Open Access Article
Open Access ArticleTriboluminescence of a new family of CuI–NHC complexes in crystalline solid and in amorphous polymer films†
Ayumu
Karimata
 a,
Pradnya H.
Patil
a,
Pradnya H.
Patil
 a,
Robert R.
Fayzullin
a,
Robert R.
Fayzullin
 b,
Eugene
Khaskin
b,
Eugene
Khaskin
 a,
Sébastien
Lapointe
a,
Sébastien
Lapointe
 a and
Julia R.
Khusnutdinova
a and
Julia R.
Khusnutdinova
 *a
*a
aOkinawa Institute of Science and Technology Graduate University, Coordination Chemistry and Catalysis Unit, 1919-1 Tancha, Onna-son, Okinawa, 904-0495, Japan. E-mail: juliak@oist.jp
bArbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences, 8 Arbuzov Street, Kazan 420088, Russian Federation
First published on 21st September 2020
Abstract
Triboluminescent compounds that generate emission of light in response to mechanical stimulus are promising targets in the development of “smart materials” and damage sensors. Among triboluminescent metal complexes, rare-earth europium and terbium complexes are most widely used, while there is no systematic data on more readily available and inexpensive Cu complexes. We report a new family of photoluminescent Cu–NHC complexes that show bright triboluminescence (TL) in the crystal state visible in ambient indoor light under air. Moreover, when these complexes are blended into amorphous polymer films even at small concentrations, TL is easily observed. Observation of TL in polymer films overcomes the limitation of using crystals and opens up possibilities for the development of mechanoresponsive coatings and materials based on inexpensive metals such as Cu. Our results may also have implications for the understanding of the TL effect's origin in polymer films.
Introduction
Triboluminescence (TL), which is also called mechanoluminescence or fractoluminescence when emission occurs by fracturing a crystal, has been known to generate light emission from organic or inorganic materials caused upon application of a mechanical stimulus such as crushing, rubbing, or grinding. This phenomenon was first recorded in 1605 by Francis Bacon who reported light emission from scraping hard sugar.1–3 A modern triboluminescent observation is the light emission that occurs upon the peeling of an adhesive tape. Currently, TL materials are considered as promising targets for the development of damage sensors4–7 or other types of “smart” materials that can find application in information storage or health care devices.8 Among known TL coordination compounds, complexes of rare earth elements, EuIII and TbIII, are the most widely known.9–11 A limited number of triboluminescent transition metal (TM) complexes have also been reported, including RuII,12 PtII,13 MnII,14–16 and CuI.17–22 Considering that Cu is one of the most abundant and inexpensive metals, TL materials based on Cu present a practical alternative to currently utilized Eu- and Tb-based materials. Until now, only a few scattered examples of triboluminescent crystalline Cu complexes have been published, featuring very diverse ligand frameworks.17–23 In all but one of these reports, however, no systematic experimental data were collected.24 All of these reports are also examples of fractoluminescence, where a crystal is required to observe the TL effect.We previously synthesized a series of photoluminescent (PL) and air-stable Cu–NHC complexes containing N4 pyridinophane ligands, which can be cross-linked into polybutylacrylate films.25 The resulting Cu-containing polymers enable sensitive detection and visualization of mechanical stress via reversible changes in PL intensity when the elastic film is stretched under UV light irradiation (Fig. 1).
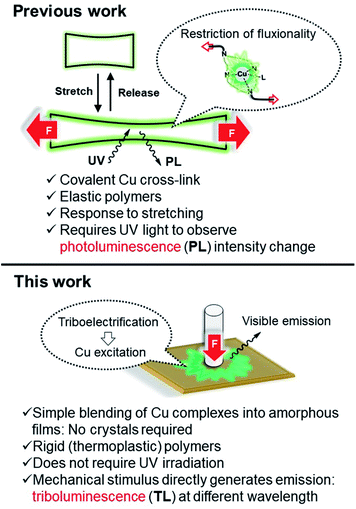 | ||
| Fig. 1 (N4)Cu(NHC)-based mechanophores in photoluminescent and triboluminescent mechanoresponsive polymers. | ||
In this work, we report a new family of air-stable CuI complexes showing TL properties not only in the crystalline state but also when physically blended into rigid, amorphous polymer films at low concentrations, when pressure is applied to the polymer. There is currently no precedent for a large family of air-stable TL copper complexes, where variation of the ligand or a counter anion would preserve their TL properties despite changes in the crystalline structure or the presence or absence of coordinating anions. For some of the complexes, bright emission can be seen even in ambient light upon grinding the crystals under air. These robust properties prompted us to transfer the TL property from the crystalline to the amorphous state, which would significantly expand the range of their possible applications. Indeed, we also observed visible light TL in an amorphous polymer film when the Cu complex was incorporated as a non-crystalline additive (Fig. 1). The ability to utilize amorphous polymer films for the observation of TL properties is, to the best of our knowledge, the first time TL has been observed in a non-crystalline bulk material. It shows that limitations associated with the use of the crystalline phase can be overcome and offers a convenient method for visualizing the application of mechanical force on polymers.
