Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Promises, facts and challenges for graphene in biomedical applications
Giacomo
Reina†
 a,
José Miguel
González-Domínguez†
a,
José Miguel
González-Domínguez†
 b,
Alejandro
Criado†
b,
Alejandro
Criado†
 c,
Ester
Vázquez
c,
Ester
Vázquez
 *b,
Alberto
Bianco
*b,
Alberto
Bianco
 *a and
Maurizio
Prato
*a and
Maurizio
Prato
 *cde
*cde
aUniversity of Strasbourg, CNRS, Immunopathology and Therapeutic Chemistry, UPR 3572, 67000 Strasbourg, France. E-mail: a.bianco@ibmc-cnrs.unistra.fr
bDepartamento de Química Orgánica, Facultad de Ciencias y Tecnologías Químicas-IRICA, Universidad de Castilla-La Mancha, 13071 Ciudad Real, Spain. E-mail: ester.vazquez@uclm.es
cCarbon Nanobiotechnology Laboratory, CIC BiomaGUNE, Paseo de Miramón 182, 20009 Donostia-San Sebastian, Spain
dBasque Fdn Sci, Ikerbasque, Bilbao 48013, Spain
eDipartimento di Scienze Chimiche e Farmaceutiche, Università di Trieste, Trieste 34127, Italy. E-mail: prato@units.it
First published on 19th July 2017
Abstract
The graphene family has captured the interest and the imagination of an increasing number of scientists working in different fields, ranging from composites to flexible electronics. In the area of biomedical applications, graphene is especially involved in drug delivery, biosensing and tissue engineering, with strong contributions to the whole nanomedicine area. Besides the interesting results obtained so far and the evident success, there are still many problems to solve, on the way to the manufacturing of biomedical devices, including the lack of standardization in the production of the graphene family members. Control of lateral size, aggregation state (single vs. few layers) and oxidation state (unmodified graphene vs. oxidized graphenes) is essential for the translation of this material into clinical assays. In this Tutorial Review we critically describe the latest developments of the graphene family materials into the biomedical field. We analyze graphene-based devices starting from graphene synthetic strategies, functionalization and processibility protocols up to the final in vitro and in vivo applications. We also address the toxicological impact and the limitations in translating graphene materials into advanced clinical tools. Finally, new trends and guidelines for future developments are presented.
Key learning points(1) Superior performances of the graphene family materials over current biomedical tools(2) Advantages, limitations and challenges of graphene-based materials for drug delivery (3) General approaches and advances for constructing graphene-based biosensors and sensing implants (4) Application of graphene in 3D scaffolds for tissue engineering (5) Future perspectives and guidelines for a proper translation of graphene in biomedicine |
1. Introduction
The increasing demand for more effective, specific and safer treatments in the biomedical domain is expanding the horizons of the research towards more innovative therapies. In this context, commercially available biomedical technologies are far from being satisfactory, because they are limited by the intrinsic properties of the materials employed. For instance, metal and silicon are among the most used materials for the fabrication of affordable and conventional implant devices. However, their poor long-term stability in physiological environments, rigid mechanical properties and high inflammatory potential, may result in strong limitations. Therefore, even when a material is considered safe, control studies in the physiological conditions should be performed to make sure the final therapy is effective and nontoxic.Among the more promising materials under current careful scrutiny, graphene has generated an increasing interest in many areas of research, including electronics, composites, energy and photonics.1 Graphene has also generated great expectations in the biomedical field, due to its extraordinary mechanical properties, accompanied by biocompatibility, transparency and electrical conductivity. More specifically, graphene offers simultaneously mechanical compatibility, cell adhesion and low toxicity properties, for applications in scaffold production, sensing and drug delivery.
A large amount of research on graphene focuses on medical applications, as there are specific properties that make graphene a strong candidate for replacing current devices, in particular its mechanical and electronic capabilities.2 Specifically, graphene's Young's modulus ranges between ∼20–40 GPa, while the tensile strength lies between ∼15–520 MPa for graphene cast into films,3 which places graphene amongst the strongest materials. This set of properties, coupled to a high flexibility, makes graphene the ideal component for flexible biomedical electronic devices or implants, acting as a structural reinforcement or as an integral element,4 since it is able to accommodate on the surrounding biological tissue without experiencing stress or fatigue. In addition, graphene possesses broadband absorption and high transparency in the visible range (∼2.3% absorption for single-layer graphene),4 which grants a unique role in medicine enabling optoelectronic stimulation. The electronic properties of graphene are very important for medical purposes (e.g. 2 × 104 cm2 V−1 s−1 carrier mobility and 1013 cm2 carrier density for mechanically exfoliated graphene), in particular to act as conducting component, electrode or support in bioelectronic devices,4 exceedingly outperforming current silicon and noble metal analogues. Biocompatibility is another important issue to take into consideration, since graphene sp2 carbon surface allows strong, non-destructive, interfacial interactions at a cellular level, which can be even improved by chemical functionalization.3,4 As a matter of fact, the tunable surface chemistry opens up many additional possibilities to obtain useful functional derivatives of graphene.2 Accordingly, a controlled chemical modification allows a precise control over the drug quantity and the release rate in specific parts of the body in drug delivery systems.5 The traditional materials for bone tissue engineering, based on metals (titanium, stainless steel, Co/Cr alloys) coated with polymers (e.g. ultra-high-molecular-weight polyethylene) or ceramics (e.g. hydroxyapatite), display long-term compatibility and durability issues.6 In the last years, many reinforcements and coatings made of graphene-based materials, applied to traditional implants, have shown promising results in terms of mechanical properties and biocompatibility. Neural and bone tissue engineering is a current huge medical business.3 Many materials are used to replace nerve grafts and bones, acting as cell promoters: small interference RNA, silicone elastomer scaffolds, natural proteins (e.g. collagen, fibronectin, keratin), polysaccharides (e.g. hyaluronan, chitosan, alginate), and more recently biodegradable synthetic polymers, including polyesters, polyurethanes or electroactive polymers (e.g. polyaniline, polypyrrole). However, graphene, in combination with many of these materials, has the ability to dramatically improve their long-term lifetime, playing a crucial role in cellular growth and activity enhancement. Moreover, the versatile and tunable surface chemistry allows the attachment of cellular growth factors. Graphene also contributes to important advantages in biosensing. Graphene derivatives are able to improve the functionality of conventional electronic components like silicon-based semiconductors or metal electrodes in sensing devices.4 The functionalization possibility and the electrical properties of graphene are able to improve the sensitivity and the range of analysis of the (bio)sensors. In addition, they can overcome the main limitations of conventional materials in sensing implants, such as the poor flexibility, the stability under the harsh biological environment and the weak mechanical strength.
A problem that is becoming more and more common in the biomedical field is that, very often, a material generically called graphene is employed. However, the broad family of graphene products includes, among others graphene oxide, reduced graphene oxide or graphene nanoplatelets.7 Besides having a different structure, these compounds possess very diverse properties and may give completely different biological responses. In addition, these graphene products, in turn, are not homogeneous species, but, rather, are represented by a very broad group of substances with different lateral sizes, oxidation state and a varied number of layers. The lack of standardized ways of reporting the characterization of graphene materials and the absence of agreement on the necessary information to report the detailed conditions used in a biological experiment, make it sometimes difficult even the reproduction of a published experiment.8
Furthermore, there is plenty of published work, in which terms such as biomedical graphene or biological graphene are used. These definitions can create some confusion, especially because they give the erroneous perception of safety, when using graphene related materials, without having really proven their absence of toxicity. However, this is still a challenging problem, even when talking about biological grade definitions for chemicals in general. Every company designs its own quality degree, including, sometimes, in the finest quality products, detailed specifications such as: (a) the purity degree, (b) presence or absence of trace metals, or (c) some application tests. On the other hand, there is not a common agreement on what is a medical grade material. For instance, the USA Food and Drug Administration suggests several materials research standards, in which not only the physicochemical characterization of the material is included, but also important information about the biological experiments related to the same material, defining, in this way, a specific grade of the material.9
Following these considerations, when preparing graphene-based materials for biomedical applications, particular attention should be paid to the characterization that will define the new products. It should therefore be necessary to establish a common background for the development of a master file for the biocompatibility evaluation of not only graphene itself but also the graphene-derived materials. Physicochemical parameters should be considered, pointing out the most useful characterization techniques in each case. The adoption of this kind of standardized data format, when reporting, could help to better compare the different graphene materials in order to clearly identify the relationships between structures and properties, favoring the development of predictive models and expanding our knowledge about the relation between bio- and nanoworld, clearing the way for clinical trials.
In this tutorial review, we will discuss the general principles that have led to the consideration of graphene materials as promising building blocks in biomedicine, with particular emphasis on the experimental procedures that make biocompatible this potentially unsafe material. In this context, we will focus on drug delivery, sensing and tissue engineering of graphene-based technologies.
