Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
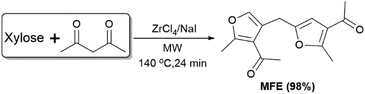
Near quantitative conversion of xylose into bisfuran†
Rui
Zhang
 a,
Aleksi
Eronen
a,
Petra
Vasko
a,
Aleksi
Eronen
a,
Petra
Vasko
 a,
Xiangze
Du
b,
Joseph
Install
a and
Timo
Repo
a,
Xiangze
Du
b,
Joseph
Install
a and
Timo
Repo
 *a
*a
aDepartment of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, P.O. Box 55, 00014 Finland. E-mail: timo.repo@helsinki.fi
bKey Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, P. R. China
First published on 31st May 2022
Abstract
Renewable and abundant carbohydrates are promising feedstocks for producing valuable chemicals. Here we report a highly efficient Zr-catalysed conversion of xylose and acetylacetone (acac) to a new type of bisfuranic monomer, 1-(4-((4-acetyl-5-methylfuran-2-yl)methyl)-2-methylfuran-3-yl)ethenone (MFE). The formation of MFE stems from the intermediate obtained through the nucleophilic addition of acac to xylose. Under optimized conditions (microwave irradiation, 140 °C, 24 min, NaI as an additive), MFE is obtained in near-quantitative yield (98%). Importantly, the reaction selectivity can be tuned by the inclusion of an additive. When NaCl is used, the reaction gives 3-(furan-2-ylmethylene)pentane-2,4-dione (FMPD, 55%), a jet-fuel precursor, and MFE (30%) with a total carbon yield of 85%. To the best of our knowledge, this is the first report on straightforward xylose transformation to a bisfuranic compound with excellent carbon efficiency. This Garcia Gonzalez (GG) reaction inclusive strategy is remarkable and could lead to many innovations in bio-based polymer synthesis.
Introduction
Using sustainable and renewable lignocellulosic biomass as a feedstock is a promising alternative towards the synthesis of value-added chemicals and materials.1–3 Hemicellulose contributes 20–35% of the total weight of biomass. Xylose, as a representative hemicellulose monomer, has been extensively studied for the production of lactic acid, xylitol and furfural.4–8 Furfural or 5-hydroxymethylfurfural (furan platform chemicals) are also employed as intermediates or starting chemicals for the synthesis of various furan derivatives via chemo-catalytic, bio-catalytic or chemoenzymatic methods.9–16 They are potential building blocks for biofuels and biochemical industries as well as important structural motifs prevalent in pharmacologically and biologically active compounds.17–20 Furan derivatives are also excellent candidates for biosourced polymers,21,22 such as curing agents for epoxy resins.23 Bisfuranic monomers have shown great potential in polymer synthesis, mimicking aromatic structures such as bisphenol A derived from petroleum-based processes.24–30 For instance, a high performance, fully bio-based epoxy thermoset could be synthesized from a syringaldehyde-derived epoxy monomer cured by a bisfuranic amine.26The one-pot production of bisfurans directly from sugars has not been reported to the best of our knowledge. The synthetic routes to such bisfuranic compounds are often complicated and circuitous. They can be prepared from 2-substituted furan chemicals (furfural, 2-furan methyl amine, 2-methyl furoate and 2-methylfuran) with aldehydes or ketones.17,31 With furfural as a starting material, the synthesis procedure would require multiple steps, such as oxidation, carboxylic acid protection and alkylation. This leads to a low overall yield of the resulting dimer structure as well as high energy demand and poor atom efficiency.27,28 In addition, the initial furfural production from xylose normally has inevitable side reactions. During the dehydration, the formation of undesired, thermodynamically favoured humins by self- and cross-polymerization of furfural and other intermediates tends to lower the furfural yield.32,33 In this respect, the synthesis of functionalized furan scaffolds, especially bisfuranic compounds, directly from xylose by a practical, cost-effective and energy-saving approach would be a very attractive strategy.
