Open Access Article
Open Access ArticleAmmonium ylides for the diastereoselective synthesis of glycidic amides†
Mario
Waser
*,
Richard
Herchl
and
Norbert
Müller
Institute of Organic Chemistry, Johannes Kepler University Linz, Altenbergerstraße 69, 4040 Linz, Austria. E-mail: Mario.waser@jku.at; Fax: +43 732 2468 8747; Tel: +43 732 2468 8748
First published on 21st December 2010
Abstract
A highly trans-selective protocol for the synthesis of glycidic amides was developed. This approach gave access to oxiranes by reacting stabilised ammonium ylides bearing an α-carbonyl group and aromatic aldehydes in moderate to good yields.
The (dia-)stereoselective synthesis of glycidic amides and esters has attracted considerable interest over the last few decades mainly due to the high potential of these compounds as synthetically useful intermediates in a variety of organic syntheses. Ylides have proven to be very powerful synthons for the formation of epoxides1–4 providing alternatives to the oxidation of α,β-unsaturated carbonyl compounds5,6 or the Darzens reaction between α-halo esters or amides and carbonyl groups.7 The synthesis of oxirane rings by reacting a sulfur ylide with an aldehyde or ketone was introduced around 50 years ago1 and a variety of applications using chiral sulfonium ylides either in stoichiometric or even in catalytic quantities have been reported.2–4 Next to sulfur ylides the use of ammonium ylides for C–C bond formation has attracted considerable interest over the last few years.8–14 Gaunt et al. showed that cinchona alkaloid catalysts are highly useful for stereoselective cyclopropanations proceeding via an ammonium ylide mechanism.8 Besides the syntheses of cyclopropanes the diastereoselective formation of epoxides in analogy to the Corey–Chaykovsky reaction has been described.11–13 However, this synthetically useful transformation has so far been limited to benzylic ammonium ylides13 and cyano-stabilised ammonium ylides11,12 giving the corresponding oxiranes in moderate yields only, whereas ester-stabilised ylides did not yield the epoxides.14 Previous studies by Aggarwal et al. clearly showed that a key factor in ylide based epoxide formation reactions is the leaving group quality of the onium group, which decreases in the order O > S > N > P.9 Furthermore, the presence of a carbonyl group α to the leaving group (stabilised ylides) significantly increases the barrier to ring closure. Altogether, these investigations clearly demonstrate why ammonium ylides are less suited for epoxide formation than sulfur ylides and rationalize why no oxirane syntheses with ammonium ylides bearing an α-carbonyl group have been reported so far.9
However, from the few reported examples it was clearly proven by Jonczyk et al.11 that cyano-stabilised ylides (being less stabilised than esters)15 undergo such reactions under biphasic conditions in moderate yield. Furthermore, less stabilised benzylic ylides give access to trans-selective formation of stilbene oxides in moderate to good yields.13 Thus, it seems reasonable that similarly stabilised ammonium ylides might be successfully employed for epoxide formation.
Based on a recent investigation of the stability of ylides,15 we reasoned that amide based ammonium ylides (being less stabilised than cyano-based ylides, but higher stabilised than benzylic), might undergo oxirane formation when reacted with aldehydes since they should be sufficiently balanced with respect to nucleophilicity and leaving group ability. Due to the high interest in glycidic amides,4 an ammonium ylide based synthetic approach would thus significantly broaden the scope of this methodology.
We therefore focused on the development of reactions between DABCO-derived amide-based ammonium ylides and aromatic aldehydes (Scheme 1).
