Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass†
Llorenç
Gavilà
ab and
Davide
Esposito
*a
aMax-Planck-Institute of Colloids and Interfaces, D-14424 Potsdam, Germany. E-mail: davide.esposito@mpikg.mpg.de
bDepartment of Chemical Engineering, Universitat Rovira i Virgili, Av Països Catalans 26, 43007, Tarragona, Spain
First published on 11th April 2017
Abstract
5-Acetoxymethylfurfural (AMF) is an important biomass derived platform chemical related to 5-hydroxymethylfurfural. Such furanic compounds can be produced via the hydrolysis of cellulose followed by dehydration of the resulting glucose units. However, the integration of these reactions in a single process remains technically challenging, and the direct use of monosaccharides is often preferred. In this work we report a new method for the synthesis of AMF based on the acetolysis of cellulose acetate in the presence of sulfuric acid. The strategy was optimized for both batch and continuous processing. Furthermore, cellulose acetate prepared by direct wood acetylation could be successfully applied as a precursor, proving the method as a robust solution for integrated biomass processing.
Large biomass exploitation for chemicals and fuel production is still awaiting integrated processing.1 One of the main problems is the scarce solubility of biomass.2 Cellulose, which is the most studied fraction for fuels and chemical production,3,4 is generally insoluble in common solvents and its solubilization can only be achieved using complex solvent systems, including the still expensive ionic liquids (ILs).5 The strong H-bond network that involves unprotected hydroxyl groups is responsible for the scarce solubility of cellulose.6 Protection or partial protection of this biopolymer disrupts the natural H-bond network, thereby modifying solubility properties. Such a strategy has a long tradition and the first cellulose derivatives, e.g. nitrocellulose, were already discovered back in the 19th century. Since then a wide range of functional groups have been used to modify the hydroxyl moieties in cellulose, including nitrates, nitrites, xanthates, formates and acetates.7
The readily available cellulose acetate has been widely used in industry due its low price. Cellulose acetate is prepared from pure cellulose, however its preparation has also been reported from crude biomass.8 Cellulose acetate solubility in organic solvents depends on the number of hydroxyl moieties substituted by acetyl groups, commonly referred to as the degree of substitution (DS). In general, common solvents like tetrahydrofuran, methyl acetate, acetone or dioxane, can be used for DS higher than 2. However, cellulose acetate characterized by a lower DS can only be dissolved by fewer solvents,9 including for instance acetic acid.
In the area of chemical conversion of cellulose into platform chemicals, almost no reports have appeared using cellulose acetates as the starting material. In turn, different products have been targeted on the basis of unprotected carbohydrates.10 Among them, 5-hydroxymethylfurfural (HMF), the product of hexose dehydration,11 is claimed to be one of the most valuable biomass-derived building-blocks, with enormous potential in the field of polymer production12 or fuel additives13 thank to its derivatives furandicarboxylic acid (FDCA) and 2,5-dimethylfuran (DMF), respectively. However, HMF production is still technically challenging when cellulose is used as the carbohydrate source. In this case, processes have been traditionally developed using aqueous reaction mixtures, although alternative solvents have been recently proposed with the aim of improving HMF yields.14 ILs, for instance, have been proved as an efficient medium to depolymerize cellulose and dehydrate the monomers, achieving the production of HMF in 54% yield.15 Nevertheless, despite the great potential, the industrial application of ILs is still costly, as mentioned above.
The use of alternative solvent systems has also opened the way to the synthesis of interesting HMF derivatives. Methoxymethylfurfural (MMF), which can be obtained in 55% yield by reacting hexoses in methanol in the presence of sulfuric acid,16 has been targeted by Avantium as a key intermediate for the production of FDCA. In addition, Mascal et al. reported the synthesis of 5-(chloromethyl)furfural (CMF) by reacting cellulose in hydrochloric acid in the presence of lithium chloride while continuously extracting the reaction mixture in dichloroethane.17 Interestingly, reaction with alkylammonium acetates can be used to transform crude CMF into acetoxymethylfurfural (AMF).18 The latter has also been proposed as a valuable HMF alternative, claiming that the acetyl moiety makes AMF hydrophobic, less reactive, and more versatile in terms of reactivity.18 Interestingly, production of AMF by continuous reaction of fructose in supercritical acetic acid has been published.19 However, we realized that the use of similar conditions for the direct conversion of cellulose derivatives into furanic compounds has not been reported, to the best of our knowledge. Indeed, the direct use of polymeric carbohydrates rather than monomers is not trivial, due to solubility issues as well as to the lower yields caused by competitive processes occurring during polysaccharide deconstruction.10 Hence, in this work, we investigated the depolymerization of cellulose acetate in acetic acid as a new method to produce AMF. Such a method takes advantage of the use of an environmentally benign solvent and offers the benefits of a homogeneous process, since cellulose acetate is readily soluble in acetic acid.
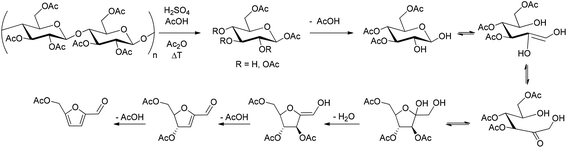
We started testing the reactivity of commercial cellulose acetate in acetic acid using an autoclave reactor. Lewis acids, as well as organic and mineral Brønsted acids are traditionally used for the transformation of unprotected sugars into HMF, and were therefore screened also in the present study (Fig. 1). Initially, all reactions were carried out in the presence of 2 equivalents of acetic anhydride, in order to preserve the reaction intermediates and the product in the acetylated state. HPLC was employed for the analysis of the products.
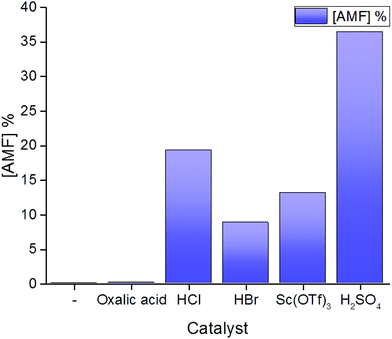 | ||
| Fig. 1 AMF yield for the acetolysis of cellulose acetate in the presence of different catalysts. General conditions: cellulose acetate, acetic anhydride, catalyst, 200 °C, 2 hours. For details of the reaction conditions please refer to the ESI.† | ||
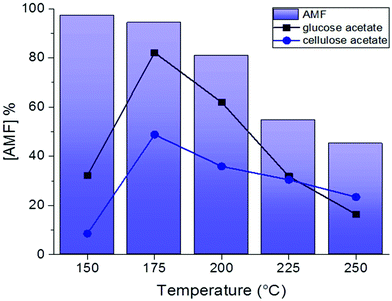
Acetic acid was used as a control experiment in the absence of an additional catalyst showing no AMF production. In a similar way, the addition of an organic catalyst such as oxalic acid, did not result in AMF production, suggesting that stronger acids are required. Scandium triflate afforded AMF in 13% yield, while hydrogen chloride and hydrogen bromide resulted in the formation of the product in a yield of 19% and 9% respectively. Among the different homogeneous catalysts screened, sulfuric acid showed the best performance, suggesting that higher dehydrating properties increase AMF yield. Although initial experiments showed that AMF can be produced in 36% yield by reaction at 200 °C for 2 hours in the presence of 2 equivalents of H2SO4 (Fig. 1), any attempt to further optimize the reaction was not successful. When increasing the reaction time over 2 hours, AMF yield decreased (Fig. 2A), possibly due to AMF degradation. A drop in AMF yield is also observed when the reaction temperature is increased from 200 °C to 220 °C (Fig. 2C), in agreement with the assumption that furfural-based condensation products are more favored at higher temperatures.20 For the same reason, increasing the concentration of cellulose acetate did not increase AMF yield (Fig. 2B), while decreasing the amount of catalyst led to a decrease in AMF yield (Fig. 2D).