Regarding the limited carbon efficiency in xylose transformations, the Garcia Gonzalez (GG) reaction is intriguing as it could enable the production of furanic compounds with high carbon yield.34 In the GG reaction, the unprotected carbohydrate directly reacts with the β-dicarbonyl compound.35,36 A Lewis acid catalyst plays an important role in product yields and distributions.20,37–43 Catalysts such as ZrCl4,34 CeCl3·6H2O,20,37 ZnCl2,38 InCl3,39 FeCl3,40 Yb(OTf)3,41 Sc(OTf)3,42 and Fe(OTf)344 have been employed to catalyse the GG reactions with varying success. However, the products are limited to polyhydroxyalkyl or C-glycosyl furans.
Recently, we reported the synthesis of substituted furanic compounds with good yields from glucose and acetylacetone (acac) via a cascade type retro-aldol condensation and GG reaction.45 Acac is seen as a sustainable component in the reaction as it is available from glucose via chemical transformation or biosynthetic routes.46,47 During the continuing research course, we observed a new type of bisfuranic compound obtained from xylose. Herein, we report a straightforward Zr-catalysed approach for the near quantitative conversion of xylose with acac to bisfuranic compound 1-(4-((4-acetyl-5-methylfuran-2-yl)methyl)-2-methylfuran-3-yl)ethenone (MFE, Scheme 1). As aforementioned, the bisfuran mimetic MFE monomer (Fig. S1–6†) with ketone groups is of interest as an underexplored potential building block for the synthesis of fully bio-based polymers.
Results and discussion
By using different Lewis acids as catalysts and NaCl as an additive, reactions of xylose together with acac gave mixtures of 3-(furan-2-ylmethylene)pentane-2,4-dione (FMPD) and MFE in moderate to good yields (Table S1 and Fig. S7–11†). Of the Lewis acids studied, ZrCl4 gave the highest total carbon yield (79%) containing 46% of FMPD and 33% of MFE. It is also worth mentioning that AlCl3·6H2O as a catalyst gave FMPD as the major product, without detectable amounts of MFE (Fig. S12–14†), but with a low total carbon yield of 43%.While studying the ZrCl4-catalysed reaction further, applied temperature (110–160 °C) and time appear to have a clear influence on the reaction outcome (Fig. S15†). The initial reaction rate is a function of temperature and a high total carbon yield (80%) is obtained after 6 min at 160 °C. Whereas the MFE yield is markedly increased with increasing temperature, the FMPD yield benefits from lower reaction temperatures and extended reaction time. The optimized yield of FMPD is 65% and is obtained at 70 °C, 14 h with a total carbon yield of 78% (Table S2†). FMPD as a jet-fuel precursor is also accessible in high yields from isolated furfural and acac by Knoevenagel condensation;48–51 the present approach enables the direct conversion of xylose to FMPD. We also studied the effect of catalyst and xylose loading (Fig. S16 and Table S3†). An increase in the catalyst amount from 2 mol% to 5 mol% showed an increase in the reaction rate whereas a further increase to 10 mol% showed no significant increase. The low substrate concentration favoured the formation of FMPD while it showed less influence on the yield of MFE. For high carbon efficiency, a reaction temperature of 140 °C and a reaction time of 24 min with 2 mmol of xylose and 5 mol% of ZrCl4 were found to be optimal (gave the highest total carbon yield of 85%; Table 1, entry 1) and used in the followed reactions.
