Synthesis of thioamide containing polybenzoxazines by the Willgerodt–Kindler reaction†
Kamer
Bayram
,
Baris
Kiskan
 * and
Yusuf
Yagci
* and
Yusuf
Yagci
 *
*
Istanbul Technical University, Department of Chemistry, 34469, Maslak, Istanbul, Turkey. E-mail: kiskanb@itu.edu.tr
First published on 9th December 2020
Abstract
Benzoxazines with thioamide linkages were successfully prepared. For this purpose, initially, thioamide containing phenolic reagents were synthesized by the Willgerodt–Kindler route using elemental sulfur, aromatic aldehydes and anilines. The obtained phenolic thioamides were then converted to benzoxazines by reacting with primary amines and formaldehyde. Moreover, the thioamide functional polymeric benzoxazine precursor was prepared with difunctional phenolic thioamide and bisaminopropyl end-functional poly(propylene glycol-block-ethylene glycol) by classical Mannich type polycondensation. The resulting precursor was solvent cast on glass plates and flexible films could be obtained after curing. All the synthesized compounds and polymers were characterized by spectral analyses. The curing behavior and thermal stabilities of benzoxazines and related polybenzoxazines were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Interestingly, DSC analyses revealed that the thioamide groups reduce the curing temperatures of benzoxazines by promoting ring opening polymerization, possibly generating thiols during heating.
Introduction
Polybenzoxazines as contender thermosets to classical phenolic resins have gained much interest in the past decade. These polymers exhibit unusual properties stemming from their unique structure. A typical polybenzoxazine contains aminomethyl bridges between phenolic moieties and therefore, several intra- and inter-molecular hydrogen bonds are generated.1,2 Moreover, the phenolic nature of these materials imparts properties already seen in phenol-formaldehyde resins and additional features.3,4 In general, most of the polybenzoxazines exhibit high thermal stability, char yield,5 and glass transition temperatures,6 low water adsorption and flammability, and limited shrinkage during production from their corresponding monomers.1 Besides, low dielectric constants7,8 and resistance against corrosive chemicals are other benefits of these materials. Consequently, many different applications related to these properties are already put into practice in the aerospace, automotive and electronic industries.9,10 Another appealing side of polybenzoxazines is their ease of synthesis from 1,3-benzoxazine monomers. Performing a simple heating procedure for 1,3-benzoxazines gives polybenzoxazines without using additives and/or curatives (Scheme 1).11 However, the non-catalytic process requires high curing temperatures in a range of 180 to 250 °C for a complete ring opening polymerization.12–14 The curing temperature range is broad because the ring opening polymerization of benzoxazine monomers is severely affected by their molecular structure and the purity of the monomer.15,16 For example, acidic hydrogen containing functional groups reduce the curing temperatures drastically. For example, carboxylic acids, phenols, and even alcohol functionalities lower the curing temperatures much below 200 °C.17–20 On the other hand, besides these functional groups, several different benzoxazine monomers were designed to alter the properties of polybenzoxazines.21–28 Hydrogen bonding interactions, rigidity, toughness, film forming ability, processibility, etc. can be tuned via the monomer design approach. Basically, a benzoxazine monomer is synthesized from a phenol, a primary amine that tolerates the synthesis conditions and formaldehyde solution or paraformaldehyde (Scheme 1). Several benzoxazine monomers with different substituents were reported and a large benzoxazine monomer library has already been developed.29–36Amide groups have attracted special attention among the different substituents located in the benzoxazine molecule because such structures can act as high performance polymers. As is well known, one of the easiest methods to synthesize an amide is the reaction of a primary/secondary amine with a carboxylic acid or its derivative. However, other synthetic approaches were applied to obtain amide-bearing benzoxazines due to difficulties of synthesizing primary amine-functional benzoxazines in one-pot. The major problem in the direct synthesis of a primary amine containing benzoxazine is related to the spontaneous formation of aminomethylols from the uncontrolled reaction between formaldehyde and amino groups. It should be noted that ensuring that the amount of primary diamine, phenol, and formaldehyde is precisely 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 moles does not prevent aminomethylol derivative formation. Hence, protecting agents were used to synthesize amino functional benzoxazines and, after the removal of the protecting groups, classical amide synthesis could be performed over these primary/secondary amines to obtain amide functional benzoxazines. Alternatively, phenolic amides were initially prepared and then converted to benzoxazines through classical benzoxazine synthesis to overcome the above-stated problems of synthesis. For this purpose, different aminophenols were reacted with acid halides to form phenolic amides as a reagent for classical benzoxazine synthesis.37–39 Besides, 3,4-dihydrocoumarines (DHC) were also used to synthesize aminophenols, as they are appropriate to synthesize benzanilide type phenolic compounds under mild reaction conditions with good yields.40 The ring opening of DHC with amines easily takes place at room temperature without using a catalyst to form the desired amide bearing phenols. Moreover, DHC were also used to obtain amide containing main chain polybenzoxazine precursors via both one-pot or sequential methods.41
2 moles does not prevent aminomethylol derivative formation. Hence, protecting agents were used to synthesize amino functional benzoxazines and, after the removal of the protecting groups, classical amide synthesis could be performed over these primary/secondary amines to obtain amide functional benzoxazines. Alternatively, phenolic amides were initially prepared and then converted to benzoxazines through classical benzoxazine synthesis to overcome the above-stated problems of synthesis. For this purpose, different aminophenols were reacted with acid halides to form phenolic amides as a reagent for classical benzoxazine synthesis.37–39 Besides, 3,4-dihydrocoumarines (DHC) were also used to synthesize aminophenols, as they are appropriate to synthesize benzanilide type phenolic compounds under mild reaction conditions with good yields.40 The ring opening of DHC with amines easily takes place at room temperature without using a catalyst to form the desired amide bearing phenols. Moreover, DHC were also used to obtain amide containing main chain polybenzoxazine precursors via both one-pot or sequential methods.41
Despite the growing number of amide based benzoxazine monomers and prepolymers, thioamide functional benzoxazines have not been investigated so far. As is known, a variety of synthetic methods have been reported for thioamides since early times ranging from the direct incorporation of sulfur as elemental sulfur to using compounds bearing P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C