2. Synthetic considerations
2.1. Preparation of CVD graphene, graphene oxide and reduced graphene oxide
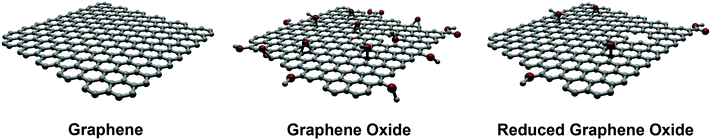
Depending on the medical application under consideration, graphene derivatives can be produced by bottom-up or top-down approaches. Generally, for the purpose of sensing, bottom-up approaches are preferred, in particular chemical vapor deposition (CVD) is the technique of choice. This procedure, which is considered inexpensive, produces high-quality monolayer and few-layer graphene with a low number of defects, being significantly useful for the production of bioelectronic devices. CVD is carried out on poly- and monocrystalline metals, particularly copper or nickel surfaces. In addition, when these substrates are unsuitable for the final use, transfer methods of graphene onto appropriate surfaces can be performed.2 A further promising approach is the synthesis of graphene nanoribbons based on the polymerization of polyaromatic monomers on surfaces.10 This chemical synthesis offers an atomic control of the structure and the dimensions of graphene nanoribbons, leading to materials with well-defined physical properties. Graphene on surfaces can also be produced by chemical or physical exfoliation of bulk graphite, followed by deposition on the substrate of interest. These latter methods are cost-effective, versatile, high-yield, and allow large-scale productions. However, the morphology and the dimensions of the obtained graphene are not very uniform. Therefore, this route is not appropriate for producing graphene-based electronic devices for biomedical applications, because of the poor quality of the resulting electronic properties. Rather, they can be used for applications in drug delivery or tissue engineering where large amounts of graphene materials are required.On the other hand, exfoliated graphene sheets are highly hydrophobic and tend to aggregate, exhibiting a low water dispersibility. To obtain dispersions of graphene in water, interesting strategies have been devised (vide infra). Recently, two other members of the graphene family have become popular: graphene oxide (GO) and reduced graphene oxide (rGO) (Fig. 1).1,2
GO represents the “hydrophilic derivative” of graphene. It can be easily synthesized by the Hummers' method and, compared to graphene, offers a richer surface chemistry due to the presence of the oxygenated groups. Structurally, GO can be considered as a single-layer graphene sheet, where the amount of C atoms has dropped to 40–60%, in favor of a higher presence of oxygen atoms. The sp3/sp2 ratio and the nature of the organic groups is strongly affected by the source of the graphite used, as well as by the synthetic protocols adopted during the GO preparation. This variability strongly influences the chemical reactivity and the macroscopic properties (dispersibility, refractive index, etc.). As already mentioned above, there is not a single and exact GO structure. The study of the morphological characteristics (by atomic force microscopy – AFM, scanning and transmission electron microscopy – TEM and SEM, and scanning transmission electron microscopy – STEM) and the structural characterization (by Raman, Fourier transform infrared spectroscopy – FTIR, solid-state nuclear magnetic resonance – NMR and X-ray photoelectron spectroscopy – XPS), give a reasonable idea of the GO structure.
In spite of the many advantages of GO, e.g., its remarkable hydrophilicity, this nanomaterial is structurally defective, electrically insulating and mechanically poorer than graphene.3 To improve its properties, the chemical or thermal reduction of GO (in order to remove oxygen functional groups and regenerate the sp2 network) has been widely studied, providing rGO. rGO can be considered as an intermediate structure between the ideal graphene sheet and the highly-oxidized GO, thus maintaining some and losing some other properties of both materials. Morphological (AFM, TEM) and functional (Raman, XPS, solid-state NMR) studies have assessed rGO as a single layer graphene sheet that still retains some functional groups after the reduction. Accordingly, the structural restoration of the graphene features is never complete, so that the final nanostructure does not quite match the ideal graphene structure.
For all these reasons, additional preparation routes, providing water-dispersible and non-defective graphene in appreciable amounts are necessary and currently thoroughly investigated.
2.2. Preparation of graphene aqueous dispersions
The application of graphene materials in nanomedicine is strongly conditioned by the need to handle and control the behavior of these nanostructures in physiological conditions, essentially complex aqueous media. While the water stabilization of graphene materials based on GO and rGO may be relatively easily achieved through mild stirring, shaking and/or sonication, the much higher hydrophobicity of graphene makes it necessary the use of external agents to induce the exfoliation and the stabilization of the sheets. The methodology is based on the simple principle of overcoming the van der Waals forces among the graphite sheets by applying counterforces either perpendicular (normal) or parallel (shear) to the graphitic crystalline planes. The mechanical exfoliation of graphite11 with molecular (e.g. pyrenesulfonic acid), macromolecular (proteins, polymers), or supramolecular (micelles, liposomes) surfactants has led to the efficient separation and stabilization of graphene sheets in water.12 A general scheme of graphite exfoliation is reported in Fig. 2. However, the excess of exfoliating agents may interact at a cellular level, often complicating the understanding of the role of graphene in the biological environment. Besides, many surfactants may cause toxicity problems, such as hemolysis. Proteins such as bovine serum albumin (BSA), used to exfoliate graphite down to few-layer graphene, can be considered as biocompatible, but this is not clearly the case for pyrene or other polyaromatic hydrocarbon (PAH) derivatives.13 Therefore, after having obtained the aqueous dispersion, it is necessary to remove the dispersants as much as possible, leaving the minimum quantity indispensable to maintain the stability of graphene in suspension, its exfoliated state and its structural integrity, ensuring not to jeopardize its true potential in nanomedicine.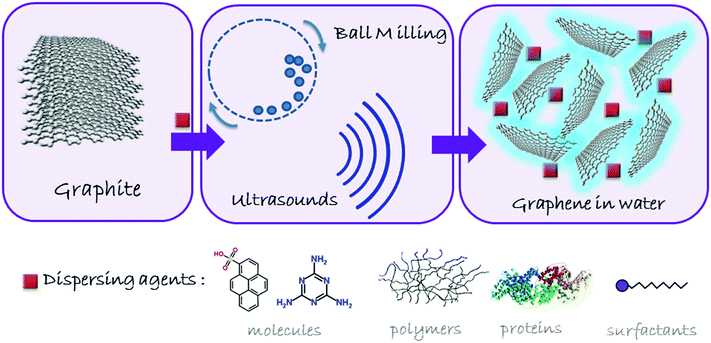 | ||
| Fig. 2 Schematic overview of the process for obtaining non-oxidized graphene in aqueous media by physical exfoliation of graphite. | ||
Ball milling is an exfoliation process ruled by shear forces in which it is possible to control the applied energy to favor shear or collision forces.11 By using a planetary mill, a high applied energy may be useful for the exfoliation process, controllable through the rotation speed and the possibility to work under wet or dry conditions.11 For instance, the successful preparation of few-layer graphene (FLG) in polar solvents, including water, can be achieved by using 2,4,6-triaminotriazine (melamine) as an intercalating agent in a planetary ball mill. This approach has been further implemented by adding dialysis steps in hot water, in order to remove the excess of melamine.14 These aqueous suspensions can be freeze-dried, resulting in a soft powder made of FLG, which can be easily re-suspended in aqueous media, including culture media, at the desired concentration, without compromising its structure.
There are a few other examples that afford liquid-phase stabilization of graphene in aqueous environments, without the use of additives. However, for the moment, these methods have not been explored in biomedical applications yet. This could be ascribed to their early stage of development, or to their low production yields, or the requirement of very strict synthetic conditions (controlled atmosphere, temperatures, etc.). An example of this approach has been recently reported by Bepete et al.15 In this work, graphene monolayers were obtained in water starting from a potassium-graphite intercalation compound (KC8), followed by exfoliation in tetrahydrofuran, eventually exchanging the organic solvent for degassed water.15
However, the implementation of graphene materials as advanced tools in biomedicine can be further improved and becomes more versatile after surface functionalization. In the last decade graphene have been successfully functionalized via non-covalent interactions16 using amphiphilic units and polymers or via covalent functionalization. In the latter case, typical reactions include cycloadditions, radical and nucleophilic/electrophilic additions.1 The properties of the final compounds strongly depend on the synthetic route and on the post-functionalization strategy adopted, in order to be tailored for the defined application.16
3. Biomedical applications of the various graphene forms
3.1. Graphene materials as platforms for drug delivery
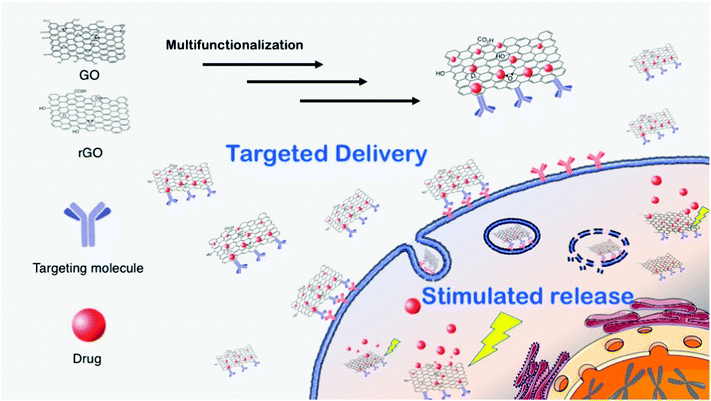
In principle, due to its high surface area, biocompatibility and versatile chemistry, graphene materials can be used as carriers for drug delivery.1,3 There is an increasing demand for the preparation of multifunctional and versatile platforms, i.e., multidrugs or theranostics (therapy and diagnostics). An extraordinary result was reported recently, showing that FLG dispersions have a specific killing action on monocytes, displaying neither toxic nor activation effects on the other immunocompetent cells. This therapeutic activity of graphene was applied against an aggressive form of cancer, namely the myelomonocytic leukemia, where the monocytes are in a malignant form. In this work it was demonstrated that FLG has the unique ability to cause specifically the necrosis of monocytic cancer cells. Moreover, the comparison between FLG and a common chemotherapeutic drug, etoposide, confirmed the higher specificity and toxicity of FLG. Since current chemotherapy treatments of leukemia still cause serious side effects, these findings may open the way to new and safer therapeutic approaches.17Graphene oxide and reduced graphene oxide can be more easily handled, especially in aqueous media, since they generally exhibit good water dispersibility and a very rich surface chemistry, which allows the development of a wide range of biomedical applications. Fig. 3 shows a scheme of GO- and rGO-based drug delivery applications.
