Synthesis of disulfide surrogate peptides incorporating an ethylene glycol bridge†
Abstract
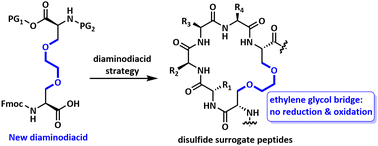
The replacement of disulfide bonds with metabolically stable isosteres by a diaminodiacid strategy has been demonstrated as an effective and flexible method to improve the stability of disulfide-rich peptides. In this study, we report a new diaminodiacid containing ethylene glycol bridge (EG-DADA), obtained primarily through the ring-opening addition reaction of aziridine derivatives with hydroxyl groups. The practicability and oxidation stability of this novel diaminodiacid have been illustrated by the synthesis of disulfide bond mimics of tachyplesin I and α-ImI. The development of EG-DADA enriches the ether-based diaminodiacids and provides a flexible option for the construction of disulfide surrogate peptides.
- This article is part of the themed collection: New Journal of Chemistry HOT Articles


 Please wait while we load your content...
Please wait while we load your content...