Open Access Article
Open Access ArticleOptimization of simultaneous production of volatile fatty acids and bio-hydrogen from food waste using response surface methodology†
Nuo Liu a,
Jianguo Jiang
a,
Jianguo Jiang *ab,
Feng Yan
*ab,
Feng Yan a,
Yiwen Xua,
Meng Yanga,
Yuchen Gaoa,
Aikelaimu Aihemaitia and
Quan Zoua
a,
Yiwen Xua,
Meng Yanga,
Yuchen Gaoa,
Aikelaimu Aihemaitia and
Quan Zoua
aSchool of Environment, Tsinghua University, Beijing 100084, China. E-mail: jianguoj@tsinghua.edu.cn
bKey Laboratory for Solid Waste Management and Environment Safety, Ministry of Education of China, Beijing 100084, China
First published on 14th March 2018
Abstract
Anaerobic digestion of food waste (FW) is commonly considered an effective and green technology to convert solid waste into valuable feedstock including volatile fatty acids (VFAs) and hydrogen. Response surface methodology (RSM) was selected to analyze the production of VFAs and hydrogen from food waste in a batch process. The effect of the three variables i.e. total solid content (TS), pH, and reaction time under each variable at three levels on VFAs and hydrogen production was assessed. The optimum conditions determined via RSM were pH = 7.0, TS = 100 g L−1, and reaction time = 3 d. The maximum VFA and hydrogen production was 26.17 g L−1 and 46.03 mL g−1 volatile solids added, respectively. The ratio of observed hydrogen (Ho) to predicted hydrogen (Hp) was x < 1.0 because of inhibition of hydrogen production by VFA accumulation. The subsequent microbial community analysis result was also consistent with the abovementioned results. The evolution of Bacteroidetes, which facilitate VFA production, has been enriched by about 16.1-times at pH 7.0 followed by 10.2-times at pH 6.0 as compared to that in the uncontrolled pH batch.
1. Introduction
In recent years, more than 60 million-ton food waste (FW) is generated per year in China due to population growth and economic development.1 FW has received public attention due to its quantity, odor, and potential for pathogenic microorganism contamination.2 FW contains a high moisture and salt/oil content; hence, conventional treatment such as incineration or sanitary landfill may cause environmental and health risks.3 Anaerobic digestion is considered as an effective alternative method for treating FW because of its ability to produce high-value products (e.g. volatile fatty acids [VFAs] and hydrogen) in an environmentally friendly way.4 Use of FW as a feedstock for anaerobic fermentation to form VFAs and hydrogen enables the production of high-value products and reduces waste material; this makes anaerobic fermentation a novel and promising approach.5Commonly, hydrolysis and acidogenesis occur during anaerobic digestion processes to produce VFAs. At first, complex compounds in food waste are broken into small compounds by enzymes, and then, the resulting small organic compounds are converted into VFAs by the action of microbes. Moreover, hydrogen is generated as a by-product.6
VFAs and hydrogen are two high-value products of anaerobic fermentation. The produced VFAs can be used as an efficient carbon source in wastewater treatment,7,8 whereas hydrogen is considered an effective alternative to the current fossil fuels. In addition, both VFAs and hydrogen may be used as precursors of chemical products.9
In previous studies, efforts have been made to maximize the VFA and hydrogen production through exploring different kinds of wastes including FW, garden waste, cattle waste, sludge, and mixtures. In addition, optimization of parameters, such as pH, temperature, and total solid content (TS), during anaerobic digestion has been considered. Typically, to optimize the parameters of an anaerobic process, the traditional approach about just one variable a time on VFA production is commonly accepted.10 However, the abovementioned method is not able to evaluate the interaction between multiple variables.
In this study, we focused on the potential of FW as a raw material to produce VFAs and hydrogen; the production was optimized using response surface methodology (RSM). The parameters, including pH, temperature, and total solid content (TS), especially their interactions, were optimized during anaerobic digestion. A Box–Behnken design for three variables, each at three levels, was used to model the process. The TS, volatile solid (VS), soluble chemical oxygen demand (SCOD), and the microbial community analysis were also assessed.
