Efficient palladium catalysis for the upgrading of itaconic and levulinic acid to 2-pyrrolidones followed by their vinylation into value-added monomers†
Abstract
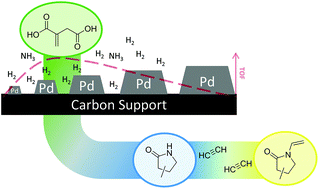
The production of monomers from bio-based platform chemicals shows great potential to reduce the chemical industry's demand for fossil resources. We herein present a two-step approach, which yields N-vinyl-2-pyrrolidone monomers from bio-based carboxylic acids, such as itaconic and levulinic acid. A highly active, heterogeneous palladium catalyst facilitating the reductive amidation of itaconic acid (TOF = 950 molPyr·molPd_surface−1 h−1) as well as the reductive amination of levulinic acid (TOF = 4000 molPyr·molPd_surface−1 h−1) was designed. Especially the reductive amidation of itaconic acid to 3- and 4-methyl-2-pyrrolidone was found to be structure sensitive. A clear trend between Pd particle size and catalyst activity could be shown by the synthesis of Pd/C catalysts with varying Pd particle sizes. The vinylation of the synthesized methyl-2-pyrrolidones with acetylene was tested using common industrial conditions (10–18 bar acetylene, 150 °C, KOH catalyst, no solvent). Similar to the industrial vinylation of 2-pyrrolidone, good yields of up to 80% N-vinyl-methyl-2-pyrrolidone were received. Therefore, and due to the excellent maximum yield of methyl-2-pyrrolidones in reductive amidation (95 mol%), the envisioned process can be a promising drop-in technology, directly replacing fossil resources in the production of an established monomer class.


 Please wait while we load your content...
Please wait while we load your content...