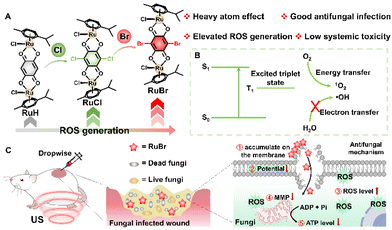
Heavy atom engineering of Ru(II) complex based sonosensitizers for enhancing antifungal therapy†
Qian
Li‡
ab,
Yida
Pang‡
bc,
Longcan
Mei‡
b,
Shiming
Liang‡
d,
Huiling
Wang
b,
Yujia
Jiao
e,
Sheng
Qiu
*a,
Hui
Chen
*e,
Xiwen
Xing
*f and
Yao
Sun
 *b
*b
aDepartment of Neurosurgery, Fifth School of Clinical Medicine of Zhejiang Chinese Medical University (Huzhou Central Hospital), Huzhou Key Laboratory of Basic Research and Clinical Translation for Neuromodulation, Huzhou 313099, P. R. China. E-mail: qius2001@126.com
bState Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan 430079, China. E-mail: sunyaogbasp@ccnu.edu.cn
cHubei Jiangxia Laboratory, Wuhan 430200, China
dNational Key Laboratory of Agricultural Microbiology, College of Biomedicine and Health, Huazhong Agricultural University, Wuhan 430070, China
eDepartment of Dermatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. E-mail: chenhui@tjh.tjmu.edu.cn
fDepartment of Biotechnology, College of Life Science and Technology, Jinan University, Guangzhou 510632, China. E-mail: xingxiwen@jnu.edu.cn
First published on 17th February 2025
Abstract
Despite the advances in antifungal therapy in the past decade, the issues of fungal resistance and the lack of effective treatments are still of major concern in clinical practice. Recently, sonodynamic therapy (SDT) has been at the forefront of the research in biomedicine, yet the employment of sonosensitizers and SDT on antifungal infections is still in its early stages. Herein, we designed and synthesized a series of Ru(II) complex-based sonosensitizers (RuH–RuBr) with enhanced ultrasound-triggered ROS generation for antifungal applications. The heavy atom (etc. Br) engineering strategy has been well employed to narrow the HOMO–LUMO energy gap of Ru(II) sonosensitizers, particularly in RuBr, which resulted in a significant boost in ROS generation (11.8 fold). In vitro results indicated that RuBr demonstrated both good anti-Candida albicans activity (MIC = 5 μM) and low mammalian cell toxicity (survival rate > 80%) under US irradiation. Further mechanism investigation suggested that RuBr initially aggregated on the fungal cell membrane and subsequently led to ultrasound-activated ROS accumulation, resulting in mitochondrial damage and triggering changes in the mitochondrial membrane potential. In vivo studies also revealed that RuBr exhibited similar antifungal performance but lower systemic toxicity when compared to the conventional clinical antifungal amphotericin B (AmB). This research offers significant insights into the design of high-performance sonosensitisers and lays the foundation for innovative antifungal therapeutic strategies.
Introduction
Fungal infections present a significant global health challenge, accounting for an estimated 1 billion cases annually, with approximately 3.75 million deaths directly attributable to these infections, exhibiting an upward trend.1,2 Unfortunately, there are only four major classes of clinical antifungal agents, a situation primarily hindered by the very similar structure between fungi and mammalian cells.3–6 The development of effective and potent antifungal agents is further complicated by the highly adaptive nature of the fungal genome and the rapid dissemination of antifungal resistance genes, leading to the emergence of “super fungi” and drug-resistant fungi.7–10 This scenario intensifies the difficulties associated with treating fungal infections. Consequently, concerted research endeavours must be undertaken to engineer highly efficacious and innocuous antifungal agents and pioneering therapeutic modalities.Recently, we and other researchers have made remarkable progress in exploring Ru(II)-based complexes for antibacterial and anticancer applications due to their outstanding properties.11–20 However, Ru(II)-based complexes are still in their initial stage for antifungal infections. Previous literature has indicated that Ru(II)-based complexes demonstrate a certain antifungal activity by generating reactive oxygen species (ROS) and disrupting the cellular structure of Candida albicans (C. albicans) with minimum inhibitory concentrations (MIC) frequently surpassing 200 μg mL−1.21 This finding suggests that the antifungal efficacy can be demonstrated by promoting the accumulation of ROS and disrupting cellular structures, which has inspired the development of reagents that generate higher levels of ROS for enhanced antifungal activity. Sonosensitizers especially Ru(II)-based have been recently highlighted for their ability to efficiently generate ROS under ultrasound (US) irradiation.22–27 This approach may not only help mitigate the emergence of fungal drug resistance, but also minimize toxic side effects by activating the complexes only under specific conditions.28–31 Moreover, the deeper penetration capabilities of sonosensitizers under ultrasound (US) irradiation may make them suitable for deep-organ sonodynamic therapy (SDT) for fungal infections.32–35 Despite their potential, current sonosensitizers are still hindered by their unsatisfactory SDT efficiency.36–39 Therefore, there is an urgent demand for developing high-performance sonosensitizers and investigating their structure–activity relationships to provide valuable insights for future design and application.
Herein, we explored the structure–activity relationship of Ru(II)-based sonosensitizers and elevated ROS generation for potent antifungal infections (Scheme 1). Based on our previous reports,12,13 we designed the sonosensitizer RuH, and then introduced the heavy atoms Br or Cl into RuH to obtain RuBr and RuCl with enhanced ROS generation for antifungal purposes. It revealed that the SDT effect of RuBr in the solution was increased 4.2-fold and 4.4-fold compared to that of RuH and Ru(bpy)3Cl2, respectively. In vitro results show that RuBr has MIC values (5 μM) for C. albicans and C. glabrata that are comparable to those of the commercial antifungal drug amphotericin B (AmB) under US irradiation conditions, with low toxicity to mammalian cells (even at 80 μM with >80% survival). The wounds of C. albicans infected mice were well-healed after 6 days following treatment with RuBr under US irradiation, which exhibited lower systemic toxicity. In short, we successfully developed a high-performance metal-based sonosensitizer (RuBr) with excellent in vivo antifungal effects as well as satisfactory biosafety.
