biomedical nanomotors be clearer and further progress be made toward their clinical applications.
From proof-of-concept to more practical application scenarios
In many current studies, the application of nanomotors is mostly about proof-of-concept trials. A large number of research groups have designed and constructed nanomotors with different structures and functions, which in general were tested in cell experiments, though several studies have been carried out in mice. This is the mainstream trend, but the ingenious design and application scenarios of these nanomotors are only limited to the applied proof-of-concept trials. Although the biomedical application of nanomotors has made certain breakthroughs at the cell and tissue level, such as cancer cell killing,82,83 antibacterial wound treatment,45 deep tissue imaging,17–19 and biological barrier penetration,12–16 the benefits of nanomotors over nanomedicines deserve more attention and exploration. Expanding the practical application scenarios of nanomotors is a key to moving nanomotors toward clinical use.The nanomotor is comparatively not smart enough
Although nanomotors are miniaturized robots at the nanoscale, due to size effects their functions are far inferior to the macroscale robots we know. Constructing intelligent nanomachines is still a great challenge. A number of teams used responsive materials such as pH sensitive materials or temperature sensitive materials to build nanomotors that can respond to surrounding environmental changes. For instance, Ma et al.84 reported Janus mesoporous silica–Pt@Au nanomotors with pH-responsive multi-phoretic propulsion. The weakly acidic H2O2 in the tumor microenvironment can trigger the disassembly and reassembly of the AuNPs, thus reconstructing the nanomotor, adjusting the contact between Pt nanoparticle and Au nanoparticle aggregates and changing the propulsion mechanisms. Wilson et al.85 fabricated a temperature-sensitive nanomotor with a polymer brush chemically growing onto the surface. When changing temperature, the opening of the stomatocyte is enlarged or narrowed, thus the access of the hydrogen peroxide fuel is controlled, and nanomotors’ movement is regulated.Intelligent nanomotors need to not only respond to a specific external stimulus, but also respond in a complex physiological environment with multiple variables. However, the tiny size of nanomotors is an advantage on one side, but also a big challenge for the further integration of smart functions. The demand for more intelligent development of nanomotors has forced nanomotors to be equipped with more components to meet this requirement. However, how to integrate multiple components at the nanoscale is always an engineering challenge in nanotechnology and materials science. Even if these functions are integrated into a tiny nanomotor, how to realize the coordination between the various functional modules is also a difficulty that needs to be overcome. In recent years, all-in-one theranostic nano-platforms have emerged due to their multiple functions like imaging and therapy.86,87 The proposal of these design schemes reflects the efforts of scientists in the exploration of intelligent nanomotors.
Summary
Nanomotors propelled by chemical fuels or external fields have shown advantages in biomedical applications such as targeted delivery, penetration of biological barriers, bio-imaging, and cancer therapy. Compared with passive nanomedicine, emerging nanomotors have overwhelming advantages due to their autonomous driving ability and multiple bio-functions. Due to advanced nanotechnology and nanomaterials, nanomotors with many novel functions have emerged in recent years, and are expected to achieve clinical translation in the near future. However, the current research on nanomotors still faces challenges regarding theoretical support, fabrication techniques, and bio-safety issues. Hopefully, we can foresee the bright prospects of nanomotors, and we anticipate that in the near future, nanomotors can bring exciting news to biomedical fields and solve