Comprehensive theoretical and experimental study of near infrared absorbing copolymers based on dithienosilole†
Abstract
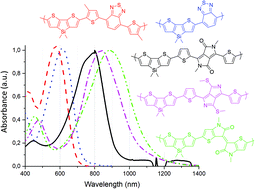
In recent contributions [H. Medlej, H. Awada, M. Abbas, G. Wantz, A. Bousquet, E. Grelet, K. Hariri, T. Hamieh, R. C. Hiorns and C. Dagron-Lartigau, Eur. Polym. J., 2013, 49, 4176; W. Khelifi, H. Awada, K. Brymora, S. Blanc, L. Hirsch, F. Castet, A. Bousquet and C. Lartigau-Dagron, Macromolecules, 2019, 52, 4820], we reported a series of low band gap copolymers with the objective of shifting the absorption from the visible to the near infrared range. This polymer family is based on the combination of the dithienosilole (DTS) electron-rich unit with different electron-withdrawing units, namely benzothiadiazole (BT), 4,7-di(thiophen-2-yl)-2,1,3-benzothiadiazole (DTBT), diketopyrrolopyrrole (DPP) and diazapentalene (DAP). In the present report, we extend this family by designing a new copolymer alternating the DTS donor with the thienoisoindigo (TII) acceptor. The experimental characterization studies are rationalized by means of DFT calculations, which provide structure–property relationships linking absorption properties of the various copolymers to the electronic structure of the ground and first excited states. To enable more complete analyses, we also carried out DFT calculations on ten supplementary copolymers based on electron-rich monomer analogues of DTS, cyclopentadithiophene (CPDT) and dithienopyrrole (DTP). Electrochemical and optical properties of the DTS-TII copolymer are compared to those of copolymers incorporating BT, DTBT, DPP and DAP accepting units. We show that this new copolymer exhibits improved near infrared light harvesting in both solution and thin films, which makes this a material of interest for a variety of optoelectronic applications.


 Please wait while we load your content...
Please wait while we load your content...