| Entry | Catalysts and additives | FMPD yielda (mol%) | MFE yielda (mol%) | Total carbon yieldb (mol%) |
|---|---|---|---|---|
| Reaction conditions: 2 mmol xylose, 5 mol% ZrCl4, 5 mL of acac, 5 mL of H2O (with 25 mmol additives if needed), microwave heating, 140 °C, 24 min, 600 rpm.a The yields of FMPD and MFE were determined by GC-FID using acetophenone as an internal standard. Yield (%) = mol of product/mol of xylose × 100%.b Total carbon yield is the sum of the yield of FMPD and MFE. | ||||
| 1 | ZrCl4_NaCl | 55 | 30 | 85 |
| 2 | ZrCl4 | 33 | 10 | 43 |
| 3 | ZrCl4_KCl | 52 | 28 | 80 |
| 4 | ZrCl4_MgCl2 | 51 | 28 | 79 |
| 5 | ZrCl4_NaBr | 35 | 40 | 75 |
| 6 | ZrCl4_NaI | 14 | 67 | 81 |
| 7 | ZrCl4_KI | 17 | 60 | 77 |
To further understand the potential of the reaction, we turned our focus back to the catalyst system. As ZrCl4 hydrolyses easily in water,52 the role of the resulting HCl was considered. However, HCl as a sole catalyst gives only traces of FMPD (Fig. S17†). In control experiments, zirconyl chloride octahydrate (ZrOCl2·8H2O) as a hydrolysis model compound of ZrCl4 gave slightly lower yields of FMPD and MFE (80%) compared with ZrCl4 (85%). To consume the formed HCl, the non-nucleophilic base N,N-diisopropylethylamine (DIPEA) was added and yielded 78% of the products (Table S4†). This suggests that the catalytic activity is largely attributed to the Lewis acidic Zr4+ cation, while the presence of HCl as a Brønsted acid could be beneficial for the following dehydration reactions.
Next, we turned our attention to the additive and found that NaCl is crucial for the reaction. Whereas it alone has no influence on the reaction (Fig. S17†), NaCl markedly enhances the Zr-catalysed reactions (Table 1, runs 1 and 2). Other salts, such as KCl and MgCl2 gave similar product distributions and carbon yields as NaCl. Surprisingly, a major change followed a halogen exchange. NaI and KI turned the reaction selectivity towards bisfuranic MFE and the total carbon yield remained high (Table 1, runs 6 and 7). The interpretation of the MFE molecular structure (xylose + 2acac − 5H2O) indicates that two moles of acac are needed for its formation through a series of dehydrations. Whereas a small amount of water is essential to dissolve xylose, its relative ratio with acac is a critical parameter. Xylose conversion in pure acac with NaI as the additive gave an MFE yield of 75%, while the addition of 10% water (volume ratio) resulted in a near-quantitative yield (98%) (Table 2, runs 1 and 2). A high acac and low water ratio is thus beneficial for the high yield of MFE. The time course for converting xylose into MFE was investigated (Fig. S18†). Xylose is very rapidly consumed when in contact with acac and 90.8% of MFE can be obtained already in 6 min of reaction. With 100% conversion of xylose, reactions after 12 min gave an MFE yield higher than 96%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios
H2O ratios
| Entry | Reaction medium (v/v) | FMPD yield (mol%) | MFE yield (mol%) | Total carbon yield (mol%) |
|---|---|---|---|---|
| Reaction conditions: 2 mmol xylose, 5 mol% ZrCl4, 25 mmol NaI, total volume of reaction medium 10 mL, microwave heating, 140 °C, 24 min, 600 rpm. | ||||
| 1 | acac | — | 75 | 75 |
| 2 | acac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9 H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 98 | 98 |
| 3 | acac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
5 | 76 | 81 |
| 4 | acac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 3 H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
25 | 48 | 73 |
| 5 | acac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
14 | 19 | 33 |
With these interesting results, we turned our attention to the product formation pathway. Xylose is known to go through a dehydration process to form furfural with Lewis acid catalysts,53–55 while FMPD can be obtained from isolated furfural and acac as previously reported.48–51 Thus, the production of furfural from xylose with ZrCl4 as a catalyst was studied using water and toluene as a biphasic reaction medium.56 In the reactions conducted with acac, the conversion of xylose is very rapid; up to 98% of xylose is converted in 2 min with 35% of FMPD formation. However, without acac, only 59% of xylose is converted. The formation of humins is also clearly observed, indicating that xylose is prone to side reactions without acac and gives a very low yield of furfural (4%). These results showed that the reaction between xylose and acac is more favoured and faster than through the dehydration pathway towards furfural or other side reactions. In addition, the amount of furfural formed over the reaction time (2–30 min) is between 4–19%, whereas the yield of FMPD is up to 35–51% (Fig. S19†). This is in agreement with the faster xylose conversion when acac is present. Thus, the reaction pathway towards FMPD through the furfural intermediate can be concluded to happen to a lesser extent.