the problems that traditional nanomedicines cannot resolve.To this end, we propose quite a few suggestions for nanomotors’ further development. First, the biomedical applications of nanomotors need more theoretical knowledge, including motion mechanism and cell–matter interactions at the cellular and subcellular levels. Starting from the study to gain a theoretical understanding of their active motion behavior will be very helpful to propose new attempts and achieve breakthroughs especially regarding their effective propulsion and motion control in a complex physiological environment. The actuation of nanomotors in a complex flow field environment in vivo should be systematically investigated. The driving engine of the nanomotor should provide power strong enough to achieve fast and efficient motion. Only by doing so can nanomotors self-propel and on-demand navigate in complex biological media such as blood, urine and interstitial fluid. Then, the application of nanomotors in biomedical fields involves knowledge in multidisciplinary fields, including biology, materials, physics, mechanical engineering, and so on. The combination and collaboration of different disciplines are expected to bring more inspiring ideas for the development of biomedical nanomotors. Additionally, the exploration of nanomotors in biomedical fields should be extended to more realistic scenarios. Current nanomotors are involved in many biomedical applications, such as diagnostics, cancer treatment, thrombus ablation, tissue repair and regeneration. However, all these previous achievements demonstrate that it is time to push nanomotors towards clinical scenarios to demonstrate their usefulness in medicine. We believe that realizing free shuttle of intelligent nanomotors in the human body to cure human diseases will hopefully soon become a great scientific achievement in the field of biomedicine.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the financial support from the National Natural Science Foundation of China (52072095 and 92163109), the Shenzhen Science and Technology Program (JCYJ20200109113408066 and KQTD20170809110344233), the Shenzhen Bay Laboratory (SZBL2019062801005), the Fundamental Research Funds for the Central Universities (Grant No. HIT.OCEF.2021032), and GuangDong Basic and Applied Basic Research Foundation (2021A1515110272).References
- S. Sánchez, L. Soler and J. Katuri, Angew. Chem., Int. Ed., 2015, 54, 1414–1444 CrossRef PubMed.
- W. Wang, W. Duan, S. Ahmed, T. E. Mallouk and A. Sen, Nano Today, 2013, 8, 531–554 CrossRef CAS.
- C. Chen, F. Soto, E. Karshalev, J. Li and J. Wang, Adv. Funct. Mater., 2019, 29, 1806290 CrossRef.
- X.-Z. Chen, M. Hoop, N. Shamsudhin, T. Huang, B. Özkale, Q. Li, E. Siringil, F. Mushtaq, L. Di Tizio, B. J. Nelson and S. Pané, Adv. Mater., 2017, 29, 1605458 CrossRef PubMed.
- R. Lin, W. Yu, X. Chen and H. Gao, Adv. Healthcare Mater., 2021, 10, 2001212 CrossRef CAS PubMed.
- W. Yu, R. Lin, X. He, X. Yang, H. Zhang, C. Hu, R. Liu, Y. Huang, Y. Qin and H. Gao, Acta Pharm. Sin. B, 2021, 11, 2924–2936 CrossRef CAS.
- D. Kagan, M. J. Benchimol, J. C. Claussen, E. Chuluun-Erdene, S. Esener and J. Wang, Angew. Chem., Int. Ed., 2012, 51, 7519–7522 CrossRef CAS PubMed.
- K. Villa, L. Krejčová, F. Novotný, Z. Heger, Z. Sofer and M. Pumera, Adv. Funct. Mater., 2018, 28, 1804343 CrossRef.
- Y. Tu, F. Peng, A. A.-M. André, Y. Men, M. Srinivas and D. A. Wilson, ACS Nano, 2017, 11, 1957–1963 CrossRef CAS PubMed.
- F. Ullrich, C. Bergeles, J. Pokki, O. Ergeneman, S. Erni, G. Chatzipirpiridis, S. Pané, C. Framme and B. J. Nelson, Invest. Ophthalmol. Visual Sci., 2013, 54, 2853–2863 CrossRef PubMed.
- W. Xi, A. A. Solovev, A. N. Ananth, D. H. Gracias, S. Sanchez and O. G. Schmidt, Nanoscale, 2013, 5, 1294–1297 RSC.
- A. Joseph, C. Contini, D. Cecchin, S. Nyberg, L. Ruiz-Perez, J. Gaitzsch, G. Fullstone, X. Tian, J. Azizi, J. Preston, G. Volpe and G. Battaglia, Sci. Adv., 2017, 3, e1700362 CrossRef.
- C. Gao, Y. Wang, Z. Ye, Z. Lin, X. Ma and Q. He, Adv. Mater., 2021, 33, 2000512 CrossRef CAS PubMed.
