Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b‘]dithiophene: a novel building-block for a planar copolymer†
Ashok
Keerthi
a,
Cunbin
An
a,
Mengmeng
Li
a,
Tomasz
Marszalek
a,
Antonio Gaetano
Ricciardulli
a,
Boya
Radha
b,
Fares D.
Alsewailem
c,
Klaus
Müllen
a and
Martin
Baumgarten
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: martin.baumgarten@mpip-mainz.mpg.de
bSchool of Physics and Astronomy, Condensed Matter Physics Group, the University of Manchester, M13 9PL Manchester, UK
cPetrochemical Research Institute, King Abdulaziz City of Science and Technology, PO Box 6086, Riyadh 11442, Saudi Arabia
First published on 26th January 2016
Abstract
A planar heteroacene building block, dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;3,4-b′]dithiophene (DTmBDT), is reported via a facile synthetic procedure. Single-crystal X-ray diffraction of Br2-DTmBDT reveals that dodecyl chains interdigitate, still enabling close π-stacking of 3.42 Å. A very high molecular weight quasi-planar copolymer PDTmBDT-DPP exhibited a high hole mobility of 0.36 cm2 V−1 s−1 in preliminary studies of organic field-effect transistors.
Conjugated molecules have become popular in the recent years for their applications mainly in organic electronics1 [organic light emitting diodes (OLEDs), organic field-effect transistors (OFETs), and organic photovoltaics (OPVs)] due to their tunable structure, solution processability and thermal stability along with their advantages of flexibility, lightweight, and low-cost for use in large-area displays, sensors, detectors, and light harvesting devices.2 In the recent years, there has been tremendous progress in the development of heteroacenes, fused conjugated molecules and their corresponding polymers towards attaining high charge carrier mobilities.3–6 However, the device performance needs to be improved with a modified synthetic design of polymers for simple processing technologies, which remains an essential task.7,8
Earlier investigations have shown that the planarization and energy levels of copolymers can be tuned by the choice of respective donor (D) and acceptor (A) comonomers.9–11 Recent developments in D–A copolymers consisting of fused planar molecules as donors and electron-acceptors such as naphthalene diimide (NDI),12,13 diketopyrrolopyrrole (DPP),14–17 and 2,1,3-benzothiadiazole (BTZ),9,18,19 have shown significant progress in OFETs and OPVs. Moreover, among the class of π-extended ring-fused heteroacenes, thiophene-based heteroacene6 donors especially those with thieno[3,2-b]thiophene (TT) units have gained much importance.19–24
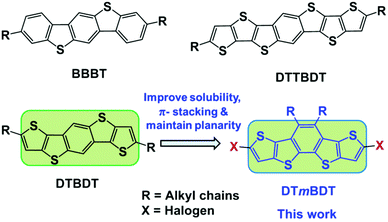
In the quest for developing heteroatom containing acenes, we have reported five-ring-fused pentacene analogs, benzo[1,2-b;4,5-b′]bis[b]benzothiophene (BBBT),25 dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;4,5-b′]dithiophene (DTBDT)26 and heptacene analog dithienothieno[2,3-d;2′,3′-d′]benzo[1,2-b;4,5-b′]dithiophene (DTTBDT)27 (Fig. 1). However, introducing solubilizing groups on the central core of these molecules will disturb the π-stacking. In this work, we present the facile synthesis of a novel didodecyl substituted tetrathiapentacene donor unit, dithieno[2,3-d;2′,3′-d′]benzo[1,2-b;3,4-b′]dithiophene (DTmBDT). The current versatile building block DTmBDT can be easily made soluble with dodecyl side chains at the central benzene ring and functionalized with halogens in the alpha positions of the outer thiophenes for further reactions. We demonstrate the potential of this highly soluble heteropentacene donor unit by copolymerization with 2,5-bis(2-octyldodecyl)-3,6-di(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (DPP) as an electron-acceptor.