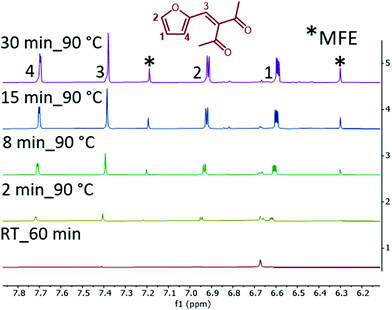
The consumption of xylose is very rapid under the applied reaction conditions, for example, 95% of xylose is converted in 2 min at a lowered 110 °C. However, the yield of FMPD and MFE is only 8 and 2%, respectively (Fig. S15†). This indicates the formation of intermediates. To follow the reaction pathway for both FMPD and MFE, nuclear magnetic resonance (NMR) studies were conducted at 90 °C and at room temperature using NaCl as the additive. The 1H NMR spectra of the organic phase showed characteristic signals for FMPD in only 2 min at 90 °C (Fig. 1; see Fig. S20† for full spectra and Fig. S21† for signal integration). The intensity of FMPD signals increased as the reaction progressed. From these observations, an alternative route through the nucleophilic addition of acac and xylose to intermediate A opens a high yield pathway to FMPD and suppresses the furfural route and humin formation (Scheme 2, route 1).
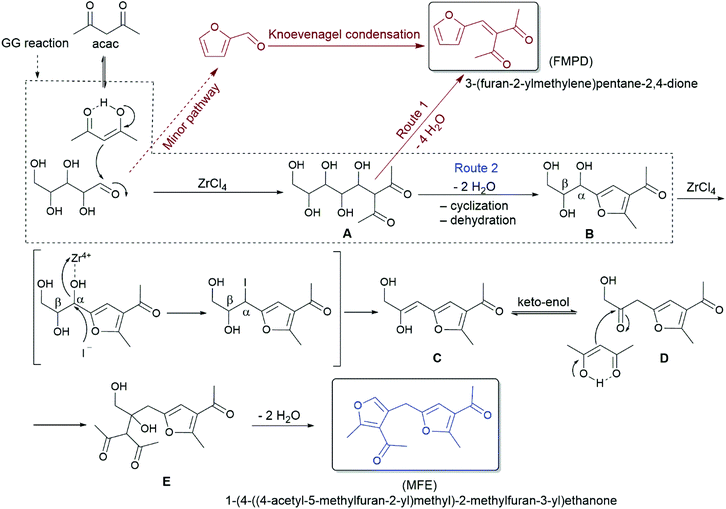 | ||
| Scheme 2 Proposed reaction pathway for the conversion of xylose and acac to FMPD and MFE (observed intermediates: B and E). | ||
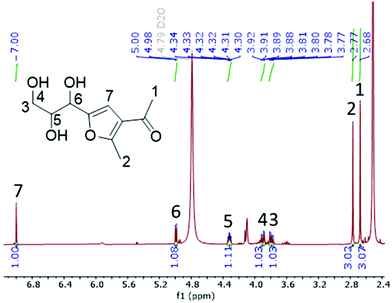
The corresponding 1H NMR spectra of the aqueous phase were recorded at room temperature and new signals appeared in the xylose_acac_ZrCl4_NaCl reaction (Fig. S22†). Using NaI as the additive, these signals could be clearly integrated and the formation of intermediate B was identified (Fig. 2 and Fig. S23†). It was further confirmed by the high-resolution electrospray-ionization mass spectra (m/z = 237.0731, Fig. S24†) and the presence of intermediate species E (m/z = 319.1152, Fig. S24†). Intermediate B originates from A by a typical GG reaction, which has been previously reported to be synthesised from xylose and acac at mild temperatures.34,43 At elevated temperatures (90 °C), the formation of MFE can be monitored accordingly (Fig. 1 and Fig. S25†). Based on these results, a reaction pathway is proposed as shown in Scheme 2 to illustrate the formation of MFE.