S and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups in the syntheses.42 Consequently, thioamides have found many applications in several fields, such as medicinal and organic chemistry. Recently, sulfur-containing polymers have attracted much attention in polymer and materials science43 because of their fascinating properties such as high refractivity44,45 and semiconductivity46 and electrochemical properties.47 Among sulfur polymers, polythioamides48,49 have received particular attention since 1999,50 when the Willgerodt–Kindler reaction51 was first utilized for polymer synthesis.52 This reaction is appealing for polymer synthesis due to the use of elemental sulfur. As is known, sulfur is a nontoxic, low cost and easily available compound.53 Moreover, the utilization of elemental sulfur as a reagent to synthesize valuable compounds has gained global attention because sulfur is produced (ca. 80 million tons annually) as a waste product in the petroleum industry.54 Typically, polythioamides are synthesized by using diamines, dicarboxylic acids and dialdehydes with sulfur in a Willgerodt–Kindler reaction. Several other examples have been reported for various purposes. For example, functional polythioamides were synthesized to recover gold by extracting Au3+ from acidic leaching solution of discarded electronic wastes.54 Similarly, polythioamides were used as collectors for extracting valuable metals from aqueous and organic solutions.55 Polythioamides with high refractive index values were synthesized by using aliphatic primary amines besides benzylic amines.44 Moreover, surface modifications of single-walled-carbon nanotubes (SWCT) were performed via the Willgerodt–Kindler reaction to obtain benzothiomorpholides on the surface.56
S groups in the syntheses.42 Consequently, thioamides have found many applications in several fields, such as medicinal and organic chemistry. Recently, sulfur-containing polymers have attracted much attention in polymer and materials science43 because of their fascinating properties such as high refractivity44,45 and semiconductivity46 and electrochemical properties.47 Among sulfur polymers, polythioamides48,49 have received particular attention since 1999,50 when the Willgerodt–Kindler reaction51 was first utilized for polymer synthesis.52 This reaction is appealing for polymer synthesis due to the use of elemental sulfur. As is known, sulfur is a nontoxic, low cost and easily available compound.53 Moreover, the utilization of elemental sulfur as a reagent to synthesize valuable compounds has gained global attention because sulfur is produced (ca. 80 million tons annually) as a waste product in the petroleum industry.54 Typically, polythioamides are synthesized by using diamines, dicarboxylic acids and dialdehydes with sulfur in a Willgerodt–Kindler reaction. Several other examples have been reported for various purposes. For example, functional polythioamides were synthesized to recover gold by extracting Au3+ from acidic leaching solution of discarded electronic wastes.54 Similarly, polythioamides were used as collectors for extracting valuable metals from aqueous and organic solutions.55 Polythioamides with high refractive index values were synthesized by using aliphatic primary amines besides benzylic amines.44 Moreover, surface modifications of single-walled-carbon nanotubes (SWCT) were performed via the Willgerodt–Kindler reaction to obtain benzothiomorpholides on the surface.56
In this paper, we report the synthesis of thioamide based mono- and di-functional benzoxazines and the main chain polybenzoxazine precursor to exploit the properties of the thioamide group. For example, resonance ability, tautomerism and hydrogen-bonding interactions of thioamides could be imparted to benzoxazines. As will be shown, besides a versatile synthetic procedure, the obtained benzoxazines exhibited latent self-catalytic behavior reducing the ring opening polymerization temperature.
Experimental
Materials
Aniline (Merck, 99.5%), sulfur (S8, colloidal powder, reagent grade, Aldrich), paraformaldehyde (Acros, 96%), benzylamine (Merck, ≥99%), furfurylamine (Fluka, ≥98%), sodium hydroxide (Acros, >97%), ethyl acetate (EtOAc, Merck, ≥99.5%), O,O′-bis(2-aminopropyl) polypropylene glycol-block-polyethylene glycol-block-polypropylene glycol (Sigma-Aldrich, Jeffamine® ED-900, St Louis, MO, USA), 4-aminophenol (p-aminophenol Merck, ≥99%), 4-hydroxybenzaldehyde (Merck, ≥98%), 1,6-diaminohexane (Fluka), 4-hydroxy-3-methoxybenzaldehyde (Vanillin) (Alfa Aesar, 99%), tetrahydrofuran (THF, VWR Chemicals, 99.7%), ethanol (EtOH, Aldrich, ≥99.5%), toluene (Carlo Erba, 99.5%), chloroform (CHCl3, Carlo Erba, 99.9%), sodium sulfide nonahydrate (Carlo Erba), N,N-dimethylformamide (DMF, Merck), and magnesium sulfate anhydrous (Aldrich, ≥99.5%).Instrumentation
The 1H NMR and 13C NMR measurements were recorded at room temperature in CDCl3 or DMSO-d6 with Si(CH3)4 as an internal standard, using a 500 MHz NMR spectrometer (Agilent NMR System VNMRS). FTIR spectra were recorded on a PerkinElmer FTIR Spectrum One spectrometer. Differential scanning calorimetry (DSC) was carried out on a PerkinElmer Diamond DSC with a heating rate of 10 °C min−1 under a nitrogen flow, covering temperatures of 30–320 °C. Thermal gravimetric analysis (TGA) was performed on a PerkinElmer Diamond TA/TGA with a heating rate of 10 °C min−1 under a nitrogen flow. Gel permeation chromatography (GPC) measurements were taken on a Tosoh EcoSEC GPC system equipped with an auto sampler system, a temperature controlled pump, a column oven, a refractive index (RI) detector, a purge and degasser unit and a TSK gel superHZ2000, 4.6 mm ID × 15 cm × 2 cm column. Tetrahydrofuran was used as an eluent at a flow rate of 1.0 mL min−1 at 40 °C. The refractive index detector was calibrated with polystyrene standards having narrow molecular weight distributions. Data were analyzed using Eco-SEC Analysis software. High-resolution LC/MS analysis was performed using an Agilent Technologies Q-TOF LC/MS System equipped with a HiP sampler, binary pump, column comp. and Q-TOF. TOF/Q-TOF Acquisition parameters are: a dual ESI ion source, gas temp 300 °C, gas flow 10 l min−1 and nebulizer 40 psig.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in a 100 mL round-bottom flask. The reaction mixture was refluxed for 24 h. The solvent was evaporated using a rotary evaporator. The resulting product was dissolved in chloroform (ca. 50 mL) and extracted with 1 M sodium hydroxide five times. Then, the solution was neutralized by washing the chloroform solution with distilled water (ca. 250 mL) three times. The solution was dried with anhydrous MgSO4 and filtered. Chloroform was evaporated under vacuum. The product was dried in a vacuum oven at a temperature of 50 °C for 24 h (yield: ca. 32% for Th-amd-MonoBz-Fr and ca. 35% for Th-amd-MonoBz-Bn).
1, v/v) in a 100 mL round-bottom flask. The reaction mixture was refluxed for 24 h. The solvent was evaporated using a rotary evaporator. The resulting product was dissolved in chloroform (ca. 50 mL) and extracted with 1 M sodium hydroxide five times. Then, the solution was neutralized by washing the chloroform solution with distilled water (ca. 250 mL) three times. The solution was dried with anhydrous MgSO4 and filtered. Chloroform was evaporated under vacuum. The product was dried in a vacuum oven at a temperature of 50 °C for 24 h (yield: ca. 32% for Th-amd-MonoBz-Fr and ca. 35% for Th-amd-MonoBz-Bn).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The reaction mixture was refluxed for 24 h. The above work-up procedure was then applied. The product was dried in a vacuum oven at a temperature of 50 °C for 24 h. (Yield: ca. 67%).