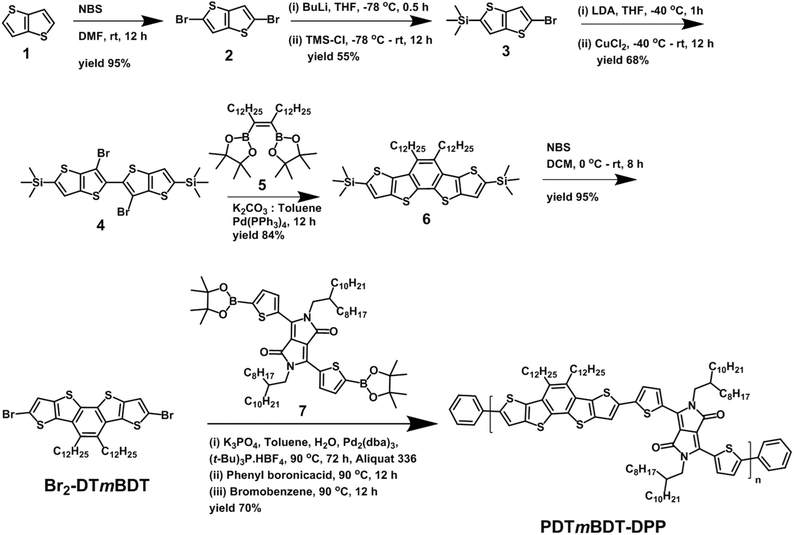
The synthetic route of PDTmBDT-DPP is sketched in Scheme 1. First thieno[3,2-b]thiophene (1) was converted to 2,5-dibromothieno[3,2-b]thiophene (2) via bromination and selective monosilylation which was carried out using n-BuLi followed by the reaction with trimethylsilylchloride to yield (5-bromothieno[3,2-b]thiophen-2-yl)trimethylsilane (3). Then the lithium diisopropylamide (LDA) induced migration of bromides of compound 3 to the β-position and dimerization using CuCl2 were accomplished in a one-pot reaction to acquire dimer 4. The planarized and ring closed derivative 6 was achieved through the Suzuki–Miyaura coupling reaction of dimer 4 and 1,2-didodecyl ethenyl diboronicester 5 in 84% yield. The disilane derivative was then converted to the dibromo derivative Br2-DTmBDT in 95% yield using N-bromosuccinimide (NBS).
According to our previous work,28 tri-tert butylphosphoniumtetrafluoroborate ((t-Bu)3P·HBF4) is an effective ligand to yield high molecular weights with Pd2(dba)3 in Suzuki couplings based on diketopyrrolopyrrol diboronicester (7). Therefore, the same polymerization conditions were used to synthesize the copolymer PDTmBDT-DPPvia the Suzuki polymerization reaction between the building block Br2-DTmBDT and DPP diboronicester 7. After purification on a Soxhlet extractor with methanol, acetone and hexane to remove the oligomeric fractions and final extraction with chloroform, the highest molecular weight polymer was achieved (Mn = 308.9 kDa and Mw = 976.4 kDa against polystyrene standards in THF). However, polydispersity (PDI = 3.2) of the polymer was found to be slightly high which might be due to aggregation of highly planar polymeric segments.
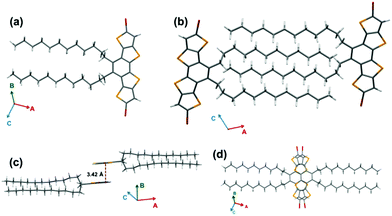
Single crystal XRD analysis of Br2-DTmBDT revealed that these crystals were packed in the P21/c (monoclinic) space group (Fig. 2). It was found that the Br2-DTmBDT is a completely planar structure and forms a dyad between two molecular planes in a face-to-face manner with a π–π distance of 3.42 Å and follows a “herringbone” pattern of dyads. Interestingly, these two molecules in one dyad only overlap through the TT units (Fig. 2d). Most surprisingly, the dodecyl chains of the neighboring molecules were packed in an interdigitating manner.