- D. Walker, B. T. Käsdorf, H.-H. Jeong, O. Lieleg and P. Fischer, Sci. Adv., 2015, 1, e1500501 CrossRef PubMed.
- J. Li, S. Thamphiwatana, W. Liu, B. Esteban-Fernández de Ávila, P. Angsantikul, E. Sandraz, J. Wang, T. Xu, F. Soto, V. Ramez, X. Wang, W. Gao, L. Zhang and J. Wang, ACS Nano, 2016, 10, 9536–9542 CrossRef CAS PubMed.
- M. Xuan, J. Shao, C. Gao, W. Wang, L. Dai and Q. He, Angew. Chem., Int. Ed., 2018, 57, 12463–12467 CrossRef CAS PubMed.
- B. Wang, K. Kostarelos, B. J. Nelson and L. Zhang, Adv. Mater., 2021, 33, 2002047 CrossRef CAS PubMed.
- J. Ye, Q. Fu, L. Liu, L. Chen, X. Zhang, Q. Li, Z. Li, L. Su, R. Zhu, J. Song and H. Yang, Sci. China: Chem., 2021, 64, 2218–2229 CrossRef CAS.
- S. Zheng, Y. Wang, S. Pan, E. Ma, S. Jin, M. Jiao, W. Wang, J. Li, K. Xu and H. Wang, Adv. Funct. Mater., 2021, 31, 2100936 CrossRef CAS.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C.-W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- A. C. Hortelao, C. Simo, M. Guix, S. Guallar-Garrido, E. Julian, D. Vilela, L. Rejc, P. Ramos-Cabrer, U. Cossio, V. Gomez-Vallejo, T. Patino, J. Llop and S. Sanchez, Sci. Robot., 2021, 6, eabd2823 CrossRef PubMed.
- D. Schamel, A. G. Mark, J. G. Gibbs, C. Miksch, K. I. Morozov, A. M. Leshansky and P. Fischer, ACS Nano, 2014, 8, 8794–8801 CrossRef CAS PubMed.
- W. Gao, R. Dong, S. Thamphiwatana, J. Li, W. Gao, L. Zhang and J. Wang, ACS Nano, 2015, 9, 117–123 CrossRef CAS PubMed.
- W. Wang, Z. Wu, X. Lin, T. Si and Q. He, J. Am. Chem. Soc., 2019, 141, 6601–6608 CrossRef CAS PubMed.
- J. Xue, Z. Zhao, L. Zhang, L. Xue, S. Shen, Y. Wen, Z. Wei, L. Wang, L. Kong, H. Sun, Q. Ping, R. Mo and C. Zhang, Nat. Nanotechnol., 2017, 12, 692–700 CrossRef CAS.
- H. Xie, M. Sun, X. Fan, Z. Lin, W. Chen, L. Wang, L. Dong and Q. He, Sci. Robot., 2019, 4, eaav8006 CrossRef PubMed.
- Y. Ji, X. Lin, Z. Wu, Y. Wu, W. Gao and Q. He, Angew. Chem., Int. Ed., 2019, 58, 12200–12205 CrossRef CAS PubMed.
- Y. Wu, T. Si, C. Gao, M. Yang and Q. He, J. Am. Chem. Soc., 2018, 140, 11902–11905 CrossRef CAS PubMed.
- A. V. Singh, M. H.-D. Ansari, P. Laux and A. Luch, Expert Opin. Drug Delivery, 2019, 16, 1259–1275 CrossRef CAS PubMed.
- S. Tan, T. Wu, D. Zhang and Z. Zhang, Theranostics, 2015, 5, 863–881 CrossRef CAS PubMed.
- C. K. Schmidt, M. Medina-Sánchez, R. J. Edmondson and O. G. Schmidt, Nat. Commun., 2020, 11, 5618 CrossRef CAS PubMed.
