Probing into the mechanisms of disinfection by-product formation from natural organic matter and model compounds after UV/chlorine treatment†
Abstract
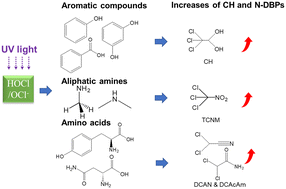
The UV/chlorine advanced oxidation process removes micropollutants efficiently, while the formation of disinfection by-products (DBPs) is a concern. The mechanisms of DBP formation triggered by UV/chlorine treatment were scrutinized in this study using natural organic matter (NOM) and seven model compounds (phenol, resorcinol, benzoic acid, methylamine, dimethylamine, asparagine, and tyrosine). It was found that compared to chlorination alone, the formation of most DBPs from NOM during post-chlorination treated by UV/chlorine increased by 10–50%, except chloral hydrate (CH) and trichloronitromethane (TCNM), which were doubled and tripled, respectively. In terms of model compounds in parallel experiments, both increases and decreases in DBP formation attributed to UV/chlorine were observed. Specifically, benzoate, the conjugate base of benzoic acid as a chlorine-resistant compound, was activated by radicals generated in the UV/chlorine process to form more DBPs, while resorcinol, as a highly reactive compound towards chlorine to form trichloromethane (TCM), was seen with an 18% decrease in TCM formation, since radicals eliminated intermediate products of resorcinol that are TCM precursors. Similarly, a 20% reduction of trichloroacetic acid (TCAA) formation was observed with phenol treated by UV/chlorine. In contrast, formation of CH from aromatic precursors was significantly increased by UV/chlorine in most cases. For model compounds containing nitrogen, UV/chlorine dramatically increased TCNM formation from methylamine and dimethylamine by about 500 and 200 times, with TCNM yields of 174 and 82 μg mgC−1, respectively, as UV/chlorine tended to oxidize aliphatic amines to nitro compounds. UV/chlorine promoted amino acids, including tyrosine and asparagine, to produce dichloroacetonitrile (DCAN) and dichloroacetamide (DCAcAm) by up to 200%. Compared to regulated DBPs such as TCM and TCAA, the significant formation of CH and nitrogenous DBPs associated with UV/chlorine treatment needs close scrutiny when considering this process being applied in practice.
- This article is part of the themed collection: Environmental Science: Water Research & Technology Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...