Results and discussion
Synthesis, characterization and photophysical properties of [(RN4)Cu(NHC)]X complexes
During our previous study, grinding a sample when trying to scrape solid material from a vial, we serendipitously found that several pyridinophane (N4)Cu N-heterocyclic carbene (NHC) complexes exhibit bright TL visible under ambient light and while being exposed to air. To further investigate the TL properties of these complexes, we synthesized a series of [(RN4)Cu(NHC)]X complexes where the counter anion X, RN4, and NHC ligands (1, 2, and 3a–d) (Scheme 1) were varied, by mixing RN4 pyridinophane ligand (R = tBu or Me) and (NHC)CuCl, followed by a counter anion exchange at ambient temperature. The complexes were isolated in 49–93% yields and characterized by single crystal (SC-XRD) and powder (PXRD) X-ray diffraction, elemental analysis, NMR, UV/vis, and IR spectroscopy. SC-XRD measurements confirmed the presence of a distorted tetrahedral geometry around the Cu atoms (Fig. 2).26 NMR spectra of 1, 2, and 3a–d confirmed a tetracoordinate structure in solution at room temperature (RT).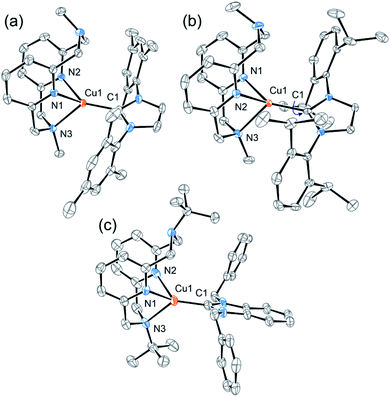 | ||
| Fig. 2 ORTEP of 1 (a), 2 (b), and 3b (c) at 50% probability level according to XRD data. Anions, solvent molecules, and hydrogen atoms are omitted for clarity. In the case of 2, the structure was inverted for clarity. The structures of 3c and 3d are present in ESI.† | ||
All complexes displayed a high photoluminescence quantum yield (PLQY) in the solid state (0.66–0.83) (Table 1 and Fig. 3). The polymethylmethacrylate (PMMA) films containing these Cu complexes also showed good PLQYs (0.51–0.79) and exceptional air stability: showing no decrease in PLQY after 30 days under air (Fig. S21, ESI†). Similar PLQYs were also obtained in Cu-containing polystyrene and polyvinyl chloride films (Table S3†). For comparison, the air stability of some previously reported NHC CuI complexes in the crystal state has been reported to vary greatly depending on the ligand structure and sterics-influencing substituents,27–29 while air stability in solution is usually limited to several hours in most cases.27–32
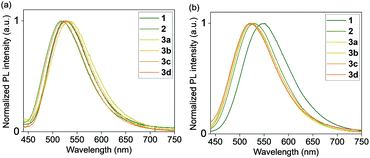 | ||
| Fig. 3 Normalized PL emission spectra of complexes 1, 2, and 3a–d (a) in the crystal state and (b) in PMMA films. Excitation at 380 nm. | ||
Bulkier Dipp-substituted NHC ligand containing complex 2 shows higher PLQY both in the solid state (Table 1) and in solution (Table S1†) compared to the less bulky mesityl-substituted NHC containing complex 1 with otherwise the same Me-substituted pyridinophane ligand. This could be due to more efficient suppression of non-radiative decay in the presence of a bulky Dipp-substituent, consistent with a smaller non-radiative decay rate constant for complex 1 compared to complex 2 (Table S2†). A similar enhancing effect of bulky alkyl groups on the photophysical properties of CuI complexes has been described previously.33 Our group also showed that there is a correlation between ligand bulk and non-radiative decay in a series of (N4)CuI complexes.34
The nature of the counter anion in the series of complexes 3a–d does not significantly affect PLQY in the solid state or PMMA films. No coordination or specific interactions with the counter anions could be observed in the crystal structures. In dichloromethane solutions, no emission is observed in complexes 3b and 3c, presumably due to the presence of coordinating anions.