- M. Xuan, J. Shao, L. Dai, J. Li and Q. He, ACS Appl. Mater. Interfaces, 2016, 8, 9610–9618 CrossRef CAS PubMed.
- A. Parodi, N. Quattrocchi, A. L. van de Ven, C. Chiappini, M. Evangelopoulos, J. O. Martinez, B. S. Brown, S. Z. Khaled, I. K. Yazdi, M. V. Enzo, L. Isenhart, M. Ferrari and E. Tasciotti, Nat. Nanotechnol., 2013, 8, 61–68 CrossRef CAS.
- L. Rao, L.-L. Bu, J.-H. Xu, B. Cai, G.-T. Yu, X. Yu, Z. He, Q. Huang, A. Li, S.-S. Guo, W.-F. Zhang, W. Liu, Z.-J. Sun, H. Wang, T.-H. Wang and X.-Z. Zhao, Small, 2015, 11, 6225–6236 CrossRef CAS PubMed.
- Z. Wu, T. Li, W. Gao, T. Xu, B. Jurado-Sánchez, J. Li, W. Gao, Q. He, L. Zhang and J. Wang, Adv. Funct. Mater., 2015, 25, 3881–3887 CrossRef CAS.
- H. Xu, M. Medina-Sánchez, V. Magdanz, L. Schwarz, F. Hebenstreit and O. G. Schmidt, ACS Nano, 2018, 12, 327–337 CrossRef CAS PubMed.
- J. Wang and W. Gao, ACS Nano, 2012, 6, 5745–5751 CrossRef CAS PubMed.
- A. C. Hortelão, R. Carrascosa, N. Murillo-Cremaes, T. Patiño and S. Sánchez, ACS Nano, 2019, 13, 429–439 CrossRef PubMed.
- D. Vilela, U. Cossío, J. Parmar, A. M. Martínez-Villacorta, V. Gómez-Vallejo, J. Llop and S. Sánchez, ACS Nano, 2018, 12, 1220–1227 CrossRef CAS PubMed.
- M. Medina-Sánchez and O. G. Schmidt, Nature, 2017, 545, 406–408 CrossRef PubMed.
- Z. Wu, L. Li, Y. Yang, P. Hu, Y. Li, S.-Y. Yang, V. Wang Lihong and W. Gao, Sci. Robot., 2019, 4, eaax0613 CrossRef PubMed.
- B. J. Nelson, I. K. Kaliakatsos and J. J. Abbott, Annu. Rev. Biomed. Eng., 2010, 12, 55–85 CrossRef CAS PubMed.
- Y. Alapan, U. Bozuyuk, P. Erkoc, A. C. Karacakol and M. Sitti, Sci. Robot., 2020, 5, eaba5726 CrossRef PubMed.
- A. Liu, Q. Wang, Z. Zhao, R. Wu, M. Wang, J. Li, K. Sun, Z. Sun, Z. Lv, J. Xu, H. Jiang, M. Wan, D. Shi and C. Mao, ACS Nano, 2021, 15, 13339–13350 CrossRef CAS PubMed.
- A. Maleki, J. He, S. Bochani, V. Nosrati, M.-A. Shahbazi and B. Guo, ACS Nano, 2021, 15, 18895–18930 CrossRef CAS PubMed.
- M. Zhou, T. Hou, J. Li, S. Yu, Z. Xu, M. Yin, J. Wang and X. Wang, ACS Nano, 2019, 13, 1324–1332 CAS.
- Z. Wu, J. Troll, H. H. Jeong, Q. Wei, M. Stang, F. Ziemssen, Z. Wang, M. Dong, S. Schnichels, T. Qiu and P. Fischer, Sci. Adv., 2018, 4, eaat4388 CrossRef CAS PubMed.
- E. V. Lengert, E. E. Talnikova, V. V. Tuchin and Y. I. Svenskaya, Skin Pharmacol. Physiol., 2020, 33, 261–269 CrossRef CAS PubMed.
