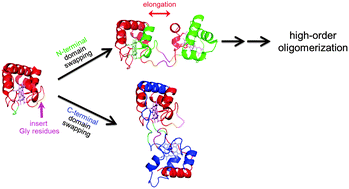
Chunguang Ren, Satoshi Nagao, Masaru Yamanaka, Hirofumi Komori, Yasuhito Shomura, Yoshiki Higuchi and Shun Hirota
Mol. BioSyst., 2015,11, 3218-3221
DOI:
10.1039/C5MB00545K,
Communication