2. Materials and methods
2.1. Substrate and inoculum
FW produced at a campus cafeteria was complex and difficult to homogenize; therefore, we analyzed its composition and prepared a mixture that represented several traditional food ingredients to simulate real FW. All food ingredients were purchased from the campus market. The FW comprised a mixture of rice (35% by weight), cabbage (45% by weight), pork (16% by weight), and tofu (4% by weight). The mixture was processed in a food grinder with tap water and stored at 4 °C. Its characteristics are presented in Table 1.| Items | Food waste | Inoculum |
|---|---|---|
| a Mean ± standard deviation of three samples.b TS, total solids.c VS, volatile solids.d VFAs, volatile fatty acids. | ||
| TS (%)b | 10.98 ± 2.04 | 4.5 ± 0.05 |
| VS (%)c | 10.95 ± 2.21 | 2.2 ± 0.04 |
| VS/TS (%) | 97.7 ± 1.11 | 48.54 ± 0.36 |
| VFAs (mg L−1)d | 829.53 ± 103.16 | 568.4 ± 116.05 |
| pH | 4.59 ± 0.17 | 6.34 ± 0.09 |
| C (% TS) | 49.97 ± 0.15 | 24.48 ± 0.05 |
| H (% TS) | 6.79 ± 0.01 | 3.45 ± 0.01 |
| N (% TS) | 3.72 ± 0.06 | 2.88 ± 0.02 |
| C/N | 13.43 ± 0.18 | 8.49 ± 0.04 |
Anaerobically digested sludge has been obtained from the Gaobeidian Wastewater Treatment Plant (Beijing, China), which has a sewage treatment capacity of 1.0 × 106 m3 per day. The sewage treatment plant uses an anaerobic-anoxic-oxic processing system to treat municipal wastewater. The sludge was obtained from the anaerobic digester after gravity thickening and anaerobic digestion were completed. The characteristics of the inoculum sludge are listed in Table 1.
2.2. Batch experiments
The TS content of each reactor was adjusted to 5, 8, or 10% with deionized water, and the substrate was composed of about 85% stimulated FW and 15% anaerobic sludge (wet weight). In all batches, 300 mL of a FW slurry was treated with 50 g sludge as measured by the VS content. All reactors were stirred at 120 rpm using a magnetic stirrer throughout the experiment. Anaerobic fermentation was conducted for 5 days. The amount of biogas produced was sampled using 0–10 mL syringes.The batch reactors were operated at the pH values 4.0, 5.0, 6.0, and 7.0 as well as at uncontrolled pH. Wherever necessary, the pH was adjusted periodically using 1.0 M HCl or NaOH to maintain the desired values. Therefore, pH 7.0 meant that the initial pH was adjusted to 7.0; the pH was then allowed to decrease at the start of fermentation using 1.0 M HCl or NaOH to maintain the pH at 6.8 to facilitate hydrogen production by bacteria.11,12 Moreover, other pH conditions were readjusted, and the pH was maintained at the designed values of about pH 6.0, pH 5.0, and pH 4.0.
2.3. Analytical methods
The pH was measured using a pH meter (DRION STAR A214; Thermo Electron, West Palm Beach, FL, USA). Elemental compositions were determined using the CE-440 elemental analyzer (Exeter Analytical Inc., North Chelmsford, MA, USA). TS and VS were analyzed in accordance with standard methods.13 The SCOD was measured using the Hach Method 8000 and a DR 5000 spectrometer (Hach, Loveland, CO, USA). VFAs (acetate, propionate, butyrate, iso-butyrate, valerate, and isovalerate) and ethanol were measured using a gas chromatograph (GC-2010 Plus; Shimadzu, Tokyo, Japan). The VFA samples were centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min, filtered through a 0.45 μm membrane, and the pH was adjusted to <2.0 using 25% H3PO4 before injection into the gas chromatograph, which was equipped with a capillary column (Stabilwax-DA, 30 m × 0.32 mm × 0.25 μm, Restek Corp., Bellefonte, PA, USA) and a flame ionization detector. The injector and detector temperatures were 220 and 250 °C, respectively. The column temperature was increased from 60 to 150 °C at the rate of 7 °C min−1, held at 150 °C for 5 min, increased to 230 °C at the rate of 20 °C min−1, and finally held at 230 °C for 10 min.
000 rpm for 15 min, filtered through a 0.45 μm membrane, and the pH was adjusted to <2.0 using 25% H3PO4 before injection into the gas chromatograph, which was equipped with a capillary column (Stabilwax-DA, 30 m × 0.32 mm × 0.25 μm, Restek Corp., Bellefonte, PA, USA) and a flame ionization detector. The injector and detector temperatures were 220 and 250 °C, respectively. The column temperature was increased from 60 to 150 °C at the rate of 7 °C min−1, held at 150 °C for 5 min, increased to 230 °C at the rate of 20 °C min−1, and finally held at 230 °C for 10 min.
