Kinetics, products and pathways for the removal of pentachlorophenol (PCP) by sulfidated nanoscale zero-valent iron (S-nZVI)†
Abstract
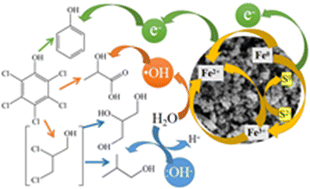
pentachlorophenol (PCP) is a kind of chlorinated aromatic contaminant that brings challenges from an environmental perspective. However, the performance of sulfidated nanoscale zero-valent iron (S-nZVI) in PCP removal remains unknown. In this study, S-nZVI was applied in the treatment of PCP-polluted water under different water chemistry conditions. The results showed that PCP was removed under the combined action of adsorption, dechlorination, and ˙OH-mediated oxidation, among which dechlorination played a leading role while oxidation lagged behind. The amount of adsorbed PCP correlated well with the PCP dechlorination rate constant (kred), except for S/Fe 0 and 0.1 and acidic initial pH (pHinitial) conditions. At neutral and alkaline pHinitial, kred increased with an increase in pHinitial due to the formation of a hydroxide layer that facilitates adsorption. At acidic pHinitial, kred increased with a decrease in pHinitial because hydrogen ions diffusing in the solution participated in the reaction. The effects of solution chemistry, such as anions, organic matter, and oxygen conditions, on PCP adsorption and transformation were also studied. Eight degradation byproducts were observed; the dechlorination products suggested that PCP dechlorination by S-nZVI occurred via a sequential pathway. PCP and its dechlorination products would then undergo ˙OH-mediated oxidation, resulting in products that are beneficial for microbes. Changes to S-nZVI after reacting and the role of sulfur were also discussed. This study confirmed that S-nZVI can be effective in the remediation of PCP-polluted groundwater. Further research is needed to optimize this remediation via coupling microbial technology and controlling the oxygen conditions.
- This article is part of the themed collection: Environmental Science: Water Research & Technology Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...