Triboluminescent properties of crystalline samples
Crystals of 1, 2, and 3a–d were found to generate intense emission upon grinding the crystalline sample with a stainless steel spatula or glass rod, or when the single crystals are compressed between glass plates, all under air (Fig. 4a–c). To obtain TL spectra, crystals of 1, 2, and 3a–d were placed in glass vials and ground by a glass tube containing a fiber optic probe (Fig. 5).35 The emission maxima of TL spectra shift to longer wavelength compared to PL spectra, consistent with literature precedents.24,36–38 Representative images showing emission generated upon grinding crystals of 1, 2, and 3a are shown in Fig. 4, and a movie showing TL during grinding a crystalline sample or compression of a single crystal under air are given in the ESI.† A high-speed camera recording of single crystal TL of 1 when it's compressed between glass plates, shows that emission is generated along the cracks in the crystal (see ESI†).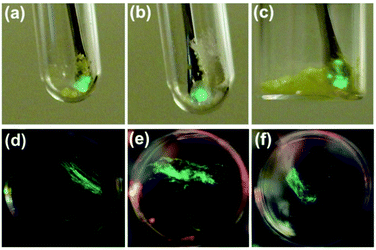 | ||
| Fig. 4 Representative images of TL in crystal of 1 (a), 2 (b) and 3a (c) under air and PMMA film containing 10 wt% of 1 (d), 2 (e), and 3a (f) under Ar. | ||
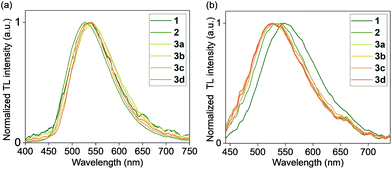 | ||
| Fig. 5 Normalized TL emission spectra of complexes 1, 2, and 3a–d (a) in the crystal state and (b) in PMMA films (1 wt%) under N2. | ||
An analysis of the relationship between space group and TL for the crystalline samples shows that both centrosymmetric and non-centrosymmetric crystals show TL properties (Table 2). The absence of polymorphs was confirmed by analyzing bulk samples by PXRD (Fig. S22–S27, ESI†), and purity was confirmed by elemental analysis and PXRD pattern comparison. The samples that were recrystallized multiple times also showed the same spectra and TL properties as the samples obtained after one recrystallization. The nature of the counter anion in complexes 3a–d does not have a strong effect on the TL emission maximum in crystals. PXRD analysis of the ground samples showed that mechanical grinding has no effect on the crystal packing, in contrast to some other known organic TL materials which showed aggregation effects and changes after grinding.39,40
| Complex | Counter anion | TL (crystal) λmaxa (nm) | TL in PMMA, λmaxa,b (nm) | Space group in crystal |
|---|---|---|---|---|
| a TL properties are measured under N2 atmosphere. b 1 wt% Cu complex loading in PMMA films. | ||||
| 1 | PF6 | 536 | 552 | P21/n (centrosym.) |
| 2 | PF6 | 527 | 543 | P212121 (non-centrosym.) |
| 3a | PF6 | 544 | 526 | Pca21 (non-centrosym.) |
| 3b | OTf | 539 | 530 | Pca21 (non-centrosym.) |
| 3c | TFA | 536 | 536 | Pca21 (non-centrosym.) |
| 3d | BPh4 | 540 | 527 | Pbca (centrosym.) |
Previous studies showed that among some classes of TL materials, non-centrosymmetric (piezoelectric) crystals are more prone to show TL, attributed to the piezoelectric effect. Upon applying mechanical force that causes material fracture, two oppositely charged surfaces are produced generating an electric field; subsequent charge recombination leads to the generation of an excited state and eventual emission.3,41–43 However, there are currently many examples of centrosymmetric crystals showing TL properties, attributed to a number of possible reasons: including the generation of oppositely charged planes in ionic crystalline compounds upon fracture, or the presence of defects or disorders in the crystal structure leading to local non-centrosymmetry.44–52 The complexes 1, 2, and 3a–d are all ionic compounds, and disordered fragments are clearly present in some of the crystal structures (1 and 3b–d), so neither of these effects can be ruled out as the source of the TL effect. However, it is clear that the centrosymmetry, or lack of it, in the parent space group of a crystal is not tied to TL emission.
