Photothermal effect promoting the reconstruction and mass-energy transfer for the enhancement of three-dimensional confinement catalysis
Tingting
Xia†
a,
Bingyuan
Zeng†
a,
Jingwen
Jiang
a,
Tenghu
Wu
b,
Weiying
Pang
a,
Wenjing
Wang
*b,
Jie
Zhang
c and
Kun
Zhao
 *a
*a
aHebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, Department of Environmental Science and Engineering, North China Electric Power University, Baoding 071003, P. R. China. E-mail: zhaokun@ncepu.edu.cn
bSchool of Life Sciences, Institute of Life Sciences and Green Development, Hebei University, Baoding 071002, P. R. China. E-mail: wangwenjing@hbu.edu.cn
cCentre for Wireless Innovation, Queen's University Belfast, BT3 9DT Belfast, UK
First published on 21st February 2025
Abstract
Photothermal catalysis, as an emerging technology, has attracted much attention owing to its high efficiency and excellent sustainability. Current industrial processes integrating solar energy with fossil fuels for large-scale chemical production remain hindered by several challenges, including dynamic catalytic site restructuring that alters product selectivity and progressive catalyst deactivation that compromises economic viability. Therefore, a reasonable structural design could enormously enhance the mass and energy transfer during catalytic reactions. Considering the complexity of the reaction micro-environment, catalytic site reconstruction plays a crucial role in dynamic photothermal catalysis. Taking into account the spatial and temporal reaction processes, this review is focused on the latest progress of the catalytic sites confined to different three-dimensional (3D) structures. Initially, we provide an introduction to the mechanism of photothermal reaction on 3D structures, focusing on the mass and energy transferring pathways and the corresponding reactions. Subsequently, several typical distribution types of the catalytic sites are discussed, emphasizing that various 3D configurations modified with different types of catalytic sites would drive different reactions, including the biological enzyme site interaction process. We further elucidate the dynamic reconstruction of active sites under varying microenvironmental conditions, primarily induced by small-molecule adsorption and weak interfacial fields. Moreover, the applications of photothermal 3D materials are discussed under realistic reaction conditions, opening a new window for filling the gap between micro-structure and macro-process in catalysis. Finally, we briefly summarized the future challenges in solar-assisted catalytic engineering.
1. Introduction
The field of catalytic science and technology has been attracting extensive attention owing to its high efficiency, prominent bridge-linking role, and interdisciplinary feature.1–3 As the central determinant of industrial catalytic processes, rational catalyst design must address both structural compatibility with reactor configurations and fundamental understanding of multiscale structure-activity relationships bridging microscopic science and macroscopic engineering.4–6 Generally, researchers consider catalyst structure and its performance, but the detailed process of mass and energy transfer is often ignored.7–10 The emerging photothermal catalysis presents huge potential in energy and materials fields in improving the energy efficiency and catalytic technique coupling with solar light.11–143D porous structures have inherent spatial-confinement effects, which endow this type of materials with many unique properties on various catalysis processes, especially for photothermal processes with different transfer pathways of the light energy and reactant species.15–17 Under the complex fluid-gas conditions, the spatially distributed catalytic site could adsorb the environmental species to form a newly reconstructed site structure with better catalytic performance.18–20 This influence should urgently be evaluated at the industry operation level. Notably, 3D photothermal materials exhibit distinct advantages:21,22 (1) tunable porous architectures enabling precise metal nanoparticle deposition and surface functionalization,23 (2) 3D arrangement of catalytic sites optimizing gas-phase reactant accessibility,24,25 and (3) nanoconfinement effects enhancing reaction kinetics through activated molecular stabilization.26
As for the applied photothermal 3D materials, in order to protect the catalytic site from being poisoned by harmful molecules, a hierarchical 3D structure in the form of a shell or hollow structure is used to pack the catalytic site.27,28 However, the reachable molecules may contact with these confined sites, under the influence of the external driven force or environmental effects (field effects and adsorbed species coordination effects), which induce active site structure transformation, leading to better mass-energy transfer.29,30 Therefore, we can utilize these external field effects to drive inner-pore catalytic reactions, aiming to improve the efficiency and selectivity by precisely manipulating the micro-environment. However, in engineering applications, the reconstruction effect under harsh conditions could cause deformations in the three-dimensional structure of the catalyst, subsequently leading to the deactivation of catalytic sites.31,32 Therefore, it is necessary to review the catalytic site confined to the 3D structure that was employed in the industry fluid reaction.
This review first emphasizes the reaction processes and mechanisms of photothermal catalysis under 3D confinement conditions. It illustrates how the spatial positions of catalytic sites affect the transfer of mass and energy during dynamic catalysis. Additionally, the influence of field-induced structural reconstruction is discussed on mass and energy transfer. Furthermore, this review summarizes the relationship between site dispersal and catalytic performance, highlighting how these factors interplay with practical industrial processes such as reactor design. Finally, we conclude and provide a perspective on the design of catalysts and their applications in industrial catalysis.
2. Mechanism of 3D photothermal catalysis
3D materials are often used for photothermal catalysis owing to their unique confinement effect, which plays a crucial role in enhancing light absorption, heat generation, and catalytic activity.33,34 Generally, as elementary structures associated with the confinement effect, pores are constructed by gathering multiple catalytic surfaces to further form a confined void, protecting the activity of catalytic sites and promoting the stability of catalytic reactions.35 Different from the quantum confinement effect,36 void confinement would influence the mass-energy transfer and light-heat synergetic process, which would lead to different mechanisms of photothermal reactions in kinetics and thermal dynamics.37Traditionally, there are three pathways involved in photothermal catalysis: photocatalysis, thermal catalysis, and photothermal catalysis.38 The photocatalyst absorbs visible light and provides enough energy to excite the electrons in the catalyst.39 As shown in Fig. 1a, when the photon energy (hv) exceeds the bandgap energy (Eg), electrons in the valence band are photoexcited to the conduction band, generating spatially separated electron–hole pairs.40,41 These photogenerated electrons and holes could then migrate to the surface sites where they participate in redox reactions with adsorbed reactants (e.g. CO2 molecules and water).42–45 Notably, there are more crystal defects in the 3D confined void, causing electrons and holes to recombine for heating effect enhancement, thus reducing the number of effective charges participating in the photocatalytic reaction.46 Especially, in terms of the electron–hole recombination process, the localized temperature at the defects increases, altering the reaction balance and influencing the catalytic reaction rate.47
 | ||
| Fig. 1 Photothermal mechanism and reaction. (a) Process and mechanism of photocatalytic carbon dioxide conversion; (b) thermal conductivity pathway (reproduced with permission from ref. 48, copyright 2018 by Elsevier); and (c) mechanism of photothermal catalytic oxidation (reproduced with permission from ref. 49, copyright 2024 by the American Chemical Society). | ||
For thermal catalysis, the catalysts reduce the activation energy needed for chemical reactions.50–52 Besides, it is essential to use materials with superior thermal conductivity to obtain high thermal catalytic efficiency.53 As shown in Fig. 1b, the diamond-foam copper material exhibited a 3D continuous diamond network structure, and its inherent confined structure effectively reduced phonon–phonon and phonon-boundary scattering.48,54 Generally, excessively large network gaps can lead to enhanced sound wave scattering, reducing phonon propagation efficiency. When the gaps are moderate in size and have a high degree of connectivity, phonons can transmit freely between the gaps, increasing the transmission speed. Thus, the thermal conductivity of this 3D material was significantly improved, which facilitated the transfer of heat generated during the reaction, preventing catalyst deactivation caused by localized high temperature.55,56 However, the excessively high thermal conductivity of the material could cause heat loss before reaching the optimal reaction temperature.57 Therefore, it is important for thermal catalysis to reasonably regulate the thermal conductivity of the 3D materials.
The photothermal catalytic process combines the excellent features of photocatalysis and thermal catalysis, overcoming the high energy consumption of traditional thermal catalysis and the low efficiency of traditional photocatalysis.58,59 The 3D confined structures used for photothermal catalysis can enhance the reaction efficiency and accelerate mass-energy transfer via the confinement effect. As shown in Fig. 1c, when volatile organic compounds (VOCs) passed through the photothermal 3D material,60 they reacted with excited electrons in the confined void and were continuously degraded into carbon dioxide (CO2) and methane (CH4) under the heat generated by photothermal conversion.49
To further exhibit the potential of photothermal confined catalysis in reported applications, photothermal synergistic catalysis, plasmonic effect, and synthetic biology method are illustrated.61,62
Photothermal synergistic catalysis, a novel catalytic approach that has emerged in recent years, involves reaction modes such as thermally assisted photocatalysis and photo-driven thermal catalysis.63,64 Two types of photothermal synergistic reactions coupling with confined materials are introduced in the methane dry reforming process.65 As shown in Fig. 2a, the first mode is photo-driven thermal catalysis, where heat is the driving force for the reaction. The 3D catalysts can absorb more light due to the photo scattering in the confined void. Finally, the confined solar light in the 3D pore structure would be transferred into heat through non-radiative relaxation, increasing the localized temperature in the micro-environment and thereby reducing the activation energy for thermal catalysis.66 As shown in Fig. 2b, the second mode is thermally assisted photocatalysis, where the reaction is primarily driven by photogenerated electrons.67 Similarly, the absorbed light can exist for a long time in a 3D structure, which could increase excited electrons. CH4 and CO2 molecules reacted with excited electrons in the confined void and were reduced to carbon monoxide (CO) and hydrogen (H2) under external heat assistance.
One remarkable phenomenon is that the plasmonic effect coupled with the confinement effect shows great potential to enhance the photothermal conversion. Metal nanoparticles (Cu, Ag, Au, etc.) possessing plasmonic effects can absorb light and generate hot charge carriers and heat on localized surfaces, which can activate reactants and reduce the activation energy of the reaction.68,69 If the metal nanoparticles are embedded in other 3D materials (e.g. semiconductors) to form the confined void, they endow the photothermal catalysis reaction with more localized heat and hot electrons (Fig. 3).70
 | ||
| Fig. 3 Design of a heterostructure with confined voids exhibiting plasmonic catalytic activity (reproduced with permission from ref. 70, copyright 2022 by Wiley). | ||
The 3D materials with photothermal properties are always used in microbial hybrid systems, which usually react on and inside the cell membranes of microorganisms.71 After absorbing light, the material generates electron–hole pairs, and then, the photogenerated electrons are transferred to the cytoplasm of microorganisms or directly bind to specific sites in the internal environment, providing energy for microbial metabolism.72–74 However, photocatalysis generates heat, which not only benefits the growth of microorganisms but also can improve the catalytic efficiency of 3D materials, thereby increasing the output of the composite system. As shown in Fig. 4a, in a constructed InP nanoparticle-S. cerevisiae Δzωf1 hybrid system, non-photosynthetic bacteria75S. cerevisiae Δzωf1 utilizes catalytic sites on its surface to generate photoelectrons for photosynthesis. The active sites generate electron–hole pairs after absorbing light, and then electrons enter the cytoplasm through soluble redox mediators, driving cofactor NADPH regeneration, and promoting the conversion of 3-dehydroshikimic acid into shikimic acid.76
 | ||
| Fig. 4 (a) Assembly of S. cerevisiae–InP hybrids and rationally designed metabolic pathways (reproduced with permission from ref. 76, copyright 2018 by Science). (b) Schematic of the M. thermoacetica/AuNC hybrid system (reproduced with permission from ref. 77, copyright 2018 by Nature). | ||
Most photothermal microbial catalysis occurs in the cytoplasm, and the electrons generated by the material shuttle into the cytoplasm, which consumes additional energy and sometimes is affected by transmembrane transport.78 Therefore, by allowing photocatalytic materials to generate electrons directly inside the cell through photoexcitation, it can improve energy utilization efficiency. As shown in Fig. 4b, the catalytic sites of AuNCs were successfully transported into the cytoplasm and evenly distributed, with no significant aggregation on the outer membrane of the cell.79 The electrons generated by intracellular AuNCs under photoexcitation are directly used by the redox mediators in the cytoplasm, which are then transferred to the energy transfer system (ETS) in the cytoplasm.77 AuNCs can also act as reactive oxygen species (ROS) inhibitors to maintain high bacterial vitality, enabling bacteria to convert products better and compensating for poor biocompatibility between three-dimensional photocatalytic materials and microorganisms.80
3. Distribution of catalytic sites in 3D structure
As for photothermal catalysts, the spatial distribution of catalytic sites directly affects the adsorption, diffusion, and reaction processes of reactant molecules on the catalyst surface.81,82 Compared to lower-dimensional structures (one-dimensional and two-dimensional), more catalytic sites are participating in the photothermal catalysis reaction in the 3D structure.83,84 Moreover, the confined micro-environments can protect the activity of the catalytic sites.853.1. Catalytic sites dispersed in the 3D structure
The catalytic sites in a one-dimensional (1D) structure are linearly arranged along a single direction, which enables the precise control of the reaction pathway.86 Two-dimensional (2D) structures possess a larger surface area and provide more abundant surface-active sites than 1D structures, thereby facilitating reactant adsorption.87 Although 1D and 2D structures may exhibit certain advantages for specific reactions, 3D structures can provide more active sites and reaction pathways (Fig. 5), resulting in higher efficiency and selectivity in complex heterogeneous catalytic reactions.88,89 Therefore, 3D structures can provide greater benefits, particularly in terms of reaction space and potential for intricate multi-step reactions.90,91 | ||
| Fig. 6 Different confinement modes of hierarchical structures (reproduced with permission from ref. 94, copyright 2022 by the American Chemical Society). | ||
Particularly, the core–shell structure, a specific hierarchical structure, is used for photothermal catalysis owing to its synergetic function between the core and the shell. Generally, the core consists of functional catalytic sites responsible for providing catalytic activity, while the shell encapsulates the core and restricts the diffusion of reactant molecules on the catalyst surface.96 In specific core–shell structures, the shell absorbs the light and converts it into hot charge carriers, which would be transferred to the core to interact with reactants.97 As shown in Fig. 7, a photothermal nano-confined core–shell structure was designed for the efficient production of hydrogen.98 Photo-driven thermal catalysis mainly involves the coupling and conversion between photons and electrons. The photothermal effect uses lattice vibrations induced by infrared light from sunlight to achieve photothermal conversion. Photogenerated electrons were excited by the shell material and migrated to the catalytic sites on the core region to react with reactants. Absorbing photons increases the intensity of lattice vibrations and increases the local temperature. The high-temperature void created by the nano-confinement effect ensured effective separation and migration of photogenerated charge carriers, thus accelerating the reaction process.