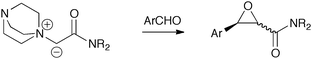 | ||
| Scheme 1 Targeted use of amide-based ammonium ylides for epoxide formation. | ||
Initial attempts were carried out reacting a small excess of benzaldehyde (1) with the diethylamide-derived ammonium salt 2 in dry THF using t-BuOK (1.2 equiv.) as the base at room temperature (Table 1, entry 1). After 24 h, full conversion of 1 was observed and the trans-configured glycidic amide 3 could be isolated in 32% yield. Noteworthy, not even trace amounts of the cis-diastereomer were obtained (judged by 1H NMR). As we had observed a significant decrease in yield (<20%) using lower quality THF, addition of molecular sieves (4 Å) to the reaction mixture was tested, resulting in an improved yield of 47% (entry 2). However, besides the target product, large amounts of benzyl alcohol and benzoic acid resulting from base mediated Cannizzaro disproportionation of 1 accompanied by unidentified decomposition products of 2 were obtained. Unfortunately, neither addition of tetrabutylammonium bromide,16 nor reducing the reaction temperature improved suppression of these side reactions. Also using other solvent/base combinations (entries 3–7 in Table 1 give a representative overview of only a few of the tested conditions), no improvement could be achieved. Noteworthy, some of the conditions which were reported to be very successful in sulfur ylide transformations (e.g. entry 6)17 gave absolutely no conversion in our case. The only significant product formation was observed when using t-BuOK in DMSO (45%, entry 7). As disproportionation of 1 was found to be the major limiting factor, 2 equiv. of aldehyde were used next (entry 8), but the yield could only be improved slightly (55%), and large amounts of the Cannizzaro products were formed.
| Entry | Solvent | Base | T (°C) | t (h) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Yielda (%) | trans b (%) |
|---|---|---|---|---|---|---|---|
| a Isolated yields. b Determined by 1H NMR of the crude product. | |||||||
| 1 | THF | t-BuOK | 0 → 25 | 24 | 1.2 | 32 | >99 |
| 2 | THF (4 Å) | t-BuOK | 0 → 25 | 24 | 1.2 | 47 | >99 |
| 3 | THF | KOH | 0 → 25 | 24 | 1.2 | 23 | >99 |
| 4 | EtOH | KOH | 0 → 25 | 24 | 1.2 | 0 | >99 |
| 5 | CH3CN | Cs2CO3 | 0 → 25 | 24 | 1.2 | 0 | >99 |
| 6 | CH3CN (aq) | KOH | 0 → 25 | 24 | 1.2 | 0 | >99 |
| 7 | DMSO | t-BuOK | 0 → 25 | 24 | 1.2 | 45 | >99 |
| 8 | THF (4 Å) | t-BuOK | 0 → 25 | 24 | 2 | 55 | >99 |
| 9 | CH2Cl2 | 50% NaOH | 0 → 25 | 24 | 1.2 | 55 | >99 |
| 10 | CH2Cl2 | 50% NaOH | 0 → 25 | 24 | 2 | 67 | >99 |
| 11 | CH2Cl2 | 50% NaOH | 0 → 25 | 24 | 3 | 77 | >99 |
| 12 | CH2Cl2 | 50% NaOH | 0 → 25 | 24 | 0.5 | 50 | >99 |
| 13 | CH2Cl2 | 50% NaOH | 40 | 8 | 3 | 76 | >99 |
| 14 | CH2Cl2 | 50% KOH | 0 → 25 | 24 | 3 | 62 | >99 |
Accordingly, even with only equimolar amounts of base the disproportionation of the aldehyde limited the yield in all these homogeneous or liquid/solid experiments (entries 1–8). Therefore we investigated biphasic conditions in analogy to those reported by Jonczyk et al.11 Carrying out the reaction in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CH2Cl2 and 50% NaOH (aq) at ambient temperature (25 °C), full conversion of 1 was observed within 24 h. The oxirane 3 was obtained in 55% yield with excellent trans-selectivity (entry 9). Although a big excess of base (∼100 equiv.) was necessary to achieve a reliable performance, the Cannizzaro reaction was well suppressed and only negligible amounts of benzyl alcohol could be detected. However, although all reactions were performed under inert conditions (Ar-atmosphere), considerable amounts of benzoic acid were formed presumably by autoxidation of 1. To overcome this limitation, the relative amount of 1 was doubled (entry 10). This increased the yield to 67% after a 24 h reaction time (longer reaction times did not improve the yield anymore). With 3 equiv. of 1 the yield was 77% (entry 11), while using an excess of ammonium salt 2, the yield dropped to 50% (entry 12). This clearly underscores that indeed the decomposition of 1 is the yield-limiting factor. Running the reaction at elevated temperature (40 °C, with excess 1) resulted in good conversion after 8 h, giving 3 in 76% (entry 13). Noteworthy, carrying out the reaction at 40 °C for a longer time (>24 h) resulted in a significant decomposition of the epoxide product. Using 50% KOH (aq) as the base resulted in a reduced yield of 62% only (entry 14). Neither lower temperature, nor varying the amount of base or solvent ratios (e.g. less H2O) and concentrations improved the yield any more.
1 mixture of CH2Cl2 and 50% NaOH (aq) at ambient temperature (25 °C), full conversion of 1 was observed within 24 h. The oxirane 3 was obtained in 55% yield with excellent trans-selectivity (entry 9). Although a big excess of base (∼100 equiv.) was necessary to achieve a reliable performance, the Cannizzaro reaction was well suppressed and only negligible amounts of benzyl alcohol could be detected. However, although all reactions were performed under inert conditions (Ar-atmosphere), considerable amounts of benzoic acid were formed presumably by autoxidation of 1. To overcome this limitation, the relative amount of 1 was doubled (entry 10). This increased the yield to 67% after a 24 h reaction time (longer reaction times did not improve the yield anymore). With 3 equiv. of 1 the yield was 77% (entry 11), while using an excess of ammonium salt 2, the yield dropped to 50% (entry 12). This clearly underscores that indeed the decomposition of 1 is the yield-limiting factor. Running the reaction at elevated temperature (40 °C, with excess 1) resulted in good conversion after 8 h, giving 3 in 76% (entry 13). Noteworthy, carrying out the reaction at 40 °C for a longer time (>24 h) resulted in a significant decomposition of the epoxide product. Using 50% KOH (aq) as the base resulted in a reduced yield of 62% only (entry 14). Neither lower temperature, nor varying the amount of base or solvent ratios (e.g. less H2O) and concentrations improved the yield any more.
Using quinuclidine-based ammonium salts instead of DABCO-derived ones gave 3 in 15% yield only, whereas quinine as the amine part did not give any product, indicating the importance of leaving group ability for this reaction.
Having developed a reliable biphasic procedure for the highly trans-selective synthesis of 3 in a reasonable yield, we next investigated the use of other amide-derived ylides in the reaction with 1 (2 equiv.)18 under these conditions (Table 2).
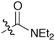
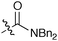
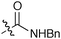
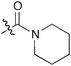
It was clearly shown that all other amides give the products in high trans-selectivity too. Furthermore, other tertiary amides (entries 2 and 4, Table 2) give yields comparable to the test reaction between 1 and 2. However, using the secondary amide 6, the corresponding glycidic amide 7 could only be isolated in a reduced yield of 24% (entry 3). In this case, an increased amount of benzoic acid was obtained, due to autoxidation of unreacted 1. Accordingly, this lower yield is due to a significantly reduced reactivity of the secondary amide-derived salt 6 (a similar tendency for sulfur ylides was reported by Aggarwal et al.4b).
Finally, reaction of 2 with other aromatic aldehydes was investigated (Table 3). All reactions were first carried out under the standard conditions using 2 equiv. of the aldehyde (cond. A) to get a clear picture about the influence of the different substituents on the reactivity (entries 1–3, 5, 7, 9). All aldehydes reacted to the trans-epoxides exclusively. However, whereas the similarly activated aldehydes 10 and 12 gave the glycidic amides 11 and 13 in good yields (entries 2–4), the conversion of the more electron-rich anisaldehyde 14 was significantly lower resulting in a reduced yield of 47% after 24 h. As expected, Cannizzaro reaction was no problem in this case but high amounts of unreacted 14 were found. The even more electron rich dimethylaminobenzaldehyde 16 showed only minor conversion, giving just traces of product under standard conditions. By contrast, the highly electron-deficient nitrobenzaldehyde 18 was fully consumed within 24 h, but giving mainly the corresponding Cannizzaro products and less than 10% of the product 19 even at reduced temperature and using less base (entries 9 and 10). Reaction of the deactivated 14 at elevated temperature (40 °C, entry 6) resulted in almost full conversion of 14 after 24 h, giving 15 in 50% accompanied with unidentified by-products. This can be rationalized by decomposition of the aldehyde 14 as well as the oxirane 15 under these harsher basic conditions. On the other hand, even under these conditions, 16 was found to be rather unreactive yielding less than 20% of 17 (entry 8).
| Entry | Ar | Aldehyde | Product | Cond.a | Yieldb (%) | trans c (%) |
|---|---|---|---|---|---|---|
| a A: 0 → 25 °C, 24 h; 100 equiv. NaOH; B: 40 °C, 24 h, 100 equiv. NaOH; C: 0 °C, 24 h, 50 equiv. NaOH b Isolated yields. c Determined by 1H NMR of the crude product. d 3 equiv. of 12 were used. e Judged by 1H NMR of the crude product. f The product decomposed during column chromatography. g Complete Cannizzaro decomposition of 18. | ||||||
| 1 | Ph– | 1 | 3 | A | 67 | >99 |
| 2 | 4-MeC6H4– | 10 | 11 | A | 68 | >99 |
| 3 | 4-ClC6H4– | 12 | 13 | A | 72 | >99 |
| 4 | 4-ClC6H4– | 12 | 13 | Ad | 82 | >99 |
| 5 | 4-MeOC6H4– | 14 | 15 | A | 47 | >99 |
| 6 | 4-MeOC6H4– | 14 | 15 | B | 50 | >99 |
| 7 | 4-Me2NC6H4– | 16 | 17 | A | <5e | >99 |
| 8 | 4-Me2NC6H4– | 16 | 17 | B | <20e,f | >99 |
| 9 | 4-NO2C6H4– | 18 | 19 | A | <10e,g | >99 |
| 10 | 4-NO2C6H4– | 18 | 19 | C | <10e,g | >99 |
Accordingly, the aptitude of different aldehydes for this type of reaction strongly depends on their electronic properties. Whereas similarly activated aldehydes like 1, 10, and 12 give the glycidic amides in good yields, the electron poor 18 is prone to rapid Cannizzaro decomposition under the highly basic conditions. On the other hand, electron rich aldehydes like 14 and 16 are significantly less reactive.
In conclusion, although ammonium ylides bearing an α-carbonyl group are less reactive in oxirane synthesis than sulfur ylides, excellent trans-selectivity and acceptable yields could be achieved by reacting amide-derived ammonium ylides with aromatic aldehydes. Key to success is the use of biphasic conditions together with a two-fold excess of aldehyde. The reaction is tolerant to different tertiary amide groups whereas secondary amides are less reactive. Using differently activated aldehydes, the outcome was strongly dependent on the electronic properties of the electrophile. Thus, it seems reasonable that this reaction is very close to the limit of what is possible with ammonium ylides. Nevertheless, to the best of our knowledge, this is the first time that stabilised ammonium ylides possessing an α-carbonyl group have been successfully employed in oxirane formation.
This work was supported by the Austrian Science Funds (FWF) Project No. P22508-N17.
Notes and references
- (a) E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 1962, 84, 867 CrossRef CAS; (b) E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 1965, 87, 1353 CrossRef CAS; (c) A. W. Johnson and R. B. Lacount, Chem. Ind., 1958, 1440 Search PubMed.
- (a) V. K. Aggarwal, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, New York, 1999, vol. 2, p. 679 Search PubMed; (b) E. M. McGarrigle, E. L. Myers, O. Illa, M. A. Shaw, S. L. Riches and V. K. Aggarwal, Chem. Rev., 2007, 107, 5841 CrossRef CAS.
- (a) O. Illa, M. Arshad, A. Ros, E. M. McGarrigle and V. K. Aggarwal, J. Am. Chem. Soc., 2010, 132, 1828 CrossRef CAS; (b) J. Zanardi, C. Leriverend, D. Aubert, K. Julienne and P. Metzner, J. Org. Chem., 2001, 66, 5620 CrossRef CAS; (c) M. Davoust, J.-F. Briere, P.-A. Jaffres and P. Metzner, J. Org. Chem., 2005, 70, 4166 CrossRef CAS.
- For syntheses of glycidic amides see: (a) Y.-G. Zhou, X.-L. Hou, L.-X. Dai, L.-J. Xia and M.-H. Tang, J. Chem. Soc., Perkin Trans. 1, 1999, 77 RSC; (b) V. K. Aggarwal, J. P. H. Charmant, D. Fuentes, J. N. Harvey, G. Hynd, D. Ohara, W. Picoul, R. Robiette, C. Smith, J.-L. Vasse and C. L. Winn, J. Am. Chem. Soc., 2006, 128, 2105 CrossRef CAS; (c) V. K. Aggarwal, G. Hynd, W. Picoul and J.-L. Vasse, J. Am. Chem. Soc., 2002, 124, 9964 CrossRef CAS.
- M. J. Porter and J. Skidmore, Chem. Commun., 2000, 1215 RSC.
- For impressive examples see: (a) T. Nemoto, H. Kakei, V. Gnanadesikan, S.-Y. Tosaki, T. Ohshima and M. Shibasaki, J. Am. Chem. Soc., 2002, 124, 14544 CrossRef CAS; (b) S. Matsunaga, T. Kinoshita, S. Okada, S. Harada and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 7559 CrossRef CAS.
- For two different stereoselective approaches see: (a) S. Arai, K. Tokumaru and T. Aoyama, Tetrahedron Lett., 2004, 45, 1845 CrossRef CAS; (b) E. J. Corey and S. Choi, Tetrahedron Lett., 1991, 32, 2857 CrossRef CAS.
- (a) M. J. Gaunt and C. C. C. Johansson, Chem. Rev., 2007, 107, 5596 CrossRef CAS; (b) C. C. C. Johansson, N. Bremeyer, S. V. Ley, D. R. Owen, S. C. Smith and M. J. Gaunt, Angew. Chem., Int. Ed., 2006, 45, 6024 CrossRef CAS; (c) C. D. Papageorgiou, M. A. Cubillo de Dios, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 4641 CrossRef CAS; (d) N. Bremeyer, S. C. Smith, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 2681 CrossRef CAS; (e) C. D. Papageorgiou, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2003, 42, 828 CrossRef CAS.
- (a) R. Robiette, M. Conza and V. K. Aggarwal, Org. Biomol. Chem., 2006, 4, 621 RSC; (b) V. K. Aggarwal, J. N. Harvey and R. Robiette, Angew. Chem., Int. Ed., 2005, 44, 5468 CrossRef CAS.
- C.-Y. Zhu, X.-M. Deng, X.-L. Sun, J.-C. Zheng and Y. Tang, Chem. Commun., 2008, 738 RSC.
- For the use of cyano-stabilised ammonium ylides see: (a) A. Kowalkowska, D. Sucholbiak and A. Jonczyk, Eur. J. Org. Chem., 2005, 925 CrossRef CAS; (b) A. Jonczyk and A. Konarska, Synlett, 1999, 1085 CrossRef CAS.
- A. Alex, B. Larmanjat, J. Marrot, F. Couty and O. David, Chem. Commun., 2007, 2500 RSC.
- For benzylic ammonium ylides see: T. Kimachi, H. Kinoshita, K. Kusaka, Y. Takeuchi, M. Aoe and M. Ju-ichi, Synlett, 2005, 842 Search PubMed.
- Y. Wang, Z. Chen, A. Mi and W. Hu, Chem. Commun., 2004, 2486 RSC.
- An extensive study about ylide stability has recently been reported: Y. Fu, H.-J. Wang, S.-S. Chong, Q.-X. Guo and L. Liu, J. Org. Chem., 2009, 74, 810 Search PubMed.
- Ammonium additives might suppress Cannizzaro reactions: G. W. Gokel, H. M. Gerdes and N. W. Rebert, Tetrahedron Lett., 1976, 17, 653 Search PubMed.
- K. Julienne and P. Metzner, J. Org. Chem., 1998, 63, 4532 CrossRef CAS.
- Using 2 equiv. of aldehyde represents a good compromise between required amount of starting materials and obtained yield.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental part including analytical and spectroscopic data of all new compounds. See DOI: 10.1039/c0cc04821f |
| This journal is © The Royal Society of Chemistry 2011 |