In the following sections, the strategies and the GO and rGO uses in the drug delivery field will be presented and discussed.
Structurally, the main difference between graphene and GO is the presence of oxygenated groups on the surface of the latter, which results in a better solubility in aqueous media, an easier handling and a richer surface chemistry.1,20 Each GO sheet is roughly composed of two different domains: one hydrophobic region composed by sp2 carbon and another hydrophilic zone, where oxygenated groups are present. Therefore, the typical reactivity of GO combines the “classical” sp2 C chemistry of graphene (1,3-dipolar additions, radical reactions, Diels–Alder reactions, etc.) with the chemistry of the oxygenated functions. Some of these reactions can be performed under mild conditions and are suitable for sensitive biological moieties, such as proteins or genetic material. However, we must keep in mind that many biomolecules adsorb strongly on GO,16 thus it is difficult to distinguish between adsorbed and covalently attached products. The general rationale for multi-functionalization is to use both covalent and non-covalent chemistry. In this case, dispersants or targeting molecules are attached via covalent bond to GO while drug molecules, generally lipophilic, interact with the sheets via π–π stacking or other low polar interactions. The covalent/non-covalent strategy is simple and versatile. In addition, there is often a positive synergistic effect on the drug uptake/release. Moreover, functionalization can increase GO dispersibility in water or in the cell culture media, decreasing its cell/tissue toxicity and inducing accumulation to target cells and tissues. For example, biocompatible polymers (chitosan, polyethylenimine, polyethylene glycol, etc.) have been widely tested. Among the non-covalent approach, doxorubicin is one of the most selected drugs to carry on. Those platforms have been successfully tested for cancer treatments in vitro. Moreover, GO is characterized by the presence of many organic groups on its surface, so that, when planning a reaction with a specific GO functional group, it is necessary to take into account that parallel or undesired reactions may occur, which can drive to false interpretations. For these reasons, control reactions should always be carried out in parallel. As an example, we described an interesting case study related to the carboxylic groups in GO coupling with amino groups, which, for its versatility, is the most performed functionalization with biomolecules in the literature.21 By comparing the products and by different control experiments, we demonstrated that amino functionalization takes place at the epoxide functionality rather than at carboxylates.
The in vivo efficacy of GO platforms is based on passive targeting and, in particular, on enhanced permeability and retention effects, common to several cancer tissues, due to hypervascularization. Unfortunately, passive targeting treatments do not always show encouraging results when the therapy is translated from in vitro to in vivo. Indeed, the in vivo treatment is more complex and the therapy can lose part of its efficacy. In addition, side effects due to unfavorable drug release can negatively alter the whole cure. For these reasons, active targeting therapies are generally preferred, where targeting moieties, such as antibodies, peptides or others are introduced on the GO surface (Fig. 3). Active targeting of GO constructs favors the accumulation of the nanomaterial in the desired tissues, enhancing its therapeutic results while decreasing the side effects.1,20 In addition, to follow the fate of the nanomaterial in the body imaging molecules can be attached to the GO surface. For this purpose, GO can be easily functionalized with organic dyes, luminescent particles or radioactive compounds. However, attention should be paid during these synthetic strategies: GO, as all graphene family members, is an efficient luminescent quencher. This feature may compromise the detection of the complex, so that non-luminescent imaging molecules, such as magnetic probes22 should be favored. The quenching property of GO can be used for feedback imaging, an approach where the luminescence signal can be detected after physical detachment of the dye from GO. This process, well-established for the luminescence-based graphene sensors,23 can be also applied to the preparation of sophisticated theranostic tools. For instance, an in situ feedback imaging system based on GO to detect in vivo apoptosis of cancer cells was recently proposed.24 For this purpose, GO was functionalized with camptothecin (the drug), folic acid (the targeting agent) and a fluorescently tagged peptide corresponding to a native substrate for caspase-3, an enzyme activated in apoptotic mechanism. Folic acid active targeting allowed the accumulation of the functionalized GO into the tumor tissues, where camptothecin is released, caspase-3 is activated, cleaving the tagged peptide from GO and inducing an enhanced fluorescence signal during the following 72 hours. This strategy provides a convenient and reliable method for in situ and real-time monitoring of the therapeutic response in animal models.
Drug release can also be tuned or stimulated by the intracellular environment. Generally, drug release in a cell is due to the change of the environmental conditions between the extracellular matrix and the cytoplasm. Therefore, drug desorption from GO can be activated by lowering the pH: this has been proven for example for doxorubicin that undergoes the protonation of the amino group of the sugar moiety leading to the destabilization of the interactions with the GO sheet. The acidic drug desorption is the prominent releasing process when the GO enters via endocytosis into the cells, where it is digested into lysosomes that have an acidic pH. However, the releasing process from GO could be incomplete or even too slow causing an inefficient effect of the whole therapy. The latest implementation in GO-based drug delivery platforms relates to the use of stimulated drug release, trigged by some external stimuli. The stimulated release is able to increase the effective drug dosage, leading to better therapeutic performances. Moreover, the stimulus is applied locally on the target cells, sensibly decreasing side effects on healthy tissues. Stimulated drug release is generally turned on by the photothermal effect.25 In fact, graphene absorbs light in the near infrared region (NIR) and converts it into heat. The thermal increase, produced inside targeted cells, activates the drug release mechanism. More importantly, heat induces cell ablation combining the advantages of chemo- with photothermal therapy.25 Despite the recent implementation, photothermal chemotherapies are far from being satisfactory. Several safety issues have been raised, due to the local heating, such that oxygenation, blood flow rate, or pH variation need to be fully understood. In addition, especially in solid tumors, the GO delivery is still not sufficiently selective.
As for GO, the combination of the covalent/non-covalent strategy is still the most exploited, allowing the grafting of dispersants, drugs, and targeting molecules. The most explored preparation strategies for an rGO platform are the post-reduction strategy and the in situ reduction/functionalization. The post-reduction approach is the most used for rGO functionalization. In this case, rGO is functionalized with target molecules after the “classical reduction” with hydrazine or ammonia. Dispersant molecules and hydrophilic drugs are usually complexed via non-covalent interaction. However, rGO prepared in this way may induce a certain degree of cytotoxicity. This side effect is mainly attributed to the reduction protocol used and in particular to residual hydrazine or ammonia. The presence of these hydrophilic chemicals can improve the rGO dispersibility in cell culture media, but enhances the toxicity of rGO, thus limiting its application in the biomedical field. To solve these problems, alternative strategies have been pursued to reduce GO. Several studies describe the preparation of rGO using vitamin C, vegetal extracts, peptides, and many more.27 rGO obtained following these “green” protocols exhibits higher biocompatibility.
3.2. Graphene on surface
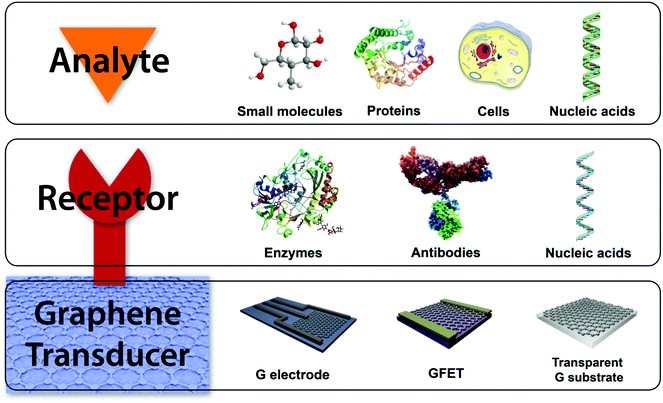
Biosensors are commonly composed of two different elements: a receptor and a transducer (Fig. 4). A receptor is the recognition element usually being a bioactive molecule with specific interaction with the target. The transducer is responsible for converting the chemical information from the recognition event into a measurable signal. However, the receptor is omitted in diverse biosensors, since the direct interaction of the analyte with the transducer produces measurable changes of its properties. Graphene and its related materials can used as the transducing platform in biosensing.4,28 Thus, chemical modification of graphene materials is a mandatory step in the production of biosensors for the attachment of the recognition elements when needed. For this purpose, covalent and non-covalent strategies have been developed, which include amide bonds with carboxylic groups of oxidized graphene materials, the use of nanoparticles, π–π interactions, and the immobilization in biomatrices.16,29 The distinctive properties of the graphene family namely zero band-gap semiconductor, high carrier mobility, quantum hall effect, high light transmittance, and large active surface areas for binding biomolecules, make them hot candidates for components on the surface of electrodes and field effect transistors (FETs) in electrochemical, FET and optical biosensors and for monitoring devices.