- X. Ji, H. Yang, W. Liu, Y. Ma, J. Wu, X. Zong, P. Yuan, X. Chen, C. Yang, X. Li, H. Lin, W. Xue and J. Dai, ACS Nano, 2021, 15, 14218–14228 CrossRef CAS PubMed.
- F. Peng, Y. Men, Y. Tu, Y. Chen and D. A. Wilson, Adv. Funct. Mater., 2018, 28, 1706117 CrossRef.
- A. Ressnerova, F. Novotny, H. Michalkova, M. Pumera, V. Adam and Z. Heger, ACS Nano, 2021, 15, 12899–12910 CrossRef CAS.
- A. Llopis-Lorente, A. Garcia-Fernandez, N. Murillo-Cremaes, A. C. Hortelao, T. Patino, R. Villalonga, F. Sancenon, R. Martinez-Manez and S. Sanchez, ACS Nano, 2019, 13, 12171–12183 CrossRef CAS PubMed.
- W. Wang, Z. Wu and Q. He, VIEW, 2020, 1, 20200005 CrossRef.
- D. Wang, C. Gao, W. Wang, M. Sun, B. Guo, H. Xie and Q. He, ACS Nano, 2018, 12, 10212–10220 CrossRef CAS PubMed.
- B. Esteban-Fernandez de Avila, C. Angell, F. Soto, M. A. Lopez-Ramirez, D. F. Baez, S. Xie, J. Wang and Y. Chen, ACS Nano, 2016, 10, 4997–5005 CrossRef CAS PubMed.
- B. Esteban-Fernandez de Avila, D. E. Ramirez-Herrera, S. Campuzano, P. Angsantikul, L. Zhang and J. Wang, ACS Nano, 2017, 11, 5367–5374 CrossRef CAS PubMed.
- X. Xu, S. Hou, N. Wattanatorn, F. Wang, Q. Yang, C. Zhao, X. Yu, H. R. Tseng, S. J. Jonas and P. S. Weiss, ACS Nano, 2018, 12, 4503–4511 CrossRef CAS.
- S. K. Srivastava, M. Medina-Sanchez, B. Koch and O. G. Schmidt, Adv. Mater., 2016, 28, 832–837 CrossRef CAS.
- M. Sun, Q. Liu, X. Fan, Y. Wang, W. Chen, C. Tian, L. Sun and H. Xie, Small, 2020, 16, e1906701 CrossRef PubMed.
- M. Xuan, J. Shao, C. Gao, W. Wang, L. Dai and Q. He, Angew. Chem., Int. Ed., 2018, 57, 12463–12467 CrossRef CAS PubMed.
- W. Wang, Z. Wu, X. Lin, T. Si and Q. He, J. Am. Chem. Soc., 2019, 141, 6601–6608 CrossRef CAS PubMed.
- G. Chen, T. Y. Ohulchanskyy, S. Liu, W.-C. Law, F. Wu, M. T. Swihart, H. Ågren and P. N. Prasad, ACS Nano, 2012, 6, 2969–2977 CrossRef CAS PubMed.
- Y. Liu, N. Kang, J. Lv, Z. Zhou, Q. Zhao, L. Ma, Z. Chen, L. Ren and L. Nie, Adv. Mater., 2016, 28, 6411–6419 CrossRef CAS PubMed.
- R. Lv, P. Yang, F. He, S. Gai, C. Li, Y. Dai, G. Yang and J. Lin, ACS Nano, 2015, 9, 1630–1647 CrossRef CAS PubMed.
- E. A. Owens, M. Henary, G. El Fakhri and H. S. Choi, Acc. Chem. Res., 2016, 49, 1731–1740 CrossRef CAS.
- Y. You, D. Xu, X. Pan and X. Ma, Appl. Mater. Today, 2019, 16, 508–517 CrossRef.
- J. Ou, H. Tian, J. Wu, J. Gao, J. Jiang, K. Liu, S. Wang, F. Wang, F. Tong, Y. Ye, L. Liu, B. Chen, X. Ma, X. Chen, F. Peng and Y. Tu, ACS Appl. Mater. Interfaces, 2021, 13, 38050–38060 CrossRef CAS PubMed.