1, v/v). The reaction mixture was refluxed for 24 h. The above work-up procedure was then applied. The product was dried in a vacuum oven at a temperature of 50 °C for 24 h. (Yield: ca. 67%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in a 250 ml round-bottom flask equipped with a reflux condenser and a magnet for stirring. The solution was refluxed for 24 h. After reflux, the solvent was removed using a rotary evaporator under reduced pressure. After cooling the content to room temperature, the oily product was dissolved in CHCl3 and washed with Na2CO3 solution (0.2 M) and deionized water, subsequently. The solution was dried with anhydrous Na2SO4 and then filtered. The solvent was removed under vacuum and a waxy polymer was obtained.
1, v/v) in a 250 ml round-bottom flask equipped with a reflux condenser and a magnet for stirring. The solution was refluxed for 24 h. After reflux, the solvent was removed using a rotary evaporator under reduced pressure. After cooling the content to room temperature, the oily product was dissolved in CHCl3 and washed with Na2CO3 solution (0.2 M) and deionized water, subsequently. The solution was dried with anhydrous Na2SO4 and then filtered. The solvent was removed under vacuum and a waxy polymer was obtained.
Results and discussion
Thioamides exhibit a larger rotational barrier for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and more charge transfer occurs from the N atom to the C
N bond and more charge transfer occurs from the N atom to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds compared to amides.57,58 Therefore, the hydrogen bonding and conformations around the S
S bonds compared to amides.57,58 Therefore, the hydrogen bonding and conformations around the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH group are different from those of amides.59 Generally, the S
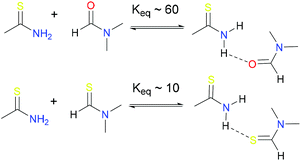
C–NH group are different from those of amides.59 Generally, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH bonds are weak hydrogen bond acceptors, on the other hand, NH protons operate as a hydrogen bond donor due their acidicity.60 For example, the formation of protonated thioformamide is more favorable than that of formamide and Keq (thioamide) is ca. 6 times less than Keq (amide) (Scheme 2).58,61 Because of these unique properties and widespread existence in nature, research interest in thioamide chemistry has recently been extended to polymer science.42
C–NH bonds are weak hydrogen bond acceptors, on the other hand, NH protons operate as a hydrogen bond donor due their acidicity.60 For example, the formation of protonated thioformamide is more favorable than that of formamide and Keq (thioamide) is ca. 6 times less than Keq (amide) (Scheme 2).58,61 Because of these unique properties and widespread existence in nature, research interest in thioamide chemistry has recently been extended to polymer science.42
Another important feature of thioamides is their ease of resonance ability and tautomerism in contrast to amides. Therefore, it seemed appropriate to investigate thioamide-based benzoxazines as these fundamental properties of thioamides can be combined with the unique features of polybenzoxazines to yield advanced materials. Thus, this motivation led us to use Willgerodt–Kindler reaction as a versatile tool to access thioamide based polybenzoxazines. For this purpose, our initial design was to synthesize dialdehyde functional benzoxazine (Bis(VaBz)-Hex) and react it with diamines and elemental sulfur to obtain main chain polybenzoxazines bearing thioamide groups in the structure in one-pot. Accordingly, Bis(VaBz)-Hex was synthesized from vanillin, 1,6-diaminohexane and paraformaldehyde (see the ESI† for experimental details and Fig. S1 and 2† for 1H NMR and FTIR spectra). Bis(VaBz)-Hex was reacted with elemental sulfur and diamines (1,6-diaminohexane and Jeffamine ED-900) under different reaction conditions (Scheme 3). Initial attempts were performed under air and at 140 °C, but all the products were completely insoluble. Therefore, the same experiments were run under nitrogen gas and at ca. 100 °C to prevent uncontrolled crosslinking. However, these attempts were also unsuccessful, a soluble thioamide bearing main chain polybenzoxazines could not be obtained in good yields, and only a minor amount of slightly soluble product was formed to perform spectral and chromatographic analysis.
 | ||
| Scheme 3 Synthesis of Bis(VaBz)-Hex and attempt to obtain thioamide containing main chain polybenzoxazine. | ||
According to gel permeation chromatography, the soluble product had a molecular weight (Mn) of ca. 1300 Da with a polydispersity index of 1.96. The spectral analysis revealed that all the oxazine rings were consumed under the stated reaction conditions (see Fig. S3† for the 1H NMR spectrum of the oligomer). In Fig. S3,† proton signals of unreacted Bis(VaBz)-Hex are visible and the presence of phenolic –OH protons clearly evidence the ring opening of oxazine. From these results, it can be inferred that the one-pot polymerization with the Willgerodt–Kindler reaction does not proceed under the reported experimental conditions. The unsuccessful polymerization observed may be attributed to the uncontrolled reactions between elemental sulfur and benzoxazines. Previously, it was reported that benzoxazines have the ability to react with sulfur through the inverse vulcanization pathway. In part, this reaction proceeds over a radical process and is accordingly coined as sulfur radical transfer and coupling (SRTC). In this reaction, S–S bonds cleave homolytically to produce sulfur radicals and then oligosulfides are formed. These oligomers abstract hydrogen atoms from Mannich bridges (Ar–CH2–N or O–CH2–N) of the oxazine ring forming –SH at the chain ends of oligosulfide that triggers the cationic ring opening of benzoxazines (Scheme 4).
The usual inverse vulcanization reactions are performed at ca. 180 °C as efficient radical formation from sulfur occurs after ca. 169 °C in bulk. Hence, S–S bond cleavage at temperatures as low as 100 °C should be limited and minor amounts of side-reactions were expected during one-pot Willgerodt–Kindler polymerization. Therefore, the classical SRTC route was considered unlikely to occur in these initial attempts. On the other hand, the catalytic effect of amines should be considered as a potential reason for network formation. Indeed, recent reports revealed that inverse vulcanization reactions could be catalyzed via pyridines and amines at lower temperatures such as 120 °C. It is likely that the diamine reagents acted as a catalyst and triggered the STRC reaction in the one-pot approach. To overcome the synthetic problems mentioned, a sequential synthetic approach was selected for the synthesis thioamide based benzoxazines. Initially, thioamides with phenolic groups were synthesized by Willgerodt–Kindler reaction as starting reagents for benzoxazine synthesis. Two types of thioamides were obtained as dihydroxythioamide and monohydroxythioamide (Scheme 5). Here, it should be noted that the reactions should be performed under nitrogen gas and with Na2S as the catalyst in successive steps. Otherwise, the yields of these reactions decrease drastically and a considerable amount of unreacted sulfur remained. The chemical structures of the formed thioamides were confirmed by 1H NMR and FTIR analyses (see Fig. S4 and 5†). The presence of thioamide NH protons is clearly visible at 11.41 and 11.20 ppm for mono- and difunctional derivatives, respectively. Moreover, the signal of the OH proton of monofunctional thioamide appears at 10.10 ppm and two different OH resonance signals are observed at 10.02 and 9.50 ppm due to two types of phenols in the structure of dihydroxythioamide. The FTIR spectra of thioamides in Fig. S5† further support the chemical structures. The N–H stretching vibrations are distinctive at 3343 cm−1 for difunctional and at 3317 cm−1 for monofunctional thioamide. Moreover, the O–H stretching vibrations appear as broad bands between 3310 and 3104 cm−1 for both thiaomides.
 | ||
| Scheme 5 Synthesis of 4-hydroxy-N-(4-hydroxyphenyl)benzothioamide (dihydroxythioamide) and 4-hydroxy-N-phenylbenzothioamide (monohydroxythioamide). | ||
After the synthesis of dihydroxythioamide and monohydroxythioamide, benzoxazine monomers were synthesized via the classical route (Scheme 6) where furfuryl and benzylamines were selected as primary amines along with paraformaldehyde. In the synthesis, a toluene/ethanol mixture was deliberately selected as the solvent to decrease the triazine formation in the synthesis of thioamide based benzoxazines. The chemical structures of the benzoxazines were confirmed by 1H NMR, 13C NMR (Fig. S6–8†) and FTIR spectral analyses. The 1H NMR spectra of the Th-amd-MonoBz-Bn, Th-amd-MonoBz-Fr and Th-amd-DiBz-Bn are presented in Fig. 1. The appearance of the proton signals at 4.95, 4.92, (O–CH2−N) and 3.98, 4.00, (Ar–CH2−N) for Th-amd-MonoBz-Bn and Th-amd-MonoBz-Fr confirms the oxazine rings, respectively. Th-amd-DiBz-Bn is anti-symmetric and the benzoxazines are not identical, thus it has two different oxazine rings. Therefore, in Fig. 1c, the proton signals are observed at 4.94, 4.87 (O–CH2−N), 3.97, 3.91 ppm (Ar–CH2−N). Moreover, the singlet peaks at 3.87, 3.86, and 3.85 ppm stem from Aryl–CH2− (benzylic) and furan–CH2− (furylic). Besides, the aromatic peaks of the furan group are visible at 6.82, 6.42, and 6.33 ppm for Th-amd-MonoBz-Fr. Moreover, the proton signals of thioamide N–H appear at 11.46, 11.48, and 11.29 ppm, as clear evidence of the preservation of the thioamide group after benzoxazine synthesis.
 | ||
Scheme 6 Synthesis of mono (Th-amd-MonoBz-Bn and Th-amd-MonoBz-Fr) and difunctional thioamide benzoxazines (Th-amd-DiBz-Bn). Reaction conditions: Toulene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (2 EtOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), reflux, 24 h. 1, v/v), reflux, 24 h. | ||
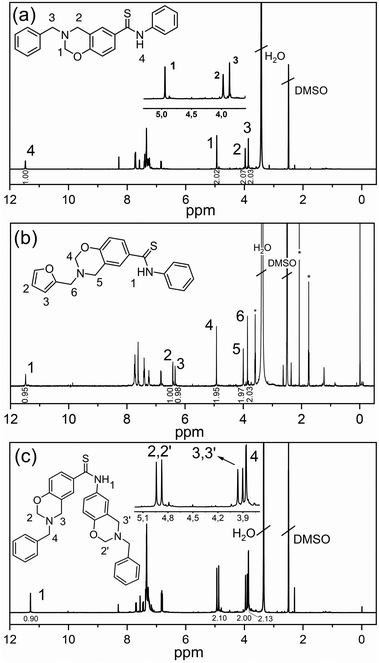 | ||
| Fig. 1 1H NMR spectra of Th-amd-MonoBz-Bn (a), Th-amd-MonoBz-Fr (b), and Th-amd-DiBz-Bn (c). *Residues of acetone and THF. | ||
The formation of thioamide functional benzoxazines was further confirmed by the FTIR spectra of the corresponding monomers (Fig. S9†). The oxazine skeletal vibrations of C–H at 917–923 cm−1 can be considered as strong evidence for the presence of oxazines in the proposed structures. Moreover, the stretching vibration of the N–H bond of thioamide appears at 3332 cm−1 for Th-amd-MonoBz-Bn. However, a similar N–H band is highly broad for Th-amd-MonoBz-Fr and Th-amd-DiBz-Bn. On the other hand, thiocarbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) bands are clearly visible at 1104–115 cm−1 and the overtones of C
S) bands are clearly visible at 1104–115 cm−1 and the overtones of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S are detectable as weak bands at ca. 2230–2238 cm−1 for the three benzoxazine monomers. In addition, the aromatic stretching vibrations of C–H bonds (3012–3115 cm−1) and aromatic C
S are detectable as weak bands at ca. 2230–2238 cm−1 for the three benzoxazine monomers. In addition, the aromatic stretching vibrations of C–H bonds (3012–3115 cm−1) and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1420–1604 cm−1) are observed as the fundamental aromatic vibrations. Besides NMR and FTIR, Q-TOF LC/MS analyses were also performed to confirm the structures. The Fig. S10 and 11† and related mass spectrum list tables match well with the molar masses of the compounds. Consequently, NMR, FTIR, and LC-MS results verify the successful synthesis of thioamide functional benzoxazines. In general, many benzoxazine monomers, especially mono-functional benzoxazines, have poor film forming properties after curing. The polybenzoxazines therefrom are usually fragile because of the low molecular weight of the network structure. Therefore, as a solution for the brittleness and improve the film forming ability, polymeric benzoxazine precursors were synthesized by using Mannich type polycondensation.62–66 In this way, polymeric benzoxazine precursors behave like a processable and cross-linkable thermoplastic by exploiting the inherent properties of thermoplastic polymers such as flexibility, high toughness and ease of film formation. The dihydroxythioamide contains two phenolic groups, which makes it suitable to synthesize the main chain polybenzoxazine precursor. Thus, this molecule was reacted with paraformaldehyde and bisamino end-functional poly(propylene oxide-b-ethylene oxide-b-propylene oxide) (PPEO) (Jeffamine ED-900, Mn ≈ 900 Da) in a toluene/ethanol mixture (2
C (1420–1604 cm−1) are observed as the fundamental aromatic vibrations. Besides NMR and FTIR, Q-TOF LC/MS analyses were also performed to confirm the structures. The Fig. S10 and 11† and related mass spectrum list tables match well with the molar masses of the compounds. Consequently, NMR, FTIR, and LC-MS results verify the successful synthesis of thioamide functional benzoxazines. In general, many benzoxazine monomers, especially mono-functional benzoxazines, have poor film forming properties after curing. The polybenzoxazines therefrom are usually fragile because of the low molecular weight of the network structure. Therefore, as a solution for the brittleness and improve the film forming ability, polymeric benzoxazine precursors were synthesized by using Mannich type polycondensation.62–66 In this way, polymeric benzoxazine precursors behave like a processable and cross-linkable thermoplastic by exploiting the inherent properties of thermoplastic polymers such as flexibility, high toughness and ease of film formation. The dihydroxythioamide contains two phenolic groups, which makes it suitable to synthesize the main chain polybenzoxazine precursor. Thus, this molecule was reacted with paraformaldehyde and bisamino end-functional poly(propylene oxide-b-ethylene oxide-b-propylene oxide) (PPEO) (Jeffamine ED-900, Mn ≈ 900 Da) in a toluene/ethanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Scheme 7).
1, v/v) (Scheme 7).
 | ||
| Scheme 7 Synthesis of main chain polybenzoxazine (poly(thioamide-benz-PPEO)) from PPEO and dihydroxythioamide. | ||
The obtained polymer (poly(thioamide-benz-PPEO)) had molecular weights of Mn ≈ 2300 Da and Mw ≈ 5220 Da according to GPC analysis and correspond to ca. 3–4 degree of polymerization. In order to obtain better insight into molecular weight of the polymer, pristine Jeffamine ED-900 was also analyzed using the same GPC device and the Mn was found to be ca. 600 Da instead of ca. 900 Da. Therefore, the actual molecular weight of poly(thioamide-benz-PPEO) could be larger than Mn ≈ 2300. However, the possible molecular mass can still be considered as low and the reason for the observed molecular weight is probably related to the bulky poly(propylene oxide) units that slow down the polymerization. Moreover, it is well known that Mannich type polycondensation reactions do not yield main chain polybenzoxazines with high molecular weights. There are only a small number of examples having large molecular masses. Hence, synthesizing polymeric benzoxazine precursors with considerably high molecular weights is still an ongoing challenge. The spectral characterization of the polymer confirms the presence of both oxazine and thioamide moieties in the main chain. The comparative 1H NMR spectra of Jeffamine ED-900 and poly(thioamide-benz-PPEO) are presented in Fig. 2. The proton signals of O–CH2−N and Ar–CH2−N appear at 4.97, 4.87 and 4.09, 4.02 ppm, respectively. Two different types of oxazines are present as repeat units in poly(thioamide-benz-PPEO) due to the anti-symmetric thioamide core. Moreover, –NH proton is clearly detectable at 11.23 ppm, evidencing the preservation of the thioamide unit. Here, it should be noted that many reaction steps take place for oxazine formation in a typical main-chain polybenzoxazine precursor synthesis. Therefore, some ring-opened structures remain in the backbone. Accordingly, the integrated peaks of O–CH2−N and Ar–CH2−N do not match for most samples. Apart from fundamental peaks related to oxazine and thiaomide groups, intense proton signals of the Jeffamine moiety are between 3.6 and 3.09 ppm, and those of the aliphatic protons are at 1.06–0.99 ppm. The FTIR spectrum of poly(thioamide-benz-PPEO) (Fig. S12†) further confirms the structure of the polymer. The IR spectrum shows the characteristic C–O band of the ether structure of Jeffamine at 1092 cm−1. Moreover, the oxazine skeletal vibrations of C–H appear at ca. 920 cm−1 providing another evidence to support the presence of the ring structure in the main chain structure. In addition, the thioamide N–H stretching vibration is detectable at around 3270 cm−1. In contrast, thiocarbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) is not visible due to the overlapping C–O bands of Jeffamine units.
S) is not visible due to the overlapping C–O bands of Jeffamine units.
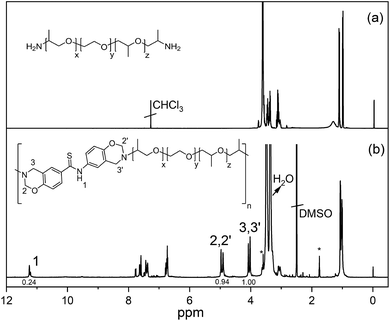 | ||
| Fig. 2 1H NMR spectra of bisamino end-functional poly(propylene oxide-b-ethylene oxide-b-propylene oxide) (PPEO) (a) and poly(thioamide-benz-PPEO) (b). *Residue of THF. | ||
Consequently, a curable, flexible and processable thioamide based benzoxazine thermoplastic was synthesized by Mannich condensation. The obtained precursor was solvent cast in Teflon molds for film fabrication and then it was heated up to 200 °C in a stepwise manner (ca. 20 °C ramp per 5 min starting from 120 °C), after evaporating the solvent. Eventually, a partially transparent, flexible cross-linked film with a smooth surface was obtained (Fig. 3). The films could absorb water because of the polyether moieties. Accordingly, water absorption of the films was tested by the water absorption 24 h per eq ASTM D570 test. Similarly, the water absorption capacities of the cured Th-amd-MonoBz-Bn, Th-amd-MonoBz-Fr and Th-amd-DiBz-Bn were also determined. The test results, as mass %, for the specimens are as follows: ≈30% for cured poly(thioamide-benz-PPEO), ≈0% for cured Th-amd-MonoBz-Bn, ≈1% for cured Th-amd-MonoBz-Fr and ≈0.3% for cured Th-amd-DiBz-Bn.
As stated previously, benzoxazines can be polymerized easily by thermal treatment and the ring opening polymerization (ROP) temperatures generally lie between 180–250 °C depending on the functional groups of the benzoxazine molecule. The ROP is an exothermic event and can be investigated by differential calorimetric analysis. In Fig. 4, the DSC traces of Th-amd-MonoBz-Bn, Th-amd-MonoBz-Fr, Th-amd-DiBz-Bn and the precursor poly(thioamide-benz-PPEO) are displayed. Moreover, the DSC characteristics of these compounds are tabulated in Table 1. Accordingly, all the benzoxazine monomers and polymeric precursors are curable and interestingly the curing temperatures are far below compared to many other benzoxazines. The on-set curing temperatures are 165, 172 and 180 °C for the monomeric benzoxazines and 181 °C for the polymeric precursor. Similarly, the maximum curing temperatures are 189, 193 and 204 °C for monomers and 211 °C for the precursor. In order to explain the observed curing temperatures, we have mixed 10 mol% Th-amd-MonoBz-Bn with a monofunctional benzoxazine (P-a) derived from aniline and phenol. Then, the DSC analysis was performed for the mixture under the same conditions as those used to analyze the monomers. The comparative DSC traces of the P-a monomer and a mixture of Th-amd-MonoBz-Bn and P-a is depicted in Fig. 5. It is obvious that the thioamide bearing bezoxazine acted as a curing promoter and reduced the curing temperature of the P-a monomer by 30 °C. As disclosed in several previous studies, the purity of a benzoxazine monomer can impact the curing temperature and extremely pure compounds exhibit high curing temperatures. Conversely, impure monomers could have lower curing temperatures depending on the amount and type of impurities. Here, it can be proposed that residues of unreacted sulfur carried from initial thioamide synthesis could reduce the curing temperatures to some extent. However, such a drastic reduction is unlikely with sulfur residues because a large drop is only possible with high amounts of sulfur, as much as 20% by mass, reported previously by separate research groups.67 Besides, the melting endotherms of elemental sulfur are not detectable in DSC thermograms that should appear as multi-endotherms between 102–117 °C in the case where residual sulfur is present.68,69 Moreover, extraction experiments were performed for cured samples to analyze the soluble unreacted possible impurities by using common organic solvents such as acetone, DMSO, DMF, chloroform and ethanol. Only a minor amount of soluble extract could be obtained from cured Th-amd-DiBz-Bn (see Fig. S13† for the 1H NMR spectrum of the soluble portion). Other benzoxazines were completely cross-linked after curing at 200 °C for 30 min in an open-air oven. These experiments reveal that all the benzoxazines are curable under the stated conditions. Consequently, the observed temperature reduction can be considered as the promoting effect of the thioamide core in benzoxazine molecules.
 | ||
| Fig. 4 DSC thermograms of Th-amd-MonoBz-Bn (a), Th-amd-MonoBz-Fr (b), Th-amd-DiBz-Bn (c), and poly(thioamide-benz-PPEO) (d). | ||
 | ||
| Fig. 5 DSC thermograms of monofunctional benzoxazine (P-a) (ΔH = −165 j g−1) (a), mixture of P-a (90 mol%) and Th-amd-MonoBz-Bn (10 mol%) (ΔH = –154 j g−1) (b). | ||
| Benzoxazine | Curing on-set (°C) | Curing end-set (°C) | Curing max. (°C) | Curing exotherm (J g−1) |
|---|---|---|---|---|
| a DSC analyses were performed under a nitrogen stream (20 mL min−1.) with a 10 °C min−1 heating rate. | ||||
| Th-amd-MonoBz-Bn | 172 | 238 | 193 | −88.01 |
| Th-amd-MonoBz-Fr | 165 | 220 | 189 | −65.77 |
| Th-amd-DiBz-Bn | 180 | 241 | 204 | −57.07 |
| Poly(thioamide-benz-PPEO) | 178 | 239 | 211 | −74.40 |
The proposed effect of the thioamide core could be related to tautomerism that is generally seen in many thiaomides (Scheme 8).70 The tautomeric properties of thioamides generate a thiol form, which can further be stabilized by the resonance effect in the case of aromatic thioamides.42,71,72 Furthermore, thiols are known as potent catalysts for curing benzoxazines and they can react efficiently with the oxazine ring even at room temperature. This reaction was coined as COLBERT (Catalytic Opening of the Lateral Benzoxazine Rings by Thiols) and the first step is the protonation of the oxazine ring by –SH of thiols.73–77 In this way, ring opening is triggered to form an iminium cation that eventually reacts with either the neighboring benzoxazine or the R–S− anion (refer Scheme 4) to form a dimer (aminophenol/benzoxazine) or a thiomethyl aminophenol.77 Accordingly, although there is no direct evidence due to the difficulties to analyze intermediates at high temperatures, the observed exothermic inflections between 150 and 170 °C in DSC traces (Fig. 4) for all three benzoxazine monomers prior to the main ROP exotherms could be the consequence of the tautomerism triggered thiol-oxazine reaction. This type of exotherm is hardly visible in the DSC thermogram of the polymeric precursor, possibly, due to the dilution effect of large Jeffamine moieties that hinder the collisions between oxazine rings and the momentarily formed thiols. Hence, the catalytic effect of the thioamide group is limited in the case of the polymeric precursor compared to the corresponding monomeric analogues.
 | ||
| Scheme 8 Tautomeric equilibrium in a thioamide (1) and resonance forms of a para-substituted thioaroyl group (2). | ||
TGA thermograms and derivative TGA thermograms are plotted to present the initial degradation temperatures, inflection points and char yields in Fig. 6 and 7, respectively. These thermal properties are also summarized in Table 2. Accordingly, polybenzoxazines derived from Th-amd-Mono(or)Bz-Bn(or)Fr exhibited different characteristics depending on the functional groups. For example, although Th-amd-MonoBz-Fr is a monofunctional benzoxazine monomer, polybenzoxazine therefrom has T5%, T10% values close to those of polybenzoxazine from monofunctional Th-amd-MonoBz-Bn and their char yield values are similar to those of polybenzoxazine derived from the difunctional Th-amd-DiBz-Bn monomer because it was reported that chemical bonding occurs between furan rings and Mannich bridges during polymerization of furan functional benzoxazines, leading to a higher degree of crosslinking in polybenzoxazines.78,79 Moreover, furan and related compounds have high tendency to form char even under hydrothermal conditions.80 In summary, char formation and extra cross-linking ability during curing of benzoxazines can be considered as the reason behind the observed high char yield of furan based polybenzoxazine. On the other hand, expectedly, the cured poly(thioamide-benz-PPEO) behaves completely differently from polybenzoxazines of monomeric benzoxazines. The char yield of this polybenzoxazine is significantly lower because the polymeric precursor contains bulky polyether segments that are prone to degrade fast at high temperatures. In contrast, although the crosslinking density is expected the lowest among these networks, obviously, polyether segments have beneficial effects on T5%, T10% and Tmax values and thus the polybenzoxazine of the precursor has high initial degradation temperatures, making this polymer suitable for high temperature applications.
 | ||
| Fig. 6 TGA thermograms of Th-amd-DiBz-Bn (a), Th-amd-MonoBz-Fr (b), Th-amd-MonoBz-Bn (c), and poly(thioamide-benz-PPEO) (d). All the samples were cured at ca. 210 °C prior to analysis. | ||
 | ||
| Fig. 7 Derivative TGA of Th-amd-MonoBz-Fr (a), Th-amd-MonoBz-Bn (b), Th-amd-DiBz-Bn (c), and poly(thioamide-benz-PPEO) (d). | ||
| Sample | T 5% (°C) | T 10% (°C) | T c (%) | T max (°C) |
|---|---|---|---|---|
| a Curing of benzoxazines was performed in TGA device at ca. 210 °C for 15 min under a N2 stream (200 mL min−1). T5%: the temperature for which the weight loss is 5% by mass, T10%: the temperature for which the weight loss is 10% by mass, Tc: the char yield at 800 °C, Tmax: the temperature for maximum weight loss that is extracted from the derivative TGA graph (Fig. 7). | ||||
| Th-amd-MonoBz-Bn | 211 | 227 | 27 | 247 |
| Th-amd-MonoBz-Fr | 212 | 225 | 41 | 229 |
| Th-amd-DiBz-Bn | 236 | 259 | 43 | 270 |
| Poly(thioamide-benz-PPEO) | 290 | 343 | 22 | 395 |
Conclusions
In this study, thioamide based benzoxazine monomers and a polymeric precursor were successfully synthesized. Initially, the synthesis of thioamide main chain polybenzoxazine was unsuccessful by the one-pot Willgerodt–Kindler reaction. However, a sequential approach that consists of the Willgerodt–Kindler and Mannich reactions and ring-closure to form oxazine rings gave the desired polybenzoxazines and monomeric benzoxazines. The polymeric precursor exhibited a good film forming ability and the cast films were flexible after curing. Interestingly, the thioamide groups affected the curing process of benzoxazines and caused an apparent reduction in the ring opening polymerization temperatures. The observed effect was attributed to the tautomerism of thioamide groups that momentarily produce a thiol tautomer, which catalyzes the ring opening of benzoxazines. In conclusion, the thioamide group was integrated into the benzoxazine structure for the first time with well-known reactions and promoting effects on curing were obtained as a benefit of thioamides. Therefore, this study could be particularly important in designing benzoxazines bearing latent self-catalytic properties. In addition, this study merges sulfur and benzoxazine chemistries to produce compounds that have many potential applications because the synthesis of various phenolic thioamides is possible by the Willgerodt–Kindler reaction and these phenolics can be easily converted to benzoxazines by altering the primary amines already present in commercial sources as diverse compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Istanbul Technical University Research Fund for financial support (Project ID: TYL-2020-42714).Notes and references
- H. Ishida and T. Agag, Handbook of Benzoxazine Resins, Elsevier, Amsterdam, 2011 Search PubMed.
- N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344–1391 CrossRef CAS.
- B. Kiskan and Y. Yagci, Isr. J. Chem., 2020, 60, 20–32 CrossRef CAS.
- C. P. R. Nair, Prog. Polym. Sci., 2004, 29, 401–498 CrossRef CAS.
- H. Ishida, US Patent 5973144, 1999.
- C. H. Lin, S. L. Chang, T. Y. Shen, Y. S. Shih, H. T. Lin and C. F. Wang, Polym. Chem., 2012, 3, 935–945 RSC.
- M. Zeng, J. Chen, Q. Xu, Y. Huang, Z. Feng and Y. Gu, Polym. Chem., 2018, 9, 2913–2925 RSC.
- K. Zhang, X. Yu and S.-W. Kuo, Polym. Chem., 2019, 10, 2387–2396 RSC.
- H. Ishida and P. Froimowicz, Advanced and Emerging Polybenzoxazine Science and Technology, Elsevier Inc., Amsterdam, Netherlands, 2017 Search PubMed.
- B. Kiskan and Y. Yagci, in Thermosets, ed. Q. Guo, Elsevier Inc., 2nd edn, 2017, ch. 17, pp. 543–576 Search PubMed.
- H. Ishida and Y. Rodriguez, Polymer, 1995, 36, 3151–3158 CrossRef CAS.
- M. W. Wang, R. J. Jeng and C. H. Lin, Macromolecules, 2015, 48, 530–535 CrossRef CAS.
- C. Liu, D. Shen, R. M. a. Sebastián, J. Marquet and R. Schönfeld, Macromolecules, 2011, 44, 4616–4622 CrossRef CAS.
- P. Chutayothin and H. Ishida, Macromolecules, 2010, 43, 4562–4572 CrossRef CAS.
- K. Zhang, X. Tan, Y. Wang and H. Ishida, Polymer, 2019, 168, 8–15 CrossRef CAS.
- L. Han, M. L. Salum, K. Zhang, P. Froimowicz and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3434–3445 CrossRef CAS.
- Z. Deliballi, B. Kiskan and Y. Yagci, Macromolecules, 2020, 53, 2354–2361 CrossRef CAS.
- R. Kudoh, A. Sudo and T. Endo, Macromolecules, 2010, 43, 1185–1187 CrossRef CAS.
- B. Kiskan, B. Koz and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6955–6961 CrossRef CAS.
- C. Zúñiga, G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Polymer, 2012, 53, 3089–3095 CrossRef.
- T. Kobayashi, M. Goto, M. Minami and F. Sanda, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 2581–2589 CrossRef CAS.
- M. Goto, T. Yajima, M. Minami, H. Sogawa and F. Sanda, Macromolecules, 2020, 53, 6640–6648 CrossRef CAS.
- M. Goto, Y. Miyagi, M. Minami and F. Sanda, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1884–1893 CrossRef CAS.
- S. N. Kolanadiyil, M. Azechi and T. Endo, Macromolecules, 2015, 48, 7466–7472 CrossRef.
- S. N. Kolanadiyil, M. Minami and T. Endo, Macromolecules, 2017, 50, 3476–3488 CrossRef CAS.
- L. Puchot, P. Verge, T. Fouquet, C. Vancaeyzeele, F. Vidal and Y. Habibi, Green Chem., 2016, 18, 3346–3353 RSC.
- A. Trejo-Machin, P. Verge, L. Puchot and R. Quintana, Green Chem., 2017, 19, 5065–5073 RSC.
- R. Ganfoud, N. Guigo, L. Puchot, P. Verge and N. Sbirrazzuoli, Eur. Polym. J., 2019, 119, 120–129 CrossRef CAS.
- N. K. Sini, J. Bijwe and I. K. Varma, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 7–11 CrossRef CAS.
- R. Andreu and J. C. Ronda, Synth. Commun., 2008, 38, 2316–2329 CrossRef CAS.
- J. C. Ronda, G. Lligadas, M. Galià and V. Cádiz, React. Funct. Polym., 2013, 73, 381–395 CrossRef CAS.
- G. Lligadas, A. Tüzün, J. C. Ronda, M. Galià and V. Cádiz, Polym. Chem., 2014, 5, 6636–6644 RSC.
- W.-H. Hu, K.-W. Huang and S.-W. Kuo, Polym. Chem., 2012, 3, 1546–1554 RSC.
- J. Wang, M.-q. Wu, W.-b. Liu, S.-w. Yang, J.-w. Bai, Q.-q. Ding and Y. Li, Eur. Polym. J., 2010, 46, 1024–1031 CrossRef CAS.
- M. Soto, M. Hiller, H. Oschkinat and K. Koschek, Polymers, 2016, 8, 278 CrossRef.