Electrochemical biosensors work by direct electron transfer between the sensing platform and the electroactive species, which can be the target molecule itself for the recognition bioelement. The modification of the traditional carbon-based electrodes for electrochemical measurements, e.g. glassy-carbon electrode (GCE), with graphene derivatives offers many advantages due to their structural and electrochemical properties: they allow an increment of the signal-to-noise ratio due to the high surface-to-volume ratio with good electron transfer characteristics, a large electrochemical potential window, high mobility and conductivity, multiple detections because of the attachment of different recognition elements and the possibility of miniaturization of sensing elements. Graphene-based FET (GFET) biosensors generally operate in a solution-gated FET configuration, due to their very stable performance, low working voltage and high transconductance. The sensing mechanism is produced by the change of the electronic properties of GFET induced by the interactions with the target element.4 Besides, another common biosensing approach in biomedical applications is the optical sensing, which is based on changes in the optical properties of the corresponding sensing platform when the recognition of the target molecule occurs.
In the following sections, the preparation of graphene-based sensors for the detection of bioactive molecules will be described with special attention to the role of graphene materials.
The detection of small molecules is very useful for clinical diagnosis. Among all the small targets, glucose is especially emphasized, since it is clinically important for diagnosis and prevention of common chronic diseases, such as diabetes. Glucose detection is usually based on the quantification of H2O2 produced during the enzymatic oxidation of glucose by glucose oxidase in the presence of O2. This subject coupled to the use of graphene-based devices has been recently reviewed in depth.28,30 In general, this quantification is performed by electrochemical sensors. As mentioned above, graphene derivatives are very useful for electrode modification due to their high surface area and electron conductivity, leading to enhanced heterogeneous electron transfer and consequently higher signal intensities. Many examples have been reported in the literature on enzymatic sensors composed by graphene-based electrodes, where glucose oxidase is attached to the graphene surface.28 For instance, the modification of a GCE with nanoparticle-functionalized rGO allows further modification with glucose oxidase, yielding a biosensor with low limit of detection (LOD, 76 μM) and high sensitivity retention. In addition, rGO-modified GCE not only detects glucose when functionalized with glucose oxidase, but can also be used for other recognition elements due to the ability of graphene materials to immobilize molecules. The use of a GO-covered electrode for electrochemical sensing also allows monitoring intracellular glucose.28 The GO surface on a borosilicate glass capillary permitted to attach glucose oxidase through amide bonds, exhibiting linear glucose-dependence with wide concentration range (10–1000 μM).
However, enzymes as glucose oxidase lose activity with changes in pH or temperature. For this reason, an increasing interest is focused on the development of non-enzymatic sensors for glucose detection. In general, the enzymeless glucose sensing devices use metal and metal oxide nanoparticles, loaded on the graphene materials, as components to catalyze the reduction of glucose.3
Small molecules secreted from living cells, related to the cell activity, can also be detected using graphene-based electrodes.3 In particular, the detection and quantification of dopamine and related metabolic derivatives is crucial due to the role that they play in several human diseases and neurological disorders. Various graphene-based sensing methods have been developed for the detection of this neurotransmitter and its metabolic derivatives.28 In this regard, an rGO-based microelectrode array was fabricated on a flexible platform by nanoimprint lithography for detection of dopamine and H2O2.4 This case proves the advantage of using graphene derivatives, since they allow the miniaturization of the device in an array of microelectrodes, which do not alter the sensing properties. The graphene-based array exhibits detection limits of 0.26 μM and 0.35 μM for dopamine and H2O2, respectively.
One of the most relevant examples of graphene biosensors is their use for the detection of nucleic acids.31,32 The interest for such targets has increased exponentially in the past years, because of their significance in gene or pathogen detection and molecular diagnosis. Electrochemical sensors are the most employed sensors for nucleic acid detection.29 Such detection is generally based on the hybridization events where complementary nucleic acids to the target are the recognition element. These devices are very sensitive because the hybridization leads to changes in the electrochemical signal enabled by the easy functionalization of graphene materials with nucleic acids by covalent and non-covalent approaches. This functionalization capability yields to a close interaction between the graphene material and the recognition element, leading to an improved electron transfer. For instance, rGO covalently linked to amino-single-stranded DNA, as the recognition motif on its carboxylic groups, afforded an impedimetric sensor for the detection of amelogenin gene, related to dental diseases. The sensing device showed a wide detection range with a LOD of 3.2 × 10−15 μM and a high specificity as compared to non-complementary DNA strands.
As above-mentioned, the most employed graphene materials as transducing component are oxidized materials due to the large defect degree, since heterogeneous electron transfer occurs at defects and edges of the corresponding material. However, their low conductivity is a disadvantage as electrode component. By combining graphene materials on a surface, it is possible to exceed the intrinsic limitations. As a representative example, an electrochemical biosensor for the detection of human immunodeficiency virus 1 (HIV1) gene was assembled, based on an double layer electrode composed by rGO on a graphene electrode.29 This sensing device achieved, for the detection of the HIV1 gene, a wide detection range (10−1–10−6 μM), a low LOD (1.58 × 10−7 μM) and selectivity in comparison to non-complementary DNA strands. The combination of rGO and graphene in a double-layered electrode showed a good electron transfer activity in comparison to segregated graphene- and rGO-based electrodes. This behavior was attributed to a combination of the large number of electroactive sites in rGO and the highly conductive nature of non-defective graphene.
Graphene is a promising material for fast gene sequencing. The translocation of double-stranded DNA through graphene nanopores was described by different research groups, opening the possibility of DNA sequencing using graphene devices, a topic that has been recently reviewed.33 However, several limitations were found, as for example the fast translocation (that avoids achieving single-base resolution) or clogging effects between DNA and graphene material due to a strong hydrophobic interaction. In this regard, the available functionalizing ability of graphene materials allows reducing the closure of graphene nanopores with DNA by hydrophilic modification with pyrene-ethylene glycol.4 Nevertheless, graphene-based nanopore devices are at an early stage, and some of their fundamental features must be still investigated. Indeed, other 2D materials and device architectures are being proposed, as for example, monolayer dichalcogenides or single-stranded DNA sequencing based on π–π interactions between nucleobases and a nanoribbon deposited on a nanochannel (as demonstrated theoretically).
Electrochemical sensors are also very popular for the detection of macromolecules such as proteins. Usually, these types of sensors are based on immunoassays taking advantage of the intrinsic sensitivity and specificity of the antibody/antigen interaction. Again, in these sensing systems, the advantage of using graphene materials as transducing platforms was evidenced.28 Apart from the improved electron transfer properties in the electrochemical measurements, multiple detections are possible with rGO-modified electrodes. A clear example is the developed electrochemical immunosensor that simultaneously detects two different antigens, the cancer carcinoembryonic marker and the squamous cell carcinoma antigen. The sensor is based on a GCE modified with covalently aminated rGO where the respective antibodies were immobilized.28
Graphene-modified surfaces present a high ability to strongly interact with bacteria and cells as well. Bacteria detection is generally performed by FET or electrochemical sensors, both functionalized with antimicrobial peptides on graphene platforms.3 Regarding cell detection, the strong interaction between cells and graphene surfaces allows the development of highly sensing and isolating devices. Recently, a microfluidic chip based on an optical graphene sensor allowed a leukemia single T-cell detection on real-time, among abundant normal immune cells.3 The sensing mechanism is based on changes of the refractive index when cells are adsorbed on the graphene material. The resulting high sensitivity of the optical sensing is explained in terms of particular optical properties of the ultra-thick rGO. Graphene sensors are also develop to address the problem of detection of circulating cancer cells, which are the responsible for the cancer metastases. For this kind of detection, there is an urgent need for the development of highly sensitive sensors, due to the very low concentrations of circulating tumor cells in real samples. An illustrative example of graphene-modified surfaces in sensing is a developed microfluidic device for detection and isolation of diverse circulating tumor cells from blood samples of pancreatic, breast, and lung cancer patients.16 The chip was composed by non-covalently functionalized GO with an aminated phospholipid derivative on flower-like gold patterns, where the corresponding specific antibodies where chemically attached. The GO/Au device showed an excellent sensitivity (3–5 cells per mL of blood) for the tested circulating cancer cells and was even able to capture them at low concentrations. The reported capture yields and detection sensitivities were much higher than the ones reported in the literature. It can be anticipated that this kind of graphene devices are paving the way for next-generation flexible implants for the in vivo analysis of cells and the identification of cancer cells.
