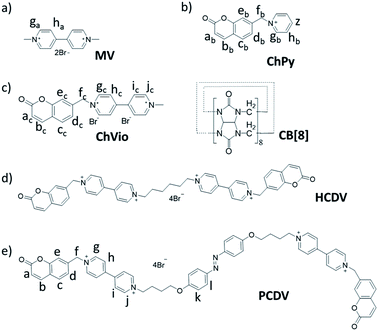
Open Access Article
Open Access ArticleMulti-mode supermolecular polymerization driven by host–guest interactions†
Dongdong
Chang
,
Dan
Han
,
Wenhao
Yan
,
Zhiyi
Yuan
,
Qiaochun
Wang
 and
Lei
Zou
and
Lei
Zou
 *
*
Key Laboratory for Advanced Materials and Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science & Technology, Shanghai 200237, PR China. E-mail: zoulei@ecust.edu.cn; Fax: +86 21 64252288; Tel: +86 21 64252758
First published on 12th April 2018
Abstract
A novel supermolecular self-assembly based on ternary host–guest interaction between cucurbit[8]uril (CB[8]), 1,1′-dimethyl-4,4′-bipyridinium dication (MV) and coumarin derivative was applied for the construction of linear supramolecular polymer with high degree of polymerization in aqueous solution. Accompanied by the introduction of azobenzene on linear ABBA type monomer the supermolecular polymerization is different and the morphology changes from linear to dendritic polymer. The successful supramolecular polymerization of linear and dendritic supramolecular polymers by non-covalent host–guest molecular recognition was confirmed by various characterization methods, such as 1H NMR spectroscopy, ROESY, transmission electron microscopy (TEM) and dynamic light scattering (DLS) measurements. Meanwhile, the supramolecular polymerization could promote the conversion of the azobenzene from cis to trans, which ultimately results in no isomerism upon UV irradiation.
1 Introduction
Supramolecular polymers, polymeric arrays of monomeric units that are brought together by reversible and highly directional secondary interactions, have been extensively applied for their versatility in the construction of intelligent artificial materials during the past few decades.1 Compared with traditional polymers, supramolecular polymers are endowed with many novel specific functions such as controllability, reversibility, responsiveness to external stimuli and ability to self-healing due to the dynamic and reversible nature of noncovalent interactions.2 With the further research, various noncovalent interactions have been widely attempted to facilitate the self-assembly of supramolecular monomers. For example, hydrogen bonding3 and metal–ligand interactions4 are usually employed as driving force for their high binding constants, macrocycle recognition5 and interlocked structures6 are frequently used as bridge to construct supramolecular polymers, etc. Supramolecular polymers can be functionalized with specific properties such as morphology adjustment,7 controllable luminescence,8 shape memory,9 self-healing,10 and so forth. Especially, the supramolecular polymers constructed in aqueous solution can offer more possibility for mimicking biocompatible or vital functional materials.Cucurbit[8]uril (CB[8]) can accommodate two guest molecules in its cavity as a significant macrocycle.11 Scherman et al. reported the CB[8]-mediated heteroternary complexation with 1,1′-dimethyl-4,4′-bipyridinium dication (MV) and an uncharged azobenzene derivative to demonstrate the formation of CB[8] supramolecular polymers from guest monomers bearing azobenzene moieties both in solution and in the solid state.12 Zhang and co-workers reported a series of CB[8]-mediated supramolecular polymers with naphthalene- and anthracene-containing monomers.13 While there were rare reports about CB[8]-mediated heteroternary complexation with MV and coumarin derivative. And plentiful researches have indicated that short spacers are of high orientation selectivity which inhibits the formation of cyclic.14 Based on above researches, we designed and synthesized two multifunctional monomers which could assemble to form multi-morphology supramolecular polymers from linear to dendritic polymerization by the introduction of azobenzene in aqueous solution based on host–guest interaction. The first monomer is 1′,1′′′-(hexane-1,6-diyl)bis(1-((2-oxo-2H-chromen-7-yl)methyl)-[4,4′-bipyridine]-1,1′-diium)bromide (HCDV; Scheme 1). HCDV contains coumarin–viologen–viologen–coumarin structure array with hexyl spacer as the flexible linkage which is suitable for supramolecular polymerization. The other is (E)-1′,1′′′-(((diazene-1,2-diylbis(4,1-phenylene))bis(oxy))bis(butane-4,1-diyl)) bis(1-((2-oxo-2H-chromen-7-yl)methyl) [4,4′-bipyridine]-1,1′-diium)bromide (PCDV; Scheme 1). PCDV is designed to comprising an azobenzene moiety in the middle of HCDV and contains two butyl spacers on either side of azobenzene as the flexible linkage. Moreover, we have demonstrated that the coumarin was able to act as a second guest for a CB[8]·MV binary complex, which led to the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heteroternary complex. Other reasons for choosing coumarin as a second guest include its high binding constant as well as its better optical and biological activity. The short length of flexible linkage of HCDV inhibits the formation of the 1
1 heteroternary complex. Other reasons for choosing coumarin as a second guest include its high binding constant as well as its better optical and biological activity. The short length of flexible linkage of HCDV inhibits the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic complex. Instead, the formation of linear supramolecular polymer is favored. The introduction of azobenzene in linkage site affects the polymerization pattern and thus forms dendritic supramolecular polymers. Apart from the transformation of polymerization pattern, the gradually introduction of CB[8] promotes the conversion of the azobenzene on the supramolecular polymer from cis to trans, which ultimately results in no isomerism upon UV irradiation. As far as we know, there were rare reports that azobenzene could be limited photoisomerization by supramolecular assemble methods.
1 cyclic complex. Instead, the formation of linear supramolecular polymer is favored. The introduction of azobenzene in linkage site affects the polymerization pattern and thus forms dendritic supramolecular polymers. Apart from the transformation of polymerization pattern, the gradually introduction of CB[8] promotes the conversion of the azobenzene on the supramolecular polymer from cis to trans, which ultimately results in no isomerism upon UV irradiation. As far as we know, there were rare reports that azobenzene could be limited photoisomerization by supramolecular assemble methods.
2 Results and discussion
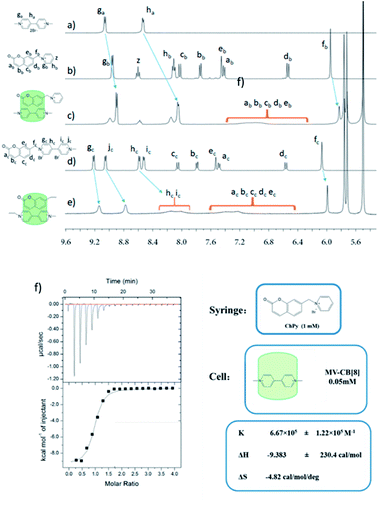
To confirm the formation of the ternary complex, 1H NMR experiments were performed in D2O (Fig. 1). After the addition of CB[8] to a solution of MV and 1-((2-oxo-2H-chromen-7-yl)methyl)pyridin-1-ium bromide (ChPy) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the 1H NMR signals of MV and ChPy shifted to upfield apparently with the signals for the protons in ChPy broadened. The process of self-assemble was also investigated by 1H NMR titration experiments which were performed in D2O by gradually increasing the ratio of ChPy to CB[8]·MV (ESI, Fig. S1†). These changes indicated the successful encapsulation of MV and ChPy in the cavity of CB[8] and thus the formation of the ternary complex. A guest molecule 1-methyl-1′-((2-oxo-2H-chromen-7-yl)methyl)-[4,4′-bipyridine]-1,1′-diium bromide (ChVio) in the cavity of CB[8] can be regarded as an AB-type monomer, with A and B representing viologen- and coumarin- moiety bound in one CB[8] respectively. Comparing the 1H NMR spectra for ChVio in the absence and presence of CB[8], the signals for protons in coumarin moiety still shifted to upfield and broadened. The peaks gc, jc, hc and ic in viologen moiety also broadened besides shifting upfield which was presumably corresponding to polymeric species. These changes revealed that a linear supramolecular polymer with a low degree of polymerization may exist for a 1
1, the 1H NMR signals of MV and ChPy shifted to upfield apparently with the signals for the protons in ChPy broadened. The process of self-assemble was also investigated by 1H NMR titration experiments which were performed in D2O by gradually increasing the ratio of ChPy to CB[8]·MV (ESI, Fig. S1†). These changes indicated the successful encapsulation of MV and ChPy in the cavity of CB[8] and thus the formation of the ternary complex. A guest molecule 1-methyl-1′-((2-oxo-2H-chromen-7-yl)methyl)-[4,4′-bipyridine]-1,1′-diium bromide (ChVio) in the cavity of CB[8] can be regarded as an AB-type monomer, with A and B representing viologen- and coumarin- moiety bound in one CB[8] respectively. Comparing the 1H NMR spectra for ChVio in the absence and presence of CB[8], the signals for protons in coumarin moiety still shifted to upfield and broadened. The peaks gc, jc, hc and ic in viologen moiety also broadened besides shifting upfield which was presumably corresponding to polymeric species. These changes revealed that a linear supramolecular polymer with a low degree of polymerization may exist for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CB[8] and ChVio.
1 mixture of CB[8] and ChVio.
The affinity between coumarin and CB[8]·MV was obtained by isothermal titration calorimetry (ITC) experiments. Titration of ChPy into an aqueous solution of CB[8]·MV gave an exothermic isotherm, and which indicated that CB[8]·MV could bind with ChPy in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with binding constant K2 value of (6.67 + 1.22) × 105 M−1 as shown in Fig. 1f. Such a high binding constant prefers to forming of a supramolecular polymer with a high degree of polymerization.
1 molar ratio with binding constant K2 value of (6.67 + 1.22) × 105 M−1 as shown in Fig. 1f. Such a high binding constant prefers to forming of a supramolecular polymer with a high degree of polymerization.
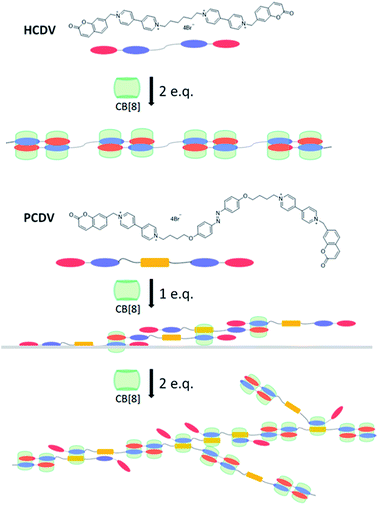
The linear supramolecular polymers with higher solubility were formed by mixing CB[8] and HCDV with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on the above assembly method. Furthermore, the introduction of azobenzene in the flexible linkage moiety generated another polymerization process and yielded dendritic supramolecular polymers whose schematic diagrams were shown in the Fig. 2. The stoichiometry between ChVio and CB[8], HCDV and CB[8] was confirmed to be 1
1 based on the above assembly method. Furthermore, the introduction of azobenzene in the flexible linkage moiety generated another polymerization process and yielded dendritic supramolecular polymers whose schematic diagrams were shown in the Fig. 2. The stoichiometry between ChVio and CB[8], HCDV and CB[8] was confirmed to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by their Job plots respectively, and the stoichiometry between CB[8] and PCDV was about 2.33
2 by their Job plots respectively, and the stoichiometry between CB[8] and PCDV was about 2.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as shown in Fig. S6 in ESI.†
1 as shown in Fig. S6 in ESI.†
To confirm the formation of supramolecular polymers and study the assembly process of the supramolecular polymers, 1H NMR titration experiments were performed in D2O. With the molar ratio of CB[8] to HCDV increased gradually, peaks of viologen and coumarin moieties in HCDV monomers broadened (Fig. S2 in ESI†), while the new peaks of the complex enhanced and the peaks of the free HCDV monomer disappeared when the molar ratio of CB[8]/HCDV was increased to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gradually. These changes suggested the formation of the supramolecular polymer in aqueous solution. Correspondingly, as the addition of CB[8] to PCDV, the signal Hk in azobenzene moiety shifted upfield obviously, indicating that the azobenzene moiety was bounded in CB[8]. On the other hand, there was an obvious decrease in peak of free PCDV monomer with δ = 8.92 when the molar ratio of CB[8]/PCDV was 1
1 gradually. These changes suggested the formation of the supramolecular polymer in aqueous solution. Correspondingly, as the addition of CB[8] to PCDV, the signal Hk in azobenzene moiety shifted upfield obviously, indicating that the azobenzene moiety was bounded in CB[8]. On the other hand, there was an obvious decrease in peak of free PCDV monomer with δ = 8.92 when the molar ratio of CB[8]/PCDV was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S3 in ESI†). When the molar ratio of CB[8]/PCDV exceeded 1.0, the peaks of the free PCDV monomer disappeared, which indicated that all PCDV monomers had been assembled to form the supramolecular polymers of CB[8] and PCDV. And when the molar ratio reached 2.0, all characteristic peaks broadened more and there were less sharp and dispersed signals in 1H NMR spectrum of PCDV-2CB[8] compared with the 1H NMR spectrum of HCDV-2CB[8]. Increased the molar ratio of CB[8] and PCDV to 2.5, the peaks of viologen and coumarin were further broadened, especially the coumarin moiety, which suggested that further supermolecular polymerization occurred. These analysis showed that there existed three assembly methods on PCDV/CB[8]. With the addition of CB[8] to PCDV, viologen and azobenzene moieties, viologen and coumarin moieties were bounded in the cavity of CB[8]. And when the molar ratio of CB[8] exceeded 2.0, two coumarin moieties were bounded in the cavity of CB[8]. The speculated process of the polymerization is depicted by a schematic diagram in the Fig. 2. On the other hand, 2D ROESY 1H NMR was employed to certify the interaction of proton signals in viologen and coumarin moieties, coumarin and coumarin15 as well as viologen and azobenzene (Fig. S4 and S5 in ESI†).
1 (Fig. S3 in ESI†). When the molar ratio of CB[8]/PCDV exceeded 1.0, the peaks of the free PCDV monomer disappeared, which indicated that all PCDV monomers had been assembled to form the supramolecular polymers of CB[8] and PCDV. And when the molar ratio reached 2.0, all characteristic peaks broadened more and there were less sharp and dispersed signals in 1H NMR spectrum of PCDV-2CB[8] compared with the 1H NMR spectrum of HCDV-2CB[8]. Increased the molar ratio of CB[8] and PCDV to 2.5, the peaks of viologen and coumarin were further broadened, especially the coumarin moiety, which suggested that further supermolecular polymerization occurred. These analysis showed that there existed three assembly methods on PCDV/CB[8]. With the addition of CB[8] to PCDV, viologen and azobenzene moieties, viologen and coumarin moieties were bounded in the cavity of CB[8]. And when the molar ratio of CB[8] exceeded 2.0, two coumarin moieties were bounded in the cavity of CB[8]. The speculated process of the polymerization is depicted by a schematic diagram in the Fig. 2. On the other hand, 2D ROESY 1H NMR was employed to certify the interaction of proton signals in viologen and coumarin moieties, coumarin and coumarin15 as well as viologen and azobenzene (Fig. S4 and S5 in ESI†).
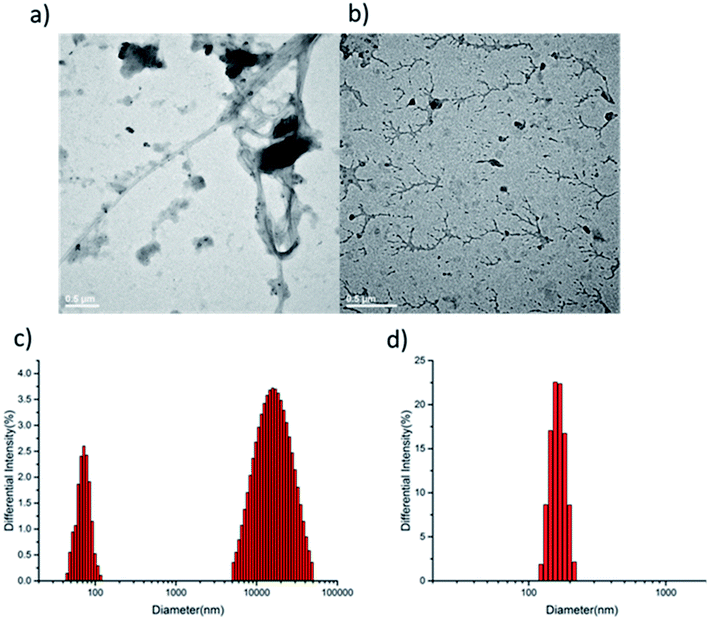
To further confirm the difference of supramolecular polymerization, transmission electron microscopy (TEM) measurements were undertaken. The TEM image of supramolecular polymer HCDV-2CB[8] and PCDV-2CB[8] gave distinctly different supramolecular self-assembled morphologies. As shown in Fig. 3a, an ultra-long curved string with more than a dozen micrometer length was observed, clearly indicating the existence of linear supramolecular polymer (for more detail morphology, see Fig. S8 in ESI†). By contrast, the TEM of PCDV-2CB[8] displayed dendritic morphology like “antler” shape with lengths of hundreds of nanometers to several micrometers in water (Fig. 3b). Such difference was consistent with the speculated polymerization process.
Dynamic light scattering (DLS) measurements in water were performed to provide the evidence for the existence of supramolecular polymer. A hydrodynamic radius of ca. 1.9 μm was found in HCDV-2CB[8], which indicated the formation of the supramolecular polymers with high degree of polymerization in water (Fig. 3c). By contrast, the PCDV-2CB[8] showed around 180 nm hydrodynamic size (Fig. 3d), but which was more evenly distributed. The results were consistent with the TEM and showed two different supramolecular polymerization process before and after the introduction of azobenzene. Meanwhile, the polymerization process of PCDV and CB[8] was also investigated by DLS. The results of PCDV-CB[8] and PCDV-2CB[8] showed that the hydrodynamic radius increased from 120 nm to 180 nm (Fig. S9†), indicating the increased degree of polymerization with the ratio of PCDV/CB[8] changed from 1.0 to 0.5. And the results were corroborated by our previous analysis of 1H NMR.
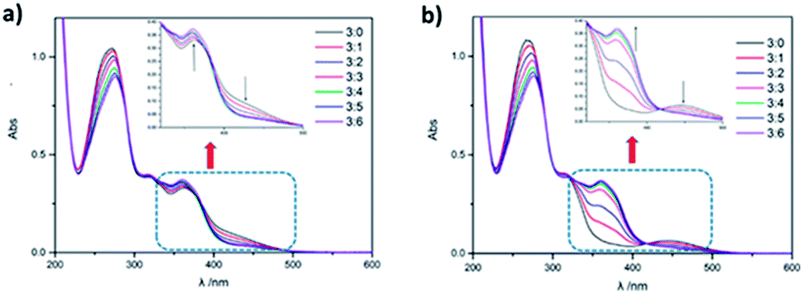
Meanwhile, the gradually introduction of CB[8] promotes the conversion of the azobenzene on the supramolecular polymer from cis to trans configuration, which ultimately results in no isomerism upon UV irradiation. The conversion process was investigated by UV/Vis spectroscopy. With the addition of CB[8] to PCDV, absorption peak at wavelength 366 nm decreased, accompanied by absorption peak at wavelength 450 nm increasing (Fig. 4a). A monomer PCDV could convert to cis from trans upon 365 nm UV irradiation. A reverse conversion happened as CB[8] was gradually added as the ratio of CB[8] to PCDV was increased from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 6
3 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 4b). When the molar ratio of CB[8]/PCDV was 2
3 (Fig. 4b). When the molar ratio of CB[8]/PCDV was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the supramolecular polymer PCDV-2CB[8] had no any response upon 365 and 254 nm (Fig. S10h in ESI†) UV irradiation. This phenomenon may be caused by strong affinity between CB[8] and two guest moiety limited isomerism of azobenzene in the middle, which was demonstrated by changing the molar ratio of CB[8] and PCDV irradiated on 365 nm UV for studying the conversion process (by UV/Vis spectroscopy shown in Fig. S10 in ESI†). And when the molar ratio of CB[8]/PCDV was smaller than 2
1, the supramolecular polymer PCDV-2CB[8] had no any response upon 365 and 254 nm (Fig. S10h in ESI†) UV irradiation. This phenomenon may be caused by strong affinity between CB[8] and two guest moiety limited isomerism of azobenzene in the middle, which was demonstrated by changing the molar ratio of CB[8] and PCDV irradiated on 365 nm UV for studying the conversion process (by UV/Vis spectroscopy shown in Fig. S10 in ESI†). And when the molar ratio of CB[8]/PCDV was smaller than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the photoisomerization happened to part of complex with the addition of CB[8] upon 365 nm UV irradiation, clearly indicating the photoisomerization of azobenzene limited by assemble of CB[8] and PCDV.
1, the photoisomerization happened to part of complex with the addition of CB[8] upon 365 nm UV irradiation, clearly indicating the photoisomerization of azobenzene limited by assemble of CB[8] and PCDV.
3 Conclusions
In summary, we demonstrated a novel supermolecular self-assembly based on ternary host–guest interaction between CB[8], MV and coumarin derivative and constructed a water soluble linear supramolecular polymer with high degree of polymerization. The higher binding constant between coumarin and CB[8]·MV was in favor of supramolecular self-assembly. Accompanied by the introduction of azobenzene on linear ABBA type monomer the supermolecular polymerization is different and the morphology changes from linear to dendritic polymer. Meanwhile, the supramolecular polymerization could promote the conversion of the azobenzene from cis to trans, which ultimately results in no isomerism upon UV irradiation. This research enriches the field of supramolecular polymers and provides a significant route for controlling molecular self-assembly with tailored properties by non-covalent interactions in the bottom-up nanofabrication of molecular devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Basic Research 973 Program (2013CB733700), National Natural Science Foundation of China (No. 21302056 and 21421004), Fundamental Research Funds for the Central Universities (222201717003).Notes and references
- (a) K. Jie, Y. Zhou, Y. Yao, B. Shi and F. Huang, J. Am. Chem. Soc., 2015, 137, 10472–10475 CrossRef CAS PubMed; (b) K. Zhang, J. Tian, D. Hanifi, Y. Zhang, A. C. Sue, T. Zhou, L. Zhang, X. Zhao, Y. Liu and Z. Li, J. Am. Chem. Soc., 2013, 135, 17913–17918 CrossRef CAS; (c) Q. Wang, M. Cheng, Y. Zhao, L. Wu, L. Wang and Y. Pan, Chem. Commun., 2015, 51, 3623–3626 RSC; (d) E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414–2477 CrossRef CAS; (e) H. Yang, B. Yuan and X. Zhang, Acc. Chem. Res., 2014, 47, 2106–2115 CrossRef CAS; (f) F. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li, F. Huang, J. Am. Chem. Soc., 2008, 130, 11254–11255 Search PubMed; (g) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS; (h) H. Chen, L. Xu, X. Ma and H. Tian, Polym. Chem., 2016, 7, 3989–3992 RSC; (i) X. Ji, D. Xia, X. Yan, H. Wang and F. Huang, Acta Polym. Sin., 2017, 9–18 CAS; (j) Q. Wang, M. Cheng, J.-L. Jiang and L.-Y. Wang, Chin. Chem. Lett., 2017, 28, 793–797 CrossRef CAS; (k) X. Wang, Y. Yang, L. Fan, F. Yang and D. Wu, Sci. China: Chem., 2018, 61, 311–318 CrossRef CAS; (l) S. Wu, J. Li, H. Liang, L. Wang, X. Chen, G. Jin, X. Xu and H.-H. Yang, Sci. China: Chem., 2017, 60, 628–634 CrossRef CAS.
- (a) X. Wang, W. Wang, G. Yin, Y. Wang, C. Zhang, J. Shi, Y. Yu and H. Yang, Chem. Commun., 2015, 51, 16813–16816 RSC; (b) P. Wei, T. R. Cook, X. Yan, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2014, 136, 15497–15500 CrossRef CAS; (c) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362–369 CrossRef CAS; (d) L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS; (e) H. Chen, X. Ma, S. Wu and H. Tian, Angew. Chem., Int. Ed., 2014, 53, 14149–14152 CrossRef CAS; (f) L. Chen, H. Chen, X. Yao, X. Ma and H. Tian, Chem.–Asian J., 2015, 10, 2352–2355 CrossRef CAS.
- (a) K. E. Thomas, L. J. McCormick, H. V. Lima and A. Ghosh, Angew. Chem., Int. Ed., 2017, 56, 10088–10092 CrossRef CAS; (b) X. Tong, J. Xiang, F. Shi and Y. Zhao, Adv. Opt. Mater., 2016, 4, 1392–1396 CrossRef CAS; (c) R. Gu, J. Yao, X. Fu, W. Zhou and D. Qu, Chem. Commun., 2015, 51, 5429–5431 RSC.
- (a) C. Lin, L. Xu, L. Huang, J. Chen, Y. Liu, Y. Ma, F. Ye, H. Qiu, T. He and S. Yin, Macromol. Rapid Commun., 2016, 37, 1453–1459 CrossRef CAS; (b) J. Cao, X. Zhang, C. Lu, Y. Luo and X. Zhang, Macromol. Rapid Commun., 2017, 38, 1700406–1700411 CrossRef; (c) P. Dydio and J. N. H. Reek, Angew. Chem., Int. Ed., 2013, 52, 3878–3882 CrossRef CAS.
- (a) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS; (b) Q. Zhang, D. Qu, X. Ma and H. Tian, Chem. Commun., 2013, 49, 9800–9802 RSC; (c) K. Ikemoto, R. Kobayashi, S. Sato and H. Isobe, Angew. Chem., Int. Ed., 2017, 56, 6677 CrossRef CAS; (d) Y. Chen and Y. Liu, Adv. Mater., 2015, 27, 5403–5409 CrossRef CAS; (e) X. Yao, X. Ma and H. Tian, J. Mater. Chem. C, 2014, 2, 5155–5160 RSC; (f) X. Yao, T. Li, S. Wang, X. Ma and H. Tian, Chem. Commun., 2014, 50, 7166–7168 RSC.
- (a) R. G. E. Coumans, J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, Chem. Eur. J., 2013, 19, 7758–7770 CrossRef CAS; (b) J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094–11125 CrossRef CAS; (c) E. M. Perez, Chem.–Eur. J., 2017, 23, 12681–12689 CrossRef CAS.
- (a) X. Yao, T. Li, S. Wang, X. Ma and H. Tian, Chem. Commun., 2014, 50, 7166 RSC; (b) Q. Zhang, X. Yao, D. Qu and X. Ma, Chem. Commun., 2014, 50, 1567–1569 RSC; (c) R. Sun, C. Xue, X. Ma, M. Gao, H. Tian and Q. Li, J. Am. Chem. Soc., 2013, 135, 5990–5993 CrossRef CAS; (d) D. Guo, S. Chen, H. Qian, H. Zhang and Y. Liu, Chem. Commun., 2010, 46, 2620–2622 RSC; (e) W. Zhang, Y. Zhang, S. Li, Y. Cui, J. Yu and Y. Liu, Angew. Chem., Int. Ed., 2016, 55, 1–6 CrossRef; (f) T. Li, X. Li, J. Wang, H. Ågren, X. Ma and H. Tian, Adv. Opt. Mater., 2016, 4, 840–847 CrossRef CAS.
- (a) Q. Zhang, D. Li, X. Li, P. B. White, J. Mecinovic, X. Ma, H. Agren, R. J. Nolte and H. Tian, J. Am. Chem. Soc., 2016, 138, 13541–13550 CrossRef CAS; (b) Y. Liu, Z. Huang, X. Tan, Z. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766–5768 RSC; (c) G. Liu, Y. Zhang, C. Wang and Y. Liu, Chem.–Eur. J., 2017, 23, 14425–14429 CrossRef CAS; (d) H. Wu, Y. Chen and Y. Liu, Adv. Mater., 2017, 1605271–1605275 CrossRef PubMed.
- (a) R. Zhao, T. Zhao, X. Jiang, X. Liu, D. Shi, C. Liu, S. Yang and E. Chen, Adv. Mater., 2017, 29, 1605908–1605913 CrossRef; (b) C. L. Lewis and E. M. Dell, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1340–1364 CrossRef CAS.
- (a) R. Tepper, S. Bode, R. Geitner, M. Jager, H. Gorls, J. Vitz, B. Dietzek, M. Schmitt, J. Popp, M. D. Hager and U. S. Schubert, Angew. Chem., Int. Ed., 2017, 56, 4047–4051 CrossRef CAS; (b) Y. Takashima, Y. Sawa, K. Iwaso, M. Nakahata, H. Yamaguchi and A. Harada, Macromolecules, 2017, 50, 3254–3261 CrossRef CAS; (c) M. Noack, R. Merindol, B. Zhu, A. Benitez, S. Hackelbusch, F. Beckert, S. Seiffert, R. Mulhaupt and A. Walther, Adv. Funct. Mater., 2017, 27, 1700767–1700775 CrossRef.
- (a) K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267–279 RSC; (b) X. Xu, F. Tian, X. Liu, R. M. Parker, Y. Lan, Y. Wu, Z. Yu, O. A. Scherman and C. Abell, Chem.–Eur. J., 2015, 21, 15516–15519 CrossRef CAS; (c) Z.-J. Yin, Z.-Q. Wu, F. Lin, Q.-Y. Qi and X. Zhao, Chin. Chem. Lett., 2017, 28, 1167–1171 CrossRef CAS.
- (a) J. Barrio, P. N. Horton, D. Lairez, G. O. Lloyd, C. Toprakcioglu and O. A. Scherman, J. Am. Chem. Soc., 2013, 135, 11760–11763 CrossRef; (b) J. Barrio, S. T. J. Ryan, P. G. Jambrina, E. Rosta and O. A. Scherman, J. Am. Chem. Soc., 2016, 138, 5745–5748 CrossRef; (c) F. Tian, D. Jiao, F. Biedermann and O. A. Scherman, Nat. Commun., 2012, 3, 1207–1214 CrossRef.
- (a) Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., 2010, 122, 6726–6729 CrossRef; (b) Y. Liu, H. Yang, Z. Wang and X. Zhang, Chem.–Asian J., 2013, 8, 1626–1632 CrossRef CAS; (c) K. Liu, Y. Liu, Y. Yao, H. Yuan, S. Wang, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2013, 52, 8285–8289 CrossRef CAS.
- (a) Y. Liu, K. Liu, Z. Wang and X. Zhang, Chem.–Eur. J., 2011, 17, 9930–9935 CrossRef CAS; (b) L. Yang, Y. Bai, X. Tan, Z. Wang and X. Zhang, ACS Macro Lett., 2015, 4, 611–615 CrossRef CAS; (c) Y. H. Ko, K. Kim, J. K. Kang, H. Chun, J. W. Lee, S. Sakamoto, K. Yamaguchi, J. C. Fettinger and K. Kim, J. Am. Chem. Soc., 2004, 126, 1932–1933 CrossRef CAS; (d) K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267–279 RSC.
- B. C. Pemberton, E. Kumarasamy, S. Jockusch, D. K. Srivastava and J. Sivaguru, Can. J. Chem., 2011, 89, 310–316 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01892h |
| This journal is © The Royal Society of Chemistry 2018 |