Experimental
Materials
C. albicans (SC5314) were provided by Prof. Leah Cowen at the University of Toronto (Ontario, Canada). Candida glabrata (C. glabrata, ATCC2001) was purchased from the Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Science and Peking Union Medical College (Jiangsu, China). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA), 1,3-diphenylisobenzofuran (DPBF), dihydroethidium (DHE), coumarin-3-carboxylic acid (3-CCA), acridine orange hydrochloride (AO), and ethidium bromide (EB) were obtained from Med Chem Express (Shanghai, China). 2,2,6,6-Tetramethylpiperidoxyl (TEMPO) and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were purchased from Thermo Scientific Chemicals (Shanghai, China). The YPD medium (YPD) and YPD agar Medium (YPDA) were purchased from Beijing Zoman Biotechnology Co., Ltd (Beijing, China). Dulbecco's modified Eagle's culture medium (DMEM) was bought from Wuhan Pricella Biotechnology Co., Ltd (Wuhan, China).Instruments
The UV-vis spectrum was collected using a UV-1900i spectrophotometer (SHIMADZU, Japan) or microplate reader (BioTek, USA). The fluorescence spectrum was recorded using an LS55 fluorescence spectrometer (PerkinElmer, USA). The zeta potential was tested using a Zetasizer Nano ZS (Malvern, Holland). Scanning electron microscopy (SEM) images were captured using a scanning electron microscope (Hitachi S4800). The ultrasonic irradiation instrument used is DJO Chattanooga 2776, and the parameters are set to 1 MHz, 50% duty cycle, 1 W cm−2 in this article. 1H NMR, 13C NMR, and 2D COSY NMR spectra were acquired on a Bruker 400 MHz magnetic resonance spectrometer. Data for 1H NMR spectra were reported as follows: chemical shifts were reported as δ in units of parts per million (ppm); multiplicities were reported as follows: s (singlet), d (doublet), t (triplet), m (multiplet); coupling constants were reported as a J value in Hertz (Hz).Synthesis of RuH, RuCl and RuBr
The synthetic methods are shown in the ESI.†Assessment of ROS production
DCFH was used as a ROS detection probe.40 DCFH (10 mM in DMSO) was incubated with sodium hydroxide (10 mM in ddH2O) for 2 h in advance. After activation, DCFH (10 mM, 0.3 μL) was exposed to US irradiation with RuH, RuCl, RuBr and Ru(bpy)3Cl2 (20 μM, 300 μL, dissolved in ddH2O containing 5% DMF) and the fluorescence spectrum were recorded every 1 min for a total of 5 min. Fluorescence profiles were recorded under the following conditions: Ex = 488 nm, Em = 500–650 nm.ESR was used to detect ROS species. TEMPO and DMPO were used as 1O2, ˙OH and O2− trapping agents respectively. The different trapping agents were mixed with RuH, RuCl, and RuBr (20 μM, 100 μL) and exposed to US irradiation for 5 min and then tested to obtain ESR spectra.
DPBF was used as a 1O2 detection probe.41 DPBF (2 mg mL−1, 0.3 μL) was exposed to US irradiation with RuH, RuCl, RuBr and Ru(bpy)3Cl2 (20 μM, 300 μL, dissolved in ddH2O containing 5% DMF) and the UV-vis spectra were recorded every 1 min for a total of 5 min. UV-vis profiles were collected from 350–500 nm.
3-CCA was used as a ˙OH detection probe.42 3-CCA (10 mM, 0.3 μL) was exposed to US irradiation with RuH, RuCl, RuBr and Ru(bpy)3Cl2 (20 μM, 300 μL, dissolved in ddH2O containing 5% DMF) and the fluorescence spectra were recorded every 1 min for a total of 5 min. Fluorescence profiles were collected under the following conditions: Ex = 395 nm, Em = 410–600 nm.
DHE was used as an O2− detection probe. DHE (10 mM, 0.3 μL) was exposed to US irradiation with RuH, RuCl, RuBr and Ru(bpy)3Cl2 (20 μM, 300 μL, dissolved in ddH2O containing 5% DMF and 250 μg mL−1 of ctDNA) and the fluorescence spectra were recorded every 1 min for a total of 5 min. Fluorescence profiles were collected under the following conditions: Ex = 536 nm, Em = 550–700 nm
Theoretical calculations
The Gaussian 09 program was used as software to perform the theoretical simulations.43 Density functional theory calculations were performed with the B3LYP exchange–correlation functional and the 6-31G basis set, including D3 dispersion corrections to account for van der Waals interactions. For ruthenium (Ru), the LANL2DZ effective core potential and associated basis set were employed. Geometry optimizations were carried out until forces converged to below 0.01 eV Å−1, and self-consistent field calculations were considered converged at an energy threshold of 10−8 eV. Solvent effects were incorporated using the Solvation Model based on Density (SMD) with water as the solvent. Energy calculations of singlet and triplet states were carried out with the same parameters.The measurement of the ROS penetration depth
To explore the penetration depth of US irradiation, the fluorescence intensity of US-activation ROS at different depths was used as a conceptual model. The prepared RuBr and Ru(bpy)3Cl2 (20 μM, 100 μL) were mixed with DCFH (10 mM, 0.1 μL) and placed on agarose blocks (containing 1% intralipid and 1% agarose) of different heights, and the US irradiation for 1 min with 1 W cm−2, 1 MHz and 50% duty cycle. Then fluorescence pictures were taken with a live imaging system for small animals (IVIS Lumina III) under the following conditions: Ex = 500 nm, Em = 520–550 nm.Stability tests
The UV-vis spectra of RuH, RuCl and RuBr were used to study their stability. The prepared RuH, RuCl and RuBr (80 μM, 100 μL) solution was irradiated by US irradiation for 30 minutes and the UV-vis spectrum were recorded at 5 min intervals using a microplate reader.Minimum inhibitory concentration (MIC) and MTT assay
For the MIC assay, C. albicans and C. glabrata were used as a fungi model. First, C. albicans or C. glabrata was cultured in YPD medium for 15 h at 28 °C with shaking (150 rpm min−1). Afterward, C. albicans or C. glabrata was centrifuged (3000 rpm, 5 min) and adjusted the OD600 = 1 with RPMI 1640 medium (buffered with 0.145 M MOPS) for experiments. RuH, RuCl, RuBr, Ru(bpy)3Cl2 and AmB were two-fold serially diluted (0, 5, 10, 20, 40, 80 μM, 50 μL) with RPMI 1640 medium in a 96-well plate, and fungi (5 × 103 CFU mL−1) were added to each well for 2 h. Then the US groups were exposed to US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min and culturing was continued for 10 h. The dark groups were cultured for 12 h without US irradiation. The MIC was determined with the naked eye and spread plate method, which is the lowest concentration of a compound to completely inhibit the growth of fungi.For the MTT assay, L929 cells (a mouse fibroblast cell line) were employed as normal cell lines. Cells (5 × 104 cells mL−2, 100 μL) were diluted with DMEM to a treated 96-well plate. Following a 24-hour culture, the cells were exposed to RuH, RuCl and RuBr. After incubation for 2 h, the US groups were exposed to US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min, after which they were returned to the incubator for a further 10 h. The dark groups were cultured for 12 h without US irradiation. The cell viability was evaluated by using the MTT assay standard protocol.44
Fungal membrane permeability
The characterisation of membrane changes in C. albicans was accomplished through the utilisation of zeta potential, SEM, and AO/EB. For the zeta potential test, C. albicans (1 × 108 CFU mL−1) was incubated with different concentrations of RuBr (0–40 μM) for 2 h and then resuspended in PBS after centrifugation for 5 min (3000 rpm min−1). Their zeta potentials were then tested using a Zetasizer Nano ZS. For the SEM and AO/EB test, the experiment was divided into 4 groups: Control, US, RuBr, RuBr + US, after different treatments for 2 h, the C. albicans cells were centrifuged. The cells were then fixed with 2.5% glutaraldehyde at 4 °C for 12 h, following which they were dehydrated using a series of ethanol (30%–100%), and finally the samples were obtained by vacuum freeze-drying. The samples were observed using SEM after being sprayed with gold. For AO/EB staining, following 30 min of staining with AO (1 μg mL−1) and EB (1 μg mL−1), the fungi were washed three times with RPMI 1640 medium prior to photography using an inverted fluorescence microscope.ROS and JC-1 assay
DCFH and JC-1 probes were used to assess ROS production and mitochondrial membrane potential changes in fungal cells, respectively. For the ROS production test, C. albicans was incubated with RuH, RuCl and RuBr (15 μM) for 2 h and treated with or without US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min, washed three times with RPMI 1640 medium and then stained with the DCFH probe for 30 min, washed three times and photographed with an inverted fluorescence microscope. For JC-1 staining experiments, RuBr (15 μM) was incubated with C. albicans for 2 h with or without US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min, washed three times with RPMI 1640 medium and then stained with the JC-1 probe for 15 min, washed three times and then photographed with an inverted fluorescence microscope.ATP assay
C. albicans cells (1 × 107 CFU mL−1) were incubated with RuBr in RPMI 1640 medium for 2 h with or without US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min. Then, the fungi were mixed with lysis buffer in the ATP assay kit on ice following the manufacturer's instructions. The luminescence intensity was recorded using a microplate reader measured with a luminometer.Hemolysis assay
Whole blood was obtained from healthy ICR mice by ocular phlebotomy, collected in anticoagulated tubes containing EDTA, thoroughly shaken and then subjected to centrifugation at 3000 rpm for 15 min three times to get blood cells. Subsequently, different concentrations of RuBr (0–80 μM, 100 μL of dissolved in PBS), 100 μL of ultrapure water (ddH2O) and 100 μL of PBS solution were mixed with 5 μL of blood cells. The samples were then subjected to incubation at 37 °C for a duration of 4 h. Thereafter, the samples were subjected to centrifugation at 3000 rpm for 15 min. Following this, the samples were placed on a horizontal surface and photographed using a mobile phone. The absorbance of the samples’ supernatant at 576 nm was subsequently measured using an enzyme meter. The haemolysis rate percentage was then calculated by the following formula: (ODsample − ODPBS)/(ODwater − ODPBS) × 100%.45Mouse wound infection model and analysis
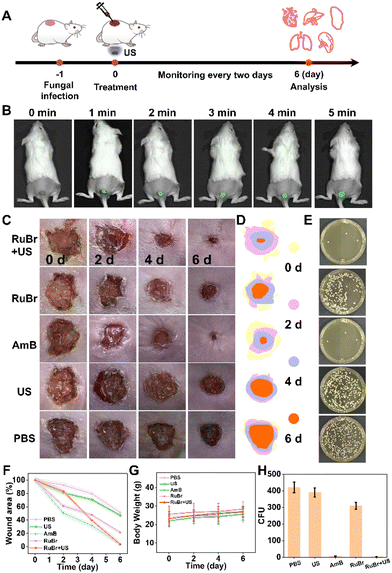
All animal experiments were conducted under the approval of the Administrative Panel on Laboratory Animal Care of Central China Normal University (no. CCNU-IACUC-2023-001). Female ICR mice (23–25 g, Hunan SJA Laboratory Animal Co., Ltd) were used to establish the infection model. The mouse wound infection model was constructed following the method described in previous reports.46 The hair on the back of the mouse was removed, and an 8 × 8 mm infected wound was cut out in a sterile environment and titrated with Candida albicans. The mice were divided into five groups: PBS, PBS + US, RuBr (300 μM), RuBr + US, and AmB (300 μM). Wound areas, body weights, and photographic records of all groups were observed consecutively on the second day. Mice were euthanized after wound healing and treated skin was excised, then ground and quantified by plate counting. All other organs were used for H&E staining, Masson staining and blood were used for haematology analysis.ROS generation in infected tissue
The DCFH probe was used to investigate the in vivo generation of ROS after US irradiation. A 50 μL PBS solution comprising DCFH (20 μM) and RuBr (300 μM) was administered dropwise into the infected region of the mouse's back wound. The US probe was then positioned on the opposite side of the infected wound on the subject's abdomen. Subsequent to varying periods of US irradiation (1 MHz, 50% duty cycle, 1 W cm−2) for 5 min, the fluorescence signals of DCFH were observed using an in vivo imaging system.Results and discussion
The synthetic schemes of RuH, RuCl and RuBr and the detailed characterization are presented in Fig. S1–S9.† The successful preparation of RuH, RuCl and RuBr was demonstrated by 1H and 13C NMR spectroscopy, and high-resolution mass spectrometry (HR-MS). We then meticulously examined the UV-visible (UV-vis) absorption spectra of RuH, RuCl and RuBr in dimethyl sulfoxide (DMSO), their maximum UV-vis absorption peaks were at 468 nm, 388 nm and 384 nm, respectively (Fig. 1A). Their stability was also evaluated through UV-vis spectroscopy. The negligible alterations of absorption spectra showed that RuH, RuCl and RuBr demonstrated good stability throughout 7 d and US irradiation over 30 min (Fig. S10†). Subsequently, the capacity of RuH, RuCl and RuBr to generate ROS under US irradiation (1.0 MHz, 1.0 W cm−2, 50% duty cycle) was evaluated. The DCFH was employed as a quantitative ROS probe. As shown in Fig. 1B and S11,† the ROS generation abilities of RuH, RuCl and RuBr surpassed that of the commercial sonosensitizer Ru(bpy)3Cl2, in which the ROS generation ability of RuBr was 4.2-fold, 1.84-fold and 4.4-fold greater than RuH, RuCl and Ru(bpy)3Cl2. These findings demonstrate that RuH, RuCl and RuBr can serve as sonosensitizers to promote the generation of ROS. Additionally, heavy atom effects may significantly enhance ROS production by metal-based sonosensitizers. In order to further explore the possible mechanism of the heavy-atom effect in boosting the yield of ROS, density functional theory (DFT) calculation was employed to calculate the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), the lowest singlet state (S1) and the lowest triplet state (T1) of RuH, RuCl and RuBr (Fig. 1C and S12†). The calculated results also confirmed that the energy gap (ΔEst) of RuBr was 0.202 eV, which was much narrower than that of RuH (0.365 eV) and RuCl (0.251 eV). This provided further evidence for the influence of the heavy-atom effect on ROS generation. In summary, both the theoretical calculation and the ROS generation test indicated that the intersystem crossing was effectively reduced and the ROS yield was well-improved by the heavy atom effect.We further evaluated ROS production in different water fractions. The results suggested that there was a significant increase in ROS production by RuBr with increasing water fractions (Fig. S13†). The increased ROS could be attributed to the chemiluminescence from the interaction of radical products by water sonolysis with RuBr.47 Additionally, the ability of US irradiation to activate ROS production of RuBr and Ru(bpy)3Cl2 at different tissue depths was also tested. The results showed that the ROS production of RuBr could be observed up to 10 cm, whereas only up to 2 cm for Ru(bpy)3Cl2 (Fig. S14†), which highlighted the promising ROS production ability of RuBr. To further elucidate the specific ROS species triggered by US irradiation, electron spin resonance (ESR) spectroscopy was employed, utilizing 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as trapping agents for singlet oxygen (1O2), superoxide anion (O2−) and hydroxyl radical (˙OH), respectively. After US irradiation, the ESR profiles of RuH, RuCl and RuBr exclusively exhibited the characteristic peaks (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of 1O2 (Fig. 1D and S15†), thereby indicating that US mainly triggered RuH, RuCl and RuBr producing 1O2. We also employed a suite of probes (DPBF, DHE, and 3-CCA) to further delineate the generation of 1O2, O2−, and ˙OH by RuH, RuCl and RuBr under US irradiation. The assessment of DPBF at 412 nm revealed a diminution in its characteristic peak intensity with the prolongation of US irradiation time (Fig. 1E and S16†). This decline was consistent with the consumption of DPBF as a consequence of 1O2 generation. The largest decrease was observed for RuBr, followed by RuCl, RuH and Ru(bpy)3Cl2, suggesting that RuBr has the best 1O2 generation capacity under US irradiation. All these results demonstrated that RuH, RuCl and RuBr, and notably RuBr, exhibited a much better capacity for 1O2 production when compared to the established sonosensitizer Ru(bpy)3Cl2. Furthermore, the absence of any significant alterations in the characteristic peaks of DHE and 3-CCA (Fig. 1F and S17†) also substantiated the selective induction of 1O2 by US activation.
1) of 1O2 (Fig. 1D and S15†), thereby indicating that US mainly triggered RuH, RuCl and RuBr producing 1O2. We also employed a suite of probes (DPBF, DHE, and 3-CCA) to further delineate the generation of 1O2, O2−, and ˙OH by RuH, RuCl and RuBr under US irradiation. The assessment of DPBF at 412 nm revealed a diminution in its characteristic peak intensity with the prolongation of US irradiation time (Fig. 1E and S16†). This decline was consistent with the consumption of DPBF as a consequence of 1O2 generation. The largest decrease was observed for RuBr, followed by RuCl, RuH and Ru(bpy)3Cl2, suggesting that RuBr has the best 1O2 generation capacity under US irradiation. All these results demonstrated that RuH, RuCl and RuBr, and notably RuBr, exhibited a much better capacity for 1O2 production when compared to the established sonosensitizer Ru(bpy)3Cl2. Furthermore, the absence of any significant alterations in the characteristic peaks of DHE and 3-CCA (Fig. 1F and S17†) also substantiated the selective induction of 1O2 by US activation.
Based on the excellent ROS generation ability, we next investigated RuH, RuCl and RuBr for antifungal infections. The MIC of these Ru(II) complexes was determined for their in vitro activity against the fungal strains C. albicans and C. glabrata (Fig. 2A and S18†). All these Ru(II) sonosensitizers demonstrated remarkable antifungal activities under US irradiation with MIC generally lower than 20 μM. By contrast, in the absence of US irradiation, the values of MIC were over 40 μM. Importantly, RuBr exhibited comparable antifungal activity (MIC = 5 μM for C. albicans and C. glabrata) compared to the first-line clinical antifungal (AmB, MIC = 5 μM). Simultaneously, the result was further supported by the colony-counting analysis (Fig. 2B and S18†). To investigate the toxicity of RuBr on mammalian cells, we employed an MTT assay to assess the viability of L929 cells (Fig. S19†). The results demonstrated that RuBr exhibited low toxicity to L929 cells, with cell survival rates exceeding 80% even after US irradiation, which could be attributed to the differences in the uptake mechanism and efficiency of Ru(II) complexes in normal cells and fungal cells.
In light of the remarkable antifungal infection performance of RuBr, in vitro experiments were subsequently carried out to investigate its potential antifungal mechanism. We suggested that the antifungal activity of RuBr may be attributed to the enhanced membrane permeability.48,49 To assess changes in membrane permeability, the changes in the membrane potential of C. albicans after the addition of RuBr at concentrations ranging from 0 μM to 20 μM were measured. It verified that the membrane potential changed from −10.63 mV to −2.53 mV (Fig. 2C). These findings supported the hypothesis that the fluctuations in the surface potential of fungal membranes might serve as the underlying mechanism for the antifungal properties of RuBr. To further validate this suggestion, scanning electron microscopy (SEM) was applied to observe the morphological changes on the fungal surface after various treatments. The fungal membranes exhibited slight wrinkles after incubation with RuBr, and a tendency for RuBr to aggregate on their surfaces was observed (Fig. 2D). Furthermore, a conspicuous distortion of the fungal surface, accompanied by a collapse of the fungal skeleton, was observed after post-treatment with RuBr and US irradiation. These alterations were indicative of compromised membrane integrity due to RuBr, with the US irradiation intensifying the disintegrative effects. Additionally, the integrity of fungal cell membranes was assessed through the use of acridine orange (AO)/ethidium bromide (EB) staining.50 AO was able to penetrate through cells with intact cell membranes, embedded in the nuclear DNA, and emitted a green fluorescence. Conversely, EB was only able to penetrate and emit a red fluorescence through cells with damaged cell membranes, and embed in the nuclear DNA. As shown in Fig. 2E, the control and US groups emitted bright green fluorescence and negligible red fluorescence. In contrast, the RuBr group displayed weak red fluorescence accompanied by green fluorescence, while the RuBr + US group manifested significant red fluorescence as well as negligible green fluorescence. These observations indicated that US irradiation induced substantial damage to the cell membrane. These results underscored the synergistic detrimental impact of RuBr in conjunction with US irradiation on the structural integrity of fungal membranes.
In order to further investigate the potential antifungal performance of RuBr under US irradiation, the DCFH probe was used to measure ROS production in C. albicans. The fluorescence images of ROS under different treatments were compared and all three Ru(II) complexes produced green fluorescence (ROS) after US irradiation in C. albicans (Fig. 3A and S20†). It is worth noting that the combination of US and RuBr led to the most ROS generation, which exhibited a 3.1-fold increase in ROS production compared to the single US groups. Previous reports indicated that Ru(II) reagents entered into the mitochondria to affect the basic mitochondrial functions.51–53 Therefore, we measured the changes in fungal mitochondrial membrane potential (MMP) by JC-1 staining experiments. JC-1 mainly aggregated in the mitochondria emitting a strong red fluorescence for all control groups (Fig. 3B). However, after the treatment of RuBr and US irradiation, the fluorescence of JC-1 in C. albicans changed from red to green, suggesting that this treatment effectively reduced mitochondrial membrane potential and triggered mitochondrial depolarization (Fig. 3B). Considering mitochondria as the key site for ATP synthesis,54 the ATP content of the fungus after different treatments was also measured. After RuBr and US irradiation, the ATP content significantly decreased and showed an 89.35% reduction (Fig. 3C). Based on the above results, the possible antifungal mechanism of RuBr could be attributed to these two pathways (Fig. 3D): (1) RuBr primarily accumulated on the membrane of C. albicans, resulting in cell membrane crumpling and collapse, and such a process could be further amplified by US irradiation; (2) the US-activated ROS production of internalized RuBr in C. albicans has the potential to disrupt the redox balance, which may ultimately result in the irreversible death of the C. albicans by destroying the MMP and reducing the ATP level.
The merits of excellent antifungal efficiency and low toxicity in mammalian cells encouraged us to utilize RuBr for in vivo antifungal therapy (Fig. 4A). Prior to conducting in vivo tests, an initial biosafety evaluation of RuBr was conducted. The haemolysis results indicated that RuBr did not undergo significant haemolysis even at a high concentration (80 μM) (Fig. S21†). Then the mice were randomly divided into five groups: (I) PBS, (II) US, (III) AmB, (IV) RuBr, and (V) RuBr + US. In order to illustrate the benefits of the US penetration depth, the ultrasound probe was positioned on the abdominal region of mice for the purpose of irradiation (Fig. S22†). Additionally, the ROS production ability of RuBr on the wound site was also measured. Fluorescence imaging well indicated a positive correlation between ROS production and the duration of US irradiation (Fig. 4B), thereby demonstrating the potential of US irradiation for deep tissue treatment. Subsequent to the initiation of treatment, alterations in the wound area and body weight of the mice were documented at two-day intervals. As demonstrated in Fig. 4C–F, the wounds in the RuBr + US and AmB groups exhibited significant healing after six days, while the wounds in the PBS, US and RuBr groups remained unhealed. The results of C. albicans colonies from the site of wound infection showed a significant reduction in the amount of fungi in the RuBr + US group after 6 days of treatment compared with the PBS- and US-treated groups, while the amount of fungi in the RuBr group without US irradiation was only slightly reduced (Fig. 4E and H). Furthermore, minimal variation in body weight was observed across all groups (Fig. 4G). These results collectively confirmed that the antifungal efficacy of the combination of RuBr and US irradiation was good analogous to that of AmB.
Finally, a histological analysis was conducted on the cellular morphology and structure of infected wounds and organs in mice after different treatments. For this purpose, hematoxylin and eosin (H&E) and Masson's staining were used. The H&E staining images confirmed extensive necrosis of subcutaneous tissues in mice of the PBS group, which was accompanied by the infiltration of a large number of inflammatory cells (Fig. 5A and S23†). In contrast, RuBr combined with US treatment resulted in a relatively intact histological structure of skin tissues, accompanied by a significant reduction in inflammatory response. Additionally, the results of individual organs revealed no substantial biotoxicity in the treated mice. Masson staining revealed that the RuBr + US group exhibited a substantial advantage in terms of neovascularization and collagen deposition. This finding indicated a remarkable recovery of the structure and function of subcutaneous tissues infected with the fungus (Fig. 5A). Furthermore, the results of the blood biochemical and routine analyses (Fig. 5B–H) indicated that there were no significant side effects on the hepatic and kidney functions of the mice. It is well known that AmB exhibits strong nephrotoxicity.55,56 In conclusion, RuBr demonstrated efficient antifungal activity as well as good biocompatibility, revealing the promising potential of RuBr combined with the US irradiation therapeutic platform for the treatment of fungal infections in deep organs.
Conclusions
In summary, we have heavy-atom engineered a series of Ru(II)-based sonosensitizers (RuH–RuBr) with enhanced ROS yields triggered by US for the treatment of antifungal infections. Among them, RuBr demonstrated the most significant enhancement in ROS production, achieving a 4.4-fold increase compared to the commercial sonosensitizer Ru(bpy)3Cl2. In vitro studies demonstrated that the combination of RuBr and US irradiation effectively inhibited C. albicans growth by damaging cell membranes and disrupting the mitochondrial membrane potential through ROS production, with a MIC of 5 μM against C. albicans and C. glabrata under US irradiation, comparable to the first-line antifungal agent AmB. In vivo antifungal experiments demonstrated that RuBr led to substantial wound healing, with significant improvements observed after six days. Histological and blood analyses indicated lower toxicity and an improved safety profile. In conclusion, our study presented RuBr as a novel antifungal agent, offering insights into the conformational relationships of sonosensitizers and paving the way for future innovations in the design of sonosensitizers for antifungal infections.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Q. L., Y, P., L. M. and S. L. contributed equally.Data availability
The author has confirmed that all the data are available in the ESI.†Data will be made available on request.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22374055, 22022404, 22204055), the National Natural Science Foundation of Hubei Province (2022CFA033), and the Fundamental Research Funds for the Central Universities (CCNU24CG013, CCNU24JCPT001, CCNU24JCPT020, CCNU30106240021). All animal experiments were conducted in agreement with the Guidelines of Institutional Animal Care and Use Committee (No. CCNU-IACUC-2023-001).References
- L. E. Cowen, Drugs from bugs in creatures of the sea, Science, 2020, 370, 6519 CrossRef PubMed.
- K. S. Ikuta, T. Meštrović and M. Naghavi, Global incidence and mortality of severe fungal disease, Infect. Dis., 2024, 24, e268 Search PubMed.
- Z. A. Kanafani and J. R. Perfect, Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact, Clin. Infect. Dis., 2008, 46, 120–128 CrossRef PubMed.
- Y. Xu, Y. Pang, L. Luo, A. Sharma, J. Yang, C. Li, S. Liu, J. Zhan and Y. Sun, De Novo designed Ru(II) metallacycle as a microenvironment–adaptive sonosensitizer and sonocatalyst for multidrug–resistant biofilms eradication, Angew. Chem., Int. Ed., 2024, 63, e202319966 CrossRef CAS PubMed.
- E. Puumala, S. Fallah, N. Robbins and L. E. Cowen, Advancements and challenges in antifungal therapeutic development, Clin. Microbiol. Rev., 2024, 37, e00142 CrossRef PubMed.
- Q. Li, H. Ye, F. Zhao, Y. Li, Z. Zhang, Q. Yan and Y. Sun, Recent advances in combatting bacterial infections via well-designed metallacycles/metallacages, Dalton Trans., 2024, 53, 3434 RSC.
- K. R. Iyer, N. M. Revie, C. Fu, N. Robbins and L. E. Cowen, Treatment strategies for cryptococcal infection: challenges, advances and future outlook, Nat. Rev. Microbiol., 2021, 19, 454–466 CrossRef CAS PubMed.
- J. Berman and D. J. Krysan, Drug resistance and tolerance in fungi, Nat. Rev. Microbiol., 2020, 18, 319–331 CrossRef CAS PubMed.
- S. R. Lockhart, A. Chowdhary and J. A. W. Gold, The rapid emergence of antifungal-resistant human-pathogenic fungi, Nat. Rev. Microbiol., 2023, 21, 818–832 CrossRef CAS PubMed.
- M. A. Schikora-Tamarit and T. Gabaldon, Recent gene selection and drug resistance underscore clinical adaptation across Candida species, Nat. Microbiol., 2024, 9, 284–307 CrossRef CAS PubMed.
- Y. Xu, W. Tuo, L. Yang, Y. Sun, C. Li, X. Chen, W. Yang, G. Yang, P. J. Stang and Y. Sun, Design of a Metallacycle–based supramolecular photosensitizer for In vivo image–guided photodynamic inactivation of bacteria, Angew. Chem., Int. Ed., 2021, 61, e202110048 CrossRef PubMed.
- Y. Xu, C. Li, X. Ma, W. Tuo, L. Tu, X. Li, Y. Sun, P. J. Stang and Y. Sun, Long wavelength-emissive Ru(II) metallacycle-based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2209904119 CrossRef CAS PubMed.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Construction of emissive ruthenium(II) metallacycle over 1000 nm wavelength for in vivo biomedical applications, Nat. Commun., 2022, 13, 2009 CrossRef CAS PubMed.
- X. Da, Y. Xu, L. Wang, X. Liu, Y. Peng, Y. Wu, W. Zhou, W. Wang, X. Wang and Q. Zhou, A self-enhanced chemiexcited PDT system for targeted and efficient treatment of deeply seated tumors, Inorg. Chem. Front., 2024, 11, 5598–5611 RSC.
- S. Kuang, F. Wei, J. Karges, L. Ke, K. Xiong, X. Liao, G. Gasser, L. Ji and H. Chao, Photodecaging of a mitochondria-localized iridium(III) endoperoxide complex for two-photon photoactivated therapy under hypoxia, J. Am. Chem. Soc., 2022, 144, 4091–4101 CrossRef CAS PubMed.
- Z. Zhang, H. Ye, F. Cai and Y. Sun, Recent advances on the construction of long-wavelength emissive supramolecular coordination complexes for photo-diagnosis and therapy, Dalton Trans., 2023, 52, 15193–15202 RSC.
- L. Wei, R. Kushwaha, T. Sadhukhan, H. Wu, A. Dao, Z. Zhang, H. Zhu, Q. Gong, J. Ru, C. Liang, P. Zhang, S. Banerjee and H. Huang, Dinuclear tridentate Ru(II) Complex with strong near-infrared light-triggered anticancer activity, J. Med. Chem., 2024, 67, 11125–11137 CrossRef CAS PubMed.
- A. Dao, S. Chen, L. Pan, Q. Ren, X. Wang, H. Wu, Q. Gong, Z. Chen, S. Ji, J. Ru, H. Zhu, C. Liang, P. Zhang, H. Xia and H. Huang, A 700 nm LED light activated Ru(II) complex destroys tumor cytoskeleton via photosensitization and photocatalysis, Adv. Healthcare Mater., 2024, 13, 2400956 CrossRef CAS PubMed.
- Z. Fan, J. Xie, R. Kushwaha, S. Liang, W. Li, A. A. Mandal, L. Wei, S. Banerjee and H. Huang, Anticancer screening of Ru(II) photoredoxcatalysts at single cancer cell level, Chem. – Asian J., 2023, 18, e202300047 Search PubMed.
- A. A. Mandal, V. Singh, S. Saha, S. Peters, T. Sadhukhan, R. Kushwaha, A. K. Yadav, A. Mandal, A. Upadhyay, A. Bera, A. Dutta and B. Kochand, S. Banerjee,Green light-triggered photocatalytic anticancer activity of terpyridine-based Ru(II) photocatalysts, Inorg. Chem., 2024, 63, 7493–7503 CrossRef CAS PubMed.
- Z. Xu, X. Ma, L. Zhang, H. Chen, D. Qing, R. Li, R. Ye and R. Wang, Antifungal activity of ruthenium(II) complex combined with fluconazole against drug-resistant Candida albicans in vitro and its anti-invasive infection in vivo, J. Inorg. Biochem., 2024, 255, 112522 CrossRef CAS PubMed.
- Y. Xu, C. Li, J. An, X. Ma, J. Yang, L. Luo, Y. Deng, J. S. Kim and Y. Sun, Construction of a 980 nm laser-activated Pt(II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization, Sci. China: Chem., 2023, 66, 155–163 CrossRef CAS.
- W. Zhu, Y. Li, S. Guo, W.-J. Guo, T. Peng, H. Li, B. Liu, H.-Q. Peng and B. Z. Tang, Stereoisomeric engineering of aggregation-induced emission photosensitizers towards fungal killing, Nat. Commun., 2022, 13, 7046 CrossRef CAS PubMed.
- C. Liang, J. Xie, S. Luo, C. Huang, Q. Zhang, H. Huang and P. Zhang, A highly potent ruthenium(II)-sonosensitizer and sonocatalyst for in vivo sonotherapy, Nat. Commun., 2021, 12, 5001 CrossRef CAS PubMed.
- Y. Wang, L. Luo, T. Zhang, J.-R. Hu, H. Wang, F. Bao, C. Li, Y. Sun and J. Li, Strategically engineered Ru(II) complexes with enhanced ROS activity enabling potent sonodynamic effect against multidrug-resistant biofilms, ACS Appl. Mater. Interfaces, 2024, 16, 52068–52079 CrossRef CAS PubMed.
- J. Shen, X. Liao, W. Wu, T. Feng, J. Karges, M. Lin, H. Luo, Y. Chen and H. Chao, A pH-responsive iridium(III) two-photon photosensitizer loaded CaCO3 nanoplatform for combined Ca2+ overload and photodynamic therapy, Inorg. Chem. Front., 2022, 9, 4171–4183 RSC.
- C. Ge, J. Zhu, A. Ouyang, N. Lu, Y. Wang, Q. Zhang and P. Zhang, Near-infrared phosphorescent terpyridine osmium(II) photosensitizer complexes for photodynamic and photooxidation therapy, Inorg. Chem. Front., 2020, 7, 4020–4027 Search PubMed.
- Y. Dou, Y. Wang, S. Tian, Q. Song, Y. Deng, Z. Zhang, P. Chen and Y. Sun, Metal-organic framework (MOF)-based materials for pyroptosis-mediated cancer therapy, Chem. Commun., 2024, 60, 6476–6487 RSC.
- S. Wen, W. Zhang, J. Yang, Z. Zhou, Q. Xiang and H. Dong, Ternary Bi2WO6/TiO2-Pt heterojunction sonosensitizers for boosting sonodynamic therapy, ACS Nano, 2024, 18, 23672–23683 CrossRef CAS PubMed.
- M. Liu, Y. Luo, J. Yan, X. Xiong, X. Xing, J. S. Kim and T. Zou, Photoactivation of boronic acid prodrugs via a phenyl radical mechanism: iridium(III) anticancer complex as an example, J. Am. Chem. Soc., 2023, 145, 10082–10091 CrossRef CAS PubMed.
- N. Singh, D. Kim, S. Min, E. Kim, S. Kim, Y. S. Zhang, H. Kang and J. S. Kim, Multimodal synergistic ferroptosis cancer therapy, Coord. Chem. Rev., 2025, 522, 216236 CrossRef CAS.
- Y. Pang, Q. Li, J. Wang, S. Wang, A. Sharma, Y. Xu, H. Hu, J. Li, S. Liu and Y. Sun, Anultrasound-activated supramolecular modulator enhancing autophagy to prevent ventricular arrythmias post-myocardial infarction, Angew. Chem., Int. Ed., 2024, 63, e202415802 CrossRef PubMed.
- G. Li, H. Lei, Y. Yang, X. Zhong, F. Gong, Y. Gong, Y. Zhou, Y. Zhang, H. Shi, Z. Xiao, Z. Dong and L. Cheng, Titanium sulfide nanosheets serve as cascade bioreactors for H2S–mediated programmed gas–sonodynamic cancer therapy, Adv. Sci., 2022, 9, 2201069 CrossRef CAS PubMed.
- L. Zuo, W. Nie, S. Yu, W. R. Zhuang, C. Liang, S. Li, D. Shi, G. Wu, X. Sui, Y. Li and H. Y. Xie, Biomimetic nanovesicle with mitochondria-synthesized sonosensitizer and mitophagy inhibition for cancer sono-immunotherapy, Nano Lett., 2023, 23, 3005–3013 CrossRef CAS PubMed.
- J. Guo, X. Pan, C. Wang and H. Liu, Molecular imaging-guided sonodynamic therapy, Bioconjugate Chem., 2022, 33, 993–1010 CrossRef CAS PubMed.
- T. Dai, W. He, C. Yao, X. Ma, W. Ren, Y. Mai and A. Wu, Applications of inorganic nanoparticles in the diagnosis and therapy of atherosclerosis, Biomater. Sci., 2020, 8, 3784 RSC.
- Z. H. Zhu, Y. Liu, C. Song, Y. Hu, G. Feng and B. Z. Tang, Porphyrin-based two-dimensional layered metal-organic framework with sono-/photocatalytic activity for water decontamination, ACS Nano, 2022, 16, 1346–1357 CrossRef CAS PubMed.
- Y. Pang, C. Li, H. Deng and Y. Sun, Recent advances in luminescent metallacycles/metallacages for biomedical imaging and cancer therapy, Dalton Trans., 2022, 51, 16428–16438 RSC.