The biogas composition (mainly H2 and CO2) was measured by the gas chromatograph, which was equipped with a thermal conductivity detector and a View the MathML source (inside diameter) stainless steel column packed with Porapak N (80–100 mesh). The injector, detector, and column temperatures were maintained at 50, 100, and 50 °C, respectively. Argon was used as the carrier gas at the flow rate of 30 mL min−1. A 0.5 mL sample was injected each time.
| Source | Sum of squares | df | Mean square | F value | P-value prob-F |
|---|---|---|---|---|---|
| a R-squared 0.9302, adj R-squared 0.8405, pred R-squared 0.0738, adeq precision 12.602. | |||||
| Model | 406.299 | 9 | 45.14434 | 10.36476 | 0.0028 |
| A-TS | 226.2601 | 1 | 226.2601 | 51.94742 | 0.0002 |
| B-pH | 45.23966 | 1 | 45.23966 | 10.38665 | 0.0146 |
| C-reaction time | 71.52363 | 1 | 71.52363 | 16.42123 | 0.0049 |
| AB | 15.38113 | 1 | 15.38113 | 3.53138 | 0.1023 |
| AC | 9.646328 | 1 | 9.646328 | 2.214716 | 0.1803 |
| BC | 0.192368 | 1 | 0.192368 | 0.044166 | 0.8395 |
| A2 | 0.374251 | 1 | 0.374251 | 0.085925 | 0.7779 |
| B2 | 10.65046 | 1 | 10.65046 | 2.445255 | 0.1619 |
| C2 | 43.59838 | 1 | 43.59838 | 10.00982 | 0.0158 |
| Residual | 30.48892 | 7 | 4.355559 | ||
| Lack of fit | 30.48892 | 5 | 6.097783 | ||
| Pure error | 0 | 2 | 0 | ||
| Cor total | 436.788 | 16 | |||
The V4 region of the 16S rRNA gene was amplified using barcoded primers (forward: GTGCCAGCMGCCGCGGTAA, reverse: GGACTACHVGGGTWTCTAAT) and a polymerase chain reaction (PCR) approach. The PCRs were run on the ABI GeneAmp® 9700 system (Applied Biosystems, Foster City, CA, USA) using the following program: 5 min of denaturation at 95 °C, followed by 30 cycles of 30 s at 95 °C (denaturation), 30 s of annealing at 58 °C, 25 s at 72 °C (elongation), and a final extension at 72 °C for 7 min. The products obtained from different samples were mixed at equal ratios for sequencing using the Illumina HISeq 2500 platform (Illumina Inc., San Diego, CA, USA). The Ribosomal Database Project (RDP) Classifier (ver. 2.2) was used for taxonomic assignments against the RDP 16S rRNA training set 9 with the confidence scores ≥ 0.8. The weighted and unweighted UniFrac distance matrices were calculated as beta-diversity metrics and visualized with principal coordinates analysis in QIIME.14
A Box–Behnken design for three variables, each at three levels, was used for the model tested by RSM. Thus, 17 group experiments were designed. The software Design Expert 8.0 was used to organize the process. All the details are shown in Table S2.†
3. Results and discussion
3.1. Model fitting analysis
The model F-value of 10.36 implies that the model is significant (Table 2). There is only a 0.28% chance that this large model F-Value can occur due to noise. Values of prob > F less than 0.0500 indicate that the model terms are significant. In this case, A, B, C, and C2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. Adeq precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. The ratio of this research is 12.602, which indicates an adequate signal. Overall, the VFA production equation fitted in terms of various factors was observed as follows:
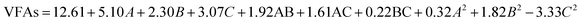 | (1) |
3.2. Response surface analysis of various factors
The optimum level of each of the various factors (TS, pH, reaction time) for VFA production and the interaction effects of these variables were analyzed using 3D response surfaces and 2D contours for two variables with the other one remaining at its optimum level (Fig. 1).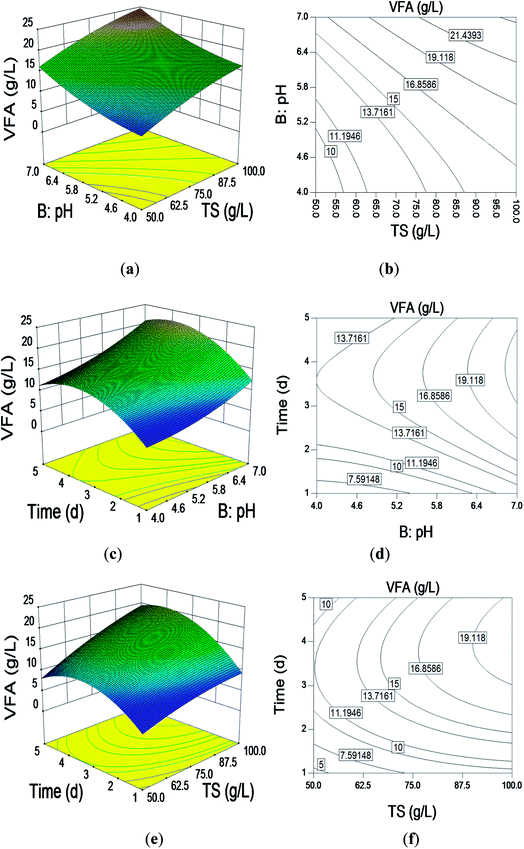 | ||