Triboluminescent properties in amorphous polymer films
Due to our interest in exploring mechanoresponsive materials such as polymer films modified with co-polymerized photoluminescent metal complexes,25 we decided to see if TL properties can also be observed when these triboluminescent Cu(NHC) complexes were blended into amorphous polymer films. Using polymer films may provide a convenient way to make bulk mechanoresponsive material or coating via simple synthetic methods, avoiding the limited repeatability and fabrication method limitations associated with the use of a crystalline solid. Would a low amount of metal complex in a bulk amorphous material be sufficient to create the conditions necessary for TL to be observed? The bigger question that can be addressed by such study: is the presence of a crystal necessary for observing visible light TL, or can an amorphous bulk material also show similar functional properties?We prepared metal loaded films by dissolving a powder of polymethylmethacrylate (PMMA), polystyrene or polyvinyl chloride and 1–10 wt% of a Cu complex in dichloromethane, then casting on a glass surface followed by slow evaporation and drying under vacuum to give transparent films. PXRD of the dried PMMA films showed that at low Cu loading, 1–10 wt%, the films were amorphous and did not show crystallinity, also confirmed by fluorescence microscopy imaging. Only at the high Cu loading of 80 wt%, some crystallinity could be observed by PXRD and fluorescence microscopy (Fig. S30 and S56, ESI†).
We found that visible TL was observed in polymer films with as little as 1 wt% Cu loading, upon rubbing the film surface with a glass rod or metal spatula, if the film was placed under a nitrogen gas atmosphere. The TL from polymer films was clearly visible by eye in the dark or in a dimmed room under inert atmosphere (Fig. 4d–f), in contrast with the control PMMA film without a Cu complex. For measurements of polymer TL spectra, only the films with a 1 wt% Cu loading were used. Their TL spectra (Table 2 and Fig. 5) displayed a red shift when compared to the PL spectra in PMMA films (Table 1). Similar to crystalline samples, the nature of the counter anion does not significantly affect the TL emission maximum. A representative movie of a triboluminescent PMMA film with 10 wt% of 3a where the TL effect is much more pronounced, generating emission by rubbing with a glass rod is shown in the ESI.†
TL was also observed in other rigid polymer films, in polystyrene and at a lower intensity, in polyvinyl chloride (Fig. S39 and S40†). This is in contrast to previously reported NHC CuI complexes cross-linked into elastomeric, stretchable polybutylacrylate films, which do not show detectable triboluminescence.25
Thus, these findings represent the first example of Cu complexes that display TL properties when simply blended into amorphous polymer films.53 There are several reports on TL polymers films containing Eu complexes. The detailed study of such Eu-containing polymers showed that TL was observed when a microcrystalline Eu complex was impregnated on the polymer surface, and TL was not observed when the film was prepared by blending, where there was an absence of a crystal phase.54 In other cases, the details of whether the crystalline phase was present were not reported.35,55,56 A crystal requirement by definition imposes a limit on its response properties and repeatability. For the first time, we definitely show that the presence of a crystalline phase is unnecessary for TL to be observed with a transition metal chromophore.
To further investigate the origin of the excited state produced by mechanical stimuli in polymer films, we recorded TL spectra under an atmosphere of various gases, including N2, Ar, He, CO2, and SF6 (Fig. 6). TL in PMMA films containing complexes 1, 2, and 3a was not observed under air but was observed in N2, Ar, and He. The absence of TL in PMMA films under air could be due to quenching of PL by oxygen (PLQY in PMMA films decrease when measured under air compared to measurements under N2), or the effect of air humidity. In addition to the broad emission peak of the Cu complexes observed in TL spectra of 1 wt% Cu PMMA samples, the sharp emission peaks characteristic of an electric discharge through the corresponding inert gas (Ar, He, or N2) were clearly observed.57,58
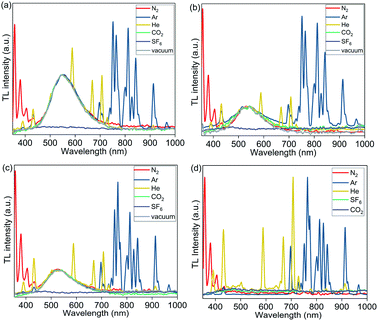 | ||
| Fig. 6 TL spectra of PMMA films containing 1 wt% of complexes (a) 1; (b) 2; (c) 3a; and (d) PMMA (without Cu complex) recorded under 1 atm of N2, Ar, He, CO2, SF6, and vacuum (0.5 Torr). | ||
Unexpectedly, under CO2 gas and under vacuum (0.5 Torr), the emission spectra showed only the TL peak of the Cu complexes, without the gas discharge spectrum components being present (with exception of complex 2, which showed small N2 discharge peaks at 0.5 Torr). TL was not observed under an SF6 atmosphere.
We then examined a control PMMA film without any metal complex. Notably, weak gas emission spectra were also observed upon rubbing pure PMMA films without Cu complex under N2, Ar, and He; however, in the absence of a Cu complex, the pure polymer emission was weak, and did not allow for the observation of TL with the naked eye even under He, Ar and N2.