As identified above, Zr4+ cation has a pivotal role in MFE formation. In general, Zr4+ cation possesses high coordinating ability57 and it interacts with the hydroxyl groups which leads to the weakening of the C–O bond. Here, interaction with the –OH group in the α-carbon of B next to the aromatic furan structure reduces the high activation energy barrier from B to C (uncatalysed reaction, 277 kJ mol−1, Fig. S26†) and opens up a possibility for a nucleophilic substitution reaction.58 Due to its good nucleophilicity, an iodide anion (I−) can substitute an activated hydroxyl group at the α-carbon (Scheme 2, route 2). Being also a good leaving group, a subsequent E1 reaction occurs and the adjacent β-proton is abstracted by a water molecule to form a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the applied acidic reaction conditions (the pH value was found to be 1.4, 0.6 and 0.4 in ZrCl4_H2O, ZrCl4_NaCl_H2O and ZrCl4_NaI_H2O medium, respectively). The resulting enol structure C undergoes a keto–enol tautomerization to form D, which is then attacked by another acac molecule to form intermediate E. A subsequent water elimination of E finally gives MFE. The labelling study using D2O confirmed that C is undergoing keto–enol tautomerisation, which is observed as exchangeable protons at the position H5 of MFE (Fig. S25†). The trapping of HCl by the DIPEA base resulted in a drop in the yield of MFE from 98% to 71%, implying that HCl is involved in assisting the cyclization and elimination reactions (Table S5†). Since I− has better nucleophilicity (when water is around) and leaving group character than Br− and Cl−, the highest yield of MFE is observed with NaI as the additive. The promoting effect of I− has also been observed in the dehydration of carbohydrates to 5-HMF and furfural.59–61 The addition of NaI also benefits the solvation of acac in water to form a more homogeneous reaction media in which xylose and acac could easily react.
C bond in the applied acidic reaction conditions (the pH value was found to be 1.4, 0.6 and 0.4 in ZrCl4_H2O, ZrCl4_NaCl_H2O and ZrCl4_NaI_H2O medium, respectively). The resulting enol structure C undergoes a keto–enol tautomerization to form D, which is then attacked by another acac molecule to form intermediate E. A subsequent water elimination of E finally gives MFE. The labelling study using D2O confirmed that C is undergoing keto–enol tautomerisation, which is observed as exchangeable protons at the position H5 of MFE (Fig. S25†). The trapping of HCl by the DIPEA base resulted in a drop in the yield of MFE from 98% to 71%, implying that HCl is involved in assisting the cyclization and elimination reactions (Table S5†). Since I− has better nucleophilicity (when water is around) and leaving group character than Br− and Cl−, the highest yield of MFE is observed with NaI as the additive. The promoting effect of I− has also been observed in the dehydration of carbohydrates to 5-HMF and furfural.59–61 The addition of NaI also benefits the solvation of acac in water to form a more homogeneous reaction media in which xylose and acac could easily react.
As a newly developed, highly efficient synthetic route for xylose transformation into a bisfuranic structure, this route could possibly open a new pathway for the synthesis of fully bio-based polymers and other valuable chemicals. The present approach offers an alternative for bio-based bisfurans without the need for processed 2-substituted furans such as furfural. The direct use of xylose reduces processing demands and opens up the potential for further valuable products to be generated directly from monosaccharides.