| Fig. 1 3D response surface and contour plots: effect of (a) and (b) TS (g L−1) and pH; (c) and (d) pH and time (d); (e) and (f) pH TS (g L−1); and time (d). | ||
The effects of pH and TS are shown in Fig. 1(a) and (b). The RSM indicated that VFA production gradually increased with the increasing TS content and pH. This finding is consistent with our previous study.4 It was clear that the higher TS content released more nutrition into the batch system, benefiting the microbial community, which then increased VFA production.15 There is a threshold TS content that is dependent on the rheology and mass transfer of the system.16 VFA production was different at different TS contents and pH values. The optimal pH for VFA production was 7.0 regardless of the TS. The maximum VFA production of 26.17 g L−1 was achieved with 100 g L−1 TS and pH 7.0.
Fig. 1(c) and (d) show the interaction of pH and reaction time. At the lowest pH and reaction time, VFA production was extremely low. The VFA production increased gradually to reach a maximum as the pH and reaction time increased, and then, it decreased. The optimal reaction time and pH for VFA production were 3 d and 7.0, respectively.
Fig. 1(e) and (f) reflect the interaction of TS and reaction time. As with the pH and reaction time, at the lowest TS content and reaction time, VFA production was extremely low. The VFA response increased gradually to reach a maximum as the TS content and reaction time increased, and then, it decreased. The reaction time is another important parameter in anaerobic digestion for VFA and hydrogen production and should be taken into consideration. The results obtained by Kim et al. showed that hydraulic reaction time contributed more to VFA production rather than the temperature of the reactor.17 Obviously, a longer reaction time provides enough time for microbes to react with the substrate; this subsequently benefits VFA production. However, there is an optimal reaction time beyond which there is no further increase in VFA production.18
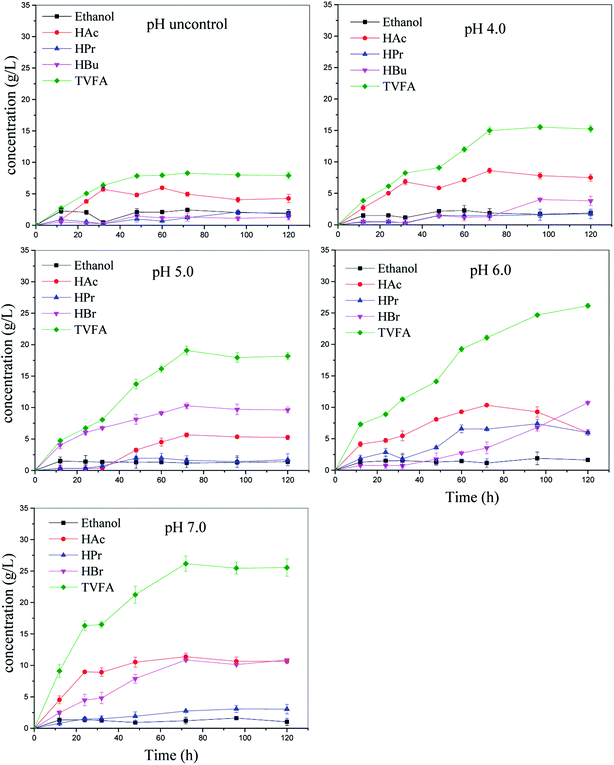
Overall, the maximum VFA concentrations were obtained at pH 7.0, TS 100 g L−1, and reaction time 3 d. This finding is consistent with our previous study.4 Other studies have reported that the maximum VFA concentration was observed at pH 6.0. This difference in the optimal pH value may be due to the characteristics of the inoculum. Wang et al. stated that the optimal pH value of kitchen waste for VFA production by anaerobic digestion was equal to 7,19 whereas a comparative study reported by Dahiya et al. showed that higher VFA production was obtained under alkaline conditions rather than under acidic or neutral conditions.6 Lee et al. have pointed out that the optimal pH value for VFA production ranges from 5.25 to 11, and it depends on the characteristics of the substrate composition and inoculum (Table 3).16 Herein, the VFA production increased with the pH value regardless of TS (Fig. 1); this meant that pH values at 7.0 were more favorable for VFA production than other pH values tested in this study. The maximum VFA production was achieved at pH 7.0, followed by at the pH values of 6.0 and 5.0. This was similar to the results reported by Dahiya et al. (2015), who discovered that a high pH reactor system enhanced the hydrolysis of carbohydrates and proteins by causing ionization and facilitating fermentation. In this study, VFA concentrations at pH 4.0 and with uncontrolled pH reactor increased more slowly during fermentation than under other conditions; this was mainly caused by the inhibition of acidogenesis at pH < 4.0.20
To evaluate the potential of the FW raw material for VFA production more convincingly, VFA production per VS content added (g g−1 VSadded) was also determined (Table S1†). According to the result, a level of 0.416 g g−1 VSadded was obtained at the TS of 50 g L−1, followed by 0.315 g g−1 VSadded at 80 g L−1 and 0.305 g g−1 VSadded at 100 g L−1. The results indicated that the VFA production increased with the increasing TS content up to a point; however, a higher TS content inhibited further degradation; this subsequently led to a lower conversion efficiency of VFA production (g g−1 VSadded). This conclusion was consistent with the research of Khan et al. who stated that the hydrolysis reduced if the organic loading rate increased beyond a certain value.21
The microbial community analysis (Fig. S1a†) also proved the abovementioned results. The result shows that Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria are the predominant phyla regardless of pH. Specially, Bacteroidetes include anaerobic rod-shaped bacteria that are widely distributed in the environment, and their fermentation products always include acetic acid, hydrogen, and ethanol.22 This phylum was enriched by about 1.2, 10.2, and 16.1 times at the pH values of 5.0, 6.0, and 7.0, respectively, as compared to the uncontrolled pH batch (Fig. S1b†).
| Substrate | Inoculum | Operational conditions | VFAs | References |
|---|---|---|---|---|
| Food waste | Dewatered sludge | pH 9.0, 35 °C | 25.93 g COD/L | 23 |
| Kitchen waste | Waste-activated sludge | pH 8.0, 37 °C | 692.4 mg COD/g VS | 24 |
| Food waste | Anaerobic digested sludge | pH 6.0, 35 °C | 24.5–25.5 g L−1 | 18 |
| Waste activated sludge | — | pH 11.0, 25 °C | 219.7 mg COD/g VS | 25 |
| Food waste | Sludge | 37 °C, microwave | 23.02 g L−1 | 26 |
| Food waste | Excess sludge | 40 °C | 867.42 mg COD/g VS | 27 |
| Food waste | Sludge | pH 7.0, 35 °C | 26.17 g L−1 (443 mg COD/g VS) | This study |
3.3. Conversion percentage of VFA/SCOD and the VFA composition
In addition, the VFA/SCOD ratio is commonly used to estimate the degree of soluble material converted into VFAs.28 Furthermore, a higher VFA/SCOD ratio of about 42.4% was achieved at pH 7.0; this indicated that the pH 7.0 enabled the anaerobic microorganisms to convert soluble compounds into VFAs better than at the other pH values reported in this study (Fig. 2).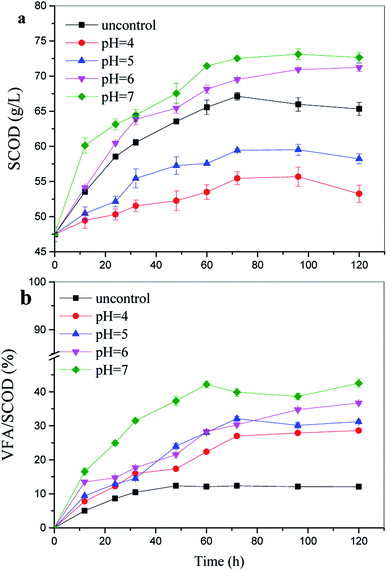 | ||
| Fig. 2 Soluble chemical oxygen demand (SCOD) concentrations and the percentage of volatile fatty acid (VFA)/SCOD at various pH values and 100 g TS/L. | ||
Then, the composition of the VFAs produced is another important parameter representing the degree of hydrolysis and fermentation.29 The main VFAs produced herein were acetic, butyric, and propionic acids (Fig. 3). Acetic and butyric acids were the most prevalent VFAs in all the reactors, accounting for >70% of the total VFA production. These results indicated that butyric-type fermentation was achieved.28 This phenomenon indicated that the pH value controlled the total VFA generation rather than the effect of the ratios of acetate, butyrate, and propionate.30 The ethanol concentration remains stable during the anaerobic digestion.
3.4. Evaluation of hydrogen production and its interaction with VFAs
CH4 was not detected in this 5 day fermentation process.31 A modified Gompertz model32 was applied to analyze the kinetics of hydrogen production during fermentation.
H = P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−exp[Rme(λ − t)/P + 1]} exp{−exp[Rme(λ − t)/P + 1]}
| (2) |
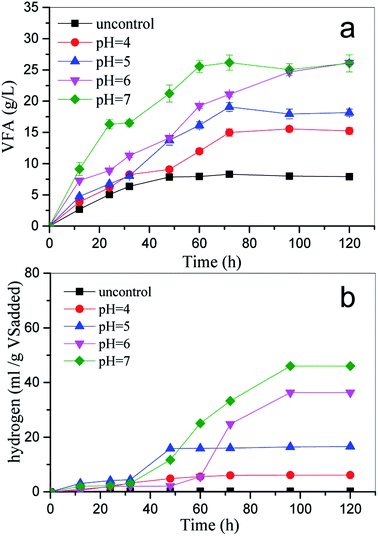
All correlation coefficients (R-square) of nonlinear analysis by the modified Gompertz model were over 0.93, suggesting that the modified Gompertz model was able to describe the cumulative hydrogen production well. Obviously, the maximum cumulative hydrogen yield was obtained at pH 7.0. This result is consistent with the results of other researchers.2
Furthermore, the predicted molar H2 production was calculated by the equation MH = VH/RT, where R = 0.08 (L atm) (mol K)−1 and T = 308 K. The predicted H2 production was calculated by the equations given below:
| C6H12O6 + 2H2O → 2ethanol + 2H2 + 2CO2 | (3) |
| C6H12O6 + 2H2O → 2HAc + 4H2 + 2CO2 | (4) |
| C6H12O6 → HBu + 2H2 + 2CO2 | (5) |
The distributions of VFA production and predicted H2 production are shown in Table 5. Acetate and butyrate were the main soluble products regardless of the pH condition. According to eqn (3)–(5), the theoretical H2 production can be predicted from the ethanol and volatile acid concentration.33 The results range from 233.7 to 651.7 mmol. The highest predicted H2 production was 651.7 mmol at pH 7.0, followed by 397 mmol at pH 6.0. It was speculated that there was no H2 loss through conversion to CH4 because no CH4 was detected. Thus, the ratio of observed hydrogen (Ho) to predicted hydrogen (Hp), i.e. Ho/Hp, theoretically should be close to 1 in the reaction system. However, it was difficult to achieve according to the result (Table 4). The gap was mainly caused by the VFA accumulation, and other reasons, such as the adsorption to the liquid phase, also influenced the Ho/Hp value.33
| pH | P (mL) | Rm (mL h−1) | λ (h) | R2 |
|---|---|---|---|---|
| a Mean ± standard error. | ||||
| Uncontrolled | 7.39 ± 0.02 | 0.63 ± 0.02 | −2.1 ± 0.27 | 0.96 |
| 4.0 | 188.43 ± 0.98 | 4.07 ± 0.08 | 8.27 ± 0.49 | 0.96 |
| 5.0 | 502.94 ± 6.44 | 15.19 ± 1.02 | 16.46 ± 1.17 | 0.94 |
| 6.0 | 1106.4 ± 8.9 | 47.93 ± 1.84 | 55.9 ± 0.45 | 0.99 |
| 7.0 | 1381.53 ± 11.3 | 38.68 ± 0.93 | 32.57 ± 0.66 | 0.98 |
| Parameters | Initial pH conditions | ||||
|---|---|---|---|---|---|
| Uncontrolled | pH 4.0 | pH 5.0 | pH 6.0 | pH 7.0 | |
| a Based on eqn (2)–(4), assuming that production of 1 mol of ethanol accompany 1 mol of H2 production, and 1 mol of acetate and butyrate production accompany 2 mol of H2 production.b The ratio of observed H2 production and predicted H2 production. | |||||
| Ethanol (mmol) | 41.74 | 40.65 | 30 | 35.43 | 38.04 |
| Acetate (mmol) | 71.67 | 125 | 160.3 | 99.83 | 232.17 |
| Butyrate (mmol) | 24.32 | 23.51 | 22.97 | 80.95 | 135.23 |
| Propionate (mmol) | 14.77 | 43.29 | 109.32 | 121.93 | 45.0 |
| Predicted H2 production (Hp) (mmol)a | 233.7 | 337.7 | 252.6 | 397 | 651.7 |
| Observed H2 production (Ho) (mmol) | 0.8 | 13.7 | 58.3 | 128.3 | 160.1 |
| Ho/Hp (%)b | 0.3 | 4.1 | 23.1 | 32.3 | 24.5 |
Moreover, in this study, the accumulation of VFA production altered and rendered the micro-environment unfavorable for the growth of hydrogen-producing microbes, mainly responsible for the gap (Fig. 4); Tapia-Venegas et al.34 achieved a higher Ho/Hp value of about 61–80% accompanied by a lower VFA production (lower 5 mg L−1).
4. Conclusions
RSM was proved to be a useful method to predict VFA production under variable factors. The optimum conditions for the reactors studied in this work analysis via RSM were pH = 7.0, TS = 100 g L−1, and reaction time = 3 d. While the ratio of Ho/Hp for hydrogen production under the optimal condition was around 0.323, lower than the theoretical value of 1.0; this was mainly caused by the effects of VFA accumulation on the microbes in the reactor. Our findings were also consistent with those of the microbial community analysis. The maximum evolution of Bacteroidetes, which facilitate VFA production, was enriched by 16.1 times at pH 7.0 as compared to that in the uncontrolled pH batch.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported financially by the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07202005).References
- Y. Jin, Y. Li and J. Li, J. Environ. Manage., 2016, 180, 291–300 CrossRef CAS PubMed.
- N. H. M. Yasin, T. Mumtaz, M. A. Hassan and N. A. Abd Rahman, J. Environ. Manage., 2013, 130, 375–385 CrossRef CAS PubMed.
- L. Zhang and D. Jahng, Waste Manage., 2012, 32, 1509–1515 CrossRef CAS PubMed.
- J. Jiang, Y. Zhang, K. Li, Q. Wang, C. Gong and M. Li, Bioresour. Technol., 2013, 143, 525–530 CrossRef CAS PubMed.
- I. K. Kapdan and F. Kargi, Enzyme Microb. Technol., 2006, 38, 569–582 CrossRef CAS.
- S. Dahiya, O. Sarkar, Y. V. Swamy and S. V. Mohan, Bioresour. Technol., 2015, 182, 103–113 CrossRef CAS PubMed.
- H. Zhang, J. Jiang, M. Li, F. Yan, C. Gong and Q. Wang, J. Environ. Manage., 2016, 166, 407–413 CrossRef CAS PubMed.
- A. Singh, S. Sevda, I. Abu Reesh, K. Vanbroekhoven, D. Rathore and D. Pant, Energies, 2015, 8, 12357 Search PubMed.
- R. R. Singhania, A. K. Patel, G. Christophe, P. Fontanille and C. Larroche, Bioresour. Technol., 2013, 145, 166–174 CrossRef CAS PubMed.
- C. P. C. Bong, L. Y. Lim, C. T. Lee, J. J. Klemeš, C. S. Ho and W. S. Ho, J. Cleaner Prod., 2018, 172, 1545–1558 CrossRef CAS.