Previous studies by Sweeting59 and earlier by Longchambon59–61 and other groups identified several possible mechanisms for TL that involve participation of the surrounding gas.3 First, an electric field generated by mechanical stimulus may lead to dielectric breakdown of the surrounding gas, leading to the excitation of the gas molecules by electron bombardment to create emission in the discharged gas. If the excited gas generates emission lines in the UV/vis region, absorption of the gas emission may cause photoluminescence of the PL material (tribophotoluminescence, TPL), leading to the observation of an emission spectrum in the PL compound.3,59 Alternatively, the electrical discharge may also directly excite a photoluminescent compound by electron bombardment.3,59
In crystalline materials mechanical stimulation typically involves crystal fracture, leading to the generation of oppositely charged planes that create the necessary electric field. By contrast, in polymers, triboelectrification has been reported to create a sufficient electric field that is able to stimulate gas discharge.62 The latter is consistent with the observation of the discharged gas spectrum in PMMA without a Cu complex. It also explains how TL can be observed in an amorphous organometallic complex when it is embedded inside a material capable of triboelectrification. The absence of TL in SF6 is consistent with its insulating properties and high dielectric strength, having many vibrational modes, high ionization potential, high mass, and high electron affinity.59 It is important to note that unlike the parent control PMMA film, we were able to observe TL in 1 wt% Cu PMMA films with the naked eye, which is unsurprising due to the complexes' broad emission peak in the visible region. However, the ability of PMMA to have sufficient triboelectrification to excite the Cu chromophore at these low loadings was not something that we could initially predict. As the TL effect does not depend on the crystallinity of the Cu complex, the emission effect is not decreased drastically over time due to the decrease of the amount of possible crystal that is left to break.
Clear observation of a gas discharge spectra in Cu-containing PMMA films suggests that TPL due to excitation by photons emitted by discharged gas contributes to the observed effect. To confirm the latter mechanism, we prepared PMMA films containing 1 wt% of 9,10-diphenylanthracene (DPA) as a simple fluorescent dye. DPA-blended PMMA films also showed emission in the expected region (432–434 nm) upon rubbing PMMA film with a glass rod (Fig. S51b†). This confirms that TPL is one of the viable mechanisms and we are currently investigating the scope of luminophores suitable for TL observation in polymer films.
According to the literature, in addition to TPL due to excitation by photons emitted by discharged gas, many PL materials also undergo direct excitation by electron bombardment.3,59 TL in amorphous Cu-blended PMMA films could involve both of these non-mutually exclusive mechanisms. This is supported by observation of TL under CO2 atmosphere, where no strong emission bands were observed in the excitation region optimal for [(N4)Cu(NHC)]+ complexes. TL is also observed under vacuum, where no gas discharge lines were detected (vide supra).
In addition, we also observed TL in PMMA films blended with EuD4TEA complex (Scheme S6†), known to be triboluminescent in the solid state.11,55,56 The TL spectra also showed both the characteristic Eu complex emission peak at 613 nm, as well as strong gas discharge lines, with TL observed under N2, Ar, He and CO2 (Fig. S52b†), but not under SF6 or air. This shows that the observed effect in polymers is rather general, further increasing its possible scope of applications, with the advantages of the current system being its lower cost, bright visible emission and greater availability of a Cu-based TL material.