- N. Ma, F.-G. Wu, X. Zhang, Y.-W. Jiang, H.-R. Jia, H.-Y. Wang, Y.-H. Li, P. Liu, N. Gu and Z. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 13037–13048 CrossRef CAS.
- T. Patiño, X. Arqué, R. Mestre, L. Palacios and S. Sánchez, Acc. Chem. Res., 2018, 51, 2662–2671 CrossRef PubMed.
- H. T. McMahon and J. L. Gallop, Nature, 2005, 438, 590–596 CrossRef CAS.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- Y. Shi and J. Massagué, Cell, 2003, 113, 685–700 CrossRef CAS PubMed.
- M. Edidin, Nat. Rev. Mol. Cell Biol., 2003, 4, 414–418 CrossRef CAS PubMed.
- S. Behzadi, V. Serpooshan, W. Tao, M. A. Hamaly, M. Y. Alkawareek, E. C. Dreaden, D. Brown, A. M. Alkilany, O. C. Farokhzad and M. Mahmoudi, Chem. Soc. Rev., 2017, 46, 4218–4244 RSC.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 CrossRef CAS PubMed.
- M. Mahmoudi, A. Tachibana, A. B. Goldstone, Y. J. Woo, P. Chakraborty, K. R. Lee, C. S. Foote, S. Piecewicz, J. C. Barrozo, A. Wakeel, B. W. Rice, C. B. Bell Iii and P. C. Yang, Sci. Rep., 2016, 6, 26960 CrossRef CAS PubMed.
- M. Geiser, B. Rothen-Rutishauser, N. Kapp, S. Schürch, W. Kreyling, H. Schulz, M. Semmler, I. Hof Vinzenz, J. Heyder and P. Gehr, Environ. Health. Perspect., 2005, 113, 1555–1560 CrossRef PubMed.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Acc. Chem. Res., 2013, 46, 622–631 CrossRef CAS PubMed.
- P. R. Leroueil, S. A. Berry, K. Duthie, G. Han, V. M. Rotello, D. Q. McNerny, J. R. Baker, B. G. Orr and M. M. Banaszak Holl, Nano Lett., 2008, 8, 420–424 CrossRef CAS.
- Y. Li and N. Gu, J. Phys. Chem. B, 2010, 114, 2749–2754 CrossRef CAS PubMed.
- Y. Li, X. Chen and N. Gu, J. Phys. Chem. B, 2008, 112, 16647–16653 CrossRef CAS PubMed.
- J. Xu, W. Han, P. Yang, T. Jia, S. Dong, H. Bi, A. Gulzar, D. Yang, S. Gai, F. He, J. Lin and C. Li, Adv. Funct. Mater., 2018, 28, 1803804 CrossRef.
- X. Deng, Z. Shao and Y. Zhao, Adv. Sci., 2021, 8, 2002504 CrossRef CAS.
- Y. Xing, M. Zhou, T. Xu, S. Tang, Y. Fu, X. Du, L. Su, Y. Wen, X. Zhang and T. Ma, Angew. Chem., Int. Ed., 2020, 59, 14368–14372 CrossRef CAS.
- Y. Tu, F. Peng, X. Sui, Y. Men, P. B. White, J. C.-M. van Hest and D. A. Wilson, Nat. Chem., 2017, 9, 480–486 CrossRef CAS PubMed.
- Q. Wang, Y. Dai, J. Xu, J. Cai, X. Niu, L. Zhang, R. Chen, Q. Shen, W. Huang and Q. Fan, Adv. Funct. Mater., 2019, 29, 1901480 CrossRef.
- X. Yan, Q. Zhou, M. Vincent, Y. Deng, J. Yu, J. Xu, T. Xu, T. Tang, L. Bian, J. Wang Yi-Xiang, K. Kostarelos and L. Zhang, Sci. Robot., 2017, 2, eaaq1155 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |