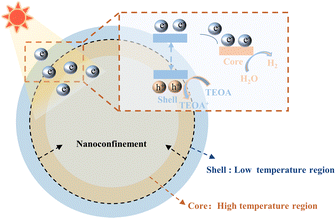 | ||
| Fig. 7 Application of the confinement effects of the core–shell structure in photothermal catalysis (reproduced with permission from ref. 98, copyright 2024 by Elsevier). | ||
The unique core–shell hierarchical structure can facilitate rapid electron transfer, accelerate molecular transport, and provide abundant active sites. The core–shell structures are not always displayed in an ideal configuration, and they often contain internal defects. After environmental condition treatments, various defects (Fig. 8a) in the core–shell hierarchical structure can significantly enhance the catalytic performance because they become catalytically active sites.99 Creating surface defect lattices on alloy catalysts can effectively regulate charge distribution, molecular orbital hybridization, and surface active centers.100 Surface defects in alloy materials with a core–shell structure are generated during the oxidation–reduction process. During the redox process (Fig. 8b), these defects can effectively promote the adsorption and dissociation of H2O molecules in the hydrogen evolution reaction (HER) and facilitate the transformation of O* (* radical) to H2O.101 OOH* is promoted through the synergistic action of two adjacent Pd sites in the oxygen evolution reaction (OER) (Fig. 8c). The introduction of defect locations significantly enhances the electrocatalytic activity for both the HER and the OER.
 | ||
| Fig. 8 (a) Different defect states induced by external conditions. Scheme for defect-promoted (b) HER and (c) OER. (Reproduced with permission from ref. 101, copyright 2024 by the American Chemical Society). | ||
The spatial confinement and distribution of catalytic sites in microbial hybrid systems resemble those in core–shell structures. The formation of this confined structure involves introducing photocatalysts into microbial culture media, where microbes internalize nanosized catalytic particles. This process necessitates that the catalysts possess a sufficiently small particle size or a near-spherical morphology, such as quantum dots (QDs). Two distinct modes of confinement and catalytic site distribution can be identified: intracellular localization within the cytoplasm and periplasmic localization. In the first mode, catalytic sites are located within the cytoplasm of microbial cells as shown in the M. thermoacetica/AuNC hybrid system (Fig. 4b). CdS@ZnS core–shell QDs were incorporated into the cytoplasm of Azotobacter vinelandii via self-assembly processes.102 In the mode of periplasmic localization, catalytic sites are confined to the periplasmic space between the inner and outer membranes. CuInS2/ZnS quantum dots were introduced into the periplasm of Shewanella oneidensis MR-1, effectively reducing the electron transfer distance and minimizing the energy loss associated with the transmembrane transport.103 Compared to QDs attached to the cell surface, the confinement structure formed by intracellular uptake of QDs enables the direct transfer of photogenerated electrons to cellular proteins, significantly enhancing the catalytic efficiency of the system.104
 | ||
| Fig. 9 Distribution of catalytic sites in 3D porous structures (reproduced with permission from ref. 111, copyright 2022 by Wiley). | ||
In photothermal catalytic reactions, the position of catalytic sites within a confined void varies, affecting the role and transfer mechanisms of micro-confined species (electrons, protons, photons).112 Photons are more easily captured and converted into chemical energy in a micro-confined environment because spatial confinement facilitates the reduction of photon loss and scattering (Fig. 10a).113 During the photothermal catalysis process, lattice vibrations intensify, causing an increase in the number of phonons. These phonons propagate through the medium and synergistically promote the reaction process with photons. Due to spatial confinement, the interactions between electrons and protons within inner pores or channels (Fig. 10b and d) and the catalytic sites may become closer, contributing to accelerated catalytic reactions.114 When catalytic sites are distributed on the surface, the transfer of electrons may be influenced by surface effects, leading to changes in the interactions with the catalytic sites. A rough surface increases phonon scattering (Fig. 10c), leading to changes in their propagation direction. Conversely, a smooth surface may maintain a certain propagation direction for phonons. The synergistic effect of light energy (photons) and thermal energy (phonons) enhances the rate and product selectivity of chemical reactions. Protons may exchange with other ions or molecules, thereby affecting their transfer rate and efficiency.
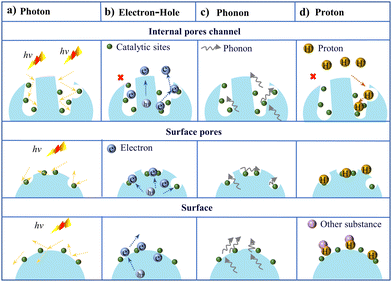 | ||
| Fig. 10 Transport characteristics of (a) photon, (b) electron–hole, (c) phonon, and (d) proton within and on the surface of porous structures. | ||
Additionally, within the core–shell structure, electrons can transition between different energy levels and can also cross energy barriers through tunneling.115 Protons primarily exhibit a behavior associated with nuclear fission and fusion reactions.116 The transfer of photons within the core–shell structure is mainly characterized by the light absorption, emission, and scattering.117 It is noteworthy that in core–shell structures, electrons, protons, and phonons are interrelated. The transfer of electrons may affect the lattice vibration and consequently alter the propagation characteristics of phonons. Meanwhile, the propagation of phonons may also influence the dynamic behavior of electrons and protons. Due to the complexity of porous structures, the transport efficiency of particles may be affected by factors such as porosity, pore size distribution, and channel connectivity. Therefore, the transport pathways of particles within porous structures are more complex than those in core–shell structures. In core–shell structures, particle transport often involves quantum mechanical effects and interactions at the atomic scale.118 In contrast, in porous structures, particle transport is more influenced by diffusion, convection, and adsorption.
When speaking of these confined voids, their influence on mass-energy transfer is still an ambiguous concept. The traditional theory which is centered on dynamics and based on momentum, mass, and energy transfer seems unable to completely explain the chemical reactions in photothermal catalysis.119 Recently, electron transfer, proton transfer, and molecular transport have been proposed for understanding the photothermal catalysis process. The efficient transfer of electrons, protons, and molecules serves as the key point for achieving high-performance mass-energy coupling. For example, the interaction between pure Co nanoparticles and the reaction medium was weak, leading to slow molecules, electrons, and momentum transferring120 (Fig. 11a). Therefore, in order to enhance the interaction, Co nanoparticles were assembled onto graphene nanosheets to form a confined void and reacted under the magnetic field, which synergistically promoted the reaction dynamics. As for the 3D networked porous materials (Fig. 11b), this type of confined void could make more catalytic sites uniformly distributed in pores.121 Compared to hollow structures, networked structures enabled fluids to flow sufficiently, which contributed to heat transferring and promoting interaction between sites and reactants. Besides, metal particles can be loaded onto the inner wall of nanotubes to form confined catalytic sites122 (Fig. 11c). Considering that the confined void could increase the fluid velocity within the nanotube, this specific structure benefited the transfer of reactant molecules, resulting in effective interaction between sites and reactants.123 To further discuss how the void confinement effect influences the photothermal catalysis process, we still choose the porous structure as an example. As a micro-reactor, the pore structure exhibits shape selectivity towards reactant molecules, allowing only those molecules that are compatible with the pore dimensions to transfer, thereby enhancing the selectivity of the photothermal catalytic reaction.124 Therefore, photothermal reactions in the confined void have a high yield.
 | ||
| Fig. 11 Distribution of catalytic sites in 3D structures: (a) under a magnetic field, the transmission of electrons, protons, and molecules and the momentum transfer in catalytic reactions are enhanced (reproduced with permission from ref. 120, copyright 2016 by the Royal Society of Chemistry); (b) porous uniform catalytic sites (reproduced with permission from ref. 121, copyright 2021 by the Royal Society of Chemistry); and (c) substrate-grown nanotubes, with catalytic sites attached to the nanotubes. | ||
The 3D confined structure exhibits significant potential in various fields. In photothermal CO2 conversion, the continuous porous structure provides abundant reaction spaces and efficient material exchange channels, enhancing the conversion efficiency of CO.125,126 Besides, in terms of photothermal water evaporation, the 3D confined structure can effectively absorb and convert light energy, thereby accelerating the water evaporation process, and giving it an advantage in applications such as solar seawater desalination.127–129 Additionally, in the electrocatalytic degradation of organic compounds, the porous structure not only increases the contact area between the catalyst and the organic compounds but also enhances electron transfer.130
3.2. Modular distribution of catalytic sites
Following the design principle, the distribution mode of catalytic sites within the confined void not only involves even distribution but also includes a modular distribution approach. Modular design optimizes the distribution of catalytic sites, reduces ineffective space, and thereby enhances the catalytic reaction efficiency.131 The catalytic sites of different modules can collaborate through the dynamic process and electron transfer to facilitate the reaction progress.132–134In a catalytic system, the tandem reaction is a process in which multiple active centers sequentially promote the consecutive occurrence of two or more independent reactions.135 Take the tandem catalysis reported by Chen136 as an example, in which the Ni–N4 site can effectively catalyze the carbon dioxide reduction reaction (CO2RR) to produce abundant CO molecules, allowing adjacent Cu nanoparticles to be covered with *CO (Fig. 12a). Moreover, the confined hydrophobic pore can protect sites from interplaying with water molecules and thereby inhibit hydrogen evolution reaction activity at the Cu nanoparticles. This site-to-nanoparticle tandem mode exhibits excellent performance in converting CO2 into C2H4.
 | ||
| Fig. 12 Modularized catalytic sites: (a) schematic of the working mechanisms of nanoparticle and site tandem catalysts (reproduced with permission from ref. 136, copyright 2023 by Wiley); (b) schematic of tandem catalysis with adjacent Ni–N4 and Fe–N4 sites (reproduced with permission from ref. 137, copyright 2023 by Wiley); (c) schematic of the dual-functional tandem catalytic mechanism of Mo and Co sites in heterostructures (reproduced with permission from ref. 138, copyright 2023 by Springer Nature Link); and (d) schematic of tandem catalysis using different catalysts (reproduced with permission from ref. 139, copyright 2023 by Nature). | ||
Additionally, the site-to-site tandem synergy is also commonly applied in catalytic reactions (Fig. 12b). By introducing another single metal atom into 3D confinement materials137 and adjusting the distance between the two single metal atoms, the introduced metal atom can synergize with the original metal site to form modular active centers, thereby optimizing the mass-energy transfer pathway.140 As shown in Fig. 12c, by using the bimetallic core–shell heterostructure, the synergistic interaction of these two types of metal sites can effectively regulate the electron transfer between the Co core and the Mo shell.138 The synergetic effect of the modules would affect the reaction energy barrier, thereby facilitating the reaction activation capacity. The design of this catalyst exhibits the cooperation of multiple metal modular centers, promoting the activation of reactants.
Similarly, there is a synergetic effect between sites on different catalysts. By loading different catalytic sites onto two distinct 3D substrate materials, these two materials can function as separate modules and synergistically engage in tandem catalytic reactions. In the tandem reaction shown in Fig. 12d, copper-based materials, and cobalt-based materials are used to synergistically promote the conversion of carbon dioxide.139 During the photothermal reaction process, light irradiation results in electron–hole separation and converts adsorbed CO2 into COOH* and CO* intermediates, while the heat from the light promotes the coupling of two CO* intermediates to form an OC–CO* intermediate.141 The coupling of two CO* intermediates to form OC–CO* does not involve electron or proton transfer and is essentially a thermal process. Increasing the temperature can accelerate the thermal motion of molecules, increasing the probability of collisions between two CO* intermediates, thereby promoting the C–C coupling process kinetically. In addition, C–C coupling also depends on the properties of different catalysts. To achieve better catalytic performance, a catalyst tandem method is adopted. The cobalt-based material reduces CO2 to CO, which is then transferred to the copper-based material for C–C coupling.142 The two modules are in tandem reaction, enabling the rapid accumulation of CO molecules, thereby inhibiting the hydrogen evolution reaction and promoting the production of C2+.
Designing a 3D confined catalyst and regulating the distribution of catalytic sites can facilitate the effective control of electron, proton, and molecular transfer within the catalytic system. Therefore, to further understand the advantages of confined catalysis, it is necessary to focus on the features of catalytic site reconstruction under field effects.
4. Site reconstruction under micro-environmental effects
Generally, the active site could be reconstructed during the reaction process, thereby affecting the efficiency and selectivity of the catalytic reaction. The external environmental effect (such as adsorbed species coordinated effect and field effect) can induce rearrangements of active sites or form new active catalytic species on the catalyst, thereby affecting the catalytic performance of the catalyst.143–145 The coordination effect could affect the number, distribution, and electron transfer of catalytic sites, altering their redox states. Besides, the field effect can facilitate the breakage or formation of targeted chemical bonds, thereby adjusting the activity and selectivity of the catalytic processes.146,147 Due to the influence of environmental effects, catalysts exhibit diverse site reconstructions, including the migration of surface modifiers, atom rearrangement, and morphological changes.148,149Additionally, the feature of site reconstruction varies from 1D structures to 3D structures. The 1D nanostructures exhibit significant quantum confinement and quantum tunneling effects, which may be further enhanced or regulated during the reconstruction process.150 Besides, in 2D materials, site reconstruction can alter the band structure and state density distribution of materials. The 3D structures with large surface areas can optimize the mass-energy transfer pathways through site reconstruction, improving the catalytic activity and selectivity.151
4.1. Spatial site surface reconstruction
The surface reconstruction effect mainly affects the surface morphology of materials, with a relatively minor impact on the internal structure.152 For instance, external environments, such as active species-coordinated environment, and magnetic field to induce electron transitions and vibrations in materials lead to surface reconstruction.As shown in Fig. 13a, these oxides will undergo irreversible surface reconstruction induced by oxygen oxidation and reduction during the oxygen evolution process. In the chemical process, the activated lattice oxygen atoms were first attacked by adsorbed water molecules, leading to the formation of hydroxide ions (OH−).153 Then, the metal-site soluble atoms leached from the oxygen-deficient surface. Therefore, the material is reconstructed from a 3D structure to a layered structure. Besides, a low-temperature oxidation technology (Fig. 13b) was employed to induce the reconstruction of catalytic sites on the amorphous surface.154 Through utilizing the intervention of O atoms to modulate the coordination environment, the activity and stability of catalytic sites were improved, thereby demonstrating remarkable performance in photocatalytic hydrogen evolution reactions. In addition, under a toxic environment, the doped copper would in situ generate to form multi-step metal sites155 (Fig. 13c). Interestingly, these generated copper sites could enrich hydrogen species on the surface to further inhibit the formation of toxic species, and reduce CO2−/OH− adsorption, which would make the catalytic sites inactivated and ultimately improve reactant activation and stabilize intermediates. In terms of magnetic field, it can modulate the electronic structure and band structure of molecules, altering the electromagnetic properties.156 As shown in Fig. 13d, during the loading of Fe onto a Cu2O-based material, the application of a magnetic field induced the valence electrons of Fe to transition to a high spin state.157 The spin rearrangement facilitated the migration of interstitial oxygen on the Cu2O surface, triggering the reconstruction of Cu clusters on the material.
 | ||
| Fig. 13 (a) A spontaneous chemical reaction process triggered by surface reconstruction (reproduced with permission from ref. 153, copyright 2023 by Wiley). (b) Low-temperature oxidation reconstruction leads to atomic rearrangement, introducing new catalytic sites (reproduced with permission from ref. 154, copyright 2024 by Wiley). (c) Poisoning reconstruction promotes the in situ growth of catalyst surfaces (reproduced with permission from ref. 155, copyright 2023 by Elsevier). (d) Magnetic field alters the valence electron orbitals of metal atoms (reproduced with permission from ref. 157, copyright 2023 by Elsevier). | ||
4.2. Spatial site complete reconstruction
During the catalytic reaction process, the structure and composition of the catalyst would change from the inside out, leading to the formation of new species or phases, thereby significantly altering the catalytic performance.158 This complete reconstruction phenomenon can modulate the formation and distribution of catalytic sites, thereby promoting the effect of utilization of internal active species, which should not have participated in the catalysis reaction.In terms of heterogeneous catalytic reactions, they generally tend to occur on the surface of materials. However, catalysts usually contain large amounts of internal components, which fail to efficiently engage in the reaction process, leading to inefficient component utilization inside catalysts. Nonetheless, the complete reconstruction of catalyst induced by external strong fields may provide a resolution to this challenge. The thermal field can enhance the molecular thermal motion of the material, leading to surface changes and internal reconstructions such as lattice distortions and site reconstructions, which could expose internal catalytic sites.159 During the pyrolysis process (Fig. 14a), the internal structure of the catalyst transformed from a core–shell structure to a hierarchical structure, and the surface catalytic sites rearranged, changing from a disordered to an ordered structure.160 It is noteworthy that catalytic sites could be distributed on each layer of the hierarchical structure, and the catalytic sites at different positions generally exhibited different efficiencies. The complete reconstructed catalyst exhibited two functional activities for both OERs and HERs. In addition to the reconstruction triggered by the thermal field, there is the reconstruction induced by the electric field and adsorbed coordinated species (Fig. 14b). As the oxidation potentials increase, specific chemical bonds within the active species break, leading to the leaching of molybdenum species and the formation of new species.161 After several cycles of the electric field, the catalyst was completely reconstructed from the outside to the inside. This unique structure avoided particle agglomeration during the catalytic process and promoted the sufficient utilization of the catalyst.
 | ||
| Fig. 14 (a) In a thermal field, the structure and site of the catalyst are reconstructed (reproduced with permission from ref. 160, copyright 2024 by Nature). (b) Schematic of geometric/phase changes, proposing the reconstruction mechanism from the perspective of crystal structure (reproduced with permission from ref. 161, copyright 2020 by Elsevier). | ||
The presence of the external environment can modulate intermolecular interactions to regulate the internal structure, surface morphology, and properties of nanospheres, thereby influencing the photothermal performance and function of the material.162,163 Site reconstruction involves structural changes within the entire catalyst system. Notably, the reconstruction is not always beneficial for catalytic reactions. Therefore, when designing and applying the 3D materials, it is crucial to utilize field effects to achieve precise control and optimization of material properties.
5. Industrial application
These analyses establish that the energy conversion efficiency in 3D architectures is fundamentally governed by spatially dependent reaction dynamics, where unique nanoconfinement effects enhance mass transfer mechanisms critical to catalytic performance. In this part, the photothermal catalytic power generation company effectively harnessed the synergies between material-based nanoreactors and chemical engineering reactors. This approach effortlessly bridged the gap from scientific theory to practical application, achieving revolutionary breakthroughs in the energy industry. In 2014, Ivanpah tower power station, the largest installed photothermal power station in the world, was officially commissioned and grid-connected. The commissioning and grid connection of Ivanpah has revolutionized the energy landscape. It demonstrates that large-scale solar power generation is not only feasible but also serves as a strong economic driving force, effectively promoting the development of the energy industry. Wikipedia, https://en.wikipedia.org/wiki/Ivanpah_Solar_Power_Facility, (accessed August 2024). At the same time, it has also expanded the industrial application scenarios of photothermal conversion in multiple fields.5.1. Industrial photothermal material preparation
In the industrial application of the photothermal effect, the design of a light-heat conversion unit is crucial due to the impact of harsh environments. Harsh conditions such as long-term temperature tolerance and poison resistance could result in photothermal material structure reconstruction, leading to a change in conversion efficiency.164,165 Indeed, these reconstructions caused by harsh conditions usually lead to material (catalyst) disability.166 In terms of catalysis, the utilization of catalysts with a 3D confined structure can effectively reduce their reconstruction effects. Especially, hollow structures exhibit advantages in industrial catalysis due to their unique structural feature.167 These catalysts possess interior cavities and specific shell structures, enabling the sufficient utilization of their internal space. The interior cavities can encapsulate high-performance catalytic components, enhancing the efficiency and selectivity through confinement effects. In the design of catalysts (Fig. 15b), 3D confined materials with appropriate pore size can accommodate sufficient active components and allow effective diffusion of reactants and products shuttling between the pores. The shell structure of the catalyst should have sufficient stability to tolerate temperature, pressure, and chemical environment changes during the reaction process.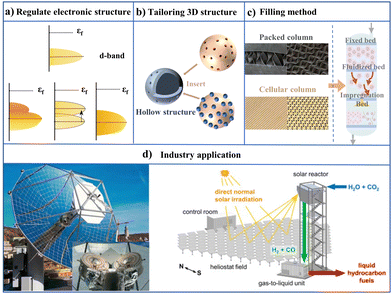 | ||
| Fig. 15 Schematic of the micro-structure engineering to macro-industry application. (a) Microscopic electronic states; (b) structural design and site distribution of catalysts; (c) integration of catalyst and the reactor; and (d) photothermal industry application (reproduced with permission from ref. 168, copyright 2020 by the American Chemical Society). | ||
5.2. Relationship between micro structure design and macro industrial process
The coupling of nanoreactors with chemical engineering reactors, serving as a bridge for connecting science and applications, plays a significant role in industries. The new generation of catalytic reaction systems necessitates the integration of interdisciplinary fields throughout the entire process, from micro to macro scales. As shown in Fig. 15a, it exhibits the electronic structure of 3D confined catalysts at the microscopic level. The introduction of additional metal atoms or doping can improve the electronic structure of catalytic materials, thereby enhancing complex reactions. Taking transition metals for example, it is useful to increase the number of metal atoms, which could broaden the d-band and facilitate charge transfer.169 When the d-band position is adjusted to be close to the Fermi level, it promotes the activation of electrons, thereby enhancing their participation in chemical reactions. Notably, by adjusting the position of the d-band center, the interaction between intermediates and active sites is optimized due to the decrease in the energy barrier of critical steps.170 In addition, the high spin state of the metal active sites is conducive to the adsorption and dissociation of intermediates.171In actual macro-engineering production, in addition to micro-structural regulation photothermal reactor packing, bed layer design is crucial for reaction efficiency and production. There are mainly two filling methods for catalysts: packed column and cellular column (Fig. 15c). The design of the bed layer falls into three major categories: (1) fixed bed catalyst loading design:172 based on the catalyst preparation method, the prepared 3D confined catalyst particles are uniformly and tightly packed into the fixed bed layer. During the packing process, it is necessary to ensure that the bed layer is even and free of gaps, allowing reactants to pass through the bed layer uniformly and make sufficient contact with the catalyst.173 After packing is completed, the bed layer needs to be fixed and sealed to prevent the catalyst particles from moving or leaking during the reaction process. (2) Design of catalyst loading in fluidized bed: based on the catalyst preparation method, 3D confined catalysts capable of suspension are prepared. According to the properties of reactants and catalysts, an appropriate fluidizing gas is selected.174 The flow rate of the fluidizing gas needs to be high enough to maintain the suspension state of the catalyst particles but avoid excessive flow rates that could lead to wear or breakage of the catalyst particles.175 In the fluidized bed reactor, precise control of parameters such as the flow rate and pressure of the flow gas is required to ensure that the catalyst particles can be uniformly suspended and efficiently catalyze the reaction. (3) Design of catalyst loading in impregnated packing bed: the supporting material is immersed in the impregnation, solution to fully load the active components of the 3D confined catalyst onto the support.176 Subsequently, the loaded support undergoes drying treatment to solidify the active components of the catalyst. The dried catalyst particles or blocks are then filled into the reactor, and corresponding operations are performed based on the type of the reactor (e.g. fixed bed or fluidized bed).
Different packing methods and the design of photothermal reactors can be used individually or in combination to accommodate the needs of various practical industrial production processes including photothermal CO2 conversion (Fig. 15d), nuclear wastewater purification and distillation, and photothermal supply equipment in space.168
6. Summary and outlook
6.1. Summary
Photothermal 3D void-confinement effects have made fundamental impacts in the field of heterogeneous catalysis during the past several decades. Although this novel fundamental theory is still developing, the related technologies are emerging and breaking through in some fields such as photothermal water evaporation and photothermal small molecule and ion capture. Once the connected techniques and cheap material design are overcome from the micro-theory to macro-engineering, the photothermal technology meets great-leap-forward development. In this review, we summarize the representative photothermal theories and the specialty of 3D photothermal effects. The related spatial site distribution brings a unique tandem reaction. The different pore structure coupling with types of catalytic sites is classified for addressing more high-value chemical production. Considering the selectivity and the efficiency, the catalytic site structure stability and the mass-energy transferring optimization are evaluated and should be paid more attention to for the future chemical industry. Maintaining structural stability of active sites remains challenging in complex fluid-phase industrial catalytic reactions. Notably, strategic regulation of coordination environments and external conditions can induce controllable reconstruction processes to generate adaptive active sites. These reconstructed sites will facilitate the transformation of the catalytic site structure to produce more valuable products. Finally, we further emphasized that the linkers are becoming increasingly important and the whole process assessment must be considered in future science-engineering interdisciplinary fields.6.2. Outlook
(1) In a complex reaction environment, such as a solid–gas reaction system with a mixed gas flow, if catalysts possess more than two types of catalytic sites, only regulating the spatial arrangement of catalytic sites to precisely control the product's selectivity often suffers from more difficulties. The detailed mechanism for more sites competitively adsorbed by gas-mixture molecules is unambiguous and difficult to be explained. The new reaction mode may be improved in the subsequent research work.(2) The interactive influence between the neighbor catalytic sites: in the confined space, there indeed exist interactive influences among the sites, which are located close to each other, but the specific mechanism of these interactions remains unclear. If the reactants and the intermediates remained in the pores for a prolonged duration, the slow diffusion of reactants can potentially lead to the formation of higher-order chemicals.
(3) Smart catalytic site transformation: artificial intelligence could assist smart catalytic site structure design and optimization. The main feature of the smart site is that this type of site can adapt to changing environments to transform its coordinated structure toward the beneficial direction in the production process. In the future, by combining machine learning and data-base, it is convenient to conclude the former investigations, and then further explore the relationship among the catalytic basic materials and the complex reaction environments with the catalytic priorities.
(4) Matching the pore morphology and the site function would achieve different selectivities for certain reactions. By regulating the mass flow and energy flow and balancing their impacts on the reaction process, the outstanding performance would ultimately be achieved.
Author contributions
Kun Zhao and Wenjing Wang proposed the topic of the review and supervised the manuscript. Tingting Xia and Bingyuan Zeng wrote and revised the manuscript. Jie Zhang and Jingwen Jiang revised the manuscript. Tenghu Wu and Weiying Pang modified and checked the format. All authors contributed to discussions and manuscript review.Data availability
The data that support the findings of this study are available from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52106141), the Fundamental Research Funds for the Central Universities (2023MS147), and the Natural Science Foundation of Hebei Province (B2022502005).References
- P. Peng, X. Gao, Z. Yan and S. Mintova, Diffusion and Catalyst Efficiency in Hierarchical Zeolite Catalysts, Natl. Sci. Rev., 2020, 7, 1726–1742 CrossRef PubMed
.
- X. Wang, G. Zhang, L. Yang, E. Sharman and J. Jiang, Material Descriptors for Photocatalyst/Catalyst Design, Wiley Interdiscip. Rev.:Comput. Mol. Sci., 2018, 8, e1369 Search PubMed
.
- W. Huang, Uniform Catalytic Nanocrystals: From Model Catalysts to Efficient Catalysts, Acc. Mater. Res., 2023, 4, 373–384 CrossRef CAS
.
- R. Cheula, A. Soon and M. Maestri, Prediction of Morphological Changes of Catalyst Materials under Reaction Conditions by Combined Ab Initio Thermodynamics and Microkinetic Modelling, Catal. Sci. Technol., 2018, 8, 3493–3503 RSC
.
- D. S. Minteer, P. Christopher and S. Linic, Recent Developments in Nitrogen Reduction Catalysts: A Virtual Issue, ACS Energy Lett., 2019, 4, 163–166 CrossRef
.
- E. Meloni, M. Martino, A. Ricca and V. Palma, Ultracompact Methane Steam Reforming Reactor Based on Microwaves Susceptible Structured Catalysts for Distributed Hydrogen Production, Int. J. Hydrogen Energy, 2021, 46, 13729–13747 CrossRef CAS
.
- Z. Zhao, X. Shen, X. Luo, M. Chen, M. Zhang, R. Yu, R. Jin and H. Zheng, Electric Field Redistribution Triggered Surface Adsorption and Mass Transfer to Boost Electrocatalytic Glycerol Upgrading Coupled with Hydrogen Evolution, Adv. Energy Mater., 2024, 14, 2400851 CrossRef CAS
.
- T. Ma, Y. Chen, A. N. Pavlenko and Q. Wang, Heat and Mass Transfer Advances for Energy Conservation and Pollution Control in a Renewable and Sustainable Energy Transition, Renewable Sustainable Energy Rev., 2021, 145, 111087 CrossRef
.
- H. Lin, Y. Yang, B. G. Diamond, T. H. Yan, V. I. Bakhmutov, K. W. Festus, P. Cai, Z. Xiao, M. Leng, I. Afolabi, G. S. Day, L. Fang, C. H. Hendon and H. C. Zhou, Integrating Photoactive Ligands into Crystalline Ultrathin 2D Metal–Organic Framework Nanosheets for Efficient Photoinduced Energy Transfer, J. Am. Chem. Soc., 2024, 146, 1491–1500 CrossRef CAS PubMed
.
- Y. Du, H. Zhang, L. Zou, X. Li, X. Lv, J. Ye, K. Deng, W. Tian and J. Ji, Manipulating 2D Membrane Interlayer Channels with Accelerated Mass-Transfer Behavior to Boost Solar Desalination, Small, 2024, 20, 2402105 CrossRef CAS PubMed
.
- J. Zhou, H. Liu and H. Wang, Photothermal Catalysis for CO2 Conversion, Chin. Chem. Lett., 2023, 34, 107420 CrossRef CAS
.
- F. Yu, C. Wang, Y. Li, H. Ma, R. Wang, Y. Liu, N. Suzuki, C. Terashima, B. Ohtani, T. Ochiai, A. Fujishima and X. Zhang, Photothermal Catalysis: Enhanced Solar Photothermal Catalysis over Solution Plasma Activated TiO2, Adv. Sci., 2020, 7, 2070092 CrossRef CAS
.
- J. Zhang, H. Chen, X. Duan, H. Sun and S. Wang, Photothermal Catalysis: From Fundamentals to Practical Applications, Mater. Today, 2023, 68, 234–253 CrossRef CAS
.
- A. Baldi and S. H. C. Askes, Pulsed Photothermal Heterogeneous Catalysis, ACS Catal., 2023, 13, 3419–3432 CrossRef CAS PubMed
.
- P. Anukunwithaya, J. J. Koh, J. C. C. Yeo, S. Liu, X. Hou, N. Liu and C. He, A Self-Regenerating 3D Sponge Evaporator with a Tunable Porous Structure for Efficient Solar Desalination, J. Mater. Chem. A, 2022, 10, 15743–15751 RSC
.
- R. Xu, H. Cui, K. Sun, X. Song, K. Yang, N. Wei, C. Hou and M. Zhao, Controllable 3D Interconnected Featured
Pore Structure of Transition Metal Borides-Carbonitride/MoS2 for Efficiently Solar Evaporation and Wastewater Purification, Chem. Eng. J., 2022, 446, 137275 CrossRef CAS
.
- W. Choi, K. Choi and C. Yu, Ultrafast Nanoscale Polymer Coating on Porous 3D Structures Using Microwave Irradiation, Adv. Funct. Mater., 2018, 28, 1704877 CrossRef
.
- S. Liu, J. Du, H. Wang, W. Jia, Y. Wu, P. Qi, S. Zhan, Q. Wu, J. Ma, N. Ren and W.-Q. Guo, How Hetero-Single-Atom Dispersion Reconstructed Electronic Structure of Carbon Materials and Regulated Fenton-like Oxidation Pathways, Water Res., 2024, 254, 121417 CrossRef CAS PubMed
.
- F. Pan, L. Fang, B. Li, X. Yang, T. O’Carroll, H. Li, T. Li, G. Wang, K.-J. Chen and G. N. Wu, OH-Immobilized Cu3 Clusters In Situ Reconstructed from Single-Metal Sites for Efficient CO2 Electromethanation in Bicontinuous Mesochannels, J. Am. Chem. Soc., 2024, 146, 1423–1434 CrossRef CAS PubMed
.
- I. Soltero, M. A. Kaliteevski, J. G. McHugh, V. Enaldiev and V. I. Fal’ko, Competition of Moiré Network Sites to Form Electronic Quantum Dots in Reconstructed MoX2/WX2 Heterostructures, Nano Lett., 2024, 24, 1996–2002 CrossRef CAS PubMed
.
- Z. Bao, N. Bing, H.-R. Yao, Y. Zhang, H. Xie and W. Yu, Three- Dimensional Interpenetrating Network Phase-Change Composites with High Photothermal Conversion and Rapid Heat Storage and Release, ACS Appl. Energy Mater., 2021, 4, 7710–7720 CrossRef CAS
.
- A. Michelson, A. Subramanian, K. Kisslinger, N. Tiwale, S. Xiang, E. Shen, J. S. Kahn, D. Nykypanchuk, H. Yan, C.-Y. Nam and O. Gang, Three-Dimensional Nanoscale Metal, Metal Oxide, and Semiconductor Frameworks through DNA-Programmable Assembly and Templating, Sci. Adv., 2024, 10, eadl0604 CrossRef CAS PubMed
.
- W. Zhang, X. Wang, X. Li, L. Zhang and F. Jiang, A 3D Porous Microsphere with Multistage Structure and Component Based on Bacterial Cellulose and Collagen for Bone Tissue Engineering, Carbohydr. Polym., 2020, 236, 116043 CrossRef CAS PubMed
.
- W. Zhang, H. Li, D. Feng, C. Wu, C. Sun, B. Jia, X. Liu and T. Ma, MOF-Derived 1D/3D N-Doped Porous Carbon for Spatially Confined Electrochemical CO2 Reduction to Adjustable Syngas, Carbon Energy, 2024, 6, e461 CrossRef CAS
.
- E. Yamada, H. Sakamoto, H. Matsui, T. Uruga, K. Sugimoto, M.-Q. Ha, H.-C. Dam, R. Matsuda and M. Tada, Three-Dimensional Visualization of Adsorption Distribution in a Single Crystalline Particle of a Metal–Organic Framework, J. Am. Chem. Soc., 2024, 146, 9181–9190 Search PubMed
.
- P. Sun, J. Liu, W. Zhang and D. Zhang, Gyroid-Structured Au Materials with 3D Interconnected Networks for Highly Efficient Heat Localization and Solar Vapor Generation, Energy Technol., 2024, 12, 2301393 CrossRef CAS
.
- W. Qu, M. Luo, Z. Tang, T. Zhong, H. Zhao, L. Hu, D. Xia, S. Tian, D. Shu and C. He, Accelerated Catalytic Ozonation in a Mesoporous Carbon-Supported Atomic Fe–N4 Sites Nanoreactor: Confinement Effect and Resistance to Poisoning, Environ. Sci. Technol., 2023, 57, 13205–13216 CrossRef CAS PubMed
.
- S. Mao, B. Zhao, Z. Wang, Y. Gong, G. Lü, X. Ma, L. Yu and Y. Wang, Tuning the Catalytic Performance for the Semi-Hydrogenation of Alkynols by Selectively Poisoning the Active Sites of Pd Catalysts, Green Chem., 2019, 21, 4143–4151 RSC
.
- Y. Tian, C. He, L. He, Z. Xu, H. Sui and X. Li, Doping Heteroatoms to Form Multiple Hydrogen Bond Sites for Enhanced Interfacial Reconstruction
and Separations, J. Hazard. Mater., 2024, 472, 134477 CrossRef CAS PubMed
.
- C. Huang, Y. Tong, J. Li, J. Peng, D. Dong, Y. Chen, M. Liu, W. Sun, Q. Wang, M. Zhu and F. He, Protonation-Induced Site and Field Reconstruction for Ultrafast Adsorptive Desulfurization over CuNC, Chem. Eng. J., 2023, 452, 139391 CrossRef CAS
.
- B. Kuchta, E. Dundar, F. Formalik, P. L. Llewellyn and L. Firlej, Adsorption-Induced Structural Phase Transformation in Nanopores, Angew. Chem., Int. Ed., 2017, 56, 16243–16246 CrossRef CAS PubMed
.
- J. C. Vivas-Báez, A. Servia, G. D. Pirngruber, A.-C. Dubreuil and D. J. Pérez-Martínez, Insights in the Phenomena Involved in Deactivation of Industrial Hydrocracking Catalysts through an Accelerated Deactivation Protocol, Fuel, 2021, 303, 120681 CrossRef
.
- M. A. Mohamed, M. F. M. Zain, L. Jeffery Minggu, M. B. Kassim, N. A. Saidina Amin, W. N. W. Salleh, M. N. I. Salehmin, M. F. Md Nasir and Z. A. Mohd Hir, Constructing Bio-Templated 3D Porous Microtubular C-Doped g-C3N4 with Tunable Band Structure and Enhanced Charge Carrier Separation, Appl. Catal., B, 2018, 236, 265–279 CrossRef CAS
.
- L. Xing, Y. Chen, Y. Yang, C. He, T. Wu, H. Xia, K. Shen, G. Tong and W. Wu, Incorporation of FexOy Nanoparticles into 3D Interlinked Porous Carbon Nanofiber Networks to Synergistically Enhance the Electrical Insulation, Electromagnetic Wave Absorbing/Shielding Performance and Thermal Conductivity, Chem. Eng. J., 2023, 469, 143952 CrossRef CAS
.
- D. Ping, S. Huang, S. Wu, Y. Zhang, S. Wang, X. Yang, L. Han, J. Tian, D. Guo, H.-J. Qiu and S. Fang, Confinement Effect and 3D Design Endow Unsaturated Single Ni Atoms with Ultrahigh Stability and Selectivity toward CO2 Electroreduction, Small, 2024, 20, 2309014 CrossRef CAS PubMed
.
- P. Pande, D. Haasmann, J. Han, H. A. Moghadam, P. Tanner and S. Dimitrijev, Electrical Characterization of SiC MOS Capacitors: A Critical Review, Microelectron. Reliab., 2020, 112, 113790 CrossRef CAS
.
- C. Dong, Q. Yu, P. R. Ye, P. Su, J. Liu and H. G. Wang, Hollow Carbon Sphere Nanoreactors Loaded with PdCu Nanoparticles: Void-Confinement Effects in Liquid-Phase Hydrogenations, Appl. Catal., B, 2024, 347, 123357 Search PubMed
.
- R. Cao, D. Xiao, M. Wang, Y. Gao and D. Ma, Solar-Driven Photocatalysis for Recycling and Upcycling Plastics, Appl. Catal., B, 2024, 341, 123357 CrossRef CAS
.
- M. F. Kuehnel, C. E. Creissen, C. D. Sahm, D. Wielend, A. Schlosser, K. L. Orchard and E. Reisner, ZnSe Nanorods as Visible-Light Absorbers for Photocatalytic and Photoelectrochemical H2 Evolution in Water, Angew. Chem., Int. Ed., 2019, 58, 5059–5063 CrossRef CAS PubMed
.
- C. Wu, Z. Xing, S. Yang, Z. Li and W. Zhou, Nanoreactors for Photocatalysis, Coord. Chem. Rev., 2023, 477, 214939 CrossRef CAS
.
- L. Kong, X. Zhang, C. Wang, F. Wan and L. Li, Synergic Effects of CuxO Electron Transfer Co-Catalyst and Valence Band Edge Control over TiO2 for Efficient Visible-Light Photocatalysis, Chin. J. Catal., 2017, 38, 2120–2131 CrossRef CAS
.
- F. Lv, W. Zhang, L. He, X. Bai, Y. Song and Y. Zhao, 3D Porous Flower-like CoAl2O4 to Boost the Photocatalytic CO2 Reduction Reaction, J. Mater. Chem. A, 2023, 11, 2826–2835 RSC
.
- S. Yang, H. Li, H. Li, H. Li, W. Qi, Q. Zhang, J. Zhu, P. Zhao and L. Chen, Rational Design of 3D Carbon Nitrides Assemblies with Tunable Nano-Building Blocks for Efficient Visible-Light Photocatalytic CO2 Conversion, Appl. Catal., B, 2022, 316, 121612 Search PubMed
.
- S. Wang, D. Song, L. Liao, M. Li, Z. Li and W. Zhou, Surface and Interface Engineering of BiOCl Nanomaterials and Their Photocatalytic Applications, Adv. Colloid Interface Sci., 2024, 324, 103088 CrossRef CAS PubMed
.
- L. Buzzetti, G. E. M. Crisenza and P. Melchiorre, Mechanistic Studies in Photocatalysis, Angew. Chem., Int. Ed., 2019, 58, 3730–3747 CrossRef CAS PubMed
.
- A. Yamakata and J. J. M. Vequizo, Curious Behaviors of Photogenerated Electrons and Holes at the Defects on Anatase, Rutile, and Brookite TiO2 Powders: A Review, J. Photochem. Photobiol., C, 2019, 40, 234–243 Search PubMed
.
- S. Yin, L. Sun, Y. Zhou, X. Li, J. Li, X. Song, P. Huo, H. Wang and Y. Yan, Enhanced Electron–Hole Separation in SnS2/Au/g-C3N4 Embedded Structure for Efficient CO2 Photoreduction, Chem. Eng. J., 2021, 406, 126776 Search PubMed
.
- L. Zhang, K. Zhou, Q. Wei, L. Ma, W. Ye, H. Li, B. Zhou, Z. Yu, C.-T. Lin, J. Luo and X. Gan, Thermal Conductivity Enhancement of Phase Change Materials with 3D Porous Diamond Foam for Thermal Energy Storage, Appl. Energy, 2019, 233–234, 208–219 Search PubMed
.
- Y. Li, Q. Zhang, Y. Chong, W.-H. Huang, C.-L. Chen, X. Jin, G. Chen, Z. Fan, Y. Qiu and D. Ye, Efficient Photothermal Catalytic Oxidation Enabled by Three-Dimensional Nanochannel Substrates, Environ. Sci. Technol., 2024, 58, 5153–5161 CrossRef CAS PubMed
.
- S. Yuan, Y. Li, J. Peng, Y. M. Questell-Santiago, K. Akkiraju, L. Giordano, D. J. Zheng, S. Bagi, Y. Román-Leshkov and Y. Shao-Horn, Conversion of Methane into Liquid Fuels—Bridging Thermal Catalysis with Electrocatalysis, Adv. Energy Mater., 2020, 10, 2002154 CrossRef CAS
.
- T. Xiao, Y. Wang, J. Wan, Y. Ma, Z. Yan, S. Huang and C. Zeng, Fe–N–C Catalyst with Fe-NX Sites Anchored Nano Carboncubes Derived from Fe-Zn-MOFs Activate Peroxymonosulfate for High-Effective Degradation of Ciprofloxacin: Thermal Activation and Catalytic Mechanism, J. Hazard. Mater., 2022, 424, 127380 CrossRef CAS PubMed
.
- P. Wu, X. Jin, Y. Qiu and D. Ye, Recent Progress of Thermocatalytic and Photo/Thermocatalytic Oxidation for VOCs Purification over Manganese-Based Oxide Catalysts, Environ. Sci. Technol., 2021, 55, 4268–4286 CrossRef CAS PubMed
.
- J. Gao, Q. Yan, L. Lv, X. Tan, J. Ying, K. Yang, J. Yu, S. Du, Q. Wei, R. Xiang, Y. Yao, X. Zeng, R. Sun, C.-P. Wong, N. Jiang, C.-T. Lin and W. Dai, Lightweight Thermal Interface Materials Based on Hierarchically Structured Graphene Paper with Superior Through-Plane Thermal Conductivity, Chem. Eng. J., 2021, 419, 129609 CrossRef CAS
.
- X. Yang, C. Li, Y. Ma, H. Chi, Z. Hu and J. Xie, High Thermal Conductivity of Porous Graphite/Paraffin Composite Phase Change Material with 3D Porous Graphite Foam, Chem. Eng. J., 2023, 473, 145364 CrossRef CAS
.
- S. Wu, T. Yan, Z. Kuai and W. Pan, Thermal Conductivity Enhancement on Phase Change Materials for Thermal Energy Storage: A Review, Energy Storage Mater., 2020, 25, 251–295 Search PubMed
.
- Y. Lin, Y. Jia, G. Alva and G. Fang, Review on Thermal Conductivity Enhancement, Thermal Properties and Applications of Phase Change Materials in Thermal Energy Storage, Renewable Sustainable Energy Rev., 2018, 82, 2730–2742 Search PubMed
.
- R. Hanus, M. T. Agne, A. J. E. Rettie, Z. Chen, G. Tan, D. Y. Chung, M. G. Kanatzidis, Y. Pei, P. W. Voorhees and G. J. Snyder, Lattice Softening Significantly Reduces Thermal Conductivity and Leads to High Thermoelectric Efficiency, Adv. Mater., 2019, 31, 1900108 CrossRef PubMed
.
- Z. Wang, Z. Yang, R. Fang, Y. Yan, J. Ran and L. Zhang, A State-of-the-Art Review on Action Mechanism of Photothermal Catalytic Reduction of CO2 in Full Solar Spectrum, Chem. Eng. J., 2022, 429, 132322 CrossRef CAS
.
- Z. Wang, Z. Yang, Z. C. Kadirova, M. Guo, R. Fang, J. He, Y. Yan and J. Ran, Photothermal Functional Material and Structure for Photothermal Catalytic CO2 Reduction: Recent Advance, Application and Prospect, Coord. Chem. Rev., 2022, 473, 214794 Search PubMed
.
- Y. Yang, S. Zhao, L. Cui, F. Bi, Y. Zhang, N. Liu, Y. Wang, F. Liu, C. He and X. Zhang, Recent Advancement and Future Challenges of Photothermal Catalysis for VOCs Elimination: From Catalyst Design to Applications, Green Energy Environ., 2023, 8, 654–672 Search PubMed
.
- Y. Wang, Q. Zhou, Y. Zhu and D. Xu, High Efficiency Reduction of CO2 to CO and CH4 via Photothermal Synergistic Catalysis of Lead-Free Perovskite Cs3Sb2I9, Appl. Catal., B, 2021, 294, 120236 Search PubMed
.
- F. Yu, C. Wang, Y. Li, H. Ma, R. Wang, Y. Liu, N. Suzuki, C. Terashima, B. Ohtani, T. Ochiai, A. Fujishima and X. Zhang, Photothermal Catalysis: Enhanced Solar Photothermal Catalysis over Solution Plasma Activated TiO2 (Adv. Sci. 16/2020), Adv. Sci., 2020, 7, 2070092 CrossRef CAS
.
- J. Zhang, Y. Li, J. Sun, H. Chen, Y. Zhu, X. Zhao, L.-C. Zhang, S. Wang, H. Zhang, X. Duan, L. Shi, S. Zhang, P. Zhang, G. Shao, M. Wu, S. Wang and H. Sun, Regulation of Energetic Hot Carriers on Pt/TiO2 with Thermal Energy for Photothermal Catalysis, Appl. Catal., B, 2022, 309, 121263 CrossRef CAS
.
- L. Wei, C. Yu, K. Yang, Q. Fan and H. Ji, Recent Advances in VOCs and CO Removal via Photothermal Synergistic Catalysis, Chin. J. Catal., 2021, 42, 1078–1095 Search PubMed
.
- J. Zhang, L. Wang, X. Zhao, L. Shi, H. Chen, S. Zhang, P. Zhang, S. Wang, L. Zhang, Y. Wang, X. Wang, Y. Zhu, H. Zhang, X. Duan, M. Wu, G. Shao, S. Wang and H. Sun, The Nature of Active Sites for Plasmon-Mediated Photothermal Catalysis and Heat-Coupled Photocatalysis in Dry Reforming of Methane7, Energy Environ. Mater., 2023, 6, e12416 CrossRef CAS
.
- L. Zhou, J. M. P. Martirez, J. Finzel, C. Zhang, D. F. Swearer, S. Tian, H. Robatjazi, M. Lou, L. Dong, L. Henderson, P. Christopher, E. A. Carter, P. Nordlander and N. J. Halas, Light-Driven Methane Dry Reforming with Single Atomic Site Antenna-Reactor Plasmonic Photocatalysts, Nat. Energy, 2020, 5, 61–70 CrossRef CAS
.
- Y. Yang, Z. Chai, X. Qin, Z. Zhang, A. Muhetaer, C. Wang, H. Huang, C. Yang, D. Ma, Q. Li and D. Xu, Light-Induced Redox Looping of a Rhodium/CexWO3 Photocatalyst for Highly Active and Robust Dry Reforming of Methane, Angew. Chem., Int. Ed., 2022, 61, e202200567 Search PubMed
.
- J. Yao, C. Wang, C. Zhang, S. Ma, L. Zhou, T. Wang, Q. Wang, H. Xu and T. Ding, Optoelectronic Tuning of Plasmon Resonances via Optically Modulated Hot Electrons, Natl. Sci. Rev., 2024, 11, nwad280 CrossRef PubMed
.
- X. Dong, L. Xu, J. Ma, Y. Li, Z. Yin, D. Chen, Q. Wang, J. Han, J. Qiu, Z. Yang and Z. Song, Enhanced Interfacial Charge Transfer and Photothermal Effect via In-Situ Construction of Atom Co-Sharing Bi Plasmonic/Bi4O5Br2 Nanosheet Heterojunction towards Improved Full-Spectrum Photocatalysis, Chem. Eng. J., 2023, 459, 141557 CrossRef CAS
.
- X. Jiang, J. Huang, Z. Bi, W. Ni, G. Gurzadyan, Y. Zhu and Z. Zhang, Plasmonic Active “Hot Spots”-Confined Photocatalytic CO2 Reduction with High Selectivity for CH4 Production, Adv. Mater., 2022, 34, 2109330 CrossRef CAS PubMed
.
- S.-V. Albers and B. H. Meyer, The Archaeal Cell Envelope, Nat. Rev. Microbiol., 2011, 9, 414–426 CrossRef CAS PubMed
.
- P. Riente, M. Fianchini, P. Llanes, M. A. Pericàs and T. Noël, Shedding Light on the Nature of the Catalytically Active Species in Photocatalytic Reactions Using Bi2O3 Semiconductor, Nat. Commun., 2021, 12, 625 CrossRef CAS PubMed
.
- B. Zhao, Y. Wang, X. Yao, D. Chen, M. Fan, Z. Jin and Q. He, Photocatalysis-Mediated Drug-Free Sustainable Cancer Therapy Using Nanocatalyst, Nat. Commun., 2021, 12, 1345 Search PubMed
.
- L. Shi, T. C. Squier, J. M. Zachara and J. K. Fredrickson, Respiration of Metal (Hydr)Oxides by Shewanella and Geobacter: A Key Role for Multihaem c-Type Cytochromes, Mol. Microbiol., 2007, 65, 12–20 CrossRef CAS PubMed
.
- J. Guo, B. L. Tardy, A. J. Christofferson, Y. Dai, J. J. Richardson, W. Zhu, M. Hu, Y. Ju, J. Cui, R. R. Dagastine, I. Yarovsky and F. Caruso, Modular Assembly of Superstructures from Polyphenol-Functionalized Building Blocks, Nat. Nanotechnol., 2016, 11, 1105–1111 CrossRef CAS PubMed
.
- J. Guo, M. Suástegui, K. K. Sakimoto, V. M. Moody, G. Xiao, D. G. Nocera and N. S. Joshi, Light-Driven Fine Chemical Production in Yeast Biohybrids, Science, 2018, 362, 813–816 Search PubMed
.
- H. Zhang, H. Liu, Z. Tian, D. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. Yang, Bacteria Photosensitized by Intracellular Gold Nanoclusters for Solar Fuel Production, Nat. Nanotechnol., 2018, 13, 900–905 Search PubMed
.
- T. J. Silhavy, D. Kahne and S. Walker, The Bacterial Cell Envelope, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414 Search PubMed
.
- Y. Yu, Z. Luo, D. M. Chevrier, D. T. Leong, P. Zhang, D. Jiang and J. Xie, Identification of a Highly Luminescent Au22(SG)18 Nanocluster, J. Am. Chem. Soc., 2014, 136, 1246–1249 CrossRef CAS PubMed
.
- B. Xiong, R. Xu, R. Zhou, Y. He and E. S. Yeung, Preventing UV Induced Cell Damage by Scavenging Reactive Oxygen Species with Enzyme-Mimic Au–Pt Nanocomposites, Talanta, 2014, 120, 262–267 CrossRef CAS PubMed
.
- G. Fleury and M. B. J. Roeffaers, Correlating Acid Site Distribution and Catalytic Activity in Dealuminated Mordenite at the Single- Particle Level, ACS Catal., 2020, 10, 14801–14809 Search PubMed
.
- Y. Fu, W. Ding, H. Lei, Y. Sun, J. Du, Y. Yu, U. Simon, P. Chen, Y. Shan, G. He and H. He, Spatial Distribution of Brønsted Acid Sites Determines the Mobility of Reactive Cu Ions in the Cu-SSZ-13 Catalyst during the Selective Catalytic Reduction of NOx with NH3, J. Am. Chem. Soc., 2024, 146, 11141–11151 CrossRef CAS PubMed
.
- L. Li, W. Han, Z. Tang, J. Zhang and G. Lu, Hard-Template Synthesis of Three-Dimensional Mesoporous Cu–Ce Based Catalysts with Tunable Architectures and Their Application in the CO Catalytic Oxidation, RSC Adv., 2016, 6, 64247–64257 Search PubMed
.
- Y. Xi, F. Dong, X. Xu, S. Wu, Z. Tang and J. Zhang, A Novel 3D Printed Technology to Construct a Monolithic Ultrathin Nanosheets Co3O4/SiO2 Catalyst for Benzene Catalytic Combustion, Nano Res., 2023, 16, 12173–12185 CrossRef CAS
.
- X. Huang, F. Dong, G. Zhang and Z. Tang, Design and Identify the Confinement Effect of Active Site Position on Catalytic Performance for Selective Catalytic Reduction of NO with NH3 at Low Temperature, J. Catal., 2023, 420, 134–150 CrossRef CAS
.
- G. Zhu, Y. Qi, F. Liu, S. Ma, G. Xiang, F. Jin, Z. Liu and W. Wang, Reconstructing 1D Fe Single-Atom Catalytic Structure on 2D Graphene Film for High-Efficiency Oxygen Reduction Reaction, ChemSusChem, 2021, 14, 866–875 Search PubMed
.
- Y. Li, T. Dong, P. Huang, J. Ji and H. Huang, Efficient HCHO Oxidation at Room Temperature via Maximizing Catalytic Sites in 2D Coralloid δ-MnO2@GO, Appl. Catal., B, 2024, 341, 123322 Search PubMed
.
- N. S. Powar, S. Kim, J. Lee, E. Gong, C. B. Hiragond, D. Kim, T. Zhang, M. Kim and S.-I. In, Unravelling the Effect of Ti3+/Ti4+ Active Sites Dynamic on Reaction Pathways in Direct Gas-Solid-Phase CO2 Photoreduction, Appl. Catal., B, 2024, 352, 124006 Search PubMed
.
- Y. He, L. Yin, N. Yuan and G. Zhang, Adsorption and Activation, Active Site and Reaction Pathway of Photocatalytic CO2 Reduction: A Review, Chem. Eng. J., 2024, 481, 148754 Search PubMed
.
- M. Liu, Y. Fu, S. Bi, S. Yang, X. Yang, X. Li, G. Z. Chen, J. He, Q. Xu and G. F. Zeng, Dimensionally-Controlled Interlayer Spaces of Covalent Organic Frameworks for the Oxygen Evolution Reaction, Chem. Eng. J., 2024, 479, 147682 CrossRef CAS
.
- C.-H. Lin, C.-H. Tsai, F.-G. Tseng, C.-C. M. Ma, H.-C. Wu and C.-K. Hsieh, Three-Dimensional Vertically Aligned Hybrid Nanoarchitecture of Two-Dimensional Molybdenum Disulfide Nanosheets Anchored on Directly Grown One-Dimensional Carbon Nanotubes for Use as a Counter Electrode in Dye-Sensitized Solar Cells, J. Alloys Compd., 2017, 692, 941–949 CrossRef CAS
.
- L. Kang, S. Liu, Q. Zhang, J. Zou, J. Ai, D. Qiao, W. Zhong, Y. Liu, S. C. Jun, Y. Yamauchi and J. Zhang, Hierarchical Spatial Confinement Unlocking the Storage Limit of MoS2 for Flexible High-Energy Supercapacitors, ACS Nano, 2024, 18, 2149–2161 CrossRef CAS PubMed
.
- J. Dai and H. Zhang, Recent Advances in Catalytic Confinement Effect within Micro/Meso-Porous, Special Issue: Emerging Crystalline Porous Materials, 2021, 17, p. 2005334 Search PubMed.
- H. Li, C. Guo, T. Zhang, P. Xue, R. Zhao, W. Zhou, W. Li, A. Elzatahry, D. Zhao and D. Chao, Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery, Nano Lett., 2022, 22, 4223–4231 Search PubMed
.
- X. Zhang, R. Bi, J. Wang, M. Zheng, J. Wang, R. Yu and D. Wang, Delicate Co-Control of Shell Structure and Sulfur Vacancies in Interlayer-Expanded Tungsten Disulfide Hollow Sphere for Fast and Stable Sodium Storage, Adv. Mater., 2023, 35, 2209354 CrossRef CAS PubMed
.
- R. Shi, J. Wang, J. Zhao, S. Liu, P. Hao, Z. Li and J. Ren, Cu Nanoparticles Encapsulated with Hollow Carbon Spheres for Methanol Oxidative Carbonylation: Tuning of the Catalytic Properties by Particle Size Control, Appl. Surf. Sci., 2018, 459, 707–715 Search PubMed
.
- P. Liu, Q. Lin, H. Pan, J. Zhao, C. Zhao and Y. Wang, Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen over Yolk– Shell Nanocatalyst Pd@HCS with Controlled Pd Nanoparticle Size, J. Catal., 2019, 377, 511–523 CrossRef CAS
.
- Y. Shen, Y. Shi, Z. Chen, S. Zhang, K. Chen, X. Luo, F. Guo, G. Wang and W. Shi, Core-Shell Nanoconfinement: Nanoreactors for Directional Electron Migration in Photothermal-Assisted Photocatalytic Hydrogen Production, Chem. Eng. J., 2024, 484, 149607 Search PubMed
.
- H. Zhou, W. Su, Y. Xing, J. Wang, W. Zhang, H. Jia, C. Hou, L. Ye and Y. Jian, Research Progress of Core-Shell Catalysts in the Field of Atmospheric Catalysis, J. Environ. Chem. Eng., 2024, 12, 113941 CrossRef CAS
.
- J. Wu, P. Li, Y.-T. (Frank) Pan, S. Warren, X. Yin and H. Yang, Surface Lattice-Engineered Bimetallic Nanoparticles and Their Catalytic Properties, Chem. Soc. Rev., 2012, 41, 8066–8074 Search PubMed
.
- C. She, S. Hong, N. Song, Z. Zhao, J. Li, Y. Niu, C. Li and H. Dong, In Situ Creation of Surface Defects on Pd@NiPd with Core–Shell Hierarchical Structure Toward Boosting Electrocatalytic Activity, Inorg. Chem., 2024, 63, 3199–3206 Search PubMed
.
- Y. Ding, J. R. Bertram, C. Eckert, R. R. Bommareddy, R. Patel, A. Conradie, S. Bryan and P. Nagpal, Nanorg Microbial Factories: Light-Driven Renewable Biochemical Synthesis Using Quantum Dot-Bacteria Nanobiohybrids, J. Am. Chem. Soc., 2019, 141, 10272–10282 CrossRef CAS PubMed
.
- B. Luo, Y.-Z. Wang, D. Li, H. Shen, L.-X. Xu, Z. Fang, Z. Xia, J. Ren, W. Shi and Y.-C. Yong, A Periplasmic Photosensitized Biohybrid System for Solar Hydrogen Production, Adv. Energy Mater., 2021, 11, 2100256 CrossRef CAS
.
- S. Koh, Y. Choi, I. Lee, G.-M. Kim, J. Kim, Y.-S. Park, S. Y. Lee and D. C. Lee, Light-Driven Ammonia Production by Azotobacter vinelandii Cultured in Medium Containing Colloidal Quantum Dots, J. Am. Chem. Soc., 2022, 144, 10798–10808 CrossRef CAS PubMed
.
- P.-C. Shi, J.-D. Yi, T.-T. Liu, L. Li, L.-J. Zhang, C.-F. Sun, Y.-B. Wang, Y.-B. Huang and R. Cao, Hierarchically Porous Nitrogen-Doped Carbon Nanotubes Derived from Core–Shell ZnO@zeolitic Imidazolate Framework Nanorods for Highly Efficient Oxygen Reduction Reactions, J. Mater. Chem. A, 2017, 5, 12322–12329 RSC
.
- F. Zhang, X. Liu, B. Wang, G. Wang and H. Wang, Bi@C Nanospheres with the Unique Petaloid Core–Shell Structure Anchored on Porous Graphene Nanosheets as an Anode for Stable Sodium- and Potassium-Ion Batteries, ACS Appl. Mater. Interfaces, 2021, 13, 59867–59881 CrossRef CAS PubMed
.
- Z. P. Chen, S. Y. Yao and L. C. Liu, 3D hierarchical porous structured carbon nanotube aerogel-supported Sn spheroidal particles: an efficient and selective catalyst for electrochemical reduction of CO2 to formate, J. Mater. Chem. A, 2017, 47, 24651–24656 RSC
.
- H. Chang, X. Liu, S. Zhao, Z. Liu, R. Lv, Q. Zhang and T.-F. Yi, Self- Assembled 3D N/P/S-Tridoped Carbon Nanoflower
with Highly Branched Carbon Nanotubes as Efficient Bifunctional Oxygen Electrocatalyst Toward High-Performance Rechargeable Zn-Air Batteries, Adv. Funct. Mater., 2024, 34, 2313491 CrossRef CAS
.
- E. Shukrun Farrell, Y. Schilt, M. Y. Moshkovitz, Y. Levi-Kalisman, U. Raviv and S. Magdassi, 3D Printing of Ordered Mesoporous Silica Complex Structures, Nano Lett., 2020, 20, 6598–6605 CrossRef CAS PubMed
.
- Y. Song, Y. Peng, H. Li, X. Sun, L. Li, C. Zhang and F. Yin, Mn3O4 Nanoparticles in Situ Embedded in TiO2 for High-Performance Na-Ion Capacitor: Balance between 3D Ordered Hierarchically Porous Structure and Heterostructured Interfaces, Chem. Eng. J., 2022, 447, 137450 CrossRef CAS
.
- J. Wordsworth, T. M. Benedetti, S. V. Somerville, W. Schuhmann, R. D. Tilley and J. J. Gooding, The Influence of Nanoconfinement on Electrocatalysis, Angew. Chem., Int. Ed., 2022, 61, e202200755 CrossRef CAS PubMed
.
- C. Weisbuch, H. Benisty and R. Houdré, Overview of Fundamentals and Applications of Electrons, Excitons and Photons in Confined Structures, J. Lumin., 2000, 85, 271–293 CrossRef CAS
.
- P. Lalanne, C. Sauvan and J. P. Hugonin, Photon Confinement in Photonic Crystal Nanocavities, Laser Photonics Rev., 2008, 2, 514–526 CrossRef CAS
.
- T. Yamada, K. Otsubo, R. Makiura and H. Kitagawa, Designer Coordination Polymers: Dimensional Crossover Architectures and Proton Conduction, Chem. Soc. Rev., 2013, 42, 6655–6669 RSC
.
- S.-J. Su, H. Sasabe, Y.-J. Pu, K. Nakayama and J. Kido, Tuning Energy Levels of Electron-Transport Materials by Nitrogen Orientation for Electrophosphorescent Devices with an ‘Ideal’ Operating Voltage, Adv. Mater., 2010, 22, 3311–3316 Search PubMed
.
- R. V. Petrescu, R. Aversa, S. Kozaitis, A. Apicella and F. I. Petrescu, Some Basic Reactions in Nuclear Fusion, Am. J. Eng. Appl. Sci., 2017, 10, 709–716 Search PubMed
.
- J. X. Xu, Y. Yuan, M. Liu, S. Zou, O. Chen and D. Zhang, Quantification of the Photon Absorption, Scattering, and On-Resonance Emission Properties of CdSe/CdS Core/Shell Quantum Dots: Effect of Shell Geometry and Volumes, Anal. Chem., 2020, 92, 5346–5353 CrossRef CAS PubMed
.
- E. E. Zhurkin, T. Van Hoof and M. Hou, Nanoscale Alloys and Core-Shell Materials: Model Predictions of the Nanostructure and Mechanical Properties, Phys. Rev. B:Condens. Matter Mater. Phys., 2007, 75, 224102 CrossRef
.
- Y. Tang, Z. Liu, Y. Li, F. Zhao, P. Fan and K. J. Chua, Mixing Process of Two Streams within a Steam Ejector from the Perspectives of Mass, Momentum and Energy Transfer, Appl. Therm. Eng., 2021, 185, 116358 Search PubMed
.
- Y. Liu, J. Zhang, X. Zhang, B. Li, X. Wang, H. Cao, D. Wei, Z. Zhou and A. K. Cheetham, Magnetic catalysts as nanoactuators to achieve simultaneous momentum-transfer and continuous- flow hydrogen production, J. Mater. Chem. A, 2016, 4, 4280–4287 RSC
.
- Y. Wu, Y. Wang, Z. Wang and X. Li, Highly Dispersed CoP on Three- Dimensional Ordered Mesoporous FeP for Efficient Electrocatalytic Hydrogen Production, J. Mater. Chem. A, 2021, 9, 23574–23581 RSC
.
- Y. Chen, Y. Tu, Y. Zhang, J. Lu and B. Fang, Fabrication and Enhanced Electrocatalytic Activity of TiO2 Nanotubes Based Three-Dimensionally Macroporous SnO2 with Mesoporous Walls, Chem. Eng. J., 2017, 311, 100–110 CrossRef CAS
.
- Y.-F. Xu, H.-S. Rao, B.-X. Chen, Y. Lin, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, Achieving Highly Efficient Photoelectrochemical Water Oxidation with a TiCl4 Treated 3D Antimony-Doped SnO2 Macropore/Branched α-Fe2O3 Nanorod Heterojunction Photoanode, Adv. Sci., 2015, 2, 1500049 CrossRef PubMed
.
- B. Lin, G. Yang, B. Yang and Y. Zhao, Construction of Novel Three Dimensionally Ordered Macroporous Carbon Nitride for Highly Efficient Photocatalytic Activity, Appl. Catal., B, 2016, 198, 276–285 CrossRef CAS
.
- H. Wang, S. Fu, B. Shang, S. Jeon, Y. Zhong, N. J. Harmon, C. Choi, E. A. Stach and H. Wang, Solar-Driven CO2 Conversion via Optimized Photothermal Catalysis in a Lotus Pod Structure, Angew. Chem., Int. Ed., 2023, 62, e202305251 CrossRef CAS PubMed
.
- S. Zhu, C. Huang, X. Li, X. Chen, H. Ye, Z. Xue, W. Hu and T. Wang, Enhanced Photothermal Conversion in 3D Stacked Metal– Organic Framework Nanosheets, Aggregate, 2024, 5, e529 CrossRef CAS
.
- Y. Bu, Y. Zhou, W. Lei, L. Ren, J. Xiao, H. Yang, W. Xu and J. Li, A Bioinspired 3D Solar Evaporator with Balanced Water Supply and Evaporation for Highly Efficient Photothermal Steam Generation, J. Mater. Chem. A, 2022, 10, 2856–2866 Search PubMed
.
- Y. Wang, C. Wang, X. Song, M. Huang, S. K. Megarajan, S. F. Shaukat and H. Jiang, Improved Light-Harvesting and Thermal Management for Efficient Solar-Driven Water Evaporation Using 3D Photothermal Cones, J. Mater. Chem. A, 2018, 6, 9874–9881 RSC
.
- X. Meng, W. Xu, Z. Li, J. Yang, J. Zhao, X. Zou, Y. Sun and Y. Dai, Coupling of Hierarchical Al2O3/TiO2 Nanofibers into 3D Photothermal Aerogels Toward Simultaneous Water Evaporation and Purification, Adv. Fiber Mater., 2020, 2, 93–104 CrossRef CAS
.
- Y.-Y. Lou, J.-M. Fontmorin, A. Amrane, F. Fourcade and F. Geneste, Metallic Nanoparticles for Electrocatalytic Reduction of Halogenated Organic Compounds: A Review, Electrochim. Acta, 2021, 377, 138039 CrossRef CAS
.
- T. Hermanns, C. Pichlo, U. Baumann and K. Hofmann, A Structural Basis for the Diverse Linkage Specificities within the ZUFSP Deubiquitinase Family, Nat. Commun., 2022, 13, 401 CrossRef CAS PubMed
.
- X. Qiu, W. Zhong, C. Bai and Y. Li, Encapsulation of a Metal–Organic Polyhedral in the Pores of a Metal–Organic Framework, J. Am. Chem. Soc., 2016, 138, 1138–1141 Search PubMed
.
- E. Gomez, X. Nie, J. H. Lee, Z. Xie and J. G. Chen, Tandem Reactions of CO2 Reduction and Ethane Aromatization, ACS, 2019.
- J. Wu, G.-G. Chang, Y.-Q. Peng, X.-C. Ma, S.-C. Ke, S.-M. Wu, Y.-X. Xiao, G. Tian, T. Xia and X.-Y. Yang, Spatial Acid–Base–Pd Triple- Sites of a Hierarchical Core–Shell Structure for Three-Step Tandem Reaction, Chem. Commun., 2020, 56, 6297–6300 Search PubMed
.
- J. Lu, J. Dimroth and M. Weck, Compartmentalization of Incompatible Catalytic Transformations for Tandem Catalysis, J. Am. Chem. Soc., 2015, 137, 12984–12989 Search PubMed
.
- B. Chen, L. Gong, N. Li, H. Pan, Y. Liu, K. Wang and J. Jiang, Tandem Catalysis for Enhanced CO2 to Ethylene Conversion in Neutral Media, Adv. Funct. Mater., 2024, 34, 2310029 Search PubMed
.
- S. Mo, X. Zhao, S. Li, L. Huang, X. Zhao, Q. Ren, M. Zhang, R. Peng, Y. Zhang, X. Zhou, Y. Fan, Q. Xie, Y. Guo, D. Ye and Y. Chen, Non-Interacting Ni and Fe Dual-Atom Pair Sites in N-Doped Carbon Catalysts for Efficient Concentrating Solar-Driven Photothermal CO2 Reduction, Angew. Chem., Int. Ed., 2023, 62, e202313868 CrossRef CAS PubMed
.
- Y. Cheng, X. Zhou, Q.-M. Pan, L.-F. Zhang, Y.-F. Cao and T. Qian, Bimetallic Active Site Nuclear-Shell Heterostructure Enables Efficient Dual-Functional Electrocatalysis in Alkaline Media, Rare Met., 2023, 42, 3024–3033 Search PubMed
.
- Y. Chen, X.-Y. Li, Z. Chen, A. Ozden, J. E. Huang, P. Ou, J. Dong, J. Zhang, C. Tian, B.-H. Lee, X. Wang, S. Liu, Q. Qu, S. Wang, Y. Xu, R. K. Miao, Y. Zhao, Y. Liu, C. Qiu, J. Abed, H. Liu, H. Shin, D. Wang, Y. Li, D. Sinton and E. H. Sargent, Efficient Multicarbon Formation in Acidic CO2 Reduction via Tandem Electrocatalysis, Nat. Nanotechnol., 2024, 19, 311–318 CrossRef CAS PubMed
.
- L. Lu, Y. Liu, J. Fan, L. Wang, Y. Lin, D. Xu, Z. Dai and M. Han, Engineering Bimetal Cu, Co Sites on 3D N-Doped Porous Carbon Nanosheets for Enhanced Oxygen Reduction Electrocatalysis, Chem. Commun., 2020, 56, 10010–10013 RSC
.
- J. Zhu, W. Shao, X. Li, X. Jiao, J. Zhu, Y. Sun and Y. Xie, Asymmetric Triple-Atom Sites Confined in Ternary Oxide Enabling Selective CO2 Photothermal Reduction to Acetate, J. Am. Chem. Soc., 2021, 143, 18233–18241 CrossRef CAS PubMed
.
- M. Sun, H. H. Wong, T. Wu, Q. Lu, L. Lu, C. H. Chan, B. Chen, A. W. Dougherty and B. Huang, Double-Dependence Correlations in Graphdiyne-Supported Atomic Catalysts to Promote CO2RR toward the Generation of C2 Products, Adv. Energy Mater., 2023, 13, 2203858 CrossRef CAS
.
- C. Hou, Z. Li, C. Fei, Y. Li, Y. Wang, T. Zhao, Y. Quan, D. Chen, X. Li, W. Bao and Y. Yang, Active Acoustic Field Modulation of Ultrasonic Transducers with Flexible Composites, Commun. Phys., 2023, 6, 1–11 CrossRef
.
- V. V. Welborn and T. Head-Gordon, Fluctuations of Electric Fields in the Active Site of the Enzyme Ketosteroid Isomerase, J. Am. Chem. Soc., 2019, 141, 12487–12492 CrossRef CAS PubMed
.
- Y. Basak, J.-H. Jeoung, L. Domnik and H. Dobbek, Stepwise O2- Induced Rearrangement and Disassembly of the [NiFe4(OH)(M3-S)4] Active Site Cluster of CO Dehydrogenase, Angew. Chem., Int. Ed., 2023, 62, e202305341 CrossRef CAS PubMed
.
- P. Li, S. Hou, Q. Wu, Y. Chen, B. Wang, H. Ren, J. Wang, Z. Zhai, Z. Yu, C. J. Lambert, C. Jia and X. Guo, The Role of Halogens in Au– S Bond Cleavage for Energy-Differentiated Catalysis at the Single-Bond Limit, Nat. Commun., 2023, 14, 7695 CrossRef CAS PubMed
.
- X. Yu, H. Zhao, P. Li and M. J. Koh, Iron-Catalyzed Tunable and Site- Selective Olefin Transposition, J. Am. Chem. Soc., 2020, 142, 18223–18230 Search PubMed
.
- X. Liu, C. Ao, X. Shen, L. Wang, S. Wang, L. Cao, W. Zhang, J. Dong, J. Bao, T. Ding, L. Zhang and T. Yao, Dynamic Surface Reconstruction of Single-Atom Bimetallic Alloy under Operando Electrochemical Conditions, Nano Lett., 2020, 20, 8319–8325 CrossRef CAS PubMed
.
- X. Zhou and C. Pu, Proton Shuttle-Assisted Surface Reconstruction toward Nonpolar Facets-Terminated Zinc-Blende CdSe/CdS Core/Shell Quantum Dots, J. Am. Chem. Soc., 2023, 145, 26287–26295 Search PubMed
.
- Y. Tian, M. R. Sakr, J. M. Kinder, D. Liang, M. J. MacDonald, R. L. J. Qiu, H.-J. Gao and X. P. Gao, One-Dimensional Quantum Confinement Effect Modulated Thermoelectric Properties in InAs Nanowires, ACS Publications.
- Z. Ma and S. Liu, A review of 3D reconstruction techniques in civil engineering and their applications, Adv. Eng. Inf., 2018, 37, 163–174 Search PubMed
.
- P. Goyal, P. Chingangbam and S. Appleby, Morphology of CMB Fields—Effect of Weak Gravitational Lensing, J. Cosmol. Astropart. Phys., 2020, 2020, 020 CrossRef CAS
.
- C. Wu, X. Wang, Y. Tang, H. Zhong, X. Zhang, A. Zou, J. Zhu, C. Diao, S. Xi, J. Xue and J. Wu, Origin of Surface Reconstruction in Lattice Oxygen Oxidation Mechanism Based-Transition Metal Oxides: A Spontaneous Chemical Process, Angew. Chem., 2023, 135, e202218599 CrossRef
.
- W. Pang, X. Du, Y. Yang, C. Long, Y. Li, C. Hu, R. Xu, M. Tian, J. Xie, W. Wang, J. Guo, B. Li, P. Zhang, D. Fu and K. Zhao, Oxygen Atom Coordinative Position Inducing Catalytic Sites Structure Reconstruction for Enhanced Photocatalytic H2 Evolution, Adv. Funct. Mater., 2024, 34, 2400466 CrossRef CAS
.
- J. Feng, J. Li, L. Qiao, D. Liu, P. Zhou, J. Ni and H. Pan, Reconstructed Anti-Poisoning Surface for Enhanced Electrochemical CO2 Reduction on Cu-Incorporated ZnO, Appl. Catal., B, 2023, 330, 122665 CrossRef CAS
.
- Y. Lu, Y. Wang, L. Zhu, L. Yang and L. Wang, Electric Field Tuning of Magnetic States in Single Magnetic Molecules, Phys. Rev. B, 2022, 106, 064405 Search PubMed
.
- M. Jiang, S. Gao, W. Shi, Z. Guo, X. Wu, Y. Wang, J. You, J. Zeng, H. Zeng, X. Hou, Y. Zhang, Q. Zhu, K. Huang and S. Feng, Magnetic- Field-Dominated Spin-Driven Lattice Deformation of 2D FeO/Cu2O Composites for CO2 Photocatalytic C–C Coupling, Chem Catal., 2023, 3, 100808 CrossRef CAS
.
- G. Liu, J. Yang, K. Zhang, H. Wu, H. Yan, Y. Yan, Y. Zheng, Q. Zhang, D. Chen, L. Zhang, Z. Zhao, P. Zhang, G. Yang and H. Chen, Recent Progress on the Development of Bioinspired Surfaces with High Aspect Ratio Microarray Structures: From Fabrication to Applications, J. Controlled Release, 2024, 367, 441–469 CrossRef CAS PubMed
.
- L. Zhang, W. Wei, Y. Zhang, C. Shen, A. van den Hengel and Q. Shi, Cluster Sparsity Field: An Internal Hyperspectral Imagery Prior for Reconstruction, Int. J. Comput. Vis., 2018, 126, 797–821 CrossRef
.
- M. Hou, L. Zheng, D. Zhao, X. Tan, W. Feng, J. Fu, T. Wei, M. Cao, J. Zhang and C. Chen, Microenvironment Reconstitution of Highly Active Ni Single Atoms on Oxygen-Incorporated Mo2C for Water Splitting, Nat. Commun., 2024, 15, 1342 Search PubMed
.
- X. Liu, J. Meng, K. Ni, R. Guo, F. Xia, J. Xie, X. Li, B. Wen, P. Wu, M. Li, J. Wu, X. Wu, L. Mai and D. Zhao, Complete Reconstruction of Hydrate Pre-Catalysts for Ultrastable Water Electrolysis in Industrial-Concentration Alkali Media, Cell Rep. Phys. Sci., 2020, 1, 100241 Search PubMed
.
- J. Qi, R. Wang, X. Chen, F. Wu, W. Shen, M. Li, R. He and X. Liu, Modulation of Intermolecular Interactions in Hole Transporting Materials for Improvement of
Perovskite Solar Cell Efficiency: A Strategy of Trifluoromethoxy Isomerization, J. Mater. Chem. A, 2024, 12, 4067–4076 RSC
.
- H. Jiang, T. Yan, W. Pang, M. Cheng, Z. Zhao, T. He, Z. Wang, C. Li, S. Sun and S. Hu, Incomplete Ionic Interactions and Hydrogen Bonds Constructing Elastomers with Water Accelerated Self- Healing and Self-Healing Strengthening Capacities, Chem. Eng. J., 2024, 489, 151074 CrossRef CAS
.
- R. Xiong, W. Ren, Z. Wang and M. Zhang, Triphenylphosphine as Efficient Antidote for the Sulfur-Poisoning of the Pd/C Hydrogenation Catalyst, ChemCatChem, 2021, 13, 548–552 CrossRef CAS
.
- R. Ahmad and A. K. Singh, Simultaneous Site Adsorption Shift and Efficient CO Oxidation Induced by V and Co in Pt Catalyst, J. Phys. Chem. C, 2017, 121, 12807–12816 CrossRef CAS
.
- W. Han, S. Wu, F. Dong, W. Han, Y. Chu, L. Su and Z. Tang, A Confined Growth Strategy to Construct 3DOM SiO2 Nanoreactor In-Situ Embedded Co3O4 Nanoparticles Catalyst for the Catalytic Combustion of VOCs: Superior H2O and SO2 Resistance, Nano Res., 2024, 17, 207–220 CrossRef CAS
.
- W. Han, Y. Wang, J. Wan and D. Wang, Eliminating Hysteresis of Perovskite Solar Cells with Hollow TiO2 Mesoporous Electron Transport Layer, Chem. Res. Chin. Univ., 2022, 38, 117–122 CrossRef CAS
.
- C. Song, X. Liu, M. Xu, D. Masi, Y. Wang, Y. Deng, M. Zhang, X. Qin, K. Feng, J. Yan, J. Leng, Z. Wang, Y. Xu, B. Yan, S. Jin, D. Xu, Z. Yin, D. Xiao and D. Ma, Photothermal Conversion of CO2 with Tunable Selectivity Using Fe-Based Catalysts: From Oxide to Carbide, ACS Catal., 2020, 10, 10364–10374 CrossRef CAS
.
- X. Wang, Z. Kang, D. Wang, Y. Zhao, X. Xiang, H. Shang and B. Zhang, Electronic Structure Regulation of the Fe-Based Single-Atom Catalysts for Oxygen Electrocatalysis, Nano Energy, 2024, 121, 109268 CrossRef CAS
.
- R. Li and D. Wang, Superiority of Dual-Atom Catalysts in Electrocatalysis: One Step Further Than Single-Atom Catalysts, Adv. Energy Mater., 2022, 12, 2103564 CrossRef CAS
.
- Z. Wang, Y. Wang, W. Wang, D. Wu, Q. Wu and H. Hu, Highly Selective Production of Singlet Oxygen by Manipulating the Spin State of Single-Atom Co–N Moieties and Electron Localization, Appl. Catal., B, 2023, 324, 122248 Search PubMed
.
- A. G. Dixon and B. Partopour, Computational Fluid Dynamics for Fixed Bed Reactor Design, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 109–130 CrossRef CAS PubMed
.
- N. Jurtz, G. D. Wehinger, U. Srivastava, T. Henkel and M. Kraume, Validation of Pressure Drop Prediction and Bed Generation of Fixed-Beds with Complex Particle Shapes Using Discrete Element Method and Computational Fluid Dynamics, AIChE J., 2020, 66, e16967 CrossRef CAS
.
- J. W. Chew, W. C. Q. LaMarche and R. A. Cocco, 100 Years of Scaling up Fluidized Bed and Circulating Fluidized Bed Reactors, Powder Technol., 2022, 409, 117813 CrossRef CAS
.
- A. Helmi, R. J. W. Voncken, A. J. Raijmakers, I. Roghair, F. Gallucci and M. van Sint Annaland, On Concentration Polarization in Fluidized Bed Membrane Reactors, Chem. Eng. J., 2018, 332, 464–478 CrossRef CAS
.
- J. Liu, C. Kong, Y. Jiang, X. Wu, X. Liao, S. Zeng, T. Wang, M. Fang and Z. Zhang, Evaluating CO2 Desorption Performance of MEA Solution with MnOx/HZ Catalytic Packings by Visualization Method, Chem. Eng. J., 2024, 479, 147650 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this paper. |
| This journal is © the Partner Organisations 2025 |