By contrast to polymer samples, crystalline samples of the pure Cu complex did not show discernible gas discharge lines. Emission of 2 was observed even under SF6 (Fig. S41b†), but at lower intensity when compared to N2. Brighter emission was seen under He, but also without the characteristic gas discharge peaks (see ESI movie and Fig. S41†). Among complexes 3a–d, three: 3b, 3c, and 3d, showed TL emission in SF6 (Fig. S42†), while complex 3a did not, suggesting that the mechanisms of TL could be dependent on the crystal packing or the nature of the counter anion. This comparison also shows that TL emission under SF6 is not correlated with either centrosymmetric or non-centrosymmetric crystal structure and different factors could be involved. Therefore, although some gas-dependent behavior is observed, leading to variable intensity in different gases, alternative mechanisms that do not involve participation of discharged gas are likely to contribute to the observed TL. In particular, according to a previous study of TL crystalline materials by Sweeting, discharge that directly excites the PL material by electron bombardment, without participation of the surrounding gas, is proposed to be operative in many TL materials.3,59 It is worth to note, however, that despite its long history, the origin of the TL phenomenon is poorly understood even at the present moment in both polymer and crystalline materials. Although various theories and multiple possible mechanisms that may co-exist are proposed in the literature, no universally agreed theory exists to explain this phenomenon as of yet.3
A natural question to ask is whether the co-polymerized copper complexes earlier reported by us, that were shown to be capable of changing their PL properties in response to stretching of the polymer film, would be considered obsolete when compared to the new TL technique of blending Cu complexes into polymer films (Fig. 1)? The Cu complexes in both applications use the same ligand framework, but the co-polymerized complexes require specialized ligands that can polymerize and require more synthetic effort. The measurements of photoluminescence (PL) intensity change need to be done under UV light irradiation. The advantages of the triboluminescence (TL) process include visible light emission without the need for external irradiation and a more straightforward sample preparation. We would submit that the two techniques are complementary. The PL co-polymerized complexes have recently been shown to display PL under air, and they have been shown to alter their response proportionally to gradually applied mechanical stress. A stretched film will have a very different PL intensity compared to its non-stretched counterpart. This response can only be observed in elastomers and is not applicable to the currently used PMMA, polystyrene or PVC samples that are rigid at RT. TL is a direct generation of emission in response to a mechanical action, and we indeed did not observe it on stretching the polymer in the previous work. TL also displays a red-shift with regard to the PL emission wavelength; thus potentially two types of mechanical stress can be differentiated by the same co-polymerized complex if the difference in λmax can be optimized, and the correct stretchable and malleable polymer matrix can be found. Two different metal probes in the same polymer could also accomplish this task; each one would ideally be sensitive to only one type of stimulus and/or have a sufficiently different λmax. We are currently exploring this research direction.
Conclusions
In summary, we report a new family of six triboluminescent (TL) Cu(NHC) complexes. These findings also significantly expand the library of known TL copper complexes, an earth-abundant and inexpensive metal. We showed that triboluminescence can be observed both in the crystal state and when the complex is amorphous and dispersed in polymer films. In the latter case, the ability of the polymer to create an electric field upon mechanical stimulus that excited the Cu complex appears to be mechanism that allows for the observation of a visual response to applying fast mechanical force. This finding may provide an explanation for the TL effect in a number of metal complexes, and it allows for the active exploration of repeatable TL effect in polymer films.The ability to utilize solution cast polymer films diversifies the range of practical applications of such TL materials for visualization of applied mechanical force and damage sensing in polymers. Currently, we are investigating the scope of luminophores and polymers that can be used to prepare triboluminescent polymer films. We seek to develop a general approach to generate visible triboluminescence in polymers under ambient conditions, as well as to create materials that can show either a PL or TL selective response to different types of mechanical stress.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP18K05247. We thank Dr Olga Gladkovskaya for synthesis of ligand precursor. We also thank Dr Takuya Miyazawa and Dr Hyung Been Kang (MEMS, OIST), Dr Michael Roy (IAS, OIST), Engineering support section and Imaging section at OIST for technical support, and Dr Julio Manuel Barros Jr (OIST) for supporting the observation of TL using a high speed camera.Notes and references
- J. I. Zink, Acc. Chem. Res., 1978, 11, 289–295 CrossRef CAS.
- P. Jha and B. P. Chandra, Luminescence, 2014, 29, 977–993 CrossRef CAS.
- Y. Xie and Z. Li, Chem, 2018, 4, 943–971 CAS.
- I. Sage and G. Bourhill, J. Mater. Chem., 2001, 11, 231–245 RSC.
- X. Wang, H. Zhang, R. Yu, L. Dong, D. Peng, A. Zhang, Y. Zhang, H. Liu, C. Pan and Z. L. Wang, Adv. Mater., 2015, 27, 2324–2331 CrossRef CAS.
- D. O. Olawale, K. Kliewer, A. Okoye, T. J. Dickens, M. J. Uddin and O. I. Okoli, J. Lumin., 2014, 147, 235–241 CrossRef CAS.
- S. Mukherjee and P. Thilagar, Angew. Chem., Int. Ed., 2019, 58, 7922–7932 CrossRef CAS.
- C. Wang, Y. Yu, Y. Yuan, C. Ren, Q. Liao, J. Wang, Z. Chai, Q. Li and Z. Li, Matter, 2020, 2, 181–193 CrossRef.
- J.-C. G. Bünzli and K.-L. Wong, J. Rare Earths, 2018, 36, 1–41 CrossRef.
- Y. Hirai, T. Nakanishi, Y. Kitagawa, K. Fushimi, T. Seki, H. Ito and Y. Hasegawa, Angew. Chem., Int. Ed., 2017, 56, 7171–7175 CrossRef CAS.
- C. R. Hurt, N. McAvoy, S. Bjorklund and N. Filipescu, Nature, 1966, 212, 179–180 CrossRef CAS.