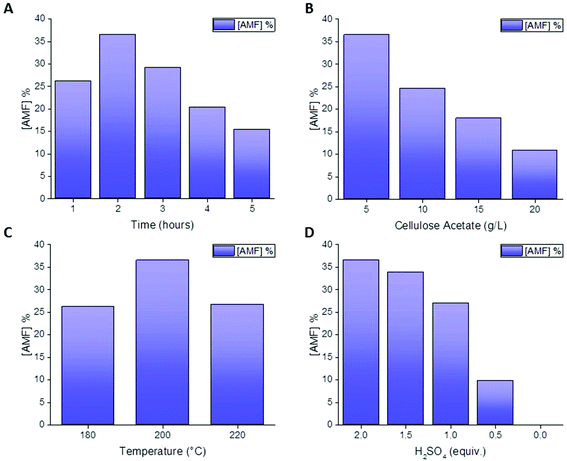 | ||
| Fig. 2 AMF yield for the conversion of cellulose acetate in acetic acid and acetic anhydride at different reaction times (A), cellulose acetate concentration (B), temperature (C) and catalyst concentration (D). For details of the reaction conditions please refer to the ESI.† | ||
Interestingly, the acetolysis of non-acetylated cellulose was performed as an additional control experiment under optimal conditions (2 eq. of H2SO4 for 2 hours at 200 °C). In this case, the desired product could be obtained in only 4%, demonstrating the crucial role of acetylation during AMF formation.
During the conversion of acetylated cellulose into AMF, the presence of acetyl groups on the hydroxyl functionality of each glucose unit (defined by the DS) does not prevent product formation. We speculated that the reaction can possibly proceed via standard cellulose hydrolysis followed by glucose–fructose isomerization (Scheme 1). For the latter step to occur, however, a partial formal deacetylation is required to generate an intermediate in which at least positions 1 and 2 on glucose are deprotected. Deacetylation can be the result of acyl transfer mediated by free hydroxyls. Alternatively, it could be mediated by traces of water present in the acetic acid, which can act as a nucleophile at high temperatures. In order to better estimate the impact of water and related acetylated species on the reaction, two additional experiments were carried out. Cellulose acetate was reacted under the optimized conditions but in the absence of acetic anhydride, which could act as a water scavenger as well as a capping agent. Alternatively, a second experiment was performed adding a slight excess of water to the reaction (10 equivalents), in order to potentially alter the DS. In both cases, we did not observe sensible changes in the yield of AMF, which was obtained in 35% and 34% yield for the reaction without acetic anhydride and with water respectively. According to these data, we concluded that slight changes in the water content, which may alter the DS in situ, do not play a major role in the reaction, and the use of acetic anhydride can be avoided in general. However, future detailed mechanistic studies will be required to confirm these assumptions.
As mentioned above, the acidic hydrolysis of cellulose acetate in acetic acid results in a light brownish clear solution with a maximum AMF yield of ca. 40%. Despite our efforts to identify potential C1–C5 byproducts of the reaction using GC-MS, NMR or HPLC-MS, we could only find traces (<5%) of acetylated glucose, fructose or levoglucosane, which did not allow us to close a mass balance. Acetic acid is an additional byproduct of the reaction, which originates via the cleavage of the acetyl groups and is added to the bulk of the solvent. Interestingly, levulinic acid and formic acid, which are among the most common byproducts reported during the aqueous degradation of HMF, were not observed. Although acetic acid has been reported to reduce the high molecular weight humin formation,21 we assumed that AMF could condense into soluble low molecular weight humin-like structures. In order to verify this hypothesis, we performed a stability experiment, reacting AMF (3 g L−1) in acetic acid (15 mL) at 220 °C for 6 hours. Interestingly, in the resulting light brownish clear solution no trace of AMF was detected by HPLC. In turn, when the solution was vacuum dried in a rotary evaporator, a solid black product was obtained. The FTIR spectra of this insoluble product (S11†), resembled the one of humic substances.22,23 In an integrated process such substances could be valorized either by gasification24 or liquefaction25 obtaining syngas and bio-oil, respectively.
So far, we have been able to show that AMF can be obtained in good yield using cellulose acetate as the starting material. Interestingly, the latter can be easily obtained by biomass fractionation protocols.8 Therefore, in order to evaluate the possible integration of this process into a biorefinery scenario, AMF production by treatment of cellulose acetate obtained by different biomass fractionation schemes was evaluated. Commercial cellulose, Organocat pulp26 and crude beech wood were acetylated (for details see the ESI†) and used for this purpose. Unlike the case of commercially available cellulose acetate, cellulose acetates with DS >2 were obtained (Table 1) via acetylation, as confirmed by NMR spectroscopy (S1–3†). Moreover, gel permeation chromatography revealed that the molecular weights of all the compounds were in the same range. This fact suggests that a previous fractionation of biomass is not required in principle, and acetylation itself could be used as an alternative wood pulping method for future exploitation of this process. The conversion of the prepared materials into AMF was tested in an autoclave reactor (Table 1) for 2 hours at 200 °C using 2 eq. of sulfuric acid as the catalyst.
| Substrate | Degree of substitutiona |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (Da) b (Da) |
Glucose units | AMF yield (%) |
|---|---|---|---|---|
| a Calculated by NMR. b Obtained by gel permeation chromatography. | ||||
| Commercial cellulose acetate | 1.75 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
127 | 36.43 |
| Acetylated cellulose | 2.67 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
36 | 39.74 |
| Acetylated organocat pulp | 2.74 | 9000 | 32 | 46.91 |
| Acetylated beech wood | 2.35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38 | 36.61 |
The as-prepared cellulose acetates afforded AMF with yields ranging from 36% to 47%. Commercial cellulose acetate resulted in a slightly lower yield, while acetylated pulp could be converted with the highest yield. All the prepared materials showed similar molecular weights, which are smaller compared to commercial cellulose acetate. However a slight change in the DS could be observed, ranging from 2.35 for acetylated wood to 2.74 for acetylated pulp. The AMF yield occurred to be higher for higher DS, going from 47% for acetylated pulp to 37% in the case of acetylated wood. However, although DS seemed to influence the AMF yield, future studies will be conducted to fully assess the role of substitution over a broad DS range, since slight variations in the DS might not be significant.
With the aim of increasing AMF yield through fine control of temperature and reaction time, the conversion of cellulose acetate into AMF was tested in a continuous flow reactor. Flow reactors are claimed to have better reproducibility and scalability, safer operability and are generally more viable for high pressure reactions.27 In this case, we focused initially on acetylated glucose as a model compound, and investigated its conversion at different temperatures (Fig. 3, black curve). It is noteworthy that AMF could be obtained in 82% yield at 175 °C in just 5 min of residence time. However, in line with our previous observations during batch studies, an increase in temperature resulted in decreased AMF yields already at 200 °C.
In order to shed light on this fact, the stability of AMF under the reaction conditions was tested and appeared to be critical above 200 °C. In fact, while AMF could still be recovered in 94% after 5 minutes at 175 °C, its recovery dropped to 55% and 45% at 225 °C or 250 °C respectively (Fig. 3, solid bars).
Based on these results, the conversion of cellulose acetate was optimized at 49% yield with a residence time of 5 minutes at 175 °C (Fig. 3, blue curve). Interestingly, higher residence time did not increase AMF yield (Fig. S12†). Comparing the flow reactor and batch results, it is important to emphasize the drastic reduction of the reaction time achieved using continuous processing. We attribute this difference to the lack of a temperature gradient and the reduced heat transfer limitations, which are in turn observed in the batch system. As a result, it was possible not only to improve the yields but also to reduce the required temperature and the processing time. In order to prove the scalability of this method, a time on stream experiment was performed running the reaction over 2 hours under the optimized conditions. The crude isolated after work up (see the ESI†) was mainly composed of AMF as confirmed by NMR analysis (Fig. S5†). Since continuous processing proved to be viable, we calculated qualitatively the costs for processing 1000 g of cellulose acetate (see the ESI†). At current laboratory prices (Sigma-Aldrich), taking into account a 49% yield, AMF can be obtained at 5.5 € g−1 against the commercial 14.3 € g−1, making the exploitation of this process and its further optimization reasonable. Furthermore, acetic acid is a convenient solvent for a variety of transformations. For instance, HMF oxidation to FDCA in acetic acid has been recently proposed,28 pointing to the development of integrated methods for the production of important HMF derivatives (e.g. FDCA) avoiding intermediate AMF separation. Finally, the presence of the acetyl functionality in AMF is expected to influence the reactivity of the furan system and to allow for the selective functionalization of the aldehyde group.
In conclusion, we demonstrated for the first time the acetolysis of cellulose acetate in the presence of sulfuric acid as an efficient method to produce AMF. The proposed method takes advantage of the good solubility of acetylated cellulose in acetic acid, enabling a homogeneous process, unlike the case of unprotected cellulose. The strategy works well also when crude acetylated biomass is used as the precursor, enabling a simple biorefinery scheme for the direct valorization of biomass into an important value added platform chemical. Finally, due to its homogeneous nature, we demonstrated that the method is compatible with continuous processing, opening a new scenario for the preparation of furans from biomass.
Acknowledgements
Open Access funding provided by the Max Planck Society.References
- S. G. Wettstein, D. Martin Alonso, E. I. Gürbüz and J. a. Dumesic, Curr. Opin. Chem. Eng., 2012, 1, 218–224 CrossRef CAS
.
- R. Rinaldi and F. Schüth, ChemSusChem, 2009, 2, 1096–1107 CrossRef CAS PubMed
.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed
.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed
.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519 RSC
.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed
.
-
T. Liebert, in Cellulose Solvents: For Analysis, Shaping and Chemical Modification, 2010, pp. 3–54 Search PubMed
.
- D. G. Barkalow, R. M. Rowell and R. a. Young, J. Appl. Polym. Sci., 1989, 37, 1009–1018 CrossRef CAS
.
- S. Fischer, K. Thümmler, B. Volkert, K. Hettrich, I. Schmidt and K. Fischer, Macromol. Symp., 2008, 262, 89–96 CrossRef CAS
.
- D. Esposito and M. Antonietti, Chem. Soc. Rev., 2015, 44, 5821–5835 RSC
.
- S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015 RSC
.
- A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G.-J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC
.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. a. Dumesic, Nature, 2007, 447, 982–985 CrossRef PubMed
.
- M. A. Mellmer, C. Sener, J. M. R. Gallo, J. S. Luterbacher, D. M. Alonso and J. A. Dumesic, Angew. Chem., Int. Ed., 2014, 53, 11872–11875 CrossRef CAS PubMed
.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed
.
- R.-J. Van Putten, J. C. van der Waal, M. Harmse, H. H. van de Bovenkamp, E. de Jong and H. J. Heeres, ChemSusChem, 2016, 9, 1827–1834 CrossRef CAS PubMed
.
- M. Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47, 7924–7926 CrossRef CAS PubMed
.
- E. S. Kang, Y. W. Hong, D. W. Chae, B. Kim, B. Kim, Y. J. Kim, J. K. Cho and Y. G. Kim, ChemSusChem, 2015, 8, 1179–1188 CrossRef CAS PubMed
.
- M. Bicker, D. Kaiser, L. Ott and H. Vogel, J. Supercrit. Fluids, 2005, 36, 118–126 CrossRef CAS
.
- I. Van Zandvoort, Y. Wang, C. B. Rasrendra, E. R. H. Van Eck, P. C. a. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745–1758 CrossRef CAS PubMed
.
-
P. Domínguez de María, Industrial Biorenewables. A critical viewpoint, Wiley-VCH Verlag, 2016 Search PubMed
.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS
.
- L. Shuai and J. Luterbacher, ChemSusChem, 2015, 133–155 Search PubMed
.
- T. M. C. Hoang, L. Lefferts and K. Seshan, ChemSusChem, 2013, 6, 1651–1658 CrossRef CAS PubMed
.
- Y. Wang, S. Agarwal and H. J. Heeres, ACS Sustainable Chem. Eng., 2017, 5(1), 469–480 CrossRef CAS
.
- P. M. Grande, J. Viell, N. Theyssen, W. Marquardt, P. Domínguez de María and W. Leitner, Green Chem., 2015, 3533–3539 RSC
.
- M. Trojanowicz, Talanta, 2016, 146, 621–640 CrossRef CAS PubMed
.
- B. Saha, S. Dutta and M. M. Abu-Omar, Catal. Sci. Technol., 2012, 2, 79–81 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc00975e |
| This journal is © The Royal Society of Chemistry 2017 |