Biocompatibility is a particularly important subject for a sensing device, because non-biocompatible devices can lead to rejection from the user, as well as degradation of the implant.6 The modification of device surfaces with graphene materials can enhance their biocompatibility. Especially, oxidized graphene materials are favorable for biocompatible implants due to their hydrophilicity. In addition, graphene functionalization can modulate biocompatibility.
Another challenge for body implants is their miniaturization. The use of graphene materials allows the reduction of electronic components to the nanoscale. A representative example of this advance on graphene sensing devices, together with good biocompatibility, is the recently reported wearable and wireless nanobiosensor for bacteria detection in saliva.3 This sensing device consisted of graphene-based electrodes and a printed antenna onto a biocompatible silk thin-film substrate, which allowed the adhesion on irregular tooth surface. The capture of bacteria by antimicrobial peptides on the graphene surface resulted in a conductivity change of the graphene film, which was wirelessly monitored by radio frequency. By assembling antimicrobial peptides on CVD graphene, this wearable sensor successfully detected around 100 Helicobacter pylori bacteria in 1 mL of human saliva. Therefore, the graphene device provided sensitive and specific detection of bacteria on biosurfaces, which can be used in diagnosis for continuously detecting bacterial infection through a non-invasive modality.
The mechanical properties of graphene materials are also fundamental for constructing body implants. This is clearly obvious in another main application of graphene-modified surfaces, related to the field of the cell and tissue monitoring by using FETs and electrodes. Metal and silicon-based devices are widely used to manufacture conventional implants. However, they have two significant limitations: (i) no flexibility, and (ii) poor chemical stability in the biological environment. Due to these factors, graphene-modified electrodes and the above-mentioned GFETs exhibit a high potential for recording the electrical activity of neuronal and cardiac cells and tissues, because of the possibility of constructing flexible electrodes, which is an important property for implants on soft tissues, in addition to the mentioned strong interaction of graphene with cells and their high biological stability.
For instance, the use of graphene derivatives allows to prepare flexible electrode microprobes, which can record electrophysiological signals for the very soft neuronal and cardiac tissues.3 There is a representative example of the modified flexible microprobe with CVD graphene, which could record electrocardiograms from the heart of an animal. The graphene-based probe has shown a high performance and good stability with no changes after use. Moreover, the hydrophilic version of this graphene device presented a higher level of sensitivity than the hydrophobic one, probably due to a better biocompatibility.
In addition, several research groups have developed GFET devices to study the electrical interaction with diverse cells, paving the way to future sensing implants.3 For instance, arrays of solution-gated FET composed by large areas of CVD graphene were used to record signals from human embryonic kidney cells. Besides, they can also quantify the release of ions by human embryonic kidney cells onto graphene transistors, helping to understand the cell–transistor coupling. More recently, GFET array on a flexible and biocompatible substrate was used to monitor variations in the electrical membrane potential of cardiac muscle cells with excellent signal-to-noise ratio and with no degradation after repeated bending.34
Graphene derivatives can also provide extraordinary optical properties, as high transparency, to sensing implants.4 For instance, the combination of the broadband transparency, good electronic conductivity and flexibility of CVD graphene with a carbon-based electrode array resulted in an efficient multifunctional neural implant on a rat brain. This implantable device could perform in vivo imaging under light stimulation, together with recording neural signal.
3.3. Graphene scaffolds for biomedical applications
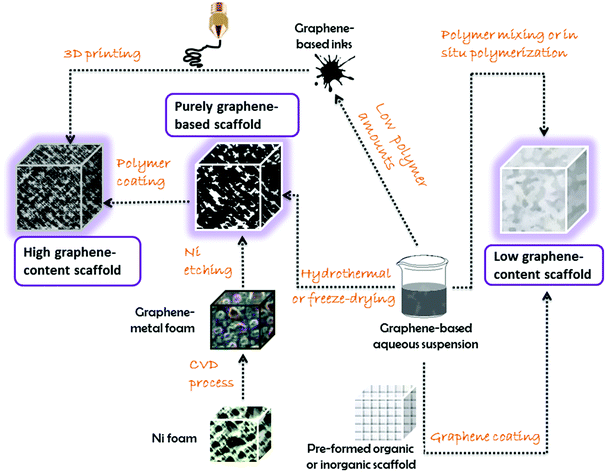
In the next sections, we will discuss the approaches according to the graphene content, resulting in: (i) nanocomposites with graphene as the minor component; (ii) nanocomposites with graphene as the major component; and (iii) only-graphene-based scaffolds.
The scaffolds described so far in the literature mostly involve gels, or cross-linked hydrogels (from which it is possible to create aerogels after removing water by freeze-drying).35,37Fig. 5 shows a general scheme of the most common trends in three-dimensional graphene-based scaffolds. These morphologies have shown truly unparalleled results and good biocompatibility, offering promising prospects in the biomedical field.
The role of the nanomaterial within the polymer network is primarily intended for reinforcing the matrix (i.e., increasing the stiffness and toughness), whose intrinsic mechanical properties are often below the requirements for medical applications,35,37 in particular when molecular loading is involved. Several examples have been designed including the radical polymerization and cross-linking of acrylic monomers to generate three-dimensional reinforced hydrogel scaffolds, suitable for medical purposes, as these are well-known for their biocompatibility and similarity to soft tissues. The most representative examples of such cross-linked hydrogels in medicine are poly(methacrylic acid), poly(acrylamide) or poly(N-isopropylacrylamide).35,37 Since the synthesis of cross-linked hydrogels is usually carried out in aqueous environment, the achievement of graphene materials stably suspended in water becomes a crucial task, if this nanomaterial is to be mixed with the matrix during the polymerization process. The graphene content in the final nanocomposite is tailored by its concentration in the polymerization medium, generally providing better mechanical consistence and higher electrical and thermal conductivities to the system than its blank counterpart. Additionally, these nanostructures may also bring along unanticipated features, such as a smart behavior based on improved responsiveness to external stimuli, enabling novel approaches in nanomedicine, as is the case of on-demand drug delivery.3,5 An illustrative case consists in the preparation of graphene/poly(methacrylic acid) nanocomposites by the in situ polymerization strategy, with water-suspended graphene from the ball milling technique (at concentrations from 0.05 to 0.2 mg mL−1).38 These scaffolds showed an unprecedented ability to release a drug upon pulsatile electrical stimulation, with an excellent control on the delivery of the drug, keeping its structural integrity, unlike the non-reinforced parent matrix. The positive effect of graphene was also evidenced in the in vivo performances of these electroresponsive scaffolds, as the nanomaterial can suppress the resistive heating effects of the electrical pulses and grant biocompatibility with minimum tissue damage.38 This example clearly shows how small amounts of graphene are able to exert a prominent stimuli-response capability, by improving the intrinsic effect of the matrix, keeping its structural shape and consistence, and eliminating unwanted side effects. This kind of three-dimensional scaffolds can be envisioned for many types of therapeutic requirements, such as those in chronic illnesses needing a precise and tailored dosage pattern.38
It is worth noting that the combination of polymers with small amounts of graphene is not the only way to achieve this kind of three-dimensional architectures. Another commonly employed method is the deposition of graphene derivatives from a liquid suspension over a preformed scaffold, without compromising its structure and porosity.3,35,36 This is the case of many examples reported for collagen, hyaluronic acid or hydroxyapatite scaffolds subjected to immersion or dip-coating in GO or rGO suspensions.36 The native scaffolds have already shown for years to have a proven osteogenic effect, but their mechanical and surface properties could be further improved, so that these graphene-based coatings provide a step ahead. These nanocomposite scaffolds have been tested both in vitro and in vivo, exhibiting osteogenic differentiation of human mesenchymal stem cells (hMSC) in spontaneous and/or accelerated mode.36
Chemical modification of graphene-based nanostructures can be also useful to link growth factors or other osteoinductive biomolecules in order to finely tune cell differentiation.36
The case of pristine (non-oxidized) graphene three-dimensional scaffolds represents a quite different approach. One of the most followed trends is to use a disposable template from which building up the graphene scaffold, followed by the removal of the template. Many research groups have used Ni foams as templates since they are affordable and also catalyze the subsequent growth of graphene sheets by the CVD technique, mostly using methane as the carbon source.35 The Ni template can be etched away with strong acids (e.g., HCl, HNO3) yielding a graphene three-dimensional porous scaffold, exhibiting high electrical conductivity due to the lack of structural defects and the low contact resistance at the sheet junctions.35
An application is represented by the recent use of Ni-templated CVD-grown graphene scaffolds for the in vitro culture of microglia and neuronal stem cells, where the medium produced by microglia within those scaffolds could promote the migration of stem cells.40 The relevance of these results relies on the fact that graphene substrates with identical surface chemistry but built in a two-dimensional fashion did not show any of these effects on neuronal stem cells,40 emphasizing the critical role that the morphology, dimensionality, accessible surface and porosity of the scaffold have on the eventual medical application.