Conclusions
We reported here a facile, environmentally benign (water is eliminated as a by-product), cost-saving and energy-effective approach for the synthesis of the bisfuranic compound MFE directly from xylose in a near-quantitative yield (98%). MFE, a bisfuran with two furanyl groups, can be used for many different types of functionalization strategies towards furan containing polymers. The direct conversion of xylose to a bisfuran is remarkable and unreported. Compared with the reported synthetic methods for similar bisfuranic structures using furfural as an isolated starting material, the GG reaction-based strategy for xylose valorisation results in very high carbon efficiency due to the rapid nucleophilic addition of acac and xylose catalysed by Zr4+. NaI as an additive assists the water elimination process for the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds to generate bisfuran rings. Notably, a fuel precursor, FMPD is also obtained in good yields in a short reaction time, which is the dominant product when NaCl is used as an additive. The developed approach is highly efficient in converting xylose into value-added new type furanic compounds, providing a new perspective for future biorefinery and bio-based polymer development.
C bonds to generate bisfuran rings. Notably, a fuel precursor, FMPD is also obtained in good yields in a short reaction time, which is the dominant product when NaCl is used as an additive. The developed approach is highly efficient in converting xylose into value-added new type furanic compounds, providing a new perspective for future biorefinery and bio-based polymer development.
Author contributions
Rui Zhang: conceptualization, investigation, methodology, data curation, formal analysis, software, writing – original draft, and writing – review & editing; Aleksi Eronen: data curation, formal analysis, and software; Dr Petra Vasko: methodology and data curation; Xiangze Du: methodology and data curation; Joseph Install: methodology; Prof. Timo Repo: conceptualization, funding acquisition, supervision, resources, project administration, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the University of Helsinki. R. Z. is grateful for financial support from CSC (China Scholarship Council) and Business Finland 43486/31/2020; A. E. received support from Magnus Ehrnrooth Foundation; P. V. is grateful to the Academy of Finland for funding (grant number 34656).References
- P. Sudarsanam, E. Peeters, E. V. Makshina, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2019, 48, 2366–2421 RSC.
- Y. Liu, Y. Nie, X. Lu, X. Zhang, H. He, F. Pan, L. Zhou, X. Liu, X. Ji and S. Zhang, Green Chem., 2019, 21, 3499–3535 RSC.
- X. Luo, Y. Li, N. K. Gupta, B. Sels, J. Ralph and L. Shuai, Angew. Chem., Int. Ed., 2020, 59, 11704–11716 CrossRef CAS PubMed.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- D. S. Naidu, S. P. Hlangothi and M. J. John, Carbohydr. Polym., 2018, 179, 28–41 CrossRef CAS PubMed.
- Y. D. Arcaño, O. D. V. García, D. Mandelli, W. A. Carvalho and L. A. M. Pontes, Catal. Today, 2020, 344, 2–14 CrossRef.
- L. Ji, Z. Tang, D. Yang, C. Ma and Y.-C. He, Bioresour. Technol., 2021, 340, 125691 CrossRef CAS PubMed.
- L. Gong, J. Zha, L. Pan, C. Ma and Y.-C. He, Bioresour. Technol., 2022, 351, 126945 CrossRef CAS PubMed.
- J. Sha, B. T. Kusema, W.-J. Zhou, Z. Yan, S. Streiff and M. Pera-Titus, Green Chem., 2021, 23, 7093–7099 RSC.
- Q. Li, J. Di, X. Liao, J. Ni, Q. Li, Y.-C. He and C. Ma, Green Chem., 2021, 23, 8154–8168 RSC.
- G. R. Dick, A. D. Frankhouser, A. Banerjee and M. W. Kanan, Green Chem., 2017, 19, 2966–2972 RSC.
- A.-D. Cheng, S.-S. Shi, Y. Li, M.-H. Zong and N. Li, ACS Sustainable Chem. Eng., 2019, 8, 1437–1444 CrossRef.