- G. L. Sharipov and A. A. Tukhbatullin, J. Lumin., 2019, 215, 116691 CrossRef CAS.
- C.-W. Hsu, K. T. Ly, W.-K. Lee, C.-C. Wu, L.-C. Wu, J.-J. Lee, T.-C. Lin, S.-H. Liu, P.-T. Chou, G.-H. Lee and Y. Chi, ACS Appl. Mater. Interfaces, 2016, 8, 33888–33898 CrossRef CAS.
- F. A. Cotton, D. M. L. Goodgame and M. Goodgame, J. Am. Chem. Soc., 1962, 84, 167–172 CrossRef CAS.
- S. Balsamy, P. Natarajan, R. Vedalakshmi and S. Muralidharan, Inorg. Chem., 2014, 53, 6054–6059 CrossRef CAS.
- J. Chen, Q. Zhang, F.-K. Zheng, Z.-F. Liu, S.-H. Wang, A. Q. Wu and G.-C. Guo, Dalton Trans., 2015, 44, 3289–3294 RSC.
- D. M. Knotter, M. D. Janssen, D. M. Grove, W. J. J. Smeets, E. Horn, A. L. Spek and G. Van Koten, Inorg. Chem., 1991, 30, 4361–4366 CrossRef CAS.
- D. M. Knotter, H. L. Van Maanen, D. M. Grove, A. L. Spek and G. Van Koten, Inorg. Chem., 1991, 30, 3309–3317 CrossRef CAS.
- F. Marchetti, C. Di Nicola, R. Pettinari, I. Timokhin and C. Pettinari, J. Chem. Educ., 2012, 89, 652–655 CrossRef CAS.
- D. M. Knotter, G. Blasse, J. P. M. Van Vliet and G. Van Koten, Inorg. Chem., 1992, 31, 2196–2201 CrossRef CAS.
- D. M. Knotter, A. L. Spek, D. M. Grove and G. Van Koten, Organometallics, 1992, 11, 4083–4090 CrossRef CAS.
- C. Pettinari, C. di Nicola, F. Marchetti, R. Pettinari, B. W. Skelton, N. Somers, A. H. White, W. T. Robinson, M. R. Chierotti, R. Gobetto and C. Nervi, Eur. J. Inorg. Chem., 2008, 1974–1984 CrossRef CAS.
- A. S. Romanov, C. R. Becker, C. E. James, D. Di, D. Credgington, M. Linnolahti and M. Bochmann, Chem.–Eur. J., 2017, 23, 4625–4637 CrossRef CAS.
- A. İncel, C. Varlikli, C. D. McMillen and M. M. Demir, J. Phys. Chem. C, 2017, 121, 11709–11716 CrossRef.
- A. Karimata, P. H. Patil, E. Khaskin, S. Lapointe, R. R. Fayzullin, P. Stampoulis and J. R. Khusnutdinova, Chem. Commun., 2020, 56, 50–53 RSC.
- Deposition numbers 2009593–2009597 contain the supplementary crystallographic data for this paper.†.
- V. A. Krylova, P. I. Djurovich, B. L. Conley, R. Haiges, M. T. Whited, T. J. Williams and M. E. Thompson, Chem. Commun., 2014, 50, 7176–7179 RSC.
- S. Shi, M. C. Jung, C. Coburn, A. Tadle, D. Sylvinson M. R., P. I. Djurovich, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2019, 141, 3576–3588 CrossRef CAS.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601–606 CrossRef CAS.
- V. A. Krylova, P. I. Djurovich, M. T. Whited and M. E. Thompson, Chem. Commun., 2010, 46, 6696–6698 RSC.
- V. A. Krylova, P. I. Djurovich, J. W. Aronson, R. Haiges, M. T. Whited and M. E. Thompson, Organometallics, 2012, 31, 7983–7993 CrossRef CAS.
- M. Nishikawa, T. Sano, M. Washimi, K. Takao and T. Tsubomura, Dalton Trans., 2016, 45, 12127–12136 RSC.
- R. Czerwieniec, M. J. Leitl, H. H. H. Homeier and H. Yersin, Coord. Chem. Rev., 2016, 325, 2–28 CrossRef CAS.
- P. H. Patil, G. A. Filonenko, S. Lapointe, R. R. Fayzullin and J. R. Khusnutdinova, Inorg. Chem., 2018, 57, 10009–10027 CrossRef CAS.
- N. Takada, J.-i. Sugiyama, R. Katoh, N. Minami and S. Hieda, Synth. Met., 1997, 91, 351–354 CrossRef CAS.