Another interesting example is represented by a copolymer made of poly(lactic acid) and poly-ε-caprolactone, dip-coated into a Ni-templated CVD-grown graphene foam. The resulting nanocomposite, with a uniform coating of the polymer phase, was used for in vitro hMSCs culture over a period of 28 days, while the results were compared to a control scaffold based on the pure (uncoated) graphene scaffold counterpart.41 Both kinds of scaffolds succeeded in the survival and proliferation of hMSCs, but, surprisingly, the uncoated graphene foam showed abnormal cell growth behavior (with a deformed and highly elongated shape) while in the polymer nanocomposite scaffold hMSCs grew normally, keeping their original morphology.41 The authors ascribed these phenomena to the differences in mechanical strength of the “graphene-only” scaffold vs. the polymer coated one, since the polymer acts as a reinforcement of the structure providing improved resistance in both compression and tension modes, at the nanoscale level and in macroscopic tests.
However, the classical approach of mixing dissolved/molten polymers (or polymerizing in situ) with powdery graphene materials has also been explored for three-dimensional polymer nanocomposites with high content in graphene,37 and those can be also applied as scaffolds for nanomedicine purposes. By controlling the rheological properties of the blends (i.e., through the polymer/graphene ratio, the solvent amount, the mixing methodology, etc.), it is possible to design gels and inks with unique processability. A particularly appealing demonstration of this approach has been reported.42 In this work, the authors fabricated a graphene ink by solution mixing with a biocompatible polymer (polylactide-co-glycolide) with a ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 wt% (graphene
25 wt% (graphene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer). This ink possesses the ideal properties to be extruded through a 3D-printing device, leading to three-dimensional honeycomb graphene scaffolds, capable of reaching the centimeter scale in a relatively easy and scalable manner. These scaffolds present enhanced electrical conductivity, mechanical flexibility, and are also biodegradable. These 3D-printed graphene scaffolds granted the viable growth and proliferation of several cell types, including adult hMSCs, which developed into neuron-like shapes. Additional in vivo tests after subcutaneous administration in mice demonstrated a good biocompatibility with no disintegration of the scaffold or migration of graphene to the main organs. More interestingly, these scaffolds have confirmed practical application in neurosurgery, where the composite could be successfully cut and sutured to a nerve bundle in a simulated chirurgical intervention to a human corpse, which provided higher expectations towards a feasible medical application. This example displays how the revolutionary application of cutting-edge processing techniques, such as 3D-printing, represents a step forward in the building of graphene-based three-dimensional scaffolds.
polymer). This ink possesses the ideal properties to be extruded through a 3D-printing device, leading to three-dimensional honeycomb graphene scaffolds, capable of reaching the centimeter scale in a relatively easy and scalable manner. These scaffolds present enhanced electrical conductivity, mechanical flexibility, and are also biodegradable. These 3D-printed graphene scaffolds granted the viable growth and proliferation of several cell types, including adult hMSCs, which developed into neuron-like shapes. Additional in vivo tests after subcutaneous administration in mice demonstrated a good biocompatibility with no disintegration of the scaffold or migration of graphene to the main organs. More interestingly, these scaffolds have confirmed practical application in neurosurgery, where the composite could be successfully cut and sutured to a nerve bundle in a simulated chirurgical intervention to a human corpse, which provided higher expectations towards a feasible medical application. This example displays how the revolutionary application of cutting-edge processing techniques, such as 3D-printing, represents a step forward in the building of graphene-based three-dimensional scaffolds.
3.4. Considerations on the impact of graphenes in the biomedical fields
Before the translation of graphene materials into clinical uses, the assessment of their safety is of paramount importance.43 Most of recent work shows negligible toxic effects of graphene materials. For instance, in vitro tests on well-established cell lines displayed a general good biocompatibility of graphenes. Unfortunately, simple cytotoxicity tests do not satisfy the strict screening criteria for translation of a new nanomaterials into valuable clinical uses. The European Scientific Committee on Emerging and Newly Identified Health Risks has recently included graphene in the category of hazard substances.44 Indeed, some of the available results indicate that graphene may cause adverse environmental and health effects, although we still have to fill in many gaps on risk-related knowledge. Evaluating the toxicity of graphene is a rather difficult task. Compared with simple molecules, where the main toxicological criteria to consider are concentration and exposure type and time, nanomaterials require the evaluation of a higher number of parameters including size, shape and agglomeration state. In case of the graphene family nanomaterials, the safety evaluation is even more complex, due to the fact that we have to deal with materials that differ also in atomic composition (graphene, GO or rGO). For this reason, there is not a unique answer to graphene toxicity but rather a more complex scenario. There is still a limited amount of data in the literature on the graphene impact on health. For the sake of clarity, in this paragraph we will try to summarize the main safety parameters that need to be addressed. First of all, the field of application must be considered. Indeed, toxicological tests should take into account the real conditions (i.e. inhalation vs. skin contact, low vs. high dose exposures) by which graphene materials come into contact with a living organism. This latter is necessary to understand the risk-to-benefit balance of a graphene-based therapy. Sensing applications require a relatively low quantity of graphene that is generally present as a single-layer on an electrode surface. In this case the assessment of the toxicity issues covers the interaction of graphene devices with cells and tissues, while longtime exposure must be investigated to avoid chronic inflammations. Applications in drug delivery and tissue engineering necessitate the use of higher quantities of material. Here, the concentration issue is more relevant. In particular, an ideal drug delivery system should guarantee an efficient delivery of the therapeutic molecules and a subsequent elimination/degradation of the material from the body. For tissue engineering instead, graphene materials integrated into a scaffold may remain for long time in the body, so long-term toxicity studies are necessary. In addition, the synthetic production methods must be carefully scrutinized, as most of the discrepant results obtained on toxicity tests can be attributed to an inefficient purification protocol.43 In most cases, the toxic response was not attributed to the graphene material but to the contaminants present in the sample.43 For instance, CVD graphene can be produced easily without the presence of functional groups but toxic metal ions may contaminate the film during the step of graphene transfer from the support. Similarly, top down preparation strategies require rigorous cleaning processes. Graphene exfoliation deals with the use of amphiphilic molecules that, if not carefully removed, may cause tissue inflammation. Modified Hummers' methods used for the preparation of GO are based on the use of manganese that may result highly toxic to cells if not completely removed. For this reason, nowadays more and more graphene material producers tailor their synthetic/purification protocols to produce “biograde” graphene materials. As we have already mentioned previously, this definition should be carefully considered since there is not a standard approved protocol that can assess the graphene “biograde” yet. In addition there is no clear knowledge of the nature of the possible contaminants (i.e. heavy metals, residual graphite, endotoxins, etc.). Morphological characteristics of the materials play also a fundamental role in influencing the toxic effects. For example, comparing GO to carbon nanotubes, it was shown that they display different toxicity for neurons.45 This effect was attributed mainly to the different shape of the two types of carbon nanomaterials.45 Size and agglomeration of graphene materials must be also taken into consideration. Indeed, the lateral size of graphene flakes can span from few nanometer up to microns. In addition, non-functionalized graphene tends to form strong multilayered aggregates while GO and rGO are generally present in single- or few-layers. About the impact of lateral size, in vivo studies using different graphene forms have highlighted that the lung is the organ with the highest risk of damage.46 In this case, larger flakes seem to be more toxic than smaller flakes.43 The accumulation of graphene materials with large size (>100 nm) into lungs resulted independent from the administration routes, mainly leading to inflammatory responses.44 On the other hand, a recent study demonstrated that intravenous administration of radiolabelled GO flakes led to significant urinary excretion.47 Studies on biodistribution, accumulation and elimination of the different types of graphene are still scarce. The published work mainly concerns GO and does not attempt to compare the effects of the different characteristics of the nanomaterials, like lateral size, oxygen content, number of layers. However, these preliminary data on how graphene nanomaterials interact with the different organs are very important to assess their safety profile and eventually for their development toward future biomedical applications. These studies certainly need more effort and should be also combined with those proving in vivo biodegradability.Among the structural characteristics of graphenes, it was reported that size is relevant on the internalization mechanism into the cells. Indeed, studies on macrophages pointed out that the intracellular localization of GO was dictated by size, thus leading to different compartmentalizations.48 Also, bigger GO flakes induced a much stronger inflammatory response with high release of pro-inflammatory cytokines.43 Less investigations have been reported on the change of agglomeration state (single-layer vs. flew-layer vs. multi-layer graphene). The oxidation state and in general surface chemistry are additional parameters that need consideration. Graphene materials are characterized by a large and complex surface area. The surface is the most important part of the flakes. In fact, the various interactions between the sheets and the small molecules, proteins or cells, are mediated by surface interactions. Graphenes with low polar surface showed a general low stability with a certain tendency to agglomerate. In addition, an apolar surface strongly adsorb less polar moieties such as hydrophobic proteins or other lipophilic molecules present in the extracellular matrix.48 In particular, as for many other nanoparticles, once in contact with biological fluids, the hydrophobic surface of graphene can strongly adsorb proteins forming a corona, which stabilizes the flakes in solution avoiding precipitation. However adverse interactions also occur affecting biodistribution or cells interaction. In particular, in the case of proteins of the complement system, acute inflammations may occur.48 This behavior is typical for graphene and some rGO. On the other hand, polar surface graphenes are characterized by a high colloidal stability in different aqueous media.48 Polar GO shows a higher influence on hemolytic activity leading to a potential thrombotoxicity.48 These results are in contrast with other studies that report almost no hemolysis after GO injection.49 Interestingly, it was shown that GO biodegradation can be modulated by dispersibility.50 In this study is has been proven that GO can be digested by peroxidases naturally present in cells. The biodegradation of graphene materials can avoid bioaccumulation thus limiting its long-term toxicity. Moreover, studies from different groups incontrovertibly demonstrated that most of the already mentioned clinical side effects may be sensibly reduced or avoided by surface functionalization.46,48,51 Summarizing, graphene toxicity still needs a thorough evaluation. The current data are still controversial. This is particularly due to the variability and inhomogeneity to the graphene samples. Most of the drawback of graphene-based therapy could be alleviated by chemical surface manipulations. A standardization of the graphene materials used is finally necessary to better address and answer to all toxicological issues.
4. Conclusions and guidelines
The biomedical applications of graphene represent a field in continuous expansion. Basic investigations demonstrate the high potential of graphene and its derivatives in many important applications, including drug delivery, tissue engineering and sensing. However, the scarce synthetic control, the lack of reproducibility and the difficult characterization render these materials weak candidates for real world applications. In fact, nanomaterial-based devices for medical use require approval by the regulatory authorities, often concerned with the potential toxicity of the new materials. In relation to the toxicity of nanomaterials, some authors have already described more than ten key factors that should be considered to define its safety grade.52 However, since the nanomaterial can change its nature during the biological tests, its physicochemical characterization should be carried out under similar experimental conditions, thus making this task even more complex. For this reason, in order to generate a standardized way of comparing characterization and results, an agreement should be reached on which crucial factors have to be considered.8 In this direction, there are some recent efforts by scientists working in nanoinformatics focused on establishing the minimal information about nanomaterial characterization.53 However as commented above, standards may also include information concerning processing of the material and the biological test already accomplished.Regarding graphene materials, there are already some physicochemical factors considered essential in every characterization: the oxygen content, the lateral size, and the number of layers.54 Nevertheless, there is still no clear conclusion on the differences in biological behavior between large and small sheets, single-, few- and multi-layer graphene samples, while the amount of oxygen can also provoke undesired effects. As stated earlier, achieving an aqueous dispersion of graphene-based nanostructures is crucial to advance in any targeted biomedical applications. GO conjugates have an easier handling in aqueous media, while pristine (non-oxidized) graphene requires the use of external chemical species to obtain stable sheets in suspension. Regardless of their intrinsic biocompatibility, in order to study the true role of graphene, these dispersion agents should be perfectly quantified, trying to minimize their possible biological effects.
Some other relevant compositional features are often ignored, such as the presence of trace of impurities, which, sometimes, can be the sole responsible of the toxicity in a graphene-based sample.55 Metals require special attention since their toxic effects may be more acute even in very tiny amounts. Some graphene production methods include the use of Ni templates or Mn species (Hummers method), and some laboratory hardware (e.g. steel sonic tips, steel milling balls) are possible sources of metal contaminations, albeit dependent on the preparation conditions.
The colloidal stability is also a critical parameter that must be taken into account when running biological experiments, since the interactions of cells with the graphene environment could change whether in a freshly-prepared suspension or after hours or days or months of preparation. For any particular application, the experimentalists should run control dispersions of graphene materials in the aqueous/liquid media of interest and continuously monitor the evolution (sedimentation) profile by means of microscopic and/or spectroscopic techniques.14 The structural characteristics of graphene at the first minutes after dispersion will certainly not be the same after a couple of hours, neither in terms of a day or more, so it is important to perfectly know what we have in suspension in a given moment and translate this knowledge to the biological experiment.
Based on the above considerations, the following information is intended to be guidelines for the development of a master file for the biocompatibility evaluation of graphene materials. Physicochemical parameters are considered and associated to the most useful characterization techniques for each case. The adoption of this kind of standardized data format, when reporting, could help to better compare the different graphene materials in order to clearly identify relationships between structures and properties, favoring the development of predictive models and expanding our knowledge about relation between bio- and nanoworld, clearing the way for clinical trials.
• Material name: accepted nomenclature (GO, rGO, FLG, graphene quantum dots, etc.)7
• Physicochemical characterization (performed for the as synthesized graphene and in biological conditions; for example size distribution should be performed in cell culture media or in biological buffers).
∘ C/O ratio (techniques: XPS, elemental analysis)
∘ Surface modification/functionalization related to surface crystallinity. Possible reactivity during storage and/or under biological conditions (techniques: XPS, elemental analysis, Raman, TGA, dynamic light scattering, zeta potential)
∘ Metal traces (techniques: X-ray electron diffraction, total reflection X-ray fluorescence, atomic adsorption, inductively coupled plasma-mass spectrometry)
∘ Size: lateral dimensions and area distribution (techniques: TEM, SEM, AFM)
∘ Number of layers (techniques: TEM; Raman, AFM)
∘ Surface area (techniques: Brunauer–Emmett–Teller analysis)
∘ Surface charge in biological conditions (zeta potential must be reported in the biological media or at the pH used for a given analysis)
• Manufacturing information
Method of preparation, starting products, reagents, conditions, knowledge of suspected impurities.
• Recommended processing methods
∘ Conditions for safe storage
∘ Dispersion agents (quantification and physicochemical characterization)
∘ Dispersion process (i.e. sonication time) and colloidal stability at short and long times
∘ Sterilization compatibility: demonstration that the sterilization process does not change the final properties of the graphene material
• Biocompatibility tests already performed with this material: these tests should provide a complete description of the method used, including interpretation and impact of their results. All biological tests should be referred to a standard reference of known activity in those tests.
∘ Cytotoxicity, genotoxicity, biodegradation, distribution and accumulation into organs, metabolism
Apart from a thorough characterization and a regulatory standardization of the graphene materials, it is necessary to remember that approval of a drug is an extremely long and selective process. In this context, we think that most of the attention should be paid to the synthetic and purification protocols. Top down methods produce materials with an intrinsic high polydispersity. Despite their successful use in the research, low quality produced nanosheets will never be approved as a drug. As for other 2D materials, the purification of graphene flakes with a controlled and uniform lateral size has not been yet achieved, so more work should be addressed in this direction. This scenario is even more random in the case of GO/rGO where exfoliation of graphite is mediated by oxidation. We deal with a growing family of Hummers' modified methods that lack standardization and of course lead to the production of a great variety of GOs. In the latter case, the surface chemistry is not at all controllable and most of the therapeutic effect/drawbacks may heavily depend on the amount and nature of the oxygenated species. The promising results obtained in drug delivery using functionalized GO/rGO platforms do not solve the problem of the intrinsic inhomogeneity of these materials, which without a standardization of the synthetic procedure, can hardly reach serious clinical consideration. Bottom up techniques, which allow the production of high quality almost monodisperse materials, have led so far to only limited amounts of materials. Graphene nanoribbons seem to be a valid alternative to graphene as the material has a controllable lateral size and surface chemistry. However, their production is still limited to very small amounts.
The road of graphene in biomedical applications is therefore very long and winding. There is still a lot to do before we can use graphene in biomedicine, but the enthusiasm of the scientific community is providing a host of very interesting breakthroughs, which place graphene in pole position for innovative diagnosis and therapy.
Acknowledgements
This work was partly supported by the Centre National de la Recherche Scientique (CNRS) by the Agence Nationale de la Recherche (ANR) through the LabEx project Chemistry of Complex Systems (ANR-10-LABX-0026_CSC) (to A. B.), by the Spanish Ministry of Economy and Competitiveness MINECO (CTQ2014-53600-R and CTQ2016-76721-R) and by the International Center for Frontier Research in Chemistry (icFRC). M. P. is the recipient of the AXA Chair (2016-2023). The authors gratefully acknowledge financial support from EU H2020-Adhoc-2014-20 Graphene Core1 (no. 696656), and from ANR (ANR-15-GRFL-0001-05). M. P. was also supported by Diputación Foral de Gipuzkoa program Red (101/16). Dr J. M. González-Domínguez greatly acknowledges MINECO for his researcher grant (Formación Postdoctoral).References
- A. Servant, A. Bianco, M. Prato and K. Kostarelos, Bioorg. Med. Chem. Lett., 2014, 24, 1638–1649 CrossRef CAS PubMed.
- S. S. Nanda, G. C. Papaefthymiou and D. K. Yi, Crit. Rev. Solid State Mater. Sci., 2015, 40, 291–315 CrossRef CAS.
- C. Cheng, S. Li, A. Thomas, N. A. Kotov and R. Haag, Chem. Rev., 2017, 117, 1826–1914 CrossRef CAS PubMed.
- P. Kang, M. C. Wang and S. Nam, Microelectron. Eng., 2016, 161, 18–35 CrossRef CAS.
- S. Merino, C. Martín, K. Kostarelos, M. Prato and E. Vázquez, ACS Nano, 2015, 9, 4686–4697 CrossRef CAS PubMed.
- H. S. Dong and S. J. Qi, Biosurf. Biotribol., 2015, 1, 229–248 CrossRef.
- A. Bianco, Carbon, 2013, 65, 1–6 CrossRef CAS.
- M. Björnmalm, M. Faria and F. Caruso, J. Am. Chem. Soc., 2016, 138, 13449–13456 CrossRef PubMed.
- U.S. Food & Drug Premarket Approval (PMA), http://www.fda.cov/medicaldevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketApprovalPMA/ucm142714.htm, accessed May 2017.
- M. Yagmurcukardes, F. M. Peeters, R. T. Senger and H. Sahin, Appl. Phys. Rev., 2016, 3, 41302 Search PubMed.
- M. Yi and Z. Shen, J. Mater. Chem. A, 2015, 3, 11700–11715 CAS.
- J. Texter, Curr. Opin. Colloid Interface Sci., 2014, 19, 163–174 CrossRef CAS.
- J. I. Paredes and S. Villar-Rodil, Nanoscale, 2016, 8, 15389–15413 RSC.
- V. León, J. M. González-Domínguez, J. L. G. Fierro, M. Prato and E. Vázquez, Nanoscale, 2016, 8, 14548–14555 RSC.
- G. Bepete, E. Anglaret, L. Ortolani, V. Morandi, K. Huang, A. Pénicaud and C. Drummond, Nat. Chem., 2017, 9, 347–352 CrossRef CAS PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- J. Russier, V. León, M. Orecchioni, E. Hirata, P. Virdis, C. Fozza, F. Sgarrella, G. Cuniberti, M. Prato, E. Vázquez, A. Bianco and L. G. Delogu, Angew. Chem., Int. Ed., 2017, 56, 3014–3019 CrossRef CAS PubMed.
- A. M. Dimiev and J. M. Tour, ACS Nano, 2014, 8, 3060–3068 CrossRef CAS PubMed.
- K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530–547 RSC.
- S. Makharza, G. Cirillo, A. Bachmatiuk, I. Ibrahim, N. Ioannides, B. Trzebicka, S. Hampel and M. H. Rümmeli, J. Nanopart. Res., 2013, 15, 2099 CrossRef.
- I. A. Vacchi, C. Spinato, J. Raya, A. Bianco and C. Ménard-Moyon, Nanoscale, 2016, 8, 13714–13721 RSC.
- L. Guo, H. Shi, H. Wu, Y. Zhang, X. Wang, D. Wu, L. An and S. Yang, Carbon, 2016, 107, 87–99 CrossRef CAS.
- Z. Li, M. He, D. Xu and Z. Liu, J. Photochem. Photobiol., C, 2014, 18, 1–17 CrossRef CAS.
- J. Tian, Y. Luo, L. Huang, Y. Feng, H. Ju and B.-Y. Yu, Biosens. Bioelectron., 2016, 80, 519–524 CrossRef CAS PubMed.
- Y.-W. Chen, Y.-L. Su, S.-H. Hu and S.-Y. Chen, Adv. Drug Delivery Rev., 2016, 105, 190–204 CrossRef CAS PubMed.
- G. Shim, M.-G. Kim, J. Y. Park and Y.-K. Oh, Adv. Drug Delivery Rev., 2016, 105, 205–227 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- J. N. Tiwari, V. Vij, K. C. Kemp and K. S. Kim, ACS Nano, 2016, 10, 46–80 CrossRef CAS PubMed.
- A. Ambrosi, C. K. Chua, N. M. Latiff, A. H. Loo, C. H. A. Wong, A. Y. S. Eng, A. Bonanni and M. Pumera, Chem. Soc. Rev., 2016, 45, 2458–2493 RSC.
- S. Viswanathan, T. N. Narayanan, K. Aran, K. D. Fink, J. Paredes, P. M. Ajayan, S. Filipek, P. Miszta, H. C. Tekin, F. Inci, U. Demirci, P. Li, K. I. Bolotin, D. Liepmann and V. Renugopalakrishanan, Mater. Today, 2015, 18, 513–522 CrossRef CAS.
- R. M. Graybill and R. C. Bailey, Anal. Chem., 2016, 88, 431–450 CrossRef CAS PubMed.
- S. Ge, F. Lan, F. Yu and J. Yu, New J. Chem., 2015, 39, 2380–2395 RSC.
- S. J. Heerema and C. Dekker, Nat. Nanotechnol., 2016, 11, 127–136 CrossRef CAS PubMed.
- B. M. Blaschke, M. Lottner, S. Drieschner, A. Bonacini Calia, K. Stoiber, L. Rousseau, G. Lissourges and J. A. Garrido, 2D Mater., 2016, 3, 25007 CrossRef.
- H.-P. Cong, J.-F. Chen and S.-H. Yu, Chem. Soc. Rev., 2014, 43, 7295–7325 RSC.
- N. Shadjou and M. Hasanzadeh, J. Biomed. Mater. Res., Part A, 2016, 104, 1250–1275 CrossRef CAS PubMed.
- M. Wang, X. Duan, Y. Xu and X. Duan, ACS Nano, 2016, 10, 7231–7247 CrossRef CAS PubMed.
- A. Servant, V. Leon, D. Jasim, L. Methven, P. Limousin, E. V. Fernandez-Pacheco, M. Prato and K. Kostarelos, Adv. Healthcare Mater., 2014, 3, 1334–1343 CrossRef CAS PubMed.
- M. C. Serrano, J. Patiño, C. García-Rama, M. L. Ferrer, J. L. G. Fierro, A. Tamayo, J. E. Collazos-Castro, F. del Monte and M. C. Gutiérrez, J. Mater. Chem. B, 2014, 2, 5698–5706 RSC.
- Z. Jiang, Q. Song, M. Tang, L. Yang, Y. Cheng, M. Zhang, D. Xu and G. Cheng, ACS Appl. Mater. Interfaces, 2016, 8, 25069–25077 CAS.
- A. Nieto, R. Dua, C. Zhang, B. Boesl, S. Ramaswamy and A. Agarwal, Adv. Funct. Mater., 2015, 25, 3916–3924 CrossRef CAS.
- A. E. Jakus, E. B. Secor, A. L. Rutz, S. W. Jordan, M. C. Hersam and R. N. Shah, ACS Nano, 2015, 9, 4636–4648 CrossRef CAS PubMed.
- A. Bianco, Angew. Chem., Int. Ed., 2013, 52, 4986–4997 CrossRef CAS PubMed.
- A. Bianco and M. Prato, 2D Mater., 2015, 2, 30201 CrossRef.
- Y. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 4, 3181–3186 CrossRef CAS PubMed.
- L. Ou, B. Song, H. Liang, J. Liu, X. Feng, B. Deng, T. Sun and L. Shao, Part. Fibre Toxicol., 2016, 13, 57 CrossRef PubMed.
- D. A. Jasim, C. Ménard-Moyon, D. Bégin, A. Bianco and K. Kostarelos, Chem. Sci., 2015, 6, 3952–3964 RSC.
- C. McCallion, J. Burthem, K. Rees-Unwin, A. Golovanov and A. Pluen, Eur. J. Pharm. Biopharm., 2016, 104, 235–250 CrossRef CAS PubMed.
- A. Sasidharan, L. S. Panchakarla, A. R. Sadanandan, A. Ashokan, P. Chandran, C. M. Girish, D. Menon, S. V. Nair, C. N. R. Rao and M. Koyakutty, Small, 2012, 8, 1251–1263 CrossRef CAS PubMed.
- R. Kurapati, J. Russier, M. A. Squillaci, E. Treossi, C. Ménard-Moyon, A. E. Del Rio-Castillo, E. Vazquez, P. Samorì, V. Palermo and A. Bianco, Small, 2015, 11, 3985–3994 CrossRef CAS PubMed.
- K. Yang, L. Feng and Z. Liu, Adv. Drug Delivery Rev., 2016, 105, 228–241 CrossRef CAS PubMed.
- L. Yan, F. Zhao, S. Li, Z. Hu and Y. Zhao, Nanoscale, 2011, 3, 362–382 RSC.
- K. C. Mills, D. Murry, K. A. Guzan and M. L. Ostraat, J. Nanopart. Res., 2014, 16, 2219 CrossRef.
- P. Wick, A. E. Louw-Gaume, M. Kucki, H. F. Krug, K. Kostarelos, B. Fadeel, K. A. Dawson, A. Salvati, E. Vázquez, L. Ballerini, M. Tretiach, F. Benfenati, E. Flahaut, L. Gauthier, M. Prato and A. Bianco, Angew. Chem., Int. Ed., 2014, 53, 7714–7718 CrossRef CAS PubMed.
- G. P. Nichols, Trends Nanotechnol. Mater. Sci., 2016, 1, 1–3 Search PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |