Possible relations between supercooled and glassy confined water and amorphous bulk ice
Abstract
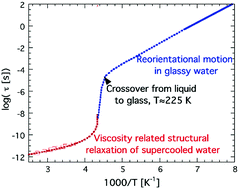
In this paper we discuss apparent contradictions in the literature between dynamical results on supercooled confined water obtained by different experimental methods. The reason for the lack of a clear glass transition of confined water is also discussed. Dielectric relaxation data and results from differential scanning calorimetry measurements provide a consistent picture, but it is still unclear why the glass transition related structural (α) relaxation disappears before the normal time-scale of a calorimetric glass transition (i.e. about 100 s) is reached. From recent results on amorphous bulk ice we propose that this anomalous phenomenon may not be an effect of confinement, but an intrinsic property of water when it transforms to a crystal-like glassy state, probably around 225 K. Thus, the results from the studies of confined water in the so-called no man's land (the temperature range 150–235 K) where bulk water rapidly crystallizes may be of more relevance for supercooled and glassy bulk water than previously thought. Furthermore, the structural difference between glassy water (or amorphous ice) and crystalline ice is likely to be rather small, due to the large degree of disorder in crystalline ice.
- This article is part of the themed collection: PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...