- J. P. Duignan, I. D. H. Oswald, I. C. Sage, L. M. Sweeting, K. Tanaka, T. Ishihara, K. Hirao and G. Bourhill, J. Lumin., 2002, 97, 115–126 CrossRef CAS.
- J.-i. Nishida, H. Ohura, Y. Kita, H. Hasegawa, T. Kawase, N. Takada, H. Sato, Y. Sei and Y. Yamashita, J. Org. Chem., 2016, 81, 433–441 CrossRef CAS.
- K. K. Neena, P. Sudhakar, K. Dipak and P. Thilagar, Chem. Commun., 2017, 53, 3641–3644 RSC.
- F. Liu, J. Tu, X. Wang, J. Wang, Y. Gong, M. Han, X. Dang, Q. Liao, Q. Peng, Q. Li and Z. Li, Chem. Commun., 2018, 54, 5598–5601 RSC.
- J. Yang, X. Gao, Z. Xie, Y. Gong, M. Fang, Q. Peng, Z. Chi and Z. Li, Angew. Chem., Int. Ed., 2017, 56, 15299–15303 CrossRef CAS.
- G. E. Hardy, W. C. Kaska, B. P. Chandra and J. I. Zink, J. Am. Chem. Soc., 1981, 103, 1074–1079 CrossRef CAS.
- B. P. Chandra, Phys. Status Solidi A, 1981, 64, 395–405 CrossRef CAS.
- B. P. Chandra and J. I. Zink, Inorg. Chem., 1980, 19, 3098–3102 CrossRef CAS.
- L. M. Sweeting and A. L. Rheingold, J. Am. Chem. Soc., 1987, 109, 2652–2658 CrossRef CAS.
- A. L. Rheingold and W. King, Inorg. Chem., 1989, 28, 1715–1719 CrossRef CAS.
- X.-F. Chen, X.-H. Zhu, Y.-H. Xu, S. Shanmuga Sundara Raj, S. Ozturk, H.-K. Fun, J. Ma and X.-Z. You, J. Mater. Chem., 1999, 9, 2919–2922 RSC.
- X.-F. Chen, C.-Y. Duan, X.-H. Zhu, X.-Z. You, S. Shanmuga Sundara Raj, H.-K. Fun and J. Wu, Mater. Chem. Phys., 2001, 72, 11–15 CrossRef CAS.
- H.-Y. Wong, W.-S. Lo, W. T. K. Chan and G.-L. Law, Inorg. Chem., 2017, 56, 5135–5140 CrossRef CAS.
- Y. Xie, J. Tu, T. Zhang, J. Wang, Z. Xie, Z. Chi, Q. Peng and Z. Li, Chem. Commun., 2017, 53, 11330–11333 RSC.
- B. V. Bukvetskii, A. S. Shishov and A. G. Mirochnik, Luminescence, 2016, 31, 1329–1334 CrossRef CAS.
- B. V. Bukvetskii, A. G. Mirochnik and A. S. Shishov, J. Lumin., 2018, 195, 44–48 CrossRef CAS.
- B. V. Bukvetskii, A. G. Mirochnik, P. A. Zhikhareva and V. E. Karasev, J. Struct. Chem., 2010, 51, 1164–1169 CrossRef CAS.
- The previous report on the Cu-impregnated polymer contains microcrystalline phase: see ref. 23.
- A. İncel, M. Emirdag-Eanes, C. D. McMillen and M. M. Demir, ACS Appl. Mater. Interfaces, 2017, 9, 6488–6496 CrossRef.
- R. S. Fontenot, W. A. Hollerman, K. N. Bhat, M. D. Aggarwal and B. G. Penn, Polym. J., 2014, 46, 111–116 CrossRef CAS.
- T. M. George, M. J. Sajan, N. Gopakumar and M. L. P. Reddy, J. Photochem. Photobiol., A, 2016, 317, 88–99 CrossRef CAS.
- K. Nakayama and R. A. Nevshupa, J. Tribol., 2003, 125, 780–787 CrossRef CAS.
- D. O. Olawale, O. O. I. Okoli, R. S. Fontenot and W. A. Hollerman, Triboluminescence: Theory, Synthesis, and Application, Springer International Publishing, 2016 Search PubMed.
- L. M. Sweeting, Chem. Mater., 2001, 13, 854–870 CrossRef CAS.
- H. Longchambon, Compt. Rend. , 1922, 174, 1633–1634 CAS.
- H. Longchambon, Compt. Rend. , 1923, 176, 691–693 CAS.
- D. Puhan, R. Nevshupa, J. S. S. Wong and T. Reddyhoff, Tribol. Int., 2019, 130, 366–377 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2009593–2009597. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc04442c |
| This journal is © The Royal Society of Chemistry 2020 |