- Y. Yan, C. Bu, Q. He, Z. Zheng and J. Ouyang, RSC Adv., 2018, 8, 26720–26727 RSC.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. L. Granados, Energy Environ. Sci., 2016, 9, 1144–1189 RSC.
- B. Liu and Z. Zhang, ChemSusChem, 2016, 9, 2015–2036 CrossRef CAS PubMed.
- R. Bielski and G. Grynkiewicz, Green Chem., 2021, 23, 7458–7487 RSC.
- J. Zhu and G. Yin, ACS Catal., 2021, 11, 10058–10083 CrossRef CAS.
- A. E. Eseyin and P. H. Steele, Int. J. Adv. Chem., 2015, 3, 42–47 CrossRef.
- J. Hidalgo-Carrillo, A. Marinas and F. J. Urbano, in Furfural: An Entry Point of Lignocellulose in Biorefineries to Produce Renewable Chemicals, Polymers, and Biofuels, World Scientific, 2018, pp. 1–30 Search PubMed.
- A. K. Misra and G. Agnihotri, Carbohydr. Res., 2004, 339, 1381–1387 CrossRef CAS PubMed.
- M. Decostanzi, R. Auvergne, B. Boutevin and S. Caillol, Green Chem., 2019, 21, 724–747 RSC.
- N. Eid, B. Ameduri and B. Boutevin, ACS Sustainable Chem. Eng., 2021, 9, 8018–8031 CrossRef CAS.
- C. Urban and N. Schäffeler, U.SPatent No. 10287389, 2019 Search PubMed.
- D. K. Ali and B. A. Sweileh, J. Polym. Environ., 2018, 26, 1940–1949 CrossRef CAS.
- H.-W. Park, M. Toan, H.-J. Kim, J.-H. Lee and S. Shin, J. Ind. Eng. Chem., 2020, 92, 184–190 CrossRef CAS.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Green Chem., 2021, 23, 501–510 RSC.
- J. K. Cho, J.-S. Lee, J. Jeong, B. Kim, B. Kim, S. Kim, S. Shin, H.-J. Kim and S.-H. Lee, J. Adhes. Sci. Technol., 2013, 27, 2127–2138 CrossRef CAS.
- V. Gaitonde, K. Lee, K. Kirschbaum and S. J. Sucheck, Tetrahedron Lett., 2014, 55, 4141–4145 CrossRef CAS PubMed.
- J. K. Cho, S. Y. Kim, D. H. Lee, B. R. Kim, B. J. Kim, J. W. Jung, S. H. Lee and J. S. Lee, U.SPatent No. 9035018, 2015 Search PubMed.
- H. Zhao, J. Ding and H. Yu, Sci. Rep., 2018, 8, 16560 CrossRef PubMed.
- L. Wang, Y. Eguchi and E. Y. X. Chen, Ind. Eng. Chem. Res., 2017, 56, 11380–11387 CrossRef CAS.
- N. Shi, Q. Liu, H. Cen, R. Ju, X. He and L. Ma, Biomass Convers. Biorefin., 2020, 10, 277–287 CrossRef CAS.
- L. Filiciotto, A. M. Balu, J. C. Van der Waal and R. Luque, Catal. Today, 2018, 302, 2–15 CrossRef CAS.
- N. Ronaghi, D. M. Fialho, C. W. Jones and S. France, J. Org. Chem., 2020, 85, 15337–15346 CrossRef CAS PubMed.
- F. G. González, in Advances in Carbohydrate Chemistry, ed. M. L. Wolfrom and R. S. Tipson, Academic Press, 1956, vol. 11, pp. 97–143 Search PubMed.
- F. G. González and A. G. Sánchez, Adv. Carbohydr. Chem., 1965, 20, 303–355 Search PubMed.
- G. Bartoli, J. G. Fernández-Bolaños, G. Di Antonio, G. Foglia, S. Giuli, R. Gunnella, M. Mancinelli, E. Marcantoni and M. Paoletti, J. Org. Chem., 2007, 72, 6029–6036 CrossRef CAS PubMed.