- F. S. Gungor, B. Bati and B. Kiskan, Eur. Polym. J., 2019, 121, 109352 CrossRef CAS.
- K. Zhang and H. Ishida, Front. Mater., 2015, 2(5), 1–9 Search PubMed.
- K. Zhang and H. Ishida, Polym. Chem., 2015, 6, 2541 RSC.
- T. Agag, C. R. Arza, F. H. J. Maurer and H. Ishida, Macromolecules, 2010, 43, 2748–2758 CrossRef CAS.
- G. Kaya, B. Kiskan and Y. Yagci, Polym. Chem., 2019, 10, 1268–1275 RSC.
- C. Durukan, B. Kiskan and Y. Yagci, Polymers, 2019, 11, 679 CrossRef CAS.
- T. Murai, Chemistry of Thioamides, Springer Nature Singapore Pte Ltd., Singapore, 2019 Search PubMed.
- W. Li, X. Wu, Z. Zhao, A. Qin, R. Hu and B. Z. Tang, Macromolecules, 2015, 48, 7747–7754 CrossRef CAS.
- Z. Sun, H. Huang, L. Li, L. Liu and Y. Chen, Macromolecules, 2017, 50, 8505–8511 CrossRef CAS.
- T. Higashihara and M. Ueda, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS.
- K. A. Mazzio and C. K. Luscombe, Chem. Soc. Rev., 2015, 44, 78–90 RSC.
- H. S. O. Chan and S. C. Ng, Prog. Polym. Sci., 1998, 23, 1167–1231 CrossRef CAS.
- K. Sanui, Y. Kishimoto and N. Ogata, Polym. J., 1971, 2, 422–425 CrossRef CAS.
- J. C. Gressier and G. Levesque, Eur. Polym. J., 1981, 17, 695–706 CrossRef CAS.
- Y. Kawai, T. Kanbara and K. Hasegawa, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1737–1740 CrossRef CAS.
- D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 7870–7880 RSC.
- T. Kanbara, Y. Kawai, K. Hasegawa, H. Morita and T. Yamamoto, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 3739–3750 CrossRef CAS.
- B. Kurpil, B. Kumru, T. Heil, M. Antonietti and A. Savateev, Green Chem., 2018, 20, 838–842 RSC.
- W. Cao, F. Dai, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2020, 142, 978–986 CrossRef CAS.
- K. Shigehiro, S. Emi, M. Ikumi, H. Kiyoshi and K. Takaki, Chem. Lett., 2003, 32, 622–623 CrossRef.
- H. R. Darabi, M. J. Tehrani, K. Aghapoor, F. Mohsenzadeh and R. Malekfar, Appl. Surf. Sci., 2012, 258, 8953–8958 CrossRef CAS.
- K. B. Wiberg and D. J. Rush, J. Am. Chem. Soc., 2001, 123, 2038–2046 CrossRef CAS.
- H.-J. Lee, Y.-S. Choi, K.-B. Lee, J. Park and C.-J. Yoon, J. Phys. Chem. A, 2002, 106, 7010–7017 CrossRef CAS.
- R. W. Newberry, B. VanVeller and R. T. Raines, Chem. Commun., 2015, 51, 9624–9627 RSC.
- N.-K. Kim, H.-J. Lee, K.-H. Choi, J.-A. Yu, C.-J. Yoon, J. Park and Y.-S. Choi, J. Phys. Chem. A, 2000, 104, 5572–5578 CrossRef CAS.
- B. K. Min, H.-J. Lee, Y. S. Choi, J. Park, C.-J. Yoon and J.-A. Yu, J. Mol. Struct., 1998, 471, 283–288 CrossRef CAS.
- T. Takeichi, T. Kano and T. Agag, Polymer, 2005, 46, 12172–12180 CrossRef CAS.
- T. Agag; and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1878–1888 CrossRef.
- A. Chernykh, J. Liu and H. Ishida, Polymer, 2006, 47, 7664–7669 CrossRef CAS.
- M. Arslan, B. Kiskan and Y. Yagci, Macromolecules, 2015, 48, 1329–1334 CrossRef CAS.
- A. Yildirim, B. Kiskan, A. L. Demirel and Y. Yagci, Eur. Polym. J., 2006, 42, 3006–3014 CrossRef CAS.
- C. R. Arza, P. Froimowicz and H. Ishida, RSC Adv., 2016, 6, 35144–35151 RSC.
- M. Arslan, B. Kiskan and Y. Yagci, Macromolecules, 2016, 49, 767–773 CrossRef CAS.
- M. Arslan, B. Kiskan, E. Cengiz, R. Demir-Cakan and Y. Yagci, Eur. Polym. J., 2016, 80, 70–77 CrossRef CAS.
- J. Leszczynski, J. S. Kwiatkowski and D. Leszczynska, J. Am. Chem. Soc., 1992, 114, 10089–10091 CrossRef CAS.
- T. Hori, Y. Otani, M. Kawahata, K. Yamaguchi and T. Ohwada, J. Org. Chem., 2008, 73, 9102–9108 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- I. Gorodisher, R. J. DeVoe and R. J. Webb, in Handbook of Benzoxazine Resins, ed. T. Agag and H. Ishida, Elsevier, Amsterdam, 2011, pp. 211–234, DOI:10.1016/B978-0-444-53790-4.00056-4.
- E. Semerci, B. Kiskan and Y. Yagci, Eur. Polym. J., 2015, 69, 636–641 CrossRef CAS.
- Z. Beyazkilic, M. Kahveci, B. Aydogan, B. Kiskan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4029–4036 CrossRef CAS.
- S. Bektas, B. Kiskan, N. Orakdogen and Y. Yagci, Polymer, 2015, 75, 44–50 CrossRef CAS.
- T. Urbaniak, M. Soto, M. Liebeke and K. Koschek, J. Org. Chem., 2017, 82, 4050–4055 CrossRef CAS.
- Y.-L. Liu and C.-I. Chou, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5267–5282 CrossRef CAS.
- O. Bayram, B. Kiskan, E. Demir, R. Demir-Cakan and Y. Yagci, ACS Sustainable Chem. Eng., 2020, 8, 9145–9155 CrossRef CAS.
- M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic methods for Bis(VaBz)-Hex and attempted polymerization procedure for the one-pot synthesis of thioamide containing main chain polybenzoxazine. NMR and FTIR spectra of Bis(VaBz)-Hex, mono and dihydroxythioamides, products of one-pot main chain polybenzoxazine synthesis experiment, 13C NMR and FTIR spectra of Th-amd-MonoBz-Bn, Th-amd-MonoBz-Fr, and Th-amd-DiBz-Bn. See DOI: 10.1039/d0py01381a |
| This journal is © The Royal Society of Chemistry 2021 |