- Y. M. Wong, T. Y. Wu and J. C. Juan, Renewable Sustainable Energy Rev., 2014, 34, 471–482 CrossRef CAS.
- K. Wang, J. Yin, D. Shen and N. Li, Bioresour. Technol., 2014, 161, 395–401 CrossRef CAS PubMed.
- APHA, AWWA and WEF, APHA, Standard methods for the examination of water and wastewater, 2005, Washington, DC, USA Search PubMed.
- Q. Wang, G. M. Garrity, J. M. Tiedje and J. R. Cole, Appl. Environ. Microbiol., 2007, 73, 5261–5267 CrossRef CAS PubMed.
- J. Yu, J. Biotechnol., 2001, 86, 105–112 CrossRef CAS PubMed.
- W. S. Lee, A. S. M. Chua, H. K. Yeoh and G. C. Ngoh, Chem. Eng. J., 2014, 235, 83–99 CrossRef CAS.
- W. Kim, S. G. Shin, J. Lim and S. Hwang, Bioprocess Biosyst. Eng., 2013, 36, 791–798 CrossRef CAS PubMed.
- S.-J. Lim, B. J. Kim, C.-M. Jeong, J.-d.-r. Choi, Y. H. Ahn and H. N. Chang, Bioresour. Technol., 2008, 99, 7866–7874 CrossRef CAS PubMed.
- Y. Wang, B. Zang, G. Li and Y. Liu, Waste Manage., 2016, 53, 62–67 CrossRef CAS PubMed.
- N. H. M. Yasin, T. Mumtaz and M. A. Hassan, J. Environ. Manage., 2013, 130, 375–385 CrossRef CAS PubMed.
- M. A. Khan, H. H. Ngo, W. S. Guo, Y. Liu, L. D. Nghiem, F. I. Hai, L. J. Deng, J. Wang and Y. Wu, Bioresour. Technol., 2016, 219, 738–748 CrossRef CAS PubMed.
- L. Niu, L. Song and X. Dong, Int. J. Syst. Evol. Microbiol., 2008, 58, 12–16 CrossRef CAS PubMed.
- H. Chen, H. Meng, Z. Nie and M. Zhang, Bioresour. Technol., 2013, 128, 533–538 CrossRef CAS PubMed.
- Y. Chen, J. Luo, Y. Yan and L. Feng, Appl. Energy, 2013, 102, 1197–1204 CrossRef CAS.
- H. Yu, Z. Wang, Q. Wang, Z. Wu and J. Ma, Chem. Eng. J., 2013, 231, 206–213 CrossRef CAS.
- Y. Zhang, X. C. Wang, Z. Cheng, Y. Li and J. Tang, Chemosphere, 2016, 144, 689–696 CrossRef CAS PubMed.
- Q.-L. Wu, W.-Q. Guo, H.-S. Zheng, H.-C. Luo, X.-C. Feng, R.-L. Yin and N.-Q. Ren, Bioresour. Technol., 2016, 216, 653–660 CrossRef CAS PubMed.
- W. Guo, Q. Wu, S. Yang, H. Luo, S. Peng and N. Ren, RSC Adv., 2014, 4, 53321–53326 RSC.
- K. Wang, J. Yin, D. Shen and N. Li, Bioresour. Technol., 2014, 161, 395–401 CrossRef CAS PubMed.
- J. B. Van Lier, K. C. Grolle, C. T. Frijters, A. J. Stams and G. Lettinga, Appl. Environ. Microbiol., 1993, 59, 1003–1011 CAS.
- K. Li, R. Liu and C. Sun, Renewable Sustainable Energy Rev., 2016, 54, 857–865 CrossRef CAS.
- D.-H. Kim, S.-H. Kim, K.-W. Jung, M.-S. Kim and H.-S. Shin, Bioresour. Technol., 2011, 102, 8646–8652 CrossRef CAS PubMed.
- W. Han, M. Ye, A. J. Zhu, H. T. Zhao and Y. F. Li, Bioresour. Technol., 2015, 191, 24–29 CrossRef CAS PubMed.
- E. Tapia-Venegas, J. E. Ramirez, A. Donoso-Bravo, L. Jorquera, J.-P. Steyer and G. Ruiz-Filippi, Int. J. Hydrogen Energy, 2013, 38, 2185–2190 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13268a |
| This journal is © The Royal Society of Chemistry 2018 |