- L. Zhang, X. Dong, D. Lu, S. Liu, D. Ding, D. Kong, A. Fan, Z. Wang and Y. Zhao, Controlled ROS production by corannulene: the vehicle makes a difference, Biomater. Sci., 2017, 5, 1236–1240 RSC.
- L. Tu, C. Li, X. Xiong, J. H. Kim, Q. Li, L. Mei, J. Li, S. Liu, J. S. Kim and Y. Sun, Engineered metallacycle–based supramolecular photosensitizers for effective photodynamic therapy, Angew. Chem., Int. Ed., 2023, 62, e202301560 CrossRef CAS PubMed.
- C. Li, L. Tu, J. Yang, C. Liu, Y. Xu, J. Li, W. Tuo, B. Olenyuk, Y. Sun, P. J. Stang and Y. Sun, Acceptor engineering of metallacycles with high phototoxicity indices for safe and effective photodynamic therapy, Chem. Sci., 2023, 14, 2901–2909 RSC.
- C. Li, L. Tu, Y. Xu, M. Li, J. Du, P. J. Stang, Y. Sun and Y. Sun, A NIR-light-activated and lysosomal-targeted Pt(II) metallacycle for highly potent evoking of immunogenic cell death that potentiates cancer immunotherapy of deep-seated tumors, Angew. Chem., Int. Ed., 2024, 63, e202406392 CrossRef CAS PubMed.
- S. Ohno, H. Uratani and H. Nakai, Implementation of nonadiabatic molecular dynamics for intersystem crossing based on a time-dependent density-functional tight-binding method, J. Phys. Chem. A, 2024, 128, 5999–6009 Search PubMed.
- S. Lei, J. Zhang, N. T. Blum, M. Li, D.-Y. Zhang, W. Yin, F. Zhao, J. Lin and P. Huang, In vivo three-dimensional multispectral photoacoustic imaging of dual enzyme-driven cyclic cascade reaction for tumor catalytic therapy, Nat. Commun., 2022, 13, 1298 CrossRef CAS PubMed.
- C. Wang, Y. Sun, S. Huang, Z. Wei, J. Tan, C. Wu, Q. Chen and X. Zhang, Self-immolative photosensitizers for self-reported cancer phototheranostics, J. Am. Chem. Soc., 2023, 145, 13099–13113 Search PubMed.
- W. Jiang, M. Zhou, S. Chen, J. Xie, M. Chen, H. Zhang, Y. Wu, X. Chen and R. Liu, Peptide-mimicking poly(2-oxazoline)spossessing potent antifungal activity and BBB penetrating property to treat invasive infections and meningitis, J. Am. Chem. Soc., 2023, 145, 25753–25765 Search PubMed.
- G. L. Sharipov, A. M. Abdrakhmanov, B. M. Gareev and L. R. Yakshembetova, Sonochemiluminescence in an aqueous solution of Ru(bpy)3Cl2, Ultrason. Sonochem., 2018, 42, 526–531 CrossRef CAS PubMed.
- C. Zhou, C. Peng, C. Shi, M. Jiang, J. H. C. Chau, Z. Liu, H. Bai, R. T. K. Kwok, J. W. Y. Lam, Y. Shi and B. Z. Tang, Mitochondria-specific aggregation-Induced emission luminogens for selective photodynamic killing of fungi and efficacious treatment of keratitis, ACS Nano, 2021, 15, 12129–12139 Search PubMed.
- M. Zhou, L. Liu, Z. Cong, W. Jiang, X. Xiao, J. Xie, Z. Luo, S. Chen, Y. Wu, X. Xue, N. Shao and R. Liu, A dual-targeting antifungal is effective against multidrug-resistant human fungal pathogens, Nat. Microbiol., 2024, 9, 1325–1339 CrossRef CAS PubMed.
- J. Lai, W. Li, S. Wei and S. Li, Natural carbolines inspired the discovery of chiral CarOx ligands for asymmetric synthesis and antifungal leads, Org. Chem. Front., 2020, 7, 2263–2268 Search PubMed.
- Z. Zhang, H. Ye, F. Cai and Y. Sun, Recent advances on the construction of long-wavelength emissive supramolecular coordination complexes for photo-diagnosis and therapy, Dalton Trans., 2023, 52, 15193–15202 RSC.
- S. De, S. Kazi, S. Banerjee, S. Banerjee, N. Sarkar, S. K. Shah, Y.-C. Kuo and S. K. A. Kumar, Metallotherapeutic complexes with high selective properties for anti-neoplastic therapy, Coord. Chem. Rev., 2024, 498, 215462 CrossRef CAS.
- G. Sahu, S. A. Patra, S. Lima, S. Das, H. Gorls, W. Plass and R. Dinda, Ruthenium(II)-dithiocarbazates as anticancer agents: synthesis, solution behavior, and mitochondria-targeted apoptotic cell death, Chemistry, 2023, 29, e202202694 CrossRef CAS PubMed.
- J. He, H. C. Ford, J. Carroll, C. Douglas, E. Gonzales, S. Ding, I. M. Fearnley and J. E. Walker, Assembly of the membrane domain of ATP synthase in human mitochondria, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 2988–2993 Search PubMed.
- A. Maji, C. P. Soutar, J. Zhang, A. Lewandowska, B. E. Uno, S. Yan, Y. Shelke, G. Murhade, E. Nimerovsky, C. G. Borcik, A. S. Arango, J. D. Lange, J. P. Marin-Toledo, Y. Lyu, K. L. Bailey, P. J. Roady, J. T. Holler, A. Khandelwal, A. M. SantaMaria, H. Sanchez, P. R. Juvvadi, G. Johns, M. J. Hageman, J. Krise, T. Gebremariam, E. G. Youssef, K. Bartizal, K. A. Marr, W. J. Steinbach, A. S. Ibrahim, T. F. Patterson, N. P. Wiederhold, D. R. Andes, T. V. Pogorelov, C. D. Schwieters, T. M. Fan, C. M. Rienstra and M. D. Burke, Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal, Nature, 2023, 623, 1079–1085 CrossRef CAS PubMed.
- D. O. Hartmann, K. Shimizu, M. Rothkegel, M. Petkovic, R. Ferraz, Z. Petrovski, L. C. Branco, J. N. C. Lopes and C. S. Pereira, Tailoring amphotericin B as an ionic liquid: an upfront strategy to potentiate the biological activity of antifungal drugs, RSC Adv., 2021, 11, 14441–14452 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00180c |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2025 |