- A. P. Kozikowski, G. Q. Lin and J. P. Springer, Tetrahedron Lett., 1987, 28, 2211–2214 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, M. Sreenivas and G. Satheesh, Synthesis, 2007, 1712–1716 CrossRef CAS.
- L. Nagarapu, M. V. Chary, A. Satyender, B. Supriya and R. Bantu, Synthesis, 2009, 2278–2282 CrossRef CAS.
- F. Rodrigues, Y. Canac and A. Lubineau, ChemComm, 2000, 2049–2050, 10.1039/B006642G.
- S. Sato, Y. Naito and K. Aoki, Carbohydr. Res., 2007, 342, 913–918 CrossRef CAS PubMed.
- V. Escande, T. K. Olszewski, E. Petit and C. Grison, ChemSusChem, 2014, 7, 1915–1923 CrossRef CAS PubMed.
- A. D. Sutton, J. K. Kim, R. Wu, C. B. Hoyt, D. B. Kimball, L. A. Silks III and J. C. Gordon, ChemSusChem, 2016, 9, 2298–2300 CrossRef CAS PubMed.
- R. Zhang, A. Eronen, X. Du, E. Ma, M. Guo, K. Moslova and T. Repo, Green Chem., 2021, 23, 5481–5486 RSC.
- Y. Zhou, Y. Ding, W. Gao, J. Wang, X. Liu, M. Xian, X. Feng and G. Zhao, Biotechnol. Biofuels, 2020, 13, 88 CrossRef CAS PubMed.
- M. Chia, T. J. Schwartz, B. H. Shanks and J. A. Dumesic, Green Chem., 2012, 14, 1850–1853 RSC.
- Z.-N. Li, X.-L. Chen, Y.-J. Fu, W. Wang and M. Luo, Res. Chem. Intermed., 2012, 38, 25–35 CrossRef CAS.
- Y. Jing, Q. Xia, J. Xie, X. Liu, Y. Guo, J.-j. Zou and Y. Wang, ACS Catal., 2018, 8, 3280–3285 CrossRef CAS.
- K. Tarade, S. Shinde, S. Sakate and C. Rode, Catal. Commun., 2019, 124, 81–85 CrossRef CAS.
- V. V. Martichonok, P. K. Chiang, P. J. Dornbush and K. M. Land, Synth. Commun., 2014, 44, 1245–1250 CrossRef CAS.
- Z. Fang and D. A. Dixon, J. Phys. Chem. C, 2013, 117, 7459–7474 CrossRef CAS.
- Y. Yang, C. W. Hu and M. M. Abu-Omar, ChemSusChem, 2012, 5, 405–410 CrossRef CAS PubMed.
- C. B. T. L. Lee and T. Y. Wu, Renewable Sustainable Energy Rev., 2021, 137, 110172 CrossRef CAS.
- L. Zhang, H. Yu, P. Wang and Y. Li, Bioresour. Technol., 2014, 151, 355–360 CrossRef CAS PubMed.
- N. K. Gupta, A. Fukuoka and K. Nakajima, ACS Catal., 2017, 7, 2430–2436 CrossRef CAS.
- J. E. Huheey, E. A. Keiter, R. L. Keiter and O. K. Medhi, Inorganic Chemistry: Principles of Structure and Reactivity, Pearson Education India, 2006 Search PubMed.
- P. H. Huy, Eur. J. Org. Chem., 2019, 10–27 Search PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- P. Körner, S. Beil and A. Kruse, React. Chem. Eng., 2019, 4, 747–762 RSC.
- K. R. Enslow and A. T. Bell, ChemCatChem, 2015, 7, 479–489 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, Fig. S1–S26 and Tables S1–S5. See DOI: https://doi.org/10.1039/d2gc00640e |
| This journal is © The Royal Society of Chemistry 2022 |