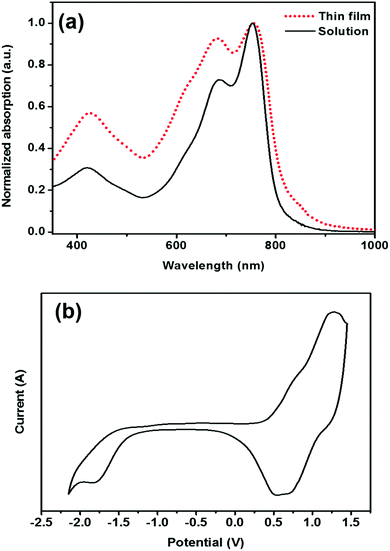
The solution state absorption spectrum of PDTmBDT-DPP in TCB shows a maximum absorption wavelength (λmax) at 753 nm with a shoulder peaking at 685 nm (Fig. 3a). The λmax is attributed to intramolecular charge transfer (ICT) between the donor and acceptor in the polymer backbone (Fig. S2a†).29 The thin film of PDTmBDT-DPP prepared by drop-casting a TCB solution onto a quartz plate displayed the same absorption wavelength but with higher intensities of the shoulder peaks at a lower absorption wavelength compared to that in solution. The optical bandgap of PDTmBDT-DPP derived from the onset of the absorption spectrum of the film is 1.51 eV. The predicted (TD-SCF) UV-vis absorption spectra suggest that the effective conjugation might be extended up to three repeating units of the copolymer (ESI, Fig. S2b†).
The electrochemical properties of the polymer were investigated by cyclic voltammetry of drop-cast films on an ITO substrate. PDTmBDT-DPP showed multiple oxidations (Fig. 3b). The first oxidation (E1/2ox1) occurred at 0.66 V with an onset potential of 0.27 V leading to an ionization potential (IP) of −5.07 eV. The first reduction (E1/2red1) of the polymer appeared at −1.54 V by showing an onset potential at 1.27 V and the electron affinity (EA) was found to be −3.53 eV. The IP and EA energy levels are calculated from the respective first oxidation and reduction onset potentials and the electrochemical energy gap (1.54 eV) is quite close to the optical bandgap. It was found from the density functional theory (DFT) calculations (B3LYP, 6-31G(d)) of the frontier orbitals that the HOMO is distributed over the two DPP units and one DTmBDT unit. However, the DPP units (Fig. S3–S5†) majorly contribute to the LUMO. Interestingly, the dihedral angles between the DPP and DTmBDT units were found to be 2–3 degrees leading to a quasi-planer polymer backbone (ESI†).
TGA analysis revealed that the polymer was stable up to 410 °C with only 2% weight loss under a nitrogen atmosphere (Fig. S9a†). DSC analysis showed that PDTmBDT-DPP has a high glass transition temperature (Tg) of 189 °C and a segmental melting point at 331 °C along with a corresponding crystallization peak at 311 °C while cooling (Fig. S9b†).
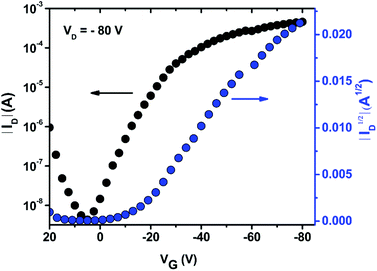
The charge carrier transport of PDTmBDT-DPP was investigated using bottom-gate bottom-contact configuration OFET devices with 50 nm-thick Au electrodes as the source and drain and 300 nm-thick SiO2 as the dielectric source. The semiconducting PDTmBDT-DPP layer was drop-cast from a chloroform solution at a concentration of 2 mg ml−1. In order to remove the residual solvent in the drop-cast thin film, annealing was performed at 100 °C for 30 min. All electrical measurements were performed under a nitrogen atmosphere. The transfer (Fig. 4) and output (Fig. S10†) characteristics indicated a typical linear saturation behavior. From the transfer plots, hole transport is observed with the mobility of 0.36 cm2 V−1 s−1 and the on/off ratio reaches 105.
The polymer organization of the PDTmBDT-DPP drop-cast film on the silicon dioxide dielectric surface of the transistor was investigated by grazing incidence wide-angle X-ray scattering (GIWAXS). The PDTmBDT-DPP film showed a high degree of order in the out-of-plane direction (along qz for qxy = 0 Å−1) as indicated by reflections up to the third order (Fig. S11†). These scattering intensities confirmed a typical lamellar organization with a high long-range order on the surface with an interlayer distance of ∼2.18 nm. A similar trend of layered behavior was observed in the AFM analysis of the OFET device (Fig. S12†). The wide-angle in-plane reflection was assigned to a π-stacking distance of 3.7 Å of the edge-on arranged conjugated backbones.30 In this edge-on organization, the π-stacking direction is oriented parallel to the surface, which is beneficial for charge carrier transport in field-effect transistors.
In summary, we have synthesized a novel fused tetrathiapentacene building block with solubilizing side chains not hindering the p-stacking and a corresponding copolymer PDTmBDT-DPP. This polymer exhibited a very high molecular weight, highly planar back-bone and excellent thermal stability up to 430 °C. For the new heteroacene with dodecyl side chains, very good π-stacking (3.4 Å) and alkyl side chain interdigitation were found from the single crystal analysis making this donor molecule itself promising for future independent OFET studies and also for the synthesis of planar copolymers with different acceptors. In first device experiments with PDTmBDT-DPP, we have demonstrated the high charge carrier nobilities of 0.36 cm2 V−1 s−1. These preliminary results are highly promising towards solution processable OFET applications, and complete investigations on device optimization and performance including OPV characterization are in progress in our laboratory.
The authors want to thank Wojciech Pisula for first OFET studies performed by M. L. Thanks to Dr Dieter Schollmeyer at Johannes Gutenberg-University, Mainz for crystal structure analysis and Jutta Schnee for helping in the synthesis of thieno[3,2-b]thiophene derivatives. B. R. would like to acknowledge the FP7 MC-fellowship. KACST is highly acknowledged for financial support.
Notes and references
- X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832–1908 CrossRef CAS.
- Y. Zhao, Y. Guo and Y. Liu, Adv. Mater., 2013, 25, 5372–5391 CrossRef CAS PubMed.
- I. Kang, H.-J. Yun, D. S. Chung, S.-K. Kwon and Y.-H. Kim, J. Am. Chem. Soc., 2013, 135, 14896–14899 CrossRef CAS PubMed.
- G. Kim, S.-J. Kang, G. K. Dutta, Y.-K. Han, T. J. Shin, Y.-Y. Noh and C. Yang, J. Am. Chem. Soc., 2014, 136, 9477–9483 CrossRef CAS PubMed.
- B. Sun, W. Hong, Z. Yan, H. Aziz and Y. Li, Adv. Mater., 2014, 26, 2636–2642 CrossRef CAS PubMed.
- K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347–4370 CrossRef CAS PubMed.
- H.-R. Tseng, H. Phan, C. Luo, M. Wang, L. A. Perez, S. N. Patel, L. Ying, E. J. Kramer, T.-Q. Nguyen, G. C. Bazan and A. J. Heeger, Adv. Mater., 2014, 26, 2993–2998 CrossRef CAS PubMed.
- H. Sirringhaus, Adv. Mater., 2014, 26, 1319–1335 CrossRef CAS PubMed.
- M. Zhang, H. N. Tsao, W. Pisula, C. Yang, A. K. Mishra and K. Müllen, J. Am. Chem. Soc., 2007, 129, 3472–3473 CrossRef CAS PubMed.
- J.-S. Wu, S.-W. Cheng, Y.-J. Cheng and C.-S. Hsu, Chem. Soc. Rev., 2015, 44, 1113–1154 RSC.
- M. E. Cinar and T. Ozturk, Chem. Rev., 2015, 115, 3036–3140 CrossRef CAS PubMed.
- X. Guo, A. Facchetti and T. J. Marks, Chem. Rev., 2014, 114, 8943–9021 CrossRef CAS PubMed.
- M. Yuan, M. M. Durban, P. D. Kazarinoff, D. F. Zeigler, A. H. Rice, Y. Segawa and C. K. Luscombe, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4061–4069 CrossRef CAS.
- P. Sonar, S. P. Singh, Y. Li, M. S. Soh and A. Dodabalapur, Adv. Mater., 2010, 22, 5409–5413 CrossRef CAS PubMed.
- C. B. Nielsen, M. Turbiez and I. McCulloch, Adv. Mater., 2013, 25, 1859–1880 CrossRef CAS PubMed.
- X. Guo, S. R. Puniredd, M. Baumgarten, W. Pisula and K. Müllen, Adv. Mater., 2013, 25, 5467–5472 CrossRef CAS PubMed.
- H. Bürckstümmer, A. Weissenstein, D. Bialas and F. Würthner, J. Org. Chem., 2011, 76, 2426–2432 CrossRef PubMed.
- C. Luo, A. K. K. Kyaw, L. A. Perez, S. Patel, M. Wang, B. Grimm, G. C. Bazan, E. J. Kramer and A. J. Heeger, Nano Lett., 2014, 14, 2764–2771 CrossRef CAS PubMed.
- L. Biniek, B. C. Schroeder, J. E. Donaghey, N. Yaacobi-Gross, R. S. Ashraf, Y. W. Soon, C. B. Nielsen, J. R. Durrant, T. D. Anthopoulos and I. McCulloch, Macromolecules, 2013, 46, 727–735 CrossRef CAS.
- C. Wetzel, E. Brier, A. Vogt, A. Mishra, E. Mena-Osteritz and P. Bäuerle, Angew. Chem., Int. Ed., 2015, 54, 12334–12338 CrossRef CAS PubMed.
- Y. Tsutsui, T. Sakurai, S. Minami, K. Hirano, T. Satoh, W. Matsuda, K. Kato, M. Takata, M. Miura and S. Seki, Phys. Chem. Chem. Phys., 2015, 17, 9624–9628 RSC.
- J. Shaw, H. Zhong, C. P. Yau, A. Casey, E. Buchaca-Domingo, N. Stingelin, D. Sparrowe, W. Mitchell and M. Heeney, Macromolecules, 2014, 47, 8602–8610 CrossRef CAS.
- J. Zhang, K. Zhang, W. Zhang, Z. Mao, M. S. Wong and G. Yu, J. Mater. Chem. B, 2015, 3, 10892–10897 CAS.
- L. Huo, T. Liu, X. Sun, Y. Cai, A. J. Heeger and Y. Sun, Adv. Mater., 2015, 27, 2938–2944 CrossRef CAS PubMed.
- P. Gao, D. Beckmann, H. N. Tsao, X. Feng, V. Enkelmann, W. Pisula and K. Müllen, Chem. Commun., 2008, 1548–1550 RSC.
- P. Gao, D. Beckmann, H. N. Tsao, X. Feng, V. Enkelmann, M. Baumgarten, W. Pisula and K. Müllen, Adv. Mater., 2009, 21, 213–216 CrossRef CAS.
- L. Chen, M. Baumgarten, X. Guo, M. Li, T. Marszalek, F. D. Alsewailem, W. Pisula and K. Müllen, J. Mater. Chem. B, 2014, 2, 3625–3630 CAS.
- C. An, T. Marszalek, X. Guo, S. R. Puniredd, M. Wagner, W. Pisula and M. Baumgarten, Polym. Chem., 2015, 6, 6238–6245 RSC.
- J.-S. Wu, C.-T. Lin, C.-L. Wang, Y.-J. Cheng and C.-S. Hsu, Chem. Mater., 2012, 24, 2391–2399 CrossRef CAS.
- I. Osaka and K. Takimiya, Polymer, 2015, 59, A1–A15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental and computational data. CCDC 1435119. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6py00023a |
| This journal is © The Royal Society of Chemistry 2016 |